PMRA Guidance Document, A Framework for Risk Assessment and Risk Management of Pest Control Products
Pest Management Regulatory Agency
12 April 2024
PDF Version (468.5 KB, 26 pages)
Document (Revision/Update) History
| Updated | Update/Rationale |
|---|---|
| 12 April 2024 | Revised update to Section 6.1.1 Assessing risks to human health |
| 28 July 2021 | Revised update to Section 7.4 Monitoring and Evaluating the Results |
| 27 January 2021 | This Guidance Document supercedes Science Policy Note SPN2000-01, A Decision Framework for Risk Assessment and Risk Management in the Pest Management Regulatory Agency |
Table of Contents
- 1.0 Executive summary
- 2.0 Purpose
- 3.0 Introduction
- 4.0 Overview of the framework
- 5.0 Identifying the issue and its context (problem formulation)
- 6.0 Assessing risk and value
- 7.0 Managing the risks
- 8.0 Involving interested and affected parties
- List of acronyms and abbreviations
1.0 Executive summary
Health Canada's Pest Management Regulatory Agency (PMRA) uses a well-defined, internationally-recognized risk assessment and risk management framework in its decision-making. This document describes the framework for developing, implementing and monitoring pesticide registration decisions, and consists of the following steps:
- Identify the issue and its context (problem formulation),
- Assess risks and value,
- Identify and analyze risk mitigation and management options,
- Select an appropriate risk management strategy,
- Implement the chosen strategy,
- Monitor and evaluate results.
Involving interested and affected parties is integral to the overall process. The PMRA Framework for Risk Assessment and Risk Management is designed to protect human health and the environment. A pesticide will not be registered unless it has acceptable risks and provides value when used according to the conditions of registration, which includes following label directions. Decisions are made on the basis of a comprehensive body of published and registrant-supplied scientific evidence and scientific methods used to determine the nature and extent of the risks posed by pesticides and by applying appropriate and effective risk management strategies.
2.0 Purpose
This document describes the framework that guides Health Canada's Pest Management Regulatory Agency (PMRA) in the assessment and management of risk and its regulatory decision-making. The PMRA uses a comprehensive body of scientific methods and evidence to determine the nature as well as the magnitude of potential risks posed by pest control products (pesticides). This approach allows for the protection of human health and the environment through the application of appropriate and effective risk management strategies.Footnote 1
3.0 Introduction
Pesticides are designed to control, destroy, attract or repel pests, or to mitigate or prevent pests' injurious, noxious or troublesome effects. Because of the properties and characteristics that make pesticides effective for their intended purposes, they also may pose risks to people and the environment.
The Pest Control Products ActFootnote 2 and its associated Regulations govern the manufacture, possession, handling, storage, transport, importation, distribution and use of pesticides in Canada. The Pest Control Products Act is administered by Health Canada's PMRA. As required under the Pest Control Products Act, a new pesticide is registered only if potential risks to human health and the environment, and value are determined to be acceptable when the product is used according to the conditions of registration, which includes following label directions. This includes consideration of active ingredients, formulants and any potential contaminants in pesticide products. All registered pesticides are regularly re-evaluated to ensure that they continue to meet current health and environmental safety standards and continue to have value. Furthermore, a special review of a registered pesticide may be conducted to address any newly identified specific aspect(s) of concern of a pesticide, and to determine its continued acceptability for registration. The PMRA applies a science-based risk assessment and risk management approach to determine the acceptability of risks to human health and the environment.
The PMRA's scientific risk-based approach to the regulation of pesticides is consistent with international standards and is similar to Health Canada's regulatory approach for other types of chemicals. This framework provides predictability and transparency to the process used to protect the health of Canadians and their environment and helps ensure risk management decision-making considers all relevant criteria in a comprehensive fashion. It also provides sufficient flexibility to incorporate alternative approaches such as RISK21 methodology and tools developed by the Health and Environmental Sciences Institute (HESI), when applicable.Footnote 3
In developing a framework based on the assessment and management of risk, the PMRA considered the following:
- the sources from which risks may arise,
- the types of activities that may cause risks to arise,
- the means available for assessing the likelihood, degree and duration of risks,
- the means available to mitigate and minimize risks,
- appropriate means to involve stakeholders in the decision-making process, and
- appropriate means to enable and facilitate interaction and cooperation with other jurisdictions and regulatory bodies.
The majority of registration decisions within the PMRA concern synthetic chemical pesticides. Accordingly, this framework is based to a large extent on the processes and approaches used to arrive at decisions about a new synthetic chemical pesticide, a major new use, post-market pesticides under re-evaluation or special review. It may also be used when considering incident reports examined during these processes. This framework also applies to registration decisions for biopesticides (microbial and pheromone pesticides), non-synthetic pesticides (plant extracts, or other naturally derived substances), and devices, with modifications specific to each situation.
4.0 Overview of the framework
The framework is divided into a number of identifiable decision steps and components, as noted below and in Figure 1:
- A clear identification of the issue and context (problem formulation),
- An assessment of the risk to human health and the environment, and the value of the product (taking into account the conditions of use),
- A methodology to mitigate and manage the risk, which involves identification and analysis of various options leading to the selection of a risk management strategy, and
- Ongoing monitoring and evaluation of the results.
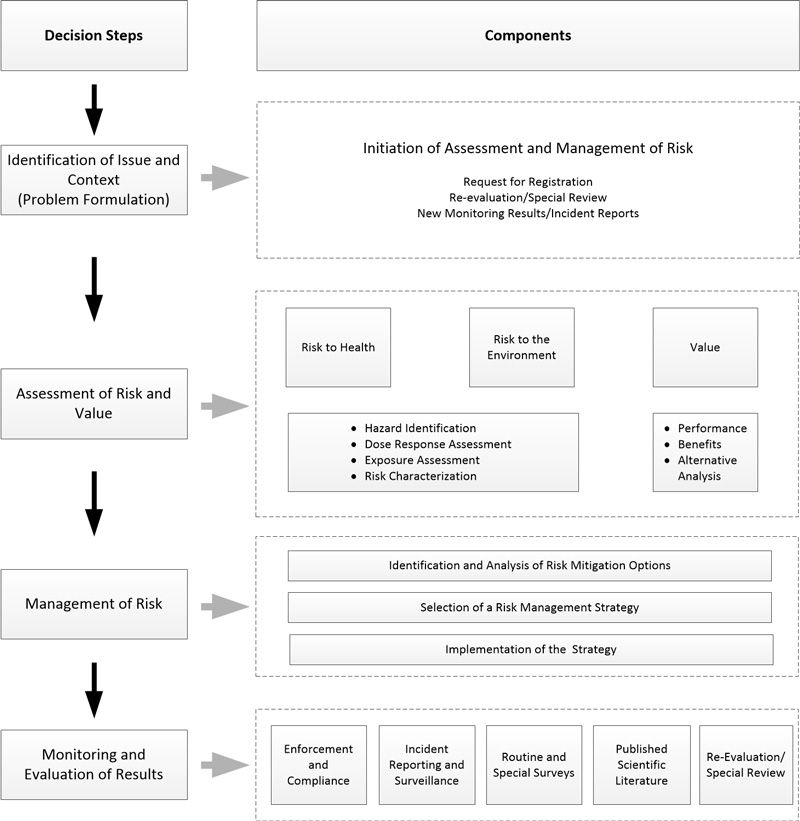
Figure 1 - Text Description
The processes shown in figure 1 consists of the following steps:
- A clear identification of the issue and context (problem formulation). This involves the initiation of assessment and risk management and can include a Request for Registration, a re-evaluation/Special Review, and may require new monitoring results and additional incident reports
- An assessment of the risk to human health and the environment; and an assessment the value of the product (taking into account the conditions of use). The assessment of human health and environmental risk can include hazard identification, dose response and exposure assessments and a risk characterization; while the value assessment may include considerations of the products overall performance, benefits, and an analysis of any alternatives.
- Once the risk is determined, a methodology to mitigate and manage that risk is developed. This involves identification and analysis of any risk mitigation options, the selection of a risk management strategy followed by the implementation of that strategy.
- Ongoing monitoring and evaluation of the results by:
- Enforcement and compliance
- Incident reporting and surveillance
- Routine and special surveys
- Published scientific literature
- Re-evaluation and special reviews
Figure 1 Process Flow Showing the Stages of Risk Assessment and Risk Management of Pest Control Products. The Process Flow is as follows: Identification of the issue and context; assessing the risks to human health and the environment, and the value of the product; management of risk, which involves identifying and analysing various options leading to selection of a risk management strategy; and ongoing monitoring and evaluation of the results.
Although the framework is presented as a series of sequential steps leading from a starting point, such as an application to register a new pesticide, to a defined end point such as the decision to register, the underlying process is highly iterative and interactive. This is particularly evident in the development of risk management options. If there is a concern that the use of a product as proposed by the applicant may be associated with an unacceptable level of risk, the PMRA will consider restrictions on use or other conditions to reduce the risk to acceptable levels. The process usually results in a number of possible risk management options. Each of these options must be described in sufficient detail to allow quantitative re-examination of the potential risks. Typically, this requires several iterations of the assessment of risk and recalculation of risk under the different options considered.
The same approach is followed for post-market assessments (re-evaluations and special reviews).
5.0 Identifying the issue and its context (problem formulation)
Since all pesticides must be authorized before they can be sold or used in Canada, the most common trigger for initiating the decision-making process is an applicant request for registration of a new pesticide. The process will also be triggered by a re-evaluation, a special review, in amending an existing product registration, or by receipt of new information and may require additional incident reporting.
This process is governed by the Pest Control Products Act, which provides the authority for decision-making on the basis of scientific risk assessment and risk management. It requires a risk-based, rigorous approach for new products that are subject to pre-market approval, and requires re-evaluation or special review to ensure that the risks associated with the use of registered pest control products remain acceptable.
The Pest Control Products Act dictates that the risks and value of a product, when used according to the conditions of registration, which includes following label directions, must be considered acceptable by the Minister of Health for it to enter and remain on the market in Canada. The legislation also includes various enforcement provisions to allow inspectors to monitor compliance with the Pest Control Products Act and its associated Regulations and address non-compliance through a variety of legislative tools. Provincial pesticide legislation also plays an important role in the overall process of pesticide regulation in Canada.Footnote 4
Pesticide registration decisions must also be considered in the context of the Toxic Substances Management Policy (TSMP),Footnote 5 and international agreements such as the Stockholm Convention on Persistent Organic Pollutants. The presence of substances such as these in existing pesticide products as active ingredients, formulants or contaminants could lead to a reassessment of their registration status and regulatory action consistent with pertinent federal policies and international commitments. It is also important to note that a person must still comply with requirements under any other relevant federal statute (for example, the Fisheries Act) when using a pesticide that is approved under the Pest Control Products Act.
As in other Organisation for Economic Co-operation and Development (OECD) countries, detailed risk and value assessment and risk management methodology or policies are not included in statute or regulation, but rather in directives and guidelines, so that they can be adapted quickly as scientific knowledge and public policy evolve.
6.0 Assessing risk and value
Assessments of health risk, environmental risk, and value are central to the PMRA's decision-making process. They provide a solid factual and contextual basis for making sound registration decisions that protect human health and the environment from unacceptable risks from pesticides. Each of the three components (health risk, environmental risk and value) must be acceptable before a pesticide can be registered. This means that products that are not effective do not have acceptable value and, therefore, would not be registered even if the health and environmental risks were acceptable. Conversely, if a product is very efficacious and useful to an important commodity, it would not be registered if health and/or environmental risks are not acceptable. The development of the required conditions of use that are feasible, is also a key part in assessing risk and value.
6.1 The risk component (human health and environment)
The risks to human health and the environment comprise the two risk components of the assessment process. This is due to the broad international consensus among regulatory agencies that the acceptability of a pesticide should be predicated on the nature, degree and duration of risk it poses. The PMRA applies a science-based approach to assessing pesticides that considers both the toxicity and the level of exposure to fully characterize risk. The extensive pre-market assessment of pesticides allows the PMRA to identify potential hazards and risks to human health and the environment prior to making the registration decision.
The PMRA's risk assessments follow a structured, predictable process that is consistent with international approaches and the Health Canada Decision-Making Framework for Identifying, Assessing, and Managing Health Risks.Footnote 6 Assessments are largely based on a required set of scientific data that must be provided by the applicant. These assessments estimate potential risks to defined populations under specific exposure conditions. They are conducted in the context of well-defined use scenarios, such as the use of a new pesticide on a particular field crop using specified application rates, methods and equipment.
Potentially exposed populations and environments are also defined and considered in the risk assessment. The data required from applicants in support of a pesticide registration are tailored to the different proposed uses. The PMRA has specified extensive and detailed data requirements and guidance for over 30 different use scenarios.Footnote 7,Footnote 8,Footnote 9
Only products with a database that fulfills all scientific requirements or have acceptable scientific waiver rationales, are allowed to progress within the evaluation process and reach the decision stage, which includes a public consultation step on all proposed major decisions. These data are generated in accordance with validated study protocols and internationally accepted OECD Principles of Good Laboratory Practice.Footnote 10,Footnote 11 Risk assessments can, and often do, use additional scientific data from other sources, such as published scientific literature, incident reports and international regulatory organizations.
Integral to the PMRA's risk assessment process is the incorporation of standard protection factors to further protect human health. These factors provide an inherent level of protection from exposures that could result in adverse effects to human health. Furthermore, the PMRA applies additional protection factors if warranted by the hazard profile of the pesticide or by the quality and completeness of the underlying data.
Re-evaluation and special review entail assessing the risks associated with the use of registered pesticides and the acceptability of these risks in light of current regulatory and scientific standards. The same steps as described for the pre-market assessment are used during re-evaluation and special review.
6.1.1 Assessing risks to human health
The purpose of assessing the risks to human health is to define the nature of potential hazards and to determine the degree and likelihood of the risk associated with a defined exposure. The PMRA considers that pesticides may pose higher risks to certain groups of people based on differences in factorsFootnote 12 such as: biology, gender, sex, behaviour, age and occupation. The PMRA's decisions about how pesticides can be used are based on protecting the most sensitive group. The Pest Control Products Act requires that pesticide evaluations consider more sensitive populations, including pregnant women, infants, children, women and seniors.
The assessment follows a four-step scientifically established and internationally accepted process:
- Hazard identification
- Dose–response assessment
- Exposure assessment, and
- Risk characterization.
Hazard identification
Animal toxicity studies are the main source of information for identifying hazards (toxic effects or potential adverse health effects) and for determining the relationship between dose and response. These studies are considered to be well-understood predictors of potential toxicity in humans. The PMRA relies heavily on a broad range of toxicology data (for example, developmental toxicity, neurotoxicity, long-term toxicity and reproductive toxicity studies) to establish reference doses for acute and repeated or chronic dietary exposure (referred to as acute reference dose (ARfD) and acceptable daily intake (ADI), respectively), non-dietary exposures, and to derive estimates of potential cancer risks, if warranted. Benchmark dose (BMD) is also used to establish the point of departure for risk assessment purposes, when applicable.
The PMRA is also an active collaborator and participant in national and global initiatives exploring the utility and implementation of approaches to reduce animal testing in regulatory decision-making. This includes implementing changes to existing data requirements to support pesticide registrations, such as the removal of the routine requirement for certain animal studies, along with exploring how to further incorporate pharmacokinetic and toxicokinetic information. As new in vitro methods evolve, these are also integrated into the hazard assessment process, as appropriate.
Dose–response assessment
With few exceptions, for example, certain cancer and mutagenic effects, most toxic effects occur only when a dose threshold has been exceeded. These differences are considered using two different approaches for assessing the acceptability of risks to human health: a margin of safety approach for "threshold" effects and in the case of cancer effects where a dose threshold has not been established, an assessment is based on the probability or likelihood of adverse effects at estimated exposure levels.
For toxic effects that have a threshold, the PMRA establishes reference values (ARfD, ADI) for assessing dietary exposures that consider both the acute and the chronic nature of the toxic effects. Other reference values to assess exposures of different durations (short-term (1–30 days), intermediate-term (1–6 months) and long-term (greater than 6 months)) are also established for non-dietary exposure scenarios for occupational and residential risk assessments.
The starting point for calculating the reference value is identifying the no observed adverse effect level (NOAEL) for each toxicity study. This is the highest level of exposure in test animals that does not cause adverse effects. Then, the lowest NOAEL from all applicable studies examined for a toxic effect observed in animals, (and that is relevant to humans), is selected. This NOAEL is usually from a study in which animal exposure is representative of the route, frequency and duration of human exposure. In addition, the NOAEL is typically selected from the test species that exhibits the greatest sensitivity to the toxic effects of the pesticide.
Reference values also consider uncertainties arising from the extrapolation of effects observed in animals to potential effects in humans, and that some humans in the population may be more sensitive to potential effects than others. Accordingly, the reference value incorporates two uncertainty (protection) factors: a 10-fold factor to account for extrapolation from animals to humans (interspecies extrapolation) and an additional 10-fold factor to account for the variation within the human population (intraspecies variability). In this way, the calculated reference value for humans is a minimum of 100-fold lower than the dose that caused no adverse effects in animal studies. In addition to these two 10-fold factors, additional factors may be applied to derive the reference value to address the severity of an effect or any concerns or uncertainties about the toxicity information.
Under certain conditions, the Pest Control Products Act requires the application of a further 10-fold factor, referred to as the PCPA factor, to provide additional protection for pregnant women, infants and children in the risk assessment. Where reliable scientific data are available, a different factor may be applied. Details on the application of the Pest Control Products Act factor (PCPA factor) are provided in Science Policy Note SPN2008-01, The Application of Uncertainty Factors and the Pest Control Products Act Factor in the Human Health Risk Assessment of Pesticides.
The assessment of a pesticide for which a dose threshold has not been established follows a different kind of assessment. Cancer assessment for pesticides is based on evidence from cancer studies in at least two species, usually the rat and the mouse, together with evidence from in vitro and in vivo genotoxicity studies. These studies are typically carried out at dose levels that are much higher than expected human exposures. In many cases these are also complemented with studies that shed light on the mechanism or biological mode of action by which the pesticide causes cancer. The outcome of the animal studies together with mechanistic considerations are used in a weight-of-evidence approach to decide if a pesticide is likely to pose a cancer hazard to humans. This type of approach is consistent with that used by other international agencies and authorities.
Exposure assessment and risk characterization
The determination of whether dietary exposure is acceptable is made by comparing the estimated human exposure to the dietary reference value (ARfD and ADI). Exposures that fall below the reference value are considered to provide sufficient margins of safety and therefore do not present health risks of concern. For non-dietary exposure scenarios, exposure estimates are compared to the appropriate NOAEL to calculate a margin of exposure. If the calculated margin of exposure is equal to, or greater than the target margin of exposure, risks are considered acceptable. Science Policy Note, SPN2014-01, General Exposure Factor Inputs for Dietary, Occupational, and Residential Exposure Assessments provides information on various exposure inputs used to estimate pesticide exposures to the population for dietary, occupational, and residential exposure scenarios.
For non-threshold effects, sophisticated statistical models are used to estimate potential risks at the lower levels of exposure seen in humans, based on high dose animal studies. A model used widely for regulatory purposes in determining cancer risk is the linearized multistage (LMS) model.Footnote 13 This model calculates the likelihood or probability of developing cancer (lifetime cancer risk) from an average daily lifetime exposure. For example, a 1 × l0−6 cancer risk means that an average daily exposure to a substance increases the likelihood of cancer by one additional case per one million exposed over a lifetime.
A lifetime cancer risk that is below 1 × 10−6 (one in a million) is generally an indicator of acceptable risk for the general population when exposure occurs through pesticide residues in or on food, and to otherwise unintentionally exposed persons. Cancer risks in the range of 1 × 10−5 to 1 × 10−6 (one in one-hundred thousand to one in a million) are considered acceptable for workers exposed occupationally to potentially carcinogenic chemicals. These risk ranges in conjnction with other factors considered in the risk assessment are used by the PMRA, as well as other jurisdictions nationally and internationally, as a guide in reaching decisions about the acceptability of lifetime cancer risk.
Both types of risk assessment, the margin of safety threshold approach and the non-threshold cancer risk assessment, provide estimates of risk arising from various types of exposures. Usually the risk estimate reflects a "typical" exposure and use situation, taking into consideration whether exposure is occasional or frequent and of short or long (life-time) duration. However, in order to afford a standard of protection, the process generally overestimates exposure and risk by using "worst case" or high-end assumptions, such as assuming that 100 percent of a crop would be treated at the maximum application rate, or that 100 percent of the pesticide deposited on the skin would penetrate through the skin.
The PMRA also aggregates exposure for a single pesticide active ingredient by combining potential exposures from all food residues and drinking water, as well as exposure from residential activities, when applicable. Cumulative risk assessments (the combined risk from several pesticides) are conducted for pesticides with a common mechanism of toxicity. Science Policy Note, SPN2018-02, Cumulative Health Risk Assessment Framework describes the PMRA's framework for assessing the cumulative health effects of pesticides.
6.1.2 Assessing risks to the environment
The purpose of the environmental risk assessment is to determine whether adverse effects from the use of pesticides may occur to organisms and to the natural environment itself. Similar to the approach for assessing the risks to human health, the environmental risk assessment assesses the exposure (environmental fate and behaviour) and hazard (toxic effects on organisms) and characterizes the risks posed by pesticides. An examination of the environmental chemistry and fate considers the potential for exposure to the pesticide. This includes the possibility for the pesticide to move into sensitive environmental compartments at the site where the pesticide is used, such as groundwater or lakes and rivers, as well as the possibility for atmospheric transport and deposition into remote regions, such as the Arctic. The hazard assessment examines effects on a vast number of plants and animals, and includes considering effects on biodiversity and the food chain. The characterization of environmental risk requires the integration of information on environmental exposure and effects to identify which, if any, organisms or environmental compartments may be at risk, as well as any uncertainties in characterizing the risk. Where risks to the environment are identified, strategies to reduce those risks are considered.
To estimate environmental exposure to pesticides, it is essential to have a detailed understanding of the pesticide's fate characteristics, including physicochemical properties, transformation rates in soils and water systems, formation of transformation products, and mobility information including the adsorption of the pesticide to soils, as well as knowing how, when, and under what conditions a pesticide is used. These fate characteristics allow the PMRA to predict the movement of the pesticide in soil, water and air, as well as the potential for uptake by plants or animals and the transfer from organism to organism through the food web to higher trophic levels. Using standard models, the PMRA calculates estimated environmental concentrations (EECs) of the pesticide in various environmental media, such as soil, water, air and food sources for animals. These estimations take into consideration the application rate(s), physicochemical properties, and environmental fate and behaviour properties of the pesticide, including the dissipation of the pesticide between applications. The reliability of the EEC predictions can be enhanced with results from field trials under conditions that reflect the Canadian environment. For post-market reviews of registered pesticides, available monitoring information (for example surface water and groundwater monitoring) is also analyzed to characterize potential exposure to the pesticide.
The PMRA reviews effects information (ecotoxicological data) for various organisms from both terrestrial and aquatic habitats, including invertebrates, vertebrates, and plants. Potential effects in non-target biota are assessed and characterized using a series of internationally recognized indicator species. Terrestrial species are represented by the following major taxonomic groups: birds; mammals; terrestrial invertebrate species including beneficial insects, arthropods, and bees; and terrestrial plants. Potential effects in aquatic biota can be characterized in both freshwater and marine species that include fish, aquatic invertebrates, algal species, and aquatic vascular plants. Acute and chronic effects endpoints are derived from laboratory and field studies that characterize the toxic response and the dose–effect relationship of the pesticide and its major transformation (degradation) products. These effects endpoints are used to predict potential effects on individuals, populations, and communities within ecosystems. The adverse effects considered include lethal (mortality) and sub-lethal effects (such as reduced growth and reproductive impairment). Effects endpoints where 50% of the test population are affected (for example, LC50) as well as the level at which no effects occur (for example, NOEC) are determined. Toxicity effects endpoints used in the environmental risk assessment may be adjusted using uncertainty factors to account for potential differences in species sensitivity as well as varying protection goals (in other words, protection at the community, population, or individual level).
The environmental risk characterization integrates the exposure (environmental fate and behaviour) and effects (ecotoxicological data) information using a tiered, or step-wise, approach. Initially, a screening level risk assessment is performed to identify non-target organisms to which the pesticide (or specific uses of the pesticide) does not pose a risk, and to identify those organisms for which there may be a potential risk. The screening level risk assessment uses simple methods, conservative exposure scenarios (for example, direct application to soil at a maximum cumulative application rate) and sensitive toxicity effects endpoints. Risks may be quantified using a risk quotient approach. A risk quotient (RQ) is calculated by dividing the exposure estimate by an appropriate toxicity value (RQ = exposure/toxicity). The RQ is then compared to the level of concern (LOC), which is set at one for the majority of organisms, with a few validated exceptions. If the screening level RQ is below the LOC, the risk is considered negligible and no further risk characterization is necessary for that organism. If the screening level RQ is equal to or greater than the LOC, then a refined risk assessment is performed to further characterize the risk. The refined assessment takes into consideration more realistic exposure scenarios (such as drift to non-target habitats) and may consider different toxicity endpoints. Refinements may include further characterization of risk based on exposure modelling, monitoring data, results from field or mesocosm studies, and probabilistic risk assessment methods. Refinements to the risk assessment may continue until the risk is adequately characterized or the available data do not permit further refinements. Where potential risks are identified, risk mitigation is also considered to see if risks can be reduced to an acceptable level. The environmental risk characterization is an iterative process incorporating more realistic effects and exposure characterizations as well as risk mitigation options, as required.
6.2 The value component
The primary consideration in the value assessment process is whether the product is efficacious, that is, "does it do what it claims to do" by the applicant. This assessment is based on different types of information including results from field studies, use history information including information from other countries where the product may be currently registered, published papers and scientific rationales. These should be representative of typical use conditions to demonstrate that the pesticide contributes to managing the specific pest or pests, as claimed on the label.
The value assessment contributes to the establishment of use conditions that are necessary to assess risks. The value assessment also allows for the development and evaluation of risk mitigation and management options by providing information on the benefits associated with the use of the pesticide. These benefits may relate to human health, safety and the environment, as well as social and economic impacts; however, as noted previously, the health and environmental risks must be acceptable before a product is considered eligible for registration, regardless of the value of the product. Similarly, if the risks are acceptable, but the product has not provided sufficient information to demonstrate acceptable value, it will not be considered acceptable for registration.
6.2.1 Assessing value
Determining the value of a pesticide is an important element of the pre-market evaluation of pest control products. The PMRA value assessments consist of two components: an assessment of the performance of a pest control product and its benefits.
The PMRA carries out a value assessment for all new pesticides corresponding to a new active ingredient or new formulation, or amendments to existing products proposing new uses, such as addition of new pests, new hosts or new application methods. The extent and focus of the value assessment is case specific. It may include a review of all the components of performance and benefits, or a review of performance only for an amendment to add a new pest to a registered pesticide.
Assessing pesticide performance involves an evaluation of the pesticide's efficacy in controlling the target pest and the potential for the pesticide to damage host crops or use sites. Pesticides that do not contribute to the management of a pest are not acceptable candidates for registration, even if they do not pose risks to human health or the environment.
Where the efficacy of a pesticide is acceptable, the assessment serves to establish appropriate label claims and directions and an application rate (or rate range) that is effective without being excessive, and with no unacceptable damage to the use site or host organism/crop (and subsequent hosts or crops) under normal use conditions.
The efficacy of a pesticide is related to the concentration or amount of the pesticide that is used and the method and timing of use. These factors can also have a significant impact on the risks that are associated with the use. The required amount, method and timing of use for successfully dealing with a pest can lead to unacceptable risks, and thus preclude registration. There is also the possibility of modifying these factors while still maintaining an acceptable level of efficacy, thus providing a significant opportunity for developing risk management options.
When data for an efficacy assessment are derived from field or laboratory trials, the trials are carried out in such a manner as to allow for a determination of the pesticide performance over a variety of conditions. In most cases, proof of performance establishes the value of the pesticide, so that the PMRA would not normally engage in an in-depth or extensive evaluation of benefits. Assessment of benefits may be undertaken in particular cases where performance alone does not sufficiently demonstrate value, or while developing risk management options.
Within the context of health, safety and environmental benefits, the PMRA assesses the compatibility of a pesticide with sustainable agricultural production systems or industrial practices. In particular, the assessment identifies existing alternative methods of control for the target pests, the fit of the pesticide with established integrated pest management (IPM) programs and the role of the pesticide in resistance management strategies.
The introduction of a pesticide with the same mode of action as other registered pesticides may accelerate the development of pest resistance (decreased effectiveness), while a pesticide with a new, unique mode of action may provide the opportunity to delay the development of resistance, or to contribute to managing resistance to other modes of action, thus increasing its value. In addition, an assessment of social and economic impacts may consider aspects such as trade implications and competitiveness, impacts on crop quality, and other indirect effects of the pest on the use site or host organism/crop.
The overall determination of value uses a weight of evidence approach in consideration of the various components of the value assessment and the available supporting information. The relative weight of each component may vary depending on the specific nature of the pest problem and its context.
During re-evaluation, value is examined under current conditions and in light of alternative pest control methods (both chemical and nonchemical) that may have been developed since the pesticide was first registered. The performance component of the value assessment is typically not included during re-evaluation because product performance has been established through its history of use. An assessment of the benefits associated with the pesticide during re-evaluation may serve to demonstrate its value in the current context, and to identify potential alternatives.
6.3 Outcome of the risk and value assessments
As discussed above, the risk and value assessments can have different outcomes, which affect the regulatory decision.
When risks to human health and the environment are acceptable and the pesticide has acceptable value, that is, the pesticide can be used safely and effectively without any modifications to its proposed or existing uses based on the conditions of registration, which includes following label directions, the registration of the pesticide can be granted. In the context of a post-market review, the registration of an existing pesticide can be maintained.
When a pesticide has acceptable value but risks are identified for human health and/or the environment, the PMRA may identify and develop risk mitigation and management options so that health and the environmental protection standards/goals are met (risks are acceptable). Mitigation options that reduce exposure may include requiring protective clothing for applicators, adding or modifying buffer zones to protect the environment, reducing application rates and lengthening pre-harvest intervals, to name a few. The extent of these mitigation measures must not reduce the performance below acceptable levels. If the mitigation measures are not feasible, the product will not be registered.
When the risks to human health and the environment are not acceptable because the potential risks cannot be mitigated through modifications of the conditions of use, or the pesticide's value is unacceptable, then the registration of a new pesticide will be denied. In the context of a re-evaluation or special review, the registration of an existing pesticide will be cancelled.
When the use of the pesticide is incompatible with specific federal policies (TSMP) and Canada's obligations under certain international agreements (for example, Stockhlom Convention on Persistent Organic Pollutants), registration of a new pesticide can be denied, and the registration of an existing pesticide can be reviewed and subsequently cancelled.
7.0 Managing the risks
Based on the results of the assessment phase, a strategy for managing a pesticide's risk is developed. This consists of the following steps:
- Identify and analyse risk mitigation and management options
- Select an appropriate risk management strategy
- Implement the risk management strategy
7.1 Identifying and analyzing risk mitigation and management options
The outcomes of the assessments of risks to human health and the environment, and the assessment of value, are the basis for the next step: identifying and analyzing risk mitigation and management options. The goal is to identify a range of options that have the potential to reduce the extent of human and environmental exposures, and to analyze these options to determine if they can achieve acceptable standards for protecting human health and the environment. The identification and analysis must focus on the nature and extent of the potential risks identified, the source of these risks, and the affected human and/or non-target organisms and associated environment(s). Both the scientists who assessed the potential risks and risk managers participate in the identification and analysis of management options. In addition, information on the agricultural production practices, including technological advances and feasibility of risk management options, are considered in conjunction with information from stakeholders.
As mentioned previously, identifying and analyzing risk mitigation and management options is a dynamic and iterative process, where risks are assessed under various risk mitigation scenarios. In many cases, the choice is not between individual risk management options, but by selecting a combination of options. For example, what may be a reasonable strategy to reduce risk to applicators or farmers (for example, certain granular formulations) may increase risks to the environment (for example, potential increased risk to birds). In this case the product would not be registered, or would not be acceptable for continued registration in the case of re-evaluation. Thus, the combination of risk management options must ensure that all risk elements are considered and are acceptable.
The range of risk mitigation and management options must also reflect legal and practical considerations. The mitigation measures, which are specified in the conditions of use and include directions on the legally binding label, must be consistent with the requirements of the Pest Control Products Act.
The risk mitigation and management options available can include requiring conditions and restrictions on the product with respect to:
- classification of use (domestic, commercial or restricted class),
- provincial permit requirement,
- professional qualification of applicator,
- specification of application technique and equipment,
- personal protective equipment,
- use conditions (use quantities, application rates, timing and frequency of application, pre-harvest intervals, restricted-entry intervals),
- crops or other areas where it can be used (use-site),
- buffer zones and other mitigative measures to protect sensitive environments and particularly vulnerable plant and animal species, and
- safe storage and disposal.
Options can also include required changes to the pesticide product, such as changes to the formulation or the physical and chemical make-up of the pesticide product. If the risks cannot be mitigated through various conditions, the registration will be denied, or cancelled in the context of a re-evaluation or special review.
The selection of risk mitigation and management options is guided by a thorough understanding of the use situation, use practices, application technology, extent of use, and geographical location. This level of detailed understanding is necessary to focus the development of options on those that are appropriate and can realistically be achieved.
The value assessment plays a significant role in defining the limits of certain mitigation and management options. Application rates, frequency, technology and practices influence the effective use of a pesticide and the value assessment provides the basis for determining these practical limits.
In addition to these regulatory approaches to risk mitigation, efforts continue to further strengthen label directions by developing standardized statements that provide clarity and consistency to facilitate pesticide users in their practices and product choice to deal with pests, which can significantly enhance understanding and compliance.Footnote 14
7.2 Selecting a risk management strategy
Selecting a risk management strategy, including selecting one or a combination of management options developed and elaborated in the previous step, involves a great deal of experience and scientific and regulatory expertise. The selection of a strategy is, to a significant degree, based on data indicating that the anticipated risks to human health and the environment are acceptable and that the value is acceptable by implementing this strategy. It also includes experience in deciding if the selected strategy is practical from both a use pattern and a compliance and enforcement perspective.
Part of the process in selecting a strategy involves recalculating margins of exposure or level of risk under various risk management options. This recalculated level of risk provides a measure of how well a management option would make the risk acceptable. Options that do not demonstrate that risks are acceptable are not further pursued.
In-depth knowledge of user groups, their level of experience in pesticide use, their past record on compliance, as well as an understanding of the feasibility of the risk management option, are essential for selecting the most appropriate strategy.
The selection of risk management options, therefore, is case-specific in seeking the optimal combination of choices that achieve an acceptable level of risk while maintaining acceptable value of the product.
7.3 Implementing the risk management strategy
The selected risk management strategy is then implemented as part of the registration, re-evaluation or special review decision. As noted previously, the PMRA specifies the pesticide registration conditions, which also include use directions on the legally binding label. Any use in contravention of the label or other specified conditions is illegal under the Pest Control Products Act.
Most pesticides require very specific measures on how the product can be used safely. In each case, the selected strategy provides the basis for specific registration conditions and restrictions. Conditions and restrictions specified on the label include domestic, commercial, or restricted categorization, which may include a permit requirement under provincial or other federal legislation; use conditions and restrictions; measures to protect users and the environment; restricted-entry and pre-harvest intervals; and buffer zones.
For pesticides used on food crops, maximum residue limits (MRLs) are specified under the Pest Control Products Act. An MRLFootnote 15 is the legal maximum amount of a pesticide residue that is expected to remain on food crops when a given pesticide is used according to the conditions of registration, which includes following label directions. Establishing science-based MRLs helps ensure that pesticides are being used properly by growers, and provides Canadians with access to a safe food supply. To verify that Canada's MRLs are respected, both domestically produced food and imported foods are examined for pesticide residues by the Canadian Food Inspection Agency (CFIA). An MRL exceedance does not automatically indicate risk issues, but indicates the need to investigate further. In the absence of an MRL specified under the Pest Control Products Act, residues on food crops are regulated under subsection B.15.002(1) of the Food and Drug Regulations, which requires that residues not exceed 0.1 ppm.
All registered pesticides are thus regulated in that they can be used only for the specified purposes under specified use conditions.
7.4 Monitoring and evaluating the results
Decisions to register pesticides reflect the state of knowledge and regulatory practices at the time the decision is made. Post-market review, monitoring and surveillance, including incident reporting, all play an essential role to help ensure the continued acceptability and value of registered pesticides.
There are several important elements to the PMRA's post-market review and monitoring. These include: the enforcement of compliance with the Pest Control Products Act and the Food and Drugs Act; the PMRA Incident Reporting Program; pesticide sales reporting; routine inspections and special monitoring (for example, for environmental levels and effects); food residue monitoring; health surveys; cyclical re-evaluation; and special review. The PMRA also has published guidelines for the advertising of pest control products in Canada.Footnote 16
The stronger the need for measures to manage the risks associated with pesticides, the stronger the need to monitor compliance with these measures. The regulatory oversight function is delivered by Health Canada’s Regulatory Operations and Enforcement Branch (ROEB). As Health Canada’s branch dedicated to compliance and enforcement, ROEB provides oversight for various acts and regulations pertaining to products and substances regulated by Health Canada.
ROEB’s Pesticide Compliance Program is responsible for promoting, verifying and enforcing compliance with the Pest Control Products Act and its Regulations. It provides oversight on all parties regulated by the Pest Control Products Act, including pesticide registrants, importers, retailers, and users. To that effect, Health Canada’s Pesticide Compliance Program conducts compliance promotion, compliance verifications (including inspections, sampling, and verifying records) and enforcement activities. When required, enforcement action is taken against regulated parties to address identified non-compliance with the Pest Control Products Act. Health Canada uses a range of enforcement tools including warning letters, compliance orders, notices of violation (NOV) under the Agriculture and Agri-Food Administrative Monetary Penalties (AMPs) Act with warning or monetary penalty, prosecution, seizure. The choice of enforcement actions is reflective of the severity of the risks posed by the identified contraventions.
Additional support mechanisms that help to ensure compliance include certification and training of users, and Best Management Practices for pesticide user sectors. These support mechanisms are largely under provincial oversight. They are encouraged and supported, and in some cases led, by the PMRA through the close interaction among all partners of the Federal/Provincial/Territorial Committee on Pesticide Management and Pesticides (F/P/T Committee).
As noted in Section 7.3, while pesticide MRLsFootnote 17 are established by the PMRA, MRL inspection, compliance and enforcement activities pertaining to food are the responsibility of the Canadian Food Inspection Agency.
The PMRA uses incident reports to identify and characterize potential risks to humans, domestic animals and the environment from the use of pesticides, which were not evident during their initial registration. Incident reports are prioritized based on the type of incident and can require a special review. Serious adverse effects such as death or life-threatening effects are evaluated immediately and mitigation measures are put into place using the post-market process, if warranted. Incident reports also inform risk assessments for new applications and re-evaluations. Monitoring incidents for unanticipated effects is an ongoing process that includes re-assessing previous conclusions, as necessary. In cases where mitigation strategies have been put in place, the PMRA also monitors incident reports to determine if the actions were effective in managing the identified risk. Reporting of pesticide incidents is mandatory for pesticide registrants, and any member of the public can also report a pesticide incidentFootnote 18 to the PMRA.
Monitoring, particularly environmental presence and effects monitoring is carried out by provincial and territorial agencies, other federal government departments and the registrants themselves. The monitoring can include a wide range of pesticides, can be regional, can apply to a part of the environment (for example, groundwater), can be use specific (a certain crop, for example), or can be narrowly focused on a single pesticide.
The Pest Control Products Act requires the PMRA to initiate re-evaluations of each registered pesticide within 15 years of its initial registration or the most recent major decision affecting the registration. As science continues to evolve, new information including published scientific literature, and new methodologies and approaches that become available over time may affect a previous regulatory decision. For this reason, the PMRA re-evaluates registered pesticides on a regular basis, to determine whether the use of these products continues to be acceptable according to current scientific standards. In addition, under certain conditions, the PMRA conducts special reviews to address a specific aspect(s) of concern of a registered pesticide.
Once a pesticide has been registered, its actual sales are tracked via the National Pesticide Sales Reporting Program. This Program collects comprehensive pesticides sales data on an annual basis and is mandatory for pesticide registrants. The sales data are useful for estimating pesticide use and provide important information for the re-evaluation and special review of pesticides, incident reporting, as well as compliance and enforcement activities.
8.0 Involving interested and affected parties
The registration decisions of the PMRA affect users and registrants, and those exposed to pesticides and pesticide residues. They are also of interest to a large number of other parties, including the Canadian public in general, other federal departments and provincial agencies and departments with health and environmental protection mandates, as well as various organizations representing the interests of pesticide users, consumers and environmental and health advocacy groups. The framework also facilitates the PMRA interaction with these affected and interested parties.
8.1 Major registration decisions – pre- and post-market and specifying MRLs
The PMRA Management of Submission Policy (MOSP)Footnote 19 sets out prescribed processes and procedures for both the PMRA and registrants/applicants, for all applications to register or amend an existing registration. Interaction and consultation with registrants occur frequently within this process including during the pre-submission phase, the screening for completeness of the submitted data, and the review of deficiencies in the initial stages of review. The PMRA further provides an opportunity for registrants to comment on the mitigation measures that the PMRA requires on the label as part of the registration process, as well as on any additional information used in the review that was not provided by the registrant, as applicable. In addition, for a new active ingredient registration or major new use, the PMRA publishes the proposed registration decision for public consultation. Major decisions, such as the registration of a new pesticide, or a major new use for an existing pesticide, are documented in a Proposed Registration Decision (PRD) followed by a Registration Decision (RD). In addition, the PMRA consults on all proposed new pesticide MRLs and amendments to existing values, which are also subject to domestic and international (World Trade Organization's (WTO) Sanitary and Phytosanitary (SPS) Measures) consultation processes. These proposed MRLs are published as proposed MRL (PMRL) documents followed by the establishment of the MRL in the online MRL database.
As part of the post-market review process, the PMRA engages various stakeholders during the early part of the review phase to gather use information as well as any additional information and data required for review. When the review is completed, registrants are also provided with an opportunity to comment on any additional information used during the review that was not provided by them, when applicable. Subsequently, the PMRA publishes the proposed re-evaluation or proposed special review decision for public consultation. Re-evaluation decisions are published as a Proposed Re-evaluation Decision (PRVD) followed by a Re-evaluation Decision (RVD). Special reviews are published as a proposed Special Review Decision (PSRD) followed by a Special Review Decision (SRD).
These various documents are published in an effort to record the basis for individual registration, re-evaluation and special review decisions, and to consult and inform other interested parties and the public about the decision. The PMRA considers all comments and information received during the consultation period before making a final registration decision. Comments received may include suggestions for significant use pattern revision; alternative risk mitigation approaches; comments on the risk assessment methods, and submission of new studies or published scientific literature.
8.2 New policies and programs
The Pest Control Products Act requires the PMRA to solicit public comments on new policies and programs, most of which are carried out by posting documents and notices on the Pesticides section of Canada.ca or, in the case of proposed new regulations or regulatory amendments, the Canada Gazette. Responses are then reviewed and, where appropriate, changes are made to reflect public input and concerns. Comments are collected via the PMRA's Public Engagement Portal, which also provides a single window through which pesticide-related inquiries may be submitted.Footnote 20
8.3 Advisory bodies
The Pest Management Advisory Council (PMAC), established in November 1998, is a forum for stakeholders to provide advice on policies and issues relating to the federal pest management regulatory system to the Minister of Health. The Council's membership includes environmental, health, labour and consumer groups, academics, pesticide manufacturers, and users.
The Federal, Provincial, Territorial Committee (F/P/T Committee), on Pest Management and Pesticides was established to help strengthen F/P/T relationships in the area of pest management and pesticides. The Committee also provides advice and direction to F/P/T governments on programs, policies and issues.
Other government departments: The PMRA works with other government departments to establish mechanisms for the exchange of information and advice about pesticides, pest management and pesticide regulatory issues. This may include Memoranda of Understanding, or the establishment of special working groups to address policy and regulatory issues. The PMRA works routinely on horizontal issues with Agriculture and Agri-Food Canada, the Canadian Food Inspection Agency, Environment and Climate Change Canada, Department of Fisheries and Oceans, as well as other branches of Health Canada. In addition, the PMRA is also an active member on the Canadian Drinking Water F/P/T committee.
International cooperation activities: The PMRA has formed strong partnerships and collaborative approaches to joint pesticide reviews, promoting international regulatory alignment, and addressing barriers to agricultural innovation and trade. For example, the PMRA is involved with several OECD initiatives, including various OECD task forces and expert group projects. The OECD Working Party on Pesticides functions as a vehicle for global cooperation and facilitates information exchange and alignment of approaches with respect to pesticide assessment. The PMRA also uses its regulatory expertise to contribute to international food safety standards through the Codex Alimentarius Committee on Pesticides Residues of the Food and Agriculture Organization of the United Nations (FAO) and the World Health Organization, in an effort to align food standards throughout the international community, where possible. Additionally, the PMRA works bilaterally with foreign jurisdictions to resolve regulatory differences where they create barriers to the free movement of safe food.
List of acronyms and abbreviations
- ADI
- Acceptable Daily Intake
- ARfD
- Acute Reference Dose
- BMD
- Benchmark Dose
- CFIA
- Canadian Food Inspection Agency
- EEC
- Estimated environmental concentration
- FAO
- Food and Agriculture Organization of the United Nations
- F/P/T
- Federal/Provincial/Territorial
- FDA
- Food and Drugs Act
- HESI
- Health and Environmental Sciences Institute
- IPM
- Integrated pest management
- LC50
- Median lethal concentration
- LOC
- Level of concern
- LMS
- Linearized multistage
- MRL
- Maximum residue limit
- MOSP
- Management of Submission Policy
- NOAEL
- No observed adverse effect level
- NOEC
- No observed effect concentration
- OECD
- Organization for Economic Co-operation Development
- PMAC
- Pest Management Advisory Council
- PMRA
- Pest Management Regulatory Agency
- PMRL
- Proposed Maximum Residue Limit
- POP
- persistent organic pollutants
- PRD
- Proposed Registration Decision
- PRVD
- Proposed Re-Evaluation Decision
- PSRD
- Proposed Special Review Decision
- RD
- Registration Decision
- RQ
- Risk Quotient
- RVD
- Re-evaluation Decision
- ROEB
- Regulatory Operations and Enforcement Branch
- SPS
- Sanitary and Phytosanitary Measures
- SRD
- Special Review Decision
- TSMP
- Toxic Substances Management Policy
- USEPA
- United States Environmental Protection Agency
- WTO
- World Trade Organization