Guidance document on classification of veterinary drugs and livestock feeds
Table of contents
- 1. Introduction
- 2. Scope and application
- 3. Guiding principles
- 4. Classification criteria
- 5. Classification decision process
- 6. Implementation
List of appendices
- Appendix A - Nutritional disorders
- Appendix B - Drug/Feed classification flowchart
- Appendix C - List of known medicinal ingredients
- Appendix D - Stress-Related Indications (or Claims)
- Appendix E-1 - Mycotoxin Detoxification Agents (MDAs)
- Appendix E-2 - Viable Microbial Products (VMPs)
- Appendix E-3: Acid based products
1. Introduction
1.1 Background
In Canada, products consumed by livestock species are largely regulated as either veterinary drugs or livestock feeds.
Veterinary drugs (henceforth referred to as drugs) are regulated under the Food and Drugs Act and Regulations by Health Canada. Within Health Canada, the Veterinary Drugs Directorate (VDD) has the mandate to evaluate and monitor the safety, quality and effectiveness, to set standards and to promote the prudent use of veterinary drugs, including veterinary natural health products, administered to food-producing and companion animals. The VDD's classification of a product is guided by the requirements of the Food and Drugs Act and Regulations.
Livestock feeds (henceforth referred to as feeds) are regulated under the Feeds Act and Regulations, which are administered by the Canadian Food Inspection Agency (CFIA). The CFIA verifies that livestock feeds manufactured and sold in Canada or imported are safe, effective and are labelled appropriately. Effective feeds contribute to the production and maintenance of healthy livestock.
1.2 Definitions
Pursuant to the Food and Drugs Act, a "drug" includes any substance or mixture of substances manufactured, sold or represented for use in
- the diagnosis, treatment, mitigation or prevention of a disease, disorder, abnormal physiological state, or its symptoms in human beings or animals,
- restoring, correcting or modifying organic functions in human beings or animals, or
- disinfection in premises in which food is manufactured, prepared or kept.
Pursuant to the Feeds Act, a "feed" is defined as any substance or mixture of substances containing amino acids, antioxidants, carbohydrates, condiments, enzymes, fats, minerals, non-protein nitrogen products, proteins or vitamins, or pelletizing, colouring, foaming or flavouring agents and any other substance manufactured, sold or represented for use:
- for consumption by livestock;
- for providing the nutritional requirements of livestock, or;
- for the purpose of preventing or correcting nutritional disorders of livestock,
- or any substance for use in any such substance or mixture of substances.
A definition and interpretation of a nutritional disorder can be found in Appendix A.
1.3 Context
The overlap of the definitions for these two types of products creates a challenge whereby nutritional and non-nutritional products may be classified as either a drug and a feed, or both. In the past, difficulties associated with the interpretation of the drug and feed definitions have resulted in significant confusion, which led to inefficiencies regarding product evaluation and approval, as well as compliance and enforcement activities. Therefore, there is a need for a clear and transparent classification process for some products intended for use in livestock species.
1.4 Purpose and objectives
The purpose of this guidance document is to present guidelines and criteria for classification in order to clarify the differences between drugs and feeds, and to assist in determining the appropriate regulatory oversight for a product destined for use in livestock species. The pursued objectives are:
- to improve the transparency of the classification process, to increase the consistency in decisions by regulators, and make these more predictable to stakeholders;
- to resolve industry confusion and to provide better guidance to manufacturers of such products regarding the appropriate regulatory department, submissions and processes for a product approval.
2. Scope and application
2.1 Scope
The classification approach presented in this document applies to a broad range of products, intended for oral consumption only and for livestock species (dairy and beef cattle, sheep, fish, goats, chickens, turkeys, ducks, geese, rabbits, horses and swine) only, and in most types of animal production (for ex., dairy cattle vs. beef cattle, broilers chickens vs. laying hens). In some situations, products may neither fit a drug nor a feed classification. Such products may be classified (and therefore regulated) as veterinary biologics, veterinary medical devices, disinfectants, pest control products or consumer products. Please note that these types of products are beyond the scope of this guidance and will not be further discussed in this document. Medicated feeds have a clear regulatory definition and are also excluded from the scope of the classification guidance.
2.2 Application
This guidance document is applicable to products (drugs and feeds) that are subject to the pre-market evaluation process (new) and to the existing products currently sold on the Canadian market (authorized for sale). The present document is intended to be used in conjunction with other regulatory tools from Health Canada and CFIA, such as feed schedules, policies, guidelines, regulations, etc.
3. Guiding principles
For a product classification to be consistent with both regulatory mandates, the following general principles will be applied:
- The primary consideration will be the respect of the definitions of a drug in the Food and Drugs Act and of a feed in the Feeds Act. Guidance will be consistent with the objectives of Health Canada and CFIA and their respective regulatory frameworks, including public health and safety.
- Classification decisions from other international veterinary regulatory authorities will be considered during the classification process.
- The potential risk to health from an ingredient or a product is generally not a factor for classification - it is taken into consideration during assessment of the product's safety (AFTER the product has been classified). The regulatory frameworks under the drug and feed legislations provide a tier of controls to mitigate the risks posed by a product. If these risks are considered unacceptable, itwill result in the product not being permitted for sale, regardless of its classification.
- The determination of a drug or feed classification can result from the consideration of only one of the most pertinent classification criterion listed below, but generally requires the consideration of multiple criteria taken together as a whole.
- Depending on the product, some criteria may not be given equal weight in support of a classification decision.
- Some products may be considered both nutritional and therapeutic, based on differences in ingredients concentrations, dosage recommendations/inclusion rates or other factors.
Based on a complete assessment of each of the criteria identified below, the differences between drugs and feeds can be identified to facilitate a regulatory classification. Please see the document Drug/Feed classification flowchart (shown in appendix B) for a step-by-step approach to the classification process.
4. Classification criteria
4.1 Route of administration
The fact that a substance or a product is administered in feed or in water does not have a significant impact on the regulatory classification of a product.
4.2 Mode of administration
4.2.1 Final product form
The product form refers to the final form in which a substance or product is available and ready for use/administration, without requiring any further manufacturing. Common product forms for therapeutic products (or "dosage" forms) include tablets, capsules, boluses, aerosols or inhalers, creams, lotions, solutions or suspensions and implants.
The form of a product is usually dependent on the route of administration of the substance in question. Most products in dosage form are considered drugs, with some exceptions, depending on other criteria (for ex., purpose, label). Products in forms that can be consumed in a voluntary manner, such as mash, liquid, pellets, flakes, free choice blocks, etc., are typically (but not exclusively) classified as feeds.
4.2.2 Forceable administration
Generally, products for forceable administration (administered orally but via gavages or drenches) are considered drugs. However, some pastes, pumps and non-injectable syringes that are:
- labelled for inclusion into feeds, and
- for which no drug claim is made,
- are regulated as feeds under the Feeds Act and Regulations. All products must indicate physical form in their product name (for ex., paste) in order to be considered feeds.
All other products for forceable administration will continue to be regulated as drugs.
4.3 Known medicinal ingredients
Some ingredients are inherently medicinal or therapeutic in nature, either due to their characteristics, their function (activity) and effect(s) on the organism or their purpose in the formulation. For example, some substances like penicillin, neomycin, or plant extracts like digitalin or digoxin have an intrinsic therapeutic function or activity. This knowledge is usually derived from scientific literature or reference textbooks. In the case of a multiple-ingredient product, if one or more ingredients in its composition have a therapeutic nature or effect, the product would be considered a drug. Also, some herbal ingredients have a generally recognized therapeutic use and no recorded nutritional use. These types of herbal ingredients are considered medicinal ingredients and classified as drugs.
To help identify ingredients currently considered as medicinal (therapeutic), please refer to Appendix C for a definition and an alphabetical listing of known medicinal ingredients.
4.4 Representation/Intended purpose
Key considerations for the classification of a product are its representation (1) and intended purpose (2):
- The representation associated with a product include indications for use (they may be explicit or implied), health claims presented as a word, sentence, a picture, a symbol, a paragraph or an implication on product labels, package inserts or advertisements (including information available on the Internet), as well as its intended purpose and history of use.
- The intended purpose of a product is the desired effect to be achieved by the administration of this product to a particular animal species during a particular situation, or under specific conditions (for ex., health or disease states, nutritional needs). The activity or properties of the ingredient(s) present in a product are also considered to provide information on the product's intended (desired) effect.
Together, these product characteristics are used to create a clear picture of what the product is and does. While the "representation for use" criterion would not be considered the only basis for a classification decision, it may be taken into account and influence the final decision because of its impact on the purpose of the product. Therefore, an indication/claim present on a product label or in the advertising material is an important but not determining factor to be considered for product classification.
However, indications (or claims) present on a product, whether they are supportable or not, will be determinants for the submission of an application to the appropriate regulatory body. For the classification of products in the "grey zone" between drugs and feeds, applications should be submitted to VDD as a single-window contact for classification.
Notes on advertising:
In classifying products, VDD and CFIA's Animal Feed Division (AFD) may also look to how the product is advertised or promoted. Although web sites alone are not extensions of the label of a product, claims made in the media, including those made on web sites of various stakeholders or social media, may be considered evidence of the intended purpose of the product for classification purposes.
4.4.1 Therapeutic indications (claims)/purposes
Therapeutic claims, indications or purposes refer to the treatment of a disease, disorder or abnormal physical state; or treatment, mitigation of its symptoms; or the modification of an organic function (such as digestion). Therapeutic claims can only be made for products which have a therapeutic purpose or intent of use (drugs), and are therefore not suitable for feeds. Examples of therapeutic (or drug) claims are:
- Treatment claims (treatment of a disease (acetonemia), a condition (intoxication) or abnormal state (wounds, oedema) or its symptoms (diarrhea);
- Control claims (control intestinal parasites, control breeding);
- Prevention claims (prevent mastitis, prevent acetonemia, prevent bloat, prevent coccidiosis), excluding prevention of nutritional deficiency claims;
- Mitigation claims (reduce severity of pneumonia, relief pain associated with colic, relief of inflammation, decrease incidence of laminitis, reduction of early mortality);
- Animal Performance claims (production claims) not supported by a nutritional purpose or mode of action (stimulate egg production, increase litter size).
Note: This list of acceptable indications for drugs is not exhaustive and others indications or claims not acceptable for feeds would require further assessment for classification.
4.4.2 Nutritional indications (claims)/purposes
Feeds are not intended to have any therapeutic purpose or activity. Products with such purposes are not considered feeds but are considered drugs. Feed products are typically fed for a reasonable amount of time as part of a balanced feeding program with the intent of meeting the nutritional requirements necessary for maintenance, growth and production of livestock. They can also include non-nutritive products such as flavours, pellet binders, preservatives, anti-caking agents and other products that facilitate the manufacture, storage or palatability of feeds. Feeds are also known to be vehicles (carriers) for delivery of therapeutic products such as medicated feeds.
Nutritional indications or purposes refer to the presence of one or more nutrient(s) or nutritive substance(s) which are scientifically recognized for providing the nutritional requirements essential for supporting growth, maintenance and production in livestock species. An intended purpose that states suggests or implies that the consumption of a specific nutrient or nutritive substance significantly reduces the incidence or prevents the development of a nutritional disease; disorder or deficiency in a healthy animal may also be acceptable in this category of claims. Examples of nutritional claims that would be acceptable for feeds include:
- Nutritional disorder prevention claims excluding disease prevention (prevention of "white muscle disease" in cattle due to selenium deficiency; prevention of anemia due to iron deficiency in neonatal piglets, prevention of scoliosis due to vitamin C deficiency in salmonid fish); More information on nutritional disorders can be found in Appendix A.
- Animal performance (Production) claims within a nutritional context (increased milk production from feeding a by-pass; amino acid (compared to unprotected source of the same nutrient));
- Increased nutrient bioavailability (organic complex minerals claiming increased bio- availability compared to a certain inorganic source; enzymes such as phytases to increase available phosphorus).
Feeds can also be non-nutritive for the following purposes or claims:
- Nutrient preservation (for ex., forage additives, mould inhibitors, feed antioxidants) not supported by a therapeutic purpose or mode of action (please see section 4.7 on Mode of Action);
- Facilitating agents intended to aid or improve the manufacturing or handling properties of a feed (for ex., pelleting aid, anti-caking agents, carriers, etc.) not supported by a therapeutic purpose or mode of action;
- Colouring agents to colour the feed or tissues/eggs of livestock;
- Flavouring agents to flavour feeds.
Note: Products making nutritional claims on their label will require registration with CFIA prior to import, manufacture and sale in Canada. These types of claims would be permitted for feeds provided that they are supported by adequate scientific evidence that has been assessed and approved by the CFIA's Animal Feed Division (AFD).
4.5 Composition
Although the composition of a product alone does not necessarily determine its classification, the presence of an ingredient, or its level, may aid in determining the correct regulatory classification of that product.
4.5.1 Type of ingredients
The components of a product or its ingredients are assessed to determine the activity or purpose of that product. The nature of each ingredient, its intrinsic characteristics, its physical and chemical properties, the degree of modification (for ex., compound, extract, isolate) and it associated function(s) and role(s) in the product are investigated in detail to provide evidence to support the intended use of a product.
4.5.2 Concentration (or level) of ingredients
The concentration, strength or level of ingredients may be useful in determining its particular role in the formulation and the intent (or purpose) of the ingredient in the product as a whole. An ingredient may have a particular function until it reaches a certain threshold or concentration, at which point it has a pharmacological or therapeutic effect (for ex., propylene glycol 1% or less = inactive ingredient vs. propylene glycol 50% or 100% = active ingredient).
Also, a nutritional ingredient such as a vitamin or a mineral can have a therapeutic effect at a higher amount, based on the concentration in the product and dosage recommendations (for ex., calcium, iron, copper, zinc, vitamin A, vitamin C, some plants and plant oils, etc.).
4.6 Directions for use
4.6.1 Dosage recommendations/Feeding rates
The recommended dosage or rate of administration of a product may assist in identifying the therapeutic or nutritional purpose of that product. For example, a very specific dosage regimen may suggest a therapeutic purpose or intended effect for a product.
The inclusion rates or dosage recommendations can sometimes be considered for classification of products as drugs, particularly for vitamin, mineral or electrolyte products. For feed products, the feeding rate is used to determine if the final amount of an ingredient or nutrient received by a particular species is within or higher than regulatory standards (for ex., supplementation levels recommended by NRC, Table 4 maximums, maximum limits prescribed in Schedule IV and V of the Feeds Regulations, etc.).
4.6.2 Duration of treatment/feeding & timing of administration
The duration of treatment and timing of administration criteria do not automatically lead to a drug or feed classification.
These specifics of directions for use are usually not a useful or relevant consideration for the classification of a product as a drug. They are quite varied for drug products since they are closely associated with the animal species and the disease (or condition) for which the drugs are indicated.
In general, products considered as feeds are part of a balanced feeding program and have to be fed for a reasonable period of time, on a continuous basis. The duration of mineral or vitamin supplementation may vary according to specific physiological periods in the production life of animals. For example, a nutritional product to be fed to dairy cattle in lactation or during gestation, or a mineral product fed during the first days of life for piglets, would be consistent with a feed supplementation regime.
4.7 Mode of action
In biological terms, a mode of action (MoA) refers to the mechanism or the manner in which an action, effect or result is obtained in an organism. The MoA and its effect are often related to the chemical structure of an ingredient or substance. A substance usually exhibits its action through an interaction between that substance and a cellular constituent, usually referred as a receptor, which results in a direct response, or which blocks the response to another agent. There are many specific receptor site(s) or enzyme(s) in a body that can trigger a biological response to a substance.
4.7.1 Therapeutic modes of action
A therapeutic mode of action is the specific biochemical interaction through which an ingredient (or combination of ingredients) produces a pharmacological effect. For example, acetyl salicylic acid (chemical substance) produces an irreversible inhibition of the enzyme cyclooxygenase (MoA), which suppresses the production of prostaglandins and thromboxanes (physiological effect), thereby reducing pain and inflammation (therapeutic effect).
Similarly to a pharmacological action, immunological and metabolic actions are also considered to be biochemical interactions leading to a particular biological response. An immunological action is an action in or on the body by stimulation and/ or mobilisation of cells and/or products from the immune system. A metabolic action is an action that involves an alteration, including stopping, starting or changing the speed of the normal chemical processes participating in, and available for, normal body functions (for ex., a partitioning agent for growth promotion).
When an ingredient or a product has an action that restores, changes or affects the physiological function(s) of an animal beyond the generally recognized physiological effects of nutrition, this action falls within the definition of a drug as per the Food and Drugs Act and is considered to be therapeutic.
Modes of action consistent with drugs:
- Indirect mechanism to stimulate appetite (stimulation of the CNS);
- Mode of action in both feed and animals (antifungal substance added at a high concentration in feeds, for prevention of moulds in the feed and mycotoxicosis in cattle);
- Any mode of action inside the animal, not related to the digestive process;
- Specific internal mechanism of action, such as:
- modification of cells or cellular functions; (for ex., insulin stimulate cells to absorb blood glucose)
- modification of a protein, enzyme, amino-acid, or its activity;
- modification of an organ or its activity; (for ex., decrease size of thyroid or decrease its activity)
- modification, elimination or blocking of a metabolic pathway;
- Bio-regulation mechanisms (bio-feedback) which controls expression and/or modification of some biological components;
- Artificial stimulation of the non-specific (innate) immune system:
- Recognition phase:
- production of an increase number of immunological cells or proteins;
- activation of the complement reactions;
- Elimination phase:
- increased phagocytic activity of immunological cells;
- increased cytotoxic activity of immunological cells;
- increase in cellular migration and in the inflammatory reaction;
- Recognition phase:
- Artificial stimulation of the non-specific (innate) immune system:
Action on the intestinal flora (substance/product not absorbed by the body and acting directly within the intestinal lumen) that may have some prophylactic/therapeutic effect.
Note: This list of acceptable modes of action for drugs is not exhaustive. Other modes of actions would require a description and further assessment for classification.
4.7.2 Nutritional modes of action
A nutritional mode of action could be defined as a process where recognized nutrients (when supplied at required levels), are digested/absorbed by the animal to maintain physiological functions, resulting in minor differences in biological activity and without a significant and lasting change or modification on the cellular, tissue, organic and metabolic reactions of the body. This process usually results in established and recognized physiological rates of growth, maintenance and/or production characteristics for healthy livestock species.
In the context of feeds, a nutrient can be defined as any substance that can be metabolized by an animal to give energy and build or maintain tissue to meet scientifically supported nutrient requirements. There are six types of nutrients: minerals, vitamins, fats, protein, carbohydrates and water.
Modes of action consistent with feeds:
- Action directly in the feed itself, before consumption, to improve or conserve the nutrient content of the feed , to aid or improve in the manufacturing or handling of the feed and to change the colour or smell of feeds (for ex., pellet binders, flow agents, colouring agents, flavouring agents, mould inhibitors, mycotoxin binders);
- Action limited to the normal digestive process of feeds (for ex., absorption of mineral and vitamins through the intestinal mucosa, improve digestibility and bioavailability of nutrients).
Note: This list of acceptable mechanisms of action for feeds is not exhaustive.
The MoA criterion may not always be a definitive one and should be evaluated in conjunction with other criteria such as the physiological particulars of the intended species (for ex., ruminants vs. non-ruminants), intent of use and concentrations of ingredients, before a final classification decision can be reached.
5. Classification decision process
The Veterinary Drug Directorate (VDD) and the Animal Feed Division (AFD) of CFIA encourage sponsors to initiate the classification process of their products by using the present guidance document, flowchart and other appendices. After self-assessing that a product would be classified as a drug, they can contact the VDD for information on submission requirements. The Veterinary Drugs section of Health Canada's website can also be consulted for additional information.
Once a product is determined to be a feed, they can proceed to submit a registration application to the AFD. Information on the registration process is available in the Regulatory Guidance section of CFIA's website.
If classification questions remain or issues arise after the review of this guidance, sponsors should contact VDD, as a single-window access for classification. VDD will collaborate with the AFD for information sharing and discussion to reach a joint classification decision. The applicant will be informed in writing of the final decision concerning the regulatory classification of a product. Drug decisions will be communicated by VDD; feed decisions will be communicated by AFD.
A classification template has been developed to facilitate the organization and presentation by the industry of the necessary information for an accurate product classification. Please contact VDD directly to obtain this template.
Classification requests from industry should include a cover letter, the completed classification template and the related supporting documentation. All classification requests should be submitted in writing (mail, e-mail or fax) to VDD.
Any classification reconsideration request should also be submitted in writing to VDD and be accompanied with a valid scientific rationale, as well as the appropriate supportive documentation. VDD will consult with AFD as needed.
6. Implementation
This guidance document will come into effect as of the online publication date. The publication, coordination and implementation of this guidance document of understanding will be the joint responsibility of Health Canada's VDD and the CFIA's AFD. For questions or additional information on the content of this document, please contact VDD.
Appendix A
Nutritional disorders
1. Definitions:
- Disease:
-
An impairment of the normal state of the living animal or plant body or one of its parts that interrupts or modifies the performance of the vital functions, which is typically manifested by distinguishing signs and symptoms, and is a response to environmental factors (as malnutrition, industrial hazards, or climate), to specific infective agents (as worms, bacteria, toxins, or viruses), to inherent defects of the organism (as genetic anomalies), or to combinations of these factors.
(Reference: Merriam -Webster Medical Dictionary)
In short, a disease refers to damage to an organ, part, structure, or system of the body such that it does not function properly or a state of health leading to such dysfunction.
Examples: heart disease, diabetes, bile duct obstruction.
- Nutrient:
- As defined under section 4.7.2 "Nutritional modes of action" (second paragraph), a nutrient can be defined as any substance that can be metabolized by an animal to give energy and build or maintain tissue to meet scientifically recognized nutrient requirements. There are six types of nutrients: minerals, vitamins, fats, protein, carbohydrates and water.
- Nutritional disorder:
- Any disorder in animals that is directly or indirectly caused by a lack of nutrients or a nutritional imbalance in the diet. Metabolic disorders and multifactorial disorders in which nutrition plays an important role are not considered to be nutritional disorders. Examples of such conditions are: post-parturient hypocalcemia (milk fever); acetonemia (ketosis) or pregnancy toxemia; metabolic acidosis; laminitis; abomasal displacement; enterotoxemia; urolithiasis (urinary calculi).
- Tonic (or conditioner):
- As defined under Subsection 2. (1) Interpretation of the Feeds Regulations 1983, a tonic or conditioner means a mineral feed formulated and represented for the correction of a specified nutrient deficiency or to aid recovery from a specified nutrient deficiency and is for use only while the condition persists (for ex., single-ingredient product of magnesium, or zinc).
2. Criteria:
Criteria for a nutritional disorder:
- Presence of deficiency, excess or imbalance of a specific nutrient in the diet;
- Cause/effect relationship between clinical signs of disorder and absence of nutrient in the diet;
- Diet supplementation prevents and/or resolves the condition
All criteria need to be met for a condition to be considered a nutritional disorder.
| Feed | Drug |
|---|---|
| Diet deficiency | Abnormal requirement from animal (deficiency due to disease state) |
| Interference between nutrients in diet | Lack of absorption from the body (due to disease state) |
| Physiological (normal) increase in animal requirements (for ex., parturition, lactation, sweating, etc.) | Abnormal requirement from animal (secondary to disease) (for ex., dehydration following diarrhea, bicarbonate in the presence of metabolic acidosis) |
| Relevant species (species with recognized nutritional need) | Species with no recognized deficiency or dietary requirement |
| Ingredients from approved and recognized feed source of vitamin/mineral | New source may have other properties or purposes |
| Scientific data supporting a nutritional purpose for a specific nutrient | Scientific data supporting a therapeutic (drug) purpose for a specific nutrient |
If one of the above drug criteria is met, the ingredient or product would be excluded from a feed classification and considered a drug.
Any treatment claim or therapeutic indication associated with these nutritional conditions (diseases) would result in the product being classified as a drug. Prevention/correction claims would be acceptable for feeds under the following situations:
- Prevention:
- when it is intended to meet nutritional requirements, and
- when it is intended to prevent a nutritional disorder caused by a deficiency in one specific nutrient, by a modification of the diet.
- Correction: when referring to the correction of a specific nutritional disorder caused by a deficiency in one specific nutrient, by modification of the diet.
- Approved nutritional sources are used for nutrient supplementation (for ex., Schedule IV of the Feeds Regulations)
Note: Products making such claims on their label will require registration with CFIA prior to import, manufacture and sale in Canada.
Appendix B
Drug/Feed classification flowchart
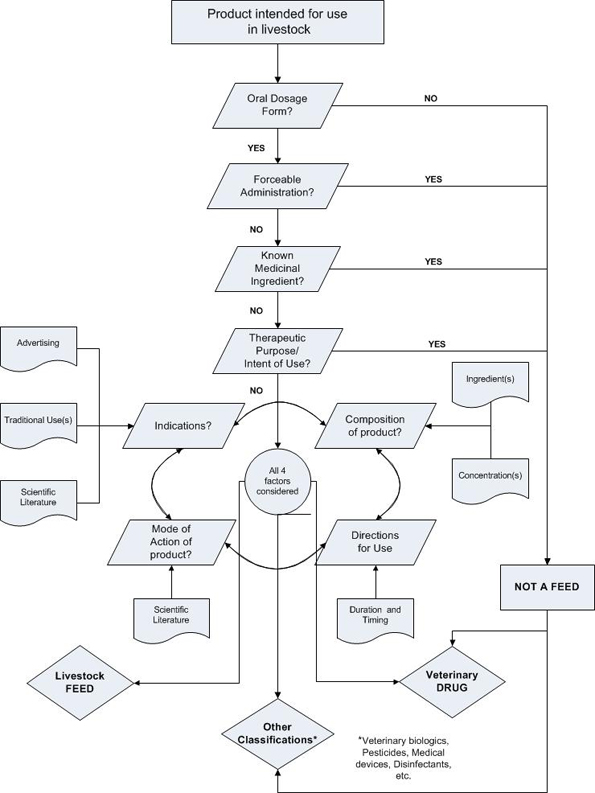
Text Description
Appendix A is composed of the Drug/Feed classification flow chart. The purposes of this graphic flowchart are to explain the logical step-by-step approach to the classification process currently used within VDD and explain the interactions between the different classification criteria. To begin, only products intended for livestock species would be considered to go through this process. Products intended for other species are not relevant for the Feed/Drug interface. Then, the following questions are asked, in the determined order, in order to exclude a probable feed classification right from the start:
- Is the product presented in an oral dosage form or administered orally? No = not a feed
- Is the product used via forceable administration? Yes = not a feed
- Does the product contain a known medicinal ingredient? Yes = not a feed
- Does the product have a therapeutic purpose or intent of use? Yes = not a feed
In order to answer this latter question, a group of four (4) classification criteria or factors need to be considered individually and as a whole;
- the indications on the product's label (which are assessed in conjunction with the subject product's advertising, traditional uses and the scientific literature associated with it);
- the composition of the product (which is influenced by the type of ingredients and their concentration);
- the mode of action (which is supported by the scientific literature); and
- the directions for use (which may or may not be influenced by the duration and or timing of the administration)
After answering the last questions, one would be in a good position to decide upon a classification of a drug (yes answer), of a feed (no answer) or another classification type. Other possible classifications for a product intended for animal use include veterinary biologics, pesticides, disinfectants, medical devices, etc.)
Appendix C
Known medicinal ingredients
1. Introduction
A known medicinal ingredient is a substance, part of a substance or a combination of substances associated with a therapeutic (medicinal) property or pharmacological effect. Based on a large number of classification requests, VDD and AFD have established a list of substances which have been classified as medicinal ingredients. These classifications were made based on the scientific knowledge current at the time of the request and related to the indications proposed by the sponsor. However, some medicinal ingredients may also have a non-medicinal purpose, depending of the concentration (level) or other factors. For your information, the list contains these "exceptions", which are ingredients listed on the Feeds Schedules IV and V in the Feed Regulations and used in feeds for non-medicinal purposes.
Important notes:
- The following list has been developed only for the purpose of this document, in order to facilitate product classification. It should not be considered as an exhaustive and complete list. However, it is our commitment to update/revise it on a regular basis.
- Classifications associated with proprietary information have not been included in this list.
- If a substance is not present in the list below, and you wish to ascertain the classification of this substance, the classification template can be submitted to VDD for classification purposes. For any questions, comments, additional guidance or to obtain the template, please contact VDD.
2. List of substances currently classified as medicinal ingredients (in alphabetical order)
| Name of medicinal ingredient | Synonym(s) | Exception(s) (as approved feed ingredients) |
|---|---|---|
| A | ||
| Achillea millefolium (whole plant) |
Yarrow, Wound wort | - |
| Activated charcoal | - | - |
| Adenosine-5-monophosphate | 5-adenylic acid | - |
| Aloe vera (whole plant) |
- | - |
| Alpha-galactosidase | - | - |
| Arabinogalactan | - | - |
| Arctium lappa (whole plant, leaf, except fruit extract (antioxidant) |
Burdock | - |
| AttapulgiteTable 1 Footnote 1Table 1 Footnote 2 (a: treatment of non-infectious diarrhea in calves) (b: 25,8 mg/kg BW and more) |
- | Schedule IV, part I, item 8.118 Anticaking agent: max 0.25% finished feed Emulsifier: max 2.5% of a liquid feed supplement |
| Arsenical | - | - |
| B | ||
| Belladone (homeopathic substance) |
Atropa belladonna, deadly nightshade | - |
| BentoniteTable 1 Footnote 1Table 1 Footnote 2 (a: prevention or treatment of diarrhea) (b: approx 400 mg/ml and more) |
Montmorillonite | Schedule IV, part I, items 8.5, 8.6, and 8.89 Anticaking agent or pelleting aid: max. 2% total diet |
| Bituminosulphonates | -- | - |
| Borage oil | Borago officinalis seed oil | - |
| Boswellia serrata | Boswellia | - |
| Bromelain | - | - |
| C | ||
| Calcium glucoheptonate | - | - |
| Calcium levulinate | - | - |
| Calcium pidolate | - | - |
| Calcium salts, when sold for the treatment of hyperphosphatemia |
- | - |
| Calendula officinalis (whole plant) |
Pot marigold | - |
| Carnitine, salts and derivatives | L-carnitine, Levocarnitine | L-carnitine: Schedule IV, part I, item 5.6.16 Swine feeds: as an amino acid, max. 0.1% total ration |
| Carya basilike | Black walnut hull powder, Juglans nigra powder |
- |
| Centella asiatica, (whole plant, extract and active principles thereof) |
-- | - |
| Cetyl myristoleate | Myristoleic acid (cetyl ester of ) | - |
| Chamazulene | Chamomille extract | - |
| Chondroitin sulfate | - | - |
| Chromium a (for regulation of glycemia) |
- | Chromium yeast dehydrated: Schedule IV, part II, item 6.38 Approved as a source of chromium for first lactation dairy cows at a level not to exceed 0.4 mg/kg chromium in the complete feed. Chromium proprionate: Schedule IV, part II, item 6.47 Approved for growing swine at level not to exceed 0.2 mg/kg of chromium in the complete feed. |
| Coenzyme Q10 | - | - |
| Copper calcium edetate | - | - |
| Copper glycinate | - | - |
| Copper sulfateTable 1 Footnote 1Table 1 Footnote 3 (for the treatment of aspergillosis and hexamitasis in poultry) |
- | - |
| Cranberry extracta, (dry) (prevent urinary tract infections) |
- | - |
| D | ||
| N, N-dimethylglycine. | Dimethylglycine hydrochloride | - |
| E | ||
| Echinacea angustifolia | Echinacea purpurea, American cone flower, Purple cone flower | - |
| Eleutherococcus senticosus | Siberian ginseng, Eleuthero | - |
| Ephedrine hydrochloride | - | - |
| Epidermal growth factor | EGF | - |
| Equisetum arvense | Horsetail, | - |
| Eucalyptus globulus (whole plant, essential oil) |
- | - |
| Eucalyptus polybractea | Blue-leaf mallee | - |
| Eupatorium perfoliatum (whole plant) |
Boneset, Feverwort, Thoroughwort | - |
| Ergot alkaloids and their salts | Claviceps purpurea | - |
| Eurycoma longifolia | Malaysian ginseng | - |
| F | ||
| Fenugreek cotyledon | - | - |
| Filipendula ulmaria | Meadowsweet | - |
| Folic acidTable 1 Footnote 1Table 1 Footnote 2 (a: prevention of birth defects in pregnant animals; treatment of pancreatic insufficiency, femoral thrombo-embolism; for use with drugs interfering with folate absorption; for prevention or treatment of enteritis) |
Vitamin B9 | - |
| G | ||
| Gamma oryzanolTable 1 Footnote 1 (to stimulate release of testosterone and growth hormone, antioxidant, antacid, stimulate immune system) |
Rice bran oil, Rice bran extract, Oryza sativa L. | - |
| Gingko biloba | Gingko, Maidenhair tree | - |
| Glucosamine hydrochloride | - | - |
| Glucosamine sulfate | - | - |
| L-GlutamineTable 1 Footnote 1 (to preserve intestinal mucosa, to help cellular repair) |
- | - |
| Green-lipped mussel | GLM, Perna canaliculus | - |
| H | ||
| Hamamelis virginiana (whole plant,extract) |
Hammelis, Witch hazel |
- |
| Harpagophytum procumbens | Devil's claw root | - |
| HempTable 1 Footnote 1 (stalks, seed, oil, which contain below 0.3% THC) (to stimulate immune system, reduce inflammation, etc.) (over 0.3 % THC = controlled substance) |
Cannabis sativa | - |
| Hesperidin | Bioflavonoid, Bioflavonoid complex, Bioflavonoid extract, Citrus bioflavonoid | Schedule V, part I, item 14.7. Neohespiridin dihydrochalcone (a derivative of hesperidin) is approved as a flavor ingredient. Not to exceed100 ppm in the feed. |
| Humic acids and their sodium saltsTable 1 Footnote 1 (immunostimulant, antiviral, antioxidant) |
Humate | - |
| Hyaluronic acid | - | - |
| Hydrogen peroxide | - | - |
| Hydroxocobalamin (crystalline form) | Vitamin B12a | - |
| I | ||
| Inosine | - | - |
| J | ||
| Juglans nigra (whole plant) |
Black walnut | - |
| K | ||
| KaolinTable 1 Footnote 2 (more than 2.5 % in product) |
- | Schedule IV, part I, item 8.87 Anticaking agent: max. 2.5% of finished feed |
| L | ||
| Larch arabinogalactan | - | - |
| Lobelia inflata | Lobelia, Indian tobacco | - |
| M | ||
| Melatonine | - | - |
| Methylsulfonylmethane | MSM | - |
| Milk Thistle | Silybum marianum | - |
| Mineral oilTable 1 Footnote 2 (concentration of 100%) |
Heavy mineral oil | Schedule IV, part I, item 8.33 Dust control/lubricant: Max. 0.6% in complete diet Max. 3% in mineral premixes and mineral feeds |
| Morinda citrifolia (whole plant, leaves, root, fruit extract) |
Noni plant | - |
| Morus nigra | Black mulberry | - |
| O | ||
| Oregano oilb (50 ppm and more in complete feed) |
- | Schedule V, part II, item 15.1 Flavouring ingredient: Less than 50 ppm in complete feed |
| Oxygen (solubilized in oral in oral dosage forms) | - | - |
| P | ||
| Pancreatic enzymes | Amylase, lipase, protease | - |
| Papaver rhoeas | Red poppy seeds, Corn poppy | - |
| Pau-d'arco | Handroanthus impetiginosus, Red lapacho, Taheebo, Trumpet bush. | - |
| Peppermint oila (to stimulate immune system and control instestinal bacterial overgrowth) |
Mentha x piperita | Schedule V, part I, item 16.20 Flavouring ingredient: Max. 100 ppm in the complete diet/feed |
| Peumus boldus | Boldea fragrans | - |
| Potassium bromide | - | - |
| Propylene glycolb (100-130 grams/cow/day around calving) |
- | Schedule IV, part I, item 8.43 Emulsifying agent Typical use rates: 0.01-0.02% |
| Psyllium (whole plant, seed, husk) |
Plantago ovata | Schedule IV, part II, item 8.55 Psyllium seed husk approved as a dietary source of fibre, not to exceed 2.0% of the total diet. |
| Q | ||
| Quercertin | Elytrigia repens, Quack grass | - |
| Quinine hydrochloride | - | - |
| Quinine sulfate | - | - |
| R | ||
| Raspberry Leaves (dry, powder) |
Leaves of Rubus idaeus | - |
| Rehmannia glutinosa | Chinese floxglove | - |
| Rumex crispus | Yellow dock | - |
| S | ||
| S-Adenosylmethionine | SAMe | - |
| Saw palmetto (whole plant, liposterolic extract, fruit extract) |
Serenoa repens | - |
| Sea buckthorn (whole plant, plant juice, fruit oil) |
Hippophae rhamnoides | - |
| Shark cartilage | Squalus acanthias | - |
| Sodium bicarbonatea | - | Schedule IV, part I, item 6.71 Mineral feed: approved source of sodium for livestock feeds; also used as an ingredient in buffer feeds for dairy cows and beef cattle on high grain diets. |
| Sodium diacetatea (to control salmonella in animals) |
- | Schedule IV, part I, item 8.55 Used in mould inhibitor products |
| Sodium hyaluronate | - | - |
| Sodium salicylate | - | - |
| Sodium selenitea (treatment of muscular dystrophy) |
- | Schedule IV, part I, item 6.77 Mineral feed: approved source of selenium. The regulatory maximum is 0.3 mg/kg in the total diet for most livestock; 0.1 mg/kg for fish and rabbits |
| Solidago virgaurea | Golden Rod | - |
| Spirulina maxima, Spirulina platensis (whole plant) |
Arthrospira platensis, Blue- Green algae, Aphanizomenon flos-aquae (AFA) | - |
| Streptococcus thermophilus extract | - | - |
| Symphytum officinale | Comfrey | - |
| T | ||
| Tannic acid | - | - |
| Taxifolin | - | - |
| Thymus nucleic acid | - | - |
| Thyroactive casein | Thyroprotein | - |
| Trimethylglycine | TMG, betaine | - |
| Tussilago farfara | Coltsfoot | - |
| U | ||
| Ulmus rubra | Slippery elm | - |
| Urtica dioica (whole plant, extracted juice) |
Common nettle, Stinging nettle, | - |
| Uva ursi (dry leaves, leaf powder, liquid extract) |
Arctostaphylos uva-ursi, Bear's grape, Bearberry |
- |
| V | ||
| Valeriana officinalis (whole plant) |
Valerian root | - |
| Verbena officinalis (whole plant) |
Vervain | - |
| Vitamin CTable 1 Footnote 2Table 1 Footnote 3 (b: 5.5 mg/g and higher ) (c: in poultry) |
L-ascorbic acid or L-ascorbate |
Schedule IV, part I, item 7.1.2 Vitamin Acceptable levels are those in accordance to NRC requirements |
| Vitamin K1 | Phytonadione | - |
| Vitex agnus-castus (whole plant) |
Monk's pepper, Chasteberry, Chastetree | - |
| W | ||
| White willow (whole plant, stem bark) |
Salix alba | - |
| Y | ||
| Yucca shidigera extracta (for osteoarthritis, for gastric disorders) |
- | Schedule IV, part II, item 8.61 Odour control agent Typical usage rates: 20-40 g/tonne of complete feed |
| Z | ||
| Zeolites | Aluminosilicate minerals | Schedule IV, part II, item 8.59 As an approved flow/anticaking agent: not to exceed 2% of the complete feed. Calcium sodium aluminosilicate and Sodium aluminosilicate are listed in part I of Schedule IV at a level not to exceed 2% of the complete feed. |
| Zinc a,b (a: for the treatment of diarrhea) (b: 2 mg/g (2,000 mg/kg) and higher) |
- | Schedule IV, part I, item 6.85 Zinc oxide, as single feed ingredient (source of zinc) Table 4 maximum: 500 mg/kg of zinc in complete feeds |
|
||
Appendix D
Stress-Related Indications (or Claims)
1. Introduction
Historically, almost all indications or claims containing the term “stress” were associated with a therapeutic purpose, hence a drug classification. The purposes of this appendix are to clarify in more detail for stakeholders:
- the acceptability of the use of the word “stress” in product indications; and
- which type of stress-related indications are considered therapeutic (drug-related), as well as which ones are considered non-therapeutic and acceptable for livestock feeds.
2. Definition
For the purpose of this document “stress” is defined as “the sum of the biological reactions to any adverse stimulus physical, mental, or emotional, internal or external, that tends to disturb the homeostasis of an organism. Should these reactions be inappropriate, they may lead to disease states. The term is also used to refer to the stimuli that elicit the reactions, e.g., heat, nutritional, lactational, confinement, transportation.”
(according to Baillère’s Comprehensive Veterinary Dictionary)
3. a) Criteria for acceptability of stress-related indications
- Clarity:
The term “stress” is too general in describing the condition of an animal. It is recommended that product labelling and advertising avoid the vague, imprecise and confusing use of the term “stress”. - Specificity:
In cases where the word stress is used, it must be accompanied with concrete and measurable qualifiers. To specify the context, the cause(s) of stress, the impact (consequences), the purpose/intent of use of the product, or the timing of administration, needs to be added in the indication.
Examples of unacceptable indications (claims) are given in table 3.1 at the end of this document.
3. b) Criteria for classification of stress-related indications
Stress-related indications (claims) will be classified as therapeutic or non-therapeutic independently of the timing of the stress event/change or of the product administration.
- An indication (claim) related to stress is considered to be therapeutic if it refers to:
- a product preventing or treating major/severe consequences (including clinical signs and diseases) of a stressful event or change; and/or
- a product directly affecting the physiological function or structure of organs in animals in a stressful situation.
In other words, claims which refer to the reduction of clinical signs or altered organ structure or function, when triggered by a stressful event, are only appropriate for veterinary drugs.
- An indication (claim) related to stress is considered non-therapeutic if it refers to:
- a product providing nutrients or supporting a nutritional intent; and/or
- a product supporting animal performance (i.e., production claims) in presence of stressful events or procedures associated with normal animal husbandry practices (e.g., vaccination, castration, dehorning, tail clipping, etc.).
Examples of therapeutic and non-therapeutic indications are also given in the table 3.1 below. Please note that this table is for illustrative purposes only and should not be considered as an exhaustive & complete list.
| Acceptable stress-related indications (therapeutic) | Acceptable stress-related indications (non-therapeutic) | Unacceptable stress-related indications |
|---|---|---|
|
|
|
4. Other Considerations
Please note that, as with all classifications, the criteria and process described in the Drug/Feed Classification flowchart (especially those indicated in the circle) will be followed for the classification of a product intended for use in periods of stress.
Appendix E-1
Mycotoxin Detoxification Agents (MDAs)
1. Background
Mycotoxins are toxic compounds produced by different types of fungus (mainly Aspergillus, Penicillium and Fusarium genera). Under favourable environmental conditions, particularly those linked to temperature and moisture, these fungi may proliferate in livestock feeds such as cereal grains, and produce mycotoxins. Attempts to reduce mycotoxin contamination have prompted the utilization of various techniques and products including heat-treatment, physical separation, soaking, de-hulling, or cleaning of seeds. The use of Mycotoxin Detoxification Agents (MDAs) is one technique that is discussed in this annex. The general purpose of MDAs is to ensure feed safety, to prevent harmful residues from entering the food chain, and to prevent animal disease. Due to product classification issues arising from the uses of MDAs, the classification criteria previously used are now being reconsidered and clarified as follows.
2. Definition
Mycotoxin Detoxifying Agents (MDAs): MDAs are substances or mixtures of substances incorporated into a feed matrix to mitigate the toxicity of known mycotoxins by reducing the animal’s exposure to mycotoxins. This may be done by reducing their reactivity through direct binding, decreasing their bioavailability, reducing their intestinal absorption, or promoting their excretion.
3. Relevant classification criteria
3. a) Mode of action
MDAs may be regulated as a veterinary drug or a livestock feed depending on the mode of action as demonstrated or claimed by the product proponent.
-
If the mode of action involves physiological functions in the animal system (such as binding to the gastrointestinal (GI) mucosa, stimulating the immune response, eliciting an endogenous enzymatic response, absorption into the systemic circulation, metabolic transformation), then the MDA may be classified as a veterinary drug.
As an example, digestives enzymes can inactivate some mycotoxins. Another example would be a combination product including a binding substance and an ingredient that helps hepatic detoxification processes.
-
If the mode of action occurs in the feed matrix prior to the ingestion of the treated feed by the animal (e.g., binding to the mycotoxin or inactivation), then the product may be classified as a livestock feed. An MDA may also be considered a feed if the mode of action occurs after solubilisation within the GI tract so long as the effects do not depend on systemic absorption.
As an example, aluminosilicates bind some mycotoxins, thus preventing their absorption across the digestive tract. Another example is the enzymatic degradation or biotransformation of mycotoxins by exogenous enzymes to reduce potential toxicity.
Products with a dual mode of action, or having other modes of action should be submitted to VDD for further assessment for classification.
3. b) Mycotoxin-related indications
- Indications related to the prevention of disease in animals or indications related to the treatment of clinical signs of mycotoxicosis (or associated diseases) would be considered therapeutic in nature. Products presenting such indications would be classified as veterinary drugs. Examples of acceptable indications for veterinary drugs are given in the table below:
Table 3-1: Therapeutic (or drug) indications
- Prevent aflatoxicosis in growing chickens
- Prevent mortality associated with Aspergillus toxins in poultry
- Reduce abortions or still births in sows associated with the ingestion of zearalenone
- Prevent vomiting, diarrhea and other ill effects of Fusarium toxins in swine
- Prevent fescue foot in horses
Indications related to reducing the contamination of feeds by acceptable modes of action in feed may be considered as non-therapeutic indications. Products presenting such indications may be acceptable for classification as livestock feeds. Examples of acceptable indications for livestock feeds are given in the table below:
Table 3-2: Non-therapeutic (including nutritional) indications
- Reduce (or minimize or prevent) exposure to Ochratoxin A in livestock feeds.
- Inactivate Vomitoxin (Deoxynivalenol) in livestock feeds
- Decrease the bioavailability of Aflatoxin B1 by binding it in livestock feeds Degrade Fumonisin B1 and Zearalenone in livestock feeds.
4. Other considerations
- Please note that MDAs will not be permitted for use in feeds that do not comply with Canadian standards for mycotoxins.
- As with all classifications, the criteria and process described in the Drug/Feed Classification flowchart will be followed.
Appendix E-2
Viable microbial products (VMPs)
1. Introduction
1.1 Background
Currently, many viable microbial products (VMPs) fall under the veterinary drug category primarily due to their claims and/or modes of action. Health Canada’s Veterinary Drug Directorate (VDD) and the Canadian Food Inspection Agency’s (CFIA) Animal Feed Division are providing different pathways for their registration by increasing the flexibility for classification. The classification criteria for VMPs have been reconsidered, recognizing that the mode of action of these products may involve modification to the gut microflora and the gut environment without necessarily classifying them as veterinary drugs. VMPs classified as feeds may fall under a new category: “Gut modifier (gastro-intestinal modifier)” which may be indicated on the label. Products under the Gut modifier category would also carry a nutritional or production/performance claim consistent with products regulated under the Feeds Act and Regulations examples of which can be found in Table E-2.1.
1.2 Purpose and Scope
The purpose of this appendix is to provide guidance and clarification concerning the revised regulatory classification of products containing viable microorganisms intended for livestock species. VMPs are fed to livestock as a source of viable cells whose main purpose in the diet is to have beneficial effects in target livestock species.
Please note that non-viable organisms and their products, as well as forage inoculants, are excluded from the scope of this appendix; refer back to the main document for appropriate classification considerations.
2. Definitions
Viable microbial products are products that contain live microorganisms (bacteria, yeast, fungi, algae and/or protozoa). These products refer to a live micro-organism biomass or single specified strain. They can include individual or multiple microbial strains and be incorporated (in a diluted form) into products such as mixed feeds, boluses and other dosage forms.
3. Relevant classification criteria
As with any product classification, the criteria outlined in the guidance document should be consulted. The following additional criteria will be considered in the classification of VMPs for use in livestock species.
3.1 Properties of Microbial Strains
Knowledge of the properties of microbial strains or strain combinations may be incorporated into the classification decision of a product. Strains with known antimicrobial properties such as: bacteriocin production, antimicrobial peptide production and/or bacteriophages, may be considered drugs.
3.2 Intended Purpose and Indications (Claims)
An indication (or claim) needs to be provided for all viable microbial products. These claims should be based on a measurable outcome and supported by valid scientific evidence. The proposed claim is a key consideration for classification, as follows:
- Therapeutic claims may include the prevention or treatment of a disease condition or state, the mitigation of clinical signs, or disease risk reduction. Products with therapeutic claims are considered drugs and may consist of viable microbial products that claim to target a particular pathogen(s) inside the gastro-intestinal tract, to reduce pathogen load, or to prevent and/or treat a particular disease condition.
- Nutritional or Production/Performance claims refer to the provision of nutrients, directly or through their improved availability, and their digestion or absorption. Products having claims that include the support of the maintenance, growth, and improved performance of animals may be associated with livestock feeds.
- General health claims are expected benefits in maintaining or promoting the health and welfare of animals. General health claims may be acceptable for the notification of Veterinary Health ProductsFootnote 1 (VHP), a category of veterinary drugs regulated under the Food and Drugs Act and Regulations.
Statements that refer to “replacement for antimicrobials” are not acceptable for any products. Product claims need to stand alone and should not reference another product or product type. Examples are provided in Table E-2.1 below.
| Food and Drugs Act; Food and Drug Regulations | Feeds Act; Feeds Regulations | |
|---|---|---|
| Therapeutic Claims – New Drug (Drug Identification Number) | General Health Claims – VHP1 (Notification Number) | Feed Claims – Feed (Registration Number) |
Treatment/Prevention of Disease:
Pathogen reduction:
|
|
Digestion:
Production and Performance:
|
|
||
Note: This table is for illustrative purposes only and should not be considered as an exhaustive and complete list. If a product’s claim or indication is not listed in the above, please contact VDD (as a single-window access for classification) for further assessment.
3.3 Dosage Forms
Consistent with the Guidance document, dosage forms that require forcible administration (eg. boluses and tablets) are regulated under the Food and Drug Regulations. Oral dosage forms that do not require forcible administration may be regulated under either the Food and Drug Regulations or the Feed Regulations depending on other classification criteria. To ensure consistent regulatory oversight and application of standards in safety, efficacy and quality, products in oral dosage form will be regulated as illustrated in Table E-2.2.
| Mode of Oral Administration | Food and Drugs Act; Food and Drug Regulations | Feeds Act; Feeds Regulations | |
|---|---|---|---|
| Therapeutic Claims – New Drug (Drug Identification Number) | General Health Claims – VHP (Notification Number) | Feed Claims – Feed (Registration Number) | |
| Forcible Administration (e.g., bolus, tablet, drench) |
Yes | Yes | No |
| Mixed in Feed | Yes | No | Yes |
| Top-Dressed on Feed | Yes | No | Yes |
| In-Water | Yes | No | Yes |
For further information on VHPs and the VHP notification pathway:
4. Other Considerations
As indicated in the Guidance document, the mode of action criterion is not always definitive and may not necessarily be used for the classification of viable microbial products. The rationale behind this approach is that the different mode(s) of action of these products are complex, not well understood, can occur in combination and vary depending on the intestinal environment. Therefore, references to mode(s) of action related to intestinal flora will no longer automatically trigger a drug classification on its own. This decision supersedes the previous classification approach elaborated by VDD and the Animal Feeds Division (AFD) in a 1997 Memorandum of Understanding.
- It is possible that for certain viable microbial products, additional criteria may need to be considered for classification (e.g., strength (concentration), dosage and conditions of use etc.)
- When proposing a claim for classification careful thought should be given to the potential supporting evidence available and the product’s intended use within a complete health management program implemented under the supervision of a veterinarian and/or animal nutritionist.
Appendix E-3
Acid-based products
1. Introduction
1.1 Background
Acid-based products (ABP) and their salts are often referred to as “acidifiers.” They contain 1 or more substances with acidic properties. They have recognized uses in oral administration to livestock.
Products with acidifying properties may be intended to act within the animal to prevent disease or target and reduce a particular pathogen. This action would be more consistent with a veterinary drug. Historically, a product would be classified as a veterinary drug when referring to the modification of the gut environment. The addition of the Gut Modifier category in feed allows for the possibility of an ABP in feed to be recognized for its ability to act locally to modify the gut.
1.2 Purpose and scope
This appendix is to provide guidance and clarification on the classification of ABP intended for oral use in livestock as either veterinary drugs, including as veterinary health products (VHPFootnote 1), or as livestock feeds.
ABP with claims related to the disinfection or sanitization of an inanimate object or surface are excluded from the scope of this appendix. They are not found at the drug-feed interface.
2. Possible classifications for ABP
2.1 Acid-based products as veterinary drugs
Acid-based products to be fed to livestock that are determined to be drugs could be further classified as either veterinary new drugs or veterinary health products (VHPs).
2.2 Acid-based products as feeds
As feed, previously all ABPs were registered as acidifiers. Typical feed purposes included the following:
- to reduce the pH of solid feed when added to a feed matrix
- to acidify water in areas with alkaline water sources
- to use as preservatives or mould inhibitors in solid and liquid feed (for example, milk replacers)
ABPs will now be divided into the following 3 categories:
- pH adjusters
- preservatives and mould inhibitors
- gut modifiers
The term ”acidifier” will no longer be used as a feed category. Each category is further defined as follows.
- pH adjusters
- added to livestock feeds, including liquid feeds, or water to adjust or maintain the pH due to its buffering capacity
- feed acidification may significantly reduce the dietary pH, which:
- improves nutrient digestibility of the feed
- may affect the activity of some digestive enzymes in the stomach (for example, pepsinogen)
- Preservatives and mould inhibitors
- may be added to feeds before and during processing to:
- decrease the pH to levels that are unfavourable for the growth of spoilage organisms (microbial or fungal) or
- prevent other undesirable chemical changes in the feed itself
- meant to work on the feed external to the animal
- may be added to feeds before and during processing to:
- Gut modifiers
- act in the gastrointestinal tract (GIT) to improve nutrient absorption and digestibility
- may be protected (for example, encapsulated) in order to pass through to the GIT to its intended target site
3. Relevant classification criteria
As with any product classification at the drug-feed interface, you should consult the criteria outlined in the guidance document. The following additional criteria could be useful to help guide the classification of ABPs.
3.1 Characteristics of acids
An essential characteristic of acids is their ability to dissociate and change their charge. This results in differing modes of action and metabolic associations.
The molecular formula, the pH of the medium or environment where the product is intended to act and the pKa value of the acid could become important criteria for classification. These characteristics can support the products’ intended purpose as nutritional, therapeutic or for general wellness.
Note that the type of acid (for example, citric, acetic or formic acid) does not determine the product’s classification. The classification or feed categorization for products that contain similar acids may change based on a number of factors, such as:
- if they are a salt
- their molecular weight
- their concentrations or formulations
- if they are being used for a different purpose
3.2 Location of action
A product that includes systemic absorption and a location of action in the animal outside of the GIT may indicate an action that is more consistent with a therapeutic or general health purpose. For this reason, such a product would most likely be classified as a veterinary drug, including VHPs. Further classification within the veterinary drug pathway will also depend on the mode of action, ingredients and intended purpose.
In general, products that act in the feed, external to the animal, are likely to be classified as a pH adjuster, preservative or mould inhibitor. Localized actions within the GIT may support a classification as a gut modifier (feed). Actions within the GIT with overt antimicrobial or antiparasitic actions to prevent or treat disease continue to be classified as veterinary new drugs.
Gut modifiers can act in several sites and induce several effects. This can lead to improved digestion, absorption and the efficient use of energy, for example, by:
- reducing gastric pH
- increasing gastric retention time
- influencing mucosal morphology
- improving metabolizable energy values of diets
Protecting an acid product through encapsulation allows for targeted action to a particular location in the GIT. This would not be consistent with pH adjusters, preservatives or mould inhibitors, where the site of action is in the feed.
3.3 Intended purpose and indications (claims)
An indication (or claim) must be provided for all ABPs, regardless of classification. These claims should be based on measurable outcomes and be supported by valid scientific evidence.
3.3.1 Veterinary drugs
Therapeutic claims, consistent with claims of veterinary new drugs, may include the prevention or treatment of a disease condition or state, the mitigation of clinical signs or disease risk reduction. Acid-based products with therapeutic claims may consist of claims to target a particular pathogen(s), reduce pathogen load or prevent and/or treat a disease.
General health claims are consistent with claims of VHPs. These may include expected benefits in maintaining or promoting the health and welfare of animals. Claims of notified VHPs are general in nature and cannot include the prevention, treatment or mitigation of a disease or disorder.
3.3.2 Feeds
Nutritional or production/performance claims are consistent with livestock feeds. These refer to the provision of nutrients (directly or through improved availability) and their digestion or absorption. This can be done by modifying the gut. Products with claims that include the support of maintenance, growth and improved performance of animals may be acceptable as livestock feeds.
Examples of claims are provided in the following table.
| Food and Drugs Act and Regulations | Feeds Act and Regulations | |
|---|---|---|
| Therapeutic claims for new drug (drug identification number) |
General Health Claims – VHP1 (Notification Number) | Feed claims for feed (registration number) |
Prevention/treatment of disease:
Pathogen reduction
Other:
|
|
pH adjuster:
Gut modifier claims:
Digestion:
Production and performance claims:
Preservative or mould inhibitor:
Other:
|
Note: This table is for illustrative purposes only. It should not be considered a complete list. If a product’s claim or indication is not listed in the table, please contact the Veterinary Drugs Directorate (as a single-window access for classification) for further assessment.
|
||
3.4 Dosage forms
Consistent with the guidance document, dosage forms that require forcible administration (for example, boluses and tablets) are regulated as veterinary new drugs or VHPs under the Food and Drug Regulations. Oral dosage forms that do not require forcible administration may be regulated under either the Food and Drug Regulations or the Feeds Regulations depending on other classification criteria. To ensure consistent regulatory oversight and application of standards in safety, efficacy and quality, products in oral dosage form will be regulated as indicated in Table E-3.2.
| New drugTable E3.2 Footnote 1 Drug identification number |
VHPTable E3.2 Footnote 1 Notification number |
FeedTable E3.2 Footnote 2 Registration number |
|
|---|---|---|---|
| Forcible administration (for example, bolus, tablet, drench) |
Yes | Yes | No |
| Mixed in feed | Yes | NoTable E3.2 Footnote 3 | Yes |
| Top-dressed on feed | Yes | Yes | Yes |
| In water | Yes | Yes | Yes |
|
|||
Footnote
- Footnote 1
-
Veterinary health products are veterinary drugs in dosage form that promote or maintain overall good health and wellbeing. VHPs cannot be sold or represented for the use in the diagnosis, treatment, mitigation of a disease, disorder or abnormal physical state, or its symptoms. Currently, notified VHPs may not be mixed in feeds where the Feeds Regulations apply.
For further information on VHPs and the VHP notification pathway: