Priority strategies to optimize testing and screening for COVID-19 in Canada: Report
This is the first report of the Testing and Screening Expert Advisory Panel. It was released in January 2021.
On this page
- Executive summary
- The Advisory Panel and reports
- Preamble
- Tests for COVID-19
- Optimizing diagnostic capacity with lab-based PCR testing
- Deploying rapid tests for screening
- Equity considerations for testing and screening
- Improving communication strategies
- Conclusions and next steps
- Key terms
Executive summary
In November 2020, the Minister of Health established a COVID-19 Testing and Screening Expert Advisory Panel. The Panel provides evidence-informed advice to the federal government on science and policy related to innovative and existing approaches to testing and screening. In this report, the Panel provides its first set of provisional advice to the Minister on COVID-19 testing and screening.
There is no single, perfect approach to COVID-19 testing and screening that will effectively address every issue the virus presents across the country. Given the diversity in geography, demographics, science and technologies available, experiences to date, as well as domestic and international data, the Panel suggests focusing on optimizing testing and screening for COVID-19. The Panel has identified the following 4 priority areas for action:
- optimizing diagnostic capacity with lab-based PCR testing
- deploying rapid tests for screening
- addressing equity considerations for testing and screening programs
- improving communications strategies
Focusing on these areas would help to:
- reduce the prevalence of infections
- protect Canada's most vulnerable populations
- limit the impact of the disease on the health care system and the economy
Optimize diagnostic capacity with lab-based PCR testing
- create higher- and lower-priority streams for specimen collection and testing where capacity is constrained
- implement 'task shifting' in the health workforce to increase capacity
Deploy rapid tests for screening
- use rapid tests in selected groups to screen for infection
- test frequently and confirm positive results from screening with PCR tests as appropriate
- use screening with rapid tests to limit outbreaks in congregate and high-risk settings, such as long-term care
- consider operational requirements for rapid test deployment
Consider equity in testing and screening measures
- leverage both lab-based PCR and rapid tests to fill in testing gaps in key geographical locations as well as with specific populations and settings
- implement context-specific strategies to improve access to testing and screening in under-served and higher-risk communities
- reduce barriers to testing for precarious (poorly paid, insecure, unprotected) workers
Improve communications strategies
- reduce language, knowledge and accessibility barriers in all forms of public health communications related to testing and screening to improve understanding and acceptance of public health messaging
- use targeted strategies to improve outreach to high-transmission and high-risk population groups
- provide clear guidance tools to help individuals identify if they need testing
The Panel anticipates providing additional guidance in subsequent reports in several additional areas. These potential areas include:
- testing and screening to support economic recovery with a focus on testing for travel, communal work settings schools and post-secondary institutions and other critical workplace settings
- surveillance and population-based approaches, such as contact tracing and use of technology that protects privacy while identifying cases and/or exposures
- engaging behavioural scientists to enhance communication strategies that target high-risk populations and youth
The Panel is also closely monitoring developments on the SARS-CoV-2 B.1.1.7 lineage reported in the United Kingdom (U.K.). We will advise the Minister as appropriate.
The Advisory Panel and reports
Mandate of the Panel
In December 2020, there were approximately 6,000 new cases of COVID-19 in Canada each day. Despite the recent approval of a COVID-19 vaccine in Canada, the Panel recognizes that the health and economic consequences of this pandemic will continue well into 2021. Improved testing and screening strategies will play an important role in reducing COVID-19 deaths and the strain on the health care system. These will also help Canadians and Canadian businesses recover from the pandemic's economic effects.
The COVID-19 Testing and Screening Expert Advisory Panel aims to provide timely and relevant guidance to the Minister on COVID-19 testing and screening. This advice is based on the best available science, data and experiences. The Panel's mandate emphasizes innovative approaches to testing and screening to:
- address existing bottlenecks within testing systems
- explore novel approaches to screening
- provide strategies to improve health equity and health communication
The Panel's mandate is to complement, not replace, evolving regulatory and clinical guidance regarding testing and screening.
The Panel's reports are intended to be responsive to federal, provincial and territorial needs as all governments seek opportunities to integrate new technologies into their COVID-19 response plans. The Panel recognizes that jurisdictions may choose to adopt some testing and screening strategies and not others based on the unique circumstances of each jurisdiction. It is in this context that the Panel sees value in communicating lessons learned as broadly as possible. These lessons include:
- exchanging strategies on testing
- shifting tasks
- enhancing communications
- ensuring equity across jurisdictions
Plan for reports
This is the first report of the Panel, issued in light of the pressure the Canadian health system is facing and the current incidence of cases. This report focuses on 4 immediate actions to optimize testing and screening. These actions involve:
- optimizing diagnostic capacity with lab-based PCR testing
- accelerating the use of rapid tests, primarily for screening
- addressing equity considerations for testing and screening programs
- improving communications strategies to enhance testing and screening uptake
Additional guidance in these areas will be issued in the future.
Consultation
The Panel consulted with more than 80 health experts, public policy experts, members of industry and others contributing to the COVID-19 response.
The Panel's decision to provide guidance rapidly resulted in focused consultation in advance of this first report. We will continue to consult with a variety of stakeholders as it prepares further reports.
Guiding principles
Public health initiatives benefit from incorporating principles to prevent unintended harm, promote equity and increase accountability. Panel discussions and engagement with stakeholders highlighted a number of key principles to consider in its guidance. These principles align with the framework outlined in the Canadian National Advisory Committee on Immunization guidance and are based on ethics, equity, feasibility and acceptability. The Panel applied these principles in framing its guidance.
This report contains the Panel's independent advice and recommendations, which were based on information presented and made available to it.
Terms
Some of the terms used in the report may not be familiar to all readers. A glossary of terms is included in an annex for reference.
Acknowledgements
The Panel expresses its appreciation to the ex officio members of the Panel and to officials at Health Canada who have been working tirelessly over the last few weeks to support the Panel. The Panel also acknowledges the support of the "shadow panel" on testing and screening, a group of students and young scientists who provided expert research and analytical assistance. Shadow panel members include Michael Liu, Matthew Downer, Jane Cooper, Sara Rotenberg, Netra U. Rajesh, Tingting Yan, and Rahul Arora.
Sue Paish, Co-Chair
Dr. Irfan Dhalla, Co-Chair
Panel members:
Dr. Isaac Bogoch
Dr. Mel Krajden
Dr. Jean Longtin
Dr. Kwame McKenzie
Dr. David Naylor
Domenic Pilla
Dr. Brenda Wilson
Dr. Verna Yiu
Dr. Jennifer Zelmer
Preamble
The global and Canadian responses to COVID-19 demonstrate the importance of testing and screening to curtail the spread of infections. Testing is only one part of a robust public health response that should also include rapid contact tracing to reduce onward transmission. The effectiveness of both testing and other strategies used to contain COVID-19 require both political and community buy-in.
Canada is at a critical juncture where testing and screening can be enhanced with new technologies to combat the spread of COVID-19, reduce the testing burden and ease anxiety. These are key pillars to managing the "second wave" while the vaccine roll-out advances. The Panel and most governments recognize that health and laboratory professional capacity is already, and will continue to be, limited. For good reason, the tightly regulated and quality controlled communicable disease landscape in Canada has required that licensed and accredited laboratories oversee the testing process in both the public and private sector.
Recently, more point-of-care (PoC) tests have been approved in Canada. While not as sensitive as comparable laboratory-based tests, most PoC tests, when properly used, may be useful tools to prevent the spread of COVID-19.
The focus of this report is on improving the use of both laboratory and PoC tests across different geographies, populations and scenarios. While all governments strive for improvement, perfection should not become the enemy of the good. Also, strategies that work in one geography or with one population may not be as effective in other scenarios.
Tests for COVID-19
The foundation of an effective public health response to COVID-19 has been referred to as a "find, test, trace, isolate and support" strategy. This has several critical elements:
- finding as many cases of COVID-19 as possible
- breaking as many chains of transmission as possible
- providing supports that encourage testing and, where appropriate, self-isolation and quarantine
- ensuring all of the above elements are executed in a timely manner
Testing is a key early step in "find, test, trace, isolate and support." A robust approach to containing COVID-19 will also incorporate comprehensive efforts to:
- identify how an individual contracted COVID-19
- provide care and support on self-isolation (case management)
- determine the individual's close contacts to recommend testing and quarantine (contact tracing)
A robust testing approach is critical. This is because some evidence suggests that up to 40% of individuals infected with COVID-19 may have no symptoms and may infect others.
There are 3 key types of tests to test for the presence of the SARS CoV-2 virus, which causes COVID-19:
- lab-based PCR
- PoC nucleic acid testing
- rapid antigen tests (RATs)
Characteristics of these 3 test types are summarized in Table 1. The advantages and disadvantages of deploying each for diagnosis and screening depend on "pretest probability," which is the likelihood that an individual has COVID-19 before being tested.
For the purposes of this report, "diagnostic testing" is testing used to identify whether an individual who is suspected to have been infected with the SARS-CoV-2 virus has been infected. Diagnostic testing is performed when a person has a reasonably high pretest probability. The person has symptoms consistent with COVID-19 or there is recent known or suspected exposure to someone with SARS-CoV-2 infection.
"Screening" involves testing individuals whose pretest probability is the same as everyone else in the relevant population (for example, a group of students or a group of health care workers. It's performed in people who are asymptomatic without known exposure to the SARS-CoV-2 virus. Screening can be used to detect asymptomatic or pre-symptomatic COVID-19 infections and to prevent outbreaks before they occur. This is especially important in settings where individuals have more social contacts (for example, students and essential workers).
Lab-based PCR tests
Lab-based PCR tests are widely used to diagnose COVID-19 infections, as they can detect genetic material from SARS-CoV-2 from patient samples. In Canada, samples are most often collected by swabbing the back of the nose (nasopharyngeal swab). Other collection methods can also be used. These include nasal swabs, throat swabs, saliva, "swish and gargle" mouth rinses and respiratory secretions.
PCR-based tests are conducted by trained professionals in accredited laboratories. These tests have:
- high specificity
- where false positives are extremely rare (approximately 1 in 200 tests)
- highest sensitivity
- where the false negative rate is acceptable, at least when the sample is collected appropriately and at the right time during the course of the infection (typically 90% to 95% sensitive)
In short, PCR-based tests allow for accurate identification of people with COVID-19 with a reasonably high degree of confidence.
Point-of-care ("rapid") tests
Point-of-care (PoC) tests detect COVID-19 antigens or nucleic acids, many within 15 minutes to 1 hour. They tests can be used to identify individuals in community or work settings with the highest levels of viral shedding, which can lead to transmission to others. They do not need to be performed by a health professional.
There are 2 major types of PoC tests:
- nucleic acid tests
- those authorized for use in Canada include the Cepheid Xpert Xpress, the Spartan Cube, the Hyris BKit and the Abbott ID NOW platforms
- are already being used in rural and remote communities across Canada
- rapid antigen tests (RATs)
- those authorized for use in Canada include the Abbott Panbio, the Becton, Dickinson and Company's BD Veritor Plus System, and the Quidel Sofia 2 test
While PoC tests are less sensitive compared to lab-based PCR, the immediate availability of results enables timely action. Despite their lower sensitivity, these tests are able to identify individuals who are shedding larger amounts of virus, which may correlate with a greater risk of transmission to others. Furthermore, repeated testing of individuals, even with these less-sensitive PoC tests, can improve the sensitivity and effectiveness of a testing strategy.
| Lab-based PCR test | PoC nucleic acid test | Antigen test | |
|---|---|---|---|
| Detects | Viral genetic material | Viral proteins | |
| Sample type | Nasal swab, nasopharyngeal (NP) swab, throat swab, saliva, respiratory secretions | Depends on test, but similar to lab-based PCR test (nasal swab, NP swab, throat swab, saliva) | Nasal swab or nasopharyngeal (NP) swab |
| Collection site |
|
PoC setting | PoC setting |
| Processing site | Laboratory | PoC setting | PoC setting |
| Typical turnaround time | about 24 hours | less than 2 hours | less than 1 hour |
Optimizing diagnostic capacity with lab-based PCR testing
Context
Lab-based PCR testing for diagnostics is currently highly constrained in many parts of the country. The constraints vary by location. Where appropriate, there is an urgent need to augment capacity throughout the testing chain:
- from sample collection
- to delivery of the sample to the lab
- to lab processing
- to reporting results
As of mid-December 2020, provinces and territories have achieved a collective lab-based PCR test processing capacity of about 160,000 per day. This is about 80% of the national target of 200,000 tests per day, as outlined in the Safe Restart Agreements. About 75% of the national capacity is used on average each day.
While efforts are being made across jurisdictions to address testing constraints, there are few shortcuts that could be safely contemplated in lab processing. Lab-based PCR tests are time-consuming to perform and involve many steps. As a consequence, turn-around times for results after specimen collection often reach 48 hours or more.
Due to lab-based PCR testing capacity, many provinces are following national consensus and focusing these tests mainly on individuals who are likely infected (with high pretest probability). These include people with symptoms or who have known exposure to someone with COVID-19.
Overall bottlenecks and limited capacity in lab-based PCR testing capacity highlight the need for more streamlined testing protocols in areas with overburdened testing systems. Careful consideration and planning as to how laboratories could plan for current and future demands on their staff is also a concern. This is considered in more detail below.
Create higher- and lower-priority streams for specimen collection and test processing where capacity is constrained
The number of individuals with lower likelihood of exposure to COVID-19 seeking testing (asymptomatic and with no known exposure to someone with COVID-19) creates pressure on testing and processing capacity in some parts of the country. This can cause an increase in turnaround times, which delays the timely initiation of case management, contact tracing and quarantine.
Case study
Ontario: Effective December 11, 2020, the province updated its testing guidelines. COVID-19 assessment centres will no longer accommodate individuals wishing to be tested before travelling. Travellers will be required to obtain tests through private laboratories for a fee. This initiative has diminished the public health human resources strain related to sample collection.
The Panel suggests provinces and territories consider implementing higher- and lower-priority streams for specimen collection and test processing where capacity is constrained. Individuals who exhibit symptoms and/or have a known exposure (a higher pretest probability) should always be a higher priority. This streamlined approach optimizes the use of existing testing capacity to expedite the delivery of results to higher-priority groups, including those in outbreak settings.
The Panel notes several leading examples of public reporting of testing performance data such as Halton's interactive dashboard. The Panel suggests that all jurisdictions publicly communicate test turnaround times and other important metrics for both higher- and lower-priority streams. By regularly sharing data about turnaround times and other key metrics, each jurisdiction may benefit from best practices that drive strong results.
Implement task shifting to increase testing capacity and processing
The Panel heard repeatedly that one of the most significant challenges constraining testing capacity is the short supply of "health human resources." These are the people who are essential in nearly every step of the process leading to the delivery of test results. Those who are available have been strained under the pressure of recent demands.
Provinces and territories have well-defined scopes of practice and regulation for health care professionals. Legislation or policy outlines which professions can collect samples, conduct diagnostic testing and report test results. In Canada, samples have mainly been collected by physicians and nurses, who are also in high demand in hospitals, primary care and long-term care settings.
Expanding sample collection and testing to other allied health professionals can help to relieve the pressure on nurses and physicians. These professionals include:
- pharmacists
- physical therapists
- occupational therapists
- licensed practical nurses
- speech language pathologists
- dentists and dental hygienists
- registered respiratory therapists
Task shifting to permit sample collection by other health professionals would have significant impacts on reducing pressure on the health care system. Qualified medical lab workers, including university-trained researchers, can also play a role in expanding capacity for test processing.
Time invested in training by experts to develop staff capable of assuming the responsibility for sample collection often requires a trainee/new employee to commit to a minimum employment time. As a result, sample collection capacity for PCR testing cannot likely be effectively increased with short-term contractors/ employees. Instead, a concerted effort can be made by public- and private-sector labs to develop a health human resources plan for the immediate and longer terms for these critical employees.
Similarly, the potential for future tests to enable home collection or self-sampling will also alleviate pressure on limited health human resources.
Case study
Manitoba: Red River College launched a micro-credential program to train individuals with a foundation in science and/or working in a laboratory setting in critical laboratory skills. The goal is to meet the immediate testing needs in response to the COVID-19 outbreak in Manitoba. The 11-hour, tuition-free course runs throughout the winter and consists of online theory and a hands-on lab.
Ontario: A new program to train medical lab workers is being rolled out at The Michener Institute. The program will prepare up to 600 lab workers in a condensed, intensive 2-day online course followed by 2 hours of in-person lab experience. The newly trained lab workers would not be certified laboratory technologists and not qualified to analyze results, but could prepare test kits.
Task shifting has been successful internationally and in several provinces and territories. Alberta, British Columbia and Quebec have taken steps to allow other health care providers to carry out COVID tests using nasopharyngeal swabs. Ontario has made legislative amendments to allow paramedics to conduct testing through the delegated scope of practice of a supervising physician.
The Panel recognizes that training large numbers of additional staff to perform sample collection and test processing is not trivial. It may also add additional burden if newly trained staff are only available for short periods of time. Therefore, the Panel recommends that jurisdictions account for the duration and intensity of commitment that newly trained staff might be able to bring to testing efforts.
In the U.K., field studies have found that RATs have higher sensitivity (73%; 95% confidence interval of 64% to 85%) when conducted by skilled research nurses compared to pharmacy test centre employees (58%; 95% confidence interval of 52% to 63%) following written instructions. Performance would be further enhanced with formal training.
Consultations with labs, educational institutions and others can inform provincial and territorial legislation or policy. Ideally, appropriate training and certification would be coordinated to enable a broader array of health professionals to collect samples accurately. All staff should receive proper training prior to task shifting and appropriate oversight should be maintained to ensure quality results.
Successful task shifting requires collaboration between health ministries, regulatory bodies and skilled workers. Key considerations for provinces and territories have been described by the World Health Organization (WHO) and include:
- identifying the key competencies required for sample collection and test processing, and which groups of workers possess the required skills
- engaging with professional associations, colleges and regulatory bodies to discuss willingness to expand scopes of practice and liability issues and to ensure competency
- identifying required changes in legislation, regulation, policies and guidelines
- addressing reimbursement mechanisms, including billing codes and federal funding
- building training resources and implementing training programs that include initial and recurring competency assessments
Case study
Canada:
Ontario: Beginning in September 2020, Ontario allowed pharmacists to collect COVID-19 samples from asymptomatic individuals. This was done to relieve the testing strain on the 150 provincial assessment centres. In November 2020, this was expanded to include asymptomatic people who meet provincial testing criteria.
Alberta has authorized a diverse array of health professionals to perform COVID-19 nasopharyngeal swabs by amending the performance of "restricted activity" in schedule 7.1, section 2 of the Government Organization Act. Professionals include:
- advance care paramedics
- registered nurses
- registered psychiatric nurses
- licensed practical nurses
- registered respiratory therapists
- occupational therapists, physical therapists
- speech language pathologists.
Quebec issued a ministerial order to allow many health care professionals to perform COVID-19 testing. Professionals include:
- acupuncturists
- hearing aid acousticians
- chiropractors
- denturologists
- occupational therapists
- veterinarians
- dispensing opticians
- optometrists
- pharmacists
- podiatrists
- medical electrophysiology technologists
- medical imaging technologists
- physiotherapy technologists
- prosthetic and dental prosthesis technologists
United Kingdom: The National Health Service (NHS) is recruiting employees from airlines who have not been working since the pandemic significantly reduced air travel. These employees may work alongside doctors, nurses and other health professionals. Many airline staff are trained in first aid or hold other clinical qualifications and have security clearance. NHS clinicians oversee the work and expert training is provided to all new recruits.
Deploying rapid tests for screening
Use rapid tests in selected groups to screen for infection
PoC tests share some things in common, such as:
- rapid turnaround times
- limited equipment requirements
- interpretation of results (read either visually or by a portable analyzer)
- less sensitive in detecting COVID-19 compared to lab-based PCR tests
However, rapid tests differ in terms of sensitivity and specificity, ease of use and other important characteristics. There are also important differences between rapid nucleic acid tests and rapid antigen tests.
Modelling suggests that the effectiveness of screening depends more on testing frequency and turnaround time than on a test's ability to identify individuals with the virus. Thus, a screening strategy that relies on rapid tests may be superior to a screening strategy that relies on lab-based PCR. Rapid antigen tests (different from rapid PCR tests) are particularly well-suited for screening. They have short turnaround times and are easy to use by a wide range of trained operators. Some RATs also have a significantly lower cost per test than other test types, which may be particularly appealing in large-scale screening applications. Modelling from school and community settings has demonstrated the value of screening with rapid tests to control disease transmission. This has resulted in success in some universities in the United States.
Case study
Nova Scotia is using RATs in pop-up clinics to test asymptomatic individuals, specifically targeting those who had attended bars and restaurants. As of November 30, 2020, 5,500 people received RAT and there were 21 positive cases. Positive results were confirmed using PCR testing.
Slovakia undertook a mass population-wide rapid testing initiative. About 20,000 medical staff and 40,000 non-medical staff performed roughly 5 million tests. Swabbing was conducted by trained medical staff. Those who chose not to participate in the program were instructed to stay home for 10 days or until the next round of the testing program. Those who participated received a certificate confirming their infection or negative status. Initial analyses demonstrated prevalence of detected COVID-19 infections decreased by about 61% within 1 week in 45 counties that were subject to 2 rounds of mass testing. However, Slovakia also imposed lockdown restrictions at the same time. It is important to note that gains have not been sustained, which illustrates that testing must be accompanied by other strategies.
Test frequently and confirm positive tests from screening
Rapid tests are being used to screen individuals with low pretest probability. These are individuals in high-risk settings who have no symptoms or known contacts with COVID-19. Rapid test results should be interpreted in the context of this pretest probability. One possible approach for this is presented in Figure 1 and described below.
Individuals who are rapid test-positive should be presumed positive for COVID-19 and public health authorities should initiate isolation and case management. In low-prevalence settings, there is a reasonable probability that a positive rapid test is a false positive. Consequently, positive test results should be confirmed by lab-based PCR or by another rapid test. The latter option will be especially useful when lab-based PCR capacity is constrained and large numbers of individuals are being screened.
In an individual with low pretest probability, a negative rapid test result is highly likely to be a true negative. However, false negatives can still occur. Negative results should not be taken as proof of no infection or as a licence to disregard public health guidelines. It is crucial to clearly communicate to all tested individuals and the public at large about the:
- limitations of rapid testing
- interpretation of positive and negative test results
- importance of maintaining public health precautions
Both false positives and false negatives can be problematic when managing outbreaks, especially in communal living situations. Therefore, lab-based PCR testing with rapid turnaround is the preferred approach. Where rapid tests are used to aid in outbreak management, specimens should also be collected for lab-based PCR testing. Expert judgment will be required on the best way to use the results of rapid tests in outbreaks.
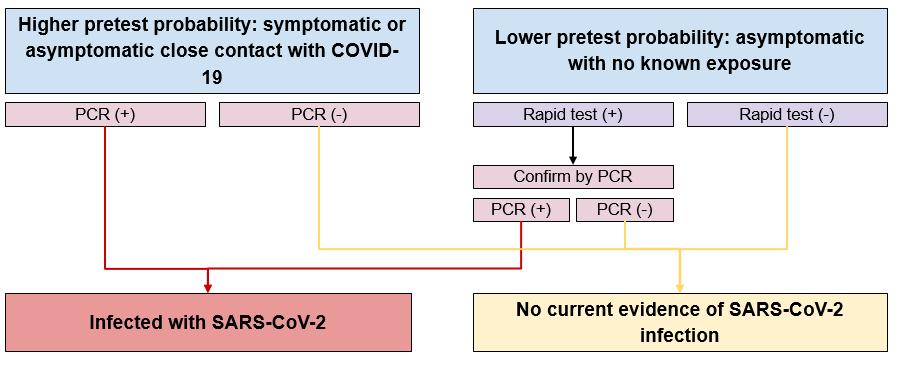
Figure 1 - Text description
Individuals with higher pretest probability are those who are close contacts with someone with COVID-19 and are either symptomatic or asymptomatic. These individuals receive a PCR test. If the result is positive, then they are infected with SARS-CoV-2. If the result is negative, then there is no current evidence of SARS-CoV-2 infection. Individuals with lower pretest probability are those who are asymptomatic with no known exposure. These individuals receive a rapid test. If the result is positive, then they are tested again using the PCR test. If the subsequent PCR test result is positive, then they are infected with SARS-CoV-2. If the subsequent PCR test result is negative or if the initial rapid test result was negative, then there is no current evidence of SARS-CoV-2 infection.
Use screening with rapid tests to limit outbreaks in congregate and high-risk settings
Canada has seen numerous outbreaks in a wider range of settings, including:
- schools
- work settings
- communal living facilities such as:
- homeless shelters
- long-term care homes
- group homes for people with disabilities
- correctional facilities
Screening programs used as part of standard practice in these settings could help identify COVID-19 infections before they spread. They could also help prevent an outbreak.
Operational considerations for using rapid tests
As of December 21, 2020, there are 7 rapid tests currently authorized in Canada. Some tests, such as the Panbio rapid antigen test, can be administered and read without additional equipment. Other tests, such as the BD Veritor rapid antigen test, require a reader device that reduces the risk of operator error. Other rapid tests such as the Cepheid Xpert Xpress have significantly higher sensitivity, comparable to lab-based PCR tests.
Provinces and territories should consider the trade-offs of specific rapid tests, including specimen collection methods. For example, repeated nasopharyngeal swabs may not be acceptable in some settings, such as schools. These types of tests may also cause "testing fatigue" in individuals due to their specific use cases and performance characteristics.
The turnaround time of rapid tests varies. This also needs to be considered prior to implementation. Depending on the rapid test used, results can be provided in about 15 minutes to 1 hour. Appropriate biosafety measures should be in place to prevent infection while obtaining and handling samples. Finally, the skill and training of operators affects the quality of samples collected and tests processed, as well as the sensitivity of the test. Jurisdictions need to ensure that operators of all PoC tests are appropriately trained.
Equity considerations for testing and screening
Context
COVID-19 has highlighted and amplified existing health inequities in Canada. Research has shown that COVID-19 has disproportionately affected some populations, in particular:
- racialized communities
- economically disadvantaged individuals
- people with disabilities
- older adults, especially those living in communal facilities such as long-term care facilities
These health inequities extend to testing and screening. Limited access to testing can be attributed to many factors, such as operating hours, inaccessible environments, centre locations, communication strategies, and the method by which appointments are allocated. Some individuals may be hesitant to get tested because of the potential for negative impacts from a positive test. These can include:
- losing a precarious job
- loss of income
- social stigma
- perceived or real impact on immigration status
Others may live in communities that lack lab resources to process large numbers of tests or where services are not provided in their primary language.
All of these factors leading to problems in access should be factored into the resourcing of a testing strategy, to ensure equity for hard-hit populations. Equitable access to COVID-19 testing and screening, which takes into consideration community transmission levels, is fundamental to any public health strategy. It also reflects legal, human rights and moral obligations.
Leverage both lab-based PCR and rapid tests to fill testing gaps in key geographies, populations and settings
Understanding the uses, advantages and risks of each type of COVID-19 test is essential to optimal deployment to promote equity in access to testing. The following recommendations concerning tests will support more equitable access.
Increase lab-based PCR testing capacity
Due to historical, structural and geographic inequities, per capita-based PCR lab testing capacity varies considerably across Canada. If the goal is similar access to testing based on need, many communities will need to be supported (for example, through surge capacity, training, procurement, financial support) to improve specimen collection and test processing ability. This is especially important in remote and Northern areas. Increasing testing capacity promises long-term benefits in respiratory infection testing beyond the COVID-19 pandemic.
Case study
Nunavut: Iqaluit and Rankin Inlet have increased their PCR testing capacity through the addition of lab-based PCR (BioFire) systems.
Deploy rapid tests to fill testing gaps
The use of both PoC nucleic acid tests and RATs provides an opportunity to quickly enhance testing capacity. However, the Panel wishes to stress that PoC testing should be done in a context-specific manner. It should not be viewed as a substitute for improving access to lab-based PCR testing. Enhancing testing capacity always needs to consider how best to meet the access needs of remote, rural and Indigenous communities.
In Northern and remote areas, where there is limited lab and human resource capacity, PoC tests provide an opportunity to increase diagnostic testing capacity. Multiple territorial governments and leaders have discussed the use of PoC, which could reduce wait times and increase testing capacity for their communities. In First Nations, Inuit and Métis communities, the Panel reiterates the need for consultation to develop Indigenous-led approaches, thus ensuring community needs are identified and met.
Implement context-specific strategies to improve access to testing and screening in under-served and higher-risk communities
The uptake of testing has varied across Canada due to several factors. Barriers to broader uptake in lab-based PCR testing include:
- unclear messaging on the importance of testing
- lack of access to testing
- lack of consistent support for workers in some work settings should they test positive
- lack of opportunity for isolation
Access to testing has hindered testing uptake, including access to testing facilities due to their hours, location, physical barriers and inaccessible environments. There is also a lack of clear, simple messaging on who should be tested.
As demand for testing exceeded supply, many jurisdictions narrowed indications for testing to symptomatic individuals and close contacts. To manage the demand for testing, jurisdictions established appointment-based models, but often the operating hours were not always practical for those with limited work flexibility. Furthermore, testing locations could be difficult to reach for those using public transportation, the use of which may increase risk of transmission to others.
The Panel suggests that all jurisdictions implement context-specific strategies to bring testing to people who need it the most, rather than placing the onus on individuals to travel to a testing centre. Efforts should be focused on supporting jurisdictions to rapidly enhance mobile testing in areas of higher test positivity in ways that work for the community. Targeted communications and outreach activities will often be required to enhance uptake in these communities.
Decentralized testing models designed to bring tests to higher-risk communities are promising. These models include mobile laboratories or mobile assessment centres. Provinces and territories should also consider expanding assessment centre hours so that those working full-time can attend, and locating assessment centres close to transit services.
Case study
Toronto has refurbished Toronto Transit Commission buses to high-prevalence neighbourhoods with limited indoor testing facilities. When patients enter the bus, their information is recorded, swabbing takes place in a tent outside, and gurneys and bench space inside provide space for further assessment and test processing.
Reduce barriers to testing for precarious workers
Many Canadians do not have secure jobs. Individuals who work in temporary positions, are "on contract," in minimum wage situations or who work in very small organizations may have limited job security. They may struggle financially to support a household. Due to the significant economic impact of COVID-19, many have used their savings and borrowed money to pay bills and cover living expenses. Further loss of income, such as unpaid leave due to illness or the need to quarantine, can be catastrophic. Canadians working in settings where there are no benefits, including no paid sick leave, may hesitate to be tested as they cannot afford to self-isolate while waiting for results and/or if they test positive. Long test turnaround times worsen this problem.
The Government of Canada introduced the Canada Recovery Sickness Benefit (CRSB). This benefit provides income support to employed and self-employed individuals who:
- are unable to work because they are sick or need to self-isolate due to COVID-19 or
- have an underlying health condition that puts them at greater risk of getting COVID-19
Applicants receive $500 for a 1-week period. In B.C., it is estimated that over 50% of the workforce does not have access to paid sick leave. This means that staying home from work if there is a positive COVID-19 test could be financially devastating.
The Panel believes that all levels of government should consider additional measures to support Canadians through isolation and quarantine. Measures could include:
- paying all or a portion of wages for an isolation period after a positive test
- funding for personal support services for those in self-isolation or quarantine, including delivering groceries
- increasing the number of isolation centres (specifically for those experiencing homelessness)
- implementing mental health support, including peer support
These initiatives have proven successful in other parts of the world.
Case study
South Korea has provided sufficient essentials for 2 weeks (food, toiletries) to self-quarantine individuals at no cost.
Improving communications strategies
Context
The COVID-19 pandemic has been characterized by rapid changes in epidemiology, evidence and tools available to respond to ongoing challenges. Public health authorities have consistently asked the public to wash hands, respect social distancing, wear masks and, if sick, stay home and self-isolate. However, the messages have changed to reflect local public health advice to minimize the spread of the virus. In some cases, the public has found this confusing.
The spread of confusing or conflicting information along with "disinformation," particularly on social media, has added to the confusion. The public is bombarded with information on COVID-19 from every media source, including social media and find it increasingly difficult to make sense of the information and keep track of what applies to them, based on where they live. This is further compounded by language barriers for those whose first language is not English or French.
Much of the Panel's guidance relies on strong public knowledge of and trust in our public health systems and guidelines. This is especially important as Canada begins to enter the vaccine deployment phase in the face of high levels of vaccine hesitancy. The public health community recognizes the need for simple and direct messages, and the Rockefeller Foundation recently created a handbook for testing and tracing messaging.
The Panel notes that it may be helpful if behavioural scientists are more consistently engaged in helping to develop communication and outreach strategies and guidelines. Their expertise can be very relevant.
Reduce language, knowledge and accessibility barriers to understanding public health messaging
Communication in multiple languages is essential as about 1 in 7 Canadians speaks a language other than English or French. Language needs vary across Canada. Multilingual campaigns need to include Indigenous languages, such as Cree, Inuktitut and Anishinaabemowin (Ojibway) or Sto:lo (Coast Salish), as well as languages spoken by people who have immigrated to Canada. Multiple stakeholders have called for multilingual COVID-19 resources to be adopted across Canada, as has been successfully used in many jurisdictions.
Timely and consistent dissemination of accurate multilingual and culturally based information is crucial to help prevent the spread of health misinformation. This should be done on a coordinated basis across the country so that the communications vehicles, words and messages are consistent across provinces and territories.
There are many situations where members of a family whose first language is not French or English live in different parts of the country. If the messaging, language and vehicles for communication differ by jurisdiction, this increases the confusion and creates lack of trust, despite best intentions.
Strong inter-provincial cooperation and coordination can improve how the pandemic is managed overall. This includes developing common outreach and communications plans.
The most effective communications approaches that were relayed to the Panel include the following:
- use plain and consistent language
- keep the messages simple, clear and understandable at all literacy levels
- use existing community networks who already have developed trust with their communities
- use spokespeople or recognized and respected figures from the community to deliver messages
- focus on what people can do to help themselves as much as on what someone else wants them to do
Case study
Australia launched a multilingual mobile app for the country's population that provided up-to-date information on COVID-19. The app allows users to:
- browse articles to find out more about COVID-19 and support in Australia
- search for topics or points of interest
- view short animations with helpful summaries of specific topics
- find useful tips and contacts to help adjusting during COVID-19
Lastly, communication strategies cannot rely only on internet-based media. In Canada, while 94 percent of Canadians have access to the internet at home, rural, remote, Northern and Indigenous communities often lack internet or it is not reliable. As a result, it is important to use a range of options, including telephone messaging, to share public health information.
Use targeted strategies to improve communication with high-transmission and high-risk population groups
It is well-established that the transmission of COVID-19 is higher in:
- certain groups, such as older children and younger adults
- health care workers and other essential workers
- racialized communities
Certain groups are also at a much higher risk of poor outcomes or death if they become infected with COVID-19. These groups include:
- people with co-morbidities
- older adults
- people with disabilities
- immunocompromised individuals
Public health messaging through televised press conferences, information web pages in English and news articles need to be designed to reach these communities. It's also important to work in partnership with communities.
Current communications strategies must be refreshed and customized to reach higher-risk communities. Other jurisdictions have had success in partnering public health with local leaders to reach specific communities.
Case study
Senegal has successfully partnered with local religious leaders to share social media and public health content on different media channels.
Strengthen tools to help individuals to identify if they need a test
Several provinces and territories have used internet-based COVID-19 assessment tools to help patients determine if they need a test. For example, Ontario's COVID-19 assessment, which is based on Health Canada's assessment, includes:
- questions on symptoms
- timeline of symptoms
- status of belonging to an "at risk group"
- evaluation of "close contact" with an individual who has tested positive for COVID-19
COVID alert is a national COVID-19 exposure notification application (app) based on Google/Apple technology. It can be used on many mobile phones. The app is a simple, user-friendly tool to inform Canadians when they have come into contact with a confirmed case of COVID-19. It is operable across provinces and territories, and is designed to minimize collection and storage of personally identifiable information.
Unfortunately, this app has not been used in all jurisdictions, which makes it difficult to evaluate this technology. As noted earlier in this report, we cannot let "perfection be the enemy of the good." It would likely help all Canadians if their province or territory encouraged them to download the app where they can. It would also be helpful if all jurisdictions used the data from this app to help inform future actions, evaluate current programs and learn from best practices across the country.
Additionally, it would be helpful to offer the assessment tools in a variety of different languages, to improve access broadly across Canadian communities. Phone-based tools can be developed as an option for those with limited broadband or who prefer phone-based communication. A number of telehealth models could be used to develop these services.
Conclusions and next steps
In this first report, the Panel presents 12 considerations to support making refinements to testing and screening approaches. The recommendations are grouped into 4 categories:
- optimizing diagnostic capacity with lab-based PCR testing
- accelerating the use of rapid tests for screening
- addressing equity considerations for testing and screening programs
- improving communications strategies to enhance testing and screening uptake
Although this report is for the federal Minister of Health, the Panel hopes that other jurisdictions will find the suggestions useful.
The Panel anticipates providing additional guidance in subsequent reports in these 4 areas as well as other areas, such as:
- testing and screening to support economic recovery with a focus on testing for travel, communal work settings, schools and post-secondary institutions, and other critical workplace settings
- surveillance and population-based approaches
- further engagement of behavioural scientists to enhance communication strategies with a focus on high-risk populations and youth
The Panel is also closely monitoring developments on the SARS-CoV-2 B.1.1.7 lineage reported in the U.K. We will advise the Minister as appropriate.
Key terms
- Antigen test:
- A test that detects the presence of a specific protein that is part of the SARS-CoV-2 virus rather than the genetic material from the virus.
- Asymptomatic person:
- An individual without symptoms of COVID-19.
- Diagnostic test:
- Tests intended to identify current infection in an individual and is performed when a person:
- has signs or symptoms consistent with COVID-19 or
- is asymptomatic but has had recent known or suspected exposure to COVID-19
- Point-of-care test:
- A test completed outside the clinical laboratory at or near where a patient is receiving care.
- Precarious worker:
- Individuals who work in temporary positions, are on contract, receive minimum wage or have limited job security.
- Pre-test probability:
- The chance that a person has COVID-19, estimated before the test result is known, based on the probability of the suspected disease in that person given their symptoms, exposure history and the prevalence in the community.
- Prevalence:
- The proportion of the population that has COVID-19 at a given time.
- Screening test:
- Tests intended to identify infected persons who are asymptomatic and without known or suspected exposure to COVID-19. Screening is usually performed to identify persons who may spread the virus so that measures can be taken to prevent further transmission.
- Sensitivity:
- The ability of the test to correctly identify those who have COVID-19 at the time the specimen was collected for laboratory analysis.
- Specificity:
- The ability of the test to correctly identify those who do not have COVID-19 at the time the specimen was collected for laboratory analysis.
- Surveillance:
- Population-wide approaches undertaken to inform public health actions. Examples of surveillance testing include sampling wastewater or surfaces to detect the presence of the virus or testing a large number of people to obtain aggregate results to determine the prevalence of the virus in a community.
- Task shifting:
- The rational re-distribution of tasks among different types of health workers (for example, nurses, pharmacists) to improve the use of resources and the provision of services.
- Turnaround time:
- The time it takes from the time a sample is collected from an individual until the test results are available.
- Use case:
- The context and circumstances in which the test is used (who will be tested, by whom, where and under what conditions) based on an understanding of the clinical performance of the test and its implications.
