Industry Guide on Mandatory Reporting under the Canada Consumer Product Safety Act - Section 14 "Duties in the Event of an Incident"
This document is not intended to substitute for, supersede, or limit the requirements under the Canada Consumer Product Safety Act. In case of any discrepancy between this guidance document and the Act, the Act will prevail. This guide may be updated from time to time.
Under the Act, you – as industry stakeholders - are responsible for making sure the consumer products you manufacture, import, sell or advertise are safe. You are also responsible for reporting health- or safety-related incidents involving any of these consumer products to Health Canada and the person from whom you received the product. Section 14 of the Canada Consumer Product Safety Act (referred to in this guide as "the Act") defines what an incident is and outlines the reporting timelines that you must meet in order to comply with the law.
The purpose of this guide is to help you understand your obligations under Section 14 of the Act.
- 1. Scope of the CCPSA
- 2. Mandatory reporting
- 3. Who must report
- 4. When mandatory reporting is required
- 5. What to report
- 6. When to report
- 7. How to report incidents to Health Canada
- 8. After you submit a 14(3) report to Health Canada
- 9. Some information on Confidential Business Information and Private Information under the Act
1. Scope of the CCPSA
The Act applies to consumer products as defined in Section 2 of the Act:
- consumer product means a product, including its components, parts or accessories, that may reasonably be expected to be obtained by an individual to be used for non-commercial purposes, including for domestic, recreational and sports purposes, and includes its packaging.
Some consumer products are not subject to the Act. These are listed in Schedule 1 of the Act and include cosmetics, drugs, medical devices, food, pest-control products and vehicles.
2. Mandatory reporting
Under section 14 of the Act, industry must report health or safety incidents involving a consumer product to the people specified under each of subsection 14(2) and 14(3). Because the law requires you to do this, it is referred to as mandatory reporting.
Mandatory reporting acknowledges the important role that you play in product safety and provides Health Canada with a broad understanding of health or safety-related incidents occurring with consumer products.
3. Who must report
If you sell, import or manufacture consumer products in Canada for commercial purposes, you must report incidents to both Health Canada and to the person who supplied you with the product. Under the Act, 'sell' includes giving away or distributing consumer products and homemade items.
For example, if you are an importer who imports from a manufacturer and sells products in Canada, you must report any incidents to both Health Canada and to the manufacturer.
If you carry out more than one activity, you report at the highest level of trade in the supply chain. For example:
- if there is an incident with a product that you import and sell in Canada, you submit an incident report as an importer
- if there is an incident with a product that you manufacture and sell in Canada, you submit an incident report as a manufacturer
4. When mandatory reporting is required
All incidents involving consumer products must be reported to Health Canada. Subsection 14(1) of the Act defines an incident. You must report anything that meets this definition.
You may learn about something that has happened with one of your consumer products in different ways such as:
- from a consumer, through product returns, complaints, or liability lawsuits or claims
- from within your organization or from other levels of the supply chain
- from reports authored by experts, test reports or information from scientific or other studies
- from governments (including Health Canada)
- from bodies that set standards or certify products
- from consumer advocates, associations or media
When something happens, this is called an 'event'. Some events are incidents, some are not. When you hear about an event involving your consumer product, you are responsible for determining if it is an incident. Even if no death or serious injury occurred, it may still meet the definition of an incident. This is because subsection 14(1) of the Act defines incident to include the reasonable potential for death or serious adverse health effects.
If there was an incident, you need to report this. If in doubt, report.
Below is guidance on interpreting the definition of an incident, which includes 4 scenarios.
4.1 Paragraph 14(1)(a) – an occurrence
An occurrence is something that happens in relation to your product.
You must report if there is:
- a death involving your consumer product
- someone suffers a serious adverse effect to their health from your consumer product
- someone may reasonably have been expected to die from your consumer product
- someone may reasonably have been expected to suffer serious adverse health effects from your consumer product
Example
A mother is pushing a stroller with her baby buckled inside. Suddenly, the front wheel shaft breaks. The stroller veers off the path, the mother falls and the stroller tips over on the road. The mother is not injured but the baby's head comes within a few centimeters of hitting the pavement. Although no one is seriously hurt, there was potential for serious injury. You are expected to report this incident. Even if no death or serious injury occurred, it still meets the definition of an incident. This is because the Act defines incident to include the reasonable potential for death or serious adverse health effects.
4.2 Paragraph 14(1)(b) – defect or characteristic
A defect is something that causes weakness or failure in the product or how it works. For example, defects can occur due to manufacturing errors or poor materials.
A characteristic is something that is inherent to the product's contents, construction, finishes or design.
You must report if a defect or characteristic of your consumer product may reasonably be expected to result in the death of an individual or in serious adverse effects on the health of an individual.
Example
You receive a complaint about a plastic part on the buckle of high chairs that you manufacture. The customer tells you that the plastic has cracked. You investigate the issue and find that there may be a problem with the manufacturing process of the plastic buckle. You recognize that if the buckle failed while in use, the restraint would be compromised, and a child in the high chair could fall and be injured. When you heard from the customer, you have become aware of a defect that may reasonably be expected to result in a child's death or serious injury. You are expected to report this as an incident.
4.3 Paragraph 14(1)(c) - incorrect or insufficient information on a label or instructions
This definition covers both:
- incorrect or insufficient information on a label or instructions
- lack of label or instructions
Examples of incorrect or insufficient information include:
- inadequate caution or warning statements
- pictures showing or encouraging unsafe use (even if written instructions are correct)
- contradictory statements on how to safely use the product
- warnings or safety directions only provided in English but not in French, or vice versa
- incorrect or incomplete instructions for assembly or use
- video tutorials demonstrating unsafe use
You must report if incorrect or insufficient information on a label or instructions – or the lack of a label or instructions – may reasonably be expected to result in death or in serious adverse effects on health.
Example
In your store, you notice that a stroller that you sell comes in a package with a photo of the stroller carrying 2 children. The stroller is designed for 1 child, so the package shows the product being used unsafely. You are expected to report this as an incident.
4.4 Paragraph 14(1)(d) – recalls or other measures
Recalls are any corrective action, communicated to consumers, taken post-production to address consumer health or safety issues associated with a consumer product.
Measures are corrective actions that you take with your products that are not communicated to consumers. The types of measures can vary but the ones that you must report to Health Canada are those taken for human health or safety reasons. Examples of measures include:
- product corrections, including new or different labelling or packaging, or changes to a product's design
- product removal or withdrawal from the supply chain
- stop sale, stop distribution, stop importation, stop manufacture
Recalls or measures may be initiated in Canada or elsewhere. As long as they are initiated for human health or safety reasons and involve a product that is available in Canada, you must report them as an incident. This is still the case when a recall or measure is initiated by a foreign entity, even if you believe the consumer product complies with the CCPSA.
Example
You learn from the manufacturer that a lawn mower that you import into and sell in Canada is being recalled in another country. The recall was initiated because the lawn mower did not meet that country's product safety requirements. As such, the recall was initiated for human health or safety reasons and involves a consumer product that you import and sell in Canada, so you must report this as an incident.
4.5 Serious adverse effects on health
Three of the four subsections in the definition of an "incident" describe serious adverse effects on health. A serious adverse effect on health:
- may include damage to the body, chronic health effects, or any injury that sends a person to hospital or requires medical treatment
- may be permanent or temporary
In determining whether a health effect is "serious" and "adverse", you should consider a combination of factors including:
- the type of effect or injury
- the part of the body harmed
- the characteristics of the person affected, including age or whether they are part of a vulnerable population
- the duration that the effect impacted the person
For example, a cut on an infant may be more serious than a similar cut on an adult. A cut on the face may be more serious than a similar cut on a leg.
Examples of serious adverse health effects include:
- threats to breathing such as choking, strangulation, suffocation, asphyxiation, aspiration, or other respiratory problem
- external physical harm, such as a serious burn or cut
- internal harm such as internal bleeding, injury to an organ, or broken bones
- poisoning
- serious allergic reactions (including anaphylaxis)
- loss of consciousness
- convulsions
- loss of sight or hearing
4.6 Other Considerations
Sometimes an occurrence happens with a consumer product that is expected as part of that product's normal use (for example, a consumer cuts herself with a knife). While such occurrences have the potential to result in a serious adverse health effect, you do not currently have to report them as an incident.
That said, when in doubt, err on the side of caution and report.
5. What to report
There are 2 types of incident reports that you may be required to submit:
- Section 14(2) report
- Applies to manufacturers, importers and sellers of consumer products for commercial purposes
- Section 14(3) report
- Applies only to manufacturers and importers of consumer products for commercial purposes
5.1 Section 14(2) report – preliminary information on the incident
A 14(2) report serves as an early indication of a possible incident to Health Canada. By providing the same information to the person from whom you received the product, Health Canada expects swift industry action to address health or safety issues.
The Act requires you to submit all information in your control related to the incident. Information typically provided in a 14(2) report includes:
- all information about the product (name, model number, UPC, serial number)
- description of how the incident happened
- details of the injury (body part, age of victim, kind of treatment required)
- where the product is sold
- complete name and contact information of the manufacturer or importer, as it appears on the product's label
- complete name and contact information of who supplied the product to you
- information on any other known events related to the product
- information on any other known incidents with this product that you have reported to Health Canada in the past
- information on products that share the same parts (components) as the one involved in the incident (if the incident relates to that part or component)
5.2 Section 14(3) report
Manufacturers and importers have an additional reporting obligation under subsection 14(3) of the Act. You must provide a written report with more details about the incident and any measures you propose to be taken with respect to the affected consumer products. A complete 14(3) report helps to ensure that the response to the incident is comprehensive and appropriate to the circumstances.
Information required in a 14(3) report includes, but is not limited to, the following:
- details about the consumer product involved in the incident
For example:- the number of products distributed
- standards to which the product is certified and any Canadian Certification Records that exist, or any other standards that the product meets
- test reports (on the product or similar products)
- warning labels and instructions on the product
- pictures and diagrams of the product
- any new information about the incident
For example:- information gathered from the consumer
- information gathered through further examination of the product involved in the incident (such as the root cause)
- details of any consumer products you manufacture or import that, to your knowledge, could be involved in a similar incident
For example:- information about other similar incidents related to the same or similar product you manufacture or import (with the number of products distributed and a clear description of the products)
- information about products that share the same component as the product involved in the incident
- clear information on any measures you have taken or propose to take related to those products. Include an explanation of how the measures will address the health or safety issue of the affected product.
For example:- Consumer advisories
- Voluntary recall of the product
- Stop sale, distribution, manufacture, advertising
- Product correction including labelling, storing, or packaging
- Changes to the product design or material
- Providing a repair kit to customers
- Monitoring for additional incidents
- Health Canada will not accept the following types of actions as measures as they do not address health or safety concerns:
- Formal investigation
- Plan to conduct a risk assessment
If you expect that you will not be able to meet the deadline for your 14(3) report, you must submit an extension request. See 6.2 Requesting an extension to your reporting timelines. If you do not submit your 14(3) report within the reporting timelines of the Act and you were not granted an extension request, you have not met your reporting obligation under the Act. This is an offence.
Not every 14(3) report will need to include corrective measures. If you assess that no measure is required, provide the reasons with relevant supporting evidence as part of your 14(3) report.
6. When to report
For the 2 types of incident reports that you may be required to submit:
- section 14(2) report are required within 2 days after the day on which you become aware of the incident
- Applies to manufacturers, importers and sellers of consumer products for commercial purposes
- section 14(3) report are required within 10 days after the day on which you become aware of the incident
- Applies only to manufacturers and importers of consumer products for commercial purposes
You must report incidents within the timelines specified by section 14 of the Act. Not reporting an incident is an offence under the Act and may result in further enforcement actions.
Figure 1: Supply Chain Reporting
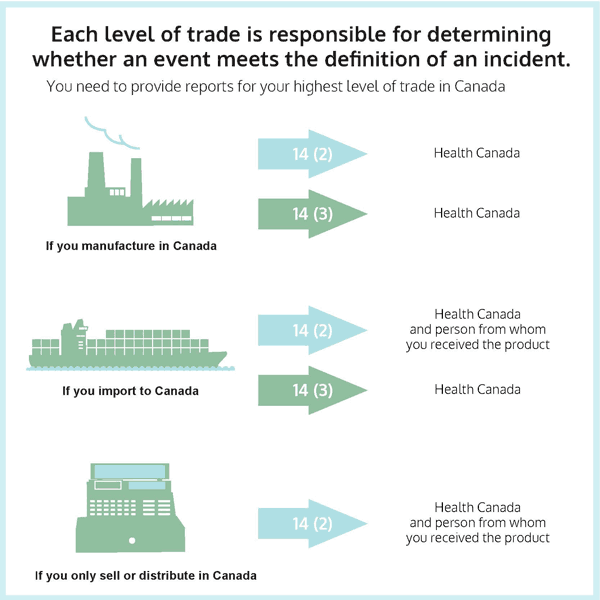
Figure 1: Text description
This graphic illustrates the situations where each level of trade is required to provide reports to Health Canada and, where applicable, to the person from whom it received the product.
The highest level of trade is the manufacturer, which is represented by an image of a factory. If you manufacture consumer products in Canada, you must provide your 14(2) and 14(3) reports to Health Canada.
The next level of trade is the importer, which is represented by an image of a ship. If you import consumer products into Canada, you must provide your 14(2) reports to both Health Canada and the person from whom you received the product, and your 14(3) reports to Health Canada.
The lowest level of trade is sale (which includes distributing and giving away), which is represented by an image of a cash register. If you only sell consumer products in Canada, you must provide your 14(2) reports to both Health Canada and the person from whom you received the product.
When you first hear about an event, you need to assess whether the event:
- involves a consumer product that you manufacture, import or sell in Canada
AND - meets the definition of an incident
You must use the best information that is available to you at the time. Do not wait for a complete investigation or for absolute certainty before first reporting an incident.
Example
You are senior personnel at an appliance manufacturing business. On Monday, you receive a complaint from a customer. The customer said that a fire originated from their newly purchased dishwasher. A fire concerns you, as it could result in serious adverse effects on health, but you find the complaint hard to believe. You want more information on what happened. On Tuesday, you ask your staff to get in touch with the consumer and to open an investigation into the matter. On Wednesday, you call other distributors or retailers to ask if they have heard any similar complaints. Some of them get back to you on Thursday that a few customers have complained of less serious but similar problems. After further discussion with your staff, on Friday, you decide to report the incident to Health Canada that day.
In this example, you have not met your reporting obligations under section 14 of the Act. Receiving the consumer complaint on Monday established that you had become aware of an incident because:
- the complaint was about one of your products
- the fire from the dishwasher could reasonably be expected to cause a house fire and lead to serious adverse effects on health
You should have provided information through a 14(2) report by Wednesday at the latest, which would have been 2 days after you received the complaint. Remember, if in doubt err on the side of caution and report to Health Canada.
To meet your 14(2) reporting obligation, you should:
- assess whether something is an incident as soon as possible
- have a process in place that enables you to carry out this assessment in a timely manner (recommended up to two days from when you first hear about an event to when you decide it is an incident or not)
At the time of submitting a 14(2) report, it is possible that you may not:
- be fully certain of all aspects or details of what happened
- be in possession of the product
- have conducted a comprehensive inspection of the product
- have completed a formal risk assessment or investigation
If in doubt, report.
Once you determine that an event is an incident, the clock begins for you to meet the reporting timelines in section 14 of the Act. The timeline to report under subsections 14(2) and 14(3) both start at the same point in time: they both begin on the day on which you become aware of an incident.
Reporting an incident to Health Canada under section 14 does not automatically mean that Health Canada will conclude that the product poses a substantial hazard or that corrective measures are necessary.
If you receive further information that makes you decide that there was no incident, then you can update Health Canada with your revised assessment. Don't forget to send this update to save yourself further questions from Health Canada. If Health Canada received a 14(2) report but not a 14(3) report, you may be seen not to be meeting your reporting obligations.
6.1 Reporting timelines
| If you become aware of an incident on: | You must provide information to Health Canada under subsection 14(2) by 11:59 p.m. of the next: | You, as manufacturer or importer, must also provide information to Health Canada under subsection 14(3) by 11:59 p.m. of the second: |
|---|---|---|
| Friday | Monday | Monday |
| Saturday | Monday | Tuesday |
| Sunday | Tuesday | Wednesday |
| Monday | Wednesday | Thursday |
| Tuesday | Thursday | Friday |
| Wednesday | Friday | Saturday |
| Thursday | Saturday | Monday |
Note 1: The report becomes overdue after 11:59 p.m. in the person's time zone. This means if you are reporting from British Columbia to Health Canada in Ottawa, the report is overdue after 11:59 PT. Note 2: If your report is due on a holiday (includes Sundays and federal holidays as well as provincial or territorial holidays applicable to your province or territory) your report is due on the day immediately following. |
||
6.2 Requesting an extension to your reporting timelines
If you do not expect to be able to submit your 14(3) report within the timelines specified by the Act, you should request an extension from Health Canada as soon as possible. Send your extension request to us in section 7 of the Consumer product incident report form for industry.
The extension request should meet all of the following:
- Clearly show that it is a request for an extension to the due date of a 14(3) report
- Include:
- Original case number provided to you by Health Canada
- Date of your extension request
- Your company's name
- Product brand and name
- Description of actions taken to date related to the incident
- Explanation of why you cannot meet your reporting deadline
- Description of proposed actions to be taken during the extended timeline in order to complete the 14(3) incident report.
- For example, you may be waiting to recover the product from the consumer in order for your engineering department to conduct a root cause assessment.
- Date when you expect to submit a complete 14(3) report to Health Canada
The actions that you outline in an extension request should describe what additional information you need to gather or testing you need to conduct to decide on any corrective measures to address health or safety concerns. Health Canada will use the information you provide in your extension request to decide whether or not to grant an extension. An extension will not be granted if the request has insufficient or unclear information.
6.2.1 Streamlining extension requests
Manufacturers and importers that have complex incident follow-up processes may provide their Health Canada regional product safety office with a document describing those processes in detail. You can reference that document each time you submit an extension request instead of re-explaining your process. This document should be up-to-date and describe in detail how you:
- receive and document incidents
- notify the relevant individuals within your organization responsible for investigating and reporting incidents
- attempt to retrieve the product and send it for inspection and analysis when it is both relevant and possible
- seek to determine the root cause of the incident
The document should also include:
- the typical timelines to complete each of those steps
- an explanation of how those timelines vary for the different categories of products that you sell
6.2.2 What to expect after you submit your request
Once your extension request is received, Health Canada will review the information you provided and use it to decide whether to grant you an extension and how long of an extension to grant. Longer extensions may be granted if you provide and reference an acceptably detailed incident follow-up process document and Health Canada is satisfied that the extra time would be appropriately used. Health Canada will communicate a decision with you in a written notice within 5 business days from the date on which the request was received by Health Canada. Because the Act specifies a 10-day timeline for meeting your 14(3) reporting obligation, Health Canada strongly encourages you to submit extension requests as soon as possible. When a request for an extension is granted, you will be provided with a new submission deadline of the 14(3) industry report.
The written notice will contain all of the following information:
- Confirmation of receipt of the extension request, including the case number and date on which it was received
- Health Canada's decision on the extension request (granted or denied) or whether more information is needed
- The reason for the decision, if denied
If denied, you may choose to re-submit your extension request with additional information as long as the request is sent before the deadline for the 14(3) report.
7. How to report incidents to Health Canada
Report incidents to Health Canada online using our Consumer product incident report form for industry.
7.1 Tips on using the online consumer product incident report form for industry
Below is additional information to help you complete the online form.
- Complete ALL applicable sections of the form
- Provide any attachments such as images, distribution lists and/or test results as additional documents
- If you already have a case number with Health Canada and need to submit additional documents, use the Form for additional documents
- There are different ways to use the form:
- Submit a 14(2) report without filling out section 7
- Submit a 14(3) report – complete section 7
- Submit an extension request - use section 7 of the 14(3) report to provide information related to your request
- If you assess that something you reported is no longer an incident, check the box "Notification – evaluated as not an incident" and be sure to include clear reasons why an event is no longer considered to be an incident as defined by the Act
- An automated reporting process is also available using Web Services Description Language (WSDL) for an Extensible Markup Language (XML) dropbox
- Contact your IT support or Health Canada (cps-spc@hc-sc.gc.ca) for further information
Require technical assistance in completing and submitting the online form? Contact Health Canada's Consumer and Hazardous Products Safety Directorate by email (cps-spc@hc-sc.gc.ca).
7.2 Provide new information on an incident you have already reported
When you want to provide new information to Health Canada:
- Open the Consumer product incident report form for industry
- In section 1 of the form, select 'Update' as the report type
- Enter the case number that Health Canada provided to you when you submitted your initial incident report
- Fill out all applicable sections on the rest of the form
If you need to submit additional documents with your 14(3) report, please use the online Form for additional documents.
If an event has been reported as an incident and you later determine that the event does not meet the criteria of an incident, you should inform Health Canada. You may provide this update to Health Canada by submitting the online form and checking the box that says: "Notification - evaluated as not an incident". This report should contain clear reasons why you no longer consider the event to meet the definition of an incident under the Act.
8. After you submit a 14(3) report to Health Canada
Health Canada evaluates the information provided in your 14(3) report to determine if a more thorough assessment is required. Health Canada may contact you if:
- further information is required to evaluate your report or to assess the product or investigate the hazard identified
- the measures stated in your report are not acceptable or do not clearly address the health or safety issue of the impacted product(s)
- the measures in your report require further follow up by Health Canada (e.g. recall)
9. Some information on Confidential Business Information and Personal Information under the Act
9.1 Confidential Business Information (CBI)
Sections 16 and 17 of the Act allow the Minister of Health to disclose confidential business information (CBI) in order to protect human health or safety or the environment.
Information about a person's business or affairs must meet ALL of the following conditions to meet the definition of CBI in the Act:
- It is not publicly available
- The person has taken reasonable steps to ensure it remains not publicly available
- It has actual or potential economic value to the person or their competitors (because it is not publicly available and making its disclosure would mean financial loss to the person or a financial gain to their competitors)
Under section 16 of the Act, the Minister may disclose CBI without the consent (or notice to) the person whose business or affairs the information relates, if both of the following conditions are met:
- The information relates to a consumer product
- The CBI is disclosed to a person or government that carries out functions relating to the protection of human health or safety, or the environment
Those who receive the information must agree in writing to keep it confidential and to only use it for functions relating to the protection of human health or safety or the environment.
Under section 17 of the Act, the Minister may disclose CBI to the public without the consent of (or prior notice to) the person whose business or affairs the information relates, if a consumer product poses a serious and imminent danger to human health or safety or the environment. Disclosing the information must be essential to addressing the danger. Subsection 17(2) requires that Health Canada notify the person by the next business day after the information is made public.
It is often possible to deal with health or safety concerns without disclosing CBI. Health Canada will consider relevant factors when determining whether to disclose information in a particular case.
9.2 Personal Information
Industry must comply with all laws that apply to the disclosure of personal information it controls.
Do not disclose personal information about a consumer when you submit incident reports under section 14 of the Act. If Health Canada requires personal information about a consumer in a specific case in order to follow up on a section 14 report, Health Canada will request the information.
Health Canada respects its obligations to safeguard information under the Privacy Act and manages personal information accordingly.
Under section 15 of the Act, the Minister of Health may disclose personal information without the consent of the individual to whom the information relates if both of the following conditions are met:
- The information is disclosed to a person or government that carries out functions related to the protection of human health or safety
- The disclosure is necessary to identify or address a serious danger to human health or safety
In addition, section 8 of the Privacy Act sets out specific instances where Health Canada may disclose personal information.
Appendix - Further information
For further information visit the resources below or contact the Health Canada Consumer and Hazardous Products Safety Directorate Office via email (cps-spc@hc-sc.gc.ca) or telephone at 1-866-662-0666 (toll-free within Canada and the United States).