Report an adverse reaction to a vaccine: Health care provider
On this page
If you are a health care professional who works in a hospital, learn about the new mandatory reporting rules.
Reporting adverse events following immunization
Health care providers who suspect an adverse reaction event following immunization to a vaccine are strongly encouraged to report it to their local public health unit using the adverse events following immunization (AEFI) form (PDF). Learn more about reporting adverse events following an immunization.
Report a side effect to Health Canada
To mail your report to the Canada Vigilance Program, use the postage-paid label.
Tracking adverse events following immunization
Health Canada and the Public Health Agency of Canada (PHAC) share the monitoring of the safety of vaccines in Canada.
PHAC manages the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS), which is a post-market vaccine safety surveillance system. CAEFISS reports are submitted by public health authorities in provinces and territories, which in turn receive them from local public health units. Nurses, doctors and pharmacists who provide immunizations or care for people who experience an adverse event from a vaccine generate most of the reports.
In addition, when manufacturers are made aware of adverse reactions to a drug or vaccine, they must report to Health Canada:
- serious adverse reactions in Canada
- unexpected serious adverse reactions in other countries
- unusual failures in efficacy for new drugs in Canada
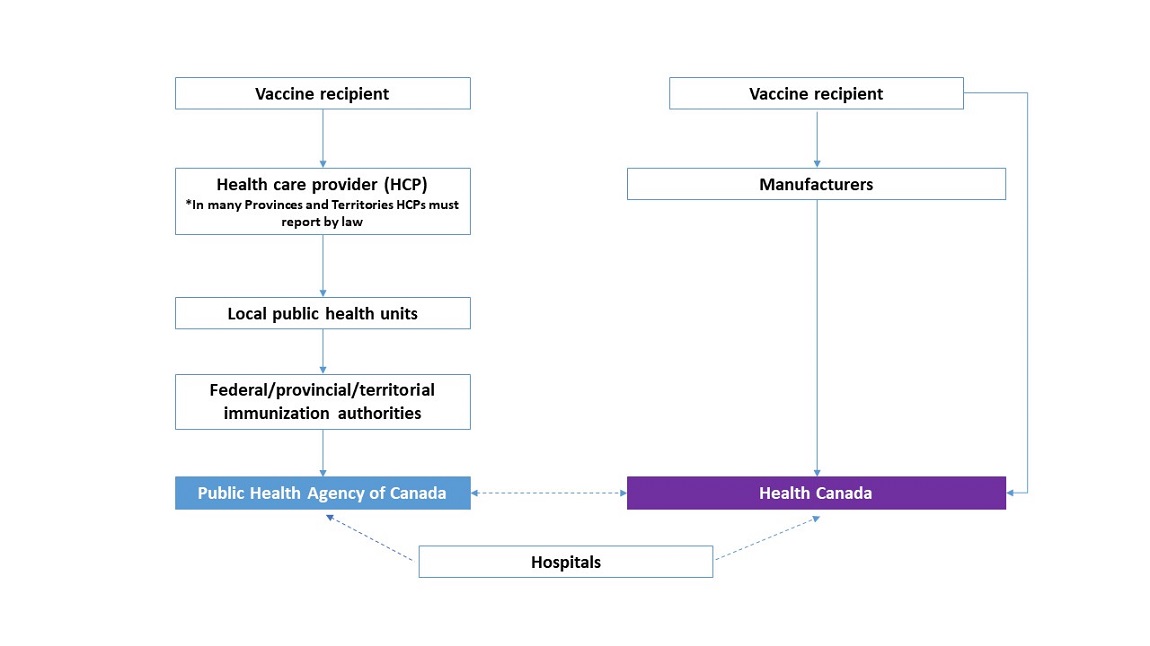
Text Equivalent
This diagram shows the reporting pathway of adverse events following immunization:
- from the source (vaccine recipient or manufacturer)
- to the Public Health Agency of Canada or Health Canada
At the top of the diagram, on the left, is a box representing the vaccine recipient. From this box, an arrow points downward to a box for the health care provider. From this box, an arrow points downward to a box for local public health units. From this box, an arrow points downward to a box for federal/provincial/territorial immunization authorities, then to a box for the Public Health Agency of Canada.
At the top of the diagram, on the right, is a box representing the vaccine recipient. From this box, an arrow points downward to a box for manufacturers. From this box, an arrow points downward to a box for Health Canada.
From the vaccine recipient box, an arrow points downward to the Health Canada box, indicating that the vaccine recipient can report directly to Health Canada. Another dotted line with double arrows points between the Public Health Agency of Canada box and the Health Canada box, indicating the Public Health Agency of Canada and Health Canada share information about adverse events.
At the bottom of the diagram is a box representing hospitals. From the left side of the box, a dotted line with a single arrow points to the Public Health Agency of Canada. From the right side of the box, a dotted line with a single arrow points to Health Canada.
Related links
- Vaccines and immunization
- MedEffect Canada
- Drugs and health products
- Adverse reaction database
- Advisories, warnings and recalls
- Drug and health product register
- Adverse reaction reporting and health product safety information - guide for health professionals