What is a cosmetic?
A "cosmetic" is any substance used to clean, improve or change the complexion, skin, hair, nails or teeth. Cosmetics include beauty preparations (make-up, perfume, skin cream, nail polish) and grooming aids (soap, shampoo, shaving cream, deodorant).
Some products that seem to be cosmetics may be classified differently and managed by different programs at Health Canada:
- Products that claim to have a therapeutic effect (e.g. to prevent or treat disease), or that contain certain active ingredients not allowed in cosmetics are considered to be drugs, for example, topical antibiotic creams.
- Products containing natural active ingredients that claim to have a therapeutic effect (for example, a topical herbal remedy to speed scar healing) are considered natural health products.
- Items that are intended to be eaten and do not have a therapeutic effect or claim are food products, such as chewing gum.
- Insect repellent lotions and sprays are pesticides.
- Products that provide a therapeutic benefit to animals, like dander-reducing creams, are veterinary drugs.

Cosmetic or drug?
A personal care product can be defined as a substance or mixture of substances which is generally recognized by the public for use in daily cleansing or grooming. Depending on the ingredients and the claims of a product, a personal care product can be regulated as a cosmetic or a drug.
A beauty product or grooming aid is usually a cosmetic, but is legally classified as a drug if it makes any claims to modify body functions, or to prevent or treat disease. A product that is authorized as a drug has a DIN (Drug Identification Number) or an NPN (Natural Product Number) on its label. If you are unsure whether a product you are using is a cosmetic or a drug, you can consult the Drug Product Database or the Licensed Natural Health Products Database to determine if your product is currently authorized as a drug or natural health product.
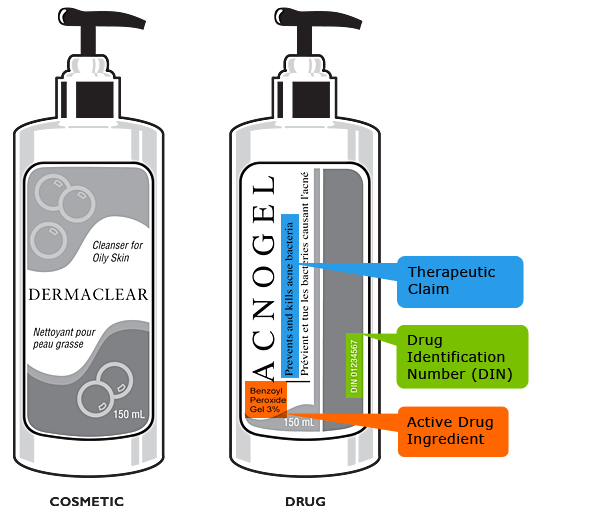
Image description
Image showing a bottle of a cosmetic product, "Dermaclear", which is similar to a bottle of a drug product, "Acnogel", and highlighting the differences between the two bottles. The bottle of the cosmetic product simply says "Cleanser for Oily Skin" on the label, while the bottle of the drug product has a therapeutic claim, "Prevents and kills acne and bacteria", a Drug Identification Number (DIN 0234567), and an active drug ingredient "Benzoyl Peroxide Gel 3%", identified on the label.
Some products that share characteristics of both "cosmetic" and "drug" are harder to classify, and Health Canada conducts assessment and consultations to properly classify them.
What safety rules do cosmetics have to meet?
Health Canada sets safety rules through the Food and Drugs Act and the Cosmetic Regulations. All cosmetics sold in Canada must:
- be free from contamination and substances that may harm you when you use the cosmetic normally and according to the directions on the label. Health Canada sets out a list of ingredients that are banned or limited in cosmetics, called the Cosmetic Ingredient Hotlist.
- be manufactured, prepared, preserved, packed and stored under clean conditions. All cosmetic manufacturers are encouraged to adhere to Good Manufacturing Practices (GMPs).
- have their composition declared to the government via Notification (In other words, manufacturers must tell the government what is in their cosmetics so that their ingredients can be monitored and checked against the Cosmetic Ingredient Hotlist.) If a safety concern arises, the cosmetic is prohibited from the market.