Hazardous substance assessment - Silicic acid, sodium salt
Important note: Hazardous substance assessments are technical documents produced by Health Canada as educational and information resources for suppliers of hazardous products under the Hazardous Products Act (HPA) and its regulations. For more information on supplier roles and responsibilities, visit supplier responsibilities.
This hazardous substance assessment was conducted according to the Hazardous Products Regulations (HPR).
Identification
Chemical name:
Silicic acid, sodium salt
CAS #:
1344-09-8
Chemical composition:
(Na2O)x.(SiO2)y
Synonyms:
Sodium silicate.
UN #:
3253
Pictograms
Figure 1.

Figure 1 - Text description
The symbol within the pictogram is an exclamation mark. This symbol indicates that hazardous products with this pictogram can cause certain health effects for example:
- skin irritation,
- eye irritation
- skin sensitization.
Figure 2.
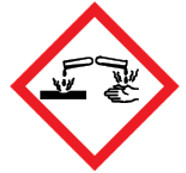
Figure 2 - Text description
The symbol within the pictogram shows a container dripping liquid onto a piece of metal and another container dripping liquid onto a hand. This symbol indicates that hazardous products with this pictogram can:
- damage or destroy metal
- cause irreversible damage to the skin (for example, burns, blisters, scarring)
- produce tissue damage in the eye or vision loss that is irreversible or not fully reversible within 21 days
WHMIS classification
Note: The classifications of silicic acid, sodium salt, depend on the concentration and molar ratio (MR) of SiO2:Na2O, which may vary from 1.5-4.0 Footnote 1,Footnote 2,Footnote 3. Sodium metasilicate, CAS # 6834-92-0, is the same as MR 1.0 Footnote 1.
- Acute Toxicity (Oral): Category 4 (MR 2.0)
- Skin Corrosion / Irritation: Category 1 (MR 1.6 at ≥52%, MR 2.4 at ≥44%, MR 0.5 at ≥90%); Category 2 (MR 2.0 at 19.5%)
- Serious Eye Damage / Eye Irritation: Category 1 (MR 2.0 at ≥19.5%); Category 2 (MR 2.4 at 19.5%)
Silicic acid, sodium salt, does not meet criteria for Physical Hazard classification.
Health hazards
Acute Toxicity (Oral):
Acute Toxicity (Oral) - Category 4 (MR 2.0)
Median lethal dose (LD50): 5,150 mg/kg (rat, MR 3.27)Footnote 3.
LD50: 1,960 mg/kg (rat, MR 2.0)Footnote 4.
LD50: 2,710 mg/kg (rat, MR 2.4)Footnote 4.
The available data meet the classification criteria for Acute Toxicity (Oral) – Category 4 at MR 2.0 [HPR 8.1.1(1)].
Acute Toxicity (Dermal):
Does not meet criteria
LD50: >4,640 mg/kg (rabbit, MR 2.0 and 2.4)Footnote 4
The available data do not meet the classification criteria for Acute Toxicity (Dermal).
Acute Toxicity (Inhalation – Gases):
Not applicable
Silicic acid, sodium salt, is not a gas. The classification criteria for Acute Toxicity (Inhalation – Gases) do not apply to this substance.
Acute Toxicity (Inhalation – Vapours):
No data available
Acute Toxicity (Inhalation – Dusts and Mists):
No data available
Skin Corrosion / Irritation:
Category 1 (MR 1.6 (≥52%), MR 2.4 (≥44%), MR 0.5 (≥90%))
Category 2 (MR 2.0 (19.5%))
Dermal corrosion or irritation caused by silicic acid, sodium salt, depends on the MR and concentration. Lower concentrations of the same MR exhibit lower corrosivity or irritancy compared to higher concentrations.
In an Organization for Economic Co-operation and Development Test Guideline (OECD TG) 404 study in rabbits, a 53.5% aqueous solution of silicic acid, sodium salt, (MR 1.6) was tested to be corrosive (based on a study summary Footnote 5). The rabbits were exposed for 4 hours. Both the edema and erythema scores were 4/4 and the effects were irreversible after 14 days. In another well conducted study, application of 0.5 mL of 82% silicic acid, sodium salt, (MR 2.4) for 4 hours on 3 rabbits’ skin under semi-occlusive conditions resulted in necrotic skin lesions in 2/3 animals (based on a study summary Footnote 5). One animal had a wound that had not healed after 14 days. Another study tested aqueous solutions (19.5%) of silicic acid, sodium salt (MR 2.4 and 2.0). Only the silicic acid, sodium salt (MR 2.0) had average scores (24- and 72-hour) of >2.3/4 for erythema and edema when applied for 24 hours on 6 rabbits’ intact skinFootnote 4. In a United States (U.S.) Department of Transportation (US DOT) skin test where rabbits were exposed to silicic acid, sodium salt, for 4 hours, a 90% solution of MR 0.5 was reported to be corrosiveFootnote 6. In a validation study for an in vitro assay (OECD TG 430: In Vitro Skin Corrosion: Transcutaneous Electrical Resistance Test Method (TER)), a 52% and a 44% aqueous solution of silicic acid, sodium salt, (MR 1.6 and 2.4, respectively) were found to be corrosive (based on a study summaryFootnote 5). Application of 0.5 mL of a 36, 80 or 99% solution of silicic acid, sodium salt (MR 3.2), using a Federal Hazardous Substances Act (FHSA) method, where exposure is for 24 hours, resulted in a primary irritation index (PII) of 3, 0 and 4, respectively, and was reported to be not corrosiveFootnote 6. Silicic acid, sodium salt (MR 3.45), at a 40.93% concentration was reported to be not irritating to human skin (based on a study summaryFootnote 5). In a 4-hour exposure in human volunteers, silicic acid, sodium salt (MR 3.45 (34.9%)), caused slight scaling of the skin in 7/20 subjects, with the results reported to be not irritatingFootnote 3. In an OECD TG 404 study, a 38.25% solution of silicic acid, sodium salt (MR 3.28), applied to the skin of a New Zealand White rabbit, was reported to be not irritatingFootnote 3.
The available data meet the classification criteria for Skin Corrosion – Category 1 for silicic acid, sodium salt, at MR 0.5 (≥90%), MR 1.6 (≥52%), and MR 2.4 (≥44%) [HPR 8.2.2(2)]; and Skin Irritation – Category 2 at MR 2.0 (19.5%) [HPR 8.2.2(3)].
Serious Eye Damage / Eye Irritation:
Category 1 (MR 2.0 (≥19.5%)); Category 2 (MR 2.4 (19.5%))
As with skin corrosion and irritation, the classification of eye irritation and serious eye damage depends on the MR and concentration of the substance.
In a study conducted similarly to OECD TG 405, administration of 0.1 mL of silicic acid, sodium salt (MR 2.0), at a concentration of 19.5% in the conjunctival sac of 6 rabbit eyes resulted in corneal opacity with scar tissue formation in 4/6 rabbitsFootnote 4. A 19.5% aqueous solution of silicic acid, sodium salt (MR 2.4), in the same study produced an average score (24-, 48-, and 72-hour) of >1/2 for iritis in 2/6 animals and a conjunctival redness score of >2/3 in all 6 animalsFootnote 4. No individual scores were available after 72 hours to determine a subcategorization. In an FHSA test, specifying albino rabbits, silicic acid, sodium salt, MR 2.0 at a 44% concentration was reported to be severely irritatingFootnote 6; however, no scores or details on the effects were provided. In a study conducted with the same FHSA method, silicic acid, sodium salt (MR 3.2), was reported to be not irritating in albino rabbits at a concentration of 36%Footnote 6 No further details on the study were provided.
The available data meet the classification criteria for Serious Eye Damage – Category 1 for silicic acid, sodium salt (MR 2.0) at ≥19.5% concentration [HPR 8.3.2(1)]; and Eye Irritation – Category 2 for MR 2.4 at a 19.5% concentration [HPR 8.3.2(3)].
Respiratory Sensitization:
No data available
Skin Sensitization:
Does not meet criteria
There is one documented case of exposure to silicic acid, sodium salt, causing contact dermatitis and contact urticaria in a 57-year-old man. He experienced an immediate hypersensitive reaction when exposed to 20% silicic acid, sodium salt (MR not specified), at the work placeFootnote 7. He tested positive in a patch test. A 20% aqueous solution of silicic acid, sodium salt (MR not specified), was then tested in 32 healthy volunteers, 22 of which (68%) had a positive response including skin irritation without ulcer formation 48 hours after the application Footnote 7. Histological findings suggested this as a case of primary irritant contact dermatitis rather than an allergic contact dermatitis.
In a mouse Local Lymph Node Assay (LLNA) study, exposure to a 2-6% concentration of sodium metasilicate was found to be not sensitizing (based on a study summaryFootnote 5).
The available data do not meet the classification criteria for Skin Sensitization.
Germ Cell Mutagenicity:
Does not meet criteria
In vivo: In an OECD TG 475 study, sodium metasilicate (MR 1.0), tested negative for chromosome aberration when administered in mice through oral feed even at dosage levels exceeding the maximally tolerated dose of 940 mg/kg (based on a study summary Footnote 5).
In vitro: In an Ames test performed according to OECD TG 471, sodium metasilicate (MR 1.0), tested negative in Salmonella typhimurium strains TA98, TA100, TA1535 and TA1537, with and without metabolic activation (based on study summary Footnote 5.
In an OECD TG 473 study, silicic acid, sodium salt (MR 3.3), tested negative in a chromosome aberration assay in Chinese hamster lung fibroblast cells (V79), with and without metabolic activation (based on a study summary Footnote 5).
The available data do not meet the classification criteria for Germ Cell Mutagenicity.
Carcinogenicity:
No data available
Silicic acid, sodium salt, has not been reviewed by the International Agency for Research on Cancer (IARC), the National Toxicology Program (NTP), or the American Conference of Governmental Industrial Hygienists (ACGIH).
It is relevant to note that sodium silicates have had a long history of safe use in numerous food-related applicationsFootnote 8. Sodium silicate and potassium silicate have been granted GRAS (Generally Recognized as Safe) status by the U.S. Food and Drug Administration (FDA) for addition to canned drinking water as a corrosion preventative at concentrations up to 100 ppmFootnote 6.
No carcinogenicity data are available for silicic acid, sodium salt.
Reproductive Toxicity:
Does not meet criteria
In a mouse study, animals were administered 12.5, 50 or 200 mg/kg/day of sodium metasilicate (MR 1.0) by gavage from day 0 to 18 of gestation (based on study summaryFootnote 5). No treatment-related effects were observed in mothers, fetuses delivered by hysterectomy, or neonates. No parturition fatalities were observed when mothers were allowed to deliver their young naturally. There was a dose-related, but not statistically significant decrease in litter size. A dose-related, but not statistically significant decrease in embryo weight and delayed ossification process were also observed. No treatment-related effects on pups' body weight gain were observed in any dose group.
In a non-standard multi-generational rat study testing silicic acid, sodium salt (MR 3.2), animals received 0, 600, or 1,200 ppm (based on SiO2 content) in drinking water Footnote 9. Mortality and clinical signs of the parental generation were not reported but there was a significant decrease in offspring born. However, the number of offspring is the accumulated total from litters from 5 generations. The results of this study cannot be considered reliable for classification due to the experimental design. Only 2 doses were tested in a very small number of F0 generation animals (6/sex/group), there was no stability testing on drinking water, and the wire-bottom cages allowed newborns to fall out of the cage.
The available data do not meet the classification criteria for Reproductive Toxicity.
Specific Target Organ Toxicity – Single Exposure:
Does not meet criteria
Oral Route of Exposure: In a single dose toxicity animal study, rats (5/sex/group) were treated with 20% silicic acid, sodium salt (MR 2.0 and 2.4), at a dose of 464, 1,000, 2,150 or 4,640 mg/kg by gavageFootnote 4. No apparent signs of toxicity were noted at the 464 mg/kg dose level. Animals treated with MR 2.0 and MR 2.4 at all other dose levels showed signs of gasping, dyspnea and acute depression. However, no confirmatory necropsies were performed. The available data do not meet the criteria for classification.
Dermal Route of Exposure: No signs of toxicity were observed in a single-dose toxicity study where rabbits were exposed to 4,640 mg/kg of 19.5% silicic acid, sodium salt (MR 2.0 and MR 2.4), dermallyFootnote 4.
Inhalation Route of Exposure: No data available
The available data do not meet the classification criteria for Specific Target Organ Toxicity – Single Exposure.
Specific Target Organ Toxicity – Repeated Exposure:
Does not meet criteria
Oral Route of Exposure: An oral exposure of weanling rats to silicic acid, sodium salt (MR 3.2), via drinking water at 789.5 and 1,587 mg/L for 180 days did not result in any adverse effectsFootnote 9. Some statistically significant differences were observed in body weight between experimental groups and controls but these were small (6% or less), not consistent, and not dose related. In a 28-day repeat-dose toxicity study in rats performed according to OECD TG 407, ingestion of silicic acid, sodium salt, of no specified MR, up to a dose level of 2,400 mg/kg/day did not result in any toxicity (based on a study summaryFootnote 5).
Dermal Route of Exposure: No data available
Inhalation Route of Exposure: No data available
The available data do not meet the classification criteria for Specific Target Organ Toxicity – Repeated Exposure.
Aspiration Toxicity:
No data available
No human data are available for silicic acid, sodium salt. This substance is not a liquid hydrocarbon.
Biohazardous Infectious Materials:
Not applicable
Silicic acid, sodium salt is not a microorganism, protein, or nucleic acid.
Physical hazards
Explosives:
Not evaluated*
* Explosives are excluded from the HPA and its regulations. Explosives are regulated under the Explosives Act. For more information, visit Natural Resources Canada.
Flammable Gases:
Not applicable
Silicic acid, sodium salt, is not a gas. The classification criteria for Flammable Gases do not apply to this substance.
Aerosols:
Not evaluated
Classification of a hazardous product in the Aerosols hazard class is product dependent.
Oxidizing Gases:
Not applicable
Silicic acid, sodium salt, is not a gas. The classification criteria for Oxidizing Gases do not apply to this substance.
Gases Under Pressure:
Not applicable
Silicic acid, sodium salt, is not a gas. The classification criteria for Gases Under Pressure do not apply to this substance.
Flammable Liquids:
Not applicable
Silicic acid, sodium salt, in pure form is not a liquid.
The classification criteria for Flammable Liquids do not apply to the pure substance.
Flammable Solids:
Does Not Meet Criteria
Silicic acid, sodium salt, is not flammable; it is used as a flame retardantFootnote 10,Footnote 11,Footnote 12,Footnote 13
The available data do not meet the classification criteria for Flammable Solids.
Self-Reactive Substances and Mixtures:
Does not meet criteria
Silicic acid, sodium salt, is a non-flammable substance, and is used as a flame retardantFootnote 10,Footnote 11,Footnote 12,Footnote 13
The available data do not meet the classification criteria for Self-reactive Substances and Mixtures.
Pyrophoric Liquids:
Not applicable
Silicic acid, sodium salt, in pure form is not a liquid.
The classification criteria for Pyrophoric Liquids do not apply to the pure substance.
Pyrophoric Solids:
Does not meet criteria
Silicic acid, sodium salt is used as a flame retardantFootnote 10,Footnote 11,Footnote 12,Footnote 13. Sodium metasilicate, a closely related substance, is hygroscopicFootnote 14. Silicic acid, sodium salt has a similar structure to silicon dioxide, which is non-flammable.
The available data do not meet the classification criteria for Pyrophoric Solids.
Self-Heating Substances and Mixtures:
No data available
No data are available to determine whether silicic acid, sodium salt, meets the classification criteria for Self-Heating Substances and Mixtures.
Substances and Mixtures which, in Contact with Water, Emit Flammable Gases:
Does not meet criteria
Silicic acid, sodium salt, forms stable mixtures in aqueous solutionFootnote 14. This substance need not be classified under substances which, in contact with water, emit flammable gases, as per the exclusion of paragraph 7.12.1(1)(c) of the HPR.
The available data do not meet the classification criteria for Substances and Mixtures which, in Contact with Water, Emit Flammable Gases.
Oxidizing Liquids:
Not applicable
Silicic acid, sodium salt, in pure form is not a liquid.
The classification criteria for Oxidizing Liquids do not apply to the pure substance.
Oxidizing Solids:
Does not meet criteria
Silicon is in the same group in the periodic table as carbon. The 2 substances behave similarly when bonding with oxygen. Silica has a stronger bond strength with oxygen than the carbon-oxygen bond strengthFootnote 15. The oxygen bonded to silicon in silicic acid, sodium salt is not available to fuel a fire.
The available data do not meet the classification criteria for Oxidizing Solids.
Organic Peroxides:
Not applicable
Silicic acid, sodium salt, is not an organic peroxide. The classification criteria for Organic Peroxides do not apply to this substance.
Corrosive to Metals:
No data available
No data are available to determine whether silicic acid, sodium salt, meets the classification criteria for Corrosive to Metals; however, of note, sodium silicate and potassium silicate have been granted GRAS (Generally Recognized as Safe) status by the U.S. FDA for addition to canned drinking water as a corrosion preventative at concentrations up to 100 ppm Footnote 6.
Combustible Dusts:
No data available
No data are available to determine whether silicic acid, sodium salt, meets the classification criteria for Combustible Dusts.
Simple Asphyxiants:
Not applicable
Silicic acid, sodium salt, is not a gas. The classification criteria for Simple Asphyxiants do not apply to this substance.
Chemicals Under Pressure:
Not evaluated
Classification of a hazardous product in the Chemicals Under Pressure hazard class is product dependent.
Regulatory and other information
Regulatory information:
Hazardous substance assessments are prepared by Health Canada as educational and information resources. Under the HPA, suppliers of hazardous products must, upon the sale or importation of a hazardous product, provide a label and safety data sheet that meet the requirements set out in the HPR.
Other information:
The information and classifications contained in these hazardous substance assessments are based on publicly available sources, such as peer-reviewed literature or reports by international bodies. New information, including proprietary information, could have an impact on the classification of substances or hazardous products containing them. It is the responsibility of the supplier to ensure the accuracy, sufficiency, and reliability of their hazardous product classifications.
Last Updated: 2022
Prepared by: Workplace Hazardous Materials Bureau, Health Canada
References
- Footnote 1
-
HERA (2005) Soluble Silicates - DRAFT - (CAS No.: 1344-09-8, 6834-92-0, 10213-79-3, 13517-24-3, 1312-76-1). Human & Environmental Risk Assessment on Ingredients of European Household Cleaning Products.
- Footnote 2
-
Centre Europeen d'etude des Silicates (2008) Soluble Silicates: Chemical, toxicological, ecological and legal aspects of production, transport, handling and application. European Chemical Industry Council, Brussels, Belgium.
- Footnote 3
-
OECD SIDS (2004) Soluble Silicates. Silicic acid, sodium salt: 1344-0908; Silicic acid (H2SiO3), disodium salt: 6834-92-0; Silicic acid (H2SiO3), disodium salt, pentahydrate: 10213079-3; Silicic acid (H2Sio3), disodium salt, nonahydrtae: 13517-24-3; Silicic acid, potassium salt: 1312-76-1. SIDS Initial Assessment Report. SIAM 18. UNEP Publications.
- Footnote 4
-
Rhone-Poulenc Inc. (1971) Initial submission: Comparative toxicology study of disilicates with cover letter dated 102392. Stauffer Chem. Co. EPA/OTS Doc #: 88-920010794. NTIS/OTS0571941.
- Footnote 5
-
European Chemicals Agency (2019) Silicic acid, sodium salt - REACH dossier. Available at: http://www.echa.europa.eu
- Footnote 6
-
Schleyer, W. L. and Blumberg, J. G. (1982) Health, Safety, and Environmental Aspects of Soluble Silicates. In: Soluble Silicates. Falcone, jr (Ed.). American Chemical Society, ACS Symposium Series, Vol. 194, pp.49-69.
- Footnote 7
-
Tanaka, T., Miyachi, Y., and Horio, T. (1982) Ulcerative contact dermatitis caused by sodium silicate. Archives of Dermatology 118:518-520.
- Footnote 8
-
Cosmetic Ingredient Review Expert Panel (2005) Final report on the safety assessment of potassium silicate, sodium metasilicate and sodium silicate. Int. J. Toxicol. 24103-117.
- Footnote 9
-
United States Coast Guard (1999) Chemical Hazards Response Information System Manual. United States Coast Guard.
- Footnote 10
-
Zhang, T., et al. (2021) Facilely produced highly adhered, low thermal conductively and non-combustible coatings for fire safety. Journal of Colloid and Interface Science, 604: 378-389.
- Footnote 11
-
Kacikova, D., et al. (2021) The influence of nanoparticles on fire retardancy of pedunculate oak wood. Nanomaterials 11(3405): 1-13.
- Footnote 12
-
Garskaite, E., et al. (2019) Surface hardness and flammability of Na2SiO3 and nano-TiO2 reinforced wood composites. RSC Advances, 9: 27973-27986.
- Footnote 13
-
Chen, S-N., et al. (2021) Enhancements on flame resistance by inorganic silicate-based intumescent coating materials. Materials, 14: 1-16.
- Footnote 14
-
Rumble, J. (2019) CRC Handbook of Chemistry and Physics. 100 Edition. J. Rumble (Eds.). CRC Press, Boca Raton, FL.
- Footnote 15
-
Cotton, F. A., Wilkinson, G., Murillo, C. A. and Bochmann, M. (1999) Advanced Inorganic Chemistry. 6th Edition. Wiley. pp. 1376.