Page 4 - Guidelines for Canadian Drinking Water Quality: Guideline Technical Document: N-Nitrosodimethylamine (NDMA)
Although quantitative data in humans have not been identified, studies conducted with laboratory animals indicate that ingested NDMA is absorbed rapidly and extensively (i.e., >90%) (Daugherty and Clapp, 1976; Diaz Gomez et al., 1977; Kunisaki et al., 1978), primarily from the lower intestinal tract (Phillips et al., 1975; Hashimoto et al., 1976; Agrelo et al., 1978; Pegg and Perry, 1981). Detection of NDMA in the urine of rats and dogs exposed by inhalation indicates that the nitrosamine is absorbed through the lungs; however, reliable quantitative information on the absorption of NDMA following inhalation was not identified. Although quantitative data were not identified, absorption through the skin may be inferred from the results of a study in which small amounts (i.e., 0.03%) of NDMA were detected in the urine of rats following epicutaneous (dermal) administration of a solution containing 350 µg NDMA (Spiegelhalder et al., 1982).
Once absorbed, NDMA and its metabolites are distributed widely (Daugherty and Clapp, 1976; Anderson et al., 1986) and likely passed to offspring through mothers' milk (Diaz Gomez et al., 1986). The nitrosamine and its metabolites have been detected in the fetuses of pregnant rodents injected with the substance (Althoff et al., 1977; Johansson-Brittebo and Tjälve, 1979). Pharmacokinetic analyses of NDMA injected intravenously into a number of laboratory species have revealed that the nitrosamine is cleared rapidly from the blood, with metabolism involving both hepatic and extrahepatic components. NDMA and its metabolites may be excreted in the urine or exhaled as carbon dioxide.
Quantitative information from studies on the metabolism of NDMA in humans was not identified. However, there appear to be differences in the rate of metabolism of NDMA between species (Jeong-Sook et al., 1987; Gombar et al., 1990). Despite potential quantitative differences in NDMA metabolism, there appear to be few qualitative differences in the metabolism of NDMA between humans and laboratory animals. The metabolism of NDMA involves either the α-hydroxylation or denitrosation of the nitrosamine (Figure 1). Both pathways are considered to proceed through a common intermediate radical [CH3(CH2·)NBN=O] generated by the action of the cytochrome CYP2E1-dependent mixed-function oxidase system (Haggerty and Holsapple, 1990; Lee et al., 1996). Along the α-hydroxylation pathway, the hydroxymethylnitrosamine (HOCH2CH3HBN=O) formed from the intermediate radical decomposes to formaldehyde (itself ultimately converting to carbon dioxide) and monomethylnitrosamine (CH3NHN=O); the monomethylnitrosamine, owing to its instability, undergoes rearrangement to the strongly methylating methyldiazonium ion (CH3N+≡N), which alkylates biological macromolecules such as DNA, RNA and proteins. It is the α-hydroxylation pathway that is believed to form the active metabolites responsible for NDMA's genotoxicity and carcinogenicity (Lee et al., 1996). Metabolic conversion of the intermediate radical via denitrosation leads to the formation of methylamine (CH3NH2) and formaldehyde (Figure 1).
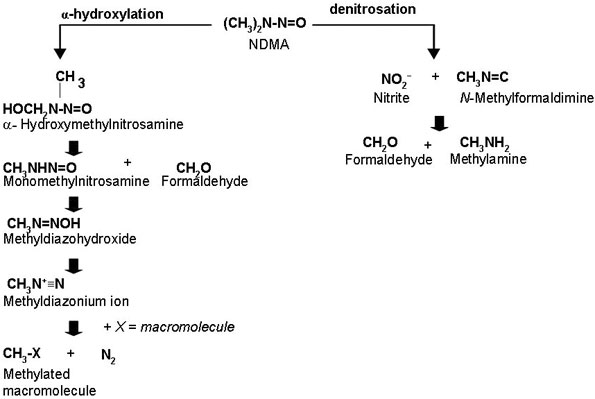
Figure 1 - Text Description
Two deaths (one adult and one child) linked to the acute ingestion of unknown quantities of NDMA in tainted lemonade, as well as a third adult fatality attributed to the consumption of at least four doses of approximately 250-300 mg of NDMA over a 2-year period, have been reported (Cooper and Kimbrough, 1980; Fussgänger and Ditschuneit, 1980; Pedal et al., 1982). Liver failure was observed in all three cases; the two acutely exposed decedents also exhibited cerebral haemorrhage.
Relevant epidemiological studies include case-control investigations in which the potential risks of cancer of the stomach (Risch et al., 1985; González et al., 1994; La Vecchia et al., 1995; Pobel et al., 1995), upper digestive tract (Rogers et al., 1995) and lung (Goodman et al., 1992; De Stefani et al., 1996) associated with the ingestion of NDMA have been assessed. In some of these reports, the estimated intake of NDMA was based upon recollection of an individual's typical diet consumed in the year preceding the onset of illness, as well as the reported levels of this nitrosamine in the foodstuffs consumed (Goodman et al., 1992; González et al., 1994; Pobel et al., 1995).
In other studies (Rogers et al., 1995; De Stefani et al., 1996), subjects were asked to recall their typical diet in the 5 and 10 years, respectively, prior to the onset of illness.
In three of four case-control studies, there was a positive relationship with evidence of exposure-response for the intake of NDMA and gastric cancer (González et al., 1994; La Vecchia et al., 1995; Pobel et al., 1995), although not in an additional study in which oral, laryngeal and oesophageal cancers were investigated separately (Rogers et al., 1995). In two case-control studies in which matching or control for confounders was more extensive than that for the investigations of gastric cancer mentioned above, there were clear exposure-response relationships for NDMA and lung cancer (Goodman et al., 1992; De Stefani et al., 1996). In almost all studies, associations between the cancers of interest and nitrate, nitrite and NDMA were examined.
A population-based cohort study of 9985 adult Finnish men and women with a follow-up period of 24 years showed a relative risk of 2.12 (95% confidence interval = 1.04-4.33) for colorectal cancer associated with NDMA intake (Knekt et al., 1999). Head and neck and stomach cancers were also studied, but the relative risks were not statistically significant. No significant association was observed between nitrate or nitrite intake and cancers of the gastrointestinal tract. There appears to be no qualitative difference between rodents and humans in the formation of DNA adducts following exposure to NDMA. In a case of suspected NDMA poisoning in a human male, methylation of liver DNA was evident at both the N7and O6 positions of guanine (Herron and Shank, 1980). Using an immunohistochemical technique, Parsa et al. (1987) detected the formation of O6-methylguanine in human pancreatic explants incubated in vitro with NDMA.
NDMA is highly acutely toxic after oral administration to rats, with lethal doses (LD50s) ranging from 23 to 40 mg/kg bw (ATSDR, 1989). It is also highly acutely toxic via inhalation; 4-h median lethal concentrations (LC50s) are 240 mg/m³ (78 parts per million [ppm]) for rats and 176 mg/m³ (57 ppm) for mice (ATSDR, 1989). A lowest-observed-effect concentration (LOEC) of 49 mg/m³ (16 ppm) was identified for inhalation in dogs exposed to NDMA for 4 h (ATSDR, 1989). In all three species, acute inhalation exposure produced haemorrhagic necrosis in the liver. An increased blood clotting time was also reported for the NDMA-exposed dogs (ATSDR, 1989). Following intraperitoneal exposure, LD50s of 43 mg/kg bw in rats and 20 mg/kg bw in mice have been reported (IARC, 1978). In other laboratory species, acute exposure to NDMA at levels of 30-60 mg/kg bw produced effects in the liver (hepatotoxicity), kidney (tumours) and testes (necrosis of the seminiferous epithelium) (Magee and Barnes, 1962; Schmidt and Murphy, 1966; Hard and Butler, 1970a,b; McLean and Magee, 1970; OME, 1991).
Hepatic effects (i.e., hepatocyte vacuolization, portal venopathy, necrosis/haemorrhage), often associated with reduced survival, have been observed in a number of mammalian species exposed orally (by gavage, unless otherwise stated) under various conditions (e.g., in rats receiving 1, 3.8 or 5 mg NDMA/kg bw per day for 30, 7-28 or 5-11 days, respectively; in mice receiving 5 mg/kg bw per day in drinking water for 7-28 days; in hamsters receiving 4 mg/kg bw per day in drinking water for 1-28 days; in guinea-pigs, cats and monkeys receiving 1 mg/kg bw per day for 30 days or 5 mg/kg bw per day for 5-11 days; in dogs receiving 2.5 mg/kg bw per day, 2 days per week, for 3 weeks; and in mink receiving 0.32 mg/kg bw per day for 23-34 days) (as reviewed in IARC, 1978; ATSDR, 1989).
In addition to effects in the liver, "congestion" (excessive blood/fluid content) in a variety of organs (i.e., kidneys, lung, spleen, myocardium) has been reported in rats receiving 3.8 mg NDMA/kg bw per day in the diet for 1-12 weeks (Khanna and Puri, 1966). Gastrointestinal haemorrhage has been observed in rats receiving dietary doses of 10 mg NDMA/kg bw per day for 34-37 days (Barnes and Magee, 1954) and in mink receiving 0.3 or 0.6 mg NDMA/kg bw per day in the diet for 23-34 days (Carter et al., 1969). Effects in the kidneys (including glomerulus dilatation and slight thickening of the Bowman's capsule) were observed in mink receiving 0.2 mg NDMA/kg bw per day from the diet (period not specified) (Martino et al., 1988).
NDMA has consistently shown potent carcinogenicity in all laboratory animal studies. As a result, there has been little attempt to study other toxic end-points, and there are inadequate data available to make a meaningful assessment of end-points other than carcinogenicity.
Although most studies would be considered limited by current standards (e.g., small group sizes, single dose levels, limited histopathological examination), there has been clear, consistent evidence of carcinogenicity in studies where rodents (i.e., rats, mice, hamsters) were exposed to NDMA orally, via inhalation or by intratracheal instillation. NDMA increased the incidence of liver and Leydig cell tumours in rats ingesting this nitrosamine from drinking water or the diet (Terao et al., 1978; Arai et al., 1979; Ito et al., 1982; Lijinsky and Reuber, 1984); increased tumour incidences were noted at concentrations of NDMA of about 5 mg/L in drinking water and 10 mg/kg in the diet. Hepatic, pulmonary and renal carcinogenicity was observed in mice administered NDMA via drinking water (Terracini et al., 1966; Clapp and Toya, 1970; Anderson et al., 1979, 1986, 1992) or through inhalation (Moiseev and Benemanskii, 1975); increases in tumour incidence were observed at concentrations of NDMA in drinking water ranging from 0.01 to 5 mg/L. Moreover, in some cases (e.g., Terracini et al., 1966), the period of exposure to NDMA was relatively short (i.e., 3 weeks). NDMA increased the incidence of liver tumours in hamsters exposed intratracheally (Tanaka et al., 1988). The administration of NDMA to pregnant rats (by intraperitoneal injection) or mice (by stomach tube) increased the frequency of hepatic and renal tumours in the offspring (Alexandrov, 1968; Anderson et al., 1989).
One study in particular stands out as the most comprehensive one to use for a quantitative risk assessment due to the exceptionally high power (2040 rats) and wide concentration range that was used. This carcinogenicity bioassay (designed to provide detailed information on exposure-response) involving lifetime exposure, 15 dose groups of 60 male and 60 female Colworth-Wistar rats were provided with drinking water containing a wide range of 15 concentrations (from 33 to 16 896 µg/L) of NDMA (Brantom, 1983; Peto et al., 1991a,b). The estimated daily intakes of NDMA ranged from 0.001 to 0.697 mg/kg bw in the males and from 0.002 to 1.224 mg/kg bw in the females. A control group of 120 males and 120 females received drinking water without NDMA. Groups of animals were taken for interim sacrifice after 12 and 18 months of study. Survival of the animals was reduced with increasing dose; animals in the highest dose group did not survive longer than 1 year. Survival in the low-dosed groups was good (up to 3.5 years). At low dose rates, it was found that the number of liver neoplasms induced was proportional to the dose rate, with no indication of a threshold. In addition, a variety of nonneoplastic abnormalities were seen in the liver at low doses; these included hyperplastic nodules and shrinkage of hepatocytes.
There is overwhelming evidence that NDMA is mutagenic and clastogenic (reviewed in IARC, 1978; ATSDR, 1989). Increased frequencies of gene mutations, chromosomal damage, sister chromatid exchange and unscheduled DNA synthesis have been observed in a wide variety of cell types, in assays conducted in the presence or absence of metabolic activation. Positive results have been observed in human as well as rodent cells. Two studies by Hakura et al. (1999, 2003) found that S9 fractions from human sources were considerably more active than those from rats in stimulating the mutagenic response to NDMA in the Ames test: the mutation rate was up to 8 times higher with some human S9 fractions.
Clear evidence of genetic effects has also been observed in in vivo studies. Clastogenic effects (e.g., micronuclei, sister chromatid exchange, chromosomal aberrations) in hepatocytes (Tates et al., 1980, 1983, 1986; Mehta et al., 1987; Braithwaite and Ashby, 1988; Cliet et al., 1989; Neft and Conner, 1989; Sawada et al., 1991), bone marrow cells (Bauknecht et al., 1977; Wild, 1978; Neal and Probst, 1983; Collaborative Study Group for the Micronucleus Test, 1986; Neft and Conner, 1989; Krishna et al., 1990; Sato et al., 1992; Morrison and Ashby, 1994), spleen cells (Neft and Conner, 1989; Krishna et al., 1990) and peripheral blood lymphocytes (Tates et al., 1983; Sato et al., 1992), as well as in oesophageal (Mehta et al., 1987) and kidney cells (Robbiano et al., 1997), have been observed in rodents (rats, mice or hamsters) administered NDMA either orally or by intraperitoneal injection. Increased frequencies of micronucleated cells were observed at doses as low as 5 mg/kg bw in rats (Trzos et al., 1978; Mehta et al., 1987). Effects in germ cells (i.e., micronucleated spermatids) were observed in mice given 6 or 9 mg NDMA/kg bw via intraperitoneal injection (Cliet et al., 1993). The inhalation exposure of female mice to NDMA at 1030 mg/m³ increased the frequency of micronucleated bone marrow cells (Odagiri et al., 1986). Evidence of genotoxicity (e.g., chromosomal aberrations, micronuclei, gene mutation, DNA strand breaks) has also been observed in the offspring of hamsters (Inui et al., 1979) and mice (Bolognesi et al., 1988) administered NDMA during gestation. In rodents (rats, mice or hamsters) administered NDMA either orally or by intraperitoneal injection, evidence of DNA damage has been observed in the liver, kidneys and lungs (Laishes et al., 1975; Petzold and Swenberg, 1978; Abanobi et al., 1979; Mirsalis and Butterworth, 1980; Brambilla et al., 1981, 1987; Bermudez et al., 1982; Cesarone et al., 1982; Barbin et al., 1983; Doolittle et al., 1984; Kornbrust and Dietz, 1985; Loury et al., 1987; Mirsalis et al., 1989; Pool et al., 1990; Brendler et al., 1992; Jorquera et al., 1993; Asakura et al., 1994; Tinwell et al., 1994; Webster et al., 1996). DNA damage in thymus (Petzold and Swenberg, 1978), sperm (Cesarone et al., 1979) and nasal and tracheal cells (Doolittle et al., 1984) has also been noted. NDMA was mutagenic at the lacI locus (in the liver) in in vivo assays involving transgenic mice (Mirsalis et al., 1993; Tinwell et al., 1994; Butterworth et al., 1998). In addition, increased unscheduled hepatic DNA synthesis has been observed in rats at doses as low as 0.1 mg/kg bw (Mirsalis and Butterworth, 1980).
Available data are inadequate as a basis for assessment of the reproductive or developmental toxicity of NDMA. Interpretation of the results of most identified investigations is complicated by the high doses administered, likely to have induced acute or repeated-dose organ toxicity. In a report by Anderson et al. (1978), time to conception in female mice provided with drinking water containing 0.1 mg NDMA/L for 75 days prior to mating was about 3 days longer than in unexposed controls; no other reproductive effects were assessed in this study. In a study conducted with male rats, a single intraperitoneal injection of 30 or 60 mg NDMA/kg bw induced testicular damage (necrosis or degeneration of the seminiferous epithelium) (Hard and Butler, 1970b).
In a single-generation study (Anderson et al., 1978) in which the reproductive effects of a number of substances were examined, groups of 20 female mice were provided with drinking water containing 0 or 0.1 mg NDMA/L for 75 days prior to mating and throughout pregnancy and lactation (estimated daily and total intakes of 0.02 mg/kg bw per day and 2 mg/kg bw, respectively). The proportion of deaths (based upon the total number of stillborn and neonatal deaths) was increased 2-fold in the NDMA-exposed animals compared with controls (i.e., 20% and 9.9%, respectively), due in large part to an increase in the number of stillborn animals. Exposure to NDMA had no effect upon maternal fluid consumption, litter size or average body weight of the weanlings, and no consistent gross or histopathological abnormalities were observed in the stillborn fetuses or dead neonates to account for the increased mortality.
The effects of NDMA on pregnant rats were studied by Nishie (1983). Single oral doses of 15 and 20 mg NDMA/kg bw given on day 18 of pregnancy resulted in lethality of 9.4% and 35.3% of pregnant rats, respectively, whereas no lethality was observed in non-pregnant rats. NDMA also increased serum a-hydroxybutyric dehydrogenase in pregnant rats on day 20 and decreased fetal weights in rats treated on days 13 and 18.
There is strong evidence that the toxicological effects of NDMA are directly dependent upon the CYP2E1-dependent metabolic conversion of this nitrosamine to highly reactive species. Lee et al. (1996) attributed the hepatotoxicity of NDMA to the methyldiazonium ion formed via the α-hydroxylation pathway; denitrosation was considered to make little contribution to the overall hepatotoxic effect of this nitrosamine in rats. The principal DNA adduct formed following exposure to NDMA is N7-methylguanine (representing about 65% of all adducts formed initially upon exposure); O6-methylguanine is a secondary adduct (representing about 7% of all adducts formed initially). Other DNA adducts formed in smaller amounts include N3-methyladenine and O4-methylthymine.
N7-Methylguanine may undergo depurination, yielding apurinic sites, which, if not repaired prior to DNA replication, can result in guanine to thymine (i.e., G → T) transversions (Swenberg et al., 1991). Two adducts formed in small amounts, O6-methylguanine and O4-methylthymine (formed at about 1% of the amount of O6-methylguanine), are strongly promutagenic by direct base mispairing. O6-Methylguanine causes guanine:cytosine to adenine:thymine (i.e., G:C → A:T) transitions, whereas O4-methylthymine causes A:T → G:C transitions (Swenberg et al., 1991; Souliotis et al., 1995).
Available data are consistent with the formation and persistence of the secondary adduct, O6-methylguanine, being associated with both the carcinogenicity and mutagenicity of NDMA (see Haggerty and Holsapple, 1990; Swenberg et al., 1991; Souliotis et al., 1995). The ability of cells to repair DNA adducts (by removing O6-methylguanine through the action of a specific O6-methylguanine DNA-methyltransferase) prior to cell division likely plays a critical role in determining the susceptibility of tissues to tumour development.
In monkeys orally dosed with 0.1 mg NDMA/kg bw, O6-methylguanine was detected in 32 tissues examined (Anderson et al., 1996). The highest levels were in the gastric mucosa and liver, but elevated levels were also present in white blood cells, the oesophagus, ovaries, pancreas, bladder and uterus. O6-Methylguanine DNA-methyltransferase activity varied over a 30-fold range; the highest activities were in the gastric mucosa, liver, kidneys and lungs. The formation of O6-methylguanine was detected in fetal liver, lung, kidney, spleen and brain in a study in which pregnant patas monkeys were administered a single gastric NDMA dose of 1 mg/kg bw (Chhabra et al., 1995).
The greater persistence of O6-methylguanine DNA adducts in the kidney compared with the liver in rats administered a single oral NDMA dose of 20 mg/kg bw parallels earlier findings in which the acute oral or intraperitoneal administration of NDMA to rats at such dose levels increased the incidence of kidney but not liver tumours (Magee and Barnes, 1962; Schmidt and Murphy, 1966; Hard and Butler, 1970a; McLean and Magee, 1970). In contrast, the long-term oral administration of low doses of NDMA (i.e., <2 mg/kg bw per day) increased the incidence of liver but not kidney tumours in these animals (Brantom, 1983; Lijinsky and Reuber, 1984; Peto et al., 1991a,b), a finding attributed to the first-pass metabolism of NDMA in the liver (Swenberg et al., 1991).
There are quantitative age- and species-related differences in hepatic O6-methylguanine, possibly associated with variations in the activity of the transferase, consistent with observed variations in the carcinogenicity of the compound among species and strains exposed under various conditions. These include greater hepatic activity in adults compared with newborn mice (Coccia et al., 1988), in rats compared with mice and between strains of mice (greater in C3H than in C57BL) (Lindamood et al., 1984).
Evidence supporting a role for O6-methylguanine formation in tumour development following exposure to NDMA was recently reviewed by Souliotis et al. (1995). G:C → A:T transitions have been observed in the ras oncogene in mouse lung tumours induced by NDMA (Devereux et al., 1991), in the livers of lacI transgenic mice administered a single dose of 4 mg NDMA/kg bw (Mirsalis et al., 1993) and in the liver, kidney and lung of lacI transgenic mice administered five daily doses of 1 mg NDMA/kg bw (Wang et al., 1998). Moreover, transgenic mice expressing high levels of O6-methylguanine DNA-methyltransferase in the liver were less susceptible than normal controls to NDMA-induced hepatocarcinogenesis (Nakatsuru et al., 1993). However, Souliotis et al. (1995) also reported that the dose-response relationship for the accumulation of O6-methylguanine in hepatic DNA in rats administered drinking water (for 28 days) containing concentrations of NDMA similar to those used in the study conducted at BIBRA Toxicology International (Brantom, 1983; Peto et al., 1991a,b) did not strictly parallel the dose-response for the development of hepatic tumours in the carcinogenicity bioassay.
There is conclusive evidence that NDMA is a potent carcinogen in experimental animals. NDMA has been classified by the International Agency for Research on Cancer in Group 2A, "probably carcinogenic to humans", which means that there is sufficient evidence of carcinogenicity in animals, but human data is limited (IARC, 1987). Based on the current weight of evidence, which includes more recent aimal studies, ,NDMA is considered highly likely to be carcinogenic to humans as determined by Environment Canada and Health Canada (2001). The mechanism by which NDMA produces cancer is well understood to involve biotransformation by liver microsomal enzymes, generating the methyldiazonium ion.
As a consequence of the clear evidence of carcinogenicity, there have been few studies of other possible toxic end-points, and existing data are inadequate to quantify health risk for NDMA by any end-point other than carcinogenicity.
There is also ample evidence that NDMA is genotoxic both in vivo and in vitro. Activation by liver microsomal S9 fractions is necessary for a positive in vitro result. The recent observation that human S9 fractions are much more active than rat S9 fractions in promoting genotoxicity in the Ames test suggests that humans may be especially sensitive to the carcinogenicity of NDMA.
There have been several case-control studies and one cohort study of NDMA in humans. Although none of these studies can be used to derive a quantitative risk of cancer, the results are supportive of a positive association between NDMA exposure and gastric or colorectal cancer. However, it should be noted that these studies did not consider drinking water as the route of exposure; instead, they used estimations of total dietary intake of NDMA.
Although there are several cancer bioassays in rodents available, one study in particular stands out as the most obvious one to use for a quantitative risk assessment due to the exceptionally wide concentration range that was used (15 dose groups between 33 and 16 896 µg/L) (Brantom, 1983; Peto et al., 1991a,b). The dose groups were also large, with 60 male and 60 female Colworth-Wistar rats at each dose level. This study was used in the IPCS (2002) and Environment Canada and Health Canada (2001) risk assessments to calculate the TD05 (i.e., the dose level that causes a 5% increase in tumour incidence over background) for hepatic cancers of various types in the male and female rats.
After successively removing higher dose groups to eliminate downturn, TD05 values were calculated by fitting a multistage model to the data and then finding the dose at which the excess risk was increased by 5% over background. For female rats, TD05 values ranged from 34 to 82 µg/kg bw per day (95% lower confidence limits [LCLs] 18 to 61 µg/kg bw per day). For males, TD05s ranged from 35 to 78 µg/kg bw per day (95% LCLs 29 to 48 µg/kg bw per day) (Health Canada, 2008).
These TD05 values were used to calculate unit risks for the current assessment. Unit risks were calculated by dividing 0.05 by the TD05 or 95% LCL on the TD05 (TDL05). An animal to human allometric scaling factor of (0.35/70)¼ was applied to the resulting unit risks. Use of this scaling factor accounts for interspecies differences in susceptibility to NDMA. Although the mechanism of action in both animals and humans appears qualitatively similar, there exists a lack of quantitative data with which to conclude that no interspecies differences exist. Further, it is possible that humans are more sensitive than laboratory animals to carcinogenicity related to N-nitroso compounds (Lijinsky, 1999).
Unit risks ranged from 2.28 × 10-3 (µg/kg bw per day)-1 (95% upper bound of 3.09 × 10-3 (µg/kg bw per day)-1, based on the TDL05) for biliary cistadenomas to 5.57 × 10-3 (µg/kg bw per day)-1 (95% upper bound of 1.04 × 10-2 (µg/kg bw per day)-1) for carcinomas. These unit risks can be used to determine a range of concentrations yielding risks of 10-4, 10-5 and 10-6. Doses in µg/kg bw per day were converted to concentrations in drinking water assuming an average human exposure via drinking water of 1.9 L/day (as indicated by the multiroute exposure assessment) and an average human adult body weight of 70 kg. The formula to convert from dose to concentration is:
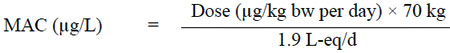
Figure 2 - Text Description
Based on the calculated unit risks, the estimated concentrations corresponding to lifetime human cancer risks of 10-4, 10-5, and 10-6 for these carcinomas are as follows:
| Risk | Concentration in drinking water (µg/L) |
|---|---|
| 10-4 | 0.4-1,0 µg/L |
| 10-5 | 0.04-0,1 µg/L |
| 10-6 | 0.004-0,01 µg/L |
The maximum acceptable concentration (MAC) for NDMA in drinking water associated with an excess lifetime cancer risk of 10-5 is 0.000 04 mg/L (0.04 µg/L).
Other organizations have set guidelines or regulations pertaining to the concentration of NDMA in drinking water. For all cases, risk estimates have been based on a carcinogenic end-point and have used the same key study to derive acceptable levels for NDMA in drinking water.
WHO has established a guideline value of 0.1 µg/L for NDMA in drinking water. This concentration corresponds to a lifetime carcinogenicity risk of 10-5 and is based on a 60-kg average weight for an adult consuming 2 L of water per day. The major difference between the WHO guideline and Health Canada's drinking water guideline in that WHO did not use an allometric scaling factor in deriving the guideline value.
The California Environmental Protection Agency (EPA) has also used the Peto et al. (1991a,b) studies to derive a public health goal of 0.003 µg/L (3 ng/L) for NDMA in drinking water, corresponding to a lifetime theoretical extra cancer risk of 10-6, assuming an average weight of 70 kg and drinking water consumption of 2 L/day. "Lifetime theoretical extra cancer risks" of 10-4 and 10-5 were calculated as 0.3 and 0.03 µg/L, respectively (OEHHA, 2006). The California EPA has set a notification level of 0.01 µg/L for NDMA in drinking water.
Most recently, the Peto et al. (1991a,b) studies on rat liver tumours were used by Australian investigators to determine a tolerable intake for NDMA using a modified benchmark dose approach (Fitzgerald and Robinson, 2007). A number of mathematical models were used to evaluate the incidence data and generate a tolerable daily intake (TDI) range of 4.0-9.3 ng/kg bw per day.
The potential for NDMA-induced carcinogenicity has also been assessed in the Priority Substances List Assessment Report on NDMA by Environment Canada and Health Canada (2001), which describes several potential sources of exposure to NDMA. Although drinking water was not identified as the most significant source of exposure to NDMA, they recommended monitoring of exposure from this source.
Although there are no longer any industrial uses for NDMA in Canada, NDMA is produced as a by-product of industrial processes that use nitrates or nitrites and amines under a range of pH conditions. NDMA can be found in both surface water and groundwater sources, but it is found in drinking water primarily from its formation during treatment, in particular by chloramination. In Canada, the daily intake of NDMA from drinking water represents a relatively low contribution compared with other sources, such as foods. A multi-route exposure assessment determined that although the inhalation route of exposure to NDMA from drinking water is insignificant, exposure through dermal absorption is significant, and it has therefore been incorporated in the calculation of the MAC.
NDMA has consistently shown potent carcinogenicity in all laboratory animal studies. As a result, there has been little attempt to study other toxic end-points, and there are inadequate data available to make a meaningful assessment of end-points other than carcinogenicity. An animal to human allometric scaling factor was incorporated into the assessment to account for interspecies differences in susceptibility to NDMA.
The estimated lifetime cancer risk from exposure to NDMA in drinking water at levels ranging from 0.004 to 0.04 µg/L (4-40 ng/L) is considered to be "essentially negligible." The guideline for a carcinogen is normally established at a level at which the increased cancer risk is "essentially negligible" when a person is exposed to that level in drinking water over a lifetime. In the context of drinking water guidelines, Health Canada has defined this term as a range from one new cancer above background per 100 000 people to one new cancer above background per 1 million people (i.e., 10-5 to 10-6) exposed to a contaminant at the MAC over a lifetime. In the case of NDMA, the MAC is established at a concentration that would present an "essentially negligible" risk of one new cancer above background per 100 000 people (i.e., 10-5) exposed to NDMA at the MAC over a lifetime, which takes into consideration treatment limitations.
NDMA can be detected and measured at very low concentrations in drinking water. The presence of NDMA in drinking water is usually associated with its formation during water treatment rather than with its presence in the source water. Consequently, it is suggested that the organic nitrogen precursors be removed or the disinfection strategy used be modified in order to minimize the formation of NDMA without compromising the efficacy of the disinfection process. Once NDMA is present in drinking water, its reduction using UV irradiation is technically feasible, but it is expensive and may be difficult for smaller utilities.
Levels of NDMA in drinking water in Canada are generally estimated to be less than or equal to 5 ng/L (0.005 µg/L), although higher levels have been reported in some communities.
In summary, a MAC of 0.000 04 mg/L (0.04 µg/L) for NDMA is proposed based on the following considerations:
- It falls within the range considered to present an "essentially negligible" risk.
- It is detectable and measurable, with a method detection limit well below the MAC.
- It is achievable at reasonable cost, by implementing strategies to minimize its formation.
As part of its ongoing guideline review process, Health Canada will continue to monitor new research in this area and recommend any change(s) to the proposed guideline that it deems necessary.