Page 3: Guidelines for Canadian Drinking Water Quality: Guideline Technical Document – Radiological Parameters
Radionuclides may be naturally present in the environment, or they may enter the environment as a result of human activities. The contribution of drinking water to total exposure to radionuclides is typically very small, as shown in Figure 1. According to WHO (2008), exposure to radiation in both food and water for the world population in general, represents only 8% of the total exposure to radiation. to radionuclides is typically very small, as shown in Figure 1. According to WHO (2008), exposure to radiation in both food and water for the world population in general, represents only 8% of the total exposure to radiation.
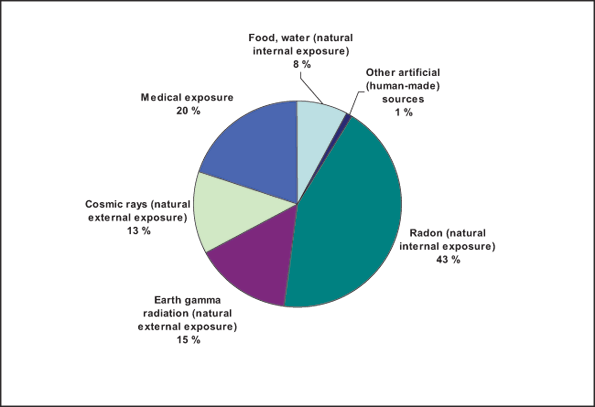
Figure 1 - Sources and distribution of average radiation exposure for the world population (WHO, 2008).
The greatest contribution to radiation exposure of the general public comes from naturally occurring radioactive elements in the Earth's crust and from cosmic radiation of extraterrestrial origin. Natural sources contribute more than 98% of the global human radiation dose, excluding medical exposures. The global average individual dose from natural sources is estimated to be about 2.4 mSv/year (UNSCEAR, 2000), which is comparable to 2.6 mSv/year for Canada (NCRP, 1987). About one-third of this dose is due to external radiation (terrestrial plus cosmic); the other two-thirds is due to the inhalation and ingestion of radionuclides in air, water, and food.
Natural radionuclides are either primordial (having half-lives comparable to the age of the Earth) or secondary (produced by the decay of primordial radionuclides). The natural radionuclides belong principally to the uranium series, the thorium series, and the actinium series, originating from 238U, 232Th, and 235U, respectively. These radionuclides are present at low concentrations in all rocks and soils. In addition, the primordial radionuclide 40K, comprising 0.012% of natural potassium, is present in the environment at significant concentrations. Because potassium is widespread in the environment and is taken into the body through ingestion as an essential nutrient, 40K is a major contributor to both internal and external exposure, providing about 180 µSv internally and 150 µSv externally to the average annual background dose (UNSCEAR, 1988). However, the absorption of elemental potassium by the body is under strict homeostatic control and is therefore not influenced by variations in environmental levels. For this reason, the dose from 40K in the body is constant and is not considered further in these guidelines.
The occurrence of natural radionuclides in drinking water is associated mainly with deep wells drilled into aquifers containing elevated mineralizations of radioactive elements, and is not necessarily correlated with surface geological features. Dissolution of these minerals takes place very slowly; however, in cases where groundwater has been in contact with the rock over hundreds or thousands of years, significant concentrations may build up in the water. These concentrations are highly variable. Concentrations are determined not only by the composition of the underlying bedrock, but also by the particular physical and chemical conditions prevailing in the aquifer. Radionuclide concentrations can vary significantly in wells just a few metres apart. Even concentrations from the same well can vary seasonally or annually, depending on groundwater flow patterns. Nor can one assume that radionuclides in groundwater will be in secular equilibrium, even if equilibrium prevails in the bedrock.
The radionuclides most frequently reported in Canadian groundwater sources are 226Ra, 222Rn, and 210Pb from the uranium series. There are many instances in Canada where elevated uranium concentrations are found in groundwater but none of its decay products are detectable. Likewise, one may see 226Ra without 238U or 210Pb without its 226Ra precursor. The radionuclides most frequently reported in Canadian surface water sources are 226Ra, tritium, 90Sr, and 137Cs.
The occurrence of natural radionuclides in shallow wells is less frequent, although it cannot be ruled out. Elevated concentrations may occur if the overburden contains a significant amount of radioactive minerals or if the overburden is being fed from deep groundwater sources containing radionuclides.
Radon-222 is a chemically inert gas formed through the radioactive decay of 226Ra. Both are members of the 238U decay series. RadonFootnote 1 has a half-life of 3.82 days. Its decay products form a series of short-lived radionuclides (all solid elements) that decay within hours to 210Pb. Because of their short half-lives, the radon daughters rapidly approach radioactive equilibrium with their radon parent. Radon is soluble in water, with its solubility decreasing rapidly with increasing temperature (51.0, 22.4, 13.0 mL/100 mL at 0°C, 25°C and 50°C, respectively) (IARC, 1988). Radon is extremely volatile and is readily released from water (NCRP, 1988).
Although most of the radon produced in soil from radium is retained in the earth, where it decays to 210Po and ultimately to 206Pb, a small portion diffuses out of soil pores and enters the atmosphere. One square metre of typical soil containing radium at 0.03 Bq/g will release between 1000 and 2000 Bq of radon to the atmosphere each day (UNSCEAR, 1988). Other sources of radon include groundwater that passes through radium-bearing rocks and soils, traditional building materials such as wallboard and concrete blocks, uranium tailings, coal residues, and fossil fuel combustion.
Environmental levels of natural radionuclides may be enhanced by industrial processes, particularly uranium mining and milling operations. This source of radionuclides is particularly important in northern Saskatchewan, where most of the uranium mining activities in Canada are now located. Earlier uranium mining activities were centred in the Elliot Lake area, with drainage through the Serpent River into Lake Huron. An additional source of natural radionuclides is the uranium refining industry, with facilities at Port Hope on Lake Ontario and Blind River on Lake Huron.
The radionuclides observed are similar to those described above for groundwater sources. However, the water bodies most likely to be affected are surface water sources such as streams and lakes. At the back end of the nuclear fuel cycle, there is the possibility of natural radionuclides being leached from nuclear wastes in shallow burial sites. These radionuclides could potentially contaminate nearby shallow wells, although there are no known instances in Canada of drinking water supplies being affected by this source of radioactivity.
Other sources include fossil fuel combustion and the production and use of phosphate rock products (such as fertilizers). The combustion of fossil fuels, such as coal, for electric power generation releases 238U and 232Th decay series radionuclides and 40K in fly ash (Tracy and Prantl, 1985). Tracy and Prantl (1985) did not report any significant pathways from this source to drinking water supplies.
Cosmogenic radionuclides, which are produced naturally by continuous cosmic ray bombardment of gases in the Earth's atmosphere, provide a small additional exposure to radiation. These radionuclides are removed to the Earth and enter surface drinking water supplies by the same processes as for nuclear weapons fallout, described in Section 4.2.1 The four important cosmogenic radionuclides, 14C, tritium, 22Na, and 7Be, together contribute a total dose to humans of about 15 µSv/year (UNSCEAR, 1988). They also provide a small natural background to the artificial radionuclides described below, particularly to the tritium and 14C concentrations in surface water.
Over the past 50 years, nuclear technologies have introduced significant quantities of artificial radionuclides into the global environment. These radionuclides contribute an additional radiation exposure over and above the natural background. The majority of these radionuclides resulted from atmospheric nuclear weapons tests conducted prior to the limited ban on atmospheric testing in 1963; additional tests conducted since that time have contributed only about 6% of the total global inventory of fallout radionuclides.
The fallout radionuclides receiving the greatest attention in environmental monitoring programs have been tritium (half-life = 12 years), 14C (half-life = 5730 years), 90Sr (half-life = 29 years), and 137Cs (half-life = 30 years), owing to their persistence in the environment and their entry into food chains leading to humans. Other fission products with short and intermediate half-lives that have been routinely detected include 95Zr (half-life = 64 days), 95Nb (half-life = 95 days), 106Ru (half-life = 368 days), 131I (half-life = 8 days), and 144Ce (half-life = 284 days). The transuranic elements 239Pu, 240Pu, 241Pu, and 241Am have also been detected in global nuclear weapons fallout. The total dose received by individuals in the North Temperate Zone (40-50°N latitude), accumulated to the year 2000, for all atmospheric weapons tests conducted between 1945 and 1980 is estimated to be about 2.1 mSv (UNSCEAR, 1982).
Radionuclides from nuclear weapons testing enter the environment mainly through the atmospheric pathway. Lower-yield tests (less than 100 kt trinitrotoluene [TNT] equivalent) inject material primarily into the troposphere, where it is transported on a time scale of days to weeks around the globe. In contrast, the enormous heat generated by high-yield thermonuclear tests can lift radioactive material into the stratosphere (greater than 10 km altitude), where it may reside for months or years before returning to the lower levels of the atmosphere. Radioactive material in the atmosphere eventually settles to the ground as fallout. This may occur as a result of dry deposition (gravitational settling) or wet deposition (rainout). As a result of vertical and horizontal air circulation and mixing, traces of fallout radionuclides can be found virtually everywhere on Earth, in water, soils, and vegetation.
Fallout radionuclides may enter drinking water supplies either by direct deposition on the surfaces of rivers and lakes or by runoff of material previously deposited on land. The impact is mainly on surface water supplies, in contrast to the situation with natural radionuclides, as described above. Fallout radionuclides percolating into soils or sediments will tend to bind to particles near the surface and thus do not reach deep-lying groundwater supplies.
In addition to global fallout from nuclear weapons testing, emissions from nuclear reactors are a potential source of artificial radionuclides in the environment. Although widespread contamination of the environment could occur in the unlikely event of a major accident, such emergency situations are beyond the scope of this document.
There are seven nuclear power generating stations in Canada: The Bruce Nuclear Generating Stations A and B, located in Kincardine, Ontario, on Lake Huron; Pickering Nuclear Generating Stations A and B, located in Pickering, Ontario, on Lake Ontario; The Darlington Nuclear Generating Station, located in Bowmanville, Ontario, on Lake Ontario; the Gentilly-2 Nuclear Generating Station, located in Gentilly, Québec, on the St. Lawrence River; and the Point Lepreau Nuclear Generating Station, located in Point Lepreau, New Brunswick, on the Bay of Fundy. There are also several non-power nuclear reactors, used for scientific research and creation of certain radioisotopes for medical uses. This includes the Chalk River Laboratories, whose activities include non-power reactors, isotope production, fuel fabrication and research, tritium processing, waste management and waste treatment, and is located in Chalk River, Ontario, on the Ottawa River (CNSC, 2010). There are an additional 12 nuclear generation sites on the U.S. side of the Great Lakes basin that could have an effect on Great Lakes water quality.
In nuclear power reactors, large quantities of fission products are formed within the fuel rods, and large quantities of activation products are found in the structural materials and cooling circuits. Under normal conditions, virtually all of these fission products are contained until they undergo radioactive decay and become stable. Low levels of radionuclides are released routinely to the environment under controlled and monitored conditions, in quantities dependent on the reactor type and design. Atmospheric releases include tritium, radioiodine, fission product noble gases (88Kr, 133Xe), activation gases (14C, 16N, 35S, 41Ar), and particulates such as 60Co, 90Sr, and 137Cs. Radionuclides released into the aquatic environment include tritium and other fission products and activated corrosion products (UNSCEAR, 1988). Tritium in aqueous and gaseous emissions is the principal radionuclide released from Canadian Deuterium Uranium (CANDU) reactors.
Aquatic releases into streams and lakes can affect surface water supplies. Many of these radionuclides are readily adsorbed onto the surfaces of suspended particulates as a result of their low water solubilities and are removed from the water column by sedimentation. Examples of such radionuclides are the isotopes of cesium, manganese, iron, cobalt, and the actinides (including thorium and uranium). Elements that tend to remain in solution in water include strontium, chromium, and antimony.
The Canadian Nuclear Safety Commission (CNSC) regulates all nuclear activities in Canada. Regulations under the Nuclear Safety and Control Act address the development, production and use of nuclear energy in Canada: the production, possession, use and transport of nuclear substances; and the production, possession and use of prescribed equipment and prescribed information. The regulations also establish dose limits for the public and nuclear energy workers, with the latter partially based on the length of exposure (CNSC, 2010).
Artificial radionuclides may also be released into the environment from non-nuclear fuel cycle activities in industry and research and from usage in diagnostic and therapeutic medicine. Canadian facilities employing radionuclides are licensed by the CNSC for radionuclide usage, and their emissions into the environment are usually insignificant. The low activities and short half-lives of the radionuclides employed generally permit disposal through dilution and discharge into municipal sewer systems. Studies conducted to assess the importance of these sources show that the majority of radionuclides contained in sewer discharge are from natural or fallout origin (Durham and Joshi, 1981).