A guide for voluntary recall of consumer products or cosmetics in Canada
Table of contents
- 1.0 Purpose
- 2.0 Introduction
- 3.0 Legislative requirements in Canada
- 4.0 What is a voluntary recall?
- 5.0 The voluntary recall process
- 5.1 Choosing a company contact and preparing for a recall
- 5.2 Deciding when to recall
- 5.3 Informing Health Canada of the decision to recall
- 5.4 Controlling the affected product(s)
- 5.5 Developing and implementing a corrective action plan
- 5.6 Identifying the level of recall and timelines to publish
- 5.7 Notifying supply chain customers and consumers
- 5.8 Determining the recall effectiveness
- 6.0 Working with Health Canada
- 6.1 Coordinate communication of a recall on the Healthy Canadians Recall and Safety Alert Database
- 6.2 Use social media and other communication tools
- 6.3 Publish a joint recall with Health Canada and the United States Consumer Product Safety Commission
- 6.4 Publish a joint recall with Health Canada and Mexico's Procuraduría Federal del Consumidor
- 6.5 Publish the recall on the Organisation for Economic Co-operation and Development GlobalRecalls portal
- 6.6 Monitor the recall
- Frequently Asked Questions
- Appendix A – Recall related links
- Appendix B – Recall checklist
- Appendix C – Health Canada Recall questions
- Appendix D – Level 1 Recall notice template – Health Canada
- Appendix E – Level 2 and 3 Recall notice template – Health Canada
- Appendix F – Level 2 and 3 Recall notice template – Joint Health Canada and CPSC
- Appendix G – Health Canada Order to Recall notice template
- Appendix H – Health Canada Regional Product Safety offices responsible for the United States of America and International countries
This document does not constitute part of the Canada Consumer Product Safety Act or its regulations or the Food and Drugs Act and the Cosmetic Regulations. In the event of any inconsistency or conflict between these Acts or regulations and this document, the Act or regulation will take precedence. This administrative document has been created to assist in understanding the topic of best practices associated with voluntary recalls. Should you require assistance in interpreting your obligations in respect to this topic, please contact your product safety advisor or legal advisor.
1.0 Purpose
This guide provides general information that may assist manufacturers, importers, distributors, wholesalers and retailers of consumer products or cosmetics on how to complete an effective voluntary recall of their products which are available in the Canadian marketplace. This general information includes, among other things, descriptions of some activities that regulated parties are not required by law to implement.
2.0 Introduction
The aim of this guide is to inform industry about some best practices they may want to follow when they have decided to voluntarily recall a consumer product or cosmetic in Canada. A voluntary recall is any corrective action, communicated to a consumer, taken post production to address consumer health or safety issues associated with a product that is negotiated with Health Canada; in contrast with a mandatory recall, which is carried out following the issuance of a mandatory recall order by Health Canada.
These recall steps include:
- Choosing a company contact and preparing for a recall;
- Communicating with the Health Canada Consumer Product Safety Program (CPSP);
- Controlling the product;
- Developing a corrective action plan;
- Identifying the level of the recall and timelines to communicate;
- Notifying supply chain customers;
- Notifying Canadians; and
- Determining the effectiveness of the recall.
Appendix A provides a list of recall-related links that may be useful in this process. This guide is not a substitute for proper product safety quality assurance. Should someone require assistance in interpreting their obligations with respect to voluntary recalls of consumer products or cosmetics in Canada, they should discuss them with their product safety advisor or legal advisor.
3.0 Legislative requirements in Canada
The Canada Consumer Product Safety Act (CCPSA) and Food and Drugs Act (FDA) have provisions to address risks posed by consumer products and cosmetics, respectively. Some products intended for use by a consumer are not subject to the CCPSA. They include drugs, medical devices, food, pest-control products and vehicles, all of which are covered by other laws in Canada. Please refer to the CCPSA or the Quick Reference Guide listed in Appendix A for a complete listing of excluded products.
A company must be aware of the legislative requirements for consumer products and cosmetics as they manage their recall. The CPSP strongly recommends that industry carry out all recalls in a voluntary manner. This voluntary approach often helps industry to provide Canadians with quick and reliable information on how they can help protect themselves from dangerous consumer products or harmful cosmetics. Protecting the health and safety of Canadians is paramount to the CPSP. For that reason, even when industry decides to carry out a voluntary recall, Health Canada may nonetheless exercise its compliance and enforcement tools to ensure that the products of concern will be removed timely and effectively from the marketplace.
Below are some examples of the legislative requirements pertaining to consumer products and cosmetics, respectively. Non-compliance with any of these requirements may result in enforcement action by Health Canada. Please note that this list is not exhaustive.
3.1 Consumer products
3.1.1 CCPSA and specifically regulated products
Under sections 5 and 6 of the CCPSA, persons manufacturing, importing, advertising or selling consumer products in Canada must ensure that their products comply with the CCPSA and its regulations.
3.1.2 Danger to human health or safety
Under sections 7 and 8 of the CCPSA, it is prohibited to manufacture, import, advertise, or sell a consumer product which is a danger to human health or safety that has been voluntarily recalled in Canada.
3.1.3 Keeping distribution records
Under section 13 of the CCPSA, a retailer must prepare and maintain documents indicating from whom they obtained the product and the location where and the period during which they sold the product. Any person who manufactures, imports, advertises, sells or tests a consumer product for commercial purposes who is not a retailer must prepare and maintain documents indicating from whom they obtained the product or to whom they sold it (or both if applicable). A CPSP inspector may request that this information be provided to them. In the event of a recall, this information will help Health Canada trace the products through the supply chain. General guidance for record-keeping requirements can be found online by reading the Guidance on Preparing and Maintaining Documents under the Canada Consumer Product Safety Act.
3.1.4 Mandatory incident reporting
Under section 14 of the CCPSA, a person who manufactures, imports or sells a consumer product for consumer purposes must provide an incident report to the CPSP after they become aware of a health or safety incident involving a consumer product for which they are responsible. An incident includes a recall or other action taken in or elsewhere based on concerns about human health or safety and must be reported. This obligation also involves reporting an incident even if it occurred after a product has already been recalled. General guidance for mandatory reporting can be found in the CPSP Industry Guide on Mandatory Reporting under the Canada Consumer Product Safety Act. Additionally, the CPSP may learn of an incident through a consumer report, in which case a person (as noted above) may be contacted to provide information regarding the issue.
3.1.5 Assist Health Canada inspectors
Under section 21 of the CCPSA, certain people, meaning an individual or organization, must provide reasonable assistance to Health Canada CPSP inspectors in their duties, which includes providing information they may reasonably require in a timely manner.
3.1.6 Order to recall by Health Canada
Under section 31 of the CCPSA, the Minister of Health has the authority to order any person who manufactures, imports or sells a consumer product in Canada to recall that product if the Minister believes on reasonable grounds that it poses a danger to human health or safety. It is important to comply with an order by the date stipulated in it as non-compliance could lead to administrative monetary penalties or prosecution and imprisonment. For information regarding orders under the CCPSA, please read the Guide to Notices of Violation and Administrative Monetary Penalties under the Canada Consumer Product Safety Act.
3.2 Cosmetics
All cosmetics sold in Canada must comply with the FDA and the associated Cosmetic Regulations.
3.2.1 Safe use
Section 16 of the FDA stipulates, among other things, that no one may sell a cosmetic that may cause injury to the health of the person using it under typical conditions of use.
3.2.2 Keeping distribution records
Under the FDA, there is no mandatory requirement regarding keeping distribution records for cosmetics, but as a best practice, persons manufacturing or importing cosmetics are encouraged to maintain a record of the person(s) to whom they sold products.
3.2.3 Mandatory incident reporting
Under the FDA, there is no mandatory reporting requirement regarding cosmetic incidents, but industry is encouraged to submit incident reports for cosmetics to the CPSP. Additionally, the CPSP may learn of an incident through a consumer report, in which case a company may be contacted to provide information regarding the issue.
3.2.4 Assist Health Canada inspectors
Under section 23 of the FDA, certain people must provide reasonable assistance to Health Canada CPSP inspectors in their duties, which includes providing information they may reasonably require in a timely manner.
4.0 What is a voluntary recall?
Health Canada's CPSP defines a recallFootnote 1 as:
Any corrective action, communicated to a consumer, taken post production to address consumer health or safety issues associated with a product.
As noted previously, a voluntary recall is a recall that is negotiated with Health Canada; in contrast with a mandatory recall, which is carried out following the issuance of a mandatory recall order by Health Canada. A regulated party might decide to initiate a recall because of a health or safety issue relating to
- their product;
- a part or component of their product, including its packaging; or
- warnings, instructions or labels that are associated with their product.
The recall process generally includes stopping all manufacturing, importing, distributing, advertising and selling of the affected products, as well as communicating to consumers the actions they should take to prevent exposure to the danger posed by the affected product(s). This action can include product correction or disposal. Sometimes these actions may be presented to the public by a company in the form of a "safety notice", "repair program" or "upgrade program", but these are all considered to be recalls and will be communicated as consumer recall notices by Health Canada.
Recalls are carried out to remove consumer products that may pose a risk such as a danger to human health or safety, or cosmetics that may pose harm or injury, from the Canadian market. Additionally, a recall will help to minimize the risk to consumers of injury or death by advising the public of the health or safety issue and the corrective action they should take if they have a consumer product or cosmetic that has been recalled.
In addition to communications by industry, the CPSP, at its discretion, may post voluntary recall notices on the Healthy Canadians Recalls and Safety Alerts Database and distribute them via listserv emails and social media channels. Recalls may be done jointly with other jurisdictions, such as the United States Consumer Product Safety Commission (CPSC) and/or Mexico's Procuraduría Federal del Consumidor (PROFECO), and are also posted on the Organisation for Economic Co-operation and Development (OECD) website.
5.0 The voluntary recall process
There are a number of best practices a company can take towards managing a timely and effective voluntary recall. While the following steps are outlined in a numbered sequence for ease of reading, you may find that many may occur at the same time. Figure 1 outlines the key steps in the recall process and Appendix B includes a short recall checklist associated with each step.
Figure 1. Key steps in the recall process

Text equivalent
Schematic representation of the eight steps in the voluntary recall process. While the steps are outlined in a numbered sequence for ease of reading, many may occur at the same time. The eight steps are explored in more detail in this document under section 5 – The voluntary recall process and include the following:
- 5.1 Choosing a contact and preparing for a recall
- 5.2 Deciding when to recall
- 5.3 Informing Health Canada
- 5.4 Controlling the product
- 5.5 Developing and implementing a corrective action plan
- 5.6 Identifying recall levels and timelines
- 5.7 Notifying supply chain customers and notifying consumers
- 5.8 Determining the recall effectiveness
5.1 Choosing a company contact and preparing for a recall
Choose a contact person within the company that has sufficient authority to communicate with the CPSP in respect to the recall. The recall contact should be knowledgeable about the legislative and regulatory requirements and should be accountable to ensure that the steps of the recall are completed. A recalling company may decide to retain outside expertise, such as a legal advisor or a reputable company that specializes in recall coordination and implementation to assist with the recall.
Companies generally find the recall process simpler if they have developed solid internal processes and quality assurance systems. In that regard, a company may wish to consider implementing the following general principles well in advance of any potential recall situation:
- Establish and maintain knowledge of applicable laws, regulations and standards.
- Establish tracking systems:
- Product traceability - Records for product design and specification, model numbers, lot numbers, serial numbers, date of manufacture, along with product changes and identifiers to differentiate old and new product.
- Product registration cards - Records of purchasers of products that send back a completed information card with useful product information and consumer contact details.
- Quality control - Records of routine testing and evaluation (internal and/or third party) associated with each production run to identify possible flaws in the design or production of the product.
- Distribution – Records, by product line, of quantities purchased or sold, dates of delivery and destinations.
- Incidents - Records of complaints, warranty returns, insurance claims, lawsuits, injuries and decisions of assessment.
- Staff training - Records of training and knowledge of internal processes.
- Develop an internal recall policy.
- Commit to prompt and effective implementation of a product recall.
- Maintain good communication with supply chain customers.
- Continue to improve the company's internal product safety program.
There are many different models that offer guidance on the steps to build into your product's lifecycle, from manufacture to sale, that may help in carrying out an effective recall should one be required. Without endorsing any particular one of them, these models include, but are not limited to the following:
- ISO 10377: Consumer product safety – Guidelines for suppliers;
- ISO 10393: Consumer product recall – Guidelines for suppliers;
- United States Consumer Product Safety Commission Recall Guidance; and
- Australian Competition and Consumer Commission Consumer Product Safety Recall Guidelines.
5.2 Deciding when to recall
5.2.1 Recalls initiated by a company
Once a company becomes aware of a health or safety incident with a consumer product to which the CCPSA applies, the company must report it to Health Canada.
Under section 14 of the CCPSA, Canadian industry must report to the CPSP after they become aware of a health or safety incident involving a consumer product for which they are responsible.
In the case of a harmful cosmetic, it is recommended that an incident report be submitted to the CPSP.
In most cases, it could be prudent to initiate a product recall when the following has been confirmed:
- An unsafe product caused or may cause death or serious negative impacts on health;
- A defect or characteristic with a product caused or may cause death or serious negative impacts on health;
- Incorrect or insufficient information on a label or instructions caused or may cause death or serious negative impacts on health;
- A product does not comply with legislative or regulatory requirements; or
- A recall has been taken for the product in another jurisdiction based on concerns about human health or safety.
5.2.2 Recalls initiated following a request from Health Canada
The CPSP may learn about health or safety issues through:
- Consumer or industry reported incidents;
- Emerging science;
- Monitoring actions of other regulators, such as recalls in other jurisdictions; and/or
- Compliance and enforcement activities, such as CPSP inspections and testing.
As a result of information received through these sources, the CPSP may contact a company to confirm that the product in question was sold in Canada. The CPSP may request that a company initiate a voluntary recall when it is confirmed that the product does not comply with the applicable legislation or regulation.
When the CPSP contacts a company and requests a recall be issued on a product, the list of recall questions, in Appendix C, will be provided to the company to assist in completing the recall process. In order to manage an effective recall, a response from the company needs to take place early on within the recall posting timelines as outlined in section 5.6 - Confirming the level of recall and timelines to publish of this document.
Under section 21 of the CCPSA, during an inspection certain people, meaning an individual or organization, must provide reasonable assistance to Health Canada CPSP inspectors in their duties, which includes providing information they may reasonably require in a timely manner. Under section 23 of the FDA, during an inspection certain people meaning, an individual or organization, must provide reasonable assistance to Health Canada CPSP inspectors in their duties, which includes providing information they may reasonably require in a timely manner.
5.3 Informing Health Canada of the decision to recall
Using the contact options in the Frequently Asked Questions (FAQ) section of the document, a company can quickly and easily notify Health Canada of their decision to recall a product for health or safety reasons. It is recommended that a company use the online Industry Incident Report Form and include the following information:
- Company contact information
- Reason for the recall
- Incident information (if applicable)
- Details about the incident gathered from the consumer or through examination of the product involved in the incident.
- Date on which the company became aware of the incident(s).
- Number of other incidents related to the same or similar product.
- Explanation of the hazard and risk (root cause analysis).
- Product description
- Product brand and name, model number, UPC, lot numbers and date codes.
- Warning labels and instructions on the product.
- Pictures and diagrams of the product.
- Standards to which the product is certified and any Canadian Certification Records that exist (if applicable).
- Test reports, if the company did testing on the product or similar products.
- Source of product
- Who the company got it from.
- Where it was manufactured.
- Corrective measures and other affected products
- Numbers of products distributed in Canada and a complete distribution list.
- Other products that may be affected (such as products that share the same component involved in the incident or that have a similar design).
- Information about corrective action, including: the proposed corrective action(s) and an explanation of how the action will address the issue.
- Whether the affected product was sold in the USA/Mexico and whether the product is being recalled by the CPSC/PROFECO or any other jurisdiction.
The list of recall questions, in Appendix C, will prompt a company to gather much of the information listed above and it is recommended that it accompany an industry incident report as a supplemental document.
Note that non-compliance to a regulatory requirement may not always result in Health Canada requesting a voluntary recall; however, industry must report any non-compliance with a regulatory requirement to Health Canada. A company cannot manufacture, import, advertise or sell a product that is not compliant with a regulation and other corrective actions may still need to be undertaken.
5.4 Controlling the affected product(s)
5.4.1 Identify affected products
Once a company has determined that a voluntary recall is appropriate, it should immediately stop manufacture, import, sale and advertisement, including online sale, of the product and identify all products that need to be recalled. In addition to those products directly affected by the problem, a company should also consider whether the risk posed by the recalled product also exists with other similar products that it markets. Specifically, determine if any other brand names/product lines, styles, colours, dimensions, UPC codes, lot numbers, item numbers, dates of manufacture or dates of import should be included. This information could form the basis of the recall notice that will help consumers identify affected products and determine the scope of products to be controlled by the company and their supply chain customers.
If the product does not have distinguishable characteristics from other similar products for which the company is responsible, then a company may be requested to expand their recall to include all similar products.
5.4.2 Isolate warehouse stock of the product to be recalled
For products that are affected by a recall, a company should identify and clearly mark stock of recalled products physically located in their warehouse or other storage facilities. These products should be isolated from other stock to prevent distribution and sale. Additionally, electronic isolation methods, such as a computer software program that flags and prevents affected inventory from being distributed should be considered. Employees should be informed of the recall and clarify expectations with respect to the remaining product stock. It is important to keep a record of internal communications.
5.4.3 Track the products that have been distributed
A company should use their product distribution records to identify supply chain customers that received the recalled product.
Under section 13 of the CCPSA, a retailer must prepare and maintain documents indicating from whom they obtained the consumer product and the location where and the period during which they sold the product. Any person who manufactures, imports, advertises, sells or tests a consumer product for commercial purposes who is not a retailer must prepare and maintain documents indicating from whom they obtained the product or to whom they sold it (or both if applicable).
These product distribution records will allow a company to more easily and quickly contact their supply chain customers regarding the recall. If a company does not have any distribution records, it should contact all of its supply chain customers to share the recall information.
It is a best practice that the product distribution list includes the following information about the supply chain customers:
- the supply chain customer name;
- the street address;
- the city;
- the province;
- the contact phone number for the supply chain customer;
- the quantity of each product that the supply chain customer received;
- the date(s) on which the products were distributed/sold to the supply chain customer; and
- the type of supply chain customer, for example, wholesaler, distributor, retailer.
This task is made easier if the company's computer system or invoicing system is set up to sort and find supply chain customers who have purchased specific items. The product distribution list should identify all your supply chain customers that must be notified of the recall and any other potential distribution channels, such as those involved in promotions or giveaways. The CPSP will ask for a copy of this list in the event that it monitors your recall.
Under section 21 of the CCPSA, during an inspection certain people, meaning an individual or organization, must provide reasonable assistance to Health Canada CPSP inspectors in their duties, which includes providing information they may reasonably require in a timely manner.
If the affected products were imported from outside of Canada, companies should provide the CPSP the name and contacts for the supplier. Health Canada may contact the exporter to determine if the same products were imported into Canada by others.
If company distribution records indicate that a recall is appropriate in the United States and/or Mexico, a best practice would be for the company to provide the information to the CPSC and/or PROFECO.
5.4.4 Instruct supply chain customers to immediately stop sale
In order to prevent further sale of recalled product, it is important to inform your supply chain customers to immediately stop sale. This stop sale notice should also include instruction to share the stop sale request with additional supply chain customers further down the supply chain that may be selling the product. As a recalling company, additional information should be provided to the supply chain customers in the form of a supply chain recall notice that provides more detailed instructions. This recall notice is outlined in section 5.7.1 – Notify supply chain customers.
5.5 Developing and implementing a corrective action plan
5.5.1 Determine corrective action options
The following are some best practices to consider when instructing your supply chain customers and consumers on how to deal with the affected product. These may include the following:
-
Product correction, such as a repair or fix, which removes the hazard. Under this option, be aware that some products cannot be brought into compliance due to their design or composition. You may wish to consider the following points:
- Determine if the product correction can be done easily and safely by the consumer. The use of videos, including those posted on YouTube or other online applications, can be helpful tools to show consumers how to complete a fix of the product.
- Determine if the product correction should be done by sending a professional to the person's premises to repair or modify the product.
- Use new or additional labelling on products to inform consumers of the hazard and how to correct it.
- Distinguish corrected products from the uncorrected, recalled products. For example, a corrected product may incorporate a new or fixed part that is identified by a part number, lot number, or an easily identifiable label that makes it clear that the replacement component is new or different. These markers often make it easy for CPSP inspectors, retailers and consumers to differentiate between recalled and corrected products.
- Verify if products with a certification mark will need to be re-certified or if the existing certification will need to be amended by the certification body. This information should be provided to the CPSP.
The company is responsible for the compliance of the corrected product to the appropriate legislation, regulation or standard. If in-stock/returned products are corrected, the company should verify compliance of the fix with the appropriate requirements before they are distributed and keep a record of the results.
-
Product replacement for a similar product of similar value. Any replacement product should meet the appropriate legislative or regulatory requirements and safety standards before they are distributed. For certified products, the certification file number and copy of the certification record demonstrating compliance of the replacement product to the appropriate safety standard should be provided to the CPSP.
-
Product disposal in such a way that unsafe products do not make their way back into the Canadian marketplace and that is carried out in a manner that is in compliance with applicable federal, provincial and municipal waste disposal requirements.
-
Product refund or some other form of compensation. This action by the company is often done in conjunction with another action by the consumer. For example, in order to receive a refund, the company may require that the consumer return the product as proof that the product is no longer being used.
5.5.2 Determine the action that supply chain customers and consumers must take
Supply chain customers: Choose the appropriate instructions for your supply chain customers, taking into account the best practices or other considerations above. If products are to be returned, determine whether to instruct supply chain customers to return the recalled products to the company's warehouse or another location. The recalling company should provide dates and timelines for their supply chain customers to return stock and identify mechanisms for return.
Consumers: Choose the appropriate actions for consumers to take after taking into account the best practices above or other considerations. Recalls may be more effective when consumers are prompted to take an action that provides an incentive to comply, such as replacement or refund. The corrective action for consumers to take should appear in the consumer recall notice.
5.5.3 Fix the cause of the recall
It is a company's responsibility to take steps to prevent similar health or safety issues from being associated with their other products. In that regard, consider these best practices:
- Determine the root cause of the issue and take action to reduce the likelihood of it happening again.
- Know the legislative or regulatory requirements, and the safety standards that exist for the products that are manufactured, imported, advertised or sold.
- Test the products regularly as part of an internal quality assurance program, or ask the supplier(s) to provide test reports to verify compliance with the CCPSA and its regulations or with the Cosmetic Regulations made under the FDA.
- Consult the Health Canada website or the nearest Regional Product Safety Office for general information on consumer product hazards.
- Consult a private sector consumer product safety specialist.
5.6 Identifying the level of recall and timelines to publish
The CPSP categorizes recalls for consumer products and cosmetics into three levels using a risk-based approach. The risk level then identifies the timeline in which a regulated party should publicize the voluntary recall in Canada. The levels are determined based on, among other things, the severity and imminence of the dangerFootnote 2 posed by the product.
If the danger is not "serious", then the voluntary recall is a level 3.
- A "serious" danger is, in the context of this document, one that could result in death or severe injury; such as, a significant, possibly permanent, injury, physical impairment or incapacity.
If the danger is "serious", then a company has to consider if it is "imminent". If the danger is "serious" and "imminent", then the voluntary recall is a level 1. If the danger is "serious" but not "imminent", then the voluntary recall is a level 2.
- An "imminent" danger is, in the context of this document, one where there is evidence, such as evidence of incidents gathered over a long period of time, or multiple incidents over a short period of time, to suggest that a serious event may occur involving the product to be recalled sooner than the established timelines for the recall.
- Specifically, a recall will be a level 1 recall if there is evidence to suggest that a serious event may occur involving the product to be recalled sooner than 10 business days from the day on which the company's decision to recall the product was made.
Figure 2 summarizes how the criteria of seriousness and imminence determine the level of recall.
Figure 2. Determination of recall levels
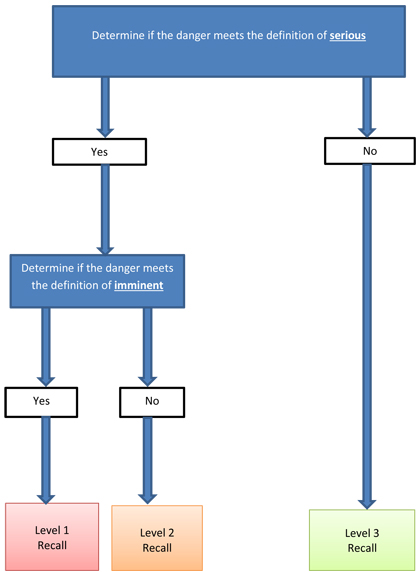
Text equivalent
Schematic representation of how recall levels are determined. The representation shows a flow chart outlining the decision process. The recall levels are determined based on, among other things, the severity and imminence of the danger posted by the product. If the danger is not "serious", then the voluntary recall is a level 3. If the danger is "serious", then a company has to consider if it is "imminent". If the danger is "serious" and "imminent", then the voluntary recall is a level 1. If the danger is "serious" but not "imminent", then the voluntary recall is a level 2.
Once a company identifies the level of recall, there are corresponding timelines within which a company should inform their supply chain customers and the public. As a best practice, no matter the level of recall, a company should inform supply chain customers to immediately stop sale as noted in subsection 5.4.4 – Instruct supply chain customers to immediately stop sale. With respect to timelines to inform the public, CPSP encourages regulated parties to:
- Level 1 recall - publicize the consumer recall notice as soon as possible and within 2 business days following the decision to recall.
- Level 2 recall - publicize the consumer recall notice as soon as possible and within 10 business days following the decision to recall.
- Level 3 recall - publicize the consumer recall notice as soon as possible and within 40 business days following the decision to recall.
Based on the information provided to Health Canada, the CPSP will discuss the level of recall with the company, so both parties are working under the same timeline to communicate the recall. In order to facilitate in this process, it is important for a company to have its rationale and supporting information available for review. For example, documents may include incident information, distribution numbers, time period sold, internal risk assessments and third party test reports. These timelines are presented as general guidance to communicate recalls; however, the CPSP may choose to deviate from them at its discretion depending on the specifics of a particular case. For example, while reviewing submitted information, the CPSP could ask the company to escalate the level of a recall should there be reasonable grounds to believe the rate of the incidents or severity will increase. At any time, the CPSP may choose to initiate compliance and enforcement action in respect to the product if they have concerns that the voluntary approach will not meet objectives.
The levels of recall and their timelines are summarized in Table 1.
| Level of Recall | Description | Example | Target timelines and actions for the recalling company |
|---|---|---|---|
| Level 1 | Clear evidence of a serious and imminent danger to human health or safety. | Toy rattle breaks leading to the release of small parts. 3 incidents in the last month.
|
Informs supply chain customers immediately to stop sale. Issues a consumer recall notice on their company website, works with Health Canada to publish the consumer recall notice on the HC website, and uses other communication tools to communicate the recall as soon as possible and within 2 business days. |
| Level 2 | Clear evidence of a serious danger to human health or safety, but no imminent danger. | Toy rattle breaks leading to small parts. 1 incident in the last year.
|
Informs supply chain customers immediately to stop sale. Issues a consumer recall notice on their company website, works with Health Canada to publish the consumer recall notice on the HC website, and uses other communication tools to communicate the recall as soon as possible and within 10 business days. |
| Level 3 | Clear evidence of danger to human health or safety that is not serious. | Office chair breaks leading to bruising. 3 reports in the last 5 years.
|
Informs supply chain customers immediately to stop sale. Issues a consumer recall notice on their company website, works with Health Canada to publish the consumer recall notice on the HC website, and uses other communication tools to communicate the recall as soon as possible and within 40 business days. |
It is expected that a company's recall will be publicized as soon as possible and within the respective timelines. To manage an effective recall, the CPSP strongly encourages that communication tools be used as identified in Table 2 of subsection 5.7.3 - Publicize the recall.
The specific date on which the recall will be publicized by a company is expected to fall within the timelines outlined in Table 1. The date of publication will be dependent on a number of factors, including:
- Identification of all affected product;
- Preparation of all the recall information in a consumer recall notice (including translation into English and French);
- Verification of any proposed product corrections and confirmation that the corrected product is ready for distribution to the consumer;
- Verification that inquiry points for the consumer (e.g., toll-free numbers) are set up and ready to be used; and
- Coordination of joint recalls with other jurisdictions.
In the case where the above factors are not ready within the timelines, consumers should still be informed of the hazard and a recall notice may be published and updated later with any missing information.
5.7 Notifying supply chain customers and consumers
5.7.1 Notify supply chain customers
As a best practice, a company should prepare a supply chain customer recall notice in English and French. The company should then distribute it to all of its supply chain customers that have purchased the recalled products as identified in the product distribution list along with instructions to remove the product from sale immediately. The supply chain customer recall notice should include:
- A heading that reads "Urgent - Product Recall";
- The date the recall notice is being sent to supply chain customers;
- The product identity (includes brand, UPC, lot number, item number, description, etc. and a picture if possible);
- The reason for the recall, including a statement of the hazard and associated risk;
- A request to immediately remove the product from sale;
- The recall action to be completed by the account (remove from sale, instructions for arranging return, etc.), and by when;
- A request that a recall be initiated by any supply chain customers who have further distributed the product;
- The name and contact number of the recalling company to call for more information;
- The date that recall action is to be completed by the supply chain customer; and
- A statement that the recall may be monitored for compliance by Health Canada.
A copy of this supply chain customer recall notice should be provided to the CPSP prior to distribution. During the recall monitoring process, the CPSP may use this notice to check that your supply chain customers were informed of the recall.
It may also be necessary for a company's supply chain customers to provide notifications to secondary or even tertiary distributors before the recall notice reaches the retail establishments that sold the affected product(s) to consumers. Having organized and up-to-date records of distribution networks and contacts will help ensure a recall quickly reaches all establishments that sell affected products.
Under section 13 of the CCPSA, a retailer must prepare and maintain documents indicating from whom they obtained the consumer product and the location where and the period during which they sold the product. Any person who manufactures, imports, advertises, sells or tests a consumer product for commercial purposes who is not a retailer must prepare and maintain documents indicating from whom they obtained the product or to whom they sold it (or both if applicable).
A company should maintain contact with its supply chain customers throughout the recall process to ensure they received and acted upon the information that was sent to them. This will help a company determine if their recall has been effective, see section 5.8 - Determining the recall effectiveness.
5.7.2 Notify consumers
The company should publicize the recall on its website and use other communication tools to communicate the recall. As a best practice, the consumer recall notice should include the following information:
- Name and location of the recalling company;
- A detailed description of the product, including name, make, model, distinguishing features, batch or serial number, etc.;
- A statement of the hazard and associated risk;
- The number and type of injuries associated with the issue;
- Dates when the product was available for sale;
- Retail locations/online sites where the product was sold;
- The number of products involved;
- The immediate action that the consumer should take;
- Who consumers should contact for further information including a telephone number, preferably toll-free with service in English and French, and hours of business;
- Links to any supporting information for the consumer, such as a repair video; and
- Picture(s) of the product.
Notices should be issued in both English and French, but may include other languages to which your target audience will respond.
It is recommended that a company keep the consumer recall notice on its company website until it has determined the recall to be effective, see section 5.8 - Determining the recall effectiveness. The time may vary depending on the type of product (short versus long life span), size and scope of the recall.
The CPSP will also publish this information on the Healthy Canadians website. Should there be a change to any company contact information provided in the recall notice, the company should inform the CPSP immediately so updates can be made to the recall notice displayed on the Healthy Canadians website. To further broaden messaging, recalls may be done jointly with other jurisdictions, such as the CPSC and/or PROFECO. For more information on this, see section 6.0 – Working with Health Canada.
5.7.3 Publicize the recall
For an effective recall, the company should publicize the recall through a number of channels. Higher risk recalls may warrant increased communication. Table 2 provides examples of distribution methods for a recall notice. Information about recalls may also be picked up and publicized by others, such as the media.
| Method | Examples |
|---|---|
| Websites | Publish on your company website |
| Share the consumer recall notice with Health Canada so the information can be published on the Healthy Canadians Recall and Safety Alert Database | |
| Social Media Platforms | Twitter - Facebook - Google+ - YouTube - Pinterest - Flickr |
| Blogger Networks | |
| Others as applicable | |
| Media/Marketing Outlets | Company media release, for example on Canada Newswire Service or other media clearinghouse |
| Video news release | |
| National news conferences | |
| Paid notices in newspapers, magazines, radio, television or online | |
| Paid notices in product catalogues, newsletters and other marketing materials | |
| Direct Notice | Mail outs to addresses or telephone calls to numbers identified through registration cards, sales records, catalogue order, loyalty program, service inquiry lists, or other means |
| Messages on credit card statements and bills | |
| Listserv e-mails and text messages to consumers | |
| Purchase and use of mailing lists for populations likely to use the affected product | |
| Notices included with product replacement parts/accessories | |
| Posters | Display signs or point-of-sale posters in retail stores that sold the product |
| Display at locations where users are likely to visit, such as stores, medical clinics, pediatricians' offices, child care centres, repair shops, equipment rental locations and others | |
| Other | Notices to repair/parts shops |
| Service bulletins | |
| Notices to child-care centres | |
| Notices to second-hand product retailers |
5.8 Determining the recall effectiveness
Recall effectiveness is the process by which the company undertaking a recall of its products summarizes the recall notification measures taken, the response from supply chain customers and consumers, and incident reports related to the recall. It is the responsibility of the company to determine whether the recall is progressing well. It is important that a company maintain ongoing communication with its supply chain customers during the recall process. Some best practices to be used to determine recall effectiveness are set out in Table 3.
| What to review | Action |
|---|---|
| The recalling company reviews the overall recall process | Make certain the recall corrective action plan has been implemented. |
| The recalling company assesses supply chain customer feedback | Verify the following:
If very few supply chain customers confirm receipt of the information or respond back, more effort may be required to reach out to them to confirm that actions have been taken. |
| The recalling company assesses consumer information feedback | Determine the following:
If very few consumers have responded, more work may be required to understand why and possibly more outreach may be necessary to ensure corrective measures, as outlined in the recall notice, are followed. |
| The recalling company reviews incident data | Determine if any additional reported incidents occurred after the recall was published. The goal of a recall is to warn consumers who have the product of the dangers associated with it, and to remove any remaining products from the marketplace. Incidents that occur in Canada after the recall may be an indication of an ineffective recall, and may warrant further examination into a company's actions to communicate it. Under section 14 of the CCPSA, Canadian industry must report to the CPSP after they become aware of a health or safety incident involving a consumer product for which they are responsible. This also includes reporting an incident even if it occurred after a product has been recalled. |
If a company determines that the recall was not effective and unsafe products remain in the marketplace or a company continues to receive incident reports about the recalled product, it should consider extending, re-announcing or modifying the recall strategy to address the effectiveness issues. Any additional steps that a company takes regarding the recall should be communicated to the CPSP.
6.0 Working with Health Canada
The CPSP can post a company's recall on the Healthy Canadians Recall and Safety Alert Database in English and French. In addition it can broaden the recall message through other channels, and may work with other international regulators to help publicize the recall when warranted.
6.1 Coordinate communication of a recall on the Healthy Canadians Recall and Safety Alert Database
The CPSP will use the information a company has provided in response to the recall questions, found in Appendix C, to populate a consumer recall notice that will be posted to the Healthy Canadians Recall and Safety Alert Database. The consumer recall notice includes the following information:
- Name and location of the recalling company;
- A detailed description of the product, including name, make, model, distinguishing features, batch or serial number, etc.;
- A statement of the hazard and associated risk;
- The number and type of injuries reported, including the country(s) where they occurred;
- Dates when the product was available for sale;
- Retail locations/online sites where the product was sold;
- The number of products involved;
- The immediate action that the consumer should take;
- Who consumers should contact for further information including a telephone number, preferably toll-free with service in English and French, and hours of business; and
- Picture(s) of the product with no text (jpg images should be 600x800 pixels or no wider/higher than 16.5 cm or 6.5 inches).
The following appendices include various recall templates used by the CPSP:
- Appendix D - Level 1 Recall notice template – Health Canada
- Appendix E – Level 2 and 3 Recall notice template – Health Canada
- Appendix F – Level 2 and 3 Recall notice template – Joint Health Canada and CPSC
- Appendix G – Health Canada Order to Recall notice template
As noted in section 5.6 - Confirming the level of recall and timelines to publish, the date on which the recall will be published to the Healthy Canadians Recall and Safety Alert Database will be discussed and communicated to all involved parties, but publication should happen as soon as possible and should not exceed the timelines outlined in Table 4. These timelines are presented as general guidance to communicate recalls; however, the CPSP may choose to deviate from them at its discretion depending on the circumstances of a particular case.
| Level of Recall | Target timelines for Health Canada |
|---|---|
| Level 1 | Communicate the recall on the HC website as soon as possible and within 2 business days. |
| Level 2 | Communicate the recall on the HC website as soon as possible and within 10 business days. |
| Level 3 | Communicate the recall on the HC website as soon as possible and within 40 business days. |
6.2 Use social media and other communication tools
Health Canada may use social media platforms, such as Facebook and Twitter, to further promote the recall. This is often the case for level 1 and level 2 recalls, or for recalls involving high volumes of product distribution which may be of interest to the general public. Additionally, for level 1 recalls, the recall notice will be published via the newswire service and highlighted on the Healthy Canadians main website page.
6.3 Publish a joint recall with Health Canada and the United States Consumer Product Safety Commission
If the product being recalled has also been sold or distributed in the United States, then a joint recall between the CPSP and the CPSC may happen. A synchronized message with other international jurisdictions provides consistency and easier accessibility for consumers to safety information. Joint postings are favourable as they permit both the CPSP and the CPSC to post the recall online at the same time in both countries which will increase the likelihood of reaching the appropriate target audience. The message may receive greater media coverage and have wider internet exposure since it would be posted on several sites at the same time.
In order for a joint recall to be started, the following factors will be considered:
- The product must have been sold or distributed in both countries.
- The product must be under the jurisdiction of both agencies.
- The regulatory agencies must agree that a recall is appropriate based on the identified product hazards and level of risk.
- The recalling company must agree to share information with both agencies and conduct a voluntary recall.
- The corrective measures/remedy (for example, refund or repair) and customer support must be available to customers in both countries.
- Both agencies must agree to move forward with a joint recall within a timeframe that is agreeable to both agencies and the recalling company.
The CPSP has target timeframes noted in Table 4 that are presented as general guidance to communicate recalls, but may choose to deviate from them at its discretion depending on the specifics of a particular case. The CPSC's timeframes may vary depending on time needed for sampling, sample assessments, and fix/remedy evaluation. As a result, coordination of a recall with the CPSC may result in longer timelines for publishing than identified in section 5.6 - Confirming the level of recall and timelines to publish. Regardless of the considerations mentioned above, an immediate stop sale of the product(s) is a best practice. The recall notice template for a joint CPSP/CPSC recall is found in Appendix F.
More information on the recall process that the CPSC uses can be found in their online Recall Handbook (PDF format) and by visiting the CPSC recalls website.
6.4 Publish a joint recall with Health Canada and Mexico's Procuraduría Federal del Consumidor
If the product(s) being recalled has been sold or distributed in Canada and Mexico, then a joint recall between the CPSP and PROFECO may be done. The CPSC may also be included, so a trilateral joint recall is possible. The benefits for the three countries to post the recall at or around the same time are similar to those outlined as for the joint postings between the CPSP and the CPSC. The recall notice will be similar to that done with the CPSC but will include reference to PROFECO. Any joint release with PROFECO will be posted on their Rapid Alert Network.
6.5 Publish the recall on the Organisation for Economic Co-operation and Development GlobalRecalls portal
Similar to the use of the CPSC and PROFECO sites to broaden recall messaging, the CPSP also adds its publicly available recalls to the Organisation for Economic Co-operation and Development (OECD) GlobalRecalls portal site. The OECD GlobalRecalls portal brings together information on product recalls being issued around the world, including the United States, Mexico, Australia, Brazil and the European Union, and is used by:
- Regulators to support corrective actions;
- Consumers to check if there are safety concerns with a product, which may be useful for cross-border shopping and online activities; and
- Businesses to track emerging hazards from around the world.
Once a company's recall is posted on the Healthy Canadians Recall and Safety Alert Database, it is posted to this portal to further increase coverage. In the recall notice templates in Appendices D-F, under the "What you should do" section a statement is provided leading consumers to the OECD GlobalRecalls portal site.
6.6 Monitor the recall
The CPSP may follow up with a company's supply chain customers, through on-site inspections, by telephone or on-line review, to verify that establishments have been notified of the recall and have taken the actions detailed in the recall notice, including removal of product from sale. The CPSP may ask for a copy of a company's product distribution list.
Under section 13 of the CCPSA, a retailer must prepare and maintain documents indicating from whom they obtained the consumer product and the location where and the period during which they sold the product. Any person who manufactures, imports, advertises, sells or tests a consumer product for commercial purposes who is not a retailer must prepare and maintain documents indicating from whom they obtained the product or to whom they sold it (or both if applicable).
Further action may also be taken by the CPSP if recall monitoring shows that supply chain customers were unaware of the recall and/or incident reports received by the CPSP after the recall was published indicate lack of awareness of the product recall. This may result in, among other actions to increase the awareness of the recall, the CPSP re-publishing the recall notice on its website or posting a consumer product update about the issue. In the event of an ineffective recall, where recalled items remain available to consumers, Health Canada may also order a company to take additional measures.
Frequently Asked Questions
-
How do I contact the Health Canada Consumer Product Safety Program?
- Report a product incident/recall using the online form.
- Submit an inquiry via email at cpsr-rspc@hc-sc.gc.ca.
- Contact a Health Canada Regional Product Safety Office. See Appendix H for the region dealing with your US state or country.
- Contact via regular mail at:
National Capital Region Consumer Product Safety Office
Consumer Product Safety Program
Health Canada
269 Laurier Avenue West
Ottawa, ON K1A 0K9
AL 4907E
-
Is there a different recall process for cosmetic products?
No, generally the best practices and processes described in this document in relation to carrying out a voluntary recall for a consumer product are the same as for a cosmetic product. Should you require assistance in interpreting your recall obligations, you may wish to discuss them with your product safety advisor or legal advisor.
- What if I do not sell directly to consumers, but my supply chain customers do, am I responsible for managing the recall?
If you are the Canadian manufacturer or importer and your products are intended to be used by consumers, you are responsible for the product recall.
-
What should I do if my recalled products carry a certification mark (such as UL, ETL, CSA, among others)?
The best practices described in this document for the recall process are the same for consumer products that carry a certification mark, but you may also wish to consider the following:
- Provide the certification file number and a copy of the certification record demonstrating compliance to the appropriate safety standard to Health Canada. The certification file number will be included under the product description in the recall notice as an additional means of product identification.
- If you have a contractual agreement about ownership of the certification mark with the Certification Body (CB), report this product safety issue to them and inform them that you are working with Health Canada on a recall of the product.
- If you do not have a contractual agreement with the CB about ownership of the certification mark, inform the higher level of trade (that owns the mark) of the issue so they can report to this product safety issue to the CB.
- If the corrective action is to repair the product, verify if the product will need to be re-certified or if the existing certification will need to be amended. You should submit this information to the CPSP.
- If the corrective action is to replace the product, you should provide the certification file number and copy of the certification record demonstrating compliance of the replacement product to the appropriate safety standard to the CPSP.
-
What if I need to update a recall notice that I already published? For example, if I have identified other brands or models of product, or need to include similar product in the recall notice.
Inform the CPSP that other products are affected by the recall and the Product Safety Officer will advise what actions are required. The CPSP may expand/update your current recall to include the new information. In those situations, generally the recall will be re-released with a new date, will link back to your original recall notice, and will include the phrase "Expanded Recall" in the title. This revised title will inform consumers that new information has been added and that they should pay particular attention to the update. You should update the information on your website and communicate using additional tools as needed. Your company should once again monitor the effectiveness of the expanded recall.
-
After the recall is published, what do I do if I continue to receive incident reports from industry or consumers that don't know their product is affected by the existing recall?
You should report any incidents related to the recall to the CPSP using the Incident Report Form. You may consider extending, re-announcing or modifying the recall strategy to address the effectiveness issues. Any additional steps that you take should be communicated to the CPSP.
Under section 14 of the CCPSA, Canadian industry must report to the CPSP after they become aware of a health or safety incident involving a consumer product for which they are responsible. This also includes reporting an incident even if it occurred after a product has been recalled.
-
My recall only involves the instructions of a product or a specific component – can the title of that recall be written so that consumers know that it is only a specific section of the whole product that is impacted?
The title of the recall is intended to be clear and easily understood by consumers and must include the name of the product. This way, consumers can more easily verify whether they have the affected product in their home. Reference to only the affected instructions or specific component of the product in the recall title is not sufficient. Details outlining the issue and risk with the affected instructions (including errors or omission of information) or the specific component will appear in the 'Hazard identified' heading of the Recall Notice (see Appendices D, E and F of this guide).
For example, the title of the recall notice related to a product with instructional issues could read as "<Product name> recalled due to lack of appropriate assembly instructions."
Appendix A – Recall related links
- Canada Consumer Product Safety Act
- Food and Drugs Act
- Canada Consumer Product Safety Act Quick Reference Guide
- Guidance on Preparing and Maintaining Documents under the Canada Consumer Product Safety Act (CCPSA) – Section 13
- Industry Guide on Mandatory Reporting under the Canada Consumer Product Safety Act – Section 14 "Duties in the Event of an Incident"
- Industry Guidance – "Danger to Human Health or Safety" Posed by Consumer Products
- Overview of Health Canada's Consumer Product Safety Program Risk Characterization Method
- Healthy Canadians Recalls and Safety Alerts Database
- United States Consumer Product Safety Commission (CPSC) Recalls Website
- Mexico's Procuraduría Federal del Consumidor (PROFECO) Website
- Organisation for Economic Co-operation and Development (OECD) Global Recalls Website
Appendix B – Recall checklist
The following is a brief summary of points for a company to remember when it has made the decision to voluntarily recall a product. It is important to stay in contact with the CPSP throughout the recall process. See the industry guide for more details on each of the sections. If you have any questions, contact a Health Canada Regional Product Safety Office.
- Reporting to Health Canada
- Complete an industry incident report.
- Answer the recall questions.
- Determine if a joint recall with the United States and/or Mexico is warranted.
- Controlling the product
- Identify affected products, which may include similar products.
- Stop manufacture, import, sale or advertisement.
- Isolate affected products.
- Track affected products.
- Developing and implementing the corrective action plan
- Identify the remedy for supply chain customers and consumers.
- Test replacement or repair.
- Change model/serial numbers of redesigned product.
- Enhance quality control measures.
- Fix the problem so it does not happen again in this or any similar products.
- Determine how returns will be processed at all levels of distribution.
- Identifying recall level and timelines
- Confirm the level of recall based on your risk evaluation as this will inform the timelines to publicize once a company has made the decision to recall.
Table 1. Recall posting timelines Level of Recall Description Target timelines Level 1 Serious and imminent danger to human health or safety As soon as possible and within 2 business days. Level 2 Serious danger to human health or safety As soon as possible and within 10 business days. Level 3 Danger to human health or safety As soon as possible and within 40 business days.
- Confirm the level of recall based on your risk evaluation as this will inform the timelines to publicize once a company has made the decision to recall.
- Notifying supply chain customers
- Request them to immediately stop sale and provide additional instructions in a supply chain customer recall notice.
- Notifying consumers
- Publish the consumer recall notice.
- Identify what products are included.
- Describe the hazard.
- Let consumers know what they need to do.
- Provide company contact information – telephone number, email and website.
- Use various communication tools to provide recall information to the public.
- Make information available in English and French.
- Publish the consumer recall notice.
- Determining the recall effectiveness
- Continue to monitor the progress of the recall.
- Stay in touch with your supply chain customers, monitor consumer response and track incidents reported after the recall.
- Keep recall information on your website until your company has deemed the recall to be effective. The time may vary depending on the type of product, size and scope of the recall.
- Inform the CPSP of any issues or changes with the recall.
Appendix C – Health Canada Recall questions
The following questions are used by Health Canada to gather information for product recalls. Some of this information will be used to draft a recall notice for posting to the Healthy Canadians website (www.healthcanada.gc.ca/cps-recalls and www.santecanada.gc.ca/rappels-spc). Additionally, social media platforms such as Facebook and Twitter may also be used by Health Canada to broaden the recall message and increase consumer awareness.
Establishment information
- Provide the name of the establishment recalling the product in Canada.
- Provide the product manufacturer's name and address.
- Provide the product supplier's name and address (if not the manufacturer).
Product information
- Provide a full description of the product, including: Name, brand, description, identifying features (e.g., model number, UPC, serial number, lot/production codes, colours/patterns) and the location of these identifying features, which could help the consumer to identify the products. If applicable, provide the model numbers or other identifying features that differ between products sold in Canada and products sold in other countries.
- Identify any similar products from the same source that may pose the same hazard.
- Provide high quality jpg image files of the recalled product(s). These will be posted to the Healthy Canadians website. There should be no text added to the image.
- What is the country of origin of the recalled product(s)?
For certified products (CSA, ULC, etc.)
- Provide the certification file number and a copy of the certification record demonstrating compliance to the appropriate safety standard.
Product hazard information
- Describe the nature of the hazard posed by the recalled product.
- Have you received any reports of incidents or injuries to Canadians? If yes, provide the number and the nature of the incidents and/or injuries. Have you received any reports of incidents or injuries internationally? If yes, provide the number and the nature of the incidents and/or injuries for each applicable country. Standard descriptions for various incidents and injuries can be found in Section 3 of the Consumer Product Incident Report - Form for Industry.
Product distribution information
- How many units of the recalled product were sold in Canada?
- To whom in Canada did you sell or distribute this product? Provide a complete distribution list including distributor/retailer names, full addresses, phone numbers and, if available, the quantities sold to each (preferably in an Excel spreadsheet format). For any sales made through online platforms that you are aware of, include any unique sellers that you distribute to.
- During what period of time (year/month) was the product sold (distribution, retail, and online) in Canada?
- A) Was this product distributed in the United States or other countries? If so, do you intend to recall the product in the United States or other countries?
For consumer products (not cosmetics):
B) If you intend on posting a joint recall with Health Canada and the Consumer Product Safety Commission (CPSC) in the United States, please contact the CPSC immediately and provide them with your permission to share recall information with Health Canada. The CPSC can only coordinate a joint recall with Health Canada after you have provided them with this permission.
Please confirm if you have provided CPSC permission to share the recall information with Health Canada.
Yes____ No ____If you have already contacted the CPSC, please provide Health Canada with the name of the CPSC person(s) contacted: ______________________________
Actions for consumers
- What are your recommended actions for consumers to take if they have the recalled product(s)? For example: return for refund, request replacement/repair kit, dispose of product, etc.
- If applicable, provide a description and product image of how consumers will be able to distinguish between the recalled products and the corrected/replacement product. Provide test reports/certification documents for replacement and corrected products.
- Provide the contact information for consumer support (e.g. phone number, e-mail address, and/or website) and the operating hours (specify the time zone) during which consumers can obtain additional information and assistance. Health Canada recommended that this service be available in both of Canada's official languages, English and French.
Additional information requested
- On what date did you make the decision to recall these products? What prompted this decision?
- On what date was the recalled product removed from distribution and sale by your company?
- On what date was the recalled product removed from online sales and advertisement?
- On what date did you notify your clients of the recall, and inform them of what to do with the recalled product (removal from sale, or other appropriate action)?
- What instructions have been or will be given to your clients regarding what to do with the recalled product? When did you initiate contact with affected clients regarding the disposition of the recalled products? If no contact has been made, when do you intend to do so? Provide a copy of the notice given to your clients.
- What will be the final fate/disposition of the recalled products that you and your clients (not end users) have in stock and those returned to you? Health Canada may request proof of disposal or export (photos, bill of lading showing export, etc.).
- Health Canada recommends that the recall notice be posted on the company website. Will other social media platforms be used? Describe the other measures you are taking to communicate the recall to the public.
Additional information requested for certified products (CSA, ULC, etc.)
- Have you reported this product safety issue to the Certification Body as required by your agreement with them?
- Do you give Health Canada permission to share the final draft recall notice with the certification body to make them aware of the upcoming recall?
Appendix D – Level 1 Recall notice template – Health Canada
Alert: <Company> recalls <product name>
- Starting date: (ENTER DATE)
- Posting date: (ENTER DATE)
- Type of communication: Consumer Product Recall
- Subcategory: (Product category)
- Source of recall: Health Canada
- Issue: Product Safety
- Audience: General Public (GP)
- Identification number: (Generated by system number)
Affected products
<Brand> <product name>
Product description
This recall involves <brand><product name>. Describe the product and include specific identifiers such as model number, lot number, UPC and certification listing number if appropriate.
Hazard identified
Include a statement on the nature of the issue with the product and the hazard it poses to the consumer.
Include information such as numbers of reports of incidents and/or injuries associated with the product and hazard.
Number sold
Approximately <xx> of the recalled products were sold in Canada.
Time period sold
The recalled products were sold from <month year> to <month year>.
Place of origin
Manufactured in <country>.
Companies <include companies as appropriate>
- Manufacturer
- Importer
- Distributor
What you should do
Consumers should immediately stop using the recalled products and <include directions for the consumers>.
For more information, consumers may contact <company> by telephone at 1-xxx-xxx-xxxx <include hours of operation and days>, by email or visit the firm's website <add website link>.
For consumer product recalls … Please note that the Canada Consumer Product Safety Act prohibits recalled products from being redistributed, sold or even given away in Canada.
For cosmetic recalls … Please note that, in addition to the requirements of the Cosmetic Regulations, the Food and Drugs Act (FDA) prohibits the sale of cosmetics that are either made with hazardous substances, or under unsanitary conditions, to users in Canada.
Health Canada would like to remind Canadians to report any health or safety incidents related to the use of this product or any other consumer product or cosmetic by filling out the Consumer Product Incident Report Form.
This recall is also posted on the OECD Global Portal on Product Recalls website. You can visit this site for more information on other international consumer product recalls.
Images
Add clear jpg image(s) of recall product and images of any other relevant information.
Images should have separate and simple captions.
Appendix E – Level 2 and 3 Recall notice template – Health Canada
<Company> recalls <product name>
- Starting date: (ENTER DATE)
- Posting date: (ENTER DATE)
- Type of communication: Consumer Product Recall
- Subcategory: (Product category)
- Source of recall: Health Canada
- Issue: Product Safety
- Audience: General Public (GP)
- Identification number: (Generated by system number)
Affected products
<Brand> <product name>
Product description
This recall involves <brand><product name>. Describe the product and include specific identifiers such as model number, lot number, UPC and certification listing number if appropriate.
Hazard identified
Include a statement on the nature of the issue with the product and the hazard it poses to the consumer.
Include information such as numbers of reports of incidents and/or injuries associated with the product and hazard.
Number sold
Approximately <xx> of the recalled products were sold in Canada.
Time period sold
The recalled products were sold from <month year> to <month year>.
Place of origin
Manufactured in <country>.
Companies <include companies as appropriate>
- Manufacturer
- Importer
- Distributor
What you should do
Consumers should immediately stop using the recalled products and <include directions for the consumers>.
For more information, consumers may contact <company> by telephone at 1-xxx-xxx-xxxx <include hours of operation and days>, by email or visit the firm's website <add website link>.
For consumer product recalls … Please note that the Canada Consumer Product Safety Act prohibits recalled products from being redistributed, sold or even given away in Canada.
For cosmetic recalls … Please note that, in addition to the requirements of the Cosmetic Regulations, the Food and Drugs Act (FDA) prohibits the sale of cosmetics that are either made with hazardous substances, or under unsanitary conditions, to users in Canada.
Health Canada would like to remind Canadians to report any health or safety incidents related to the use of this product or any other consumer product or cosmetic by filling out the Consumer Product Incident Report Form.
This recall is also posted on the OECD Global Portal on Product Recalls website. You can visit this site for more information on other international consumer product recalls.
Images
Add clear jpg image(s) of recall product and images of any other relevant information.
Images should have separate and simple captions.
Appendix F – Level 2 and 3 Recall notice template – Joint Health Canada and CPSC
<Company> recalls <product name>
- Starting date: (ENTER DATE)
- Posting date: (ENTER DATE)
- Type of communication: Consumer Product Recall
- Subcategory: (Product category)
- Source of recall: Health Canada
- Issue: Product Safety
- Audience: General Public (GP)
- Identification number: (Generated by system number)
Joint recall
Joint recall with Health Canada, the United States Consumer Product Safety Commission (US CPSC) and <company>
Affected product
<Brand> <product name>
Product description
This recall involves <brand><product name>. Describe the product and include specific identifiers such as model number, lot number, UPC and certification listing number if appropriate.
Hazard identified
Include a statement on the nature of the issue with the product and the hazard it poses to the consumer.
Include information such as numbers of reports of incidents and/or injuries associated with the product and hazard (for both Canada and the US).
Number sold
Approximately <xx> of the recalled products were sold in Canada and about <xx> were sold in the United States.
Time period sold
The recalled products were sold from <month year> to <month year> in Canada and the United States. <If different time periods for each country are identified, two separate sentences can be used.>
Place of origin
Manufactured in <country>.
Companies <include companies as appropriate>
- Manufacturer
- Importer
- Distributor
What you should do
Consumers should immediately stop using the recalled products and <include directions for the consumers>.
For more information, consumers may contact <company> by telephone at 1-xxx-xxx-xxxx <include hours of operation and days>, by email or visit the firm's website <add website link>.
Consumers may view the release by the US CPSC on the Commission's website.
Please note that the Canada Consumer Product Safety Act prohibits recalled products from being redistributed, sold or even given away in Canada.
Health Canada would like to remind Canadians to report any health or safety incidents related to the use of this product or any other consumer product or cosmetic by filling out the Consumer Product Incident Report Form.
This recall is also posted on the OECD Global Portal on Product Recalls website. You can visit this site for more information on other international consumer product recalls.
Images
Add clear jpg image(s) of recall product and images of any other relevant information.
Images should have separate and simple captions.
Appendix G – Health Canada Order to Recall notice template
Health Canada orders <Company> to recall <product name>
- Starting date: (ENTER DATE)
- Posting date: (ENTER DATE)
- Type of communication: Consumer Product Recall
- Subcategory: (Product category)
- Source of recall: Health Canada
- Issue: Product Safety
- Audience: General Public (GP)
- Identification number: (Generated by system number)
Affected products
<Brand> <product name>
Product description
This recall involves <brand><product name>. Describe the product and include specific identifiers such as model number, lot number, UPC and certification listing number if appropriate.
Hazard identified
Include a statement on the nature of the issue with the product and the hazard it poses to the consumer.
Include information such as numbers of reports of incidents and/or injuries associated with the product and hazard.
Number sold
Approximately <xx> of the recalled products were sold in Canada.
Time period sold
The recalled products were sold from <month year> to <month year>.
Place of origin
Manufactured in <country>.
Companies <include companies as appropriate>
- Manufacturer
- Importer
- Distributor
What you should do
Consumers should immediately stop using the recalled products and <include directions for the consumers>.
For more information, consumers may contact <company> by telephone at 1-xxx-xxx-xxxx <include hours of operation and days>, by email or visit the firm's website <add website link>.
Please note that the Canada Consumer Product Safety Act prohibits recalled products from being redistributed, sold or even given away in Canada.
Health Canada would like to remind Canadians to report any health or safety incidents related to the use of this product or any other consumer product or cosmetic by filling out the Consumer Product Incident Report Form.
This recall is also posted on the OECD Global Portal on Product Recalls website. You can visit this site for more information on other international consumer product recalls.
Images
Add clear jpg image(s) of recall product and images of any other relevant information.
Images should have separate and simple captions.
Appendix H – Health Canada Regional Product Safety offices responsible for the United States of America and International countries
Figure 1. Health Canada Regional Product Safety Offices Responsible for the United States of America
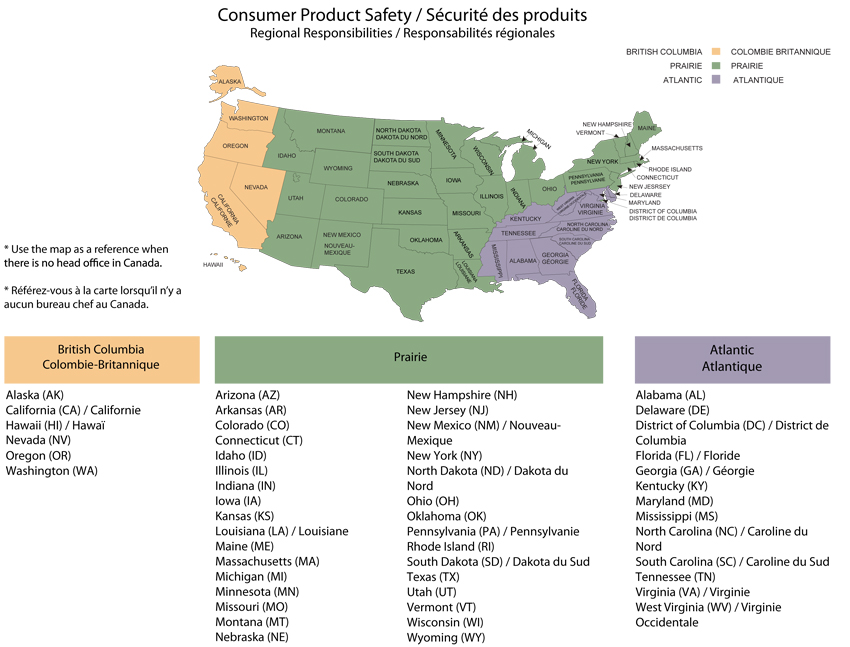
Text equivalent
Graphic of a map showing the Health Canada Regional Product Safety Offices responsible for the various American states. The British Columbia region works primarily with the west coast states, The Prairie region works primarily with the central and northeastern states and the Atlantic region works primarily with the southern and southeastern states.
Figure 2. Health Canada Regional Product Safety Offices Responsible for International Countries
Text equivalent
Graphic of a map showing the Health Canada Regional Product Safety Offices responsible for international countries. The British Columbia region works with Asia, the Prairie region works with central and South America, Africa and Australia, and the Atlantic region works with Europe.