Data on cannabis adverse reactions: 2018-2019 annual report

Download the alternative format
(PDF format, 800 KB, 22 pages)
Organization: Health Canada
Date published: December 2022
Cat.: H131-1E-PDF
ISBN: 2817-0555
Pub.: 220647
Adverse reactions associated with cannabis reported to Health Canada between October 17, 2018 and December 31, 2019.
Contents
- Key highlights
- 1.0 Introduction
- 2.0 Adverse reactions with cannabis
- 3.0 Clinical evaluation of serious and medically important cases
- 4.0 Note to readers
- 5.0 Reporting an adverse reaction involving a cannabis product
- 6.0 Contact us
Key highlights
- This is the first annual report pertaining to adverse reaction reports associated with legal cannabis products that were submitted to the Canada Vigilance Program and analysed by the Controlled Substances and Cannabis Branch within Health Canada.
- The purpose of this report is to provide an overview of the adverse reaction reports collected by Health Canada during the first year since the coming into force of the Cannabis Act and its Regulations, covering the reporting period of October 17, 2018 to December 31, 2019.
- Between October 17, 2018 and December 31, 2019, a total of 219 adverse reaction reports were reported to Health Canada's Canada Vigilance Database, associated with cannabis as a suspect ingredient. Of these, 151 adverse reaction cases were associated with legal cannabis products that were used for either medical or non-medical purposes.
- Of the 151 adverse reaction cases associated with legal cannabis products, 77 were reported as serious, with hospitalization as the most frequently reported reason for seriousness. An additional 11 non-serious cases were deemed to be medically important and were further reviewed.
- No trends were observed in the number or seriousness of cases month-over-month or on a cumulative basis during the reporting period. The majority of cases originated from consumers (76%), and were submitted to Health Canada by cannabis licence holders (62%). Just under a quarter (24%) of cases were reported by health care practitioners.
- The majority of cases involved young to middle aged adults (25% and 24% for those aged 18-44 years and 45-64 years, respectively); however, cases also frequently involved older adults (28% were 65 years of age and older). Cases more frequently involved females as compared to males (55% and 39%, respectively; 6% did not have sex reported).
- Most cases involved cannabis products for self-reported medical purposes (87%) and frequently involved cannabis oil products consumed orally (64%).
- The most frequently reported medical events were headache (n=23), nausea (n=19), hallucination (n=15), dizziness (n=13), anxiety (n=12), dyspnoea (n=11) and malaise (n=9). Other events of interest included drug interactions (n=8) and suspected product quality issues (n=8).
- One new important risk was identified during this period, of an interaction between orally ingested cannabidiol (CBD)-dominant cannabis oil products and the anti-coagulant drug warfarin, resulting in a potential increased risk of bleeding. This signal was assessed in a comprehensive case series and was published in Health Canada's InfoWatch Newsletter in October 2020.
- Other cases were associated with known or previously identified risks (that is, characterised in Health Canada's document Information for Health Care Professionals). These included: hypersensitivity or allergic reactions (for example, localised or systemic hypersensitivity reactions such as pruritus, urticaria, oedema; anaphylaxis), psychiatric reactions (for example, panic attack, anxiety, hallucination), neurologic reactions (for example, headache, light-headedness), and gastrointestinal reactions (for example, nausea, vomiting).
- Important potential risks observed during this reporting period but requiring further evidence and which will continue to be monitored include: cannabis-drug interactions; alterations in blood glucose levels; alterations in haematological parameters; and, elevations in liver enzymes.
- Adverse reactions involving inhalable cannabis extracts (for example, vaping products), particularly those suspected of being associated with vaping-associated lung illness (VALI) or other serious respiratory-related harms, continue to be monitored on a priority basis as well as cases which involve special populations (for example, older adults, pediatric populations).
1.0 Introduction
This report describes the findings of the domestic case reports of adverse reactions associated with cannabis products submitted to Health Canada's Canada Vigilance Program and analyzed by the Office of Cannabis Science and Surveillance of the Controlled Substances and Cannabis Branch. This work forms part of the Vigilance Framework for Cannabis that has been in place since the coming into force of the Cannabis Act (October 17, 2018).
This report summarizes all adverse reactions reported to Health Canada between October 17, 2018 and December 31, 2019, associated with a cannabis product as defined under the Cannabis Regulations, intended for human consumption:
A cannabis product means cannabis of only one of the classes set out in Schedule 4 to the Act — or a cannabis accessory that contains such cannabis — after it has been packaged and labelled for sale to a consumer at the retail level. It does not include:
- cannabis that is intended for an animal
- a cannabis accessory that contains cannabis that is intended for an animal
- health products containing cannabis or for use with cannabis
As outlined in Section 248 of the Cannabis Regulations, licence holders (LHs) who sell or distribute a cannabis product must within 15 days after becoming aware of a serious adverse reaction to the cannabis product, provide Health Canada with a detailed report containing all information in their possession that is associated with the use of the cannabis product by the individual who experienced the reaction. Under the Regulations:
- an adverse reaction is defined as "a noxious and unintended response to a cannabis product"
- a serious adverse reaction is defined as "a noxious and unintended response to a cannabis product that requires inpatient hospitalization or a prolongation of existing hospitalization, causes congenital malformation, results in persistent or significant disability or incapacity, is life-threatening or results in death"
Consumers, patients, health care practitioners (HCPs), medical cannabis clinics and other reporters, such as provincial and territorial authorized retailers, may submit adverse reaction reports with cannabis products on a voluntary basis to Health Canada. Reports may also be received from market authorization holders of licenced health products that submit adverse reaction reports for other suspect health products (such as prescription or non-prescription drugs, or natural health products) in which cannabis is also identified as co-suspect. Adverse reactions with cannabis may involve cannabis that is: (i) a cannabis product produced by a federal LH; (ii) cultivated in the homeFootnote 1; (iii) undefined (cannabis as a substance not otherwise specified); (iv) from illegal sources.
For the purposes of this report, case reports suspected of involving a legal cannabis product (that is, identifiable by product brand name and/or LH) are classified according to the intended use of the cannabis product(s) as described in the case report:
- Cannabis use for medical purposes includes case reports described as having a medical authorization document; or a reported medical or therapeutic purpose, without mention of a medical authorization document. This definition aligns with the definition of medical use within the Canadian Cannabis Survey. This is broader in scope than the regulatory definition of cannabis for medical purposes under the Cannabis Regulations; that is, a medical document from a HCP (physician, nurse practitioner) authorizing the use of cannabis for medical purposes.
- Cannabis use for non-medical purposes: If there is no reported medical or therapeutic indication or reason for use provided in the case report, minimal details or the intended use is for non-medical purposes, the case report is classified as 'non-medical use of cannabis'.
Adverse reaction case reports are collected and housed in the Canada Vigilance Database. The majority of adverse reactions reported to Health Canada are reported spontaneously (report type = spontaneous) from consumers, patients, HCPs or from LHs (referred to as "market authorization holder" in the Canada Vigilance Database). However, reports may also originate from studies (report type = study), including real-world observational studies, human studies involving cannabis that fall outside of the definition of a clinical trial, or other organized data collection systems (for example, surveys of patients or HCPs).
Health Canada conducts near-time monitoring, detection, assessment and associated activities for cases of adverse reactions involving cannabis products as part of the Vigilance Framework for Cannabis. Health Canada also monitors cases involving cannabis as a substance for broader issues of public health importance such as vaping-associated lung illness (VALI), cases involving pediatric populations and other potential emerging safety issues.
This report does not cover adverse reactions associated with health products, including drugs containing cannabis, which are regulated under the Food and Drugs Act and its Regulations. A summary of adverse reactions associated with other health products and medical devices received by Canada Vigilance in 2019 are described in the following report: Adverse reactions, medical device incidents and health product recalls in Canada: 2019 summary report.
The purpose of this report is to provide a descriptive analysis of the types of adverse reactions involving cannabis products under the Cannabis Act and the Cannabis Regulations, submitted to Health Canada between October 17, 2018 and December 31, 2019. This reporting period is reflective of the first year of legalization and regulation and the transitional period into the second year of regulation (with amendments to the Cannabis Regulations), covering the period of October 17, 2019 to December 31, 2019Footnote 2. During this period, only initial classes of cannabis products (dried cannabis, fresh cannabis, and cannabis oil) were available for sale to adult Canadians through provincial and territorial-authorized retailers, or from LHs directly for medical purposes. As such, no observations about the newer classes of cannabis products (cannabis extracts, edible cannabis or cannabis topicals) were made during this reporting period as such products were not available for legal purchase.
1.1 Considerations
Certain caveats should be considered when interpreting the adverse reaction data in this report:
- Adverse reactions are generally spontaneously reported to Health Canada and cannot be used to determine the incidence or prevalence of adverse reactions to cannabis in the general population.
- Serious adverse reactions have a greater representation in this dataset as LHs have a regulatory obligation to report these to Health Canada under the Cannabis Regulations. The submission of non-serious adverse reactions by LHs to Health Canada as individual case reports is voluntaryFootnote 3; therefore, these cases are likely underreported and underrepresented in this dataset.
- Reporting of adverse reactions by consumers, HCPs, medical cannabis clinics and retailers is voluntary. Therefore, both serious and non-serious cases from these sources are likely underreported.
- Individuals experiencing serious outcomes or using cannabis products for medical purposes may be more motivated to report or seek out medical attention.
- Several factors may influence the number of cases reported to Health Canada such as: consumer/patient medical history; reason for cannabis use; length of time a product is on the market; media coverage; awareness, motivation and ability to report; and nature of reports (spontaneous reports versus studies or other organized data-collection systems).
- This report includes information on cannabis for medical and non-medical purposes; however, the number of cases elsewhere (for example, online databases, other reports) may not directly align with what is presented in this current annual summary report due to different dates of extraction from the Canada Vigilance Database.
2.0 Adverse reactions with cannabis
2.1 Adverse reaction cases associated with cannabis as a substance
Between October 17, 2018 and December 31, 2019, a total of 219 adverse reaction reports were submitted to Health Canada involving cannabis as a substance.
The majority of these cases involved legal cannabis products (68%, n=151), and most were classified as being used for self-reported medical purposes (88%, n=132) relative to non-medical purposes (13%, n=19). The remaining cases involved cannabis of an undetermined or illegal nature (for example, described as 'marijuana' without additional details; often co-suspected with multiple other co-suspected substances).
Case reports involving cannabis products for medical and non-medical purposes from the legal marketplace form the basis for the remaining portions of this Annual Report.
2.2 Adverse reaction cases associated with legal cannabis products for medical or non-medical purposes
Figure 1: Cases by reporting month and seriousness
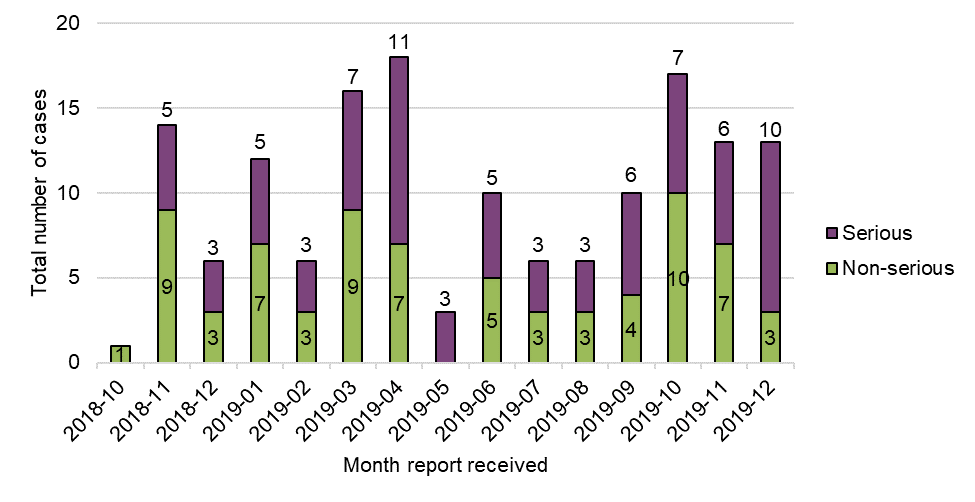
Figure 1 - Text Description
| Month report received | Total number of cases by seriousness | ||
|---|---|---|---|
| Non-serious | Serious | Grand total | |
| 2018-10 | 1 | 0 | 1 |
| 2018-11 | 9 | 5 | 14 |
| 2018-12 | 3 | 3 | 6 |
| 2019-01 | 7 | 5 | 12 |
| 2019-02 | 3 | 3 | 6 |
| 2019-03 | 9 | 7 | 16 |
| 2019-04 | 7 | 11 | 18 |
| 2019-05 | 0 | 3 | 3 |
| 2019-06 | 5 | 5 | 10 |
| 2019-07 | 3 | 3 | 6 |
| 2019-08 | 3 | 3 | 6 |
| 2019-09 | 4 | 6 | 10 |
| 2019-10 | 10 | 7 | 17 |
| 2019-11 | 7 | 6 | 13 |
| 2019-12 | 3 | 10 | 13 |
| Grand total | 74 | 77 | 151 |
Caveat(s):
- Cases are presented according to the initial date of receipt by the Canada Vigilance Program. The actual date of the adverse reaction may not align with the month that the case was received (lag time between event and reporting).
- Seriousness is based on the initial report and may be subject to change if additional information is submitted to Health Canada.
Overall, no temporal trends were observed in the total number of adverse reaction cases with legal cannabis products, including number of serious cases and non-serious cases on a cumulative basis (n=77 serious, n=74 non-serious), or month-over-month. The greatest number of cases (combined serious and non-serious) were reported in April 2019 (n=18), October 2019 (n=17) and March 2019 (n=16), while the lowest number of cases were reported in May 2019 (n=3) (excluding October 2018 as an incomplete month). The greatest number of serious cases was reported in April 2019 (n=11) and December 2019 (n=10).
Of note, it is not mandatory for LHs to submit non-serious adverse reaction reports to Health Canada as individual case reports. Therefore, it is possible that there are additional non-serious adverse reactions that have not been reported to Health Canada. However, as recommended in Health Canada's Cannabis adverse reaction reporting guide for licence holders, case reports that are medically important, as well as any clusters of cases of interest regardless of seriousness, are still encouraged to be submitted to Health Canada. In addition, all adverse reactions, including those that are serious and non-serious, must be included in an annual summary report that LHs have to prepare and maintain on an annual basis (calendar year) under the Cannabis Regulations.
Figure 2: Cases by reason for seriousness in serious cases
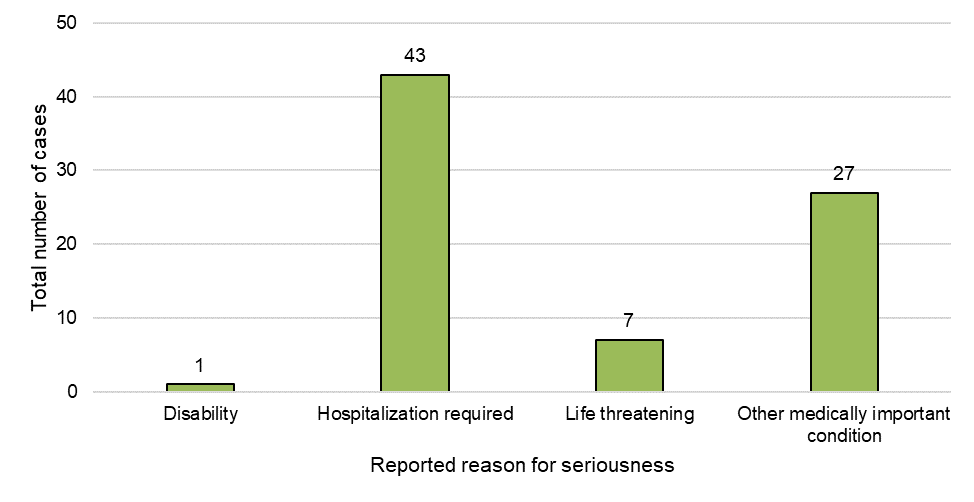
Figure 2 - Text Description
| Reported reason for seriousness | Total number of cases |
|---|---|
| Disability | 1 |
| Hospitalisation required | 43 |
| Life threatening | 7 |
| Other medically important condition | 27 |
| Grand total | 78 |
Caveat(s):
- Each serious case may have more than one reason for seriousness as multiple reasons can be selected by the reporter.
Of the 77 cases reported as serious to Health Canada, a total of 78 responses for seriousness, spanning four categories, were represented (Figure 2). The most frequently reported reason for seriousness was 'hospitalization required' (55%, n=43) followed by 'other medically important condition' (35%, n=27). The latter category may involve an emergency department visit, outpatient visit with a doctor or HCP or may require a medical intervention to prevent one of the outcomes listed under the serious definition in the Cannabis Regulations. No fatal cases were reported with cannabis products for medical or non-medical purposes; however, seven cases reported 'life threatening events', and one reported disability.
An additional 11 cases that were reported as non-serious were considered medically important by Health Canada during case screening and were further reviewed.
Figure 3: Reports by initial reporter type and report source
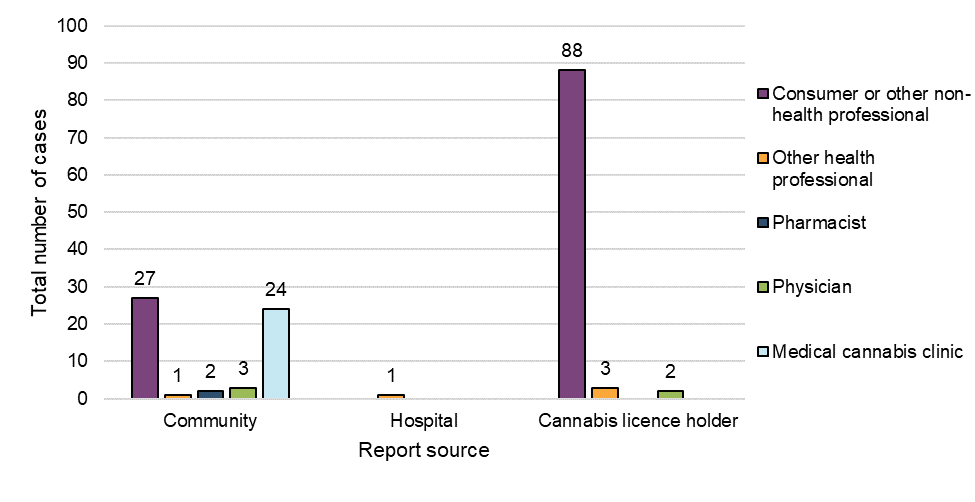
Figure 3 - Text Description
| Report source | Initial reporter type | |||||
|---|---|---|---|---|---|---|
| Consumer or other non-health professional | Other health professional | Pharmacist | Physician | Medical cannabis clinic | Grand total | |
| Community | 27 | 1 | 2 | 3 | 24 | 57 |
| Hospital | 0 | 1 | 0 | 0 | 0 | 1 |
| Cannabis licence holder | 88 | 3 | 0 | 2 | 0 | 93 |
| Grand total | 115 | 5 | 2 | 5 | 24 | 151 |
Caveat(s):
- Report source is reflective of where the report was submitted from to Health Canada (community, hospital, or cannabis licence holder [referred to as "market authorization holder" in the Canada Vigilance Database]).
- For purposes of this figure, each report source is further subdivided according to initial reporter type (consumer, HCPs [physician, pharmacist, other], medical cannabis clinics).
- Reports from HCP sources are considered 'medically confirmed' as per international guidelinesFootnote 4; therefore, are distinct from consumer reports.
- Reports from medical cannabis clinics are considered 'medically confirmed' as they fall under the scope of a medical practice and medical oversight by clinics who have patients using cannabis for medical purposes and who may experience an adverse reaction.
The majority of the adverse reaction cases involving cannabis products used for medical or non-medical purposes were submitted to Health Canada by LHs (62%, n=93), followed by community reporters (38%, n=57; reports submitted directly to Health Canada by voluntary reporters). Only one case originated from a hospital.
Most adverse reaction cases were initially reported by patients or consumers (76%, n=115), whereas 24% (n=36) were reported by HCPs, the majority being from medical cannabis clinics (n=24). Health care practitioners appeared more inclined to report directly to Health Canada, whereas consumers appeared more inclined to report to LHs.
2.3 Demographics
Figure 4: Cases by age group
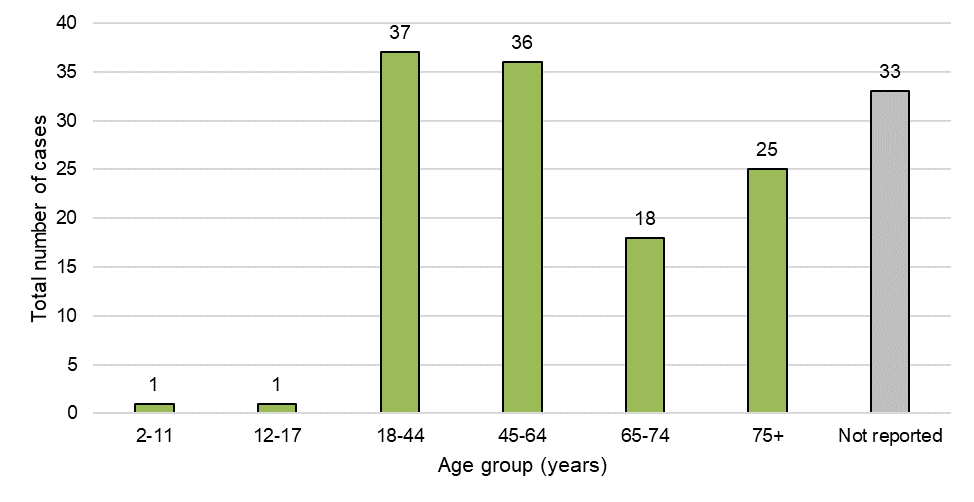
Figure 4 - Text Description
| Age group (years) | Total number of cases |
|---|---|
| 2-11 | 1 |
| 12-17 | 1 |
| 18-44 | 37 |
| 45-64 | 36 |
| 65-74 | 18 |
| 75+ | 25 |
| Not reported | 33 |
| Grand total | 151 |
Caveat(s):
- In cases where the year of birth and the date of reaction are listed in the report, then the age is calculated.
- In cases where only the year of birth is listed without a date of reaction, the date the report was submitted is used to calculate the age.
- Age was classified using the World Health Organization's Vigilyze database (Vigibase) age groupings.
Twenty five percent (25%) of adverse reaction cases involved adults aged 18-44 years (n=37) and 24% involved adults aged 45-64 years (n=36). A large proportion of cases also involved older adults (65-74 years: 12%; 75+ years: 17%). Of note, a large proportion of cases (22%, n=33) did not report age. Cases involving pediatric patients (<18 years; n=2) only involved cannabis products used for medical purposes.
Figure 5: Cases by sex
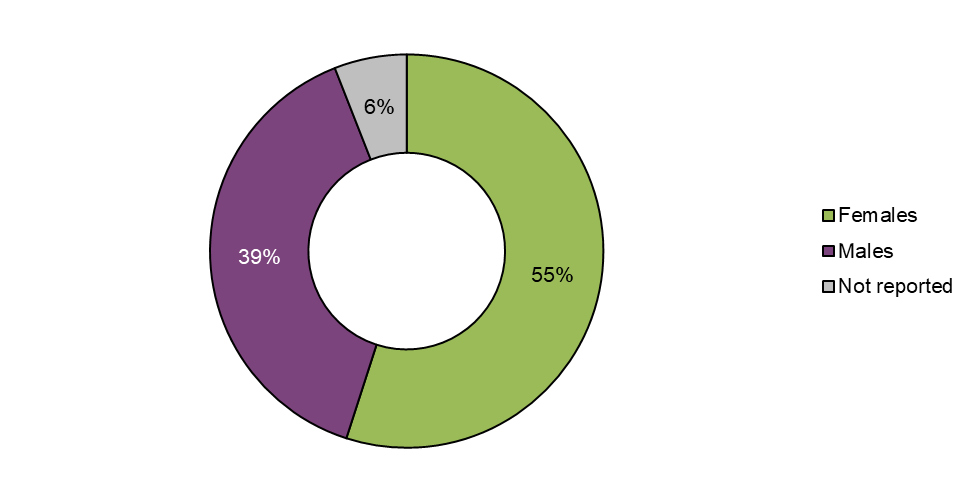
Figure 5 - Text Description
| Sex | Total number of cases | Proportion (%) of total |
|---|---|---|
| Females | 83 | 55 |
| Males | 59 | 39 |
| Not reported | 9 | 6 |
| Grand total | 151 | 100 |
Just over half of the cases involved females (55% females, n=83; 39% males, n=59). In 6% (n=9) of cases sex was not reported.
2.4 Suspected cannabis products
Overall, the majority of adverse reaction cases reported cannabis as a sole suspect product, meaning that no other products were reported as co-suspects (96%, n=145). Products may include prescription or non-prescription drugs, natural health products or other types of health products regulated under the Food and Drugs Act. However, one or more suspect cannabis product may exist within a case. In fact, 26 cases reported use of multiple cannabis products (range: two to seven products), with the majority reporting two products (85%, n=22). Notably, all suspect products are based on the suspicion of the reporter and the involvement of other products or factors cannot always be ruled out. These considerations form part of the clinical evaluation in the clinical summary portion of this report.
Cannabis oil products were more frequently reported in serious cases (83%, n=64), whereas dried cannabis products were more frequently reported in non-serious cases (57%, n=42).
Figure 6: Cases by route of administration
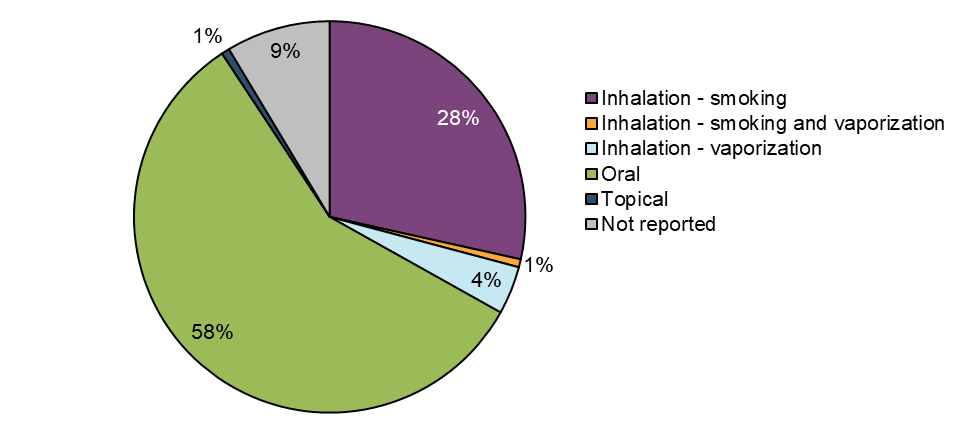
Figure 6 - Text Description
| Route of administration | Total number of cases | Proportion (%) of total |
|---|---|---|
| Inhalation - smoking | 43 | 28 |
| Inhalation - smoking- vaporization | 1 | 1 |
| Inhalation – vaporization | 6 | 4 |
| Oral | 87 | 58 |
| Topical | 1 | 1 |
| Not reported | 13 | 9 |
| Grand total | 151 | 100 |
Caveat(s):
- This figure describes route of administration of suspect cannabis products in cases, which is coded separately from dosage form (that is, a cannabis oil product may be ingested, applied topically, etc.).
- Oral administration refers to consuming cannabis by mouth and may involve ingestion, buccal administration, sublingual administration, etc., meaning that absorption may occur at the level of the gastrointestinal tract as well directly into the bloodstream through oral mucosal tissue.
- Inhalation refers to consuming cannabis through the respiratory tract and may involve smoking or vaporization.
- Cases may involve more than one suspect cannabis product; therefore, multiple routes of administration may appear for a single case.
Suspect cannabis products involved in case reports were most frequently consumed via the oral route (58%, n=87). The inhalation of dried cannabis via smoking was the second most common route of administration among cases (28%, n=43). A small number of cases involved vaporization of dried cannabis (4%, n=6). Of note, cannabis extracts, including those intended for inhalation, were not legally available in the marketplace during this reporting period (ending December 31, 2019) and therefore are not captured in these data.
Nearly all adverse reaction cases were associated with cannabis products reported as being used for medical purposes (87%, n=132), relative to use for non-medical purposes (13%, n=19). Just over half of the cases involving cannabis products used for medical purposes were serious (53%, n=70 serious; 47%, n=62 non-serious). In contrast, most cases involving cannabis products used for non-medical purposes were reported as non-serious (n=37%, n=7 serious; 63%, n=12 non-serious).
Figure 7: Cases by product class and reason for use
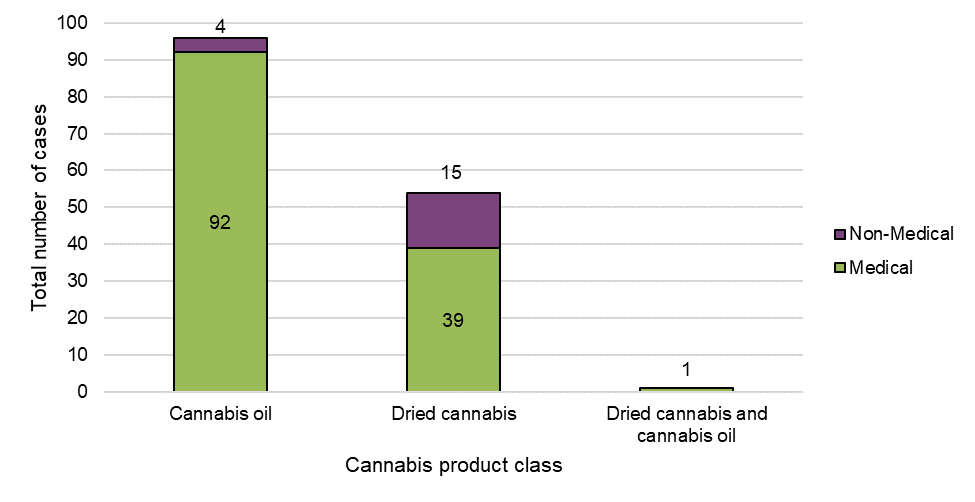
Figure 7 - Text Description
| Cannabis product class | Reason for use | ||
|---|---|---|---|
| Medical | Non-medical | Grand total | |
| Cannabis oil | 92 | 4 | 96 |
| Dried cannabis | 39 | 15 | 54 |
| Dried cannabis and cannabis oil | 1 | 0 | 1 |
| Grand total | 132 | 19 | 151 |
Cannabis oil was the most frequent class of suspect cannabis product reported in adverse reaction cases overall (64%, n=96), followed by dried cannabis (36%, n=54). No cases were reported with fresh cannabis. One single case involved both dried cannabis and cannabis oil; otherwise, cases involved either one or more suspect cannabis oil products, or dried cannabis products.
As outlined above, cases involving cannabis oil products were nearly exclusively reported as being used for medical purposes. Suspect cannabis oil products, as well as use for medical purposes, often co-existed in serious cases suggesting a potential interaction between these factors.
Most cannabis oil products in the adverse reaction cases were 'CBD-dominant' or 'CBD-leaning', whereas dried cannabis products were typically 'THC-dominant'Footnote 5. Moreover, older adults (65 years and older) were frequently involved in adverse reaction cases with cannabis oil products, in contrast to younger adults where both dried cannabis and cannabis oil products were comparably involved (data not shown).
Figure 8: Breakdown of individual medical events by system organ class (SOC)
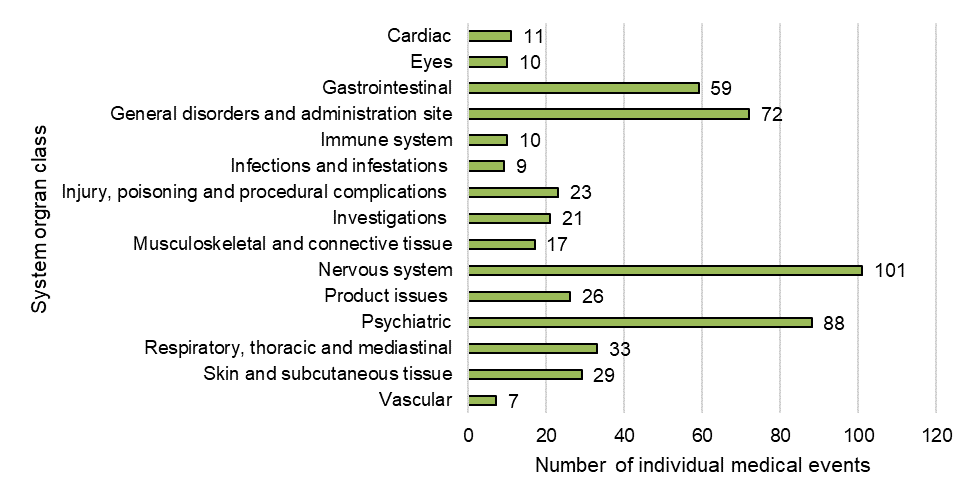
Figure 8 - Text Description
| System organ class | Number of individual medical events |
|---|---|
| Cardiac disorders | 11 |
| Eye disorders | 10 |
| Gastrointestinal disorders | 59 |
| General disorders and administration site conditions | 72 |
| Immune system disorders | 10 |
| Infections and infestations | 9 |
| Injury, poisoning and procedural complications | 23 |
| Investigations | 21 |
| Musculoskeletal and connective tissue disorders | 17 |
| Nervous system disorders | 101 |
| Product issues | 26 |
| Psychiatric disorders | 88 |
| Respiratory, thoracic and mediastinal disorders | 33 |
| Skin and subcutaneous tissue disorders | 29 |
| Vascular disorders | 7 |
Caveat(s):
- This figure focuses on the top 15 System Organ Classes (SOCs) reported across all adverse reaction cases.
- Each case may describe one or more individual medical event, reflective of signs, symptoms, diseases, diagnoses, investigations and procedures.
- Events are coded according to MedDRA, which provides standardized medical terminology in hierarchical groupings. The highest level grouping is the SOC.
- One adverse reaction case may be represented across multiple SOCs, and is influenced by how individual events (signs, symptoms, observations or diagnostics) are reported.
Overall, 541 individual medical events (representing 243 unique types of individual medical event categories) were reported across 151 adverse reaction cases. When grouped by SOC the most frequent categories were:
- nervous system disorders (19%, n=101)
- psychiatric disorders (16%, n=88)
- general and administrative site conditions (13%, n=72)
- gastrointestinal disorders (11%, n=59)
Figure 9: Frequency of individual medical events
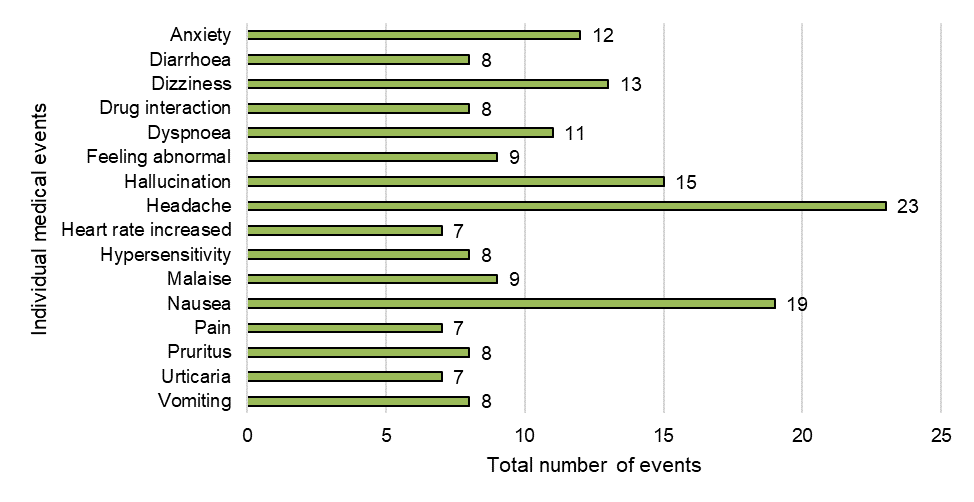
Figure 9 - Text Description
| Individual medical event | Total number of events |
|---|---|
| Anxiety | 12 |
| Diarrhoea | 8 |
| Dizziness | 13 |
| Drug interaction | 8 |
| Dyspnoea | 11 |
| Feeling abnormal | 9 |
| Hallucination | 15 |
| Headache | 23 |
| Heart rate increased | 7 |
| Hypersensitivity | 8 |
| Malaise | 9 |
| Nausea | 19 |
| Pain | 7 |
| Pruritus | 8 |
| Urticaria | 7 |
| Vomiting | 8 |
Caveat(s):
- This figure focuses on the top 15 medical events reported across all adverse reaction. More than 15 events are observed in this figure due to several events being counted an equal number of times. Other individual medical events were reported but do not appear in this figure.
- Individual medical events are coded using MedDRA terminology based on the verbatim described in the case report.
- Each case can have multiple individual medical events reported therefore the number of individual medical events exceeds the total number of unique cases.
- Several types of hallucination were combined to create an all-inclusive hallucination category. These included auditory hallucination, visual hallucination, mixed hallucination, hypnagogic hallucination and pseudohallucination.
As noted in Figure 9, the most frequently reported individual medical events in 2018-2019 were:
- headache (n=23)
- nausea (n=19)
- hallucination (n=15)
- dizziness (n=13)
- anxiety (n=12)
Other events of interest included drug interactions (n=8) and suspected product issues (n=8).
Figure 10: Frequency of individual medical events by cannabinoid dominance
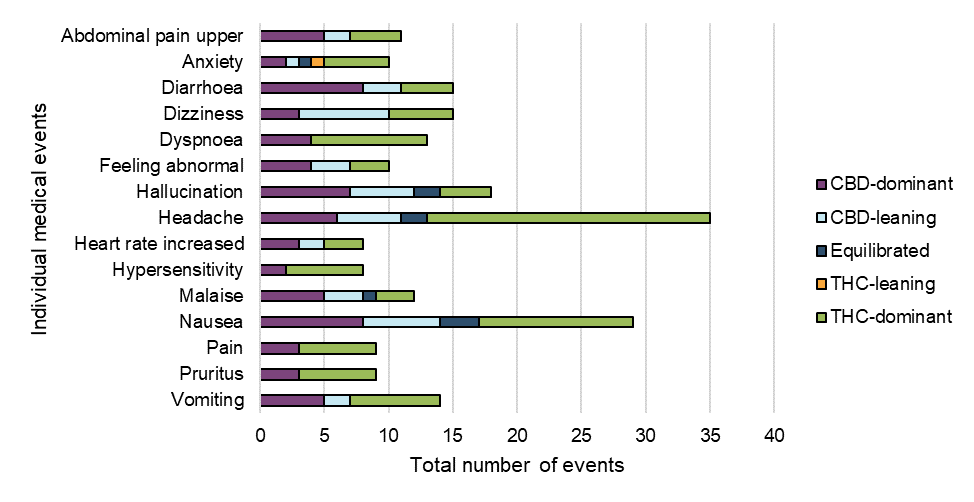
Figure 10 - Text Description
| Individual medical event | Cannabinoid dominance | |||||
|---|---|---|---|---|---|---|
| CBD-dominant | CBD-leaning | Equilibrated | THC-leaning | THC-dominant | Grand Total (excluding unclassified cases) | |
| Abdominal pain upper | 5 | 2 | 0 | 0 | 4 | 11 |
| Anxiety | 2 | 1 | 1 | 1 | 5 | 10 |
| Diarrhoea | 8 | 3 | 0 | 0 | 4 | 15 |
| Dizziness | 3 | 7 | 0 | 0 | 5 | 15 |
| Dyspnoea | 4 | 0 | 0 | 0 | 9 | 13 |
| Feeling abnormal | 4 | 3 | 0 | 0 | 3 | 10 |
| Hallucination | 7 | 5 | 2 | 0 | 4 | 18 |
| Headache | 6 | 5 | 2 | 0 | 22 | 35 |
| Heart rate increased | 3 | 2 | 0 | 0 | 3 | 8 |
| Hypersensitivity | 2 | 0 | 0 | 0 | 6 | 8 |
| Malaise | 5 | 3 | 1 | 0 | 3 | 12 |
| Nausea | 8 | 6 | 3 | 0 | 12 | 29 |
| Pain | 3 | 0 | 0 | 0 | 6 | 9 |
| Pruritus | 3 | 0 | 0 | 0 | 6 | 9 |
| Vomiting | 5 | 2 | 0 | 0 | 7 | 14 |
Caveat(s):
- This figure focuses on the top 15 medical events after stratification by cannabinoid dominance. This figure excludes cases without sufficient information for assignment of cannabinoid dominance (that is, unclassified), therefore, the top 15 events in this figure may differ from those observed in Figure 9. Other individual medical events were reported during the reporting period, but do not appear in this figure.
- This figure was manually created by Health Canada by classifying each suspect cannabis product to a cannabinoid dominance based on available product details and assigning all individual medical events within a case to all reported suspect cannabis products and their cannabinoid dominance (weighted equally for all events). Therefore, this may over-estimate the correlation between cannabinoid dominance and individual medical events and is not reflective of causality.
While the following data are preliminary, with many events reported across more than one type of cannabis product, and any conclusions may be limited, certain medical events appeared to be more frequently reported with THC-dominant or THC-leaning products, whereas others were more frequently reported with CBD-dominant or CBD-leaning products. For example, the events of headache and dyspnoea were more frequently observed with THC-dominant products, whereas the events of dizziness and diarrhoea were more frequently reported with CBD-dominant or CBD-leaning products.
It is important to note that these are reported events only and other factors may be contributing to these events including: the age and health status of patients (including pre-existing health conditions and use of concomitant medications); prior exposure to cannabis (for example, cannabis naïve consumers); dosage; route of administration; and knowledge or awareness of effects of cannabis and cannabinoids.
Figure 11: Primary reason for reporting (case level)
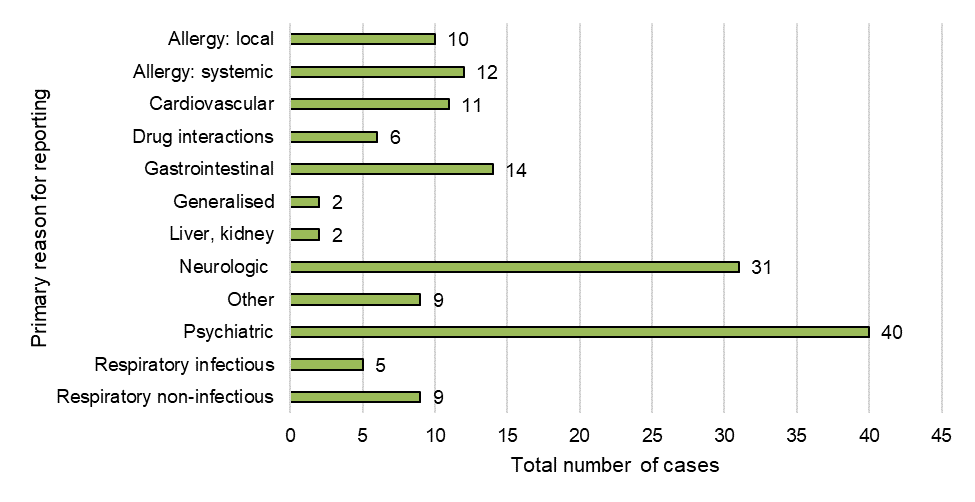
Figure 11 - Text Description
| Primary reason for reporting | Total number of cases |
|---|---|
| Allergy/ hypersensitivity – local | 10 |
| Allergy/hypersensitivity – systemic | 12 |
| Cardiovascular condition(s) | 11 |
| Drug interactions | 6 |
| Gastrointestinal condition(s) | 14 |
| Generalised condition(s) | 2 |
| Liver/kidney condition(s) | 2 |
| Neurologic condition(s) | 31 |
| Other | 9 |
| Psychiatric condition(s) | 40 |
| Respiratory -Infectious (Respiratory, other) | 5 |
| Respiratory non-infectious conditions (irritation, etc.) | 9 |
| Grand total | 151 |
Caveat(s):
- This figure was manually created by assigning one primary reason (type of medical event) at the case level based on overall case details (including verbatim narrative), rather than by frequency of individual medical event extracted from all case reports (all events).
- If more than one reason existed, the event with greater severity was selected.
As described above, breakdowns of individual medical events by SOC is useful for overall data presentation; however, it is not always sufficient to represent clinical conditions or events that may have multi-system involvement (that is, involve multiple SOCs), which is important for ongoing monitoring and detection of new safety signals. As such, Health Canada conducted a manual review of case-level details in order to assign a primary reason for reporting to each case. This allows Health Canada to identify and highlight cases of clinical interest that may span across multiple SOCsFootnote 6, or that may fall under generalized SOC categories that may lack specificityFootnote 7, to aid in signal monitoring and case identification for further assessment. Using this methodology, psychiatric, neurologic (nervous system) and gastrointestinal conditions were the most common primary reason for reporting at the case level, which is consistent with the SOCs (according to frequency of individual medical events, described above). However, other adverse reactions of interest that were identified included allergy or hypersensitivity conditions (systemic and localized) and respiratory conditions (infectious and non-infectious).
3.0 Clinical evaluation of serious and medically important cases
3.1 Summary of serious and medically important adverse reactions
Health Canada reviewed all adverse reaction cases that were reported as serious, as well as those that were non-serious but deemed medically important (n=88).
| Causality Assigned | Number of cases |
|---|---|
| Certain | 0 |
| Probable | 12 |
| Possible | 61 |
| Unlikely | 5 |
| Unassessable | 10 |
| Grand total | 88 |
- Overall, the majority of the cases were assigned a causality of 'Possible' (69%, n=61), meaning there was a reasonable possibility that the cannabis product contributed to the adverse reaction but the contribution of other factors could not be ruled out (for example, concomitant medications, co-morbidities, etc.).
- There were 12 cases assigned a causality of 'Probable' (14%), meaning there was sufficient information to judge that the cannabis product probably contributed to the adverse reaction and the contribution of other factors was considered to be unlikely.
- There were 10 cases determined to be 'Unassessable' (11%), meaning there was insufficient or contradictory information to assign causality.
- There were 5 cases assigned a causality of 'Unlikely' (6%), meaning that factors in the case made the causal relationship improbable, or a clear alternative cause was identified.
- Among the 12 cases assigned a causality of 'Probable', both dried cannabis products used by inhalation and cannabis oil products used orally were involved. These products had varying concentrations of cannabinoids and other ingredients (for example, terpenes, carriers) and were all used for medical purposes.
- Among the 'Probable' cases, there were different primary reasons for reporting, including: psychiatric conditions (n=4); allergy or hypersensitivity conditions (systemic and local (n=3)); neurological conditions (n=2); drug interactions (n=2); and liver or kidney condition (n=1).
3.2 Important identified risks during the reporting period
- During this reporting period, one 'new' important riskFootnote 8 was identified by Health Canada: that of increased bleeding risk (increased international normalised ratio [INR]) associated with an interaction between orally ingested CBD-dominant cannabis oil products and the anti-coagulation medication warfarin. This signal was considered to be new and not previously well-characterised; therefore, an in-depth assessment (case series) was completed that included a review of both domestic and foreign case reports of adverse reactions, as well as data from the published literature (case reports and scientific literature).
- It was concluded that there is an increased risk of bleeding events (increased INR) with cannabis products used in combination with warfarin likely due to a pharmacokinetic interaction, particularly with CBD-dominant cannabis products ingested orally. There also exists a small number of published case reports of interaction with warfarin with inhaled cannabis (commonly THC-dominant) as well as edible cannabis products; however, product details were frequently lacking. Cannabis products are not pure isolated cannabinoids; therefore, even with CBD-dominant or THC-dominant products, both cannabinoids are present in varying concentrations and may be involved; the contribution of other cannabinoids is unknown at this time. A summary of this signal was published as an article in Health Canada's October 2020 InfoWatch Newsletter.
- Other serious or medically important cases with 'Probable' causality were considered to be previously identified health effects, for example, described in Health Canada's Information for Health Care Professionals document. These included:
- hypersensitivity or allergic reactions (for example, localised or systemic hypersensitivity reactions such as pruritus, urticaria, oedema, anaphylaxis)
- psychiatric reactions (for example, anxiety, hallucination)
- neurologic reactions (for example, headache, light-headedness)
- gastrointestinal reactions (for example, nausea, vomiting, stomach pain)
3.3 Important potential risks during the reporting period
'Important potential risks'Footnote 9 observed in adverse reaction data with cannabis products during this reporting period that continue to be monitored includes:
- alterations in hematological parameters (for example, decreased hemoglobin, uncontrolled bleeding event in absence of blood thinning medication)
- alterations in blood glucose parameters
- alterations in liver or kidney parameters (for example, elevated liver enzymes)
- cannabis – drug interaction with topiramate
- cannabis – drug interaction with clobazam
3.4 Missing information during the reporting period
This section highlights potential risks identified on a preliminary basis with limited information available, and continue to be monitored.
- cannabis – drug interactions with unspecified muscle relaxants drugs; cannabis and unspecified antidepressant drugs
- cannabis (CBD-dominant) and possible sudden onset of sleep
- alterations in immune parameters (for example, severe immune-related cutaneous reactions)
4.0 Note to readers
Adverse reaction reports with cannabis submitted to Health Canada are received and entered into the Canada Vigilance Database. The Marketed Health Products Directorate (MHPD) of the Health Products and Food Branch (HPFB) collects, monitors and analyses adverse reactions submitted to the Canada Vigilance Database, amongst other activities, and codes and houses adverse reaction reports for cannabis. The Controlled Substances and Cannabis Branch (CSCB) is responsible for the monitoring, detection, prioritization, evaluation and aggregate reporting of adverse reactions associated with cannabis as part of the Vigilance Framework for Cannabis (pharmacovigilance).
Voluntary reports from the public may be received via the online reporting form, through the toll-free number or through the printable form via electronic fax or mailing to Health Canada. Mandatory reports are submitted by LHs in order to meet their regulatory reporting obligations for serious adverse reactions under the Cannabis Regulations and are submitted through fax or mail, unless the company is registered to submit electronic reports directly to the Canada Vigilance Database (specific format must be met). Cannabis complaints and/or product quality issues may also be referred via the Cannabis Reporting Form from Health Canada. Incidents involving cannabis accessories (for example, mechanical, physical or electrical issue or failure of a cannabis accessory and associated injuries) can be reported via the Consumer Product Incident Reporting Form from the Healthy Environments and Consumer Safety Branch.
All cannabis adverse reaction cases are coded in the following manner:
- Case reports are translated into electronic data into the Canada Vigilance Database. All individual medical events are coded using the Medical Dictionary for Regulatory Activities (MedDRA), which is developed, maintained and updated by the ICH as an international set of standardized medical terms for symptoms, signs, diseases, syndromes and diagnoses.
- Case reports involving cannabis as a substance in a suspected role are coded as 'cannabis sativa' at the active ingredient level, irrespective of the identity of the cannabis product (legal, illegal, unspecified, undetermined).
- Case reports involving a legal cannabis product in a suspected role (identified either by product name or LH) are classified according to the intended use: cannabis product used for medical purposes ('medical cannabis') or used for non-medical purposes ('non-medical cannabis'), based on the information in the report. Cannabis use for medical purposes includes reports described having a medical authorization document; or, a reported medical or therapeutic purpose or indication, without mention of a medical authorization document. If there is no reported reason for use provided in the report, minimal details or intended use for non-medical purposes, the report is classified as 'non-medical cannabis'.
- Case reports are coded as serious as reported if at least one criterion for seriousness is selected: death, life-threatening, admitted to the hospital, lengthened hospital stay, disability, or birth defect. Serious - other medically important condition may also be selected by the reporter.
- According to international pharmacovigilance guidelines (ICH guidelinesFootnote 10), medically important conditions may also be considered serious under certain circumstances and therefore are an option to select when reporting an adverse reaction to Health Canada, and any adverse reaction case identified as such are further reviewed. However, these cases fall outside of the regulatory definition of a serious adverse reaction under the Cannabis Regulations.
- Cannabinoid dominance is a value assigned by Health Canada to each individual suspect cannabis product across all cases, based on available information. In the event that the concentrations are missing, using the product name the cannabinoid concentrations are verified from online information using the LH's website, provincial or territorial store websites, or other available resources. In the event that a product is not able to be identified (for example, Unknown Cannabis Oil by LH) and the cannabinoid concentrations are not reported, then the cannabinoid dominance is assigned as "unassessable".
The criteria used for assigning cannabinoid dominance is as follows:
- "THC-dominant": THC:CBD ratio greater than 1.5:1
- "THC-leaning": THC:CBD ratio in between 1.5:1 and 1.2:1
- "Equilibrated": THC:CBD ratio in between 1.2:1 and 1:1.2
- "CBD-leaning": THC:CBD ratio in between 1:1.2 and 1:1.5
- "CBD-dominant": THC:CBD ratio greater than 1:1.5
Health Canada conducts routine monitoring, detection, assessment and associated activities for all cannabis adverse reaction reports, which involves:
- Screening of all new cannabis case reports to ensure they are coded appropriately according to MedDRA; classified as either cannabis for medical or non-medical purposes (legal classes); and, product names are accurate.
- Case reports involving a suspected non-compliance (that is visual mould present, metallic taste, unusual odour, etc.) are referred to the Compliance Directorate for verification.
- Non-serious reports are screened by Health Canada and those deemed to be medically important events are included for further assessment (causality assessment).
- All serious and medically important case reports undergo further investigation and assessment:
- follow-up is conducted for additional information on product details or clinical details of cases to aid in assessment
- cursory causality assessment is conducted on all routine serious and medically significant cases
- all fatal and life-threatening cases are considered priority reports and undergo a comprehensive individual causality assessment
- causality assessment is based primarily on the World Health Organization (WHO-UMC) causality system
- Any cases involving new or unexpected adverse reactions of interest undergo preliminary assessment to determine if they should be further evaluated (signal prioritization).
- A case series evaluation (signal assessment) is conducted in the event of a cluster or related cases involving new adverse reactions of interest. These comprehensive assessments involve the determination of biological plausibility based on published literature, domestic as well as international adverse reaction data (WHO Vigibase).
5.0 Reporting an adverse reaction involving a cannabis product
Licence holders must submit serious adverse reaction reports, as defined by the Cannabis Regulations, involving a cannabis product and are encouraged to voluntarily submit non-serious adverse reaction reports involving a cannabis product. More information can be found in the Cannabis adverse reaction reporting guide for licence holders.
Consumers and HCPs are encouraged to report all adverse reactions to a cannabis product directly to CSCB. Consumers and HCPs may also send a report to the LH of the cannabis product.
Report an adverse reaction to Health Canada
6.0 Contact us
Any questions or comments on this report, including any requests for the data used to support this report, should be directed to cannabis_oss-cannabis_bss@hc-sc.gc.ca.
Footnotes
- Footnote 1
-
Under the Cannabis Act and its Regulations adults are allowed to legally grow up to a maximum of four cannabis plants. This is in addition to any plants that may be authorized for personal and designated production for medical purposes. However, rules surrounding home growing for non-medical purposes may vary based on the rules and regulations of individual provinces or territories.
- Footnote 2
-
Extended to the end of the calendar year for purposes of this Phase 1 report.
- Footnote 3
-
As per Section 248 of the Cannabis Regulations all adverse reactions, including non serious adverse reactions, must be maintained in an annual summary report by the LH, of which can be requested by Health Canada.
- Footnote 4
-
World Health Organization (2012). Safety monitoring of medicinal products. Reporting system for the general public. Available from: https://www.who.int/publications/i/item/9789241503198
- Footnote 5
-
CBD-dominant, CBD-leaning, THC-dominant, THC-leaning, and equilibrated categories were manually assigned by Health Canada. Please refer to Section 4: Note to Readers for further details.
- Footnote 6
-
For example, allergy or hypersensitivity reactions usually span across multiple SOCs, including SOC skin and subcutaneous disorders; SOC immune system disorders; and may involve others such as SOC cardiac disorders, SOC investigations, and SOC gastrointestinal disorders in instances of anaphylaxis.
- Footnote 7
-
For example, drug interaction is classified under the SOC 'general disorders and administration site conditions'.
- Footnote 8
-
Risk for which there is adequate evidence of association.
- Footnote 9
-
Risk for which there is suspicion of an association but no confirmation based on current information, requiring ongoing monitoring.
- Footnote 10
-
https://www.ema.europa.eu/en/documents/scientific-guideline/international-conference-harmonisation-technical-requirements-registration-pharmaceuticals-human-use_en-12.pdf