Types of research with cannabis
The Cannabis Act (the Act) and the Cannabis Regulations (the Regulations) authorize activities with cannabis, including research.
The purpose of this page is to help you decide what cannabis licence you may need for a research study.
On this page
- Research studies
- Research studies involving human participants
- Other types of research studies
- Contact us
Disclaimer: You need to read these pages along with the Act and the Regulations. If there are differences, the Act and the Regulations are correct. If there are differences between this page and the Cannabis Tracking and Licensing System (CTLS), this page is correct.
Research studies
You can refer to the decision tree below to help decide what type of cannabis licence you may need. You can find more information about each type of study below.
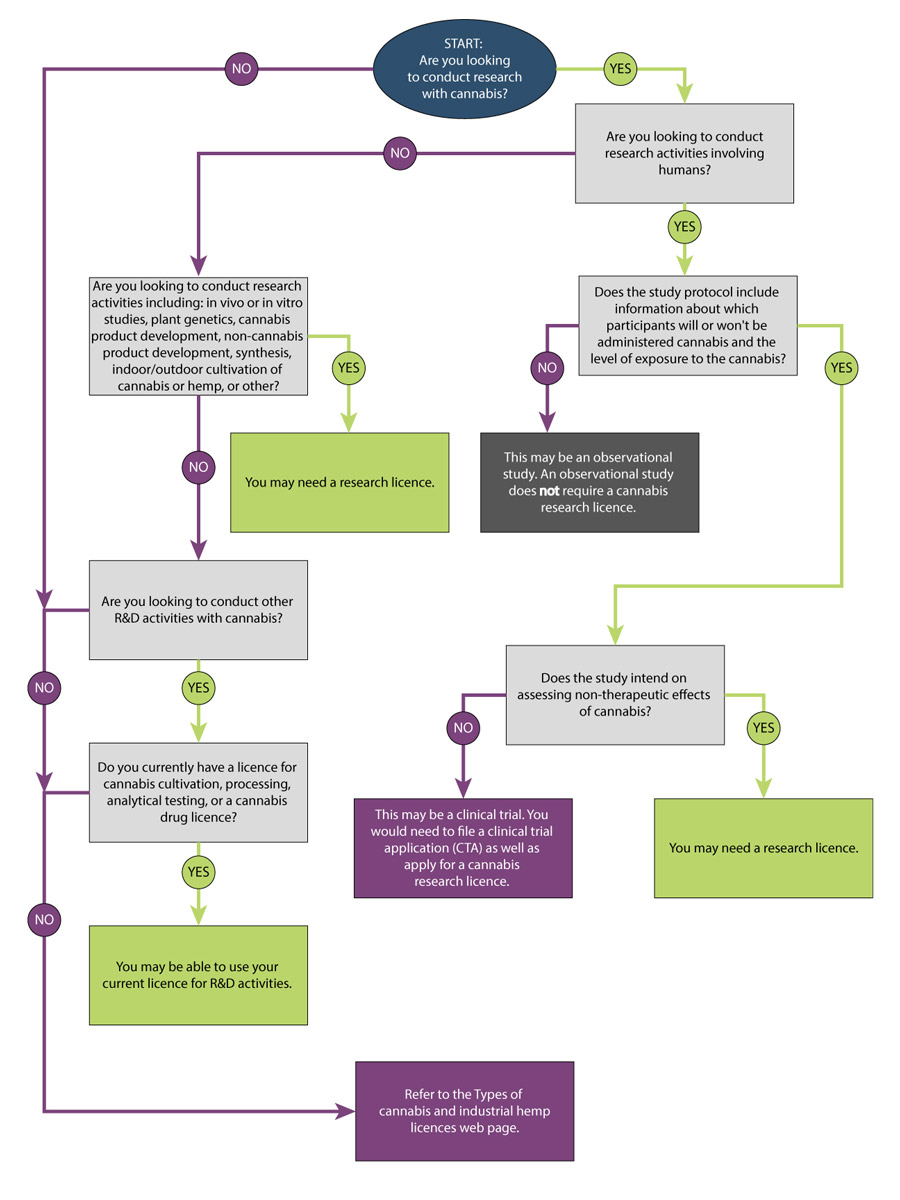
Figure 1 - Text description
Start: Will you be conducting research with any of the following:
- With humans: Go to With humans
- With animals
- You may need a research licence. You may also need to obtain other authorizations from Health Canada.
- With neither animals nor humans: Go to With neither animals nor humans
With humans
- Does the study protocol include information about which participants will or will not be administered cannabis and the level of exposure to the cannabis?
- Yes: Does the study intend on assessing non-therapeutic effects of cannabis?
- Yes: You may need a research licence.
- No: This may be a clinical trial. You would need to file a clinical trial application as well as apply for a cannabis research licence.
- No: This may be an observational study. An observational study does not require a cannabis research licence.
- Yes: Does the study intend on assessing non-therapeutic effects of cannabis?
With neither animals nor humans
- Will you possess more than 30 g of dried cannabis or its equivalent for your research?
- Yes: Go to Are you looking to conduct research activities including: in vivo or in vitro studies, plant genetics, cannabis product development, non-cannabis product development, synthesis, indoor/outdoor cultivation of cannabis or hemp, or other?
- No: Will your research involve cultivating, propagating or harvesting cannabis or industrial hemp?
- Yes: Go to Are you looking to conduct research activities including: in vivo or in vitro studies, plant genetics, cannabis product development, non-cannabis product development, synthesis, indoor/outdoor cultivation of cannabis or hemp, or other?
- No: You may not need a cannabis research licence. Refer to the Types of cannabis and industrial hemp licences to find out if a different cannabis licence may be more suitable.
Are you looking to conduct research activities including: in vivo or in vitro studies, plant genetics, cannabis product development, non-cannabis product development, synthesis, indoor/outdoor cultivation of cannabis or hemp, or other?
- Yes: Do you currently have a licence for cannabis cultivation, processing, analytical testing or a cannabis drug licence?
- Yes: You may be able to use your current licence for research and development activities.
- No: You may need a research licence.
- No: You may not need a cannabis research licence. Refer to the Types of cannabis and industrial hemp licences to find out if a different cannabis licence may be more suitable.
To conduct research and development with cannabis, you may need to apply for a research licence. Refer to the Cannabis licensing application: Research licence for more information on research licence applications.
As an adult or an organization, you may conduct cannabis research without a licence if you meet all these criteria:
- possess 30 g or less of dried cannabis or its equivalent for your research
- are not administering or distributing cannabis to a human or animal
- will not conduct research at a dwelling-house or at a site authorized for personal or designated production (for example, at a university)
While conducting this research, you may:
- produce cannabis, including the use of organic solvents to alter the chemical or physical properties of cannabis
- distribute cannabis to analytical testing or research licence holders
However, you are not permitted to cultivate, propagate or harvest cannabis without a licence.
If you have a cannabis drug licence or a licence for cultivation, processing or analytical testing, you are already authorized to conduct certain research activities (such as chemical characterization of cannabis or certain in vitro tissue culture studies). These activities must be within your authorized activities according to the Regulations. If you want to conduct research outside of your authorized activities, you need to apply for a research licence.
For more information on research activities, refer to Types of cannabis and industrial hemp licences.
Research studies involving human participants
There are 3 types of research studies with cannabis involving human participants:
- non-therapeutic research on cannabis (NTRC)
- clinical trials
- observational studies
NTRC and clinical trials are both interventional studies where researchers administer and distribute cannabis to study participants. NTRC studies investigate non-therapeutic effects of cannabis, while clinical trials can investigate therapeutic and non-therapeutic effects of cannabis.
Therapeutic effects can include:
- the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms
- the restoration or correction of organic functions in humans
Non-therapeutic research on cannabis (NTRC)
NTRC are interventional, non-therapeutic studies with cannabis regulated under the Act and its regulations.
The purpose of NTRC is to:
- increase the knowledge on cannabis and its non-therapeutic effects (for example, sensory-emotive, physiological, psychological effects)
- gain knowledge to inform public health and public safety measures, public education, and policy
- further research and development of cannabis products
An example of an NTRC study is assessing participants in a driving simulation test to find out how long they are impaired for after researchers have administered cannabis.
If you want to conduct an NTRC study, you need to apply for a research licence under the Act and its regulations. For the NTRC application requirements, refer to Cannabis licensing application: Research licence.
Clinical trials
Clinical trials with cannabis are interventional studies regulated under the Food and Drugs Regulations.
Clinical trials aim to gather evidence to help bring new therapeutic products to market or inform clinical practice. Researchers may use them to:
- discover or verify the therapeutic effects of cannabis
- understand how the human body processes cannabis
- find out the safety and efficacy of cannabis
An example of a clinical trial with cannabis could include studying whether a specific form of cannabis could ease pain.
These questions can help you decide if your research study may be a clinical trial:
- Is the intent of the study to discover or verify the effects of cannabis for therapeutic purposes?
- Are there factors that would exclude the study from the NTRC framework (for example, participants younger than 18 years of age)?
- Is the purpose of the study to use the results to support an application for market authorization under the Food and Drugs Regulations?
If you answer yes to any of these questions, your study may be a clinical trial under the Food and Drugs Regulations.
If you want to conduct a clinical trial with cannabis, you will need both:
- a No Objection Letter from the Office of Clinical Trials
- a cannabis research licence from the Controlled Substances and Cannabis Branch
For more information on clinical trials with cannabis, please see the Clarification of requirements when conducting clinical trials with cannabis.
Important: You can choose to study cannabis for non-therapeutic purposes under the Act and its regulations (as NTRC) or the Food and Drugs Act and its regulations (as clinical trials). Each framework has specific requirements you must follow. If the study involves investigational drugs or pediatric patients, it must follow the Food and Drugs Act framework. A cannabis research licence is required for both clinical trials and NTRC.
Observational studies
Observational studies with cannabis are non-interventional studies that are limited to recording observations and analyzing data. These types of studies do not require approval from Health Canada. A researcher conducting an observational study is not permitted to conduct any activities with cannabis that involve possessing, distributing or administering cannabis to participants.
While you do not need authorization from Health Canada to conduct observational studies with cannabis, you should check with your Research Ethics Boards about applicable ethics requirements.
An example of an observational study could be asking participants to fill out a questionnaire on how they felt after consuming cannabis products that both:
- are available on the legal, regulated market or under the cannabis for medical purposes framework
- they decided to consume or their health-care professional recommended
Other types of research studies
Research studies for veterinary purposes
For research studies to be conducted using a veterinary drug, the objective is to ensure that there is appropriate oversight for the overall health and safety of study animals.
In order to conduct veterinary drug research using drugs containing cannabis, the investigator or the sponsor is required to submit an Experimental studies certificate application. If the study includes food-producing animals, Health Canada also considers relevant information to address food safety. Depending on the details of the study being considered, you may need to obtain other authorizations before you begin.
For any questions about veterinary drug research involving cannabis, contact vdd.vetdrugs-medsvet.dmv@hc-sc.gc.ca.
Contact us
For questions about a specific research licence application, email sp-licensing-cannabis-licences-sp@hc-sc.gc.ca. Use the subject line "Questions about APP #". Include your application ID found in the CTLS.
For all other questions, refer to the Cannabis and industrial hemp contact information to find the appropriate email address.