Paper on the Allergen Control Activities within the Canadian Food Inspection Agency
For further information or to obtain additional copies, please contact the Food Allergen Program.
This document can be made available in alternate format(s), upon request.
© Her Majesty the Queen in Right of Canada, represented by the Minister of Health, 2003
ISBN: 0-662-32959-7
Cat. No.: H39-649/2002E-IN
Table Of Contents
- Activity 1: Developing Science-based Strategies and Work plans to Deal with Risks
- Activity 2: Establishing/Aligning Domestic and International Legal and Operating Mechanisms
- Activity 3: Informing the Public and Fostering Industry Cooperation
- Activity 4: Assessing Compliance With Food Safety and Labelling Requirements
- Activity 5: Identifying and Responding to Threats to Food Safety
- Infrastructure
List of Exhibits
Executive Summary
In April 1997, the Canadian Food Inspection Agency Act established the Canadian Food Inspection Agency (CFIA) reporting to the Minister of Agriculture and Agri-food Canada. Health Canada is responsible for assessing the effectiveness of the Agency's activities related to food safety. The objective of this paper is to provide information on the allergen control activities within the Agency from its creation on April 1, 1997 until the end of May 2001.
Generally, our assessment reports would typically involve the development of questions in areas such as program design, delivery and implementation, leading to a description of the effectiveness of the Agency's activities. In the case of allergen control activities, it became evident that our document would be largely descriptive in nature, without a full assessment of the Agency's effectiveness.
This decision was based on the fact that a majority of the high profile food allergen cases (i.e. Class 1 recalls) tend to involve the bakery and confectionary products (at both the domestic and import levels) which fall under the jurisdiction of the Bureau of Food Safety and Consumer Protection (BFSCP) in the CFIA. As a result of an internal review in March 2000, the BFSCP was created to manage program activities pursuant to the Food and Drugs Act and to provide a horizontal approach to risk prioritization throughout the CFIA. The BFSCP provides a forum for developing science-based strategies dealing with food safety risks across all food programs. Since this Bureau has recently been redesigned, we felt that a full assessment of allergen control activities within the Agency would be premature at this time.
This paper describes the nature of the allergen control activities within the CFIA and provides some information on the new risk-based approach used by the Agency. As part of the information-gathering, we interviewed key Agency staff in the programs and operations areas and reviewed several Agency program manuals to get an idea of how allergen control activities were described. We also performed a quantitative analysis of food recall tables in order to obtain an idea of the main food allergens that resulted in recalls. We interviewed key staff at Health Canada who are involved in various aspects of allergen control activities such as developing methods for detecting allergens and providing advice on health risk assessments. We also spoke to representatives from two allergy associations and from one industry association, namely the Food and Consumer Products Manufacturers of Canada, to find out their views and concerns.
This paper represents a summary of the information gathered on CFIA's allergen control activities. We outlined five major food safety activities within the Agency and linked these to the type of allergen control activities that we observed within the Agency. The following areas are discussed:
- developing science-based strategies and work plans to deal with risks;
- establishing and aligning domestic and international legal and operating mechanisms;
- providing information to the public and fostering industry cooperation;
- assessing compliance with food safety and labelling requirements; and
- identifying and responding to threats to food safety which we linked to food safety recalls.
We also covered compliance and enforcement activities, special concerns related to imported food products, food recalls based on the presence of allergens, laboratories' role in testing for allergens and some infrastructure activities within the CFIA that support allergen control activities.
In summary, the Canadian Food Inspection Agency is involved in a series of activities to deal with the harmful effects of undeclared food allergens. Through the work of the Science Committees, the Agency is adopting a horizontal science-based approach across all sectors and food divisions to deal with the potentially harmful effects posed by known food allergens. The Agency is also involved in activities to inform and educate the public and to support and promote industry cooperation. Other activities support food safety labelling as well as enforcement and compliance efforts. Through the course of our information gathering, we also identified some practices within the Agency's main food safety activities linked to allergen controls, that we felt merited a review and potential update.
For instance, it would be valuable to perform trend analyses on a regular basis on the underlying reason(s) for allergen recalls. This would provide needed information to help determine the level of risk and to assist in the development of risk mitigation strategies within the program areas of the CFIA to reduce the number of food allergen recalls. As well, the results of any industry sector national assessment reports could be used as benchmarks to measure the changes in the level of compliance within selected industry sectors.
Since compliance and enforcement are key activities within the Agency, these could be applied effectively to the area of undeclared food allergens to eliminate repeat offenders such as those involved in imported European chocolate. Finally, the development of close links among government departments and agencies and allergy and industry associations would strengthen the overall food allergy network and provide opportunities to increase collaboration in such areas as the development of guidelines or standards for precautionary labelling. Such action helps to ensure that there is more direction in identifying products that have come into contact with known food allergens, thereby providing a greater level of assurance to the allergic consumer on the contents of the product. The subject of precautionary labelling requires further exploration by Health Canada in collaboration with the CFIA.
We feel that this paper contributes to the overall knowledge of food allergen controls and could be useful in any review and update of policies and practices related to this topic.
Introduction
1. Each year a number of people experience adverse reactions to foods that they have consumed. In Canada and the United States, an estimated 1-2 percent of the adult population is allergic to one or more particular foods. In children, the incidence is somewhat higher, at approximately 5-8 percent. Unfortunately, there are no reliable data to confirm the percentage of the population that is susceptible to food allergies. Some children outgrow their food allergies at a later stage, especially those who are allergic to egg, milk, soy and wheat; however, some food allergies continue into adulthood and can be quite serious in nature such as those associated with peanuts. Some adults who have previously been able to tolerate certain foods quite well can develop severe allergic reactions to them. There is currently no cure for a food allergy, other than to avoid eating the offending food.
2. Symptoms of a food allergy can appear immediately after the food is consumed or can be delayed. Immediate reactions can range in severity from a skin rash or a slight itching of the mouth, to migraine headaches and to anaphylactic shock and death. Anaphylactic reactions occur when the body's sensitized immune system overreacts in response to the presence of a particular allergen. Anaphylaxis affects multiple body systems: skin, upper and lower respiratory, gastro-intestinal and cardiovascular. Symptoms may include any of the following: itchy eyes, nose and face; flushing of face and body; swelling of eyes, face, lips, tongue and throat; hives; vomiting; diarrhea; wheezing; a feeling of foreboding, fear and apprehension; weakness and dizziness; inability to breathe; loss of consciousness; and coma. In its most severe form, a reaction can result in death. There are other forms of adverse reactions to food that are not deemed to be true allergic reactions. Some examples include lactose and monosodium glutamate intolerance.
3. Variability in responses is common in individuals who have food allergies; quite often the magnitude of the response is in direct proportion to the amount of allergenic food eaten by the allergic individual. Individuals that have asthma tend to have much more serious reactions to food allergens. While the exact threshold doses (i.e. the amount of allergenic protein necessary to elicit an allergic response) for specific allergenic foods are unknown, some test results have indicated that it can take as little as 1-2 mg (or in some cases less) of the offending food to elicit allergic reactions in sensitive individuals.
4. Reactions to foods may vary from one country to another, depending on people's diet patterns. For instance, in Canada and the United States, there are similarities among the foods most commonly reported as causing severe adverse reactions. Health Canada and the Canadian Food Inspection Agency (CFIA) have jointly identified 10 food products which are primarily responsible for about 90 percent of severe adverse food reactions among the Canadian population. These products are peanuts, tree nuts, soy, milk, eggs, fish, crustacea and shellfish, sesame seeds, sulphites, and wheat. Although sulphites are not considered to be true allergens, they do produce an adverse reaction that can lead to anaphylactic shock and death. It is the seriousness of these adverse reactions that has resulted in the inclusion of sulphites on the priority allergen list.
5. As well, consumption of wheat, rye, oats, barley and triticale can seriously affect quality of life by increasing the risk of lymphoma and osteoporosis in individuals with celiac disease. In the case of wheat, a variety of cereals has been implicated in IgE-mediated allergic reactions and anaphylactic reactions to wheat have been reported in children. This has resulted in the inclusion of wheat on the priority allergen list.
6. Consumers largely depend on the accurate formulation and labelling of products to avoid eating foods that contain ingredients to which they may be allergic. Other appropriate measures for controlling food allergens include proper design and cleaning of equipment, preventing cross-contamination by allergens during manufacturing, and the appropriate use of "re-work". (Re-work is recycled processed food that is reintroduced into the production line.)
Legislation affecting Food Allergens in Canada
7. There are a number of laws that deal with the labelling of food products in Canada. The Agency is responsible for administering the labelling provisions of the Food and Drugs Act and the Consumer Packaging and Labelling Act. Other acts such as the Canada Agricultural Products Act, the Meat Inspection Act and the Fish Inspection Act that the Agency administers, contain regulations pertaining to labelling.
8. In particular, the Food and Drug Regulations state that all ingredients must be declared on the labels of food products. Division 1 of these Regulations require almost all prepackaged food to list all ingredients and components (i.e., the ingredients of ingredients). Under subsection B.01.009(1), the components of 36 ingredients or groups of ingredients do not have to be declared on the label of a product. Among the items on this list are ingredients that can create problems for allergic individuals. For instance, margarine may contain milk products and meat may contain wheat fillers. These regulations are being revised by Health Canada to ensure that the ten most common food allergens on the priority allergen list, (as recognized by the Codex Alimentarius Commission in 1997), are consistently labelled in order to protect consumers.
9. The proposed labelling regulations will require (among other things) manufacturers to identify previously exempt components and ingredients on labels, as outlined in section B.01.009 of the Food and Drug Regulations. Where one or more (allergenic) ingredients or components of ingredients are added either directly to a food or indirectly as a result of use in one or more of the ingredients of that food, that ingredient, or component of an ingredient, or product thereof, shall be shown in the list of ingredients by its common name as if it were an ingredient of that food. Sulphites or sulphiting agents must be labelled when they are added and present at a level of 10 parts per million (ppm) or higher. It is further proposed that there be an identification of the plant source in the common name of each of the following foods: hydrolysed plant proteins, starches and modified starches, flour and gluten.
10. The Canadian Food Inspection Agency Act states in sub-section 11(3) that the Agency is responsible for ". . . the enforcement of the Food and Drugs Act as it relates to food, as defined in section 2 of that Act . . .", in other words, "for . . . any article manufactured, sold or represented for use as food or drink for human beings." Health Canada has developed a Policy on Food Allergens for Compliance Purposes that the CFIA uses as a guideline to assess whether a food product poses a significant risk to the consumer and whether it should be recalled. In addition, further advice is provided by Health Canada through the health risk assessments, requested by the CFIA on a case by case basis.
11. Specifically, the Health Canada policy document on food allergens provides recommendations on compliance actions such as product recalls and public alerts that can be taken when the following situations are presented:
- when peanut protein is detected in a food at levels greater than or equal to 1 ppm and is not declared on the label, Health Canada would support a recall and public alert;
- when tree nuts (named), sesame seeds, soy, cow's milk, eggs, fish, crustaceans and shellfish, wheat and sulphites (in excess of 10 ppm) are added as ingredients/additives to a food without any declaration of their presence on the product label, Health Canada would support a recall; and
- when trace quantities of tree nuts (named), sesame seeds, soy, cow's milk, eggs, fish, crustaceans and shellfish, and wheat are present in a food as a result of cross contamination at some stage in the food production process (and without label warning), this will be assessed on a case by case basis by Health Canada through a health risk assessment.
12. This policy was developed in 1998, and was based on the best available knowledge at the time regarding assessment of risks associated with exposure to substances known to be the cause of the most frequent and severe allergic or allergic-type reactions in Canada. The policy recognizes that there are numerous unknowns in regard to quantitative risk assessment of food allergens and that research laboratories are continuing to improve analytical detection methods for food allergens. As new data becomes available, the policy will be further refined.
Approach of this Paper
13. The objective of this paper is not to look at the effectiveness of the Agency's activities in this case, in relation to food allergen controls, but to describe and document the effort that the Agency devotes to various activities with a view to reducing risk to the public from food allergens. We adopted this objective because, after an extensive information- gathering exercise, it became apparent that most of the Agency's allergen-related recall activities (about 60 percent) concentrate on bakery and confectionery products which are the responsibility of the newly created Bureau of Food Safety and Consumer Protection (BFSCP) within the Agency. More than 80 percent of Class 1 allergen recalls in a four-year period in both the domestic and imported foods sector have occurred in the non-federally registered sectorFootnote 1. The BSFCP has recently undergone a program redesign employing a new risk-based approach. Given the recent significant changes at the Bureau, we felt that it was premature to assess the effectiveness of its activities. The BFSCP's new risk-based approach is described in paragraph 21 of this paper.
14. We had considered using a small "market-basket" survey of foods sold in retail stores to determine how well the food industry is performing with respect to ensuring that product labels accurately indicate the presence of all known food allergens. This survey could have been directed to those allergens that are frequently involved in the cross-contamination of food products. This type of survey would have been feasible, provided that a brief validation process was applied to the available methodologies and would have involved collaboration between CFIA and Health Canada. While the CFIA was interested in the potential results of such a survey, they indicated that it was not a strategy they would choose to pursue at this time. This does not preclude such a survey being conducted in the future. As well, we chose not to deal with biotechnology issues such as genetically modified organisms (GMOs) and their allergen-related concerns as this would have broadened the scope of our review considerably outside the area of known allergens. Instead, we concentrated on the Canadian priority food allergen list.
15. This paper summarizes our observations of CFIA's involvement in allergen control activities as well as the information that we gathered through data review and interviews to understand the roles of staff and the nature and scope of allergen control activities within the Agency from its creation on April 1, 1997 to the end of May 2001.
16. The information in this paper has certain limitations because it is based on preliminary work and does not explore any particular assessment questions. It is also largely qualitative, with some quantitative analysis of data in the area of allergen-related food recalls. Nonetheless, we felt this information would help to provide a broad overview of the allergen control activities of the CFIA. As well, this information would be useful to both Health Canada and the Agency in reviewing and updating policies and practices that would impact on controls of food allergens. (See the "About this Paper" section at the end of this document for more details on the information-gathering process.)
Summary of CFIA's Food Safety Activities Linked to Allergen Controls
17. We identified five main activities that are at the core of the CFIA's food safety work. We subsequently linked the allergen control activities that we identified through interviews and data reviews, to each of these five main activities. (Refer to Appendix 1). These activities are:
- developing science-based strategies and work plans to deal with risks;
- establishing and aligning domestic and international legal and operating mechanisms;
- providing information to the public and fostering industry cooperation;
- assessing compliance with food safety and labelling requirements; and
- identifying and responding to threats to food safety (with a focus on food safety recalls).
18. This section of the paper provides a brief overview of each activity, along with identified outputs or products. We have also included a section entitled "Infrastructure" which describes some features that help support allergen control activities within the CFIA.
Activity 1: Developing Science-based Strategies and work plans to Deal with Risks
19. CFIA's role in managing risks associated with food allergies is both to promote and determine compliance on the part of industry with all applicable regulations and guidelines. Another role is to encourage the development of plans to control allergens in all domestic and imported goods offered for sale in Canada.
20. As a result of an internal review within the CFIA of the Consumer Food Products Program in March 2000, the Bureau of Food Safety and Consumer Protection (BFSCP) was created to manage program activities pursuant to the Food and Drugs Act and to provide a horizontal approach to risk prioritisation throughout the CFIA. The BFSCP provides a forum for developing science-based strategies dealing with food safety risks across all food programs.
21. The Bureau has adopted a risk-based approach which uses a risk-estimation model that takes into consideration the probability and severity of risks in terms of their consequences and the industry's approach to controlling them. The approach and strategy for managing allergen-related risks involve identifying risks through an environmental scanning process. These risks are then discussed and prioritized by the CFIA Science Committee that deals with allergen issues.
22. The main purpose of the Science Committee is to provide a forum to bring together programs, operations, regulatory staff and laboratories from different parts of the Agency. This committee also includes representatives from Health Canada. The approach is horizontal, with participants sharing information and best practices across all programs and examining risk-management options that can be used by both the federally registered and non-federally registered sectors, across all divisions and food commodities. Risk-management options are prepared and sent to a national steering committee for review and advice. As well, the committee provides specific scientific and technical expertise to the Bureau of Food Safety and Consumer Protection.
23. Work plans and projects, such as the industry sector national assessment on food importers which focussed on labelling and allergen controls, are the products of this activity. The CFIA also collected data on the bakery sector where there is potential exposure to food allergens if food ingredients have not been properly labelled or accidental product contamination has occurred during the manufacturing process.
24. The industry sector national assessment project was conducted in cooperation and consultation with the food importers sector. The aim of the resultant CFIA report on food importers which was completed in November 2000, was to understand the level of control that the industry has adopted, to foster food allergy awareness and to promote best practices. CFIA used the information from the national assessment to develop tools such as allergen checklists that could be used by the industry to develop allergen prevention programs. This information was made available to industry on the CFIA website.
25. The CFIA food importers sector assessment report indicates that importers did not always have product information. For example, information on some ingredients, components and additives, including allergens, were not always available. Information on processing and handling was also lacking. However, when information was available, it was usually up-to-date and complete. Although 86 percent of importers were aware of allergy concerns, only a few had either instituted a formal policy on allergen awareness and control, or provided training programs for employees on appropriate labelling and allergen controls. The ratings that CFIA gave on this sector indicated that importers needed to improve their labelling and allergen controls to ensure that they received accurate product information from their suppliers, and that this information appeared on labels.
26. The findings of reports such as the above mentioned national sector industry assessment are valuable as they provide information on how well the industry is performing with respect to allergen controls and the improvements that are needed (whether these are based on additional training needs or better controls for cross-contamination) to reduce the risk posed by allergens to human health. The CFIA Science Committee will determine the feasibility of using the national assessments of industry sectors to obtain further information on compliance when they convene to develop their list of work plan priorities.
Activity 2: Establishing/Aligning Domestic and International Legal and Operating Mechanisms
27. The CFIA participates in domestic and international fora that discuss issues related to allergen controls. On the domestic side, the Agency's allergen technical specialists work with Health Canada in a variety of ways. For example, they are involved in Science Committees and technical meetings. They also request health risk assessments in accordance with the Health Canada Policy on Food Allergens for Compliance Purposes. These specialists also liaise with the provinces, especially with respect to distributing information on allergy alerts.
28. CFIA meets with international organizations such as the Codex Alimentarius Commission and participates in developing various standards and guidelines for food products (e.g. general standards for labelling pre-packaged foods). The Agency also works with the Canadian Importers and Exporters Association and exporting countries to increase their awareness of food allergen issues. The Bureau of Food Safety and Consumer Protection has contacts with the United States Food and Drug Administration (US FDA) and the Food Allergy Research and Resource Program (FARRP), a leading food allergy research group in the United States.
29. Partnerships and standards are the products of this activity. As a result of CFIA's participation on various fora, partnerships are created at the federal/provincial/territorial level and with other government departments (e.g. Health Canada, Department of Foreign Affairs and International Trade) and with other countries. When a standard is approved by the Codex Alimentarius Commission, endorsing countries must find ways of implementing it (e.g. through enacting legislation, policies, etc.).
Activity 3: Informing the Public and Fostering Industry Cooperation
Public Information
30. The CFIA communicates with the general public and consumer groups to make them more aware of allergen-related issues. For example, the CFIA provides information on these issues through its website. It also publishes allergy alerts through the Canada News Wire Service and distributes information to a range of individuals and groups inside and outside the Agency. CFIA also publishes fact sheets that both identify products containing undeclared food allergens, and provide general information, advice and warnings to the public.
31. The Agency's program staff in the regional offices have done some work with local allergy associations by disseminating information about allergens through meetings, educational material, workshops and the CFIA website.
Industry Cooperation
32. The food industry plays a key role in promoting consumer safety by ensuring that products do not become contaminated with allergens during the manufacturing process, and that the ingredients and products purchased from suppliers clearly identify any known allergens. The CFIA encourages the industry to declare all ingredients on its products (including the ten priority allergens) and follow good manufacturing and importing practices. CFIA offers educational sessions and guidelines for industry through direct meetings, consultation, and information on its website. Information transfer is also provided through CFIA's participation in academic or trade sponsored workshops.
33. More specifically, to support the industry's efforts, the Agency publishes documents on its website such as the Guide to Food Labelling and Advertising, which refers directly to allergens. To help importers in obtaining product information from their suppliers, the Agency has prepared two resources: a "Tool for Managing Allergen Risks in Domestic and Imported Food Products"; and an "Allergen Checklist for Food Suppliers and Manufacturers".
Other Stakeholders
34. The allergy and industry associations consulted called our attention to various issues. These issues centred on some on-going labelling problems with imported food products (in particular imported chocolate products with undeclared nut allergens), the use of precautionary labelling, the need to clearly define allergens in ingredient listings (ie. milk rather than whey) and the need for science-based organisations to get more involved in conducting molecular research on how to prevent the human body from reacting to food allergens.
35. Both industry and allergy groups are heavily involved in mounting education and awareness sessions in various fora across the country and are working closely with their members and partners to get their messages out. They are aware of the emerging issues surrounding food allergens and are familiar with the on-going concerns of their members.
36. The representatives from the two allergy associations would like to see a closer partnering relationship that would involve the sharing of information among representatives of government, industry, and the allergy associations. One industry representative from the Food and Consumer Products Manufacturers of Canada (FCPMC) had a similar view. Since the allergy associations have well-developed networks, they serve as highly effective partners, especially in transferring allergen-related information to the affected population and public institutions (i.e. school programs).
37. The allergy associations are also concerned about the terms used in precautionary labelling (labelling which indicates that a food product may inadvertently contain substances capable of causing severe adverse reactions). The associations feel that the industry should standardize the use of terms related to precautionary labels as well as use these judiciously. For instance, some labels may state "may contain nuts" while others may indicate that "this product was made in a facility that has peanuts in production." Such terminology is subject to consumer misinterpretation and does not assist people, who are sensitive to allergens, to appropriately identify the level of risk with the product. This often leads to limitations in consumer choice.
38. The FCPMC provides large and small companies with advice and guidance in a number of areas, including developing allergen-prevention plans. It has prepared an Allergy Beware instructional manual for its members that defines food allergies, and describes such items as:
- the responsibilities of food manufacturers and their employees in relation to allergen controls;
- how to minimize cross-contamination and mislabelling;
- the potential allergen issues related to the ingredients being purchased from suppliers; and
- the need to choose equipment that is easy to dismantle and clean.
39. The CFIA has expressed interest in including industry representatives in its Science Committee meetings, especially the meetings of the Food Composition committee that deals with allergen issues. The Director of the Bureau of Food Safety and Consumer Protection indicates that since the Bureau is now responsible for allergen control issues, it is the key contact or focal point for food allergen issues, including any involvement on Science Committees within the Agency.
Activity 4: Assessing Compliance With Food Safety and Labelling Requirements
40. The CFIA has inspection programs and projects related to assessing allergen threats to food safety. Inspection programs include such activities as verifying the accuracy of labels on domestic and imported products, and inspections and audits of domestic manufacturers and importers to assess their compliance with the applicable legislation.
41. Within the federally registered sectors of Dairy, Eggs and Processed Products, the label review process is conducted under the Canada Agricultural Products Act, and the label verifications are carried out as part of routine inspections. The Dairy, Egg, and Processed Products Programs verify ingredients as part of their scheduled inspections. In the Meat Program, registration of labels and process is mandatory, and label reviews are performed by operations staff.
42. Domestic manufacturers under federally registered programs such as Egg, Dairy and Processed Products are inspected for compliance according to a scheduled frequency. In the BFSCP, work can be characterised as being both proactive and reactive in nature. From the proactive side, projects are identified by the Science Committees based on a prioritisation of risk and seek to understand the nature of the industry's adherence to policies and standards. From the reactive side, investigations are carried out, often as a result of such incidents as consumer complaints. This activity results in the production of various reports, compliance ratings, detection methods and training programs for staff. CFIA collaborates with Health Canada on developing and refining detection methods.
43. As mentioned earlier, there are two divisions in the Bureau of Consumer Protection and Food Safety, namely the Food Safety Investigation Program and the Fair Labelling Practices Program. The focus of the Fair Labelling Practices Program is to develop policy dealing with consumer protection against product misrepresentation, mislabelling and fraud in the food sector and to provide this to the various commodity groups within the CFIA to enforce within their areas of jurisdiction. The Food Safety Investigation Program deals more specifically with health and safety issues arising from improper labelling of food products which is largely compliance oriented.
44. In the area offices that we visited, we were advised that the CFIA operations staff are integrating food safety functions with the labelling review aspects of their jobs. At the national level, these two programs are quite distinct and require joint planning in order to ensure that investigators in the field offices consider both aspects of the labelling issue in their label review functions. This type of planning would help to ensure that a consistent approach was taken across the country to the important aspect of label reviews. As well, this approach would provide greater certainty that the health and safety issues resulting from undeclared food allergens would be identified early in order to protect the consumer. We were advised that CFIA plans to strengthen the links between these two programs at the national level and train its inspectors in order to ensure that they consider concurrently the health and safety issues (arising from undeclared allergens) and misrepresentation/fraud issues during the label review process.
45. In the labelling activity, there are other challenges related to the control of imported food products which arise because other countries often have different standards for manufacturing, processing, and re-work of ingredients. The Agency has placed "A Good Import Practices Protocol" on its website to encourage importers to review their labels for allergens. The CFIA has dealt with the concern over imported products through a variety of strategies. These include, for example, letters to importers, working with embassies, the Quality Management Program for Importers (QMPI) initiative and monitoring of products on the history of enforcement action (alerts, complaints, non-compliant labels, etc., for federally registered products). The Agency has, as noted earlier, also carried out a national assessment of imported food products.
Activity 5: Identifying and Responding to Threats to Food Safety
46. This activity includes investigating complaints, incidents and recalls; sampling and analyses of products as necessary; taking enforcement and compliance actions; and providing central co-ordination of recalls. Investigations can occur as a result of provincial referrals, trade complaints, consumer complaints, CFIA's audits and inspections, industry sector national assessments and the notification of foreign recalls.
47. The Agency's Enforcement and Investigations Services Division has been operating since August 1999 to provide national consistency for CFIA's enforcement actions across all programs and food commodity areas. A policy document has been produced and training has been provided to staff across Canada. As well, a tracking system is under development.
48. The Office of Food Safety and Recall (OFSR) co-ordinates recalls at the corporate level to ensure the timeliness and appropriateness of actions taken. Accordingly, the OFSR is involved in investigations, decision-making, verification and follow-up activities. Its responsibility is to provide advice and direction, access to technical resources, and a fast turn-around time in recall situations. Laboratory results, reports, and enforcement and compliance actions are products of this activity.
49. Compliance and enforcement are issues of fundamental concern in the food allergens area, especially because of repeat offenders in both the domestic manufacturing and food importers sector. In particular, there have been frequent recalls of the same types of imported food products over the past year because they contained milk protein and/or peanuts, which had not been declared on their labels. The BFSCP is currently working with the CFIA enforcement division to develop an action plan that will involve specific enforcement measures for violations of the labelling provisions of the Food and Drugs Act. It is anticipated that this approach would likely lead to greater care on the part of the importers to ensure that product labels accurately reflect the contents.
Food Recalls
50. We performed an analysis of various allergen-related recalls (Class 1, 2 and 3) over a four- year periodFootnote 2. The data indicates that the total number of allergen recalls had risen from 91, 125, 104 to 199 for the period of 1997-2001 for a total of 519 recalls (see Exhibit 1).
51. Fifty-eight percent of these allergen recalls were at the Class 1 level. It appears that the high numbers for allergen recalls is consistent with an overall increase of recalls for all hazards for the 1999-2001 period. The increase in allergen-related recalls for the year 2000-2001 may be partly explained by the fact that CFIA introduced a new counting scheme for allergen recalls based on incidents which was to facilitate allergen incident investigation and tracking of subsequent recalls.Footnote 3
52. We also reviewed this same data in order to determine whether variances exist between domestic and imported products. We found that, on average, the percentage of Class 1, 2, 3 recalls due to allergens in imported foods was very similar to the domestic sector (refer to Exhibit 2).
Allergen Related Recalls (1997-2001)
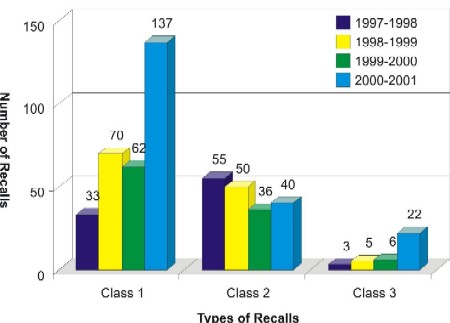
This is a bar graph showing the numbers of allergen-related recalls for the years 1997 to 2001. The recalls are further categorised into Class I, Class 2 and Class 3 (a definition of these classes of recall has been provided in paragraph 50).
The numbers of Class 1 recalls for the years 1997 to 1998; 1998 to 1999; 1999 to 2000 and 2000 to 2001 are respectively 33, 70, 63 and 137.
The numbers of Class 2 recalls for the years 1997 to 1998; 1998 to 1999; 1999 to 2000 and 2000 to 2001 are respectively 54, 50, 37 and 40.
The numbers of Class 3 recalls for the years 1997 to 1998; 1998 to 1999; 1999 to 2000 and 2000 to 2001 are respectively 3, 5, 6 and 22.
53. Of the imported food product recalls (as in the case of the domestic sector), the majority are within the jurisdiction of the non-federally registered sector which includes bakery and confectionery products. In fact, more than 80 percent of Class 1 allergen recalls in a four- year period in both the domestic and imported foods sector have occurred in the non- federally registered sector. In particular, bakery and confectionary products accounted for, on average, more than 60 percent of the allergen recalls.
54. The Agency has indicated that a small import task force has been formed for the non- federally registered sector which will examine the situation with respect to undeclared food allergens in imported foods and attempt to formulate appropriate guidelines for this sector. As well, the Agency is developing specific strategies which would aim to reduce the number of recalls related to undeclared allergens in imported chocolate products.
Exhibit 2
| Year | Class 1 | Class 2 | Class 3 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| I | D | I | D | I | D | I | D | |
| 97-98 | 14 | 19 | 20 | 35 | 1 | 2 | 35 | 56 |
| 98-99 | 39 | 31 | 28 | 22 | 0 | 5 | 67 | 58 |
| 99-00 | 30 | 32 | 18 | 18 | 4 | 2 | 52 | 52 |
| 00-01 | 76 | 61 | 13 | 27 | 20 | 2 | 109 | 90 |
| Total | 159 | 143 | 79 | 102 | 25 | 11 | 263 | 256 |
| % ratio | 50.8 | 49.2 | ||||||
55. In a review of Class 1 recalls of all types (e.g. microbiological, chemical, extraneous, and other) over the same four-year period, we observed that the majority of these recalls are allergen-related, compared with the other recall types. For example, we observed the following percentages for Class 1 allergen-related recalls in each year compared to other types of recalls: 75% in 1997-98; 69.2% in 1998-1999; 58.5% in 1999-2000 and 75.7% in 2000-2001. (See Exhibit 3). Allergen recalls are higher on average compared to other hazards (about 70% ).
56. We were not able to shed any light on the reasons or triggers for changes in recall activity over this four-year period. The high numbers of Class 1 allergen recalls could perhaps be due to the following factors:
- increased attention to particular allergens;
- increased consumer awareness and reporting;
- changing consumption patterns;
- the effects of enforcement activities;
- improved consumer complaint reporting;
- greater awareness on the part of industry of the risks posed by allergens; or
- better data to improve the effectiveness of health risk assessments and/or improvements in allergen detection methods.
Class 1 Recalls (Comparison of Hazards)
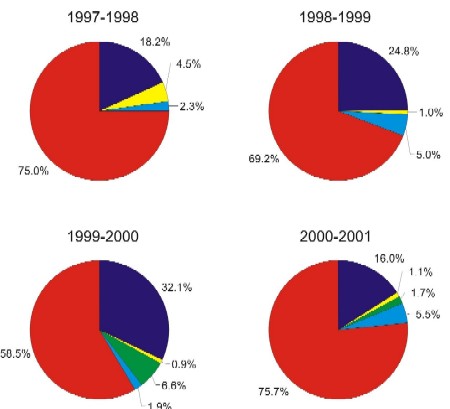

This has 4 pie charts showing the percentage of Class 1 recalls that involve a particular type of hazard. The category of hazards depicted are microbiological, chemical, extraneous material, allergen and other. Each pie chart represents a year of Class 1 recalls - 1997 to 1998; 1998 to 1999; 1999 to 2000 and 2000 to 2001.
In 1997 to 1998, the percentage of Class 1 recalls involving a microbiological hazard was 18.2%, chemical was 2.3%, allergen was 75.0% and other was 4.5%. There were no Class 1 recalls involving extraneous material in 97/98.
In 1998 to 1999, the percentage of Class 1 recalls involving a microbiological hazard was 24.8%, chemical was 5.0%, allergen was 69.2% and other was 1.0%. There were no Class 1 recalls involving extraneous material in 98/99.
In 1999 to 2000, the percentage of Class 1 recalls involving a microbiological hazard was 32.1%, chemical was 1.9%, extraneous material was 6.6%, allergen was 58.5% and other was 0.9%.
In 2000 to 2001, the percentage of Class 1 recalls involving a microbiological hazard was 16.0%, chemical was 5.5%, extraneous material was 1.7%, allergen was 75.7% and other was 1.1%.
57. We also reviewed the same data in order to determine the key allergens that were implicated in the recalls. We found on average, a higher percentage of recalls involving peanuts/tree nuts, dairy, sulphites, and eggs compared to other allergens (see Exhibit 4). It is noted that CFIA only has analytical methods for peanuts/tree nuts, dairy, sulphites and eggs. There are no lab tests for the other possible allergens. Health Canada is in the process of developing further allergen analytical methods but they are not yet available for routine use.
58. At the time of our interviews and information gathering, the CFIA did not have any trend analyses completed for recalls. This capability to do trend analyses could be useful to CFIA program staff in developing strategies for reducing allergen recalls as they would provide information on the underlying or root causes for the recalls. The Agency indicated that it would analyse trends in allergen recalls as part of the environmental scan work it is doing in preparation for upcoming Science Committee meetings.
Exhibit 4
| Allergens | 1997-98 | 1998-99 | 1999-00 | 2000-01 | Total Recalls | Overall %* |
|---|---|---|---|---|---|---|
| Peanuts & Tree Nuts | 15 | 32 | 21 | 35 | 103 | 34 |
| Dairy | 1 | 2 | 7 | 70# | 80 | 26 |
| Sulphites | 1 | 11 | 16 | 17 | 45 | 15 |
| Egg | 5 | 7 | 12 | 6 | 30 | 10 |
| Soya | 4 | 5 | 2 | 5 | 16 | 5 |
| Wheat | 0 | 2 | 1 | 4 | 7 | 2 |
| Seafood | 1 | 0 | 0 | 0 | 1 | ~1 |
| Sesame Seed | 1 | 0 | 0 | 0 | 1 | ~1 |
| Multiple Allergens | 5 | 11 | 3 | 0 | 19 | 6 |
| Total recalls by year | 33 | 70 | 62 | 137 | 302 | 100 |
* percentages are estimates based on a review of CFIA's data
# increase could be due to improved method detection and targeted enforcement activity
Laboratory Work
59. The laboratories' role in allergen control is to contribute to the safety of Canada's food supply by analysing food samples for contaminants and to provide expert scientific advice to support the Agency's programs. The laboratories are also involved in validating and adapting methodologies for detecting allergens in foods. The development of appropriate detection and testing methodologies is a key factor in controlling allergens.
60. Without these methodologies, finding trace amounts of allergens in food products would be difficult. The CFIA laboratories that conduct tests on food allergens are located in Longueuil, Burnaby and Ottawa.
61. Health Canada (HC) and CFIA have had discussions on the need for closer CFIA/HC collaboration on detection and sensitivity of methods and the respective limitations of the science (i.e. detection ability, validation and threshold limits) and have taken proactive steps in addressing the issues at a research level. Progress in this area would facilitate the closure of some testing methodology gaps. Recently, Health Canada and CFIA researchers have been working successfully on method development that would permit more effective testing of the priority allergens. In particular, Health Canada has made progress on the detection of hazelnut, egg protein and seafood, and on evaluating the recent development of (consumer) individual test kits. These test kits have been demonstrated to be effective as a result of internal validation within the CFIA and are being used by designated Agency laboratories.
62. Health Canada and the CFIA continue to refine and evaluate methods in order to clear any technical problems prior to the transfer of the method from the research area to CFIA operations. Moreover, both organizations are moving towards having a system that would standardize the methodologies that are currently developed and evaluate those that are already available through the newly appointed Allergen Method Committee. There is an intention to create a compendium of methodologies that may gather all the internally developed methods and also provide guidelines for the evaluation and validation of other techniques. Another action that is being implemented for the long-term is the focus on alternative methodologies to Enzyme-Linked Immunosorbent Assay (ELISA) techniques in order to allow confirmation of the identity and level of allergen.
Infrastructure
63. The items that fall under infrastructure are training and program manuals as well as information management systems. All of these are important tools to support the Agency's efforts in the area of food allergen control activities. They contribute to the accessibility of information on food allergens within the CFIA, which in turn, helps staff administer their programs more effectively by taking this element into account. In this section, we discuss some observations that relate to these items.
Manuals
64. We examined CFIA manuals to determine what type of training material was available to staff as well as the type of information that was available about allergen controls in the program manuals.
65. The Agency has two training manuals that deal with allergen controls, namely, the Ontario Area Reference Guide, and the Food Allergies-2nd edition, November 2000. Since both manuals are essentially local area manuals and neither has been endorsed by National Headquarters, the Agency may wish to review the content to ensure the information is equivalent. The CFIA also uses the Workshop Manual on Food Safety and Recall to train personnel involved in investigation activities that may lead to recalling products containing undeclared food allergens.
66. We reviewed several of the Agency's program manuals to determine what references they contained to allergens as hazards, and the extent to which they addressed allergen controls. These manuals include: the Processed Products Establishment Inspection Manual, the Dairy Products Inspection Manual, the Food Safety Enhancement Program Manual, the Meat Hygiene Manual and the Fish, Seafood and Production Inspection and User Manuals. We did not review the Processed Products Inspection Manual as it was dated June 1993, prior to the formation of CFIA.
67. Some of these CFIA manuals contain useful information on allergens, allergen controls and inspection procedures. As the Agency reviews its various manuals, it could consider the type of references to allergen controls that may be required within each of these, depending on the risks that exist. The updating of the allergen specific information in the program manuals would ensure that staff have the necessary information to administer the programs and that this information is consistent across programs.
Database Management Systems
68. We identified a variety of database management systems within the CFIA that contain information relevant to allergen controls. These systems track fraud and labelling issues, information on investigations/inspections, lab samples and resource use. Two other systems, one for tracking enforcement activities and the other to follow up on the results of inspections, were being worked on at the time of our review. The inter-linkages among the existing systems were missing which could provide better cross referencing and accessibility of data amongst the various CFIA programs. This would enhance knowledge management within the CFIA. The CFIA advises that it is updating its electronic database capacity and determining where inter-linkages are required.
Conclusion
69. It is evident that food allergens can present a significant health hazard to the sensitive consumer. From 1997 to 2001, about 58 percent of the allergen-related food recalls that CFIA was involved in were at the Class 1 level. A Class 1 recall occurs when there is a reasonable probability that the use of, or exposure to an allergen will cause serious health consequences or death. As well, the percentage of Class 1 recalls attributed to allergens was higher over the past four years in comparison to the other types of recalls (microbiological, extraneous and chemical) as indicated by Exhibit 3 (about 70 percent on average).
70. In summary, the Canadian Food Inspection Agency is involved in a series of activities to deal with the potentially harmful effects of undeclared food allergens. Through the work of the Science Committees, including representatives from Health Canada, the Agency is adopting a horizontal science-based approach across all sectors and food divisions to deal with the risks posed by known food allergens. The Agency is also involved in activities to inform and educate the public and to support and promote industry compliance. Other activities support food safety labelling, laboratory analyses as well as enforcement and compliance efforts. Through the course of our information gathering, we also identified some practices within the Agency's main food safety activity areas linked to allergen controls, that we felt merited a review and potential update.
71. For instance, trend analyses could provide information to help determine the identity and level of allergen(s) and associated levels of risk. This would assist in the development of appropriate strategies to reduce the number of food allergen recalls. The results of any industry sector national assessment reports could be used as benchmarks to both measure the changes in the level of compliance within the imported food sector as well as to assess the compliance of other relevant sectors. This would provide information to the Agency on where improvements were made, as well as the gaps in food allergen awareness that still exist in the industry sectors. Enforcement activities could be applied effectively to the area of undeclared food allergens to eliminate repeat offences such as those involved in imported European chocolate.
72. Finally, we noted that the development of closer links among allergy associations, industry associations, government departments and agencies would benefit the allergic consumer by strengthening the overall allergy network and providing increased opportunities for collaboration in such areas as the development of standards or guidelines that could be employed for precautionary labelling. The subject of precautionary labelling requires further exploration by Health Canada in collaboration with the CFIA.
73. We believe that reviewing and updating these practices would have positive long-term outcomes in terms of reducing risks to the public from undeclared food allergens and improving the health of Canadians.
CFIA Management Response
74. The CFIA has reviewed the paper on "The Canadian Food Inspection Agency Activities Related to Food Allergen Controls" and is pleased to provide the following response.
75. Health Canada's (HC) Bureau of Food Safety Assessment has provided information regarding the Canadian Food Inspection Agency's (CFIA) activities related to food allergen controls. The paper presents information gathered on the CFIA's allergen control activities that took place between April 1997 and May 2001. This information, however, does not cover the activities of the Agency's recently reviewed and redesigned Bureau of Food Safety and Consumer Protection (BFSCP). The activities of this Bureau include protecting consumers from health hazards (including allergens) associated with food products, and assisting consumers in protecting themselves from food-related health hazards. A full assessment was not feasible at the time of the evaluation due to this redesign.
76. Although a full food safety assessment was not conducted, the paper does outline areas for consideration such as utilizing trend analyses on a regular basis in order to understand the underlying reasons for allergen recalls, and applying more effective compliance and enforcement procedures with respect to repeat offenders. The Agency is in full agreement with these observations and has taken actions to address both of these issues. For example, trend analysis is being used as input for the development of operational strategies and work plans, and the Agency is developing enhanced compliance and enforcement procedures aimed at decreasing the number of repeat violations.
77. The Agency looks forward to continued work with Health Canada on future food safety assessments.
About this Paper
78. The majority of this information-gathering exercise involved interviewing CFIA staff at their Headquarters office and in several regions. We obtained information from the Office of Food Safety and Recall to understand the nature of recalls related to allergens and how the Agency collects and uses this data. As we did not have direct access to the CFIA Issues Management System database where the recall data are stored, we were not able to do any analysis of the root causes of the recalls (i.e. such as mislabelling, cross-contamination etc.).
79. Key personnel in the Dairy Program and the Processed Products Program provided information on the controls that the Agency's program staff use. We also obtained information from the Bureau of Food Safety and Consumer Protection on their Fair Labelling Practices Program and their Food Safety Investigation Program. As well, we reviewed the relevant program and training manuals and legislation.
80. We did not interview staff in the other registered programs relating to meat, eggs or fish because of the relatively few allergen-related recalls in these areas. However, we did review their manuals and related legislation. We also interviewed program and operations staff in two CFIA area offices in order to better understand their roles and responsibilities in dealing with undeclared allergens in food products. We interviewed Health Canada staff and CFIA laboratory staff in order to understand the methodologies that they are using and those being developed.
81. CFIA allergen technical specialists were interviewed in order to understand their roles and responsibilities with respect to allergen control activities. We also gathered information on compliance and enforcement activities through interviews with key staff at the corporate and operations levels and reviewed policy documents in this area.
82. We interviewed representatives from two allergy associations to obtain their views on controls that were required to protect the affected consumer. We also met with one industry representative from the Food and Consumer Products Manufacturers of Canada in order to obtain an industry perspective on food allergen controls. In order to gain a perspective of the major allergen control activities within the United States, we reviewed the literature and met with a representative of the U.S. Food and Drug Administration who was responsible for food allergen control issues.
Project Team
Senior Project Manager Irene Roberts
Food Safety Auditor Shirley Chalouh
Food Safety Auditor Lucien Comeau
Food SafetyAuditor Michel Cloutier
Appendix 1
Activity #1. Develop Science-based Strategies and Work Plans to Deal with Risks
|
Linked to these outputs:
|
Activity #2. Establish/Align Domestic and International Legal and Operating Mechanisms
|
Linked to these outputs:
|
Activity #3. Inform the Public and Foster/Support Industry Co-operation
|
Linked to these outputs:
|
Activity #4. Assess Food Safety / Labelling Compliance Efforts
|
Linked to these outputs:
|
Activity # 5. Identify/Respond to Threats to Food Safety
|
Linked to these outputs:
|