Annual Compliance and Enforcement Report Fiscal Year: 2018-2019
Table of Contents
- Acronyms
- 1. What we do
- 2. Executive Summary
- 3. Compliance and Enforcement Key Activities
- 4. Conclusion
- 5. Contact Information
- Appendix A – Examples of Product Types in "Product Categories"
Acronyms
- CBSA
- Canada Border Services Agency
- CCPSA
- Canada Consumer Product Safety Act
- CPSP
- Consumer Product Safety Program
- CVI
- Compliance Verification Inspection
- FDA
- Food and Drugs Act
- MIR
- Mandatory Incident Reporting
- US CPSC
- United States Consumer Product Safety Commission
1. What we do
1.1 Mission
To identify, assess, manage, and communicate health or safety risks to Canadians associated with consumer products and cosmetics.
1.2 Vision
A Canada where Canadians are confident that consumer products and cosmetics are safe or can be used safely and that is recognized as a world leader in the reduction of health risks posed by consumer products and cosmetics.
1.3 Program Description
The Consumer Product Safety Program (CPSP) is responsible for the administration and enforcement of the Canada Consumer Product Safety Act (CCPSA), and regulations made under it, as well as cosmetic-related provisions of the Food and Drugs Act (FDA) and the Cosmetic Regulations.
Health Canada promotes, monitors, verifies, and enforces compliance with the CCPSA and the FDA. CPSP reviews reports submitted by industry and consumers and regularly monitors the marketplace to look for potentially dangerous products. CPSP gathers information, both domestically and internationally, about injuries, emerging issues and new science related to consumer product safety. CPSP also conducts routine sampling and testing of products in the marketplace and works closely with the Canada Border Services Agency (CBSA) and other regulators domestically and internationally to verify the compliance of products being imported into Canada.
CPSP uses this information to identify possible risks to Canadians posed by consumer products or cosmetics. We conduct risk assessments to identify issues that may result in serious injury. This allows CPSP to target the Program's compliance and enforcement resources towards products that pose the greatest potential risk to Canadians. CPSP uses a triage-based approach to identify product-related health and safety issues for follow up compliance and enforcement activity. CPSP monitors issues that do not require immediate attention so that we are prepared to take action if the risk changes over time.
Finally, CPSP provides credible and reliable information that facilitates public education and provides tools for informed decision-making by the public.
CPSP's authorities do not include products excluded from the CCPSA in Schedule 1 such as explosives, drugs, food, medical devices, ammunition, natural health products, and tobacco products, nor do they include products regulated under the FDA beyond cosmetics.
1.4 What Compliance and Enforcement Activities We Do
CPSP's compliance and enforcement activities focus on promoting and verifying compliance, as well as on correcting non-compliances with regulatory obligations, prohibitions, product standards, and reporting and notification requirements.
1.5 How Compliance and Enforcement Activities Are Conducted
The safety of consumer products and cosmetics is regulated through a post-market approach in Canada. Despite the fact that there is no pre-approval required for industry members to sell their products in Canada, industry is still responsible for ensuring their compliance with the legislation, including that the consumer products and cosmetics they manufacture, import, sell, or advertise in Canada do not pose a danger to human health or safety.
CPSP directs its resources to where the human health risks are greatest. This approach is similar to risk-based approaches used by our major trading partners. Tools used by CPSP to identify and manage these risks include:
- Planning targeted inspections;
- Sampling and testing products;
- Following up on incidents and complaints involving consumer products;
- Attending trade shows;
- Communicating the risks to Canadians;
- Reviewing records from establishments, such as test reports;
- Negotiating and, if necessary, ordering corrective measures such as stopping sale and recalling a product; and
- Working with our other federal and foreign counterparts (such as the CBSA and the US CPSC, respectively) to prevent the importation of non-compliant consumer products and cosmetics into Canada.
2. Executive Summary
The Consumer Product Safety Program (CPSP) of Health Canada helps protect Canadians by assessing the health risks and safety hazards associated with consumer products and cosmetics. From April 1, 2018 to March 31, 2019, CPSP conducted the following compliance and enforcement (C&E) activities:
- Carried out 1,105 planned product-based inspections across 12 different product categories;
- Carried out 54 planned establishment-based, compliance verification inspections;
- Responded to 2,063 referrals from the Canada Border Services Agency (CBSA) leading to the refusal of 526 (25.5%) shipments;
- Reviewed 2,635 consumer complaints and incident reports (1,611 from Industry, 1,024 from Consumers);
- Identified a total of 792 different non-compliant products requiring corrective action, 233 of which resulted in a recall (149 recalls being a result of reported incidents and 84 recalls being a result of CPSP product inspections);
- Carried out 4,343 monitoring inspections to ensure recalled products were not for sale; and
- Received and processed 56,755 cosmetic notifications.
Key highlights included:
- Compared to 2017-2018, CPSP saw changes in the following areas:
- A 15% decrease in consumer reports received and a 19% increase in industry reports received; and
- an overall trend in the collaboration between Canada and the United States for recalls is in a steady decline over the last several years, with a sharp 37% drop over the last year.
3. Compliance and Enforcement Key Activities
3.1 Following-up on Consumer Complaints and Mandatory Incident Reports
CPSP reviews reports submitted by industry and consumers to look for potentially dangerous products. Under section 14 of the CCPSA, industry must report to Health Canada after it becomes aware of a health or safety incident involving its consumer product. An incident can include:
- An occurrence, a product defect, or incorrect or a lack of information that resulted or may result in death or serious negative impacts on health; or
- A product recall in another jurisdiction based on concerns about human health or safety.
When reports are received by CPSP, they are reviewed and triaged to determine if action is required to address the risk. Product returns or complaints from consumers will often trigger reporting requirements for industry, which leads to the submission of reports describing the incident and any corrective measure(s) that the company is implementing.
In 2018-2019, 2,635 reports were received by the program: 1,024 from consumers and 1,611 from industry. Table 1 shows the most frequently reported product categories for those submitted reports.
| Product Category | Percent |
|---|---|
| Housewares | 22 |
| Appliances | 20 |
| Children's Products | 18 |
| Electronics | 12 |
| Home and Automotive Maintenance | 8 |
| Grooming Products and Accessories | 7 |
| Outdoor Living | 5 |
| Clothing, Textiles, and Accessories | 3 |
| Sports, Recreation, and Hobby | 3 |
Of the 2,635 reports received, 149 (5.7%) resulted in the posting of a recallFootnote 1. Table 2 shows the most frequently recalled product categories.
| Product Category | Percent |
|---|---|
| Children's Products | 20 |
| Housewares | 17 |
| Home and Automotive Maintenance | 14 |
| Sports, Recreation, and Hobby | 13 |
| Outdoor Living | 9 |
| Clothing, Textiles, and Accessories | 9 |
| Appliances | 7 |
| Electronics | 6 |
| Grooming Products, and Accessories | 4 |
Table 2 shows similar trends from previous years, except Grooming Products, Clothing, textiles and accessories have decreased nearly 50%, whereas Home and Automative Maintenance products have increased by 60%.
There is no incident reporting requirement for cosmetics under the FDA and Cosmetic Regulations, although the Program does encourage consumers and industry members to report when they have health or safety concerns related to a cosmetic. Under section 30 of the Cosmetic Regulations, manufacturers and importers must notify Health Canada within 10 days after they first sell a cosmetic in Canada. Failure to notify may result in a product being denied entry into Canada or removed from sale. Over the course of 2018/2019, the Program received 56,755 cosmetic notifications, which is a slight increase from the 56,612 notifications received in 2017/2018.
3.2 Collaboration with the United States
Canada actively collaborates with the US CPSC on consumer product safety as both countries share similar distribution networks, general approaches to consumer product health and safety standards, and enforcement activities. Information gathered on products of concern in the United States is used by CPSP to determine if compliance or enforcement activities should be initiated in Canada. When Canadian establishments are initiating a product recall and are present in both jurisdictions, CPSP reminds them of their reporting requirements to the US CPSC, and vice-versa.
In 2018-2019, 60 joint recalls were posted by Health Canada and the US CPSC. The most frequent product types jointly recalled and their relative percentages are shown in Table 3.
| Product Category | Percent |
|---|---|
| Sports, Recreation, and Hobby | 20 |
| Electronics | 17 |
| Children's Products | 15 |
| Outdoor Living | 15 |
| Housewares | 13 |
| Appliances | 12 |
| Home and Automotive Maintenance | 7 |
| Grooming Products and Accessories | 2 |
| Clothing, Textiles, and Accessories | 0 |
The overall trend for the past few years for collaboration between Canada and the United States is a decrease, with a sharp 37% drop over the last year. Figure 1 illustrates the overall number of joint recalls over the past 7 fiscal years showing the general trend.
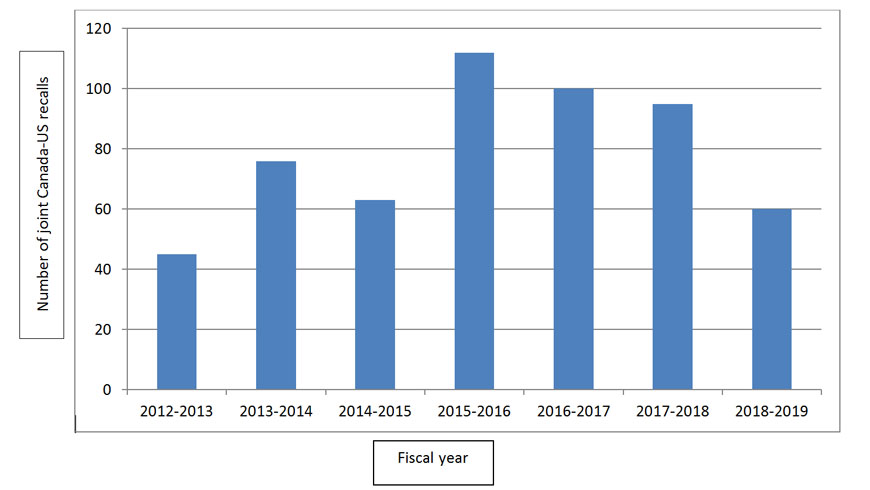
Figure 1 - Text Description
Graphic showing the number of joint Canada-US recalls from fiscal years 2012 to 2019. In 2012-2013 there were 45 recalls, in 2013-2014 there were 76 recalls, in 2014-2015 there were 63 recalls, in 2015-2016 there were 112 recalls, in 2016-2017 there were 100 recalls, in 2017-2018 there were 95 recalls and in 2018-2019 there were 60 recalls.
Health Canada and the US CPSC continue to work towards posting recalls with Mexico's Consumer Protection Federal Agency (Profeco). In 2018-2019, 2 recalls were posted jointly by all three regulators, compared to 2017-2018, where 5 recalls were posted jointly. This continues the decline of recalls posted jointly by all three regulators over the last few years.
3.3 Targeted Inspections
One of the key tools that CPSP uses to support the goal of targeted oversight is planned, targeted inspections. CPSP uses a risk-based approach to monitor and verify industry compliance with the regulations for consumer and cosmetic products on a cyclical basis. Sampling and inspecting for compliance is targeted to those regulated product categories and establishments where CPSP intelligence indicates elevated levels of non-compliance. In many cases, inspectors can identify a higher probability of non-compliance based on previous market analysis, information gathered from other Health Canada inspectors or in the field using readily identifiable indicators, such as labelling or packaging issues, missing certification marks, or design issues that may lead to regulatory violations. Due to this targeted approach, higher rates of non-compliance are expected than if sampling was random.
3.3.1 Establishment Inspections
The purpose of conducting establishment inspections is to evaluate industry's ability to identify and report incidents to CPSP and evaluate record-keeping practices. The results of these inspections also help CPSP assess how well a company's internal product safety quality system is functioning and also how to more effectively focus CPSP's resources.
In 2015-2016, CPSP piloted a Compliance Verification Inspection (CVI) approach with inspections of 15 establishments at the retail head office level. In 2016-2017, this approach was repeated and expanded to 69 establishments at the importer, distributor and retail level. Similarly, in 2017-2018 this establishment inspection project was repeated and manufacturers were added with 58 establishments at all levels being carried out. During this fiscal year, 2018/2019, 48 establishments at all levels were carried out. CPSP concluded that this approach can be effective in helping establishments comply, and in identifying establishments at higher risk of non-compliance. Higher-risk establishments will be more likely to be selected as targets for product inspections. CVI is now being evolved to a more compliance education approach in hopes to improve industry compliance to the legislation.
While a specific establishment inspection project on mandatory incident reporting was expected to start in 2017-2018, it was deferred to 2018-2019 in an effort to improve the process and guidance to industry based on the lessons learned in 2016-2017. The Industry Guide on Mandatory reporting was updated in 2018 and in 2018-2019, 6 Mandatory Incident reporting (MIR) were carried out.
3.3.2 Product Inspections
In addition to establishment inspections, CPSP conducts inspections targeted to specific product types to monitor and verify industry compliance with the CCPSA and the FDA. Compliance is verified by either conducting product sampling and testing, or by requesting documents, such as test reports or safety information, to evaluate compliance with the relevant requirements. Projects are chosen every year depending on factors such as emerging trends, the level of risk a product poses, the type of product, industry's previous level of compliance, the hazards posed by the product and the vulnerability of product users.
In 2018-2019, projects for 12 different product categories were completed resulting in 1,105 inspections. In total, 1,272 products were assessed leading to 643 enforcement actions, including 84 product recalls. Table 4 describes those 12 different targeted inspection product categories and also gives details on the means by which compliance determinations were made. For more details on the results of the individual projects see the Enforcement Summary Reports published online. Publication of these reports is part of the Government of Canada's commitment to regulatory transparency and openness.
The cyclical enforcement product-based projects (Table 4) included in this annual report are those that had enforcement summary reports approved by March 31, 2019. Other CE projects that started in 2018-2019 but did not have enforcement summary reports approved by March 31, 2019 are not included in the calculations.
The reason they are not included is that the corrective actions had not yet been finalized, even though we know some recalls were posted. These projects ran over many months and/or started late in current fiscal year, and the results for those will be captured in the next annual report.
| Project | Number of Inspections | Evaluation Means (and applicable legislation) | Number of Samples Assessed | Findings of Non-compliance (%) | Recall | Stop Sale | Stop Distribution | Trader Commitment | Other |
|---|---|---|---|---|---|---|---|---|---|
| Started in 2017-2018 and completed in 2018-2019 | |||||||||
| USB Chargers (Standards Project) | 114 | Sampling and testing (Canada Consumer Product Safety Act) and (sections of the CSA Standard C22.2 No. 60950-1-07 – Information Technology Equipment – Safety – Part 1: General Requirements) | 67 | 40 (60%) | 40 | 0 | 0 | 0 | 0 |
| Consumer Chemicals and Containers | 15 | Document review and sampling and testing (Consumer Chemicals and Containers Regulations, 2001) | 46 | 33 (72%) | 10 | 0 | 11 | 12 | 0 |
| Mechanical requirements of toys | 35 | Onsite assessment and sampling and testing (Toys Regulations) | 29 | 12 (41%) | 11 | 0 | 0 | 1 | 0 |
| Second-hand children's products | 490 | (Canada Consumer Product Safety Act) | 488 | 320 (66%) | 0 | 252 | 0 | 68 | 0 |
| Carriages and Strollers (mechanical and chemical testing) | 30 | Sampling and testing Carriages and Strollers Regulations | 30 | 18 (60%) | 5 | 1 | 6 | 6 | 0 |
| Started in 2018-2019 and completed in 2018-2019 | |||||||||
| Vaping Liquids | 270 | Sampling and testing (Canada Consumer Product Safety Act) and (Consumer Chemicals and Containers Regulations, 2001) |
497 | 180 (36%) | 1 | 58 | 0 | 121 | 0 |
| Lighters | 11 | Sampling and testing (Lighters Regulations) | 9 | 9 (100%) | 4 | 0 | 1 | 4 | 0 |
| Cellulose Insulation | 10 | Sampling and testing (Cellulose Fibre Insulation) Regulations) | 10 | 6 (60%) | 0 | 0 | 5 | 1 | 0 |
| Cosmetics (Baby Wipes) | 30 | Sampling and testing (Food and Drugs Act and Cosmetic Regulations) | 30 | 3 (10%) | 0 | 0 | 0 | 3 | 0 |
| Kettles | 5 | Sampling and testing (Kettles Regulations) | 5 | 0 (0%) | 0 | 0 | 0 | 0 | 0 |
| Lasers (Standards Project) | 51 | Sample and testing (International Electrotechnical Commission Standard IEC 60825-1:2014 Ed.3, Safety of laser products- Part 1: Equipment classification and requirements) | 24 | 3 (13%) | 0 | 3 | 0 | 0 | 0 |
| Toys (Retail) | 44 | Onsite assessment and sampling and testing (Toys Regulations) | 37 | 19 (51%) | 13 | 6 | 0 | 0 | 0 |
| Total | 1,105 | 1,272 | 643 (51%) | 84 | 320 | 23 | 216 | 0 | |
Based on the inspections that were conducted, the following projects ranked among those categories with the highest levels of non-compliance:
- Lighters with 100% non-compliance
- Consumer Chemicals and Containers with 72% non-compliance
- Second hand children's products with 66% non-compliance
- Carriages and Strollers (mechanical and chemical testing) with 60% non-compliance
- USB Chargers with 60% non-compliance
- Cellulose Insulation with 60% non-compliance
With respect to these product categories, high-risk violations, among other non-compliances, were identified and a number of product recalls were required. As noted in Table 4, a wide range of enforcement actions were taken with USB chargers, toys, second hand children's products, products subject to the CCCR, 2001 and vaping products. Given the broad type and volume of products available for sale within these product categories, these compliance projects continue to be carried out regularly to verify compliance and educate industry on the product requirements.
Overall, while working with industry to address all non-compliance, CPSP continues to focus its more serious enforcement actions, such as recall, on products where a high risk has been identified.
Other notable projects completed over 2018-2019 include the following listed below. These were surveys to see what was on the Canadian market and hence, no enforcement actions were taken.
a) Market Survey of Residential Smoke Detectors
C&E-2018-011 Residential Detectors: The purpose of this market survey was to update our knowledge of the residential detector products available on the Canadian marketplace and determine which certification marks were visible on the product packaging.
The products included in this survey were all found to have certification marks present on their packaging or product itself. However, the compliance approach should be to verify the validity of the certification marks by requesting compliance information from the responsible establishments and/or certification bodies. Due to the variety of certification marks found on the products, following up with the responsible establishments or certification bodies will allow Health Canada inspectors to better identify valid certification marks on these products and confirm compliance to the Residential Detectors Regulations.
b) Market Survey of Liquid Laundry Detergent Packets
C&E-018-018 Liquid Laundry Detergent Packages: The purpose of this Market Survey project was to review liquid laundry detergent packets. In addition to compliance to the Consumer Chemicals and Containers Regulations, 2001, a voluntary industry standard is available for this product, specifically Standard Safety Specification for Liquid Laundry Packets (ASTM F3159-15e1). Of the 29 products evaluated, 27 appeared to meet the ASTM standard based on visual assessment, resulting in a 93% compliance rate.
Health Canada continues to evaluate whether the performance criteria of this consensus-based safety standard are sufficient to mitigate the risk associated with these products.
c) Market Survey of Ice Hockey Helmets and Face Protectors
C&E-018-007 Market Survey of Ice Hockey Helmets and Face Protectors for Ice Hockey and Box Lacrosse Players: The purpose of this survey was to gather information on the ice hockey helmets and face protectors currently available on the Canadian marketplace, with the intention of using the information to guide future action and projects that may be taken within each of these product areas. Similar to past years, the presence of CSA labelling on ice hockey helmets and face protectors being sold in Canada remained high. However, verification of compliance to the current standard was made more challenging in that some products did not have the year of manufacture included in the label as required by the CSA standards that the regulations reference. Ongoing engagement with the CSA should be maintained in an effort to share such issues since they are the Certification Body providing the product certification listing.
3.3.3 Recall Monitoring Inspections
Whether an establishment agrees to voluntarily recall a product or has been ordered to do so (for consumer products under the CCPSA only), they should contact their supply chain accounts to ensure that the product is no longer offered for sale. Through recall monitoring inspections, CPSP verifies that selected supply chain accounts have received the notification of the recall from the responsible establishment and have taken the necessary steps to remove the product from sale; further minimizing the hazard in the Canadian marketplace. In 2018-2019, 4,343 recall monitoring inspections were conducted.
3.3.4 Import Admissibility Recommendations
CPSP works with the CBSA to identify non-compliant products being shipped into the country. By stopping non-compliant products at the border, CPSP is able to prevent these products from reaching the market and thus reduce the potential for risk to Canadians. In 2018-2019, CPSP received 2,063 referrals from the CBSA for shipments that were flagged as being potentially non-compliant and requiring input from CPSP, about which 40% were cosmetics. Of these referrals, 526 were recommended for refusal and 293 of those refusals involved cosmetics. Shipments may be refused for a variety of reasons, including that the products are prohibited, or do not meet specific regulatory requirements.
4. Conclusion
By continuing to implement its key compliance and enforcement activities, CPSP is able to quickly identify and act on product safety risks while keeping Canadians informed about health and safety issues related to consumer products and cosmetics.
Compared to 2017-2018, CPSP saw some the following trends with various activities conducted in 2018-2019:
- A 15% decrease in consumer reports received (about 180 less) and a 19% increase in industry reports received (about 260 more). While there were particular events throughout the year that contributed to higher than usual reports, we still see a consistent rise in the overall number of reports from Industry received by the Program.
- Cosmetic notifications received went up only by 0.3% (about 140 more).
With respect to product inspections carried out in 2018-2019, there were 1,272 products assessed which resulted in a 51% non-compliance rating. This is a much higher non-compliance rating than the 32% seen in 2017-2018, however product categories change from year to year within the established 6-year cycle of rotating consumer chemical categories (see examples in Appendix A). Therefore, it is not easy, nor correct, to compare the data from the previous year. As mentioned in section 3.3, a systematic bias is also applied during inspection and sampling.
All information gathered from compliance and enforcement activities during 2018-2019 and the reports received will be used in the planning of future years' activities. These data serve as timely and relevant intelligence that helps in targeting existing and upcoming product safety risks as efficiently as possible.
5. Contact Information
Any questions or comments on this report should be directed to cps-spc@hc-sc.gc.ca.
Appendix A – Examples of Product Types in "Product Categories"
- Appliances: Kitchen appliances; heating and cooling appliances; laundry and cleaning appliances.
- Children's Products: Nursery products; baby gear; toys.
- Clothing, Textiles, and Accessories: Clothing; household textiles; footwear.
- Electronics: Televisions and home theatres; electronic cables, batteries and chargers; computers and peripherals; cellphones and accessories.
- Home and Automobile Maintenance: Construction materials; tools.
- Housewares: Furniture; home décor; lighting; household cleaning; kitchenware.
- Outdoor Living: Outdoor furniture and decorations; pools and accessories; lawn and garden.
- Grooming Products and Accessories: Beauty and body care; beauty accessories; oral care.
- Sports, Recreation, and Hobby: Sports and outdoor activities; play structures; hobby or crafts.