Consultation: Registration of Clinical Trials and Public Disclosure of Results: Draft Guidance and Public Search Portal
From Health Canada
Current status: Closed
This consultation ran from February 23, 2023 to April 24, 2023.
Health Canada has developed a draft guidance document and is proposing a clinical trials search portal. This portal would help make more Canadian authorized trial information available to the public and improve accessibility of this information.
Health Canada is seeking your feedback on:
- Draft guidance describing policy expectations for sponsors of authorized clinical trials to register and report the results of their trials with international registries
- Proposed mock-ups of a new clinical trials search portal
Who was the focus of this consultation
We engaged with:
- health professionals
- patients and their caregivers and patient advocate organisations
- researchers investigating drugs, medical devices and natural health products (NHPs) in humans
Goals of the consultation
Health Canada is committed to improving the transparency of Canadian clinical trial information. This consultation advances priority work under the Department's Forward Regulatory Plan for 2022-2024 to modernize the clinical trials regulatory framework.
In spring 2021, Health Canada held a consultation on its proposed Clinical Trials Regulatory Modernization Initiative to gather feedback from stakeholders. A description of the transparency proposals was included. Stakeholder feedback has informed the development of the draft guidance document and proposed public search portal.
The draft guidance outlines Health Canada's policy expectations for sponsors of authorized clinical trials investigating drugs (pharmaceutical, biologic and radiopharmaceutical), medical devices and NHPs. This policy should give sponsors time to adjust to the process for registration of trials and reporting results using international registries, before regulations are proposed in Canada.
To complement this work and improve public accessibility, Health Canada is building a search portal to replace the existing Clinical Trials Database. All Canadian authorized clinical trials would be included, with phased implementation. During the first phase of the portal, the department will publish information about authorized drug trials. Future phases will include information about trials investigating medical devices and NHPs.
Consultation: Health Canada's Clinical Trials Regulatory Modernization Initiative
Forward Regulatory Plan 2022-2024: Modernization of the Regulation of Clinical Trials
Key questions for your input
Ideas and inputs were sought around 3 themes/topics:
- Draft guidance: Registration of Clinical Trials and Public Disclosure of Results: Transparency of Health Canada-authorized clinical trials
- Series of mock-up images of the proposed clinical trials portal
Mock-up 1
Image Text View or download 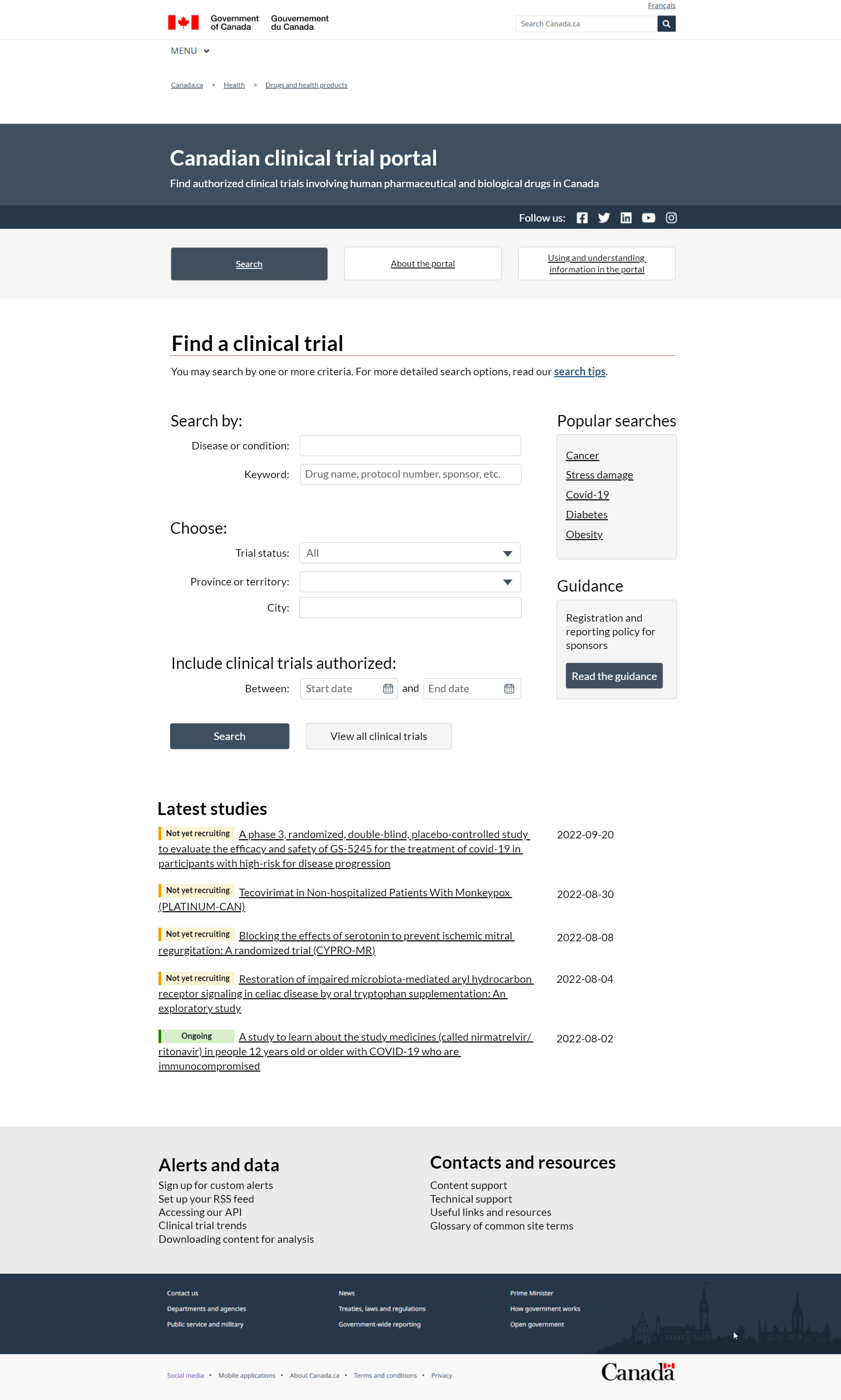
FIGURE 1: Clinical trial portal home page – Text description
This is an image of the new Canadian Clinical Trial Portal home page. The top header with a dark blue background says the portal's purpose is to help people find authorized clinical trials on pharmaceutical and biological drugs for human use in Canada. The header to the right has social media links to Health Canada's Facebook, Twitter, LinkedIn, YouTube and Instagram channels. Under the header, there are 3 buttons so you may navigate quickly between the Search, About the portal and Using and understanding information in the portal pages. The Search button, which is dark blue, indicates you are on that page. The About the portal link takes you to corporate information. The Using and understanding information page gives instructions on how the interface works and how to navigate the features of the system.
Under the 3 navigation buttons is the main search interface for the Clinical Trial Portal, where you can search for a Canadian clinical trial. The Search tips page gives you tips to help you with your search. You may search for clinical trials by:
- specific words
- status and location
- a certain date range
The first criteria under Search by is Disease or condition. It is an open text box with autofill so you can match the medical condition to the terms in the database. The second criteria is Keyword, which allows you to search all the content contained within a record. This could be by drug name, protocol number, sponsor or anything else that is of interest. The second section titled Choose lets you refine your search further by Trial status and/or by Province or territory. You are also able to drill down to City, for more relevant results. City is conditional based on the province or territory you choose. The final search criteria is by date, which means you can include Clinical trials authorized between 2 dates of your choice. Two buttons follow the search criteria. The first button, called Search, runs the search based on the entered criteria, taking you to a new page with your search results. The second button, called View all clinical trials, allows you to skip the search and see all the available Canadian clinical trials in a new page.
To the right of the search criteria section is a highlight box with the top search terms. This Popular searches box lists the top 5 searched terms by users in a set time period. We have given you an example to show you how this works. Our example lists 5 hyperlinks for 5 conditions as possible popular search options:
- cancer
- stress damage
- COVID-19
- diabetes
- obesity
Clicking one of these links will take you to a results page pre-filtered on that specific keyword. Beneath the Popular searches section on the right is another highlight box called Guidance. The box reads Registration and reporting policy for sponsors. Below this is a button that says Read the guidance. Clicking this button will take you to the guidance document on the registration of clinical trials and public disclosure of results.
The next section on the page located below the search criteria is called Latest studies. This section will highlight the last 5 authorized clinical trials to receive their no objection letter. Each study is labelled with its status, official protocol title and date of authorization. The title is hyperlinked to the record if you want more detail.
The footer is the final section of the clinical trial portal home page. Here, there are 2 sections organized in 2 columns. Each column has a set of useful hyperlinks to help you understand and use the system. The first section on the left is called Alerts and data and contains 5 options:
- sign up for custom alerts
- set up your R-S-S feed
- access our A-P-I
- see clinical trial trends
- download content for analysis
The second section in the footer has Contacts and resources, where you can find:
- contact information
- technical support
- useful links and resources
- glossary of common site terms
The standard Canada.ca footer is under this section.
Mock-up 2
Image Text View or download 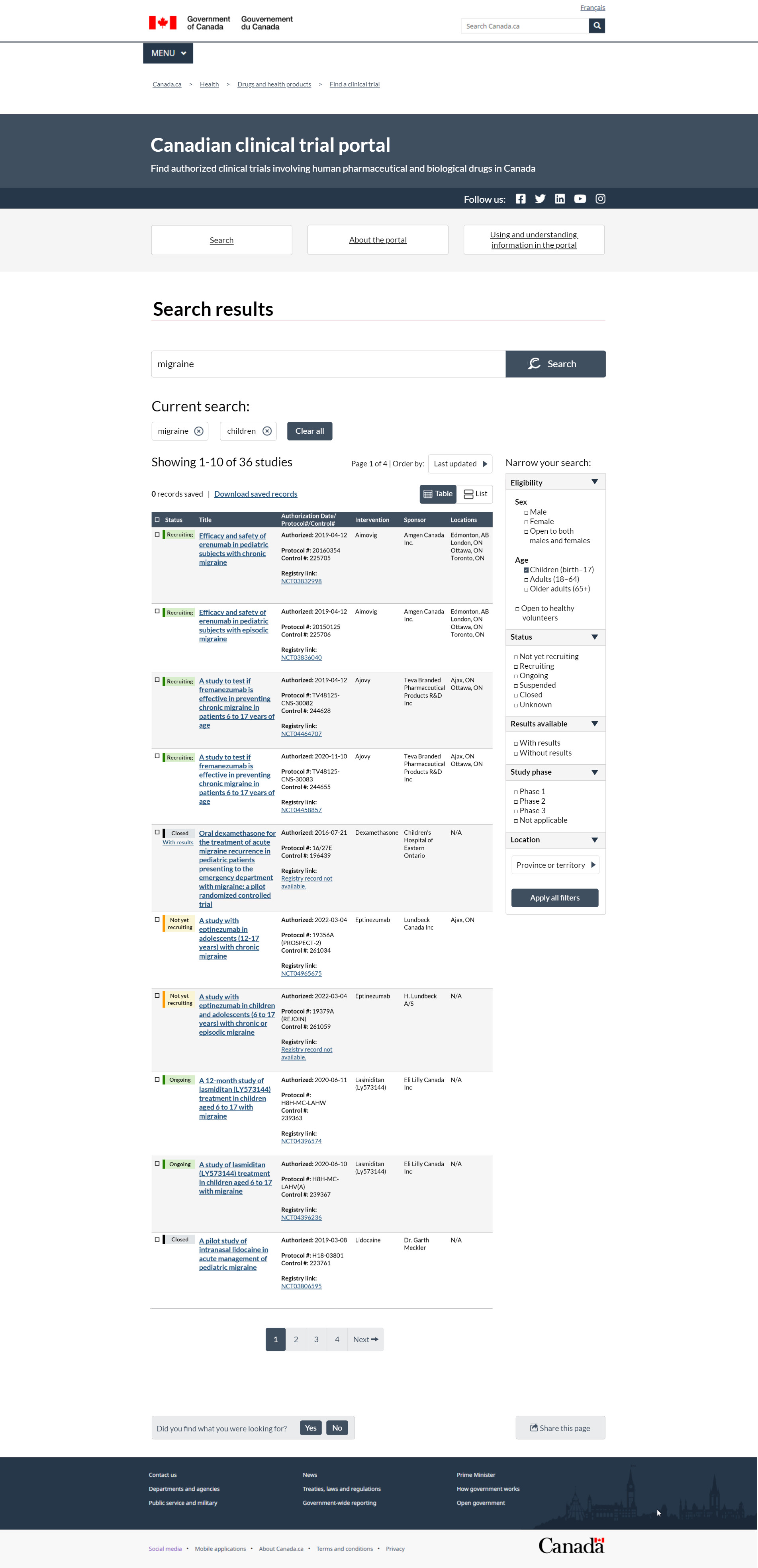
FIGURE 2 – Search results page, table view
This image represents the Search results page for the new Canadian clinical trial portal. At the top is a header with a dark blue background. It says the portal's purpose is to help people find authorized clinical trials on pharmaceutical and biological drugs for human use in Canada. The header to the right has social media links to Health Canada's Facebook, Twitter, LinkedIn, YouTube and Instagram channels. Under the header are 3 buttons that help you move quickly between the Search, About the portal and Using and understanding information in the portal pages. The Search button takes you to the main search. The About the portal link takes to you to corporate information. The Using and understanding information page tells you how the interface works and how to navigate the portal.
In this example, we show what the results look like if you manually entered the term 'migraine' from the Search page and then used the filters on the Search results page to choose the age group for children.
Under the header is the title of this page, called Search results. The search box follows the page title. It has a search button to the right where you can apply the search terms. The input box contains the word 'migraine', which would have been entered on the clinical trial Search page. Under the search box, there is a heading called Current search. Under this heading are 2 boxes, one with the term migraine and the other with the word children. You can refine your search by excluding either term by clicking on the X in the box. To the right of the current search terms is a Clear all button, if you need to reset the search. The results are below the search terms. The total number of results are listed first. In this example, the search results are showing 1 to 10 of 36 studies. It is organized on 4 pages and you are currently on page 1. To the right of the results total is a drop-down. You can use the drop-down to order your results in 5 different ways, by:
- last updated
- date authorized
- status
- intervention
- sponsor name
Status, Intervention and Sponsor name are organized in alphabetical order. The Last updates and Date authorized options are listed in chronological order from newest to oldest. Beneath this section, there is a box to the right that reads 0 records saved as well as a hyperlink to Download saved records. At the end of this section is a toggle feature. It displays the results in a table view, which is the current view of this page, or in a list that stacks information instead of displaying it side by side. The results table is made up of 10 rows, 1 record per row and 8 columns. In the dark blue header in column 1, there is a checkbox where you can choose all the displayed records to make them available for download. Column 2 is the trial's overall Status. Column 3 is the short Title. Each record's short Title is hyperlinked to the more detailed record page. Column 4 gives you the:
- authorized date, which is the date of the no objection letter
- protocol number
- control number
- registry link, which is the hyperlinked ClinicalTrials.gov record number
The Intervention column identifies the name of the drug being tested. The Sponsor column identifies the name of the company or organization that is responsible for the clinical trial. The last column lists each of the Locations where a clinical trial is taking place.
A set of filters called Narrow your search to the right of the table gives the search results. You can use any of the 5 filters to refine your search further. The first filter called Eligibility includes the following options:
- sex: male, female or both
- age: children from birth to age 17, adults aged 18 to 65 or older adults over the age of 65
- if the study is open to healthy volunteers
In this example, children was chosen. The second filter is Status where you were able to refine your search results using 1 or more of the 6 statuses available:
- not yet recruiting
- recruiting
- ongoing
- suspended
- closed
- unknown
You can also narrow the search if results are available and by Study phase. Study Phase has 4 options: phase 1, 2 or 3 or if phase is not applicable. The final filter is Location where you can drill down by province and territory. City is a contextual field that appears based on the province or territory chosen. This is followed by an Apply button that allows you to activate other search parameters.
The content in the results table shows you what search results might look like for 3 different scenarios. In the second row, we have the first example of a clinical trial recruiting participants. In the first column of this row, you can choose the record for download using the select box. We did not choose it for this example. In the next column, the status of this record is Recruiting. There is a green box behind the status, and each status is coded with a corresponding colour. In the third column, the title of this study is called Efficacy and safety of erenumab in pediatric subjects with chronic migraine. The title is hyperlinked to take you to the record view of this clinical trial. In column 4, we tell you that the study was authorized on April 4, 2019, its protocol number is 20160354 and its control number is 225705. The registry record number is also listed. It is NCT03832998 and is linked to ClinicalTrials.gov, where the clinical trial was registered. The type of intervention is in column 5, and for this trial it is Aimovig. The sponsor is Amgen Canada Inc., noted in column 6. And in the last column, clinical trials are taking place in Edmonton, Alberta; London, Ontario; Ottawa, Ontario; and Toronto, Ontario.
The second example is a closed clinical trial. In column 1, you can choose the record for download using the select box. It has not been chosen in this example. In column 2, the status of this record is Closed with results. Behind the status is a box, where the words with results are hyperlinked to the record view Results section. In column 3, is the title of this study called Oral dexamethasone for the treatment of acute migraine recurrence in paediatric patients presenting to the emergency department with migraine: a pilot randomized controlled trial. The title is hyperlinked to take you to the record view of this clinical trial. In column 4, you will note that the study was authorized on July 21, 2016, its protocol number is 16–27E and its control number is 196439. This particular record did not have a registry link. The type of intervention is in column 5, which for this trial is dexamethasone. The sponsor Children's Hospital of Eastern Ontario is given in column 6. And in column 7, there are no locations indicated as this is a closed trial. It is labelled not applicable.
In the third and final example is a study that is not yet recruiting. In column 1, you can choose the record for download using the select box. It is has not been chosen in this example. In column 2, the status of this record is Not yet recruiting. Behind the status is a yellow box. In column 3, the title of this study is called A study with eptinezumab in children and adolescents 6 to 17 years with chronic or episodic migraine. The title is hyperlinked to take you to the record view of this clinical trial. In column 4, you will note that the study was authorized on March 4, 2022, its protocol number is 19356A (PROSPECT-2) and its control number is 261059. The registry record number is also listed. It is NCT04965675 and is linked to ClinicalTrials.gov, where the clinical trial was registered. The type of intervention is in column 5, which for this trial is eptinezumab. The sponsor is Lundbeck Canada Inc., noted in column 6. Finally, in column 7, the clinical trial is taking place in Ajax, Ontario.
At the end of the results table is page navigation where you can view all the results. The navigation is followed by a feedback mechanism where you can click either yes or no and give written feedback if there was an issue. You can also share the results of your search through a variety of social media channels by using the Share this page button, which is to the right of the Did you find your what you were looking for feedback mechanism.
Mock-up 3
Image Text View or download 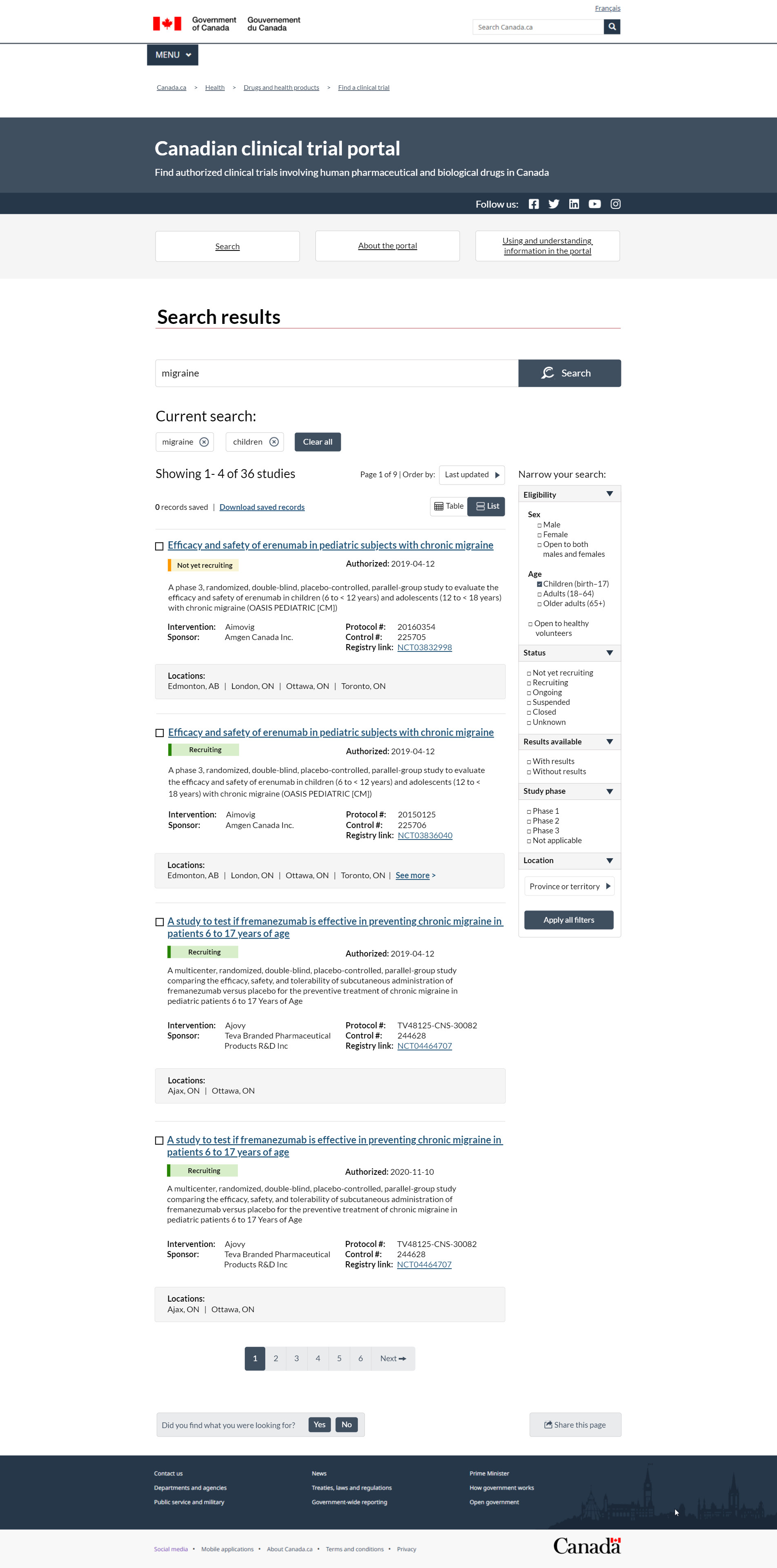
FIGURE 3 – Search results page, list view
This image represents the Search results in a list view. The top header with a dark blue background says that the portal's purpose is to help people find authorized clinical trials involving human pharmaceutical and biological drugs in Canada. The header to the right has social media links to Health Canada's Facebook, Twitter, LinkedIn, YouTube and Instagram channels. Under the header are 3 buttons so you can navigate quickly between the 3 pages. The Search button takes you to the main search. The About the portal button takes to you to corporate information. The Using and understanding information button takes you to instructions on how the interface works and how to navigate the system.
In this example, we show what the results look like if you manually entered the term migraine from the Search page, then used the filters on the Search results page to choose the age group children.
Under the header we see the title of this page: Search results. The search box has a search button that you can use to apply the search terms. The input box contains the word migraine, which would have been entered on the clinical trial Search page. Under the search box is a second heading called Current search. Under this heading are 2 boxes. One has the term migraine and the other has the word children. You can refine the search results by excluding either term by clicking on the X in the box. To the right of the current search terms is a Clear all button to reset the search. The results are below the search terms. The total number of results are listed first. In this example, the search results are showing 1 to 4 of 36 studies. It is organized on 6 pages and you are currently on page 1. To the right of the results total is a drop-down. The drop-down allows you to order your results 5 different ways:
- last updated
- date authorized
- status
- intervention
- sponsor name
Status, Intervention and Sponsor name are organized in alphabetical order. The Last updates and Date authorized options are listed in chronological order from newest to oldest. A box under and to the right of this section reads 0 records saved alongside a hyperlink to Download saved records. At the end of this section is a toggle to display the results in either a table or in a list that stacks information instead of displaying it side-by-side. This page is currently toggled on to list view, as indicated by the dark blue slider. The results list is made up of 4 rows, 1 record per row.
A set of filters labelled Narrow your search can be found to the right of the list of search results. There are 5 filters in this section. Your first option is to narrow your search by eligibility. This includes:
- sex: male, female or both
- age: children from birth to age 17, adults aged 18 to 65 or older adults over the age of 65
- if the study is open to healthy volunteers
Children has been selected in this example. The second filter is Status where you are able to refine your search results by studies that are:
- not yet recruiting
- recruiting
- ongoing
- suspended
- closed
- unknown
You can also filter your search if results are available and by Study phase. Study Phase has 4 options available, if the trial is in phase 1, 2 or 3 or if the phase does not apply. The final filter is Location where you are able to drill down by province and territory. City is a contextual field that appears based on the province or territory chosen. You can use an Apply button to enhance your search.
The main results list is in paragraph format, intended to be easily scalable for different devices. The Short title is the main record heading. It is hyperlinked text that opens the record view on a new web page. In the next row, you can see the colour-coded Status. To the right of the status is the Authorized date, which is the date the trial received its no objection letter. The Official protocol title follows the status and authorized date. After this section are 2 columns. Column 1 contains the Intervention name and Sponsor. Column 2 gives the Protocol number, Control number and Registry link. The Registry link opens the record as it appears on the ClinicalTrials.gov website. In a highlight box under the 2 columns you will find the Locations for where a clinical trial is taking place. A line separates each record. The records can be navigated with pagination similar to the table view.
This image has examples of 2 different statuses. The first is an example that is a Not yet recruiting record. Before the short title is a select box that can be used to choose this record for download. The short title of the study is the Efficacy and safety of erenumab in pediatric subjects with chronic migraine. On the next line are the status and authorized date. The study status is Not yet recruiting. The status has a yellow box behind it. The authorized date is April 12, 2019. The official protocol title is next. This record is called A phase 3 randomized double-blind placebo controlled parallel group study to evaluate the efficacy and safety of a erenumab in children 6 to less than 12 years and adolescents 12 to less than 18 years with chronic migraine (OASIS PAEDIATRIC [CM]). The intervention name is Aimovig. The sponsor is Amgen Canada Inc. The protocol number is 20160354 and the control number is 225705. The registry link is NCT03832998. It opens the record in the ClinicalTrials.gov website, where the study was originally registered. The locations where this trial is running are Edmonton, Alberta; London, Ontario; Ottawa, Ontario; and Toronto, Ontario.
The second example in the results list is a recruiting trial. This record is similar to the previous record, but its reference numbers are different. Before the short title is a select box that you can use to choose this record for download. The short title of the study is the Efficacy and safety of erenumab in pediatric subjects with chronic migraine. On the next line are the status and authorized date. The study status is Recruiting. The status has a green box behind it. The authorized date is April 12, 2019. The official protocol title is next. This record is called A phase 3 randomized double-blind placebo controlled parallel group study to evaluate the efficacy and safety of a erenumab in children 6 to less than 12 years and adolescents 12 to less than 18 years with chronic migraine (OASIS PAEDIATRIC [CM]). The intervention name is Aimovig. The sponsor is Amgen Canada Inc.
The protocol number is 20150125 and the control number is 25706. The registry link is NCT0386040. It opens the record in the ClinicalTrials.gov website, where the study was originally registered. The locations where this trial is running are Edmonton, Alberta; London, Ontario; Ottawa, Ontario; and Toronto, Ontario. This clinical trial has more locations that could not fit in the highlight box. You can use the See more hyperlink to find all of the contacts and locations for this trial.
At the end of the results list is page navigation to allow you to view all the results. There are 6 pages in the navigation. After this section is a feedback mechanism where you can click either yes or no and provide written feedback if there was an issue. You can also share the results through a variety of social media channels by using the Share this page button to the right of the Did you find your what you were looking for feedback mechanism.
Mock-up 4
Image Text View or download 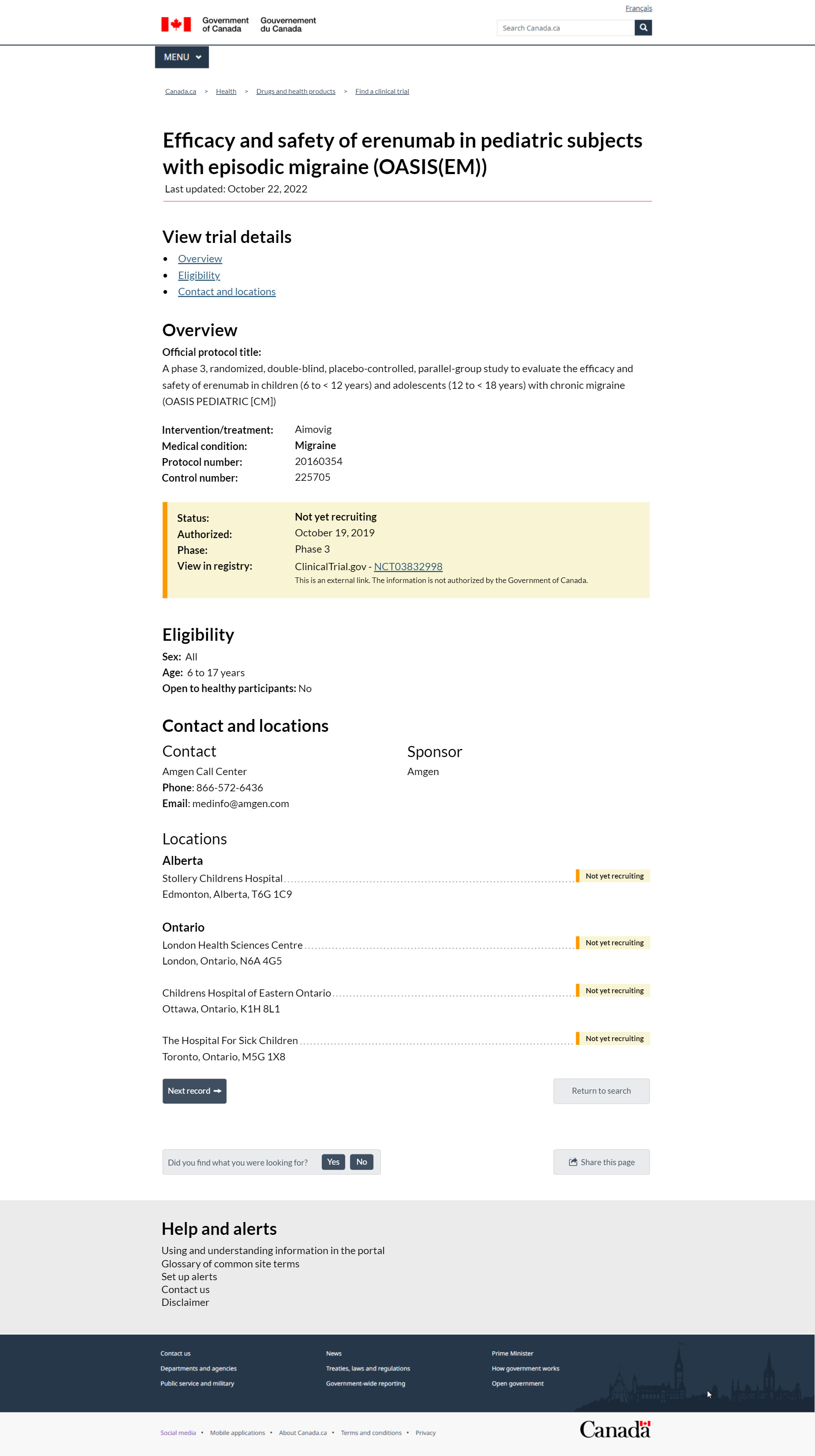
FIGURE 4 – Record view
This is a view of a record in the Clinical Trials Portal. The title of this record is Efficacy and safety of erenumab in pediatric subjects with chronic migraine (OASIS(EM)). It was last updated October 22, 2022. The first heading in this record is View clinical trials. You can go to any of the linked headings listed in this section. In this example, we can quickly access the Overview, Eligibility or Contact and location sections. In the Overview section, we start with the official protocol title, A phase 3 randomized double-blind placebo controlled parallel group study to evaluate the efficacy and safety of a erenumab in children 6 to less than 12 years and adolescents 12 to less than 18 years with chronic migraine (OASIS PAEDIATRIC [CM]). The intervention being tested is Aimovig and the medical condition being treated is migraine. The protocol number is 20160354 and the control number is 225705. In a large yellow status box, the status is listed as Not yet recruiting. The box also has the authorized date or the date when the no objection letter was received, which was October 19, 2019. This study is in Phase 3. The registry link is NCT03832998. Clicking the registry number opens the record in the ClinicalTrials.gov website where the study was registered. There is a note that tells you this is an external link. The information is not authorized by the Government of Canada.
The Eligibility criteria for this record is the next heading. The eligibility criteria for this trial are that it takes both men and women, it is open to children aged 6 to 17 years old and it is not open to healthy participants. The next section, Contact and location, lists the contact as the Amgen Call Center. The phone number is 866-572-6436. You can also reach the call center by sending an email to medinfo@amgen.com. The sponsor listed for this trial is Amgen. There are several locations where this trial is taking place. Under this, we have listed the locations in Canada where the clinical trial is taking place. For each location, a more specific status has been identified to the right using both a label and corresponding colour. In Alberta, this trial will be available at Stollery Children's Hospital in Edmonton, Alberta. It is not yet recruiting. In Ontario, there are 3 locations:
- London Health Sciences Centre in London
- Children's Hospital of Eastern Ontario in Ottawa
- Hospital for Sick Children in Toronto
None of the locations in Ontario is recruiting yet. Each status box is yellow. Results would follow this section, if available.
Under the location, you will find 2 buttons. The Next record button on the left allows you to navigate from one record to the next from your search results. A Return to search button on the right allows you to restart your search if you wish to search for a new set of parameters.
Next is a feedback mechanism where you can say if you found what you were looking for. You can click either yes or no and provide written feedback if there was an issue. You can also share the results through a variety of social media channels using the Share this page button. This button is to the right of the Did you find your what you were looking for feedback mechanism.
After these buttons there is a footer for the page called Help and alerts. Under this title are 5 hyperlinks that take you to 5 web pages:
- using an understanding information in the portal
- the glossary
- the alerts and notification system
- the contact information for the clinic for the critical trial portal
- content disclaimer
This section is followed by the Canada.ca standard footer.
- Clinical trial information practices
The input that was gathered through this process will be used to:
- further the development of the clinical trials portal
- finalize the policy for registration and reporting of results for authorized clinical trials in Canada
Related information
Contact us
Bureau of Policy, Science and International Programs
Pharmaceutical Drugs Directorate
Health Products and Food Branch
Health Canada
1600 Scott Street
Holland Cross, Tower B
2nd Floor, Address Locator 3102C5
Ottawa, Ontario
K1Y 4N7