Certified Product Information Document - Chemical Entities (CPID-CE)
This HTML document is not a template. Its purpose is to display the information as found on the form for viewing purposes only.
Word document (DOC Version - 121 K)
(Version: 2017/10/30)
Summary of Product Information
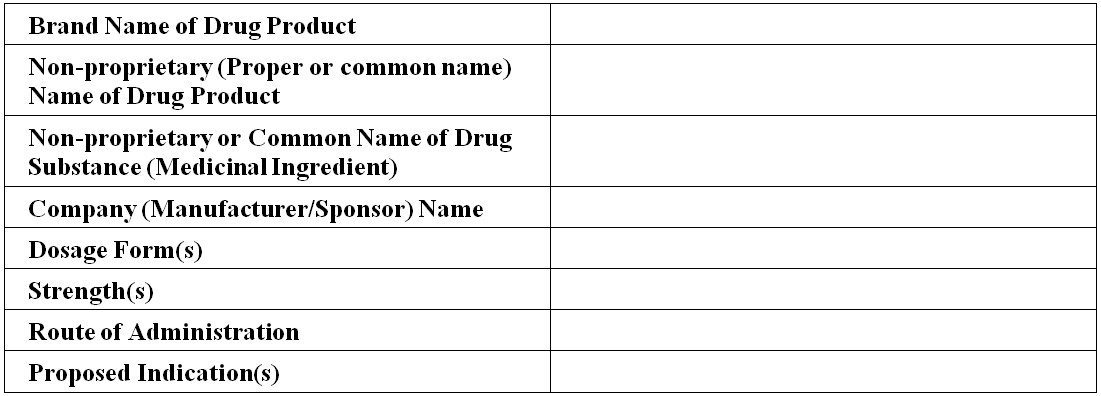
Figure 1 - Text Description
- Brand name of drug product
- Non-proprietary (Proper or common name) Name of Drug Product
- Non-proprietary or Common Name of Drug Substance (Medicinal Ingredient)
- Company (Manufacturer/Sponsor) Name
- Dosage Form(s)
- Strength(s)
- Route of Administration
- Proposed Indication(s)
(a) Sponsor's Date of CPID:
(b) Administrative Summary: (Health Canada use only)
Summary of Product Information

Figure 2 - Text Description
- DocuBridge Identifier
- Control Number
- Internal Version and/or Date of Acceptance
2.3.S Drug Substance (Name, Manufacturer)
2.3.S.1 General Information
2.3.S.1.2 Structure
(a) Structural formula, including relative and absolute stereochemistry:
(b) Molecular formula:
(c) Molecular mass:
2.3.S.1.3 General Properties
(a) Physical form (for example [e.g.], polymorphic form, solvate, hydrate):
(b) Solubilities and Dose/Solubility Volume over the physiological pH range (1.2-6.8):
(c) pKa:
2.3.S.2 Manufacture (name, manufacturer)
2.3.S.2.1 Manufacturer(s) (name, manufacturer)
(a) Name, address, and responsibility of each manufacturer, including contractors, and each proposed production site or facility involved in manufacturing and testing:

Figure 3 - Text Description
- Name and Address
- Responsibility (for example, manufacturing, packaging, labelling and testing)
- MF # or CEP #
2.3.S.2.2 Description of Manufacturing Process and process controls (name, manufacturer)
(a) Flow diagram showing reactants, solvents and reagents:
Name and address of sites manufacturing the API starting material(s) and/or intermediates:
Name and chemical structure of API starting material/intermediate:
Manufacturer:
Manufacturing site address:
S.3.2 Impurities
Potential impurities not routinely controlled in the drug substance:
2.3.S.4 Control of the Drug Substance
2.3.S.4.1 Specification (name, manufacturer)
(a) Specification for the drug substance:
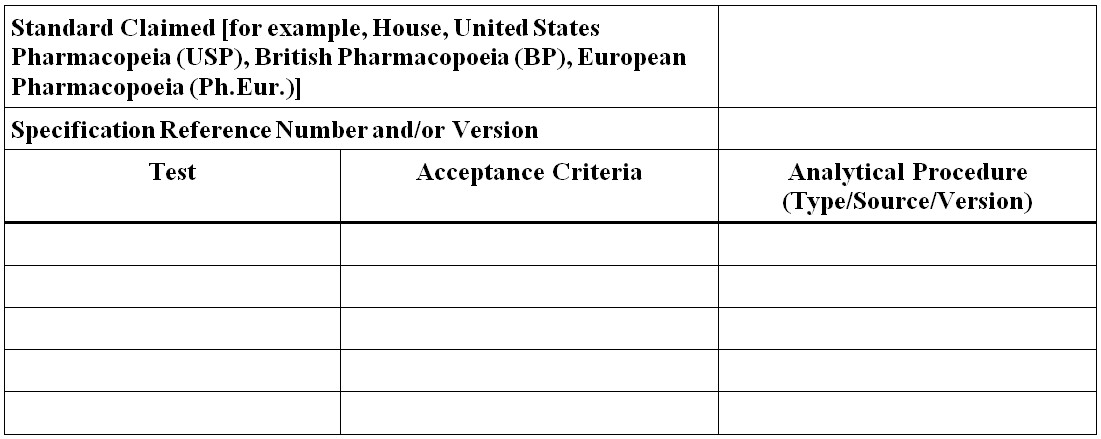
Figure 4 - Text Description
- Standard Claimed [for example, House, United States Pharmacopeia (USP), British Pharmacopoeia (BP), European Pharmacopoeia (Ph.Eur.)]
- Specification Reference Number and/or Version
- Test
- Acceptance Criteria
- Analytical Procedure (Type/Source/Version)
2.3.S.6 Container Closure System
(a) Description of the container closure system(s) for the storage and shipment of the drug substance:
2.3.S.7 Stability
2.3.S.7.1 Stability Summary and Conclusions
(a) Proposed storage conditions and re-test period (or shelf life, as appropriate):

Figure 5 - Text Description
- Container Closure System
- Storage Conditions
- Re-test Period
2.3.P Drug Product (Name, Dosage Form)
2.3.P.1 Description and Composition of the Drug Product (name, dosage form)
(a) Composition of the dosage form:
(i) Composition, that is (i.e.), list of all components of the dosage form, and their amounts on a per unit basis (including overages, if any):
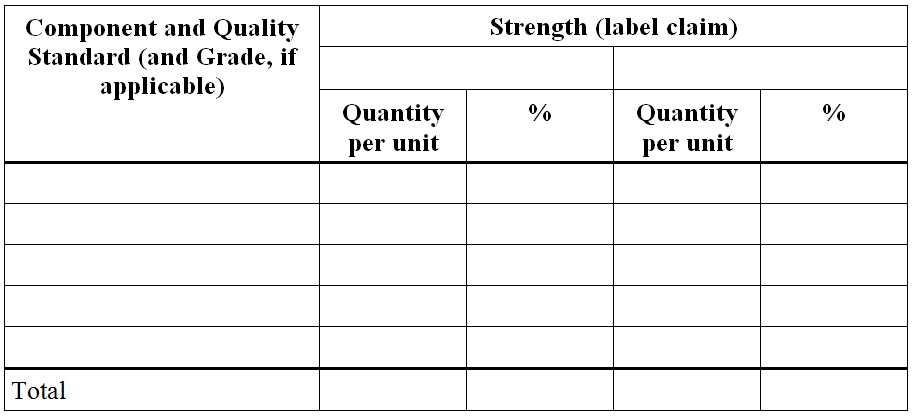
Figure 6 - Text Description
- Component and Quality Standard (and Grade, if applicable)
- Strength (label claim)
- Quantity per unit
- %
- Quantity per unit
- %
- Total
(ii) Composition of all components that are mixtures (e.g., colourants, coatings, capsule shells, imprinting inks):
(b) Description of accompanying reconstitution diluent(s), if applicable:
2.3.P.3 Manufacture (name, dosage form)
2.3.P.3.1 Manufacturer(s) (name, dosage form)
(a) Name, address, and responsibility of each manufacturer, including contractors, and each proposed production site or facility involved in manufacturing and testing:

Figure 7 - Text Description
- Name and Address
- Responsibility (for example, manufacturing, packaging, and testing)
- MF #
2.3.P.3.2 Batch Formula (name, dosage form)
(a) List of all components of the dosage form to be used in the manufacturing process, and their amounts on a per batch basis (including overages, if any):

Figure 8 - Text Description
- Strength (label claim)
- Master Production Document, Reference Number and/or Version
- Batch Size(s) (number of dosage units)
- Component and Quality Standard (and Grade, if applicable)
- Quantity per batch
- Quantity per batch
2.3.P.3.3 Description of Manufacturing Process and Process Controls (name, dosage form)
(a) Flow diagram of the manufacturing process:
(b) Narrative description of the manufacturing process, including equipment type and working capacity, process parameters:
2.3.P.3.4 Controls of Critical Steps and Intermediates (name, dosage form)
(a) Summary of controls performed at the critical steps of the manufacturing process and on isolated intermediates:
2.3.P.3.5 Process Validation and/or Evaluation (name, dosage form)
(a) Summary of process validation information, including any commitments, for the critical steps in the manufacturing process (e.g., protocol number, parameters):
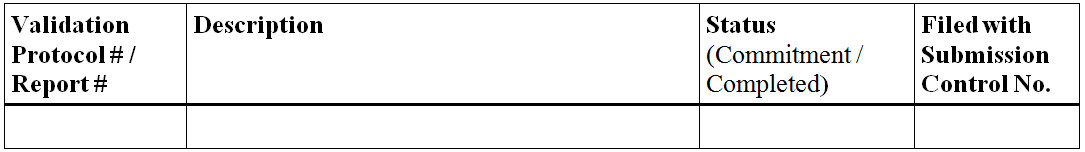
Figure 9 - Text Description
- Validation Protocol # / Report #
- Description
- Status (Commitment / Completed)
- Filed with Submission Control No.
2.3.P.5 Control of Drug Product (name, dosage form)
2.3.P.5.1 Specification(s) (name, dosage form)
(a) Specification(s) for the drug product:
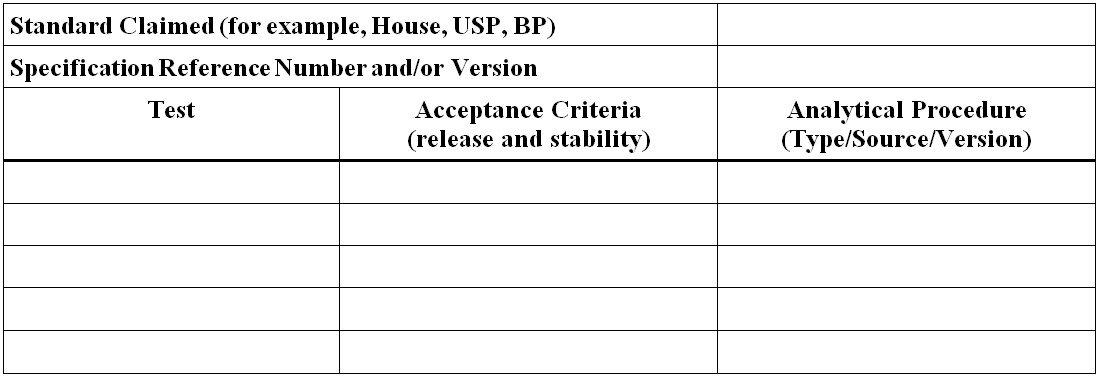
Figure 10 - Text Description
- Standard Claimed (for example, House, USP, BP)
- Specification Reference Number and/or Version
- Test
- Acceptance Criteria (release and stability)
- Analytical Procedure (Type/Source/Version)
2.3.P.7 Container Closure System (name, dosage form)
(a) Description of the container closure systems, including unit count or fill size, container size or volume:
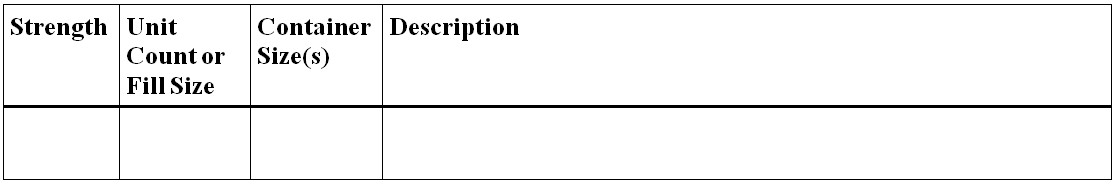
Figure 11 - Text Description
- Strength
- Unit Count or Fill Size
- Container Size(s)
- Description
2.3.P.8 Stability (name, dosage form)
2.3.P.8.1 Stability Summary and Conclusions (name, dosage form)
(a) Proposed storage conditions and shelf life (and in-use storage conditions and in-use period, if applicable):

Figure 12 - Text Description
- Container Closure System
- Storage Conditions (and In-use Storage Conditions, if applicable)
- Shelf Life (and In-use Period, if applicable)
2.3.P.8.2 Post-approval Stability Protocol and Stability Commitment (name, dosage form)
(a) Stability protocol for commitment batches:
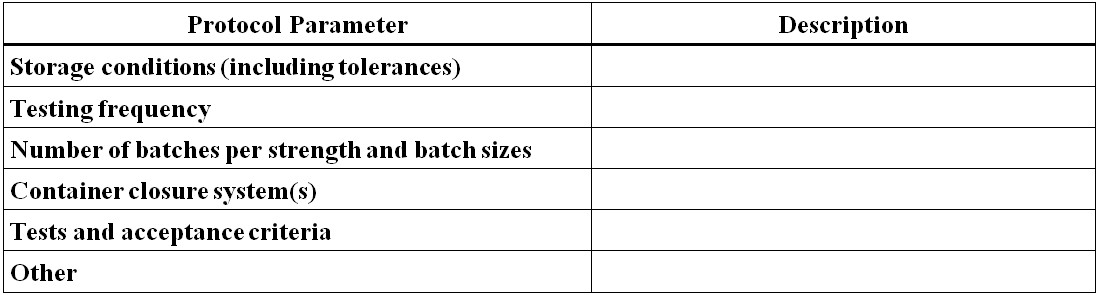
Figure 13 - Text Description
- Protocol Parameter
- Storage conditions (including tolerances)
- Description
- Testing frequency
- Description
- Number of batches per strength and batch sizes
- Description
- Container closure system(s)
- Description
- Tests and acceptance criteria
- Description
- Other
- Description
- Storage conditions (including tolerances)
(b) Stability protocol for continuing (i.e., ongoing) batches:
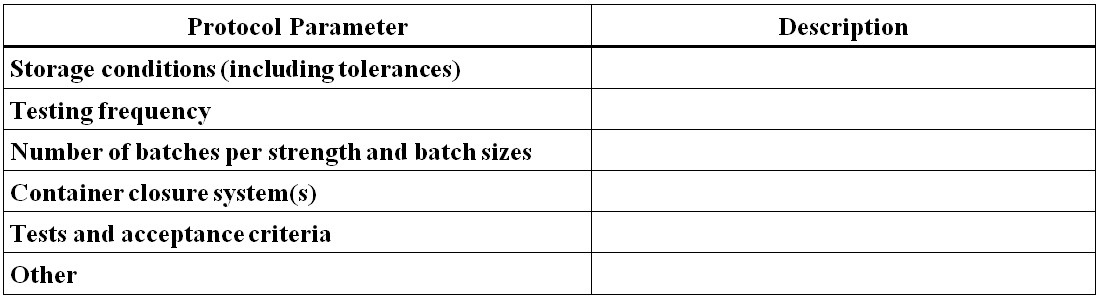
Figure 14 - Text Description
- Protocol Parameter
- Storage conditions (including tolerances)
- Description
- Testing frequency
- Description
- Number of batches per strength and batch sizes
- Description
- Container closure system(s)
- Description
- Tests and acceptance criteria
- Description
- Other
- Description
- Storage conditions (including tolerances)
2.3.P.8.3 Stability Data (name, dosage form)
(a) Bracketing and matrixing design for commitment and/or continuing (i.e., ongoing) batches, if applicable: