ARCHIVED - 2016 Protecting Canadians from Unsafe Drugs Act Transparency Needs-based Assessment: What We Heard
Introduction:
The Protecting Canadians from Unsafe Drugs Act (Vanessa's Law) amended the Food and Drugs Act in order to improve safety oversight of drugs and medical devices throughout their lifecycle. Among others, this Act introduced important new transparency measures including:
- an obligation on the Minister of Health to make publicly available all orders issued with respect to therapeutic products and positive and negative regulatory decisions;
- an obligation on therapeutic product authorization holders to make required information about their clinical trials publicly available on a website; and,
- an authority for the Minister of Health to disclose confidential business information in certain circumstances.
As part of Health Canada's Regulatory Transparency and Openness Framework, Health Canada conducted an online needs-based assessment from March 25 to May 25, 2015, targeting patients and their caregivers, healthcare professionals, heath technology assessors, pharmaceutical and medical device industry, academia and Canadians.
The main goal of this consultation was to help us better understand the information needs of the respondent groups so that we continue to develop the best and most valuable ways of sharing information about drugs and medical devices with Canadians.
The secondary goal was to identify if there were any differences for information needs between the different targeted respondents so that we can adjust the ways that information is being disseminated (e.g. patients versus healthcare professional information needs).
Health Canada is committed to providing greater access to credible, timely, useful information that will allow Canadians to make well-informed decisions concerning their health and that of their families. This feedback is being used in the regulatory, guidance and protocol development, and as a starting point for more targeted consultations with specific stakeholders.
This document summarizes what we heard from our stakeholders. To request a copy of the full data set of the consultation please contact lrm-mlr@hc-sc.gc.ca.
Participation summary
During the course of the consultation, 530 individuals and organizations participated in the consultation. The breakdown by respondent is shown in Figure 1. Healthcare professionals represented the largest group, representing 42% of all respondents, followed by the general public at 18%, and patients and their caregivers being the third largest group of respondents at 11%. Other respondents included health technology assessors, academic researchers, federal government and provincial or territorial governments. Approximately 5% of the respondents self-identified themselves as 'others'.
Figure 1: Breakdown of respondent groups
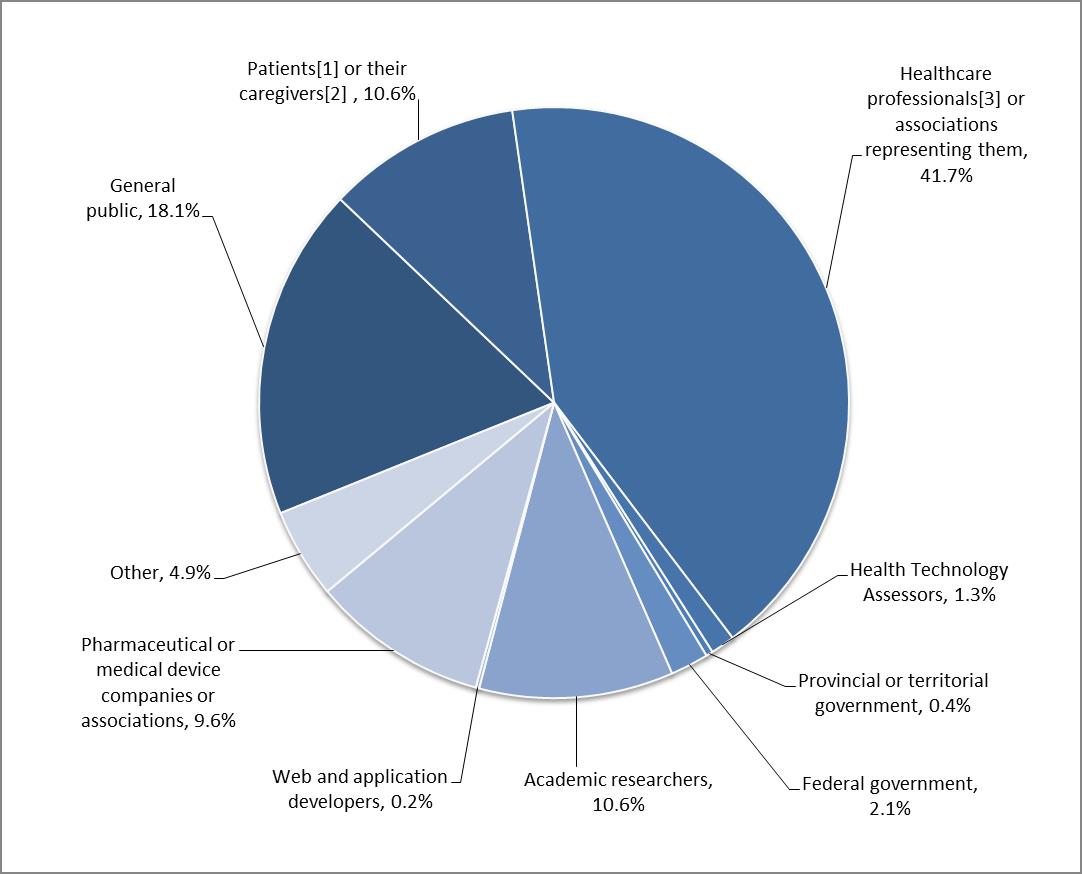
- [1] People who are diagnosed with or are being treated for a disease or a medical condition
- [2] People who help another individual with their disease or medical condition, such as a parent of a child, or someone caring for an elderly patient
- [3] Pharmacists, nurses, nurse practitioners, physicians, dentists) or associations representing them
Results:
Section 1: Current online sources and information needs for drugs and medical devices
In the first section of the consultation, Health Canada sought information to determine the most common sources of online information about drugs and medical devices; the reasons why these sources were chosen; most common purpose of the respondents' search, and how much time was spent searching for this information.
Figure 2: Sources of online information about drugs and medical devices
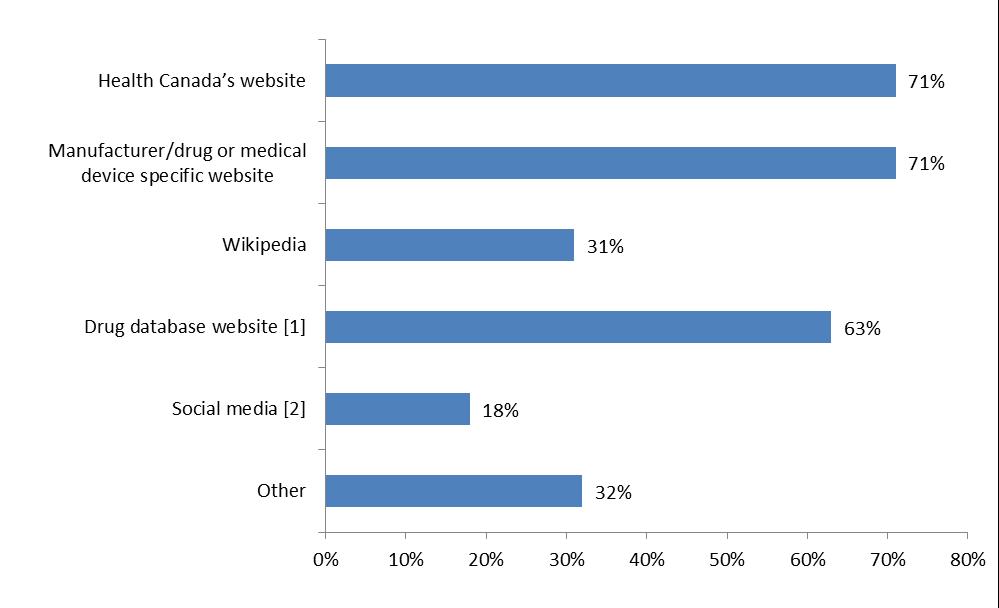
- [1] e.g., online Compendium of Pharmaceuticals and Specialties (e-CPS)
- [2] e.g. Facebook, personal blogs, support group sites
From a list of common websites (Figure 2), respondents were asked to select all sites used to conduct to their searches over the past year. Overall, Health Canada and the drug or medical device manufacturer websites were the most used sites; both categories were selected by 71% respondents. Drug product database (e.g. Compendium of Pharmaceuticals, e-CPS) was chosen as the third most common site (63%). When the same information was filtered by respondent groups, two variations stood out: 1) healthcare professionals chose drug databases as the most used websites (87%); 2) the general public and patient and their caregivers use social media website as a source of information at 39% and 46%, respectively.
Health Canada asked the respondents to select the most common reasons for selecting a particular website. The most common response was that the information came from a reputable source and it was easy to access. When the response was filtered by respondent groups, approximately 30% of the patients and the general public chose their site because the information was provided by others who share the experience, coping strategies and issues (e.g. support group-based information).
Health Canada also asked the respondents to select the most common reason for their web search. From the pre-selected choices, respondents' top search was for information regarding an existing drug or medical device. Health Canada also asked how much time the respondents spent looking for that information. The majority of the respondents (65%) spent less than 4 hours per month on the search. There were no variations identified when the data was filtered by respondent groups.
Section 2: Information needs concerning clinical trials
The second section of the consultation asked various questions concerning the use of online clinical trial information. Clinical trial registries are online platforms used by the public to access information about clinical trials including drug/ medical device names, diseases- related, trial locations, contact information and summary of the clinical trial results, once the trial is concluded.
Based on the information provided, over 61% of all respondents search for clinical trial information online (Figure 3). The most common search was for specific drug information. When the data was filtered by respondent groups, only 33% of healthcare professionals look for clinical trial information online, in contrast to 95% of academic researchers.
Figure 3: Clinical trial information sought online

- [1] e.g., recruiting stage or completed stage
Health Canada asked the respondents who look for clinical trial information online whether they use clinical trial registries. Out of the 325 respondents that searched for clinical trial information online, 229 used a clinical trial registry, with more than 97% of those using ClinicalTrials.gov from the United States Food and Drug Administration as their primary registry.
Section 3: Information needs for drugs that are reviewed by Health Canada
Health Canada evaluates and monitors the safety, efficacy and quality of thousands of drugs, medical devices, and other therapeutic products available to Canadians. Currently, Health Canada publishes a large volume of information related to specific drugs and medical devices that are approved for sale in Canada. This information includes approvals, summary basis of decision, safety alerts and recalls, which are all published in both official languages. As part of this consultation, Health Canada sought to determine the stakeholders' preferred type of information and preferred methods of access to information (Figure 4).
Figure 4: Ranking of types of information, by importance, to be published on Health Canada's website for prescription drugs and professional-use medical devices
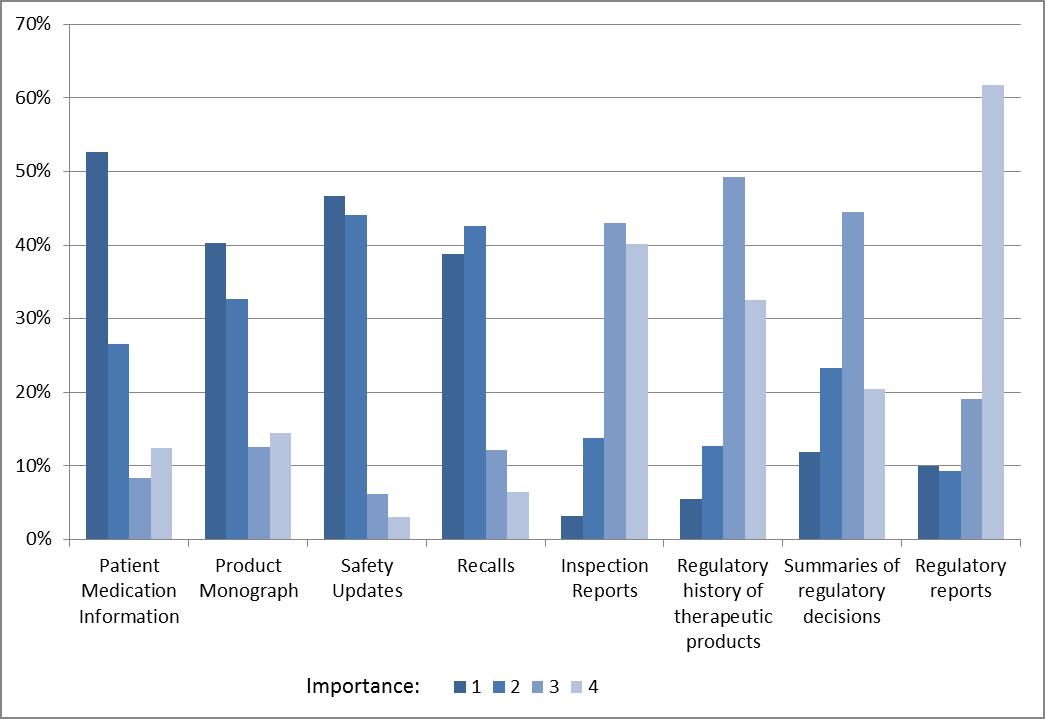
Respondents identified safety updates and recalls as the most important information to be published on Health Canada's website for prescription drugs. Plain language information such a Patient Medication or Device Information and scientific/technical information were also identified as being important. Only one respondent group, health technology assessors were interested in seeing full regulator reports (e.g. reviewer's reports). Similar results were seen for non-prescription drugs and "consumer" medical devices.
Section 4: Academic researchers, scientific and medical experts, and research organizations who are seeking access to information to answer specific scientific questions
The last section of the consultation focused on the ability to obtain confidential business information. Under section 21.1(3)(c) of the Food and Drugs Act, the Minister of Health has discretionary authority to disclose confidential business information about a therapeutic product to a person who carries out functions relating to the protection or promotion of human health or the safety of the public, if the purpose of the disclosure is related to the protection or promotion of human health or the safety of the public. To ensure that the information sharing is carried out as intended Health Canada asked the respondents if they agree with the following three proposed principles:
- 1. Information sharing will take place with qualified researchers and established bodies who have a formal plan to use the information to advance medical or scientific knowledge;
- 2. Health Canada will ensure that patients' privacy is protected and that participants' informed consent for clinical trials is respected (if available);
- 3. Prior to disclosure, Health Canada will ensure that the information will not be shared or used for commercial interests.
Figure 5: Respondents' agreement with proposed principles
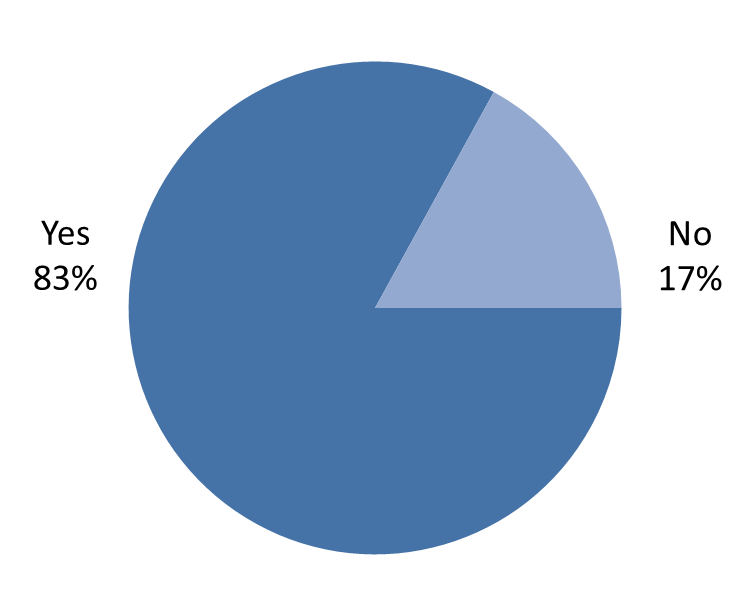
Overall, 83% of the 353 respondents agreed with the proposed principles (Figure 5).
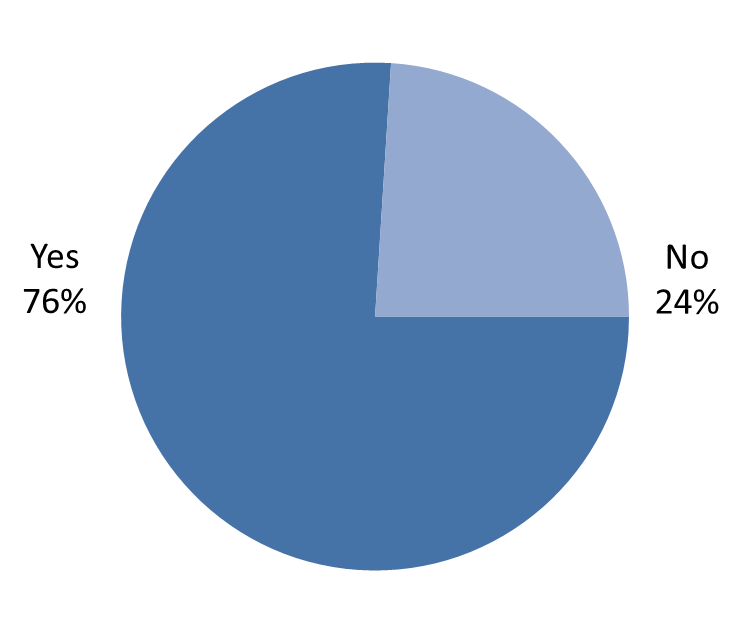
Health Canada also asked whether industry-based confidential business information disclosure platforms are used to obtain the information needed to conduct research. Only 33 respondents (10%) have used industry-based platforms, but from the 33 who have, 25 (76% of those who have used confidential business information) responded that the information obtained met their research needs (Figure 6).
Figure 6: Respondents indicating that the confidential business information obtained met their research needs
Health Canada wishes to thank all individual and organizations that have taken time to participate in this consultation. To request a copy of the full data set of the consultation please send a request to lrm-mlr@hc-sc.gc.ca.