Guidance document: Reconsideration of Decisions Issued for Human Drug and Natural Health Product Submissions
Foreword
Guidance documents provide assistance to industry and health care professionals on how to comply with policies, governing statutes and regulations. Guidance documents also provide assistance to Health Canada staff on how the Department's mandates and objectives should be implemented in a manner that is both fair and consistent.
Guidance documents are administrative instruments and do not have the force of law. As such, they allow for flexibility in approach. Alternatives to the principles and practices described in this document may be acceptable provided they are supported by adequate justification. Alternate approaches should be discussed in advance with the relevant program area to ensure that applicable statutory or regulatory requirements would be met.
To enable the Department to adequately assess the safety, efficacy or quality of human drugs and natural health products, Health Canada may request information or define conditions in addition to those described in this guidance. Health Canada is committed to ensuring that such requests are justifiable and that decisions are clearly documented.
Version : Reconsideration of Decisions Issued for Human Drug and Natural Health Product Submissions | Date : 2025/xx/xx
Replaces: Reconsideration of Decisions Issued for Human Drug and Natural Health Product Submissions | Date : 2019/04/01
| Change | Nature of and/or reason for change |
|---|---|
The document has been edited to update terminology and Directorate names. |
Document edited to reflect current terminology and Directorate names. |
The option to submit documents via Facsimile or electronic platforms other than email was removed |
This option to submit by Facsimile or ePost will be discontinued as they were no longer being used for submission of documents. |
In this guide
- 1.0 Overview
- 2.0 Decisions Eligible for Reconsideration
- 3.0 Possible Outcomes of a Request for Reconsideration
- 4.0 The Reconsideration Process
- 4.1 Overview
- 4.2 Detailed Outline of Reconsideration Process
- 4.2.1 Letter of Intent
- 4.2.2 Request for Reconsideration
- 4.2.3 Decision Regarding Process Path
- 4.2.4 Internal Review
- 4.2.5 External Reconsideration Panel
- 4.2.6 The Reconsideration Meeting
- 4.2.7 Making Recommendations to the Director General
- 4.2.8 Decision by the Director General
- 4.2.9 Follow-up Action
- 5.0 Roles and Responsibilities
- 6.0 Information Formats and Address
- 6.1 Human Drugs
- 6.2 Natural Health Products
- Appendix A: Reconsideration Process Map
- Appendix B: Performance Targets
- Appendix C: Request for Human Drugs Reconsideration Template
- Appendix D: Request for Natural Health Products Reconsideration Template
Abbreviations
- BRDD:
- Biologic and Radiopharmaceutical Drugs Directorate
- DG:
- Director General or his/her delegate
- eCTD:
- Electronic Common Technical Document
- FDALO:
- Food and Drugs Act Liaison Office
- IAS:
- Issue Analysis Summary
- NNHPD:
- Natural and Non-prescription Health Products Directorate
- OSIP:
- Office of Submissions and Intellectual Property
- PDD:
- Pharmaceutical Drugs Directorate
- RR:
- Request for Reconsideration
Different terms - same meaning
Different terms are used for human drug submissions, including hard-surface biocides, and natural health product applications, based on their respective regulations. To make this document easier to follow, the terms identified below will be used throughout in place of their synonyms.
| Term in guidance document | Synonym |
|---|---|
| Applicant | Sponsor |
| Submission | Application |
| Review bureau | Review centre; Assessment division |
Note: Reconsideration advisor referred to in this document is a senior directorate staff not previously involved in the submission, drawn from the Bureau of Policy, Science, and International Programs in the Pharmaceutical Drugs Directorate, the Office of Business Integration in the Biologic and Radiopharmaceutical Drugs Directorate, or the Director General's Office in the Natural and Non-prescription Health Products Directorate.
1.0 Overview
When Health Canada issues certain negative decisions related to the submission review process for a human drug, including hard-surface biocides, or a natural health product, the applicant may request a reconsideration of the decision. This document provides Guidance regarding the process.
It is noteworthy that this Guidance covers the reconsideration for products that fall under two different regulatory frameworks; drug submissions under the Food and Drug Regulations and product licence applications under the Natural Health Product Regulations. The steps of the reconsideration process are uniform for both product categories, with a few exceptions which are highlighted in this Guidance. All timelines indicated are in calendar days. Should a deadline fall on a weekend or a statutory holiday, the deadline will be extended to the next business day.
The reconsideration process is a non-adversarial dispute resolution process to ensure that decisions about a specific submission were made correctly, and in keeping with existing scientific standards and regulations. The applicant and review staff are given an opportunity to be heard by scientific and regulatory experts who were not involved in the original submission. These experts will reconsider the decision and make a recommendation to the Director General (DG).
Highlights
- If an applicant objects to a decision eligible for reconsideration, they must file a Letter of Intent within 30 days of receiving the negative decision letter.
- The DG, or delegate of the Health Canada directorate that reviewed the original submission, retains the final regulatory authority to make a decision following the reconsideration process.
- Working with the Food and Drugs Act Liaison Office (FDALO), the DG has final authority on whether to proceed with the reconsideration process, and to determine whether outside expertise will be required.
- The reconsideration process re-examines the decision based on information that was included in the original submission. Information that was not available to reviewers when the original decision was made will not be accepted for the reconsideration process.
- To avoid a duplication of effort, applicants are discouraged from refiling a submission while undergoing a reconsideration process for the same submission.
- The reconsideration process may not proceed if the applicant files a Notice of Application to the Federal Court to challenge the decision that is the subject of the reconsideration.
2.0 Decisions eligible for reconsideration
To request a reconsideration process, the applicant must file a Letter of Intent within 30 days of receiving the negative decision.
For human drugs under the Food and Drug Regulations, if the applicant does not submit the request within this time frame, the Minister of Health's decision will become final on the 31st day after the date of the negative decision letter.
For natural health products under the Natural Health Product Regulations, if the applicant does not submit a reconsideration request within 30 days, the refusal stands.
For both product categories, if the applicant withdraws from the reconsideration process once it has commenced, the negative decision letter originally issued by the review bureau is the final decision. The applicant cannot request that the reconsideration process be reinstated at a later date.
This Guidance sets out which decisions on human drug, including hard-surface biocides, and natural health product submissions are eligible for reconsideration as noted in Tables 1, and 2. The decisions can be made by 1 of the following directorates:
- Pharmaceutical Drugs Directorate (PDD);
- Biologic and Radiopharmaceutical Drugs Directorate (BRDD);
- Natural and Non-prescription Health Products Directorate (NNHPD).
Examples of issues that could lead to a Request for Reconsideration (RR) include, but are not limited to, disputes on:
- interpretation of submitted scientific data;
- applied methodology;
- relative weight given to data and its impact on the risk/benefit assessment;
- the application of Guidance or internal processes.
- Health Canada's refusal to accept a science-based alternate approach to one set out in a Guidance document;
FDALO will confirm in writing if the applicant is eligible for the reconsideration process based on (1) whether the request is received within 30 days of the negative decision letter, and (2) if the decision is listed in Tables 1 or 2.
Table 1: Decisions eligible for reconsideration - human drug submissions
- Rejection of a Priority Review Request under the Priority Review Policy
- Rejection of a Request for Advance Consideration under the Notice of Compliance with Conditions Policy
- Screening Rejection Letter (including New Drug Letter)
- Notice of Deficiency - Withdrawal Letter
- Notice of Non-compliance - Withdrawal Letter
- Not Satisfactory Notice
- Notice of Insufficient Information Withdrawal
- Notice of Refusal
Table 2: Decisions eligible for reconsideration - Natural health product submissions
- Notice of Refusal to Issue a Licence for Product Applications
- Notice of Refusal to Amend a Licence for Product Applications
2.1 Issues not eligible for reconsideration
The reconsideration process is meant to deal with regulatory and scientific disputes in a specific submission. Complaints related to the following issues are not eligible for reconsideration:
- negative decisions based on submissions containing falsified information;
- allegations of bias;
- complaints about service delivery.
3.0 Possible outcomes of a request for reconsideration
The following are outcomes of a reconsideration process:
- original decision is upheld;
- original decision is partially amended but the submission remains refused;
- original decision is amended.
3.1 Decision upheld
For human drug submissions, if the original decision is upheld, the reconsideration decision letter becomes the Minister of Health's final decision. No further action is taken by the directorate.
For natural health product reconsideration decisions, if the original decision is upheld, a letter with accompanying reasons will be sent notifying the applicant that the refusal has been maintained.
3.2 Decision partially amended
For both product categories, where the original negative decision contains a number of objections and the reconsideration process results in only some of the objections being amended, the submission will remain refused. The file will not go back into review. The applicant may refile the submission, making the necessary changes to address the issues that remain refused.
3.3 Decision amended
Drug decisions that are fully amended through the reconsideration process are returned to the review bureau for completion. For complex submissions requiring further review beyond the issues that were part of the reconsideration process, the review process is re-instated. See Section 4.2.8 for more information.
For the natural health product reconsideration process, if the original refusal is overturned, the Minister of Health will either issue or amend the Product Licence, as appropriate, if the requirements of section 7 of the Natural Health Product Regulations are met. See section 4.2.9 of this guidance for more information.
4.0 The reconsideration process
This section provides a chronological overview and a detailed outline of the steps of the process.
4.1 Overview
FDALO consults with reconsideration advisorFootnote 1 in the relevant directorate to make decisions or recommendations to the DG on certain steps outlined as follows. FDALO ensures the reconsideration advisor has not previously been involved in the submission.
- Within 30 days of receipt of a negative decision, the applicant is responsible for filing a Letter of Intent to request a reconsideration of the decision.
- FDALO sends the applicant an Eligibility Letter to confirm acceptance or rejection of the request within 5 days of receiving the Letter of Intent. (See Section 2.0 for eligibility criteria.)
- If eligible, the applicant has 45 days from the date of the Eligibility Letter to submit a full RR that includes background and rationale for the objection.
- Reconsideration advisor screens the RR to verify that there is no new data (including but not limited to studies or references that were not included in the original submission). The advisor may consult with review bureau staff to verify whether or not new information is included.
- Within 20 days of receiving the RR, FDALO sends the applicant an Invitation Letter confirming the type of reconsideration process selected. The process is approved by the DG based on a recommendation by FDALO and reconsideration advisor. It can be either of the following options or a combination of the 2 processes:
- Internal Review: carried out by 1 or more scientific and regulatory experts from Health Canada who were not previously involved in the submission. The applicant can choose to have the reconsideration process based on a review of a written submission, a teleconference or an in-person meeting.
- External Reconsideration Panel of external experts: selected if there are no experts available within Health Canada who were not previously involved with the review, or if outside expertise is required.
- After deliberating on the issues under reconsideration, the Internal Review staff or External Reconsideration Panel prepares a recommendation for the DG.
- The DG issues a reconsideration decision letter which is sent to the applicant with the recommendation report prepared by the Internal Review staff or External Reconsideration Panel.
- There are 3 available process options, all with different performance targets. The time frame for each begins when the RR is received by Health Canada and the reconsideration decision is issued.
- Internal Review: decision based on written submission - 55 days.
- Internal Review: decision based on a teleconference or in-person reconsideration meeting - 90 days.
- External Reconsideration Panel: decision based on a panel meeting - 145 days.
4.2 Detailed outline of reconsideration process
This section of the Guidance provides a detailed description of key steps of the reconsideration process. Additional supporting materials can be found in the appendices, which include:
- Appendix A: Reconsideration Process Map;
- Appendix B: Performance Targets;
- Appendix C: Request for Human Drugs Reconsideration Template;
- Appendix D: Request for Natural Health Products Reconsideration Template;
4.2.1 Letter of intent
Within 30 days of receiving the negative decision letter (see eligible decisions in Section 2.0), the applicant must submit a Letter of Intent that explicitly makes a request for a reconsideration of the decision.
Within 5 days of receiving the Letter of Intent, FDALO will send the applicant an Eligibility Letter to confirm if the request is accepted or refused, with supporting rationale.
4.2.2 Request for reconsideration
Within 45 days of the date of the Eligibility Letter, the applicant must submit a RR.
The RR should be filed using the same filing format as the original submission and include the completed template (see Appendix C or D). See Section 6.0, "Information Formats and Address," for more information.
The RR should contain the following information:
- a copy of the negative decision letter for which the reconsideration is requested;
- numbered paragraphs with the applicant's position and rationale for each issue(s) under dispute, cross-referenced with points in the negative decision letter.
The information should be a brief, high-level summary of the issue(s) in dispute. The RR should not include new issues or information that was not in the submission when the original decision was made. FDALO will communicate with the applicant if new information is found and will act as an intermediary if there is a dispute between the review bureau and applicant in this matter. If new information is found, the applicant will be given the option of withdrawing the RR or proceeding without including the new information. In the case of withdrawal, the applicant can choose to refile a new submission.
FDALO may grant the applicant a reasonable extension of time to file the RR. The applicant must make this request in writing and identify the additional time required, as well as the rationale.
4.2.3 Decision regarding process path
FDALO and the directorate's reconsideration advisor will review the RR and will recommend a process to the DG for approval. Within 20 days of receiving the RR, FDALO will send the applicant an Invitation Letter indicating the process selected.
Efforts to ensure fairness, impartiality and responsible stewardship of resources will be made when selecting a reconsideration process. The process could involve an Internal Review by Health Canada experts or an External Reconsideration Panel of external experts. The choice is related to the type of issues under consideration and the availability of expertise within Health Canada. Factors that could lead to selecting an external Reconsideration Panel include:
- internal expertise, not previously involved, is not available for the reconsideration;
- Health Canada determines an external perspective is required.
In exceptional circumstances, a combination of the 2 processes may be used if the complexity of the case warrants it.
If either party has concerns about what process is being proposed, they can contact FDALO directly.
4.2.4 Internal review
FDALO and reconsideration advisor will determine if there are experts, on the matters under reconsideration, within Health Canada who have not previously been involved with the submission. One or more experts will be identified for each Internal Review process. A recommendation is made to the DG for Internal Review staff, based on their credentials to conduct a subsequent re-examination of the issues under reconsideration.
The applicant can choose whether the reconsideration be based on a written submission, teleconference or in-person meeting. The review bureau participates according to the choice made by the applicant.
If a written submission is chosen, the only document the applicant has to prepare is the RR.
4.2.4.1 Natural health product class I applications
NNHPD offers applicants a simplified and expedited route of applying for Product Licences for products that comply with all parameters of an individual NNHPD monograph as set out in the Guidance Document: Natural Health Products Management of Applications Policy. Objections to Natural Health Product Class I applications will be subject to an Internal Review process to maintain an expedited review of these applications through the reconsideration phase. The reconsideration decision will be based on a review of written submissions made by the applicant, the negative decision letter, and information available at the time of the initial submission. No teleconference or in-person meeting will be held. A recommendation will be sent to the NNHPD DG for a final decision. The final decision letter and recommendation report by Internal Review staff will be sent to the applicant.
For NHP Class II and III Applications, the timelines for the reconsideration process as outlined in this Guidance will apply.
4.2.5 External reconsideration panel
There are a number of tasks related to the selection of panel members and the development of the questions for the panel to deliberate.
4.2.5.1 Selecting members for a panel
If an External Reconsideration Panel is selected, the applicant and the review bureau are asked to provide nominee(s) within 7 days of the date of the Invitation Letter. It is preferable for each party to list more than 1 nominee to make allowances for the nominee's availability and interest in participating in the process. The parties can rank nominees according to preference. FDALO and the relevant directorate's reconsideration advisor will select from each list, taking into consideration the rank order, each nominee's experience, expertise, analytical skills relevant to issues under reconsideration and/or availability.
FDALO and reconsideration advisor will make a recommendation for the DG's approval of the overall membership of the External Reconsideration PanelFootnote 2, based on:
- 1 member being selected from nominations by the applicant;
- 1 member being selected from nominations by the review bureau; and
- a Chair being recommended by FDALO in consultation with the reconsideration advisor.
4.2.5.2 Eligibility of external panellists
Any person who conducted the review of the submission, or reviewed information related to the submission on behalf of the directorate or applicant, is not eligible to be a member of the External Reconsideration Panel. Potential external panel members who have worked for other applicants on a similar product, or made public statements about a product or similar products, will be reviewed for conflict of interest. To ensure that the nominees can comply with conflict of interest requirements, the applicant and review bureau must not contact the nominees and must not provide them with any material for review. The nominees must not have publicly expressed their views regarding the product or issues in question and must not have been involved with the applicant for the product. The nominees must complete a conflict of interest declaration.
4.2.5.3 Selection of external panellists
FDALO will contact all nominees to determine whether they are interested and available to participate on the panel. Nominees will be asked to provide a current curriculum vitae and must satisfy all conflict of interest and security clearance requirements. FDALO will complete the screening process and make recommendations to the DG, who will make the final determination on the panel membership. If either of the parties has concerns about how conflict of interest guidelines were applied to a nominee, they should direct these concerns to FDALO. Each situation will be assessed on its individual merits. Upon request, FDALO will endeavour to explain the basis for its screening process. External panel members are contracted by Health Canada and are not volunteers.
4.2.5.4 Questions for the external reconsideration panel
Within 14 days of the date of the Invitation Letter, the applicant and the review bureau are required to submit proposed questions for the External Reconsideration Panel to deliberate. These questions help the External Reconsideration Panel focus its deliberations on the specific issues under dispute.
The questions must be formulated in a manner to draw upon the scientific expertise of the panellists so that they can provide the DG with a response to a scientific issue. The questions should not be leading in nature (i.e. worded so as to suggest the desired answer). General questions that do not touch on the scientific or regulatory merits of the submission, such as "Should a specific product be approved?," will not be accepted.
FDALO, in consultation with reconsideration advisor, will consider questions provided by the applicant and review bureau to formulate questions for the panel. FDALO will attempt to build consensus between the applicant and the review bureau. FDALO will share the proposed questions with the applicant and review bureau, who may suggest minor revisions. If the parties cannot arrive at a consensus, the DG will decide on the final questions while considering the suggestions by the parties.
4.2.6 The reconsideration meeting
The format for the reconsideration meeting, whether it is carried out by Internal Review or an External Reconsideration Panel, is the same.
FDALO, in consultation with reconsideration advisor, will ensure all relevant background material provided by the applicant and review bureau is sent to Internal Review staff or External Reconsideration Panel members with enough time for review and consideration.
FDALO will communicate the time frames and submission deadlines for the exchange of presentations between the applicant and review bureau. Fourteen days prior to the reconsideration meeting, the parties should submit a presentation to FDALO of the key points they wish to make in the meeting. FDALO will facilitate the exchange of presentations between the review bureau and the applicant to allow for full advance disclosure of each party's perspective prior to the reconsideration meeting. Based on this exchange, the parties may make minor modifications to their respective presentations and submit final presentations 4 days before the meeting. No additional changes will be accepted after the submission of the final presentations.
The reconsideration meeting will be held within 60 days of the date when panel members have been selected and their availability confirmed. This date is considered firm and both the sponsor and Health Canada representatives are expected to make themselves available.
Fourteen days prior to the meeting, the applicant and Review Bureau will provide a list of their representatives (up to 6 individuals) who are expected to participate in the reconsideration meeting. The list should indicate each participant's title and role (e.g. presenter), as well as whether they will participate via teleconference or in person.
FDALO moderates the meeting. The applicant and review bureau will each make formal presentations to the External Reconsideration Panel/Internal Review staff. The DG and reconsideration advisor are also present. The applicant will be given the option of presenting first or second. Each party will have approximately 45 minutes to complete their presentation.
The purpose of this meeting is to provide both parties the opportunity to explain and share their perspectives. Each presentation should be a brief overview of the issue(s) under dispute. The panel, reconsideration advisor and DG may ask questions following each party's presentation. If the applicant or review bureau has clarifying questions for the other, these will be allowed at the discretion of the Panel Chair and time permitting.
Following the Internal Review meeting, the reconsideration advisor may consult with areas of expertise within Health Canada as needed. In these situations, details of this activity will be captured and noted in the Issue Analysis Summary (IAS), which is shared with the applicant.
4.2.7 Making recommendations to the director general
The Internal Review staff or Panel Chair will submit, within 14 days, a report to FDALO with recommendations for each issue under reconsideration and/or question posed. The reconsideration advisor will analyze the report and may contact the External Reconsideration Panel or Internal Review staff to request clarification on their recommendations if necessary.
The reconsideration advisor prepares an IAS summarizing key points in the report. The IAS, containing recommendations, is presented to the DG with the report for approval within 14 days of the meeting.
The IAS report includes a summary of the process, information considered in the analysis, recommendations and details regarding follow-up actions required. In the case of an internal review, the panel report may be considered as the IAS.
4.2.8 Decision by the director general
The DG, taking into consideration the IAS, will issue a reconsideration decision letter within 5 days of receipt of the IAS. These are three possible decisions. Section 3.0 of this Guidance provides detail on these outcomes.
- original decision is upheld;
- original decision is partially amended but the submission remains refused;
- original decision is amended.
The decision letter and supporting documents such as the IAS and Panel Report are sent to the applicant by the DG.
4.2.9 Follow-up action
Once the DG's decision is issued, FDALO will forward all documents generated through the reconsideration process to the relevant directorate. It is the responsibility of the directorate to ensure appropriate follow-up actions are taken.
Specific follow-up actions will depend on the nature of the reconsideration decision.
If the drug reconsideration process results in an amendment of 1 or more issues, then an amended decision letter is prepared by the review bureau for the DG's signature. If the decision was to refer the submission back to the review bureau for re-evaluation or implementation, the review bureau is responsible for ensuring that the reconsideration decision is implemented correctly.
The review bureau will ensure that a new target date for completing the review is set and communicated to the applicant within 14 calendar days of the reconsideration decision. The new target will be set on a case-by-case basis, depending on such factors as the number of issues involved, the remaining information to be reviewed and the complexity of the issues.
A natural health product reconsideration outcome in favour of the applicant will result in the Product Licence Application being returned to the review process and a licence being issued or amended, as appropriate, if the requirements of section 7 of the Natural Health Products Regulations are met. If the natural health product reconsideration results in upholding the refusal to issue or amend the product licence, the applicant will be sent a final notice setting out the reasons for refusal.
5.0 Roles and responsibilities
This section of the Guidance document outlines the roles and responsibilities of each party involved in the reconsideration process.
| Party | Responsibilities with request for reconsideration process |
|---|---|
DG (or delegate) of: NNHPD |
|
FDALO |
In consultation with reconsideration advisor:
|
Drug or Natural Health Product Licence Applicant |
|
Reconsideration Advisor Not Previously Involved in the Submission from: Bureau of Policy, Science, and International Programs, PDD Office of Business Integration, BRDD Director General's Office, NNHPD |
|
OSIP |
|
External Reconsideration Panel Internal Review Staff |
|
Regulatory Project Management Division, PDD Office of Regulatory Affairs, BRDD Bureau of Licensing, NNHPD |
|
Review Bureau (PDD, BRDD, NNHPD) |
|
6.0 Information formats and address
6.1 Human drugs
The Letter of Intent, RR and other information (such as presentations for the meeting, proposed External Reconsideration Panel questions and background material) should be sent electronically via the Common Electronic Submission Gateway or via mail to the Office of Submissions and Intellectual Property, to the attention of FDALO.
Where the original submission was made using the Electronic Common Technical Document (eCTD) format, all reconsideration documents should be submitted using this format. Applicants should refer to the latest version of Guidance Document: Preparation of Drug Regulatory Activities in the Electronic Common Technical Document (eCTD) Format and Guidance Document: Creation of the Canadian Module 1 Backbone for preparing submissions in eCTD format.
Where the original submission was not made in the eCTD format, reconsideration documents should be sent electronically in Portable Document Format, better known as PDF. The information should be organized in folders and should be named according to Appendix D, "Common Technical Document (CTD) Format"of Guidance Document: Preparation of Drug Regulatory Activities in the Common Technical Document (CTD) Format.
6.2 Natural health products
For natural health products, Letters of Intent, the RR and other information should be sent via mail or facsimile to FDALO at:
Food and Drugs Act Liaison Office
200 Eglantine Driveway
Address Locator 1915C
Ottawa, Ontario
K1A 0K9
Fax: 613-946-3585
Requests for reconsideration and other information can also be sent directly to FDALO through ePost. To use this secure platform, provide our office with a valid email address in the letter of intent.
Appendix A: Reconsideration Process Map
Figure 1 - Appendix A: Reconsideration Process Map (part 1)
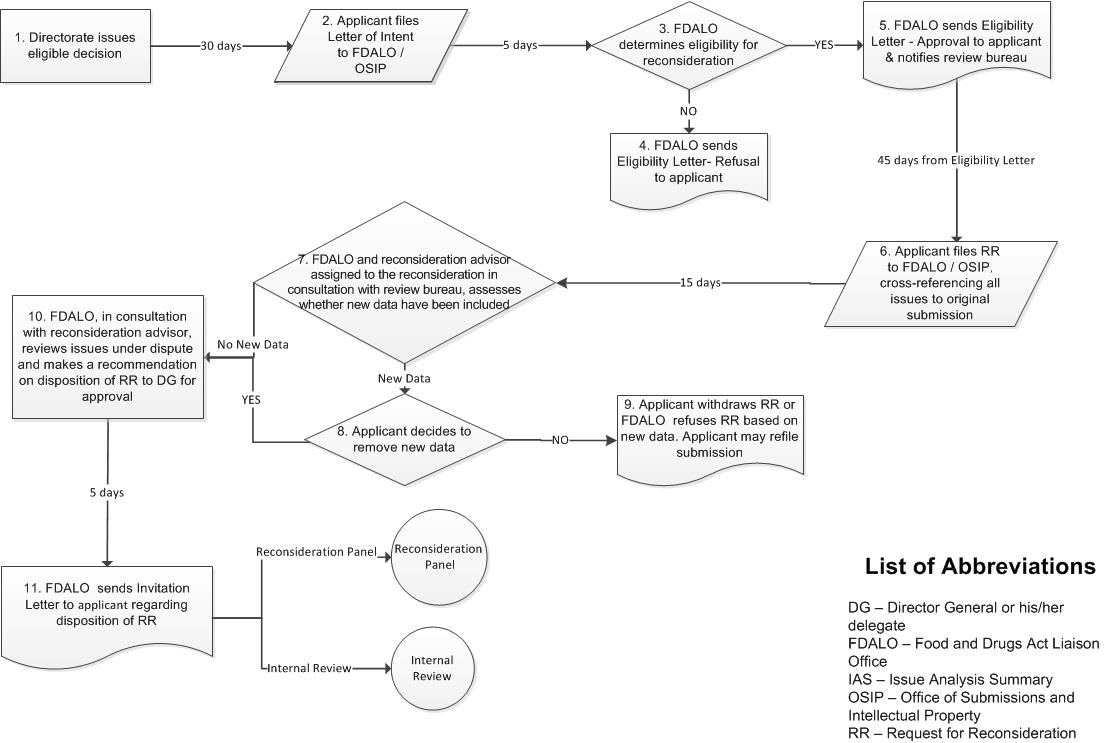
Figure 1 (part 1) - Text Description
List of Abbreviations
- DG
- - Director General or his/her delegate
- FDALO
- - Food and Drugs Act Liaison Office
- IAS
- - Issue Analysis Summary
- NON/w
- - Notice of Noncompliance - withdrawal
- OSIP
- - Office of Submissions and Intellectual Property
- RR
- - Request for Reconsideration
- Step 1: Directorate issues eligible decision. 30 days.
- Step 2: Applicant files Letter of Intent to FDALO or OSIP accordingly. 5 days.
- Step 3: FDALO determines eligibility for reconsideration.
- Step 4: If not eligible, FDALO sends Eligibility Letter- Refusal to applicant.
- Step 5: If eligible, FDALO sends Eligibility Letter - Approval to applicant, notifies review bureau. 45 days from Eligibility Letter.
- Step 6: Applicant files RR to FDALO or OSIP according to type of submission, cross-referencing all issues to original submission. 15 days.
- Step 7: FDALO and reconsideration advisor assigned to the reconsideration in consultation with review bureau, assesses whether new data have been included.
- Step 8: If new data is included: Applicant decides to remove new data.
- Step 9: If applicant decides not to remove new data: Applicant withdraws RR or FDALO refuses RR based on new data. Applicant may re-file submission.
- Step 10: No new data or applicant removes new data: FDALO, in consultation with reconsideration advisor, reviews issues under dispute and makes a recommendation on disposition of RR to DG for approval. 5 days.
- Step 11: FDALO sends Invitation Letter to applicant regarding disposition of RR, two options (A or B).
- A. Reconsideration panel.
- B. Internal Review.
- If A: 7 days.
Figure 1 - Appendix A: Reconsideration Process Map (part 2)
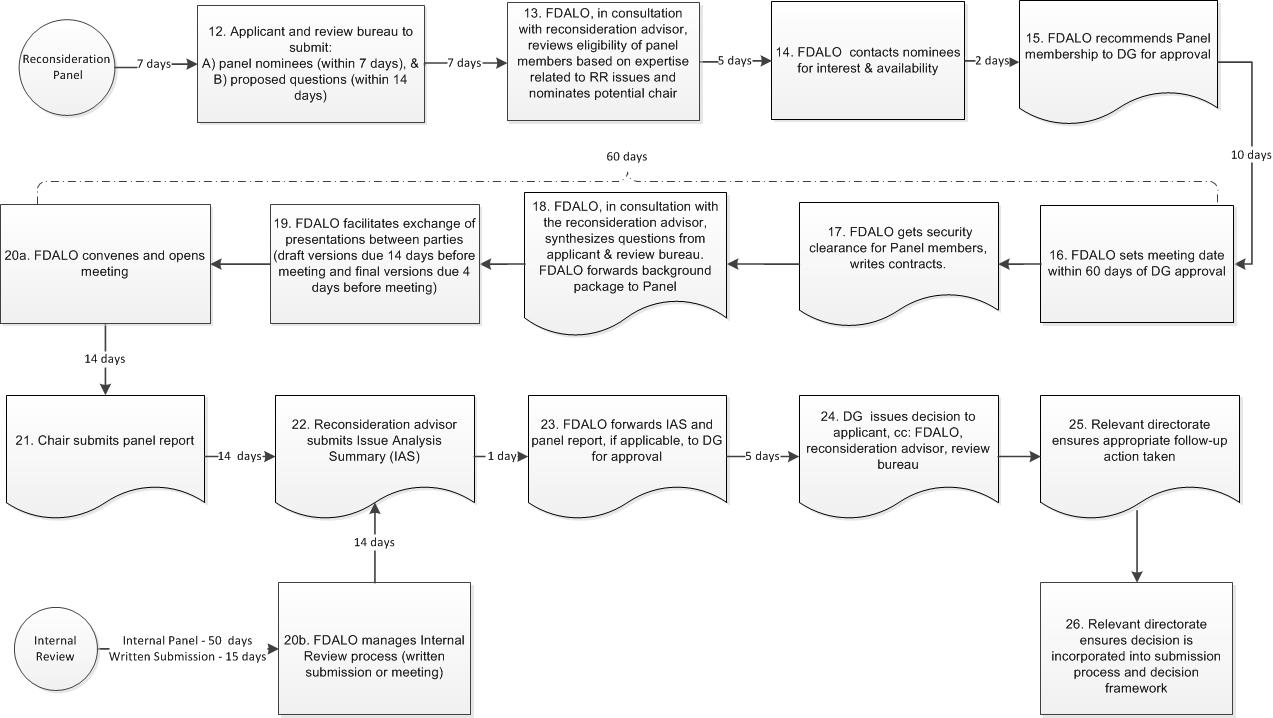
Figure 1 (part 2) - Text Description
- Step 12: Applicant and review bureau to submit: panel nominees (within 7 days), & proposed questions (within 14 days). 7 days.
- Step 13: FDALO, in consultation with reconsideration advisor, reviews eligibility of panel members based on expertise related to RR issues and nominates potential chair. 5 days.
- Step 14: FDALO contacts nominees for interest & availability. 2 days.
- Step 15: FDALO recommends Panel membership to DG for approval. 10 days. Note that steps 16-20 are expected to take 60 days.
- Step 16: FDALO sets meeting date within 60 days of DG approval.
- Step 17: FDALO gets security clearance for Panel members, writes contracts.
- Step 18: FDALO, in consultation with the reconsideration advisor, synthesizes questions from applicant & review bureau. FDALO forwards background package to Panel.
- Step 19: FDALO facilitates exchange of presentations between parties (draft versions due 14 days before meeting and final versions due 4 days before meeting).
- Step 20a: FDALO convenes and opens meeting. 14 days.
- Step 20b: FDALO manages Internal Review process (written submission or meeting). 14 days. There is no step 21 for this process flow.
- Step 21: Chair submits report. 14 days.
- Step 22: Reconsideration advisor submits Issue Analysis Summary (IAS). 1 day.
- Step 23: FDALO forwards IAS to DG for approval. 5 days.
- Step 24: DG issues decision to applicant, cc: FDALO, reconsideration advisor, review bureau.
- Step 25: Relevant directorate ensures appropriate follow-up action taken.
- Step 26: Relevant directorate ensures decision is incorporated into submission process and decision framework.
- If 20B: Internal review. Internal review flow. Internal Panel expected to take 50 days, reconsideration based on written submission only is expected to take 15 days.
- Step 22: Reconsideration advisor submits Issue Analysis Summary (IAS). 1 day.
- Step 23: FDALO forwards IAS to DG for approval. 5 days.
- Step 24: DG issues decision to applicant, cc: FDALO, reconsideration advisor, review bureau.
- Step 25: Relevant directorate ensures appropriate follow-up action taken.
- Step 26: Relevant directorate ensures decision is incorporated into submission process and decision framework.
Appendix B: Performance targets
This table highlights key steps in the reconsideration process and is provided as a quick reference. Full details are contained in the appropriate sections of this Guidance.
| Section | Step in reconsideration process | Output of step | Performance targets/deadlinesFootnote 3 (calendar days) |
|---|---|---|---|
| 4.2.1 | Applicant files Letter of Intent. | Letter of Intent filed to OSIP, which forwards it to FDALO for human drugs. Letter of Intent sent directly to FDALO for natural health products. | 30 (from date of the negative decision letter) |
| 4.2.1 | FDALO issues Eligibility Letter. | Eligibility Letter issued with rationale. If reconsideration allowed, Letter of Intent forwarded to the relevant directorate. | 5 (from date of receipt of Letter of Intent) |
| 4.2.2 | Applicant files RR. | RR filed to relevant directorate. | 45 (from date of the Eligibility Letter) |
| 4.2.2 | OSIP processes RRs for human drugs. FDALO processes RRs for natural health products. | FDALO forwards RR to reconsideration advisor in relevant directorate. | 5 (from date of receipt of RR by OSIP) |
| 4.2.3 | FDALO (in consultation with the relevant directorate) assesses the RR for new information. | If information is complete with no new information, recommendation made to DG on selection of reconsideration process. If the information contains new information, the applicant is given the option of removing the new information or of withdrawing request. |
10 (from date of receiving RR from OSIP or mail) |
| 4.2.3 | FDALO sends Invitation Letter to applicant with decision on the reconsideration process (External Reconsideration Panel and/or Internal Review). | FDALO issues Invitation Letter to applicant. | 5 (from date of DG decision on reconsideration process) |
| Internal Review Process | |||
| 4.2.4 | FDALO identifies Internal Review staff who have not previously been involved in the submission. | Internal Review staff confirmed | 15 (from date of Invitation Letter) |
| 4.2.6 | FDALO convenes Reconsideration meeting. | Meeting is held with presentations to Internal Review staff and DG. | 35 teleconference or in person meeting (from date of confirmation of Internal Review staff) For written submission (no meeting is held) |
| 4.2.7 | Relevant directorate's reconsideration advisor analyzes issue(s) and prepares IAS. | IAS forwarded to FDALO. | 14 (from date of the meeting or analysis of written submission) |
| External Reconsideration Panel Process | |||
| 4.2.5.1 | Applicant and review bureau submit nominees. | Nominees received. | 7 (from date of Invitation Letter) |
| 4.2.5.2 | FDALO, in consultation with the relevant directorate, reviews potential conflict of interest and eligibility of nominees. | Potential panel members identified. | 7 (from date of receiving nominees' names from applicant and review bureau) |
| 4.2.5.3 | FDALO contacts nominees for interest and availability. Conflict of interest form is completed by nominees. | Members' interest and eligibility is confirmed. | 12 (after consulting with the relevant directorate) |
| 4.2.5.3 | FDALO receives DG approval on panel membership. | Membership list approved. | 2 (from date when "draft" membership is completed) |
| 4.2.5.4 | Applicant and review bureau submit proposed questions and background material for reconsideration. | Questions are submitted to FDALO. | 14 (from date of Invitation Letter) |
| 4.2.6 | FDALO contacts panel members to determine a meeting date and to confirm meeting logistics. FDALO convenes reconsideration meeting. | Meeting is held with presentations to panel members and DG. | 70 (from date of confirmation of nominees' interest) |
| 4.2.7 | Chair submits report. | Report submitted to FDALO | 14 (from date of meeting) |
| 4.2.7 | Relevant directorate's reconsideration advisor reviews panel's report. | Relevant directorate's reconsideration advisor prepares IAS and forwards it to FDALO. | 14 (from date when the report was submitted) |
| Both External Reconsideration Panel & Internal Review Processes | |||
| 4.2.7 | FDALO receives IAS/Panel Report from Internal Review staff/External Reconsideration Panel. | FDALO forwards IAS/Panel Report to DG. | 1 (from date of receipt of the IAS/Panel Report) |
| 4.2.8 | DG makes a decision on the reconsideration. | Decision letter and IAS/Panel Report are sent to the applicant. | 5 (from date of receipt of the IAS/Panel Report from FDALO) |
| 4.2.9 | Follow-up action. | Where appropriate, the relevant directorate will contact applicant to communicate new target dates. | 14 (from date of reconsideration decision by DG) |
Note: Every effort will be made to meet these targets; however, unforeseen delays can occur as a result of conflict of interest and security clearance requirements, and the need to accommodate schedules of external experts.
Appendix C: Request for Human Drugs Reconsideration Template
- Request for Human Drugs Reconsideration Template [PDF version, 646 KB]
Appendix D: Request for Natural Health Products Reconsideration Template
- Request for Natural Health Products Reconsideration Template [PDF version, 183 KB]