Frequently Asked Questions - Common Electronic Submissions Gateway
Frequently Asked Questions
1. How does the Common Electronic Submissions Gateway (CESG) work?
The CESG allows Trading Partners provide regulatory transactions to Health Canada electronically, i.e. an "electronic" courier. The CESG has been the mandatory method of transmission for regulatory transaction in eCTD format since January 2017, however, transactions exceeding a size limit of 10GBshould continue to be submitted on media.
See Figure 1 for an illustration of the CESG High Level Architecture:
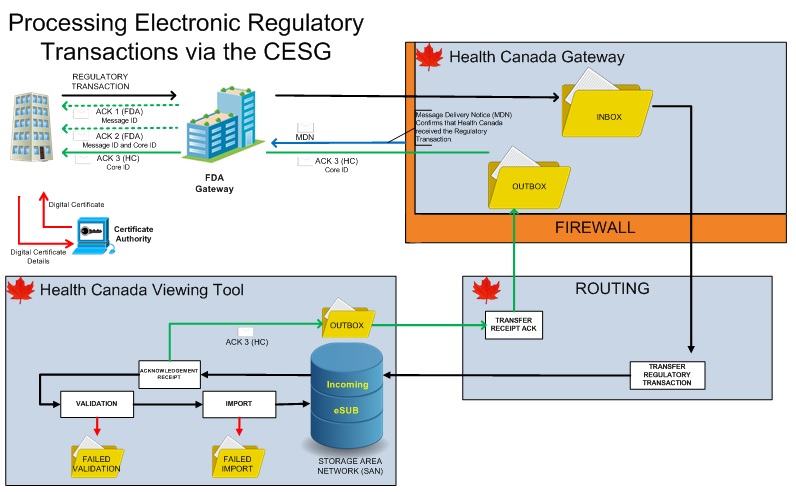
Figure - Text Description
The Trading Partner (TP) requests a digital certificate from the Certificate Authority (CA). The CA assigns a digital certificate to the TP.
The TP transmits a regulatory transaction. It is first received by the Food & Drug Administration (FDA) portion of the gateway. The TP receives two FDA Acknowledgements once the transmission is complete.
After passing through a firewall, the regulatory transaction is then routed to the inbox of the Health Canada portion of the gateway. The regulatory transaction routed to the Storage Area Network in the Health Canada Viewing Tool.
An Acknowledgement Receipt is created and sent to the outbox. This Acknowledgement Receipt is then routed back through the Health Canada and FDA portions of the gateway and sent to the Trading Partner. A MDN is also sent to the FDA Gateway confirming that the Health Canada received the regulatory transaction.
The regulatory transaction then undergoes Validation and Import. If it fails validation, the regulatory transaction is placed in the Failed Validation folder. If it fails Import, the regulatory transaction is placed in the Failed Import folder. After a successful Import, the regulatory transaction is stored in the eSub portion of the Storage Area Network.
2. How do I become a Trading Partner to use the CESG?
Please refer to the Food and Drug Administration (FDA) User guide for registration process information.
3. Is it required to also send a letter of authorization to Health Canada (Appendix E of the FDA user guide) when signing up for ESG account?
There is no requirement to send the authorization letter to Health Canada when signing up for ESG account, only to FDA as per the user guide.
4. I am already registered as a Trading Partner with the Food and Drug Administration (FDA), do I need a Health Canada specific account?
If you are already registered with the FDA as a Trading Partner and now wish to do business with Health Canada via the CESG, you do not need to create a new account. You can use your existing account and simply select "HC" as the Review Centre
5. What do the various notifications being received by the CESG mean?
When using the CESG to submit to the “HC” Centre, you will receive three messages. The first message ‘Message Disposition Notifications (MDN)' will be issued by the FDA, indicating that the FDA portion of the gateway has successfully received your regulatory transaction and will include your Message ID. The second message is a new Acknowledgment (ACK) from the FDA which contains the Message ID and Core ID. The third message (Health Canada ACK Receipt, see figure 4) will be issued by Health Canada, indicating that your regulatory transaction has been successfully received by the Health Canada portion of the CESG and contains the Core ID.

Figure - Text Description
Illustrates how the Message Disposition Notification (MDN), FDA Acknowledgement and Health Canada Acknowledgment Receipt appear in the Inbox of the WebTrader.

Figure - Text Description
Image of the FDA Acknowledgement Receipt which includes the Message ID, Core ID and Date & Time stamp.
6. Where can I find the time stamp that Health Canada received the regulatory transaction?
The time stamp that Health Canada uses as the receipt date/time can be found in the Health Canada ACK Receipt and is highlighted in Figure 4:

Figure - Text Description
Image of the Health Canada Acknowledgement Receipt with the Date stamp highlighted. In this example the Date & Time that is highlighted is 03202019142628, which can be read as March 20, 2019 at14:26:28 PM.
The time stamp "03202019142628" can be read as"Month/Day/Year/Hour/Minute/Second" or "March 20, 2019 at 02:26:28 PM".
7. How are transactions received after end of business, on the weekend, or on a Statutory Holiday handled by Health Canada?
These transactions are handled in a manner that is consistent with current practices. Any regulatory transaction received after 5:00 pm Eastern Standard Time, on a weekend, or on a Statutory Holiday is considered received on the next Health Canada business day.
8. What regulatory transactions must be sent via CESG?
At this time, the following regulatory transactions are mandatory via the CESG.
- Transactions in eCTD format
- Transactions for Regulatory Enrolment Process (REP) XML files (eCTD and non-eCTD format)
9. What folder structure should be used when sending regulatory transactions via CESG?
Health Canada requires that the Trading Partner includes the top level folder when sending a transaction via CESG. For an illustration of the acceptable folder structure, see Figure 5.

Figure - Text Description
Illustrates the acceptable folder structure for sending regulatory transactions via CESG including the Top Level Folder and Sequence Number Folder. In this example the Top Level Folder is e004567 and the Sequence Number Folder is 0000.
10. Can the revised regulatory transaction that failed validation be resent via CESG?
Yes.
11. What should be done if the Health Canada ACK Receipt is not received?
The Trading Partner should notify Health Canada via email at hc.cesg-pcde.sc@canada.ca.
12. What should be done if the Health Canada ACK Receipt is received with missing information?
The Trading Partner should verify that the top level folder was included when sending the regulatory transaction. If it was not included, please resubmit with the top level folder. If included, please provide the ACK to Health Canada via email at hc.cesg-pcde.sc@canada.ca.
13. If the Health Canada ACK Receipt was not received should the transaction be resent?
Please do not resend a transaction if the Health Canada ACK is not received right away. Depending on the file size, it may take one hour or more to receive an Acknowledgement Receipt. Trading Partners should not resend transactions unless they are requested by Health Canada. If you would like to confirm whether your transaction has been received, please contact hc.cesg-pcde.sc@canada.ca.
14. Are electronic Signatures (e-Signatures) accepted by the Health Products and Food Branch (HPFB) and can they be used when submitting to the CESG?
Yes, e-signatures are accepted at HPFB in accordance with the Health Products and Food Branch Electronic Signatures Policy. They are coordinated with the directorates and handled on a case-by-case basis. The Policy, which was co-authored with industry, is provided upon request by emailing hc.cesg-pcde.sc@canada.ca.
15. What are some of the CESG terminology differences between the FDA and Health Canada?
When using the CESG you will notice differences in the terminology used by the FDA and Health Canada. Table 1 provides a comparison of similar terms used by FDA and Health Canada.
| FDA Term | Health Canada Term |
|---|---|
| FDA ESG (FDA Electronic Submissions Gateway) | CESG (Common Electronic Submissions Gateway) |
| Transaction Partner | Trading Partner |
| Submission | Regulatory Transactions |
Please note that, in some cases, these terms may be used interchangeably when corresponding with Health Canada and/or the FDA.
16. Who do I contact for more information on the Common Electronic Submissions Gateway (CESG)?
Please refer to Inquiries and Support for details on where to direct your questions.
17. What is the maximum allowed path length, including the file name, for regulatory transactions sent via CESG?
The maximum allowed path length in transactions sent via CESG is 200 characters.
18. Can I send multiple transactions for a particular dossier at the same time?
No. Regulatory transactions (sequences) for a dossier should be sent individually. Sponsors should ensure they have received the Health Canada acknowledgement for one transaction prior to sending a subsequent transaction. If eCTD transactions are not received in the correct order, this will result in a missing transaction and an eCTD error "Sequence Numbering" (A07) will be generated during validation.
19. Is there a recommended time of day to send Regulatory Transactions?
Consistent with the FDA Frequently Asked Questions regarding process and policies of the Electronic Submissions Gateway (ESG), Health Canada recommends that large regulatory transactions (between 5GB and 10GB) be sent after 4:30 PM EST. This will allow your transaction to be processed and delivered to Health Canada overnight. For more information on providing large transactions refer to the FDA Frequently Asked Questions and Section 5.7 of the FDA ESG User Guide. Sponsors providing files greater than 10 GB should continue to use media. For detailed information on how to file using media, refer to Section 3.2 (ii) of the Guidance Document - Preparation of Regulatory Activities in eCTD Format.