Regulatory Directive DIR2018-01, Policy on Cancellations and Amendments Following Re-evaluation and Special Review
Pest Management Regulatory Agency
7 March 2018
ISSN: 1498-5926 (PDF version)
Catalogue number: H113-3/2018-1E-PDF (PDF version)
This Regulatory Directive, DIR2018-01, Policy on Cancellations and Amendments Following Re-evaluation and Special Review, has been prepared taking into consideration the comments received in response to Regulatory Proposal PRO2016-04, Policy on Cancellations and Amendments Following Re-evaluation and Special Review, published on 21 December 2016. Open "Summary of Public Comments" to view the comments received along with the responses by PMRA.
Table of Contents
- 1.0 Purpose
- 2.0 Context and Legal Framework
- 3.0 Definitions
- 4.0 Scope
- 5.0 Health and Environmental Considerations for Determining Cancellation and Amendment Timelines
- 6.0 Procedures
- 7.0 Delay of the Implementation Date of Re-evaluation or Special Review Decision
- 8.0 Failure to Implement Required Changes
- 9.0 Policy Implementation
- Appendix I Timelines for Amendment and Cancellation of Pest Control Products
- Appendix II Legislative Authority
1.0 Purpose
The purpose of this policy is to provide a framework for the cancellation of pesticide products or amendments to pesticide product uses, labels, or other conditions of registration following a re-evaluation or special review decision. The policy also outlines the process, the associated timelines as well as how the timelines for cancellation or amendment of pesticide products are established.
This policy is intended to enhance transparency of the process and associated timelines when regulatory action is required to remove products from the market, change approved uses, or introduce amendments to labels. It is also intended to facilitate efficient and effective implementation of re-evaluation and special review decisions. Standardized timelines aim to clarify expectations, obligations and communications around the implementation of regulatory decisions.
2.0 Context and Legal Framework
Pesticides in Canada are regulated by Health Canada's Pest Management Regulatory Agency (PMRA) under the authority of the Pest Control Products Act, to prevent unacceptable risks to human health and the environment. Before pesticides are approved, an extensive body of data and information must be provided by the applicant. The PMRA conducts human health and environmental risk assessments, as well as an assessment of a product's value, by applying established science-based standards.
Furthermore, the Pest Control Products Act contains two separate mechanisms for the post-market review of registered pest control products, namely re-evaluations and special reviews under sections 16 and 17 respectively. Under s.16(1), the Minister may initiate a re-evaluation in circumstances where there has been a change in information requirements, or a change in the procedures used to evaluate risks or value, since the pesticide was initially registered. In addition, the Minister must initiate a re-evaluations of a pesticide no later than one year after 15 years since its most recent major decision affecting the registration including its initial registration (s.16(2)). Therefore, pesticides in Canada undergo periodic reviews to ensure that they continue to meet current standards for continued use in Canada.
A special review is initiated when new information provides reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable, or when an Organisation for Economic Co-operation and Development (OECD) member country prohibits all uses of an active ingredient for health or environmental reasons (s.17).
During a re-evaluation or special review, pursuant to s.20(1), the Minister may amend or cancel the registration if a registrant fails to provide information required under s.16(3), s.18(1) or para. 19(1)(a) of the Pest Control Products Act, or if the Minister has reasonable grounds to believe that the amendment or cancellation is necessary to deal with a situation that endangers human health or safety or the environment, taking into account the precautionary principle (s. 20(1)(b); s.20(2)). The Pest Control Products Act also provides the authority to amend or cancel the registration of a pest control product when, after conducting the necessary scientific evaluations and consultations, the Minister does not consider the risks or value of the product to be acceptable (para. 21(2)(a) and (b)). In these circumstances a phase-out period may be implemented as part of the decision, commensurate with the level of risk.
When the re-evaluation or special review decision is to cancel a registration of a pest control product, continued possession, handling, storage, distribution and use of stocks may be allowed, subject to any conditions considered necessary (para. 21(5)(a)). The implementation date of cancellation may be delayed if no suitable alternatives to the use of the pesticide is available, and a determination is made that human health and environmental risks and value of the product are considered acceptable until the effective date of the amendment or cancellation (s.21(3)).
This policy has undergone a 60-day public consultation period as Regulatory Proposal PRO2016-04, Policy on Cancellations and Amendments Following Re-evaluation and Special Review which was published on 21 December 2016. Comments received during this consultation period were taken into consideration for the preparation of this document.
3.0 Definitions
In the context of re-evaluation and special review,
- Amendment is a change to the conditions of registration of a product as authorized or required by the Pest Control Products Act (s.20, s.21). Changes can include additional risk mitigation measures, or cancellation of certain uses. When required by re-evaluation or special review these changes are primarily enacted by revising the approved product label, typically through an applicationFootnote 1 (also refer to Appendix I.a)
- Cancellation is a termination of a product registration as authorized or required by the Pest Control Products Act (s.20, s.21), for example, due to risks of concern or failure to provide required data (also refer to Appendix I.b)
- Phase-out Period is a limited time by which a cancellation or amendment is implemented.
The relevant sections of the Pest Control Products Act are found in Appendix II.
4.0 Scope
This policy applies to the cancellation of pesticide products or amendments to product uses, labels, and other conditions of registration during or following a re-evaluation or special review. This policy does not address voluntary amendments (s.7(1)) or voluntary discontinuations (s.22(1)) by the registrant.
5.0 Health and Environmental Considerations for Determining Cancellation and Amendment Timelines
The primary consideration for the implementation timelines for cancellation and amendment is based on the risks of concern to human health or the environment, i.e., whether risks are considered imminent and serious, taking into account the following factors:
- Potential magnitude of harm, i.e., seriousness of the effect of concern, including reversibility;
- Likelihood of the effect occurring, i.e., whether an effect of concern is likely to happen based on how the product is being used;
- The population exposed to the product, for example, trained pesticide applicators, the general public, or bystanders; and
- Information from post-market surveillance considered as part of the re-evaluation or special review, for example, incident reports, poison control centre data, or monitoring data.
In cases where no imminent and serious risks to human health or the environment are identified, the implementation timelines outlined in Section 6.0 of this document are applied to re-evaluation and special review decision requirements.
Implementation may be expedited when risks of concern are considered to be imminent and serious. Such circumstances involve a significant likelihood of serious effects occurring, for example, adverse effects reported in incident reports submitted to the PMRA involving death or serious bodily harm. In these circumstances, other appropriate measures may also be required, such as requiring the registrant to over-sticker labels on existing stocks with risk mitigation statements, or issuing an immediate product recall in accordance with the Pest Control Products Act (s.21(5)(b)).
The implementation date of amendment or cancellation may be delayed if no suitable alternatives to the use of the pesticide exist, so long as the human health and environmental risks, as well as value of the product, are considered to be acceptable until the effective date of the amendment or cancellation (s.21(3)). The suitability of potential alternatives as replacements is determined by factors such as whether they can provide a reasonable level of control of the pest, the economic impact of the change, or whether the change would promote misuse of other products or practices.
6.0 Procedures
6.1 Cancellation Timelines
Product cancellations may be required due to failure to provide information required for re-evaluation or special review under the Pest Control Products Act; or when products do not meet current standards to protect human health and/or the environment. Where risks of concern are not considered imminent and serious, existing stocks of the products are to be phased-out following timelines outlined below (also refer to Appendix I.b Cancellation Timelines):
- One (1) year of sale by registrant from the date of re-evaluation or special review decision, followed by;
- One (1) year of sale by retailer from the last date of sale by registrant, followed by;
- One (1) year of permitted use from the last date of sale by retailer.
In other words, existing stocks of the product will be phased-out in Canada within three (3) years from a re-evaluation or special review decision date, following a sequential timeline provided to each level of supply chain as described above. Any remaining product must be appropriately disposed of. The implementation timeline is intended to allow a limited opportunity to exhaust existing stocks at each level of supply chain (at registrant, retail, and user levels), to minimize potential risks associated with disposing of large quantities of existing product, and to transition to suitable alternatives.
Subsequent to the decision, if at any point the PMRA determines that imminent and serious risks to human health and/or the environment may exist, an expedited phase-out will be implemented on a case-by-case basis, commensurate with the likelihood and severity of the risk as per Section 5.0 of this document.
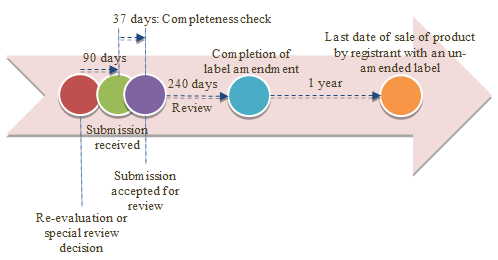
6.2 Amendment Timelines
When an amendment to a registration is determined to be necessary as a result of the product not meeting current standards for human health and/or environmental protection, such as the need for additional risk mitigation measures or the cancellation of certain uses (refer to Appendix I.a):
- The PMRA notifies registrants of the need to amend their product registration and update product labels to reflect the required amendments. The PMRA also communicates the required process and implementation timelines.
- Registrants submit an application. The PMRA reviews the applications within the performance standard (i.e., 37 calendar days for a completeness check followed by 240 calendar days for review).
When there are no imminent and serious risks to human health or environment, registrants will generally have up to two (2) years from the date of the decision to transition to selling the product with the newly amended labels.
Subsequent to the decision, if at any point it is determined that imminent and serious risks to human health and/or the environment may exist, expedited timelines will be determined on a case-by-case basis commensurate with the likelihood and severity of the risk.
7.0 Delay of the Implementation Date of Re-evaluation or Special Review Decision
During the public consultation period following the publication of a proposed decision, registrants or users may request a delay of the implementation date of re-evaluation or special review decision with evidence supporting a lack of suitable alternatives. Delay of the implementation date of up to an additional two (2) years may be considered by the PMRA on a case-by-case basis as per section 21 (3) of the Pest Control Products Act.
During phase-out periods and thereafter, registrants wishing to revise the conditions of re-evaluation and special review decisions may submit a new application to the PMRA for review, as per the PMRA's Management of Submissions Policy (DIR2017-01). These submissions may be for either new product registrations, or to amend any existing registrations. Such submissions must be accompanied by relevant information/data to support the submission.
8.0 Failure to Implement Required Changes
Failure to make the required changes to product labels and/or phase-out products when required to do so could result in regulatory actions in accordance with the Pest Control Products Act.
9.0 Policy Implementation
This policy is in effect as of the date of this regulatory directive.
Appendix I Timelines for Amendment and Cancellation of Pest Control Products
a) Amendment Timelines
Figure 1. Amendment Timelines
b) Cancellation Timelines
Figure 2. Cancellation Timelines

Appendix II Legislative Authority
This Appendix lists the sections of the Pest Control Products Act which are relevant to the amendment or cancellation of registration of pest control products in the context of re-evaluation or special review.
Section 7
- An application to register a pest control product or to amend the product's registration must be made to the Minister in the form and manner directed by the Minister and must include any information or other thing that is required by the regulations to accompany the application.
Section 16
- The Minister may initiate the re-evaluation of a registered pest control product if the Minister considers that, since the product was registered, there has been a change in the information required, or the procedures used, for the evaluation of the health or environmental risks or the value of pest control products of the same class or kind.
- Without limiting the generality of subsection (1),
- if a decision of a type referred to in paragraph 28(1)(a) or (b) was made in relation to a pest control product on or after April 1, 1995, the Minister shall initiate a re-evaluation of that product no later than one year after 15 years have elapsed since the most recent decision of that type; and
- if the most recent decision of a type referred to in paragraph 28(1)(a) or (b) was made in relation to a pest control product before April 1, 1995, the Minister shall initiate a re-evaluation of that product no later than April 1, 2005 or the date that is one year after 15 years have elapsed since that decision, whichever date is later.
- Re-evaluation of a pest control product is initiated by the Minister delivering a notice in writing to the registrant explaining the reasons for initiating the re-evaluation and, if considered necessary by the Minister, requiring the registrant to provide information in the form and within the period specified in the notice.
Section 17
- The Minister shall initiate a special review of the registration of a pest control product if the Minister has reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable.
- Without limiting the generality of subsection (1), when a member country of the Organisation for Economic Co-operation and Development prohibits all uses of an active ingredient for health or environmental reasons, the Minister shall initiate a special review of registered pest control products containing that active ingredient.
- Without limiting the generality of subsection (1), the Minister shall initiate a special review of the registration of a pest control product if a federal or provincial government department or agency has provided information to the Minister that relates to the health or environmental risks or the value of the product and if, after considering the information provided, the Minister has reasonable grounds to believe that the health or environmental risks of the product are, or its value is, unacceptable.
- Any person may request a special review of the registration of a pest control product by making a request to the Minister in the form and manner directed by the Minister.
Section 18
- A special review of a pest control product is initiated by the Minister delivering a notice in writing to the registrant explaining the reasons for initiating the special review and, if considered necessary by the Minister, requiring the registrant to provide information in the form and within the period specified in the notice.
Section 19
- During an evaluation that is done in the course of a re-evaluation or special review,
- the Minister may, by delivering a notice in writing, require the registrant to provide, in the form and within the period specified in the notice, additional information that the Minister considers necessary for the evaluation;
Section 20
- The Minister may cancel or amend the registration of a pest control product if
- the registrant fails to satisfy a requirement under subsection 16(3) or 18(1) or paragraph 19(1)(a).
- in the course of a re-evaluation or special review, the Minister has reasonable grounds to believe that the cancellation or amendment is necessary to deal with a situation that endangers human health or safety or the environment, taking into account the precautionary principle set out in subsection (2).
- Where there are threats of serious or irreversible damage, lack of full scientific certainty shall not be used as a reason for postponing cost-effective measures to prevent adverse health impact or environmental degradation.
- The Minister may rescind any action taken under subsection (1) if the circumstances that prompted it cease to exist.
- If the registration of a pest control product is cancelled or amended under paragraph (1)(a), the Minister may, for a prescribed period, refuse to consider any application made in respect of that product by the registrant.
Section 21
- If the Minister does not consider that the health or environmental risks or value of a pest control product are acceptable, the Minister shall
- amend the registration if the Minister considers that the health and environmental risks and value of the product would be acceptable after the amendment; or
- cancel the registration.
- The Minister may delay the effective date of the amendment or cancellation if
- no suitable alternative to the use of the pest control product is available; and
- the Minister considers that the health and environmental risks and value of the product are acceptable until the effective date of the amendment or cancellation.
- A delay is subject to any conditions that the Minister considers necessary for carrying out the purposes of this Act.
- When cancelling the registration of a pest control product under this section or any other provision of this Act, the Minister may
- allow the continued possession, handling, storage, distribution and use of stocks of the product in Canada at the time of cancellation, subject to any conditions, including disposal procedures, that the Minister considers necessary for carrying out the purposes of this Act;
- require the registrant to recall and dispose of the product in a manner specified by the Minister; or
- seize and dispose of the product.
Section 22
- A registrant who intends to discontinue the sale of a pest control product for one or more uses for which it is registered shall notify the Minister of that intention in the form and manner directed by the Minister.
- The Minister may deliver a notice in writing to the registrant requiring the registrant to explain the reasons for the discontinuation.
- On receipt of notification under subsection (1), the Minister shall cancel or amend the registration, as the case may be, as of a date to be determined by the Minister and, pending that date, may impose any conditions that the Minister considers necessary for carrying out the purposes of this Act.
Footnotes
- Footnote 1
-
Submission category as prescribed in Regulatory Directive DIR2017-01, Management of Submissions Policy