Directory of supplemented food caution identifier specifications
Download the alternative format
(PDF format, 107 KB, 7 pages)
Organization: Health Canada
Supersedes: 2022-07-20
Published: 2024-07-17
Overview
This document contains tables that outline formatting specifications of the variations of the supplemented food caution identifier that are acceptable for use on prepackaged products. It also:
- identifies key supplemented food caution identifier features
- sets out the layout of information presented as well as required graphic standards for each supplemented food caution identifier variation.
This document is incorporated by reference in the Food and Drug Regulations.
For graphic illustrations of the variations of the supplemented food caution identifier, please refer to the Compendium of templates for supplemented food facts tables, supplemented food caution identifiers and lists of cautionary statements.
Who this document is for
- Food industry
- Other interested stakeholders
In this document
- Reference diagrams
- Table 1: Unilingual standard format
- Table 2: Bilingual standard format
- Table 3: Bilingual compact format
Reference diagrams
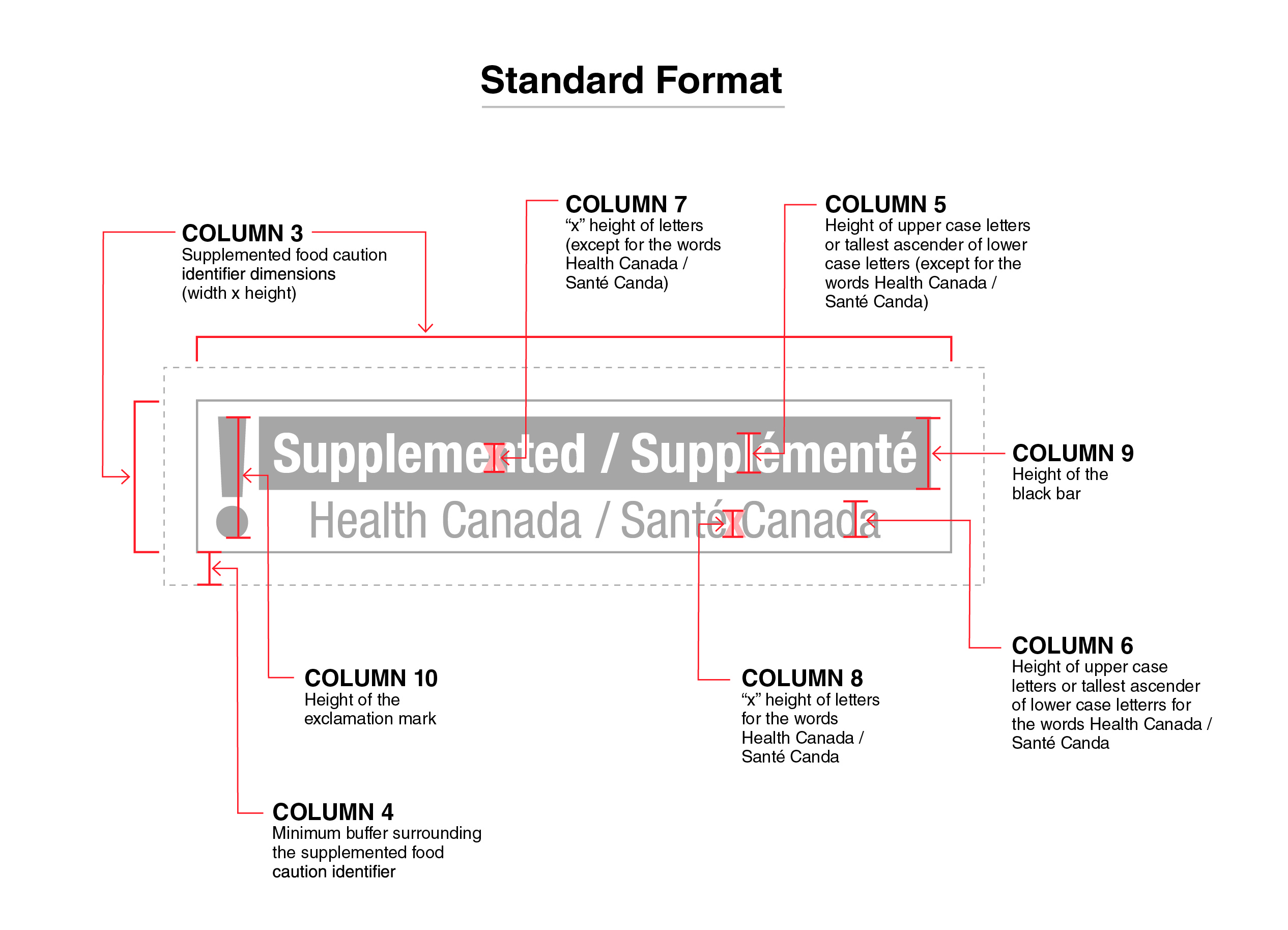
Figure 1 - Text Description
This figure shows the standard format of the supplemented food caution identifier for the principal display panel of prepackaged products. This identifier is bilingual, with the English text shown first, followed by the French text.
There is a white rectangular box outlined by a thin black line. Centred vertically on the left side of the box is a black exclamation mark. To the right of the exclamation mark is a horizontal bar. There is a small amount of white space between the exclamation mark and the bar as well as between the end of the bar and the thin black line that outlines the box. The bar is black and contains the word "Supplemented" followed by a forward slash and the word "Supplémenté", with both words in white, bold, lower case letters, except that the first letter of each word is in upper case. Centred below the black bar are the words "Health Canada" followed by a forward slash and the words "Santé Canada", with all words in black lower case letters, except that the first letter of each word is in upper case.
All elements of the supplemented food caution identifier are greyed out and there is a dotted rectangular box around the identifier. There is text shown around the outside of the supplemented food caution identifier with red arrows pointing to the identifier specifications. This is described below.
Starting at the top left of the identifier, there are two sets of arrows pointing to square brackets representing the width and height of the identifier followed by the words "Column 3 Supplemented food caution identifier dimensions" open parenthesis "width" by "height" closed parenthesis.
Centered above the identifier, there is an arrow pointing to the letter x placed over the word "Supplemented" followed by the words "Column 7" open quotation mark x closed quotation mark "height of letters" open parenthesis "except for the words Health Canada" forward slash "Santé Canada" closed parenthesis.
At the top right of the identifier there is an arrow pointing to the letter l in the word "Supplémenté" followed by the words "Column 5 Height of upper case letters or tallest ascender of lower case letters" open parenthesis "except for the words Health Canada" forward slash "Santé Canada" closed parenthesis.
To the right of the identifier, there is an arrow pointing to a black bar followed by the words "Column 9 Height of the black bar".
At the bottom left of the identifier, there is an arrow pointing to the space between the identifier and the dotted rectangular box followed by the words "Column 4 Minimum buffer surrounding the supplemented food caution identifier".
Above it, there is another arrow pointing to the exclamation mark within the identifier followed by the words "Column 10 Height of the exclamation mark".
Towards the right, at the bottom of the identifier, there is an arrow pointing to the letter x between the word "Santé" and the word "Canada" followed by the words "Column 8" open quotation mark x closed quotation mark "height of letters for the words Health Canada" forward slash "Santé Canada".
At the bottom right of the identifier, there is an arrow pointing to the letter d in the word "Canada" followed by the words "Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada" forward slash "Santé Canada".
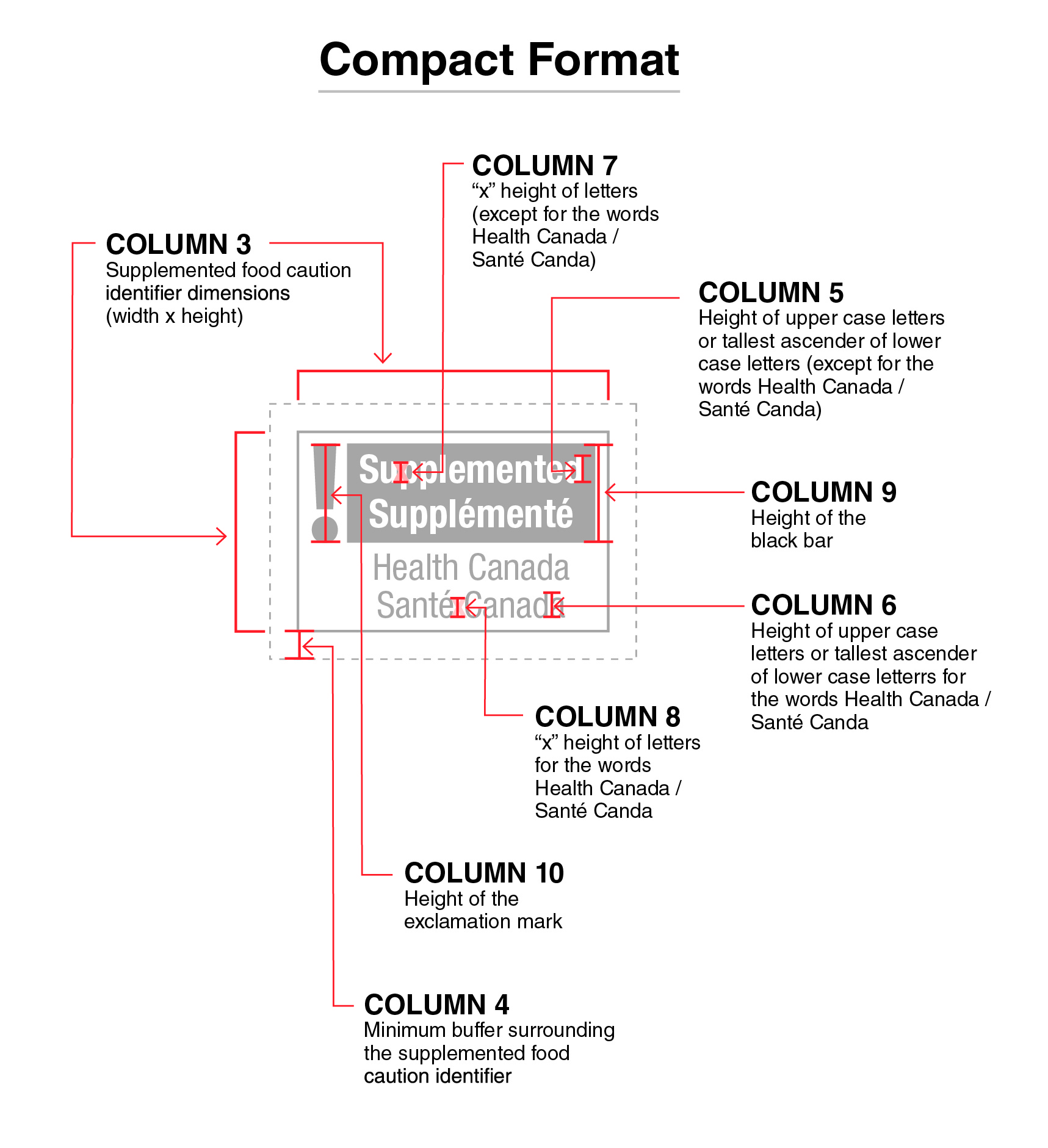
Figure 2 - Text Description
This figure shows the compact format of the supplemented food caution identifier for the principal display panel of prepackaged products. This identifier is bilingual, with the English text shown first, followed by the French text.
There is a white rectangular box outlined by a thin black line. At the top left of the box is a black exclamation mark. To the right of the exclamation mark is a horizontal bar. There is a small amount of white space between the exclamation mark and the bar as well as between the end of the bar and the thin black line that outlines the box. The bar is black and contains two lines of text in white, bold, lower case letters, except that the first letter of each word is in upper case. On the first line is the word "Supplemented" and on the second line is the word "Supplémenté". Centred below the black bar are two lines of text in black lower case letters, except that the first letter of each word is in upper case. On the first line are the words "Health Canada" and on the second line are the words "Santé Canada".
All elements of the supplemented food caution identifier are greyed out and there is a dotted rectangular box around the identifier. There is text shown around the outside of the identifier with red arrows pointing to the identifier specifications. This is described below.
Starting at the top left of the identifier, there are two sets of arrows pointing to square brackets representing the width and height of the identifier followed by the words "Column 3 Supplemented food caution identifier dimensions" open parenthesis "width" by "height" closed parenthesis.
Centered above the identifier, there is an arrow pointing to the letter x placed over the word "Supplemented" followed by the words "Column 7" open quotation mark x closed quotation mark "height of letters" open parenthesis "except for the words Health Canada" forward slash "Santé Canada" closed parenthesis.
Over the top right of the identifier there is an arrow pointing to the letter d in the word "Supplemented" followed by the words "Column 5 Height of the upper case letters or tallest ascender of lower case letters" open parenthesis "except for the words Health Canada" forward slash "Santé Canada" closed parenthesis.
To the right of the identifier, there is an arrow pointing to a black bar followed by the words "Column 9 Height of the black bar".
At the bottom left of the identifier, there is an arrow pointing to the space between the identifier and the dotted rectangular box followed by the words "Column 4 Minimum buffer surrounding the supplemented food caution identifier".
Above it, there is another arrow pointing to the exclamation mark within the identifier followed by the words "Column 10 Height of the exclamation mark".
Towards the right, at the bottom of the identifier, there is an arrow pointing to the letter x between the word "Santé" and the word "Canada" followed by the words "Column 8" open quotation mark x closed quotation mark "height of letters of the words Health Canada" forward slash "Santé Canada".
At the bottom right of the identifier, there is an arrow pointing to the letter d in the word "Canada" followed by the words "Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada" forward slash "Santé Canada".
Table 1: Unilingual Standard Format
Item |
Column 1 Range of principal display surface |
Column 2 Supplemented food caution identifier in Schedule K.2 of the Food and Drug Regulations |
Column 3 Supplemented food caution identifier dimensions (width x height) |
Column 4 Minimum buffer surrounding the supplemented food caution identifierFootnote a |
Column 5 Height of upper case letters or tallest ascender of lower case letters (except for the words Health Canada / Santé Canada) |
Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada / Santé Canada |
Column 7 "x" height of letters (except for the words Health Canada / Santé Canada) |
Column 8 "x" height of letters for the words Health Canada / Santé Canada |
Column 9 Height of the black bar |
Column 10 Height of the exclamation mark |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | > 600 cm² | 1(ES) and 1(FS) | 4.42 cm x 1.61 cm | 3.0 mm | 4.2 mm | 3.8 mm | 3.0 mm | 2.6 mm | 7.6 mm | 12.7 mm |
| 2 | > 450 cm² to ≤ 600 cm² | 2(ES) and 2(FS) | 3.79 cm x 1.38 cm | 2.7 mm | 3.7 mm | 3.1 mm | 2.7 mm | 2.3 mm | 6.5 mm | 10.9 mm |
| 3 | > 250 cm² to ≤ 450 cm² | 3(ES) and 3(FS) | 3.10 cm x 1.13 cm | 2.2 mm | 3.0 mm | 2.5 mm | 2.2 mm | 1.8 mm | 5.4 mm | 8.9 mm |
| 4 | > 100 cm² to ≤ 250 cm² | 4(ES) and 4(FS) | 2.50 cm x 0.92 cm | 1.7 mm | 2.4 mm | 2.0 mm | 1.7 mm | 1.4 mm | 4.3 mm | 7.2 mm |
| 5 | > 30 cm² to ≤ 100 cm² | 5(ES) and 5(FS) | 1.87 cm x 0.69 cm | 1.3 mm | 1.8 mm | 1.6 mm | 1.3 mm | 1.1 mm | 3.2 mm | 5.4 mm |
| 6 | ≤ 30 cm² | 6(ES) and 6(FS) | 1.65 cm x 0.65 cm | 1.1 mm | 1.6 mm | 1.6 mm | 1.1 mm | 1.1 mm | 3.0 mm | 5.1 mm |
ES: English standard
|
||||||||||
Table 2: Bilingual Standard Format
Item |
Column 1 Range of principal display surface |
Column 2 Supplemented food caution identifier in Schedule K.2 of the Food and Drug Regulations |
Column 3 Supplemented food caution identifier dimensions (width x height) |
Column 4 Minimum buffer surrounding the supplemented food caution identifierFootnote a |
Column 5 Height of upper case letters or tallest ascender of lower case letters (except for the words Health Canada / Santé Canada) |
Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada / Santé Canada |
Column 7 "x" height of letters (except for the words Health Canada / Santé Canada) |
Column 8 "x" height of letters for the words Health Canada / Santé Canada |
Column 9 Height of the black bar |
Column 10 Height of the exclamation mark |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | > 600 cm² | 1(BS) | 6.29 cm x 1.28 cm | 2.5 mm | 3.4 mm | 3.2 mm | 2.5 mm | 2.3 mm | 6.1 mm | 10.1 mm |
| 2 | > 450 cm² to ≤ 600 cm² | 2(BS) | 5.40 cm x 1.10 cm | 2.1 mm | 2.9 mm | 2.7 mm | 2.1 mm | 1.9 mm | 5.2 mm | 8.7 mm |
| 3 | > 250 cm² to ≤ 450 cm² | 3(BS) | 4.50 cm x 0.93 cm | 1.7 mm | 2.4 mm | 2.2 mm | 1.7 mm | 1.5 mm | 4.4 mm | 7.2 mm |
| 4 | > 100 cm² to ≤ 250 cm² | 4(BS) | 3.60 cm x 0.76 cm | 1.4 mm | 1.9 mm | 1.9 mm | 1.4 mm | 1.3 mm | 3.5 mm | 5.9 mm |
| 5 | ≤ 100 cm² | 5(BS) | 2.97 cm x 0.63 cm | 1.1 mm | 1.6 mm | 1.6 mm | 1.1 mm | 1.1 mm | 2.9 mm | 4.9 mm |
BS: Bilingual standard
|
||||||||||
Table 3: Bilingual Compact Format
Item |
Column 1 Range of principal display surface |
Column 2 Supplemented food caution identifier in Schedule K.2 of the Food and Drug Regulations |
Column 3 Supplemented food caution identifier dimensions (width x height) |
Column 4 Minimum buffer surrounding the supplemented food caution identifierFootnote a |
Column 5 Height of upper case letters or tallest ascender of lower case letters (except for the words Health Canada / Santé Canada) |
Column 6 Height of upper case letters or tallest ascender of lower case letters for the words Health Canada / Santé Canada |
Column 7 "x" height of letters (except for the words Health Canada / Santé Canada) |
Column 8 "x" height of letters for the words Health Canada / Santé Canada |
Column 9 Height of the black bar |
Column 10 Height of the exclamation mark |
|---|---|---|---|---|---|---|---|---|---|---|
| 3 | > 250 cm² to ≤ 450 cm² | 1(BC) | 2.17 cm x 1.40 cm | 1.5 mm | 2.0 mm | 1.9 mm | 1.5 mm | 1.4 mm | 6.9 mm | 6.9 mm |
| 4 | > 100 cm² to ≤ 250 cm² | 2(BC) | 1.75 cm x 1.14 cm | 1.2 mm | 1.6 mm | 1.6 mm | 1.2 mm | 1.1 mm | 5.6 mm | 5.6 mm |
| 5 | ≤ 100 cm² | 3(BC) | 1.66 cm x 1.09 cm | 1.1 mm | 1.6 mm | 1.6 mm | 1.1 mm | 1.1 mm | 5.3 mm | 5.3 mm |
BC: Bilingual compact
|
||||||||||
Related information
- About supplemented foods and their labels
- Supplemented foods: Regulations and compliance
- Guidance document for Supplemented foods regulations