Overview of the licence application process: Cannabis research licences
On this page
- 1.0 Who is this for
- 2.0 Foreword and disclaimer
- 3.0 Diagram of licence application process
- 4.0 Licence application checklists
- 5.0 Contact us
1.0 Who is this for
Use the content in these pages to apply for a cannabis research licence. For other licences, refer to:
- Application requirements for micro-cultivation, nursery and standard cultivation, micro-processing and standard processing and sale for medical purposes
- Application requirements for analytical testing licences
- Application requirements for cannabis drug licences
- Industrial hemp licensing application guide
2.0 Foreword and disclaimer
2.1 Foreword
The Cannabis Act sets out that an applicant must file a licence application in the form and manner set by the Minister of Health, and include all the required information. These pages set out:
- the application process, including the requirements of a licence application
- the information that the applicant must submit
Under the Cannabis Act, the Minister of Health may require more information that pertains to the information in the application and that is necessary to process your licence application. If you don't submit the information requested, the Minister may refuse to consider your application.
2.2 Disclaimer
You need to read these pages along with the Cannabis Act and the Cannabis Regulations. If there are differences between these pages and the Cannabis Act and the Cannabis Regulations, the Act and the Regulations are correct. If there are differences between the Cannabis Tracking and Licensing System (CTLS) and these pages, these pages are correct.
Health Canada's CTLS is a Secure Web Portal and a single point of access for licence application submissions. You can access the CTLS directly at Health Canada's Secure Web Portal. You should familiarize yourself with the use of this system and should refer to the CTLS Getting Started Guide for more information.
3.0 Diagram of licence application process
Important: You can only start your authorized cannabis activities after you've received your research licence from Health Canada. Upon the issuance of your licence, it's possible that your authorized activities may be restricted by terms and conditions.
When starting the application process, refer to the Before you start applying for a licence section for more information about what you should consider before applying. It may also be helpful to use the application checklist that can guide you through this process.
Figure 1 provides an overview of the licence application process and the things you need to do as an applicant.
Figure 1: Overview of licence application process
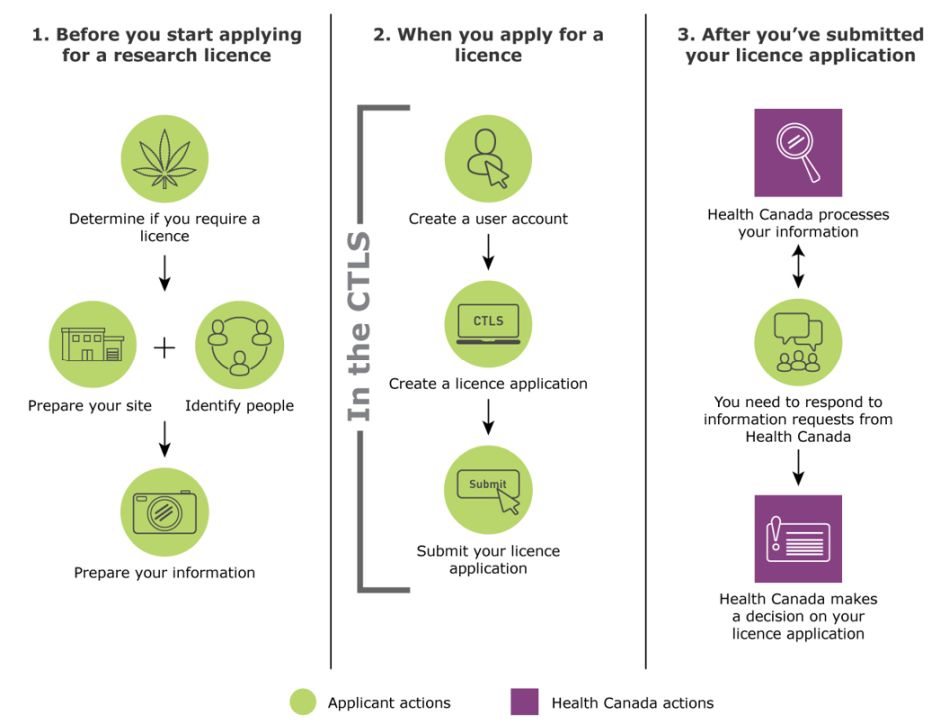
Figure 1 - Text Description
1. The key steps you need to do before you start applying for a licence are to:
- Determine if you require a licence
- Prepare your site and identify people
- Prepare your information
2. The key steps you need to do when you apply for a licence are to:
- Create a user account in the CTLS
- Create a licence application in the CTLS
- Submit your licence application in the CTLS
3. The key steps after you've submitted your licence application are:
- Health Canada processes your information
- You need to respond to information requests from Health Canada
- Health Canada makes a decision on your licence application
4.0 Licence application checklists
The Applying for a research licence checklist is a tool that can guide you through the licence application process. It summarizes the key tasks:
- before you start applying for a licence
- when you're applying for a licence
- after you've submitted your licence application
The checklist is provided in a web page format and in a printable PDF format. The printable PDF format includes the suggested naming convention for each document listed in the checklist.
Important: Health Canada recommends using a document naming convention for all the information you have to submit. We can process your application more efficiently when you follow this document naming convention. You'll obtain your application ID (APP#) once you start your application in the CTLS. It generally follows the layout: "MainCategory_APP-#_SubCategory_YYYY-MM-DD.PDF"
- MainCategory: Main category of the information type
- APP-#: Your application number, which is available after you've created a licence application in the CTLS
- SubCategory: Sub-category of the information type
- YYYY-MM-DD: The date (year-month-date) of when your document was created
- PDF: Document file type
- For your corporate profile, if applicable:
- "CompanyName_DocumentCategory_YYYY-MM-DD.PDF"
- CompanyName: Name of your corporation, cooperative or partnership
- DocumentCategory: Category of the information type
- YYYY-MM-DD: The date (year-month-date) of when your document was created
- PDF: Document file type
- "CompanyName_DocumentCategory_YYYY-MM-DD.PDF"
Checklist: Applying for a research licence
View printable version of the checklist (PDF).
Section 1: Before you apply
- Determine if you require a research licence
- Determine your research licence model
- Single (one protocol, one site or multiple sites)
- Multiple (more than one protocol, one site)
- Not applicable to non-therapeutic research on cannabis (NTRC)
- Institution-wide (one institution, one site)
- Familiarize yourself with the legislation
- Know what business supports apply to you
- Self-identify as an Indigenous affiliated applicant. If so, you may want to contact the Navigator services at navig@hc-sc.gc.ca.
- Prepare your site
- Identify people
- All identified people need to create their own CTLS account and give you their CTLS account ID
Section 2: Information to prepare
- For an individual applicant
- Copy of a government-issued photo ID
- For corporation, cooperatives or partnerships
- Corporate organizational chart
- If you're a corporation: copy of your certificate of incorporation, amalgamation or amendment
- If you're a cooperative or a partnership: Copy of your business name registration or partnership agreement
- Academic institution or research centre
- Organizational chart
- Site details
- All research licences to conduct activities excluding those that authorize activities in relation to non-therapeutic research on cannabis (NTRC):
- All institution-wide research licences:
- Clinical trials:
- No objection letter (If available. Not required for the application submission)
- In vivo (animal) studies:
- Experimental studies certificate (If available. Not required for the application submission)
- Research licences that authorize activities in relation to non-therapeutic research on cannabis (NTRC):
- All risk categories:
- Application form
- Cannabis information form (Required for all categories. For Category 1 studies, if not available at the time of licence application submission, this may be submitted after licence issuance)
- Category 1 only:
- Category 2 and 3:
- Full protocol
- Cannabis research and evidence dossier
- If obtained before licence application submission, a copy of the Research ethics board, Institutional review board or Independent ethics committee letter of approval or certificate of ethics review
- Category 3 only:
- Informed consent form
- All risk categories:
- Physical security
- Site plan
- Description of physical security
- Physical security measures framework for institutions
- Institution-wide site attestation
- Identified people
- Licence holder as an individual
- Responsible person ID
- Alternate responsible person ID, if applicable
- Record keeping
Important: If you've reached the document upload limit in CTLS, combine similar documents together. For example, you may append PDF documents together. It's recommended to first append documents under the same category together (such as a site plan with a physical security plan). If you have any questions or need further instructions, email sp-licensing-cannabis-licences-sp@hc-sc.gc.ca. Subject line should be "Document upload limit for APP #".
Proposed document naming conventions
- For an individual applicant
- Copy of a government-issued photo ID: "Name_LH_IDType_YYYY-MM-DD.PDF"
- For corporation, cooperatives or partnerships
- Corporate organizational chart: "CompanyName_CorporateOrgChart _YYYY-MM-DD.PDF"
- Certificate of incorporation: "CompanyName_Certificate-of-Incorporation _YYYY-MM-DD.PDF"
- Certificate of amalgamation: "CompanyName_Certificate-of-Amalgamation _YYYY-MM-DD.PDF"
- Certificate of amendment: "CompanyName_Certificate-of-Amendment_YYYY-MM-DD.PDF"
- Business name registration: "CompanyName_BusinessNameRegistration_YYYY-MM-DD.PDF"
- Partnership agreement: "CompanyName_PartnershipAgreement_YYYY-MM-DD.PDF"
- Academic institutionor research centre
- Organizational chart: InstitutionName_OrgChart_YYYY-MM-DD.PDF
- Site details
- All research licences to conduct activities excluding those that authorize activities in relation to non-therapeutic research on cannabis (NTRC):
- Research protocol: "ResearchProtocol_APP-#_YYYY-MM-DD.PDF"
- All institution-wide research licences (IRLs):
- Research project administration framework for institutions: "ResearchFramework_APP-#_YYYY-MM-DD.PDF"
- Clinical trials:
- No objection letter: "NOL_APP-#_YYYY-MM-DD.PDF"
- In-vivo (animal) studies:
- Experimental studies certificate: "ESC_APP-#_YYYY-MM-DD.PDF"
- Research licences that authorize activities in relation to non-therapeutic research on cannabis (NTRC):
- All risk categories:
- Application form: "ApplicationForm_APP-#_YYYY-MM-DD.PDF"
- Cannabis information form: "CannabisInformationForm_APP-#_YYYY-MM-DD.PDF"
- Category 1 only:
- Abridged protocol: "NTRC_AbridgedProtocol_APP-#_YYYY-MM-DD.PDF"
- Category 2 and 3:
- Full protocol: "NTRC_FullProtocol_APP-#_YYYY-MM-DD.PDF"
- Cannabis research and evidence dossier: "CRED_ APP-#_YYYY-MM-DD.PDF"
- Research ethics board, Institutional review board or Independent ethics committee letter of approval or certificate of ethics review: "ProofREBApproval_APP-#_YYYY-MM-DD.PDF"
- Category 3 only:
- Informed consent form: "ICF_ APP-#_YYYY-MM-DD.PDF"
- All risk categories:
- All research licences to conduct activities excluding those that authorize activities in relation to non-therapeutic research on cannabis (NTRC):
- Physical security
- Site plan: "SitePlan_APP-#_YYYY-MM-DD.PDF"
- Description of physical security: "PhysicalSecurityPlan_APP-#_YYYY-MM-DD.PDF"
- Physical security measures framework for institutions: "PhysicalSecurityFramework_APP-#_YYYY-MM-DD.PDF"
- Institution-wide site attestation: "IRL_SiteAttestation_APP-#_YYYY-MM-DD.PDF"
- Identified people
- Licence holder as an individual ID: "Name_LH_ID_YYYY-MM-DD.PDF"
- Responsible person ID: "Name_RP_ID _YYYY-MM-DD.PDF"
- Alternate responsible person ID, if applicable: "Name_ARP_ID_YYYY-MM-DD.PDF"
- Record keeping
- Record keeping attestation: "RecordKeepingAttestation_APP-#_YYYY-MM-DD.PDF"
- Institution-wide responsible person attestation: "Name_IRL_RP_Attestation_APP-#_YYYY-MM-DD.PDF"
- Institution-wide alternate responsible person attestation: "Name_IRL_ARP_Attestation_APP-#_YYYY-MM-DD.PDF"
Section 3: Create a licence application
- Create an account in the CTLS, if you don't already have one
- If you're applying as a part of a corporation, a cooperative, or a partnership, create a corporate profile, if you don't already have one
- Create a new licence application in the CTLS
Section 4: Submit your information in the CTLS
5.0 Contact us
For questions related to a specific licence application, contact Health Canada by email at sp-licensing-cannabis-licences-sp@hc-sc.gc.ca. Include your Application ID found in the CTLS. The subject line should be "Questions about APP #".
For questions about the CTLS, contact Health Canada by email at hc.ctls-sscdl.sc@hc-sc.gc.ca or by phone at 1-866-337-7705 (toll free).
For general questions, contact Health Canada by email at cannabis@hc-sc.gc.ca or by phone at 1-866-337-7705 (toll free)