Guidelines for Canadian drinking water quality: Guideline technical document – Total coliforms
Guidelines for Canadian drinking water quality: Guideline technical document - total coliforms

Download the entire report
(PDF format, 504 KB, 40 pages)
Organization: Health Canada
Type: Guidelines
Published: 2020-06-19
Related topics
Table of contents
- Part I. Overview and application
- Part II. Science and technical considerations
- 4.0 Significance of total coliforms in drinking water
- 5.0 Analytical methods
- 6.0 Sampling for total coliforms
- 7.0 Treatment technology
- 7.1 Municipal scale
- 7.1.1 Level of treatment necessary
- 7.1.2 Physical removal
- 7.1.3 Disinfection
- 7.1.3.1 Chemical disinfection
- 7.1.3.2 UV light disinfection
- 7.1 Municipal scale
- 7.2 Residential scale
- 8.0 Risk assessment
- 9.0 Rationale
- 10.0 References
- Appendix A: Decision tree for routine microbiological testing of municipal scale systems
- Appendix B: Decision tree for routine microbiological testing of residential scale systems
- Appendix C: List of acronyms
Part I. Overview and application
1.0 Guidelines
The maximum acceptable concentration (MAC) for total coliforms in water leaving a treatment plant and in non-disinfected groundwater leaving the well is none detectable per 100 mL.
Total coliforms should be monitored in the distribution system because they are used to indicate changes in water quality. Detection of total coliforms from consecutive samples from the same site or from more than 10% of the samples collected in a given sampling period should be investigated.
2.0 Executive summary
Total coliforms are a group of bacteria that are naturally found on plants and in soils, water, and in the intestines of humans and warm-blooded animals. Because total coliforms are widespread in the environment, they can be used as one of the many operational tools to determine the efficacy of a drinking water treatment system.
Health Canada completed its review on the usefulness of total coliforms as part of a source-to-tap approach to producing microbiologically acceptable drinking water. This guideline technical document reviews and assesses available literature on the uses of total coliforms in drinking water quality management, including as indicators of groundwater vulnerability, the adequacy of disinfection, and changes in distribution system water quality.
From this review, the guidelines for total coliforms in water leaving a treatment plant and in non-disinfected groundwater leaving the well is reaffirmed as a maximum acceptable concentration of none detectable in 100 mL of water. This MAC does not apply to distribution systems where total coliforms are used to indicate changes in water quality.
2.1 Significance of total coliforms in drinking water systems and their sources
Monitoring of total coliforms should be used, in conjunction with other indicators, as part of a source-to-tap approach to producing drinking water of an acceptable quality. Total coliforms are naturally found in both fecal and non-fecal environments, so they are commonly present in both surface water and groundwater under the direct influence of surface water (GUDI) sources. Consequently, monitoring total coliforms in these sources does not provide information on the quality of the source water from the perspective of health risk. Protected groundwater systems, on the other hand, should not contain total coliforms. As their presence indicates that the groundwater may be vulnerable to contamination from the surrounding environment, detection of total coliforms in the water leaving the well should trigger further actions.
Monitoring for total coliforms at the treatment plant and in the distribution and storage system provides information on the adequacy of drinking water treatment and on the microbial condition of the distribution system. The presence of total coliforms in water leaving any treatment plant signifies that treatment has been inadequate and therefore additional actions need to be taken. These should include actions such as notifying the responsible authorities, investigating the cause of the contamination, and implementing corrective actions; which could include issuing a boil water advisory.
The presence of total coliforms in the distribution and storage system, when water tested immediately post-treatment is free of total coliforms, indicates water quality degradation, possibly via bacterial regrowth or post-treatment contamination. In municipal-scale systems, the detection of more than 10% of samples in a given sampling period, or of consecutive samples from the same site, that are positive for total coliforms indicates changes in the quality of the water and a need for follow-up actions to be initiated. In residential-scale systems where there is little or no distribution system, the presence of any total coliforms should trigger follow-up actions to investigate the cause of the positive results.
2.2 Treatment technology
Surface water or GUDI systems that meet the guidelines for enteric protozoa (minimum 3 log or 99.99% removal and/or inactivation) and enteric viruses (minimum 4 log or 99.99% removal and/or inactivation) and groundwater systems that meet the guidelines for enteric viruses (minimum 4 log or 99.99% removal and/or inactivation), will be capable of achieving the proposed MAC of none detectable per 100 mL for total coliforms.
For municipal-scale systems, it is important to apply a monitoring approach which includes the use of multiple operational and water quality verification parameters (e.g., turbidity, disinfection measurements, Escherichia coli [E. coli], total coliforms), in order to verify that the water has been adequately treated and is therefore of an acceptable microbiological quality. For residential-scale systems, regular monitoring of bacterial indicators (e.g., total coliforms and E. coli) combined with monitoring of critical processes, regular physical inspections and a source water assessment can be used to confirm the quality of the drinking water supply.
2.3 International considerations
The MAC and distribution system guidance for total coliforms are consistent with drinking water guidelines established by other countries and international organizations. The World Health Organization, the European Union, the United States Environmental Protection Agency (U.S. EPA) and the Australian National Health and Medical Research Council have all established provisions that state that total coliforms should be absent immediately after disinfection, and that their presence is indicative of inadequate treatment. They also include specific recommendations for total coliforms designed to minimize microbial risks from the drinking water distribution system.
3.0 Application of the guidelines
Note: Specific guidance related to the implementation of drinking water guidelines should be obtained from the appropriate drinking water authority in the affected jurisdiction.
Monitoring for total coliforms should be used, in conjunction with other indicators, as part of a source-to-tap approach to producing drinking water of an acceptable quality. The number, frequency, and location of samples for total coliform testing will vary according to the type and size of the system and jurisdictional requirements. For decision-making, the focus is the presence of total coliforms, regardless of quantity. However, although quantitative results are not precise, they can be used to provide an indication of the magnitude of a problem and thus inform the public health response.
3.1 Municipal-scale drinking water supply systems
3.1.1 Monitoring total coliforms in water leaving the treatment plant
Total coliforms should be monitored at least weekly in water leaving a treatment plant. If total coliforms are detected, it indicates a serious breach in treatment and is therefore unacceptable. These tests should be used in conjunction with other indicators, such as residual disinfectant and turbidity monitoring as part of a source-to-tap approach to producing drinking water of acceptable quality. While the required frequency for all testing at the treatment plant is prescribed by the responsible authority, best practice commonly involves a testing frequency beyond these minimum recommendations based upon the size of the system, the number of consumers served, the history of the system, and other site-specific considerations.
3.1.2 Monitoring total coliforms in water distribution and storage systems
In municipal-scale distribution and storage systems, the number of samples collected for total coliform testing should reflect the size of the population being served, with a minimum of four samples per month. The sampling points and testing frequencies for total coliforms, residual disinfectant, and turbidity in treated water within distribution and storage systems will be specified and/or approved by the responsible authority. As an important part of a source-to-tap approach to ensuring safe drinking water, incorporating total coliforms into a distribution and storage system monitoring strategy can, over time, provide an enhanced knowledge of water quality throughout the system as well as overall system condition. The approach should take into account the particular characteristics of the distribution and storage system and historic knowledge of the overall system such as age, layout, or materials. This strategy allows for the detection of changing conditions, intrusion of contaminants, or areas of declining water quality, which can then be investigated further.
3.1.3 Notification
The presence of any total coliform bacteria in water leaving a treatment plant indicates a serious breach in treatment and is therefore unacceptable. This situation should be corrected immediately. The system owner should notify all responsible authorities and immediately reanalyze the coliform-positive sample(s) for Escherichia coli (E. coli), resample, and test the positive site(s) to confirm the presence or absence of both E. coli and total coliforms (see Appendix A). Guidance on analytical methods for E. coli and the actions that are required if the presence of E. coli is confirmed are outlined in sections 5.0 and 3.0, respectively, of the Guidelines for Canadian Drinking Water Quality: Guideline Technical Document for E. coli (Health Canada, 2020a).
In a distribution system, coliform bacteria are operational indicators. Their presence indicates water quality degradation, possibly via bacterial regrowth or post-treatment contamination. Detection of total coliforms (in the absence of E. coli) in more than 10% of samples in a given sampling period, or from consecutive samples from the same site, should be investigated and appropriate corrective actions taken. Some or all of the corrective actions listed in section 3.1.4 may be necessary.
3.1.4 Corrective actions
The degree of response to the presence of total coliforms (in the absence of E. coli) should be discussed with the appropriate authorities and will depend on:
- A risk-based assessment of the significance and extent of the problem, taking the history of the entire system into account
- The history and variability of the quality of the raw water supply
- The documented historical effectiveness of the treatment process
- The integrity of the distribution system, including the existence and effectiveness of a cross-connection control program
Knowledge of the history of the system, including the past frequency and locations of total coliform-positive samples, enables qualified personnel to consider appropriate actions when total coliforms are detected in the absence of E. coli.
If corrective actions are deemed necessary, the owner of the drinking water treatment system, in consultation with the responsible authorities, should carry out appropriate corrective actions, which could include the following measures:
- Verify the integrity and the optimal operation of the treatment process
- Verify the integrity of the distribution system
- Verify that the required disinfectant residual is present throughout the distribution system
- Increase the disinfectant dosage, flush the water mains, clean treated water storage tanks (municipal reservoirs and domestic cisterns), and check for the presence of cross- connections and pressure losses. Water should be dechlorinated before being discharged into the environment. The responsible authority should be consulted regarding the methods available, as well as the correct procedure, for carrying out dechlorination
- Sample and test sites adjacent to the site(s) of the positive sample(s). Tests performed should include total coliforms, E. coli, disinfectant residual, and turbidity. At a minimum, one sample upstream and one sample downstream from the original sample site(s) plus the finished water from the treatment plant as it enters the distribution system should be tested. Other samples should be collected and tested following a sampling plan appropriate for the distribution system
- Conduct an investigation to identify the problem and prevent its recurrence, including a measure of raw water quality (e.g., bacteriology, colour, assimilable organic carbon [AOC], turbidity, conductivity) and variability
- Continue selected sampling and testing (e.g., bacteriology, disinfectant residual, turbidity) of all identified sites during the investigative phase to confirm the extent of the problem and to verify the success of the corrective actions
If enhanced health surveillance indicates that a waterborne outbreak may be occurring or if conditions exist that could result in a waterborne outbreak, then the necessity of issuing a boil water advisoryFootnote 1 should be discussed immediately with qualified operations personnel at the water utility and with the responsible authority. In the event that an incident that may have contaminated the distribution system or interfered with treatment is known to the owner, consumers should be notified immediately to boil the drinking water. A boil water advisory should be rescinded only after a minimum of two consecutive sets of samples, collected 24 hours apart, show negative results that demonstrate full system-wide integrity (including acceptable bacteriological quality, disinfection residuals, and/or turbidity). Additional negative results may be required by the local responsible authority. Further information on boil water advisories can be found in Guidance for Issuing and Rescinding Boil Water Advisories in Canadian Drinking Water Supplies (Health Canada, 2015).
Minimum treatment of supplies derived from surface water or GUDI sources should include adequate filtration (or technologies providing an equivalent log removal/inactivation) and disinfection to ensure the removal/inactivation of enteric protozoa and enteric viruses. For groundwater sources not under the direct influence of surface waters, adequate treatment is recommended to ensure the removal/inactivation of enteric viruses, unless exempted by the responsible authority based on site-specific considerations including historical and on-going monitoring data. In all systems with a distribution system, a disinfectant residual should be maintained at all times. The appropriate type and level of treatment should take into account the potential fluctuations in water quality, including short-term water quality degradation, and variability in treatment performance.
3.2 Residential-scale
3.2.1 Testing requirements
Sampling frequencies for residential-scaleFootnote 2 systems will be determined by the authority having jurisdiction for the system and should include times when the risk of contamination is greatest, for example, early spring after the thaw, after an extended dry spell, or following heavy rains. Owners of private supplies (such as private wells) should be encouraged to have their water tested for total coliforms during these same periods. New or rehabilitated wells should also be tested before use to confirm the microbiological quality.
3.2.2 Notification
No samples from residential-scale water supplies should contain coliforms. If a sample contains total coliform bacteria, it should be immediately reanalyzed and the positive site resampled and tested to confirm the presence or absence of both E. coli and total coliforms. If resampling confirms that the system is contaminated with E. coli, the actions required are outlined in the guideline technical document for E. coli.
Responses to total coliform-positive samples in the absence of E. coli can vary from jurisdiction to jurisdiction. As a precautionary measure, some jurisdictions will always advise the owner to boil the drinking water or use an alternative safe source as an interim measure until corrective actions are taken. In other jurisdictions, advice on interim measures is site-specific and depends on such factors as the historical water quality data, the health status of the users, and delays in investigation. Regardless of whether a boil water advisory is issued, the source of the coliforms needs to be investigated, and appropriate actions need to be taken (see Appendix B). These may include some or all of the corrective actions outlined in the following sections.
3.2.3 Corrective actions for disinfected supplies
The first step is to verify the physical condition of the drinking water system, as applicable, including water intake, well, well head, pump, treatment system (including chemical feed equipment, if present), plumbing, and surrounding area. Any identified faults should be corrected before proceeding. If all the physical conditions are acceptable, some or all of the following corrective actions may be necessary:
- In a chlorinated system, verify that a disinfectant residual is present throughout the system. Increase the disinfectant dosage, flush the system thoroughly, and clean treated- water storage tanks and domestic cisterns. Water should be dechlorinated before being discharged into the environment. The responsible authority should be consulted regarding the methods available, as well as the correct procedure, for carrying out dechlorination
- For systems where the disinfection technology does not leave a disinfectant residual, such as ultraviolet (UV), it may be necessary to shock-chlorinate the well and plumbing system; further information on shock-chlorination is available in the factsheet on wells, available at Well water and health: Protect and maintain your well
- Ensure that the disinfection system is working properly, and maintained according to manufacturer's instructions
After the necessary corrective actions have been taken, samples should be collected and tested for both total coliforms and E. coli to confirm that the problem has been corrected. If total coliforms are detected after implementing these corrective actions, a boil water advisory should be issued, if one is not already in place. Alternatively, a source of water known to be safe should be used until the situation is corrected. The presence of total coliforms after corrective actions suggests that the system remains vulnerable to contamination. If the problem cannot be corrected, additional treatment or a new source of drinking water may need to be considered. In some instances, in residential-scale systems, the presence of coliform bacteria may be the result of bacterial regrowth within the distribution system biofilm as opposed to the intrusion of contaminants, and therefore a boil water advisory may not be necessary. This determination would need to be made by qualified personnel using knowledge of the history of the system and other site-specific considerations.
Minimum treatment of supplies derived from surface water or GUDI sources should include adequate filtration (or technologies providing an equivalent log reduction credit) and disinfection to ensure the removal/inactivation of enteric protozoa and enteric viruses. For groundwater sources not under the direct influence of surface water, adequate treatment is recommended to ensure the removal/inactivation of enteric viruses, unless exempted by the responsible authority based on site-specific considerations including historical and on-going monitoring data.
3.2.4 Corrective actions for non-disinfected wells
The first step, if it has not already been taken, is to verify the physical condition of the well, well head, pump, plumbing, and surrounding area. Any identified faults should be corrected before proceeding. If all the physical conditions are acceptable, then the following corrective actions should be carried out:
- Shock-chlorinate the well and plumbing system. Further information on this topic is available in the factsheet on wells (available at Corrective actions for non-disinfected wells)
- Flush the system thoroughly and retest to confirm that the water is free of total coliform contamination. Confirmatory tests should be done no sooner than either 48 hours after tests indicate the absence of a chlorine residual or five days after the well has been treated. Local conditions may determine acceptable practice. Water should be dechlorinated before being discharged into the environment. The responsible authority should be consulted regarding the methods available, and the correct procedure, for carrying out dechlorination
If total coliforms are detected after implementing these corrective actions, a boil water advisory should be issued, if one is not already in place. Alternatively, a source of water known to be safe should be used until the situation is corrected. The presence of total coliforms after shock-chlorination and flushing suggests that the well remains vulnerable to contamination. If the problem cannot be reasonably identified or corrected, an appropriate disinfection device or well reconstruction or replacement should be considered. In some instances, in residential-scale systems, the presence of coliform bacteria may be the result of bacterial regrowth within the distribution system biofilm as opposed to on-going contamination, and therefore a boil water advisory may not be necessary. This determination would need to be made by qualified personnel using knowledge of the history of the system and other site-specific considerations.
A single negative total coliforms test result does not necessarily indicate that the problem has been corrected. A minimum of two consecutive total coliform-negative samples should be obtained. An additional test should be taken after three to four months to ensure that the contamination has not reoccurred. Over the long term, only a history of bacteriological and operational monitoring in conjunction with regular physical inspections and a source water assessment can be used to confirm the quality of a drinking water supply.
Part II. Science and technical considerations
4.0 Significance of total coliforms in drinking water
4.1 Description
Total coliforms belong within the family Enterobacteriaceae and have traditionally been defined based on their phenotypic characteristics, for example, their ability to ferment lactose, producing gas and acid (Leclerc et al., 2001; Rompré et al., 2002). Standard Methods for the Examination of Water and Wastewater (APHA et al., 2017) characterizes them as follows:
- All facultative anaerobic, Gram-negative, non-spore-forming, rod-shaped bacteria that ferment lactose with gas and acid formation within 48 hours at 35°C
- Many facultative anaerobic, Gram-negative, non-spore-forming, rod-shaped bacteria that develop red colonies with a metallic (golden) sheen within 24 hours at 35°C on an Endo- type medium containing lactose
- All bacteria possessing the enzyme β-galactosidase, which cleaves a chromogenic substrate (e.g., ortho-nitrophenyl-β-D-galactopyranoside), resulting in the release of a chromogen (e.g., ortho-nitrophenol)
These definitions are not to be regarded as identical; rather, they refer to three groups of coliforms that are roughly equivalent. All three groups contain various species of the genera Escherichia, Klebsiella, Enterobacter, Citrobacter, Serratia, and many others (Leclerc et al., 2001). It is important to note that non-fecal origins and/or adaptations to the environment have been found for organisms from all genera (Table 1) (Leclerc et al., 2001; Brennan et al. 2010; Byappanahalli and Ishii, 2011). Since phenotypic traits can be influenced by stress conditions, they cannot be consistently relied upon to detect all total coliforms all the time (e.g., injured organisms). Molecular characterization methods (see Section 5.2), including typing and sequencing, address this problem, and have led to a revised definition of total coliforms based on genetic relatedness (Fricker and Fricker, 1994; Maheux et al., 2014; Zhang et al., 2015). The total coliform group consists of 20 genera and over 80 species, including anaerogenic (producing little or no gas) lactose-fermenting microorganisms (Stevens et al., 2003; Brenner et al., 2005; Carrero-Colón et al., 2011; Maheux et al., 2014; APHA et al., 2017). Although not included in the coliform group, members of the genus Aeromonas can ferment lactose and possess β-galactosidase; therefore, they can yield false-positive total coliform reactions. Aeromonas species are ubiquitous in the environment, and have been found in lakes, rivers, marine waters, sewage effluents, drinking waters, and other places (Allen et al., 1983; Nakano et al., 1990; Poffe and Op de Beeck, 1991; Payment et al., 1993; Ashbolt et al., 1995; Bernagozzi et al., 1995; Chauret et al., 2001; El-Taweel and Shaban, 2001). Information on excluding false-positives resulting from the presence of Aeromonas is included in Section 5.0.
A subset of the total coliform group, known as the thermotolerant coliforms, (coliforms that have the ability to ferment lactose at 44 to 45ºC previously referred to as fecal coliforms) were routinely used as fecal indicators since they were considered more fecal-specific than total coliforms. By definition, thermotolerant coliforms include the portion of the total coliform group capable of forming gas within 24 hours at 44.5°C or that produce a blue colony on m-FC broth within 24 hours at 44.5°C (APHA et al., 2017). This group includes members of the genera Escherichia, which are fecal specific, as well as organisms that are found in both fecal and non-fecal environments such as Klebsiella, Enterobacter, and Citrobacter. Advances in E. coli detection methods have made thermotolerant coliform testing in drinking water quality management redundant.
| Coliform Group | ONPGTable 1 Footnote b | Faecal origin | Non-faecal origin |
|---|---|---|---|
| Budvicia | + | - | + |
| Citrobacter | + | + | + |
| Enterobacter | + | + | + |
| Erwinia | + | - | + |
| Escherichia | + | + | +Table 1 Footnote c |
| Klebsiella | + | + | + |
| Leclercia | + | - | + |
| Pantoea | + | - | + |
| Serratia | + | - | + |
Table 1 Footnotes
|
|||
4.2 Sources
The total coliform group is composed of various genera with similar characteristics. The natural niches for members of this group range from being predominantly fecal specific, such as E. coli, to being widely distributed in the water, soil, and vegetation (Leclerc et al., 2001; Rompré et al., 2002). Many coliform bacteria are not specific to any one source and are present in both fecal and non-fecal environments. A comparison of total coliforms within a specific environment has shown that some members of the coliform group can consistently be found in higher concentrations in that source. For example, analysis of the coliform complement of fecal matter found Klebsiella, Citrobacter, and Enterobacter present in small numbers compared with E. coli (Edberg et al., 2000). In contrast, the majority of coliforms isolated from a distribution system were Klebsiella, and to a lesser extent Enterobacter, Pantoea, Escherichia, Citrobacter, Leclercia, and Serratia (Geldreich, 1987; Edberg et al., 2000; Blanch, 2007).
4.2.1 Groundwater
Total coliforms have been detected in various groundwater sources in Canada (Simpson, 2004; Payment and Locas, 2005; Locas et al., 2007, 2008; Hynds et al., 2012, 2014; Maier et al., 2014) and elsewhere (Amundson et al., 1988; Francy et al. 2000; Borchardt et al., 2003; Embrey and Runkle, 2006; Allevi et al., 2013; Atherholt et al. 2013, 2015, 2017), indicating that the groundwater is likely being impacted by surface or subsurface contamination, which may include fecal sources (DeSimone et al., 2009; Kozuskanich et al. 2011). Microbial contamination of groundwater sources can occur through various means, including infiltrating surface water, storm water, or point sources (e.g., septic tank effluents). The presence and concentration of total coliforms in groundwater can vary significantly, and is dependent on how efficiently these organisms can be transported from surface and subsurface sources. Transport of total coliforms is affected by a number of factors, including:
- Aquifer type and geology (Embrey and Runkle, 2006; DeSimone et al., 2009; Toccalino and Hopple, 2010; Atherholt et al., 2013; Somaratne and Hallas, 2015)
- Well depth (Glanville et al., 1997; Gonzales, 2008; Allevi et al., 2013)
- Well integrity (Bacci and Chapman, 2011; Swistock et al., 2013; Hynds et al. 2012, 2014; Somaratne and Hallas, 2015)
- Precipitation (Reid et al., 2003; Bacci and Chapman, 2011; Hynds et al., 2012; O'Dwyer et al., 2014; Procopio et al., 2017)
A number of studies have highlighted the impact of aquifer type on microorganism transport (Scandura and Sobsey, 1997; Powell et al., 2003; Embrey and Runkle, 2006; Borchardt et al., 2007; DeSimone et al., 2009; Toccalino and Hopple, 2010; Borchardt et al., 2011; Levison and Novakowski, 2012; Atherholt et al., 2013; Kozuskanich et al., 2014; Somaratne and Hallas, 2015). In general, groundwater supplies in karst and fractured bedrock aquifers are considered highly vulnerable to contamination because groundwater velocity and microorganism transport can be rapid. This was observed in a South Australian study, where higher coliform detections and levels were reported for karst and fractured rock aquifers; subtyping results showed that a variety of coliform species, including some of potentially fecal origin (e.g., Hafnia), were present in these groundwater supplies (Somaratne and Hallas, 2015).
Well characteristics, such as depth and integrity, can affect coliform presence. Shallow wells have been associated with higher total coliform detections (Sworobuk et al., 1987; Glanville et al., 1997; Gonzales, 2008; Allevi et al., 2013). Wells with integrity issues, such as cracked casings, have been correlated with higher total coliform concentrations (Lamka et al., 1980; Sworobuk et al., 1987; Bacci and Chapman, 2011; Swistock et al., 2013; Hynds et al., 2012, 2014; Somaratne and Hallas, 2015).
Rainfall events can have a significant impact on groundwater quality, by facilitating transportation of coliforms (and other microorganisms) from the surface (Goss et al., 1998; Fenlon et al., 2000; Samarajeewa et al., 2012; Méric et al., 2013) and subsurface (Krapac et al., 2002; Karathanasis et al., 2006; Bradbury et al., 2013; Hynds et al., 2014). Several studies have noted the impact of precipitation on coliform detection in groundwater (Reid et al., 2003; Bacci and Chapman, 2011; Hynds et al., 2012; Page et al., 2012; O'Dwyer et al., 2014). In a study by Procopio et al. (2017), the authors assessed the impact of antecedent precipitation on coliform detection rates in domestic wells. Total coliforms were detected more frequently when the cumulative precipitation in the 10 days prior to sampling exceeded 34.5 mm. An impact of seasonality was also noted by the authors, with increased coliform detection rates in the summer and autumn. This is consistent with the findings of others, who also noted increased coliform detections during specific times of the year (Raina et al., 1999; Rutter et al., 2000; Reid et al., 2003; Richardson et al., 2009; Atherholt et al., 2017).
4.2.2 Drinking water distribution systems
The drinking water distribution system is a complex environment, consisting of both resident and transient populations of microorganisms: the combined genetic material of these microorganisms is known as the microbiome. The microbiome is impacted by numerous factors, including nutrient availability, predation, and disinfectants (Zhang et al., 2017). Researchers have shown that microbiomes are very heterogeneous, as well as time and site-specific (within and between distribution systems) (Gomez-Alvarez et al., 2012; Chao et al., 2013, 2015; Delafont et al., 2013: Wang et al., 2014; Zhang et al., 2017).
Coliforms have been isolated from a number of drinking water distribution systems in Canada (Coulibaly and Rodriguez, 2003, 2004; Besner et al., 2007; Gagnon et al., 2007; Locas et al., 2007; Payne et al., 2010) and elsewhere (LeChevallier, 1990; LeChevallier et al.,1996; Karim et al., 2003; Kilb et al., 2003; Batté et al., 2006; Mosher, 2011). They can be found in bulk water samples, and are associated with biofilm (Morin et al., 1996; Camper et al., 1999; Lee and Kim, 2003: Lee et al., 2005; Batté et al., 2006; Sakyi et al., 2012; Lee, 2013). Several studies have demonstrated the potential for coliforms, and other microbial contaminants, to enter the distribution system (Karim et al., 2003; LeChevallier et al., 2003; Besner et al, 2010, 2011; Yang et al., 2011; Ebacher et al., 2012; Fontanazza et al., 2015). Their presence in the distribution system is indicative of bacterial regrowth, system integrity issues (e.g., watermain break, system leaks, negative pressure events) and/or post-treatment contamination (e.g., cross-connections, non-sanitary construction/repairs) (Kirmeyer et al. 1999).
Once total coliforms are present in the distribution system, they can colonize and grow in the biofilm on pipe surfaces or in deposits. A number of factors can influence bacterial regrowth in the distribution system, including: type and concentration of organic and inorganic nutrients; type and concentration of residual disinfectant; biofilms and sediments; and distribution system conditions (e.g., disinfectant residual decay, water temperature, residence time, hydraulic conditions, pipe material and diameter, pH, corrosion rate) (LeChevallier et al., 1991b, 1996, 2015a; Besner et al., 2001, 2002, 2010, 2011; Escobar and Randall, 2001; Blanch et al., 2007; Lee, 2013; Prest et al., 2016a,b). For a comprehensive review of the impact of these factors on microbial growth in the drinking water distribution system, please refer to van der Kooij and van der Wielen, 2014; WHO, 2014; LeChevallier et al., 2015b; Prest et al., 2016a.
Biodegradable organic matter (BOM) impacts distribution system water quality by providing a source of nutrients that contributes to bacterial regrowth and biofilm development. Levels of BOM (e.g., assimilable and biodegradable organic carbon) are only one component influencing changes in water quality in the distribution system (Prest et al., 2016a,b). Other compounds have been identified as having roles in controlling microbial growth in the distribution system, including phosphorus, ammonia, manganese, iron and humic substances (Camper, 2004; Prest et al., 2016a,b; Health Canada, 2020b).
Pipe material can also impact bacterial regrowth and biofilm formation in the drinking water distribution system, although there does not appear to be a consensus regarding which materials most favour bacterial adhesion and growth (Haas et al., 1983; Schwartz et al., 1998; Niquette et al., 2000; Donlan, 2002; U.S. EPA, 2002; van der Kooij et al., 2005; White et al., 2011; Camper, 2014). In contrast, some have not observed any significant difference in bacterial concentrations or diversity among different pipe materials (i.e., stainless steel, Polyvinyl chloride [PVC] and polyethylene [PE]) (Pedersen, 1990; Zacheus et al., 2000; Wingender and Flemming, 2004).
Biofilms provide a habitat for the survival of microbial indicators, including total coliforms, and fecal pathogens that may have passed through drinking water treatment barriers or entered the distribution system directly via an integrity breach (Leclerc, 2003). Enteric pathogens have been detected in biofilms (Park et al., 2001; Howe et al., 2002; LeChevallier et al., 2003; Chang and Jung, 2004; Berry et al., 2006; September et al., 2007; Gomez-Alvarez et al., 2015; Revetta et al., 2016); although these organisms cannot grow in this environment, they can accumulate and be released over an extended period of time (Howe et al., 2002; Warnecke, 2006; Wingender and Flemming, 2011). Additionally, opportunistic premise plumbing pathogens (OPPPs), such as Legionella pneumophila and non-tuberculous mycobacteria (e.g., M. avium, M. intracellulare), have adapted to grow and persist in distribution and plumbing system biofilms. The potential for the multiplication of OPPPs in distribution system and plumbing system biofilms is of concern to the water utilities, especially in light of the fact that their presence does not correlate with that of microbial indicators (Falkinham et al., 2015; Wang et al., 2017).
It is difficult to eliminate total coliforms once they have colonized biofilm matrices in distribution systems as the biofilm can shield the coliforms from disinfection and other eradication measures (Martin et al., 1982; Geldreich and Rice, 1987). Detachment of colonized biofilm into the bulk water can result in total coliform detections in the distribution system (McMath, 1999). For example, activities such as hydrant tests and fire-fighting can cause surges in water mains resulting in the sloughing of biofilm and a subsequent rise in total coliform bacterial counts (Kirmeyer et al., 1999).
Overall, in the complex environment of the distribution system, total coliforms remain a useful indicator of potential bacterial regrowth and/or post-treatment contamination. Additional information on the drinking water distribution system can be found in Guidance on monitoring the biological stability of drinking water in distribution systems (Health Canada, 2020b).
4.3 Role of total coliforms in maintaining drinking water quality
The best means of safeguarding against the presence of waterborne pathogens in drinking water is the application of the source-to-tap approach. This approach should include an assessment of the entire drinking water system, from the watershed or aquifer and intake through the treatment and distribution chain to the consumer, to assess the potential effects on drinking water quality and public health. Total coliforms are one of several indicators that are used as part of this approach. Their role in maintaining drinking water quality varies depending on where they are being measured in the drinking water system.
4.3.1 Role in source water monitoring
Because total coliforms are present in both fecal and non-fecal environments, they are not good indicators of fecal contamination. Consequently, monitoring total coliforms in raw surface water or GUDI sources does not provide information on the quality of the source water from the perspective of health risk. Other means of assessing surface waters and GUDI sources should be used to identify potential sources of fecal contamination in the watershed or aquifer that may affect the quality of the water.
4.3.1.1 Groundwater considerations
Total coliform presence in groundwater sources can be used to indicate that the groundwater source may be vulnerable to contamination. Groundwater is an important source of drinking water throughout the world (Chilton and Seiler, 2006), and it is often consumed with little or no treatment (Pedley et al., 2006). In Canada, 10% (3.3 million) of the population relies on a groundwater source (Statistics Canada, 2013a,b). Consuming fecally contaminated groundwater that is untreated or inadequately treated has been linked to illness, disease outbreaks (Yoder et al., 2008; Borchardt et al. 2011, 2012; Brunkard et al., 2011; Zhou et al. 2012; Gunnarsdóttir et al., 2013; Hilborn et al. 2013; Jack et al. 2013; Cho et al. 2014; Wallender et al. 2014; Beer et al., 2015; Guzman-Herrador et al. 2015). Uhlmann et al. (2009) estimated that individuals drinking water from (mostly untreated groundwater) private wells, in a region of British Columbia, were 5.2 times more likely to develop acute gastrointestinal illness (AGI) than those drinking water from community (treated surface and groundwater) supplies. Borchardt et al. (2012) determined that between 6 and 22% of self-reported AGI cases in 13 Wisconsin communities were attributable to viruses in tap water from non-disinfected community groundwater supplies. Murphy et al. (2016a) estimated the burden of endemic AGI annually in Canada from tap water. The authors estimated that 103,230 AGI cases per year, or 0.003 cases/person-year, are due to the presence of pathogens in drinking water from private and small community water systems (defined by the authors as those serving less than 1000 people). Small community water systems relying on groundwater are estimated to account for 13,034 AGI cases per year, with the highest incidence, 0.027 cases/person-year, amongst systems without treatment (Murphy et al., 2016a).
There is some research supporting a link between the presence of pathogens and total coliforms in groundwaters (Abbaszadegan et al., 2003; Locas et al., 2007). Abbaszadegan et al. (2003) assessed the occurrence of viruses and microbial indicators in groundwater samples from 35 states in the United States (U.S.). Groundwater sources were located in a variety of hydrogeological settings and some were known to be under the influence of surface water. The authors reported that, overall, there was no significant correlation between microbial indicators, including total coliforms, and the presence of viruses. However, for sites where repeated sampling was conducted, there was an increased likelihood that samples testing positive for viruses would also test positive for microbial indicators, including total coliforms (i.e., positive predictive value increased) (Abbaszadegan et al., 2003). Borchardt et al. (2003) tested water samples from wells located in various geological strata, including highly vulnerable strata (e.g., fractured dolomite), for the presence of enteric viruses and water quality indicators. Twenty-five percent of the virus-impacted well samples were positive for total coliforms (Borchardt et al., 2003). Borchardt et al., (2004) did not detect any microbial indicators, including total coliforms, in municipal groundwater wells of varying susceptibility to surface water infiltration, in which enteric viruses were detected (using reverse transcription polymerase chain reaction [RT-PCR]). During an investigation of a large groundwater outbreak of gastrointestinal illness on South Bass Island, Ohio, no virus-indicator relationships were observed (Fong et al., 2007; O'Reilly et al., 2007). Similarly, no coliforms were detected in an untreated groundwater supply associated with a community campylobacteriosis outbreak in Finland (Kuusi et al., 2005).
A meta-analysis conducted by Hynds et al. (2014) focused on studies of groundwater systems in Canada and the U.S., between 1990 and 2013, that had reported contamination by enteric pathogens. No significant correlation was observed between enteric pathogens and total coliforms in groundwater. More recently, Fout et al. (2017) conducted a meta-analysis which included raw data from 12 international groundwater studies of 718 public drinking water systems located in different hydrogeological environments. Correlations between virus and indicator occurrence were assessed at the sample and well level. The authors concluded that total coliforms were statistically associated with both culturable virus and virus detected using RT-PCR, at the well level (Fout et al., 2017). Fout et al. (2017) also noted that the strength of the associations between microbial indicators and viruses changed depending on the hydrogeological setting, indicating the need for site-specific assessments. However, because total coliforms only indicate a vulnerability to contamination, they may be present without pathogens being detected (Borchardt, 2003, Marrero-Ortiz 2009).
The absence of total coliforms in a single sample does not necessarily indicate that the groundwater is less vulnerable to fecal contamination. There is some research that suggests groundwater sources should be sampled multiple times (i.e., 10 or more) to determine their sanitary status (Atherholt, 2003). Fout et al. (2017) observed that repeat sampling for indicators, including total coliforms, improved their positive predictive value, and resulted in an improved understanding of the well's susceptibility to virus contamination. This supports the recommendation that a history of bacteriological sampling, together with a site-specific assessment and other contaminant monitoring, is needed to understand the quality of the groundwater. Collection and analysis of larger water samples may also be beneficial for determining whether a groundwater is vulnerable to contamination (Fujioka and Yoneyama, 2001; Atherholt 2003). In an investigation of three outbreaks in Finland caused by Campylobacter from groundwater sources, Hanninen (2003) showed that large volumes (1000 to 2000 mL) of water needed to be collected to detect indicator bacteria (E. coli) in the well water. Although collecting larger samples can provide additional information, there can be difficulties associated with analyzing large volumes of water using the current standard methods.
Given the body of literature detailing the lack of correlation between total coliforms and enteric pathogens in groundwater sources, it is clear that they are not good indicators of pathogen presence in these sources. However, since their prevalence and concentrations appear to increase in situations of increased susceptibility to intrusion (e.g., after significant rainfall events, in vulnerable aquifer and geological strata types, in wells with compromised integrity), total coliforms are useful as indicators of the susceptibility of groundwater sources to contamination.
4.3.2 Role in treatment and distribution system monitoring
Distribution system contamination has been linked to a number of disease outbreaks (Craun and Calderon, 2001; Lee et al., 2002; Blackburn et al. 2004; Nygard et al. 2004, 2007; Hunter et al. 2005; Liang et al. 2006; NRC, 2006; Blokker et al., 2014). Although these outbreaks were associated with events that affected the integrity of the distribution system (e.g., watermain breaks), low level and/or intermittent contamination also occurs and contributes to endemic illness (Egorov et al., 2002; Hunter et al., 2005; Nygård et al., 2004, 2007; Messner et al., 2006; Córdoba et al., 2010; Lambertini et al., 2012; Murphy et al., 2016b). Messner et al. (2006) estimated the cases of highly credible gastrointestinal illness (HCGI) associated with drinking water among community water supplies in the U.S. The authors estimated that half of the HCGI cases (i.e., 0.02-0.06 cases per person-year) could be attributed to the distribution system. Lambertini et al. (2012) estimated the risk of AGI due to virus contamination of the distribution system in 14 municipal groundwater systems where UV disinfection had been implemented, without a chlorine residual. The AGI risk from distribution system contamination was calculated, and ranged from 0.0180 to 0.0611 episodes/person-year. These findings highlight the importance of distribution system integrity. Murphy et al. (2016b) estimated the burden of endemic AGI annually in Canada from tap water. The authors estimated that 334,966 AGI cases per year were associated with the consumption of tap water from municipal systems that serve over1000 people in Canada; and that over 35% of these cases were attributable to the distribution system. It is clear that controlling water quality within the distribution system has a significant impact on public health.
The use of total coliforms for predicting the presence or absence of enteric pathogens in treated drinking water is hindered by the fact that enteric pathogens have different removal rates through physical processes, and dissimilar resistance to disinfectants (Hijnen and Medema, 2010). As evidence of this, enteric pathogens have been detected in filtered, treated drinking water meeting existing regulatory standards and have been linked to waterborne disease outbreaks (LeChevallier et al., 1991a; Craun et al., 1997; Marshall et al., 1997; Nwachuku et al., 2002; Aboytes et al., 2004). Despite this, as operational indicators, total coliforms provide information on the adequacy of drinking water treatment and on the microbial condition of the distribution system. In a treated drinking water system, where each barrier in the drinking water system has been controlled to ensure that it is operating adequately based on the quality of the source water, total coliforms can be used as part of the verification process to show that the water has been adequately treated and is of an acceptable microbiological quality as it leaves the treatment plant. The presence of any total coliform bacteria in water leaving a treatment plant shows inadequate treatment, is unacceptable, and should be corrected immediately.
If total coliforms are absent from water leaving the treatment plant but are detected in the distribution system, bacterial regrowth or post-treatment contamination may have occurred. Several studies (LeChevallier et al., 1987; Le Chevallier and McFeters, 1990; Edberg et al., 1994) have documented that Enterobacter and Klebsiella frequently colonize the interior surfaces of water mains and storage tanks when conditions are favourable. Post-treatment contamination (e.g., through cross-connections, back siphonage, low pressure events), contamination of storage reservoirs, and contamination of watermains from repairs have been identified as causes of distribution system contamination linked to illness (Craun and Calderon, 2001; Hunter, 2005). A U.S. study comparing water systems for the presence of outbreaks and violations of the U.S. EPA's revised Total Coliform Rule found no significant difference in total coliform violations between areas with and without outbreaks of waterborne illness (Nwachuku et al., 2002). Therefore, although the presence of total coliforms in the absence of E. coli is of no immediate public health significance, total coliform detection should trigger an investigation and corrective actions in order to maintain the overall bacteriological quality of the water. Corrective actions, such as routine distribution system flushing, have been reported to help limit microbial regrowth in the distribution system (Lehtola, 2004).
Flushing (e.g., conventional flushing, unidirectional flushing) and chlorination are important corrective actions in response to fecal contamination or microbiological water quality deterioration issues (Szabo and Minamyer, 2014). However, if not properly implemented, flushing techniques can mobilize and spread deposits and contamination within the distribution system instead of facilitating their controlled removal (Hill et al., 2018). It is therefore important that water utilities identify and implement the most appropriate flushing technique for correcting or providing help with the particular microbiological water quality issue. Guidance for water utilities on managing water quality in the drinking water distribution system can be found in Guidance on monitoring the biological stability of drinking water in distribution systems (Health Canada, 2020b).
Despite their limitations, the presence of total coliforms in the drinking water distribution system is an indication of deterioration in microbiological quality. Other indicators can be used in conjunction with total coliform testing to assess the conditions that favour microbiological growth or intrusion of untreated water into the distribution system. These other indicators include turbidity, disinfectant residual, E. coli, aerobic endospores, enterococci, and coliphage monitoring (LeChevallier et al., 2006; Cartier, 2009; Health Canada, 2020a, 2013).
4.3.3 Considerations for residential-scale systems
Murphy et al. (2016a) estimated that private wells accounted for over 75% (78,073) of the estimated 103,230 AGI cases per year due to the presence of pathogens in drinking water from private and small community water systems in Canada. In North Carolina, DeFelice et al. (2016) estimated the total number of emergency department visits for AGI per year attributable to microbiological contamination of unregulated private wells. The authors estimated that over 7% of emergency department visits from AGI, between 2007 and 2013, were related to microbial contamination of drinking water; and that of those cases related to drinking water, almost 99% were associated with the consumption of water from contaminated private wells. Their analyses also revealed a linkage between the prevalence of total coliforms and emergency room visits for AGI in one county. A 3% increase in visits was noted for every 10% increase in total coliform prevalence in private wells, providing support for the usefulness of this bacterial group as a meaningful indicator in untreated well water (DeFelice et al., 2016).
In disinfected residential-scale systems, total coliforms are considered operational indicators. Their presence provides evidence of the inadequacy of disinfection or deterioration of water quality in the system. The presence of total coliforms in non-disinfected wells indicates that the well is either prone to surface water infiltration and therefore at risk of fecal contamination, or that bacterial regrowth is occurring within the well or plumbing system (if the sample is not taken directly from the well). Implementation of corrective actions, such as shock- chlorination and flushing, provides valuable information on the source of the total coliform bacteria. Microbial regrowth problems should be solved after these actions have been taken. The continued presence of total coliforms after shock-chlorination is probably the result of infiltration, indicating that the system is vulnerable to contamination with pathogenic microorganisms. The extent of the contamination (e.g., how many samples tested positive and the locations where they were collected) can also be used to aid in determining the cause of the contamination, interim protective measures, and the necessary corrective actions. Examples of corrective actions are outlined in section 3.2.
5.0 Analytical methods
All analyses for total coliforms should be carried out as directed by the responsible authority. In many cases, the responsible authority will recommend or require the use of accredited laboratories. In some cases, it may be necessary to use other means to analyze samples in a timely manner, such as non-accredited laboratories or on-site testing using commercial test kits by trained operators. To ensure reliable results, a quality assurance (QA) program, which incorporates quality control (QC) practices, should be in place. In addition to the QA/QC program, any test kits used should meet minimum requirements for accuracy, detection (sensitivity), and reproducibility, and be used according the manufacturer's instructions.
5.1 Culture-based methods
The Standard Methods for the Examination of Water and Wastewater (APHA et al., 2017) outlines the methods used for routine monitoring of total coliforms in Canadian drinking water: culture-based methods for the detection of total coliforms, including the presence-absence technique, membrane filtration (MF) technique, the multiple tube filtration (MTF) technique and an enzymatic substrate test (APHA et al., 2017). Formulations of the enzyme substrate are available commercially for use in a MTF, multi-well or presence-absence format (APHA et al., 2017). ISO Methods 9308-1, 9308-2 and 9308-3 are the specified methods of water analysis for total coliforms under the European Union (EU) Council Directive (EU, 1998; ISO, 1998, 2012, 2014). The U.S. EPA has approved several methods for the detection of total coliforms in water (U.S. EPA, 2017). Table 2 summarizes the features of the above methods.
5.1.1 Performance of culture based detection methods
A number of comparison studies have been conducted to assess culture-based methods, and have reported varying detection rates (Covert et al., 1989; Brenner et al., 1993; Hallas et al., 2003; Bernasconi et al., 2006; Chao, 2006; Olstadt et al., 2007; Maheux et al., 2008). A wide range of false positive rates have also been reported, ranging from 10% to 37.5%; and false negative rates as high as 48.6% have been reported (Covert et al., 1989; Brenner et al., 1993; Hallas et al., 2003; Bernasconi et al., 2006; Chao, 2006; Olstadt et al., 2007; Maheux et al., 2008, 2014). Maheux et al. (2015) compared four commercially-available culture-based methods (chromogenic test kits), and observed that their ability to detect total coliforms can vary significantly. The authors reported a detection difference of 47.2% between the best and worst performing methods when assessing total coliform strains of known origin. When these methods were used to assess well water samples (from Quebec), only two showed concordance - these two methods detected significantly more total coliforms than the others. Zhang et al. (2015) compared isolates detected using 12 coliform culture-based methods, approved by the U.S. EPA, by 16S rRNA gene sequencing. The authors reported a wide range of variability among the culture-based methods in detecting total coliforms, with false positive rates between 9.1% and 65.7%; and false negative rates ranged from 0.9% and 28.2%. These studies highlight the importance of analytical quality control measures, such as the use of multiple detection methods, when appropriate, performing repeat counts or duplicate analyses, and regular verification using known positive and negative controls.
There is a need to further evaluate the efficacy of these testing methods, and to improve their sensitivity and specificity. Water utilities should establish performance measures regarding method sensitivity and specificity when selecting analytical methods for internal analysis or when purchasing laboratory services.
5.2 Molecular methods
Given the issues with culture-based methods, molecular detection methods have been explored with limited success (Bej et al., 1990; Fricker and Friker, 1994; Tantawiwat et al., 2005; Liu and Stahl, 2007; Shaban et al., 2008; Worakhunpiset and Thampoophasiam, 2009; Fatemeh et al., 2014). The most significant challenge associated with drinking water analysis is the stricter limit for indicator organism presence, and therefore, the need for method sensitivity at very low concentrations. No molecular methods have been approved for drinking water compliance monitoring, and researchers continue to optimize approaches to detection (Figueras and Borrego, 2010; Maheux et al., 2014, 2017; Molina et al., 2015). Maheux et al. (2014) developed a polymerase chain reaction (PCR)-based assay, paired with a bacterial concentration and recovery procedure, aimed at detecting total coliforms in potable (well) water samples. The authors examined three gene targets: lacZ, wecG and 16S rRNA, along with two culture-based methods. The 16S rRNA molecular assay proved to be as sensitive as the culture-based methods, and only required five hours for analysis (Maheux et al., 2014). More work is needed in this area to develop standardized methods that can be used accurately, reliably and affordably.
5.3 Online methods
A number of biological sensor technologies are available for real-time detection of microorganisms or microbial activity, including: adenosine triphosphate (ATP), flow cytometry and multi-angle light scattering (Miles et al., 2011; Storey et al., 2011; Samendra et al., 2014). ATP measurements have been applied at full-scale to address increased biological activity in the distribution system and associated low chlorine residuals (Shurtz et al., 2017). The other technologies can inform operators that water quality is degrading and a situation requires action (see Section 7.3), however, further improvements are needed before widespread use materializes (Miles et al., 2011; Samendra et al., 2014). Online monitoring tools are promising for future applications and development is progressing rapidly (Samendra et al., 2014; Ikonen et al., 2017). These and other monitoring methods and tools are further discussed in Guidance on monitoring the biological stability of drinking water in distribution systems (Health Canada, 2020b).
| Method | Organization/ Manufacturer | Media | Basis for detection | Detection criteria | Time to obtain results |
|---|---|---|---|---|---|
| Presence-absence (P-A) | |||||
| 9221 D.2 (Presumptive Phase) | Standard Methods | P-A culture broth | Lactose fermentation | Yellow color (with or without gas production) | 24-48 h |
| 9221 D.3 (Confirmed Phase) | Standard Methods | Brilliant green lactose bile broth (BGLB) | Lactose fermentation | Gas and/or acid production | Up to 48 h |
| Multiple tube fermentation (MTF) | |||||
| 9221 B.2 (Presumptive Phase) | Standard Methods | Lauryl tryptose broth | Lactose fermentation | Gas and/or acid production | 24-48 h |
| 9221 B.3 (Confirmed Phase) | Standard Methods | BGLB | Lactose fermentation | Gas and/or acid production | Up to 48 h |
| Membrane filtration (MF) | |||||
| 9222 B, C | Standard Methods | Endo-type agar medium | β-galactosidase enzyme | Red colonies with golden-green metallic sheen | 24 h |
| 9308-1, 2, 3 | ISO Methods | Chromocult®Coliform agar | β-galactosidase enzyme | Red colonies | 24 h |
| 9222 H | Standard Methods | m-ColiBlue24®broth | β-galactosidase enzyme | Red colonies | 24 h |
9222 I 1604 |
Standard Methods U.S. EPA |
MI medium or broth | β-galactosidase enzyme | Blue-white colonies that fluorescence under longwave ultraviolet (UV) light | 24 h |
| Enzyme substrate [MTF, multi-well (e.g., Quanti-Tray®), presence-absence] | |||||
| 9223 B | Standard Methods | Various substrate media available commercially: Colilert® Colilert-18® Colisure® |
β-galactosidase enzyme | Yellow or red/magenta colonies depending on substrate used | 18-24 h |
| N/A | EMD Millipore | Readycult® Fluorocult® |
β-galactosidase enzyme | Blue-green color | 24 h |
| N/A | CPI International, Inc. | Modified Colitag® | β-galactosidase enzyme | Yellow color | 16 h |
| N/A | Veolia Water Solutions and Technologies | TECTATM EC/TC | β-galactosidase enzyme | Fluorescence detected by TECTATM instrument | 2-18 h |
6.0 Sampling for total coliforms
6.1 Sample collection
Proper procedures for collecting samples must be observed to ensure that the samples are representative of the water being examined. Detailed instructions on the collection of samples for bacteriological analysis are given in Standard Methods for the Examination of Water and Wastewater (APHA et al., 2017).
Storage temperature can have a significant impact on microbial populations within samples and thus, affect recovery of coliforms (Ahammed, 2003; Pope et al., 2003). This is an especially important consideration when collecting samples in remote locations; and for Canadian utilities in the winter months, when there is an increased likelihood of sample freezing. Commercial devices are available for verifying that the proper transport temperatures are being achieved. The sample should be transported to the laboratory in a cooler containing ice or cooling packs (at 5 ± 3°C), to minimize changes in populations and concentrations (Dutka and El-Shaarawi, 1980; McDaniels et al., 1985; ISO, 2006). As well, samples should be protected from direct contact with the ice or cooling packs to prevent freezing during transport. Water utilities should record any collection or storage related issues and report them to the laboratory, so that results can be properly interpreted. Any on-going problems with sampling and/or transportation should be discussed with the responsible authority.
Studies of the effects of holding time on the detection of coliforms in water samples have been limited and contradictory. Ahammed et al. (2003) observed a sharp decline in coliform counts in various water samples between 12 and 48 hours of storage, at both ambient and refrigerator temperatures. A similar effect was noted by Toranzos and McFeters (1997) after 24-30 hours versus six hours of storage. McDaniels et al., (1985) and Ferguson (1994) indicated that holding times can be critical for members of the coliform group when their concentrations are low. In contrast, Bushon et al. (2015) reported no significant difference in total coliform detections in groundwater samples tested after eight hours versus those tested within 18-30 hours. These different findings may be related to a number of factors, including: variations in survival rates among coliform isolates; sampling location; detection methods used; and how polluted water samples are (i.e., presence and density of competitor organisms) (Ahammed et al., 2003; Pope et al., 2003; Maier et al., 2014). To avoid unpredictable changes in the bacterial flora of the sample, examination should be started as soon as possible after collection. Where on-site facilities are available or when an accredited laboratory is within an acceptable travel distance, analysis of samples within six to eight hours is suggested (Payment et al., 2003; APHA et al., 2017). Ideally, for total coliform analysis of drinking water samples, the holding time between the collection of the sample and the beginning of its examination should not exceed 30 hours (APHA et al., 2017). In remote areas, up to 48 hours may be an acceptable time interval; however, the implications of the extended holding time should be discussed with the responsible authorities. When delays are anticipated, a delayed incubation procedure should be employed or consideration given to on-site testing. The delayed incubation procedure is described in APHA et al. (2017). Alternatively, if normal transportation time exceeds the above recommendations, the sample should be processed and arrangements made to have another sample collected as soon as the first sample is received. Thus, if the late sample contains coliforms, a repeat sample will already have been received or will be in transit. Samples should be labelled with the time, date, location, type of sample (e.g., raw water, distribution system), sampler's name, and identification number (if used), along with the disinfectant residual measurements and any special conditions. In most cases, much of this information, along with the identification number linked to the sample bottle, is recorded on accompanying submission forms and, in cases where samples are collected for legal purposes, chain-of-custody paperwork. When examination will be delayed, it is particularly important to record the duration and temperature of storage, as this information should be taken into consideration when interpreting the results.
A minimum volume of 100 mL of water should be examined to obtain a reliable estimate of the number of organisms (using MTF or MF) or to obtain an accurate presence-absence result at the expected low levels in treated drinking water. For the MTF method, a test series consisting of one 50-mL volume and five 10-mL volumes is suggested by the World Health Organization (WHO, 1972) for water expected to be of good quality. Examination of larger volumes in groundwaters with very low levels of contamination, can increase both the test sensitivity and the test reliability. Smaller volumes, dilutions, or other MTF combinations may be more appropriate for waters of poor quality. Hargy et al. (2010) demonstrated that large sample volumes were useful in improving the detection of total coliforms. The authors examined 100 mL and 20 L drinking water distribution system samples (n = 252), obtained from three utilities, using a commercially available capsule filter in conjunction with a substrate-based indicator reagent. Total coliforms were detected in 18 of the 20 L samples, but in only two of the corresponding 100 mL samples. The authors concluded that the microbiological quality of distribution systems may be better characterized by collecting larger volume samples (Hargy et al., 2010). An increased concentration of microorganisms is generally detected after periods of stagnation related to disinfectant decay and bacterial regrowth in the distribution system, and is reflective of the conditions under which OPPPs might be present (NRC, 2006; Lautenschlager et al., 2010; Wang et al., 2017). Thus, improved detection of total coliforms in the drinking water distribution system may be aided by not flushing and disinfecting the tap prior to sampling (NRC, 2006; Lautenschlager et al., 2010; Wang et al., 2017).
6.2 Sampling frequency considerations
The WHO lists the following factors that should be taken into account when determining sampling frequency for municipal-scale systems (WHO, 1972, 1976, 2004):
- Past frequency of unsatisfactory samples
- Source water quality
- The number of raw water sources
- The adequacy of treatment and capacity of the treatment plant
- The size and complexity of the distribution system
- The practice of disinfection
These variables preclude application of a universal sampling frequency formula. Instead, the sampling frequency and location of sampling points should be decided after due consideration of local conditions, for example, variations in raw water quality and a history of treated water quality. The sampling frequency should meet all jurisdictional requirements.
As a minimum, water leaving a treatment plant should be tested daily for disinfectant residual and turbidity and at least weekly for total coliforms as part of the verification process in a source-to-tap approach. In many systems, the water leaving the treatment plant will be tested for these indicators well in excess of the minimum requirements. For supplies where weekly total coliform testing is impractical (e.g., in small supplies), total coliform sampling may be reduced and other means of verifying the microbiological quality may be used, such as residual disinfectant determinations and good process control.
In a distribution system, the number of samples for bacteriological testing should be increased in accordance with the size of the population served. The general practice of basing sampling requirements on the population served recognizes that smaller water supply systems may have limited resources available for monitoring. However, because small water supplies have more facility deficiencies (Schuster et al., 2005) and are responsible for more disease outbreaks than large ones (Schuster et al., 2005), emphasis should also be placed on identified problems based on source-to-tap assessments. Minimum recommended sampling frequencies for total coliform testing in the distribution system are provided in Table 3. However, systems should conduct a site-specific assessment to ensure that their sampling frequency meets the requirements of the responsible authority. The WHO also provides guidance on the minimum number of samples for indicator testing in drinking water distribution systems (WHO, 2014).
| Population served | Minimum number of samples per monthTable 2 Footnote 1 |
|---|---|
| Up to 5000 | 4 |
| 5000-90,000 | 1 per 1000 persons |
| 90 000+ | 90 + (1 per 10,000 persons) |
Table 2 Footnotes
|
|
Disinfectant residual and turbidity analyses tests should be conducted when bacteriological samples are taken in the distribution system. Further information on monitoring for turbidity can be found in the Guidelines for Canadian drinking water quality: guideline technical document - turbidity (Health Canada, 2012). Routine verification of the concentration of the disinfectant residual and the bacteriological quality of the water ensures that immediate remedial action can be taken if water of doubtful quality enters the distribution system. The preceding frequencies (Table 3) are only general guides. For small systems, additional guidance may need to be considered by the responsible authority. In supplies with a history of high-quality water, it may be possible to reduce the number of samples taken for bacteriological analysis. Alternatively, supplies with variable water quality may be required to sample on a more frequent basis. Sampling frequencies in residential-scale and private systems may vary from jurisdiction to jurisdiction but should include times when the risk of contamination is greatest, for example, spring thaw, heavy rains, or dry periods. New or rehabilitated wells should also be sampled initially to confirm acceptable bacteriological quality.
Even at the recommended sampling frequencies for total coliforms, there are limitations that need to be considered when interpreting the sampling results. Simulation studies have shown that it is very difficult to detect a contamination event in a distribution system unless the contamination occurs in a watermain, in a reservoir, at the treatment plant, or occurs for a long duration at a high concentration (Speight et al., 2004; van Lieverloo et al., 2007). Therefore, even if the analytical result indicates the absence of coliforms, intrusion may be occurring in the distribution system. Some improvement in detection capabilities were found when sampling programs were designed with the lowest standard deviation in time between sampling events (van Lieverloo et al., 2007), such as samples collected every five days, regardless of weekends and holidays. There are also some limitations that are inherent when analyzing for parameters that are considered rare events, such as total coliforms. Hrudey and Rizak (2004) have reported that because the rate of total coliform positives in most distribution systems is usually lower than the false-positive rate for the method, it is difficult to determine whether the results obtained are true positives. In addition, the low rate of positive samples means that it is difficult to see statistically significant differences in total coliform positive rates, for example, before and after a corrective action, unless a very large number of samples are evaluated (Rosen et al., 2009). These limitations highlight the importance of implementing a source-to-tap approach, as opposed to relying on a single parameter for determining the microbiological quality of the drinking water.
6.3 Location of sampling points
In municipal-scale systems, the location of sampling points must be selected or approved by the responsible authority. The sampling locations selected may differ depending on the monitoring objectives. For example, fixed sampling points may be used to help establish a history of water quality within the distribution system, whereas sampling at different locations throughout the distribution system may provide more coverage of the system. A combination of both types of monitoring is common (Narasimhan et al., 2004). Some information is available on how to select statistically based random sampling sites (Speight et al., 2004).
In general, samples should be taken at the point where the water enters the system and from representative points throughout the distribution system. If the water supply is obtained from more than one source, the location of sampling points in the system should ensure that water from each source is periodically sampled. Distribution system drawings can provide an understanding of water flows and directions and can aid in the selection of appropriate sampling locations. The majority of samples should be taken from potential problem areas: low-pressure zones, reservoirs, dead ends, areas at the periphery of the system farthest from the treatment plant, and areas with a poor previous record. In residential-scale systems, samples are generally collected from the locations recommended by the responsible authority. More extensive sampling may be necessary depending on the system and results from previous samples.
Given the presence of biofilm in drinking water distribution systems, consideration should be given to including biofilm sampling as part of total coliform detection, as it would likely provide a more accurate assessment of potential risk (WHO, 2011, 2014; Botsaris et al., 2015; Wang et al., 2017). Careful consideration should also be given to identifying locations within the distribution system where the risk of microbial contamination (either because of regrowth, intrusion or biofilm dislodgement) is likely highest (e.g., areas where disinfectant residual decay is anticipated to occur), and to sample a variety of these locations. The composition of the sampling tap also needs to be considered when determining sampling locations, as it can affect microbial test results (Geldreich et al, 1985; Cox and Giron, 1993; Goatcher et al, 1992; Geldreich and LeChevallier, 1999). The U.S. EPA's Revised Total Coliform Rule addresses this issue by allowing the collection of samples from dedicated (distribution system) sampling stations - this also ensures spatially representative sampling (U.S. EPA, 2013; LeChevallier, 2014). Detection may be improved by rotating among sampling sites throughout the distribution system (WHO, 2014). Additional guidance on selecting sampling points within the drinking water distribution system can be found in Guidance on monitoring the biological stability of drinking water in distribution systems (Health Canada, 2020b).
7.0 Treatment technology
The application of a source-to-tap approach, including watershed or well-head protection, optimized treatment barriers, and a well-maintained distribution system, is the best approach to reduce the presence and associated health risks of waterborne pathogens to an acceptable level. Total coliforms are one of several indicators that are used as part of the source-to-tap approach.
An array of options is available for treating source waters to provide high-quality drinking water in municipal- and residential-scale systems. The quality of the source water will dictate the degree of treatment necessary. Generally, minimum treatment of supplies derived from surface water or GUDI sources should include adequate filtration (or technologies providing an equivalent log reduction credit) and disinfection to ensure removal/inactivation of enteric protozoa and enteric viruses. All groundwaters should receive adequate treatment for the removal and/or inactivation of enteric viruses, unless exempted by the responsible authority based on site- specific considerations including historical and on-going monitoring data. In systems with a distribution system, a disinfectant residual should be maintained at all times.
7.1 Municipal-scale
In general, all drinking water supplies should be disinfected, and a disinfectant residual should be maintained throughout the distribution system at all times. In addition, surface water or GUDI sources should include physical removal methods, such as chemically assisted filtration (coagulation, flocculation, clarification, and filtration) or technologies that provide an equivalent log reduction credit for microorganisms. It is essential that the removal and disinfection targets are achieved before drinking water reaches the first consumer in the distribution system. Adequate process control measures and operator training are also required to ensure the effective operation of treatment barriers at all times (U.S. EPA, 1991; Health and Welfare Canada, 1993; AWWA, 1999).
7.1.1 Level of treatment necessary
Most source waters are subject to fecal contamination, as such, treatment technologies should be in place to achieve a minimum 4 log (99.99%) removal and/or inactivation of enteric viruses and a minimum 3 log (99.9%) removal and/or inactivation of enteric protozoa in accordance with the guideline technical documents on enteric viruses and protozoa (Health Canada, 2019a, 2019b). Depending on the source water quality, a higher log reduction may be necessary to produce safe drinking water. Groundwater, classified as less vulnerable to fecal contamination, should not have protozoa present when using procedures determined by the responsible authority. Therefore the minimum treatment requirements for protozoa would not apply. However, even groundwater sources will have a degree of vulnerability and should be periodically reassessed. In general, protozoa and enteric viruses are more difficult to inactivate or remove than bacterial pathogens. Therefore, water that is treated to meet the guidelines for enteric viruses and enteric protozoa should have an acceptable bacteriological quality, including meeting the MAC for total coliforms of none detectable in 100 mL of water leaving the treatment plant.
7.1.2 Physical removal
The physical removal of coliform bacteria can be achieved using various types of filtration. Although physical removal of bacteria is achievable, Smeets et al. (2006) found that most filtration technologies achieved less than 6 log reduction in bacteria on their own; thus, disinfection is a critically important additional treatment barrier (see Section 7.1.3).
Physical log removal credits for several filtration technologies are outlined in Table 4. More detailed information on filtration techniques can be found in the guideline technical document on turbidity (Health Canada, 2012).
| Technology | Bacterial log removals | |||
|---|---|---|---|---|
| Minimum | Mean | Median | Maximum | |
| Conventional filtration | 1.0 | 2.1 | 2.1 | 3.4 |
| Direct filtration | 0.8 | 1.4 | 1.5 | 3.3 |
| Slow sand filtration | 1.2 | 2.7 | 2.4 | 4.8 |
| Microfiltration (pore size - 0.1-1 µm) |
0 | Not given | Not given | 4.3 |
| Ultrafiltration (pore size - 0.01-0.1 µm) |
Not given | >7 | Not given | Not given |
|
||||
7.1.3 Disinfection
The commonly used drinking water disinfectants are chlorine, chloramine, UV light, ozone, and chlorine dioxide. Disinfection is typically applied after treatment processes that remove particles and organic matter (Health Canada, 2019c). This strategy helps to ensure efficient inactivation of pathogens and minimizes the formation of disinfection by-products (DBP). Also, when describing microbial disinfection of drinking water, the term "inactivation" is used to indicate that the pathogen is no longer able to multiply within its host and is therefore non-infectious, although it may still be present. Primary disinfection is a critically important barrier to reduce bacteria in drinking water. Thus operators should understand and maintain the required CT/IT values (where CT = concentration of disinfectant x contact time, and IT = intensity of UV light x contact time, see sections 7.1.3.1 and 7.3.1.2 for descriptions of the CT and IT concepts, respectively) for primary disinfection, in addition to disinfectant residual requirements for secondary disinfection. Typically, bacterial reduction goals will be achieved if primary disinfection for viruses is achieved.
7.1.3.1 Chemical disinfection
Currently, chlorine is the most widely used disinfectant in the drinking water industry. It is a strong oxidant capable of inactivating bacteria and viruses present in bulk water, although, as with most chlorine-based disinfectants, it is not as effective for the control of protozoans.
Chlorine is also less effective for inactivating organisms present in biofilms. In comparison with chlorine, chloramine is a weaker oxidant. This property is advantageous in that the disinfectant resides longer in a distribution system. It is therefore easier to maintain a disinfectant residual, and the disinfectant is better able to penetrate into the biofilm found in the pipes and reservoirs, leading to superior coliform control (LeChevallier, 1990; LeChevallier, 2003). However, chloramine is less efficient at controlling a sudden pulse of contamination (Snead et al., 1980), and it can lead to nitrification. Chlorine dioxide is as effective as, and in some instances more effective than, chlorine. However, this compound is difficult to work with and therefore is not widely used. Ozone, compared with chlorine-based disinfectants, is more efficient for the inactivation of bacteria, viruses, and protozoa, although ozone treatment can result in an increase in biodegradable organic compounds that can promote bacterial regrowth in the distribution system. Ozone is highly effective at the point of treatment, but an additional disinfectant (usually chlorine or chloramine) needs to be added to supply a residual.
Maintaining a disinfectant residual will limit the growth of organisms within the distribution system and, depending on the residual concentration, contact time, and the pathogens present, a disinfectant residual also may afford some protection against contamination from intrusion (Besner et al., 2007). The disappearance of the residual may also provide an immediate indication of the entry of oxidizable matter into the system or a malfunction of the treatment process.
The efficacy of chemical disinfectants can be predicted based on knowledge of the residual concentration of disinfectant, temperature, pH, and contact time (AWWA, 1999). This relationship is commonly referred to as the CT concept, where CT is the product of "C" (the residual concentration of disinfectant, measured in mg/L) and "T" (the disinfectant contact time, measured in minutes). To account for disinfectant decay, the residual concentration is usually determined at the exit of the contact chamber rather than using the applied dose or initial concentration. The contact time, "T", is often calculated using a T10 value, such that 90% of the water meets or exceeds the required contact time. The T10 values can be estimated based on the geometry and flow conditions of the disinfection chamber or basin. Hydraulic tracer tests, however, are the most accurate method to determine the contact time under actual plant flow conditions.
CT values for 99% inactivation of E. coli using chlorine, chlorine dioxide, chloramine, and ozone are provided in Table 5. For comparison, CT values for Giardia lamblia and for viruses have also been included. In a well-operated treatment system, the CT provided will result in a much greater inactivation than 99%. From Table 5, it is apparent that, in comparison with most protozoans and viruses, coliform bacteria are easier to inactivate using the common chemical disinfectants. Also, chloramines have a much higher CT value than any of the other disinfectants listed. This means that to achieve the same level of inactivation with chloramine, a higher disinfectant concentration or a longer contact time, or a combination of both, is necessary. Thus, chloramine is not recommended as a primary disinfectant because of the high CT values required for inactivation of bacteria.
| Disinfectant agent | pH | E. coliTable 3 Footnote a (mg·min/L) |
Giardia lambliaTable 3 Footnote b (mg·min/L) |
VirusesTable 3 Footnote b (mg·min/L) |
|---|---|---|---|---|
| Free chlorine | 6-7 | 0.034-0.05 | 65-93 | 4.0Table 3 Footnote c |
| Chloramines | 8-9 | 95-180 | 1470 | 857 |
| Chlorine dioxide | 6-7 | 0.4-0.75 | 17c | 5.6Table 3 Footnote c |
| Ozone | 6-7 | 0.02 | 1.3 | 0.6 |
Table 3 Footnotes
|
||||
7.1.3.2 UV light disinfection
UV light disinfection is highly effective for inactivating many types of pathogens.
Further information on inactivation of specific protozoan and viral pathogens can be found in the guideline technical documents on enteric viruses and enteric protozoa (Health Canada, 2019a, 2019b).
Similar to ozone, UV light is highly effective at the point of treatment, but an additional disinfectant (usually chlorine or chloramine) needs to be added to supply a residual. When using UV light for the inactivation of E. coli (and other bacteria), the bacteria can undergo photo repair (i.e., photoreactivation) (Harris et al., 1987; Schoenen and Kolch, 1992; Zimmer and Slawson, 2002) and, to a lesser extent, dark repair. However, the amount of repair is not considered significant in drinking water treatment and distribution.
For UV disinfection, the product of light intensity "I" (measured in mW/cm2 or W/m2) and time "T" (measured in seconds) results in a computed dose (fluence) in mJ/cm2 for a specific microorganism. This relationship is referred to as the IT concept. Log inactivations using UV light disinfection are listed in Table 6, and show that, of the representative organisms, bacteria (in this instance, E. coli) and protozoa require comparable doses of UV light to achieve the same level of inactivation, whereas certain viruses are much more resistant. E. coli, because of its importance as a public health indicator, has been used as a representative bacterial species. For comparison, UV light doses for representative protozoa and viruses have also been included.
| Log inactivation | E. coliTable 6 Footnote a,Table 6 footnote d | CryptosporidiumTable 6 Footnote a | Adenovirus, Table 6 Footnote a Table 6 footnote b Table 6 footnote c, Table 6 footnote d | RotavirusTable 6 Footnote a,Table 6 footnote c,Table 4 footnote d | GiardiaTable 6 Footnote a |
|---|---|---|---|---|---|
| 1 | 1.5-5 | 2.5 | 42-58 | 7.1-10 | 2.1 |
| 2 | 2.8-9 | 5.8 | 83-111 | 15-20 | 5.2 |
| 3 | 4.1-14 | 12 | 129-167 | 23-29 | 11 |
| 4 | 5.0-18 | 22 | 167-186 | 36-40 | 22 |
|
|||||
With regards to UV disinfection, Sommer et al. (2000) found the dose (fluence) required to inactivate eight pathogenic E.coli strains differed significantly. The authors reported a dose of 1.2 mJ/cm2 was required to achieve 6 log reduction for the most susceptible strain, whereas 12.5 mJ/cm2 was required for the most resistant strain. Up to 30 mJ/cm2 was necessary when photoreactivation for 2 hours was considered. Typically, photoreactivation is not a concern for drinking water applications, however, it may be a consideration for potable water use in food production facilities that have their own private water supply (i.e., a semi-public system). One strain was also found to repair under dark conditions; a dose of approximately 10 mJ/cm2 was required to achieve 6 log reduction for this strain.
Many jurisdictions require a minimum UV dose of 40 mJ/cm2 on the basis that 4 log inactivation can be achieved for most pathogens, including bacteria, viruses and protozoan (oo)cysts (ÖNORM, 2001, 2003; DVGW, 2006a,b,c; Ontario Ministry of Environment, 2006; Nova Scotia Environment, 2012). The responsible jurisdiction should be contacted to confirm the UV dose requirements.
7.2 Residential-scale
Residential-scale treatment is also applicable to small drinking water systems. This could include both privately owned systems and systems with minimal or no distribution system that provide water to the public from a facility not connected to a municipal supply (previously referred to as semi-public systems).
Groundwater is a common source of drinking water for small or individual water supplies, and the consumption of fecally contaminated groundwater has been associated with illness (see Section 4.3.3). As a result, small groundwater supplies providing drinking water to the public (i.e., semi-public systems) that are vulnerable to fecal contamination should be treated.
In cases where an individual household obtains its drinking water from a private well, the vulnerability of the source to fecal contamination should be assessed. Although it is difficult for homeowners to conduct a detailed assessment of the vulnerability of their well to fecal contamination, steps can be taken to minimize the likelihood of a well becoming contaminated. General guidance on well construction, maintenance, protection and testing is typically available from provincial/territorial jurisdictions. When considering the potential for fecal contamination, well owners should have an understanding of the well construction, type of aquifer material surrounding the well and location of the well in relation to sources of fecal contamination (e.g., septic systems, sanitary sewers, animal waste, etc.). This information can be obtained from records provided to the homeowner during well and septic system construction, and well log databases, aquifer vulnerability maps, and regional groundwater assessments that are generally available from the responsible drinking water authority. If insufficient information is available to determine if a well is vulnerable to fecal contamination, treatment of the well is a way to reduce risk. In general, surface water is not recommended as a private or semi-public water supply unless it is properly filtered, disinfected and monitored for water quality.
Various options are available for treating source waters to provide high-quality pathogen-free drinking water. These include filtration and disinfection with chlorine-based compounds or alternative technologies, such as UV light. These technologies are similar to the municipal treatment barriers, but on a smaller scale. Many of these technologies have been incorporated into point-of-entry devices, which treat all water entering the system, or point-of-use devices, which treat water at only a single location-for example, at the kitchen tap. To minimize the potential health risks from the use of microbiologically-contaminated drinking water, it is important to note that in the absence of a point-of-entry system, all points of water used for drinking, food and beverage preparation, hygiene or washing dishes should be equipped with a point-of-use treatment device.
Specific guidance on technologies that can be used in small systems should be obtained from the responsible drinking water authority in the affected jurisdiction. Health Canada does not recommend specific brands of drinking water treatment devices, but it strongly recommends that consumers look for a mark or label indicating that the device has been certified by an accredited certification body as meeting the appropriate NSF International (NSF)/American National Standards Institute (ANSI) standard. These standards have been designed to safeguard drinking water by helping to ensure the safety of material and performance of products that come into contact with drinking water.
Certification organizations provide assurance that a product conforms to applicable standards and must be accredited by the Standards Council of Canada (SCC). Accredited organizations in Canada (SCC, 2019) include:
- CSA Group
- NSF International
- Water Quality Association
- UL LLC
- Truesdail Laboratories Inc
- Bureau de Normalisation du Québec (available in French only)
- International Association of Plumbing and Mechanical Officials
An up-to-date list of accredited certification organizations can be obtained from the SCC web site.
Private and semi-public supplies that use liquid chlorine should use hypochlorite solutions that are certified as meeting NSF/ANSI/CAN Standard 60 (NSF/ANSI/CAN, 2018) and follow the handling and storage recommendations for hypochlorite outlined in (Health Canada, 2016).
NSF/ANSI Standard 55 (Ultraviolet Disinfection Systems) (NSF/ANSI, 2019) provides performance criteria for two categories of certified systems, Class A and Class B. UV systems certified to NSF/ANSI Standard 55 Class A are designed to deliver a UV dose at least equivalent to 40 mJ/cm2 in order to inactivate microorganisms, including bacteria, viruses, Cryptosporidium oocysts, and Giardia cysts, from contaminated water. However, they are not designed to treat wastewater or water contaminated with raw sewage and should be installed in visually clear water. Also, systems certified to NSF Standard 55 Class B systems are intended for a drinking water supply that is already disinfected, tested, and deemed acceptable for human consumption.
Reverse osmosis (RO) membranes have a pore size smaller than bacteria and could provide a physical barrier to remove them. However, NSF/ANSI Standard 58 (NSF/ANSI, 2018a) does not include a claim for bacterial reduction. It is important to note that RO systems are intended for point-of-use installation only. This is because water treated by a RO system may be corrosive to internal plumbing components. These systems also require larger quantities of influent water to obtain the required volume of drinking water and are generally not practical for point-of-entry installation.
Ultrafiltration membranes also have a pore size smaller than bacteria and could also provide a physical barrier to bacteria, although there is currently no NSF/ANSI standard for residential-scale ultrafiltration systems. Semi-public systems that require higher capacity may refer to ultrafiltration membranes certified under NSF/ANSI Standard 419 (NSF/ANSI, 2018b). Although units are not certified for bacterial reduction, those with a pore size of <0.1 µm should be effective.
The NSF/ANSI Standard 62 (NSF/ANSI, 2018c) for Drinking Water Distillation Systems includes reduction claims for bacteria. To meet this standard, a distillation system must provide a minimum 6 log reduction of bacteria and bacterial spores. Distillation systems should only be installed at the point-of-use as the water they have treated may be corrosive to internal plumbing components.
Periodic testing for E. coli and total coliforms by an accredited laboratory should be conducted on both the water entering the treatment device and the treated water to verify that the treatment device is effective. Treatment devices lose their removal capacity through usage and time and need to be maintained and/or replaced. Consumers should verify the expected longevity of the components in their treatment device according to the manufacturer's recommendations and establish a clearly defined maintenance schedule. Treatment devices should be inspected and serviced in accordance with the maintenance schedule and manufacturer's recommendations.
7.3 Distribution system
A well-maintained distribution system is a critical component of the multi-barrier approach for management of drinking water safety (Fisher et al., 2000). Distribution system optimization is a complex process involving numerous concomitant goals (e.g., microbial, DBPs, corrosion, physical integrity).
Distribution system water quality is known to deteriorate due to a variety of issues, including long-term biofilm regrowth and short-term transients/intrusions that may result due to normal day-to-day operations (e.g., start/stop pump; open/close valve), as well as accidental cross-contamination or intentional contamination. Contamination events are difficult to detect due to the low frequency of microbial indicator sampling (Hall et al., 2007; Storey et al., 2011; Ikonen et al., 2013). Distribution system water quality should be regularly monitored. Several instruments and water quality probes, including those for chlorine, conductivity, flow, pH, pressure, temperature, total/dissolved organic carbon and turbidity, provide reliable means by which changes in the distribution system can be measured in real-time (Hall et al., 2007; Miles et al., 2011; Rosen and Bartrand, 2013; Durand et al., 2016; Ikonen et al., 2017). The most sensitive sensors are reported to be total organic carbon and free/total chlorine, while the five most commonly used by water utilities in the U.S., Netherlands, Belgium and Australia include: flow, turbidity, pH, pressure and chlorine (van der Gaag and Volt, 2008). Online monitoring shows promise to better understand distribution system dynamics such that problems are rapidly detected, quick response is taken and drinking water is transported to the consumer with minimum loss of quality (van der Gaag and Volt, 2008; U.S. EPA, 2010; Storey et al., 2011; Rosen and Bartrand, 2013; Samendra et al., 2014; WHO, 2014; Durand et al., 2016; Ikonen et al., 2017).
In addition to regularly monitoring distribution system water quality, source water protection measures should be implemented, along with treatment optimization, maintenance of physical/hydraulic integrity of the distribution system, and minimization of negative- or low-pressure events (Karim et al., 2003; Fleming et al., 2006; Friedman et al., 2009; WHO, 2014). Treatment optimization should include biofilm reduction strategies, including: optimized removal of natural organic matter; maintenance of an effective disinfectant residual; maintenance of low levels of biostability indicators in treated water (e.g., AOC, biodegradable dissolved organic carbon [BDOC], biofilm formation rate); controlling corrosion; and managing water temperatures (Prévost et al., 2005; van der Kooij and van der Wielen, 2014; LeChevallier et al., 2015a,b; Prest et al., 2016a,b; Health Canada, 2019c). Operations and maintenance programs should be in place (e.g., watermain cleaning, cross-connection control, asset management) and strict hygiene should be practiced during watermain repairs to ensure drinking water is transported to the consumer with minimum loss of quality (Kirmeyer et al., 2001, 2014). Further details on monitoring and managing biostability in the drinking water distribution system can be found in Guidance on monitoring the biological stability of drinking water in distribution systems (Health Canada, 2020b).
8.0 Risk assessment
The adoption of a risk-based approach, such as a multi-barrier approach, is essential to the effective management of drinking water systems (CCME, 2004). This approach should include assessment of the entire drinking water system, from the watershed or aquifer and intake through the treatment and distribution chain to the consumer, to assess potential effects on drinking water quality and public health.
A health-based risk assessment is not relevant for total coliforms since they are only used as an indicator and their presence in drinking water is not considered a risk to human health. Risk assessments have been done for specific microbiological organisms that have health implications, such as enteric viruses and the enteric protozoa Cryptosporidium and Giardia (Health Canada, 2019a, 2019b)
The detection of total coliform bacteria in drinking water provides important information about a drinking water system and therefore, they should be routinely used as part of a source-to-tap approach. In groundwaters that are less vulnerable to fecal contamination, the presence of total coliforms signals that intrusion into the groundwater has occurred from surface water sources, that contamination occurred after construction of a new well or after repair or replacement of any part of the well or pump, or that growth of total coliform bacteria is occurring in the system. Total coliform bacteria are susceptible to the processes commonly used in drinking water treatment. Therefore, the presence of total coliforms in water leaving a municipal- or residential-scale treatment plant indicates a problem with the treatment. In a municipal-scale distribution system, total coliforms may result from bacterial regrowth within distribution system biofilms, so occasional detections may occur. Occasional detections (e.g., up to 10% of samples) in municipal-scale systems do not necessarily indicate a degradation in water quality and are considered acceptable. In residential-scale distribution systems, fewer samples are taken; therefore, the presence of total coliform bacteria should be investigated to determine their source and the implications for the water quality. Monitoring for total coliforms, when used in conjunction with a source-to-tap approach, is used as part of the verification that the drinking water system is supplying water that is of acceptable microbiological quality.
8.1 International considerations
Other countries and international organisations use total coliforms in drinking water quality management. The Drinking Water Inspectorate of England and Wales has included, in its regulations, a mandatory value of zero coliforms per 100 mL in water leaving treatment plant, a mandatory value of zero coliforms per 100 mL in 95% of samples for water in service reservoirs, and a non-mandatory value of zero coliforms per 100 mL at the consumer's tap. In these regulations, non-mandatory values do not need to be met, but exceedances need to be investigated and actions taken only if they represent a health risk (DWI, 2000). These regulations are based on the EU's Council Directive on the quality of water intended for human consumption (Council of the European Union, 1998).
The WHO Guidelines for Drinking Water Quality (2017) specify that total coliforms should be absent immediately after disinfection, and that their presence is indicative of inadequate treatment. These Guidelines also mention that the presence of total coliforms in distribution systems may relate to regrowth and possible biofilm formation. However, no guideline values are specified for total coliforms; instead, guideline values for thermotolerant coliforms and E. coli are established (WHO, 2017).
The Australian Drinking Water Guidelines do not establish a guideline value for total coliforms; instead, guideline values for thermotolerant coliforms and E. coli are established (NHMRC, 2016).
The U.S. EPA (2013) revised the Total Coliform Rule to include specific provisions designed to minimize microbial risks from the drinking water distribution system, including the establishment of a total coliform treatment technique requirement, and monitoring requirements for total coliforms according to site-specific plans. Under this treatment technique, a water system that exceeds a specified frequency of total coliform occurrence must conduct an assessment to determine if any sanitary defect exists and, if found, correct them. This change means that, instead of triggering a non-acute Maximum Contaminant Level violation, the presence of total coliforms at a level that exceeds the treatment-technique trigger indicates potential pathways of contamination exist in the distribution system.
The U.S. EPA Groundwater Rule establishes a risk-based approach to identify subsurface sources that are susceptible to fecal contamination, and requires corrective action to address deficiencies. Systems not providing 4 log virus reduction must monitor for E. coli, enterococci or somatic coliphage within 24 hours of receiving notice of a total coliform positive result (U.S. EPA, 2006).
9.0 Rationale
Total coliform bacteria are widely distributed in surface waters and groundwaters under the direct influence of surface water, soil, and vegetation, and they are also susceptible to drinking water treatment. They are not usually found in protected groundwater sources.
Sampling and analysis for total coliforms is an easy, relatively quick, inexpensive way of monitoring water. Because total coliforms are widespread in the environment, they can be used as one of the many operational tools to determine the efficacy of a drinking water treatment system. Total coliforms can also be used to indicate issues with groundwaters that are less vulnerable to fecal contamination since total coliforms should not be found in these sources. Total coliforms can colonize and grow within the biofilm that builds up on surfaces in drinking water systems. The maximum acceptable concentration (MAC) for total coliforms in water leaving a treatment plant and in non-disinfected groundwater leaving the well is none detectable per 100 mL. In a distribution system, total coliforms should be monitored because they are used to indicate changes in water quality. Detection of total coliforms from consecutive samples from the same site or from more than 10% of the samples collected in a given sampling period should be investigated.
The sampling scheme for the MAC to be applied in distribution systems takes into account that occasional detection of total coliforms is expected to occur in a municipal-scale distribution system and does not necessarily indicate degradation of water quality. The sampling requirements are based on practical considerations and established practice.
The absence of total coliforms, when used in conjunction with a source-to-tap approach, is used as part of the verification that the drinking water system is producing water that is microbiologically acceptable.
10.0 References
Abbaszadegan, M., LeChevallier, M. and Gerba, C. (2003). Occurrence of viruses in US groundwaters. J. Am. Water Works Assoc., 95: 107-120.
Aboytes, R., Di Giovanni, G.D., Abrams, F.A., Rheinecker, C., Mcelroy, W., Shaw, N. and LeChevallier, M.W. (2004). Detection of infectious Cryptosporidium in filtered drinking water. J. Am. Water Works Assoc., 96(9): 88-98.
Ahammed, M.M. (2003). Effect of holding time and temperature on bacterial counts. Indian J. Environ. Health, 45(3): 209-212.
Allen, D.A., Austin, B. and Colwell, R.R. (1983). Aeromonas media, a new species isolated from river water. Int. J. Syst. Bacteriol., 33: 599-604.
Allevi, R.P., Krometis, L-H., Hagedorn, C., Benham, B., Lawrence, A.H., Ling, E.J. and Ziegler, P.E. (2013). Quantitative analysis of microbial contamination in private drinking water supply systems. J Water Health, 11(2): 244-255.
Amundson, D., Lindholm, C., Goyal, S.M. and Robinson, R.A. (1988). Microbial pollution of well water in southeastern Minnesota. J. Environ. Sci. Health Part A Environ. Sci. Eng., 23(5): 453-468.
APHA, AWWA and WEF (2017). Standard methods for the examination of water and wastewater. 23rd edition. American Public Health Association, American Water Works Association and Water Environment Federation, Washington, DC.
Ashbolt, N.J., Ball, A., Dorsch, M., Turner, C., Cox, P., Chapman, A. and Kirov, S.M. (1995). The identification and human health significance of environmental aeromonads. Water Sci. Technol., 31: 263-269. Atherholt, T., Feerst, E., Hovendon, B., Kwak, J. and Rosen, J.D. (2003). Evaluation of indicators of fecal contamination in groundwaters. J. Am. Water Works Assoc., 95: 119-131.
Atherholt, T.B., Bousenberry, R.T., Carter, G.P., Korn, L.R., Louis, J.B., Serfes, M.E. and Waller, D.A. (2013). Coliform bacteria in New Jersey domestic wells: Influence of geology, laboratory, and method. Groundwater, 51(4): 562-574.
Atherholt, T.B., Korn, L.R., Louis, J.B. and Procopio, N.A. (2015). Repeat sampling and coliform bacteria detection rates in New Jersey domestic wells. Groundwater Monit. Rem., 35(2): 70-80.
Atherholt, T.B., Procopio, N.A. and Goodrow, S.M. (2017). Seasonality of coliform bacteria detection rates in New Jersey domestic wells. Ground Water, 55(3): 346-361.
AWWA (1999). Water quality and treatment: a handbook of community water supplies. American Water Works Association. McGraw-Hill, New York, New York.
Bacci, F. and Chapman, D.V. (2011). Microbiological assessment of private drinking water supplies in Co. Cork, Ireland. J. Water. Health., 9(4): 738-751.
Batté, M., Féliers, C., Servais, P., Gauthier, V., Joret, J-C. and Block, J-C. (2006). Coliforms and other microbial indicators occurrence in water and biofilm in full-scale distribution systems. Water Sci. Technol., 54(3): 41-48
Beer, K.D., Gargano, J.W., Roberts, V.A., Hill, V.R., Garrison, L.E., Kutty, P.K., Hilborn, E.D., Wade, T.J., Fullerton, K.E. and Yoder, J.S. (2015). Surveillance for waterborne disease outbreaks associated with drinking water - United States, 2011-2012. MMWR Morb. Mortal. Wkly. Rep., 64(31): 842-848.
Bej, A.K., Steffan, R.J., DiCesare, J., Haff, L. and Atlas, R.M. (1990). Detection of coliform bacteria in water by polymerase chain reaction and gene probes. Appl. Environ. Microbiol., 56(2): 307-314.
Bernasconi, C., Volponi, G. and Bonadonna, L. (2006). Comparison of three different media for the detection of E. coli and coliforms in water. Water Sci. Technol., 54(3): 141-145.
Bernagozzi, M., Bianucci, F. and Sacchetti, R. (1995). Prevalence of Aeromonas spp. in surface waters. Water Environ. Res., 67(7): 1060-1064.
Berry, D., Xi, C. and Raskin, L. (2006). Microbial ecology of drinking water distribution systems. Curr. Opin. Biotechnol., 17(3): 297-302.
Besner, M.C., Gauthier, V., Barbeau, B., Millette, R., Chapleau, R. and Prévost, M. (2001). Understanding distribution system water quality. J. Am. Water Works Assoc., 93: 101-114.
Besner, M.C., Gauthier, V., Servais, P. and Camper, A. (2002). Explaining the occurrence of coliforms in the distribution system. J. Am. Water Works Assoc., 94: 95-109.
Besner, M.C., Gauthier, V., Trépanier, M., Martel, K. and Prévost, M. (2007). Assessing the effect of distribution system O&M on water quality. J. Am. Water Works Assoc., 99(11): 77-91.
Besner, M.C., Ebacher, G., Jung, B.S., Karney, B., Lavoie, J., Payment, P. and Prévost, M. (2010). Negative pressures in full-scale distribution system: Field investigation, modelling, estimation of intrusion volumes and risk for public health. Drink. Water Eng. Sci., 3(2): 101-106.
Besner, M.C., Prévost, M. and Regli, S. (2011). Assessing the public health risk of microbial intrusion events in distribution systems: Conceptual model, available data, and challenges. Water Res., 45(3): 961-979.
Blackburn, B.G., Craun, G.F., Yoder, J.S., Hill, V., Calderon, R.L., Chen, N., Lee, S.H., Levy, D.A. and Beach, M.J. (2004). Surveillance for waterborne-disease outbreaks associated with drinking water--United States, 2001-2002. MMWR Surveill. Summ., 53(8): 23-45.
Blanch A.R., Galofré, B., Lucena, F., Terradillos, A., Vilanova, X. and Ribas, F. (2007). Characterization of bacterial coliform occurrences in different zones of a drinking water distribution system. J. Appl. Microbiol., 102:711-721.
Blokker, M., Smeets, P. and Medema, G. (2014). QMRA in the drinking water distribution system. Procedia Eng., 89: 151-159.
Borchardt, M.A., Bertz, P.D., Spencer, S.K. and Battigelli, D.A. (2003). Incidence of enteric viruses in groundwater from household wells in Wisconsin. Appl. Environ. Microbiol., 69: 1172-1180.
Borchardt, M.A., Haas, N.L. and Hunt, R.J. (2004). Vulnerability of drinking-water wells in La Crosse, Wisconsin, to enteric-virus contamination from surface water contributions. Appl. Environ. Microbiol., 70(10): 5937-5946.
Borchardt, M.A., Bradbury, K.R., Gotkowitz, M.B., Cherry, J.A. and Parker, B.L. (2007). Human enteric viruses in groundwater from a confined bedrock aquifer. Environ. Sci. Technol., 41(18): 6606-6612.
Borchardt, M.A., Bradbury, K.R., Alexander, E.C., Kolberg, R.J., Alexander, S.C., Archer, J.R., Braatz, L.A., Forest, B.M., Green, J.A. and Spencer, S.K. (2011). Norovirus outbreak caused by a new septic system in a dolomite aquifer. Ground Water, 49(1): 85-97.
Borchardt, M.A., Spencer, S.K., Kieke, B.A., Lambertini, E. and Loge, F.J. (2012). Viruses in nondisinfected drinking water from municipal wells and community incidence of acute gastrointestinal illness. Environ. Health Perspect., 120(9): 1272-1279.
Botsaris, G., Kanetis, L., Slaný, M., Parpouna, C. and Makris, K.C. (2015). Microbial quality and molecular identification of cultivable microorganisms isolated from an urban drinking water distribution system (Limassol, Cyprus). Environ. Monit. Assess., 187(12).
Bradbury, K.R., Borchardt, M.A., Gotkowitz, M., Spencer, S.K., Zhu, J. and Hunt, R.J. (2013). Source and transport of human enteric viruses in deep municipal water supply wells. Environ. Sci. Technol., 47(9): 4096-4103.
Brennan, F.P., O'Flaherty, V., Kramers, G., Grant, J. and Richards, K.G. (2010). Long-term persistence and leaching of Escherichia coli in temperate maritime soils. Appl. Environ. Microbiol., 76(5): 1449-1455.
Brenner, J., Kreig, R. and Stanley, T. (2005). Volume two: The probacteria, part A. introductory essays. In: Bergey's manual of systematic bacteriology. Garrity, G. (ed.). Springer US, New Delhi, India, pp. 587-848. Brenner, K.P., Rankin, C.C., Roybal, Y.R., Stelma Jr., G.N., Scarpino, P.V. and Dufour, A.P. (1993). New medium for the simultaneous detection of total coliforms and Escherichia coli in water. Appl. Environ. Microbiol., 59(11): 3534-3544.
Brunkard, J.M., Ailes, E., Roberts, V.A., Hill, V., Hilborn, E.D., Craun, G.F., Rajasingham, A., Kahler, A., Garrison, L., Hicks, L., Carpenter, J., Wade, T.J., Beach, M.J., Yoder Msw, J.S. and CDC (2011). Surveillance for waterborne disease outbreaks associated with drinking water---United States, 2007--2008. MMWR Surveill. Summ., 60(12): 38-68.
Bushon, R.N., Brady, A.M.G. and Lindsey, B.D. (2015). Holding-time and method comparisons for the analysis of fecal-indicator bacteria in groundwater. Environ. Monit. Assess., 187(11).
Byappanahalli, M.N. and Ishii, S. (2011). Environmental sources of fecal bacteria. In: The fecal bacteria. Sadowsky, M.J. and Whitman, R.L. (eds.). American Society for Microbiology (ASM) Press, Washington, DC, pp. 93-110.
Camper, A.K. (2004). Involvement of humic substances in regrowth. Int. J. Food Microbiol., 92(3): 355-364.
Camper, A. (2014). Organic matter, pipe materials, disinfectants and biofilms in distribution systems. In: Microbial growth in drinking-water supplies: Problems, causes, control and research needs. van der Kooij, D. and van der Wielen, P.W.J.J. (eds.). IWA Publishing, London, UK, pp. 73-94.
Camper, A.K., Butterfield, P., Ellis, B. and Abernathy, C. (1999). Coliforms in biofilms: An overview. In: Proceedings of the Water Quality Technology Conference. Tampa, Florida.
Carrero-Colón, M., Wickham, G.S. and Turco, R.F. (2011). Taxonomy, phylogeny and physiology of fecal indicator bacteria. In: The fecal bacteria. Sadowsky, M.J. and Whitman, R.L. (eds.). American Society for Microbiology (ASM), Washington, DC, pp. 23-38.
Cartier, C., Besner, M.C., Barbeau, B., Lavoie, J., Besjardins, R. and Prévost, M. (2009). Evaluating aerobic endospores as indicators of intrusion in a distribution system. J. Am. Water Works Assoc., 101: 46-58.
CCME (2004). From source to tap: guidance on the multi-barrier approach to safe drinking water. Produced jointly by the Federal-Provincial-Territorial Committee on Drinking Water and the CCME Water Quality Task Group.
Canadian Council of Ministers of the Environment, Winnipeg, Manitoba. Available at: www.ccme.ca/assets/pdf/mba_guidance_doc_e.pdf
Chang, Y. and Jung, K. (2004). Effect of distribution system materials and water quality on heterotrophic plate counts and biofilm proliferation. J Microbiol. Biotechnol., 14(6): 1114-1119.
Chao, W.L. (2006). Evaluation of colilert-18 for the detection of coliforms and Escherichia coli in tropical fresh water. Lett. Appl. Microbiol., 42(2): 115-120.
Chao, Y., Ma, L., Yang, Y., Ju, F., Zhang, X.X., Wu, W.M. and Zhang, T. (2013). Metagenomic analysis reveals significant changes of microbial compositions and protective functions during drinking water treatment. Sci. Rep., 3: 3550.
Chao, Y., Mao, Y., Wang, Z. and Zhang, T. (2015). Diversity and functions of bacterial community in drinking water biofilms revealed by high-throughput sequencing. Sci. Rep., 5: 10044.
Chauret, C., Volk, C., Creason, R., Jarosh, J., Robinson, J. and Warnes, C. (2001). Detection of Aeromonas hydrophila in a drinking-water distribution system: a field and pilot study. Can. J. Microbiol., 47: 782-786.
Chilton, J. and Seiler, K.P. (2006). Groundwater occurrence and hydrogeological environments. In: Protecting groundwater for health: Managing the quality of drinking-water sources. Schmoll, O., Howard, G., Chilton, J. and Chorus, I. (eds.). IWA Publishing, London, UK.
Cho, H.G., Lee, S.G., Kim, W.H., Lee, J.S., Park, P.H., Cheon, D.S., Jheong, W.H., Jho, E.H., Lee, J.B. and Paik, S.Y. (2014). Acute gastroenteritis outbreaks associated with ground-waterborne norovirus in South Korea during 2008-2012. Epidemiol. Infect., 142(12): 2604-2609.
Córdoba, M.A., Del Coco, V.F., Minvielle, M.C. and Basualdo, J.Á. (2010). Influencing factors in the occurrence of injured coliforms in the drinking water distribution system in the city of La Plata, Argentina. J. Water Health, 8(2): 205-211
Council of the European Union (1998). Council directive 98/83/EC of 3 November 1998 on the quality of water intended for human consumption. Off. J. Eur. Commun., L330: 32.
Coulibaly, H.D. and Rodriguez, M.J. (2003). Portrait of drinking water quality in small Quebec municipal utilities. Water Qual. Res. J. Can., 38(1): 49-76.
Coulibaly, H.D. and Rodriguez, M.J. (2004). Development of performance indicators for small Quebec drinking water utilities. J. Environ. Manage., 73(3): 243-255.
Covert, T.C., Shadix, L.C., Rice, E.W., Haines, J.R. and Freyberg, R.W. (1989). Evaluation of the autoanalysis colilert test for detection and enumeration of total coliforms. Appl. Environ. Microbiol., 55(10): 2443-2447.
Cox, W. and Giron, J.J. (1993). Gimmicks and gadgets -- taking large core samples made easy with a shop vacuum. Opflow, 19(7): 5.
Craun, G.F., Berger, P.S. and Calderon, R.L. (1997). Coliform bacteria and waterborne disease outbreaks. J. Am. Water Works Assoc., 89(3): 96-104.
Craun, G.F. and Calderon, R.L. (2001). Waterborne disease outbreaks caused by distribution system deficiencies. J. Am. Water Works Assoc., 93: 64-75.
DeFelice, N.B., Johnston, J.E. and Gibson, J.M. (2016). Reducing emergency department visits for acute gastrointestinal illnesses in North Carolina (USA) by extending community water service. Environ. Health Perspect., 124(10): 1583-1591.
Delafont, V., Brouke, A., Bouchon, D., Moulin, L. and Hechard, Y. (2013). Microbiome of free-living amoebae isolated from drinking water. Water Res., 47(19): 6958-6965.
DeSimone, L.A., Hamilton, P.A. and Gilliom, R.J. (2009). The quality of our nation's waters-Quality of water from domestic wells in principal aquifers of the United States, 1991-2004-Overview of major findings. U.S. Geological Survey (USGS), Circular 1332, Washington, DC.
Donlan, R.M. (2002). Biofilms: Microbial life on surfaces. Emerg. Infect. Dis., 8(9): 881-890.
Durand, J., Deshommes, E., Nour, S. and Prévost, M. (2016). Monitoring water quality in distribution systems: Laboratory and field validation of on-line multi-parameter probes. 17th Canadian National Conference on Drinking Water. Ottawa, ON.
Dutka, B.J. and El-Shaarawi, A. (1980). Microbiological water and effluent sample preservation. Can. J. Microbiol., 26: 921-929. DVGW (2006a). German national standard:
DVWG W 294-1:2006: UV disinfection equipment for water supply systems - part 1: Design and construction, function and operation. Deutscher Verein des Gas und Wasserfachs, Bonn, Germany. DVGW (2006b). German national standard:
DVWG W 294-2:2006: UV disinfection equipment for water supply systems - part 2: Testing (construction, function and efficiency). Deutscher Verein des Gas und Wasserfachs, Bonn, Germany. DVGW (2006c). German national standard:
DVWG W 294-3:2006: UV disinfection equipment for water supply systems - part 3: Measuring windows and sensors for monitoring UV disinfection equipment - requirements, testing and calibration. Deutscher Verein des Gas und Wasserfachs, Bonn, Germany.
DWI (2000). Water, England and Wales: the water supply (water quality) regulations 2000 no. 3184. Drinking Water Inspectorate, London, UK. Available at: www.dwi.gov.uk/regs/si3184/3184.htm#sch1pA
Ebacher, G., Besner, M.C., Clément, B. and Prévost, M. (2012). Sensitivity analysis of some critical factors affecting simulated intrusion volumes during a low pressure transient event in a full-scale water distribution system. Water Res., 46(13): 4017-4030.
Edberg, S.C., Patterson, J.E. and Smith, D.B. (1994). Differentiation of distribution systems, source water, and clinical coliforms by DNA analysis. J. Clin. Microbiol., 32: 139-142.
Edberg, S.C., Rice, E.W., Karlin, R.J. and Allen, M.J. (2000). Escherichia coli: the best biological drinking water indicator for public health protection. J. Appl. Microbiol., 88: 106S-116S.
Egorov, A., Paulauskis, J., Petrova, L., Tereschenko, A., Drizhd, N. and Ford, T. (2002). Contamination of water supplies with Cryptosporidium parvum and Giardia lamblia and diarrheal illness in selected Russian cities. Int. J. Hyg. Environ. Health, 205(4): 281-289.
El-Taweel, G.E. and Shaban, A.M. (2001). Microbiological quality of drinking water at eight water treatment plants. Int. J. Environ. Health Res., 11: 285-290.
Embrey, S.S. and Runkle, D.L. (2006). Microbial quality of the Nation's ground-water resources, 1993-2004. U.S. Geological Survey (USGS), 2006-5290, Washington, DC.
Escobar, I.C. and Randall, A.A. (2001). Assimilable organic carbon (AOC) and biodegradable dissolved organic carbon (BDOC): complementary measurements. Water Res. 35: 4444-4454.
EU (1998). Council directive 98/83/EC on the quality of water intended for human consumption. Official Journal of the European Communities, L 330(5.12): 32-54.
Falkinham, J.O., Pruden, A. and Edwards, M. (2015). Opportunistic premise plumbing pathogens: Increasingly important pathogens in drinking water. Pathogens, 4(2): 373-386.
Fatemeh, D., Reza, Z.M., Mohammad, A., Salomeh, K., Reza, A.G., Hossein, S., Maryam, S., Azam, A., Mana, S., Negin, N., Reza, K.A. and Saeed, F. (2014). Rapid detection of coliforms in drinking water of Arak City using multiplex PCR method in comparison with the standard method of culture (most probable number). Asian Pac. J. Trop. Biomed., 4(5): 404-409.
Fenlon, D.R., Ogden, I.D., Vinten, A. and Svoboda, I. (2000). The fate of Escherichia coli and E. coli O157 in cattle slurry after application to land. J. Appl. Microbiol. Symp. Suppl., 88(29): 149s-156s.
Ferguson, C.M. (1994). Refrigerated autosampling for the assessment of bacteriological water quality. Water Res., 28(4): 841-847.
Figueras, M.J. and Borrego, J.J. (2010). New perspectives in monitoring drinking water microbial quality. Int. J. Environ. Res. Public Health, 7(12): 4179-4202.
Fisher, I., Angles, M., Chandy, J., Cox, P., Warnecke, M., Kastl, G. and Jegatheesan, V. (2000). Biofilms - a sticky situation for drinking water? Water (Australia), 27(2): 33-37.
Fleming, K.K., Dugandzic, J.P., LeChevallier, M.W. and Gullick, R.W. (2006). Susceptibility of distribution systems to negative pressure transients. AWWA Research Foundation and American Water, US.
Fong, T., Mansfield, L.S., Wilson, D.L., Schwab, D.J., Molloy, S.L. and Rose, J.B. (2007). Massive microbiological groundwater contamination associated with a waterborne outbreak in Lake Erie, South Bass Island, Ohio. Environ. Health Perspect., 115(6): 856-864.
Fontanazza, C.M., Notaro, V., Puleo, V., Nicolosi, P. and Freni, G. (2015). Contaminant intrusion through leaks in water distribution system: Experimental analysis. Procedia Eng., 119(1): 426-433.
Fout, G.S., Borchardt, M.A., Kieke, B.A. and Karim, M.R. (2017). Human virus and microbial indicator occurrence in public-supply groundwater systems: Meta-analysis of 12 international studies. Hydrogeol. J., 25(4): 903-919.
Francy, D.S., Helsel, D.R. and Nally, R.A. (2000). Occurrence and distribution of microbiological indicators in groundwater and stream water. Water Environ. Res., 72(2): 152-161.
Fricker, E.J. and Fricker, C.R. (1994). Application of the polymerase chain reaction to the identification of Escherichia coli and coliforms in water. Lett. Appl. Microbiol., 19(1): 44-46.
Friedman, M., Hanson, A., Dewis, K., Kirmeyer, G., LeChevallier, M., Gagnon, G., Truelstrup Hansen, L., Krentz, C., Mosher, M., Payne, S.J., Rosen, J., Hargy, T., Sobrinho, J., Besner, M-C. and Prevost, M. (2009). Strategies for managing total coliform and E. coli in distribution system. Water Research Foundation, Denver, CO.
Fujioka, R.S. and Yoneyama, B.S. (2001). Assessing the vulnerability of groundwater systems to fecal contamination. J. Am. Water Works Assoc., 93: 62-71.
Gagnon, G.A., Murphy, H.M., Rand, J.L., Payne, S.J., Springthorpe, S., Matias, F., Nokbeh, R. and Sattar, S.S. (2007). Coliforms in distribution systems; integrated disinfection and antimicrobial resistance. AWWA, AWWA Research Foundation, IWA, Denver, CO.
Geldreich, E.E., Taylor, R.H., Blannon, J.C. and Reasoner, D.J. (1985). Bacterial colonization of point-of-use water treatment devices. J. Am. Water Works Assoc., 77(2): 72-80.
Geldreich, E.E. and Rice, E.W. (1987). Occurrence, significance, and detection of Klebsiella in water systems. J. Am. Water Works Assoc., 79: 74-80.
Geldreich, E.E. and LeChevallier, M.W. (1999). Microbial water quality in distribution systems. 5th. Letterman, R.D. (ed.). McGraw-Hill, New York, US.
Glanville, T.D., Baker, J.L. and Newman, J.K. (1997). Statistical analysis of rural well contamination and effects of well construction. Trans. Am. Soc. Agric. Eng., 40(2): 363-370.
Goatcher, L.J., Simpson, K.A. and Emde, K.M.E. (1992). Are all positive microbiological samples created equal? Proceedings from the 1992 AWWA Water Quality Technology Conference. American Water Works Association, Denver, CO.
Gomez-Alvarez, V., Revetta, R.P. and Santo Domingo, J.W. (2012). Metagenomic analyses of drinking water receiving different disinfection treatments. Appl. Environ. Microbiol., 78(17): 6095-6102.
Gomez-Alvarez, V., Humrighouse, B.W., Revetta, R.P. and Santo Domingo, J.W. (2015). Bacterial composition in a metropolitan drinking water distribution system utilizing different source waters. J. Water Health, 13(1): 140-151.
Gonzales, T.R. (2008). The effects that well depth and wellhead protection have on bacterial contamination of private water wells in the Estes Park Valley, Colorado. J. Environ. Health, 71(5): 17-23.
Goss, M.J., Barry, D.A.J. and Rudolph, D.L. (1998). Contamination in Ontario farmstead domestic wells and its association with agriculture: 1. results from drinking water wells. J. Contam. Hydrol., 32(3-4): 267-293.
Gunnarsdottir, M.J., Gardarsson, S.M. and Andradottir, H.O. (2013). Microbial contamination in groundwater supply in a cold climate and coarse soil: Case study of norovirus outbreak at Lake Mývatn, Iceland. Hydrol. Res., 44(6): 1114-1128.
Guzman-Herrador, B., Carlander, A., Ethelberg, S., Freiesleben de Blasio, B., Kuusi, M., Lund, V., Löfdahl, M., MacDonald, E., Nichols, G., Schönning, C., Sudre, B., Trönnberg, L., Vold, L., Semenza, J.C. and Nygård, K. (2015). Waterborne outbreaks in the Nordic countries, 1998 to 2012. Euro Surveill., 20 (24).
Haas, C.N., Meyer, M.A. and Paller, M.S. (1983). Ecology of acid-fast organisms in water supply, treatment, and distribution systems. J. Am. Water Works Assoc., 75(3): 139-144.
Hall, J., Zaffiro, A.D., Marx, R.B., Kefauver, P.C., Krishnan, E.R. and Herrmann, J.G. (2007). On-line water quality parameters as indicators of distribution system contamination. J. Am. Water Works Assoc., 99(1): 66-77.
Hallas, G., Giglio, S., Capurso, V., Monis, P.T. and Grooby, W.L. (2003). Evaluation of chromogenic technologies for use in Australian potable water. J. Appl. Microbiol., 105(4): 1138-1149. Hänninen M.L.,
Haajanen, H., Pummi, T., Wermundgen, K., Katila, M.L., Sarkkinen, H., Miettinen, I. and Rautelin, H. (2003). Detection and typing of Campylobacter jejuni and Campylobacter coli and analysis of indicator organisms in three waterborne outbreaks in Finland. Appl. Environ. Microbiol., 69: 1391-1396.
Hargy, T.M., Rosen, J., Lechevallier, M., Friedman, M. and Clancy, J.L. (2010). A high-volume sampling method for total coliform and E. coli. J. Am. Water Works Assoc., 102(3): 79-86.
Harris, D.G., Adams, V.D., Sorensen, D.L. and Curtis, M.S. (1987). Ultra-violet inactivation of selected bacteria and viruses with photoreactivation of bacteria. Water Res., 21: 687-692.
Health and Welfare Canada (1993). Water treatment principles and applications: a manual for the production of drinking water. Canadian Water and Wastewater Association, Ottawa, Ontario.
Health Canada (2012). Guidelines for Canadian drinking water quality: guideline technical document - turbidity. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No. H144-9/2013E-PDF). Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-turbidity.html.
Health Canada (2015). Guidance for Issuing and Rescinding Boil Water Advisories in Canadian Drinking Water Supplies. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario (Catalogue No. H128-1/09-578-1E-PDF). Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidance-issuing-rescinding-boil-water-advisories-canadian-drinking-water-supplies.html.
Health Canada (2016). Guidelines for Canadian drinking water quality: Guideline technical document - bromate. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No. H144-37/2017E-PDF). Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-bromate.html.
Health Canada (2019a). Guidelines for Canadian drinking water quality: guideline technical document - enteric viruses. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No. H129-6/2019E-PDF). Available at: https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-enteric-viruses.html.
Health Canada (2019b). Guidelines for Canadian drinking water quality: guideline technical document - enteric protozoa: Giardia and Cryptosporidium. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (Catalogue No H144-13/10-2018E-PDF). Available at: https://www.canada.ca/en/health-canada/services/environmental-workplace-health/reports-publications/water-quality/enteric-protozoa-giardia-cryptosporidium.html.
Health Canada (2019c). Guidance on natural organic matter in drinking water. Document for public consultation. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at: https://www.canada.ca/content/dam/hc-sc/documents/programs/consultation-organic-matter-drinking-water/NOM20190129-eng.pdf
Health Canada (2020a). Guidelines for Canadian drinking water quality: guideline technical document - Escherichia coli. Water, Air and Climate Change Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. (https://www.canada.ca/en/health-canada/services/publications/healthy-living/guidelines-canadian-drinking-water-quality-guideline-technical-document-escherichia-coli.html)
Health Canada (2020b). Guidance on monitoring the biological stability of drinking water in distribution systems. Document for public consultation. Water and Air Quality Bureau, Healthy Environments and Consumer Safety Branch, Health Canada, Ottawa, Ontario. Available at:
Hijnen, W.A., Beerendonk, E.F. and Medema, G.J. (2006). Inactivation credit of UV radiation for viruses, bacteria and protozoan (oo)cyst in water: A review. Water Res., 40: 3-22.
Hijnen, W.A. and Medema, G.J. (2010). Elimination of micro-organisms by drinking water treatment processes-A review. KWR Watercycle Research Institute. IWA Publishing, London, UK.
Hilborn, E.D., Wade, T., Hicks, L., Garrison, L. and Gargano, J. (2013). Surveillance for waterborne disease outbreaks associated with drinking water and other nonrecreational water - United States, 2009 - 2010. Morb Mortal Wkly Rep, 62(35): 714-720.
Hill, A.S., Friedman, M., Hallett, M., Salo-Zieman, V., Booth, S., Hanson, A., Gupta, K., Akagi, Y., Kochiss, C., Koperski, L., Igoe, P., Kirby, L. and Harper, W. (2018). Use of Flushing as a Corrective Action Under the Revised Total Coliform Rule. Report #4653. Water Research Foundation, Denver, Colorado.
Hoff, J.C. (1986). Inactivation of microbial agents by chemical disinfectants. U.S. Environmental Protection Agency, Cincinnati, Ohio (EPA/600/S2-86/067).
Howe, A.D., Forster, S., Morton, S., Marshall, R., Osborn, K.S., Wright, P. and Hunter, P.R. (2002). Cryptosporidium oocysts in a water supply associated with a cryptosporidiosis outbreak. Emerg. Infect. Dis., 8(6): 619-624.
Hrudey, S.E. and Rizak, S. (2004). Discussion of: rapid analytical techniques for drinking water security investigations. J. Am. Water Works Assoc., 96(9): 110-113.
Hunter, P.R., Chalmers, R.M., Hughes, S. and Syed, Q. (2005). Self-reported diarrhea in a control group: a strong association with reporting of low-pressure events in tap water. Clinical Infect. Dis., 40: e32-e34.
Hynds, P.D., Misstear, B.D. and Gill, L.W. (2012). Development of a microbial contamination susceptibility model for private domestic groundwater sources. Water Resour. Res., 48(12).
Hynds, P.D., Thomas, M.K. and Pintar, K.D. (2014). Contamination of groundwater systems in the US and Canada by enteric pathogens, 1990-2013: A review and pooled-analysis. PLoS One, 9(5): e93301.
Ikonen, J., Pitkänen, T. and Miettinen, I.T. (2013). Suitability of optical, physical and chemical measurements for detection of changes in bacterial drinking water quality. Int. J. Environ. Res. Public Health, 10(11): 5349-5363.
Ikonen, J., Pitkänen, T., Kosse, P., Ciszek, R., Kolehmainen, M. and Miettinen, I.T. (2017). On-line detection of Escherichia coli intrusion in a pilot-scale drinking water distribution system. J. Environ. Manage., 198: 384-392.
ISO (1998). Method 9308-3: Water quality - detection and enumeration of Escherichia coli and coliform bacteria. part 3: Miniaturized method (most probable number) for the detection and enumeration of E.coli in surface and waste water. International Organization for Standardization, Geneva, Switzerland. ISO (2006). Water quality - Sampling for microbiological analysis.
ISO 19458: 2006. ISO (2012). Method 9308-2: Water quality - enumeration of Escherichia coli and coliform bacteria. part 2: Most probable number method.International Organization for Standardization, Geneva, Switzerland.
ISO (2014). Method 9308-1: Water quality - enumeration of Escherichia coli and coliform bacteria. part 1: Membrane filtration method for waters with low bacterial background flora. International Organization for Standardization, Geneva, Switzerland.
Jack, S., Bell, D. and Hewitt, J. (2013). Norovirus contamination of a drinking water supply at a hotel resort. New Zealand Med. J., 126(1387): 98-107.
Karathanasis, A.D., Mueller, T.G., Boone, B. and Thompson, Y.L. (2006). Effect of soil depth and texture on fecal bacteria removal from septic effluents. J. Water. Health., 4(3): 395-404.
Karim, M.R., Abbaszadegan, M. and Lechevallier, M. (2003). Potential for pathogen intrusion during pressure transients. J. Am. Water Works Assoc., 95(5): 134-146.
Kilb, B., Lange, B., Schaule, G., Flemming, H.C. and Wingender, J. (2003). Contamination of drinking water by coliforms from biofilms grown on rubber-coated valves. Int. J. Hyg. Environ. Health, 206(6): 563-573.
Kirmeyer, G., Friedman, M., Martel, K., LeChevallier, M.W., Funk, J., Jackman, M. and Harbour, J. (1999). Pathogen intrusion into potable water.In: Proceedings of the 1999 AWWA Water Quality Technology Conference, Tampa, Florida. American Water Works Association, Denver, Colorado.
Kirmeyer, G.J., Friedman, M., Martel, K., Howie, D., LeChevallier, M., Abbaszadegan, M., Karim, M., Funk, J. and Harbour, J. (2001). Pathogen intrusion into the distribution system. AWWA Research Foundation and American Water Works Association, Denver, CO.
Kirmeyer, G.J., Thomure, T.M., Rahman, R., Marie, J.L., LeChevallier, M.W., Yang, J., Hughes, D.M. and Schneider, O. (2014). Effective microbial control strategies for main breaks and depressurization. Water Research Foundation, Denver, CO.
Kozuskanich, J., Novakowski, K.S. and Anderson, B.C. (2011). Fecal indicator bacteria variability in samples pumped from monitoring wells. Ground Water, 49(1): 43-52.
Kozuskanich, J.C., Novakowski, K.S., Anderson, B.C., Crowe, A.S. and Balakrishnan, V.K. (2014). Anthropogenic impacts on a bedrock aquifer at the village scale. Ground Water, 52(3): 474-486.
Krapac, I.G., Dey, W.S., Roy, W.R., Smyth, C.A., Storment, E., Sargent, S.L. and Steele, J.D. (2002). Impacts of swine manure pits on groundwater quality. Environ. Pollut., 120(2): 475-492.
Kuusi, M., Nuorti, J.P., Hänninen, M-L., Koskela, M., Jussila, V., Kela, E., Miettinen, I. and Ruutu, P. (2005). A large outbreak of campylobacteriosis associated with a municipal water supply in Finland. Epidemiol. Infect., 133(4): 593-601.
Lambertini, E., Borchardt, M.A., Kieke, B.A., Spencer, S.K. and Loge, F.J. (2012). Risk of viral acute gastrointestinal illness from nondisinfected drinking water distribution systems. Environ. Sci. Technol., 46(17): 9299-9307.
Lamka, K.G., LeChevallier, M.W. and Seidler, R.J. (1980). Bacterial contamination of drinking water supplies in a modern rural neighborhood. Appl. Environ. Microbiol., 39(4): 734-738.
Lautenschlager, K., Boon, N., Wang, Y., Egli, T. and Hammes, F. (2010). Overnight stagnation of drinking water in household taps induces microbial growth and changes in community composition. Water Res., 44(17): 4868-4877.
LeChevallier, M.W. (1990). Coliform regrowth in distribution systems: a review. J. Am. Water Works Assoc., 82: 74-86.
LeChevallier, M.W. (2003). Conditions favouring coliform and HPC bacterial growth in drinking-water and on water contact surfaces. In: Heterotrophic plate counts and drinking-water safety: The significance of HPCs for water quality and human health. J. Bartram, J. Cotruvo, M. Exner, C. Fricker and A. Glasmacher (eds.). IWA Publishing, London, United Kingdom. pp. 177-198.
LeChevallier, M.W. (2014). Conducting self-assessments under the revised total coliform rule. J. Am. Water Works Assoc., 106(9): 90-102.
LeChevallier, M.W. and Au, K.K. (2004). Water treatment and pathogen control: Process efficiency in achieving safe drinking water. IWA Publishing, London, United Kingdom, on behalf of the World Health Organization, Geneva, Switzerland.
LeChevallier, M.W. and McFeters, G.A. (1990). Microbiology of activated carbon. In: Drinking water microbiology: progress and recent developments. G.A. McFeters (ed.). Springer-Verlag, New York, New York. pp. 104-119.
LeChevallier, M.W., Norton, W.D. and Lee, R.G. (1991a). Occurrence of Giardia and Cryptosporidium spp. in surface water supplies. Appl. Environ. Microbiol., 57(9): 2610-2616.
LeChevallier, M.W., Schiemann, D.A. and McFeters, G.A. (1987). Factors contributing to the reduced invasiveness of chlorine-injured Yersinia enterocolitica. Appl. Environ. Microbiol., 53: 1358-1364.
LeChevallier, M.W., Schulz, W. and Lee, R.G. (1991b). Bacterial nutrients in drinking water. Appl. Environ. Microbiol., 57: 857-862.
LeChevallier, M.W., Welch, N.J. and Smith, D.B. (1996). Full-scale studies of factors related to coliform regrowth in drinking water. Appl. Environ. Microbiol. 62: 2201-2211.
LeChevallier, M.W., Gullick, R.W., Karim, M.R., Friedman, M. and Funk, J.E. (2003). The potential for health risks from intrusion of contaminants into the distribution system from pressure transients. J. Water Health, 1(1): 3-14.
LeChevallier, M.W., Karim, M.R., Weihe, J., Rosen, J.S. and Sabrinho, J. (2006). Coliphage as a potential indicator of distribution system integrity. J. Am. Water Works Assoc., 98: 87-96.
LeChevallier, M.W., Schneider, O.D., Weinrich, L.A., Jjemba, P.K., Evans, P.J., Hooper, J.L. and Chappell, R.W. (2015a). An operational definition of biostability in drinking water. Water Research Foundation, Denver, CO.
LeChevallier, M.W., Schneider, O.D., Weinrich, L.A., Jjemba, P.K., Evans, P.J., Hooper, J.L. and Chappell, R.W. (2015b). Guidance manual for control of biostability in drinking water. Water Research Foundation, Denver, CO.
Leclerc, H. (2003). Relationships between common water bacteria and pathogens in drinking-water. In: Heterotrophic plate counts and drinking-water safety: The significance of HPCs for water quality and human health. Bartram, J., Cotruvo, J., Exner, M., Fricker, C. and Glasmacher, A. (eds.). IWA Publishing, London, UK, pp. 80-118.
Leclerc, H., Mossel, D.A.A., Edberg, S.C. and Struijk, C.B. (2001). Advances in the bacteriology of the coliform group: their suitability as markers of microbial water safety. Annu. Rev. Microbiol., 55: 201-234.
Lee, D. and Kim, S. (2003). Bacterial species in biofilm cultivated from the end of the Seoul water distribution system. J. Appl. Microbiol., 95(2): 317-324.
Lee, D., Lee, J.and Kim, S. (2005). Diversity and dynamics of bacterial species in a biofilm at the end of the Seoul water distribution system. World J. Microbiol. Biotechnol., 21(2): 155-162.
Lee, S.H., Levy, D.A., Craun, G.F., Beach, M.J. and Calderon, R.L. (2002). Surveillance for waterborne-disease outbreaks--United States, 1999-2000. MMWR Surveill Summ, 51(8): 1-47.
Lee, Y. (2013). Factors controlling bacterial growth in drinking water distribution pipes. Asian J. Chem., 25(3): 1629-1634.
Lehtola, M.J., Nissinen, T.K., Miettinen, I.T., Martikainen, P.J. and Vartiainen, T. (2004). Removal of soft deposits from the distribution system improves the drinking water quality. Water Res. 38: 601-610.
Levison, J.K. and Novakowski, K.S. (2012). Rapid transport from the surface to wells in fractured rock: A unique infiltration tracer experiment. J. Contam. Hydrol., 131(1-4): 29-38.
Liang, J.L., Dziuban, E.J., Craun, G.F., Hill, V., Moore, M.R., Gelting, R.J., Calderon, R.L., Beach, M.J. and Roy, S.L. (2006). Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking--United States, 2003-2004. MMWR Surveill Summ, 55(12): 31-65.
Liu, W.T. and Stahl, D.A. (2007). Chapter 12: Molecular approaches for the measurement of density, diversity, and phylogeny. In: Manual of environmental microbiology. Hurst, C.J., Garland, J.L., Lipson, D.A., Mills, A.L. and Stetzenbach, L.D. (eds.). Third. pp. 139-156.
Locas, A., Barthe, C., Barbeau, B., Carrière, A. and Payment, P. (2007). Virus occurrence in municipal groundwater sources in Quebec, Canada. Can. J. Microbiol., 53(6): 688-694.
Locas, A., Barthe, C., Margolin, A.B. and Payment, P. (2008). Groundwater microbiological quality in Canadian drinking water municipal wells. Can. J. Microbiol., 54(6): 472-478.
Maheux, A.F., Huppé, V., Boissinot, M., Picard, F.J., Bissonnette, L., Bernier, J-T. and Bergeron, M.G. (2008). Analytical limits of four ß-glucuronidase and ß-galactosidase-based commercial culture methods used to detect Escherichia coli and total coliforms. J. Microbiol. Methods, 75(3): 506-514.
Maheux, A.F., Boudreau, D.K., Bisson, M., Dion-Dupont, V., Bouchard, S., Nkuranga, M., Bergeron, M.G. and Rodriguez, M.J. (2014).Molecular method for detection of total coliforms in drinking water samples. Appl. Environ. Microbiol., 80(14): 4074-4084.
Maheux, A.F., Dion-Dupont, V., Bouchard, S., Bisson, M., Bergeron, M.G. and Rodriguez, M.J. (2015). Comparison of four ß-glucuronidase and ß-galactosidase based commercial culture methods used to detect Escherichia coli and total coliforms in water. J. Water Health, 13(2): 340-352.
Maheux, A.F., Bouchard, S., Bérubé, E. and Bergeron, M.G. (2017). Comparison of MI, chromocult®coliform, and compass CC chromogenic culture-based methods to detect Escherichia coli and total coliforms in water using 16S rRNA sequencing for colony identification. J. Water Health, 15(3): 353-359.
Maier, A., Krolik, J., Randhawa, K. and Majury, A. (2014). Bacteriological testing of private well water: A trends and guidelines assessment using five years of submissions data from southeastern Ontario. Can. J. Public Health, 105(3): e203-e208.
Marrero-Ortiz, R., Riley, K.R., Karpiscak, M.K. and Gerba, C.P. (2009). Groundwater quality of individual wells in small systems in Arizona. J. Am. Water Works Assoc., 101: 89-100.
Marshall, M.M., Naumovitz, D., Ortega, Y. and Sterling, C.R. (1997). Waterborne protozoan pathogens. Clin. Microbiol. Rev., 10(1): 67-85.
Martin, R.S., Gates, W.H., Tobin, R.S., Grantham, D., Sumarah, R., Wolfe, P. and Forestall, P. (1982). Factors affecting coliform bacteria growth in distribution systems. J. Am. Water Works Assoc., 74: 34-37.
McDaniels, A.E., Bordner, R.H., Gartside, P.S., Haines, J.R. Brenner, K.P. and Rankin, C.C. (1985). Holding effects on coliform enumeration in drinking water samples. Appl. Environ. Microbiol., 50: 755-762.
McMath, S.M., Sumpler, C., Holt, D.M., Delanoue, A. and Chamberlain, A.H.L. (1999). The face of environmental coliforms in a model water distribution system. Lett. Appl. Microbiol., 28: 93-97.
Méric, G., Kemsley, E.K., Falush, D., Saggers, E.J. and Lucchini, S. (2013). Phylogenetic distribution of traits associated with plant colonization in Escherichia coli. Environ. Microbiol., 15(2): 487-501.
Messner, M., Shaw, S., Regli, S., Rotert, K., Blank, V. and Soller, J. (2006). An approach for developing a national estimate of waterborne disease due to drinking water and a national estimate model application. J. Water Health, 4(SUPPL. 2): 201-240.
Miles, S.L., Sinclair, R.G., Riley, M.R. and Pepper, I.L. (2011). Evaluation of select sensors for real-time monitoring of Escherichia coli in water distribution systems. Appl. Environ. Microbiol., 77(8): 2813-2816.
Molina, F., López-Acedo, E., Tabla, R., Roa, I., Gómez, A. and Rebollo, J.E. (2015). Improved detection of Escherichia coli and coliform bacteria by multiplex PCR. BMC Biotechnol., 15(1).
Morin, P., Camper, A., Jones, W., Gatel, D. and Goldman, J.C. (1996). Colonization and disinfection of biofilms hosting coliform-colonized carbon fines. Appl. Environ. Microbiol., 62(12): 4428-4432.
Mosher, M. (2011). Coliform bacteria from a drinking water distribution system: Microbial source tracking, characterization and biofilm formation. MSc thesis. Dalhousie University, Halifax, NS.
Murphy, H.M., Thomas, M.K., Schmidt, P.J., Medeiros, D.T., McFadyen, S. and Pintar, K.D. (2016a). Estimating the burden of acute gastrointestinal illness due to Giardia, Cryptosporidium, Campylobacter, E. coli O157 and norovirus associated with private wells and small water systems in Canada. Epidemiol. Infect., 144(7): 1355-1370.
Murphy, H.M., Thomas, M.K., Medeiros, D.T., McFadyen, S. and Pintar, K.D. (2016b). Estimating the number of cases of acute gastrointestinal illness (AGI) associated with Canadian municipal drinking water systems. Epidemiol. Infect., 144(7): 1371-1385.
Nakano, J., Kameyama, T., Venkateswaran, K., Kawakami, J. and Hashimoto, J. (1990). Distribution and characterisation of hemolytic and enteropathogenic motile Aeromonas in aquatic environment. Microbial Immunol., 34: 447-458.
Narasimhan, R., Brereton J., Abbaszadegan, M., Alum, A. and Ghatpande, P. (2004). Sample collection procedures and locations for bacterial compliance monitoring. Awwa Research Foundation, Denver, Colorado.
NHMRC (2016). Australian drinking water guidelines 6, 2001 version 3.3 - updated November 2016. National Health and Medical Research Council (NHMRC), National Resource Management Ministerial Council, Commonwealth of Australia, Canberra, Australia
Niquette, P., Servais, P. and Savoir, R. (2000). Impacts of pipe materials on densities of fixed bacterial biomass in a drinking water distribution system. Water Res., 34(6): 1952-1956. Nova Scotia Environment (2012).
Nova Scotia treatment standards for municipal drinking water systems. Available at : www.novascotia.ca/nse/water/docs/Treatment_Standards_for_Municipal_Drinking_Water_Systems.pdf.
NRC (2006). Drinking water distribution systems: Assessing and reducing risks. National Research Council. The National Academies Press, Washington, DC.
NSF/ANSI (2018a). Standard 58-Reverse osmosis drinking water treatment systems. NSF International, Ann Arbor, Michigan.
NSF/ANSI (2018b). Standard 419-Public drinking water equipment performance - filtration. NSF International, Ann Arbor, Michigan
NSF/ANSI (2018c). Standard 62-Drinking water distillation systems. NSF International, Ann Arbor, Michigan.
NSF/ANSI (2019). Standard 55-Ultraviolet microbiological water treatment systems. NSF International, Ann Arbor, Michigan.
NSF/ANSI/CAN (2018). Standard 60-Drinking water treatment chemicals - health effects. NSF International, Ann Arbor, Michigan.
Nwachuku, M., Craun, G.F. and Calderon, R.L. (2002). How effective is the TCR in assessing outbreak vulnerability? J. Am. Water Works Assoc., 94(9): 88-96.
Nygård, K., Vold, L., Halvorsen, E., Bringeland, E., Røttingen, J.A. and Aavitsland, P. (2004). Waterborne outbreak of gastroenteritis in a religious summer camp in Norway, 2002. Epidemiol. Infect., 132(2): 223-229.
Nygård, K., Wahl, E., Krogh, T., Tveit, O.A., Bøhleng, E., Tverdal, A. and Aavitsland, P. (2007). Breaks and maintenance work in the water distribution systems and gastrointestinal illness: A cohort study. Int. J. Epidemiol., 36(4): 873-880.
O'Dwyer, J., Dowling, A. and Adley, C.C. (2014). Microbiological assessment of private groundwater-derived potable water supplies in the mid-west region of Ireland. J. Water. Health., 12(2): 310-317.
Olstadt, J., Schauer, J.J., Standridge, J. and Kluender, S. (2007). A comparison of ten USEPA approved total coliform/E. coli tests. J. Water Health, 5:267-282.
ÖNORM (2001). Austrian national standard: ÖNORM M 5873-1E, plants for disinfection of water using ultraviolet radiation: Requirements and testing, part 1: Low pressure mercury lamp plants. Austrian Standards Institute, Vienna, Austria.
ÖNORM (2003). Austrian national standard: ÖNORM 5873-2E, plants for disinfection of water using ultraviolet radiation: Requirements and testing, part 2: Medium pressure mercury lamp plants. Austrian Standards Institute, Vienna, Austria.
Ontario Ministry of Environment (2006). Procedure for disinfection of drinking water in Ontario. Ontario Ministry of Environment, PIBS 4448e01.
O'Reilly, C.E., Bowen, A.B., Perez, N.E., Sarisky, J.P., Shepherd, C.A., Miller, M.D., Hubbard, B.C., Herring, M., Buchanan, S.D., Fitzgerald, C.C., Hill, V., Arrowood, M.J., Xiao, L.X., Hoekstra, R.M., Mintz, E.D., Lynch, M.F., Bannerman, T., Bradley, B., Bratka, L., Carmean, J., Fugitt, R., Golden, S., Koch, E., Murphy, M., Salehi, E., Smith, F., Osborn, N.C., Wertenbach, J.M., Young, S.M., Baker, M., Bowman, C., Kolaz, H., Messer, B., Nabors, S., White, D., Achen, M., Agin, J., Brisker, T., Byrum, B., Flynn, T., Gerhardt, T., Gosnell, C., Kallis, T., Kirchner, C., McDonnell, D., Ott, M., Panico, P., Utter, C., Bopp, C.A. and Browne, L.H. (2007). A waterborne outbreak of gastroenteritis with multiple etiologies among resort island visitors and residents: Ohio, 2004. Clin. Infect. Dis., 44(4): 506-512.
Page, R.M., Scheidler, S., Polat, E., Svoboda, P. and Huggenberger, P. (2012). Fecal indicator bacteria: Groundwater dynamics and transport following precipitation and river water infiltration. Water Air Soil Pollut., 223(5): 2771-2782.
Park, S.R., Mackay, W.G. and Reid, D.C. (2001). Helicobacter sp. recovered from drinking water biofilm sampled from a water distribution system. Water Res., 35(6): 1624-1626.
Payment, P. and Locas, A. (2005). Evaluation et contrôle de la qualité virologique des eaux souterraines. Report to the Ministère de l'Environnement du Québec, programme d'aide à la recherche et au développement en environnement (PARDE).
Payment, P., Franco, E. and Siemiatycki, J. (1993). Absence of relationship between health effects due to tap water consumption and drinking water quality parameters. Water Sci. Technol., 27: 137-143.
Payment, P., Waite, M. and Dufour, A. (2003). Chapter 2: Introducing parameters for the assessment of drinking water quality. In: Assessing microbial safety of drinking water. Dufour, A. et al. eds. World Health Organization and The Organization for Economic Co-operation and Development. IWA Publishing, London, UK. pp. 47-77.
Payne, S.J., Besner, M.C., Lavoie, J., Krentz, C., Truelstrup Hansen, L., Friedman, M., LeChevallier, M.W., Prévost, M. and Gagnon, G.A. (2010). Molecular techniques and data integration: Investigating distribution system coliform events. J. Water Supply Res. T., 59(5): 298-311. Pedersen, K. (1990). Biofilm development on stainless steel and PVC surfaces in drinking water. Water Res., 24(2): 239-243.
Pedley, S., Yates, M., Schijven, J.F., West, J., Howard, G. and Barrett, M. (2006). Pathogens: Health relevance, transport and attenuation. In: Protecting groundwater for health managing the quality of drinking-water systems. Schmoll, O., Howard, G., Chilton, J. and Chorus, I. (eds.). IWA Publishing, London, UK, pp. 49-80.
Poffe, R. and Op de Beeck, E. (1991). Enumeration of Aeromonas hydrophila from domestic wastewater treatment plants and surface waters. J. Appl. Bacteriol., 71: 366-370.
Pope, M.L., Bussen, M., Feige, M.A., Shadix, L., Gonder, S., Rodgers, C., Chambers, Y., Pulz, J., Miller, K., Connell, K. and Standridge, J. (2003). Assessment of the effects of holding time and temperature on Escherichia coli densities in surface water samples. Appl. Environ. Microbiol., 69(10): 6201-6207.
Powell, K.L., Taylor, R.G., Cronin, A.A., Barrett, M.H., Pedley, S., Sellwood, J., Trowsdale, S.A. and Lerner, D.N. (2003). Microbial contamination of two urban sandstone aquifers in the UK. Water Res., 37(2): 339-352.
Prest, E.I., Hammes, F., van Loosdrecht, M.C.M. and Vrouwenvelder, J.S. (2016a). Biological stability of drinking water: Controlling factors, methods, and challenges. Front. Microbiol., 7: 45.
Prest, E.I., Hammes, F., Kötzsch, S., Van Loosdrecht, M.C.M. and Vrouwenvelder, J.S. (2016b). A systematic approach for the assessment of bacterial growth-controlling factors linked to biological stability of drinking water in distribution systems. Water Sci. Technol. Water Supply, 16(4): 865-880.
Prévost, M., Laurent, P., Servais, P. and Joret, J. (2005). Biodegradable organic matter in drinking water treatment and distribution. American Water Works Association, Denver, CO.
Procopio, N.A., Atherholt, T.B., Goodrow, S.M. and Lester, L.A. (2017). The likelihood of coliform bacteria in NJ domestic wells based on precipitation and other factors. Ground Water, doi:10.1111/gwat.12518.
Raina, P.S., Pollari, F.L., Teare, G.F., Goss, M.J., Barry, D.A. and Wilson, J.B. (1999). The relationship between E. coli indicator bacteria in well-water and gastrointestinal illnesses in rural families. Can. J. Public Health, 90(3): 172-175
Reid, D.C., Edwards, A.C., Cooper, D., Wilson, E. and Mcgaw, B.A. (2003). The quality of drinking water from private water supplies in Aberdeenshire, UK. Water Res., 37(2): 245-254.
Revetta, R.P., Gomez‐Alvarez, V., Gerke, T.L., Santo Domingo, J.W. and Ashbolt, N.J. (2016). Changes in bacterial composition of biofilm in a metropolitan drinking water distribution system. J. Appl. Microbiol., 121(1): 294-305.
Richardson, H.Y., Nichols, G., Lane, C., Lake, I.R. and Hunter, P.R. (2009). Microbiological surveillance of private water supplies in England: The impact of environmental and climate factors on water quality. Water Res., 43(8): 2159-2168.
Rompré, A., Servais, P., Baudart, J., de-Roubin, M. and Laurent, P. (2002). Detection and enumeration of coliforms in drinking water: current methods and emerging approaches. J. Microbiol. Methods, 49: 31-54.
Rosen, J.S. and Bartrand, T. (2013). Using online water quality data to detect events in a distribution system. J. Am. Water Works Assoc., 105(7): 22-26.
Rosen, J.S., Sobrinho, J.A.H. and LeChevallier, M. (2009). Statistical limitations in the usefulness of total coliform data. J. Am. Water Works Assoc., 101: 68-81.
Rutter, M., Nichols, G.L., Swan, A. and De Louvois, J. (2000). A survey of the microbiological quality of private water supplies in England. Epidemiol. Infect., 124(3): 417-425.
Sakyi, P.A., Asare, R., Anani, C. and Dampare, S.B. (2012). Induced growth of coliform and HPC bacteria in drinking-water pipes. J Environ Prot, 3: 508-517.
Samarajeewa, A.D., Glasauer, S.M., Lauzon, J.D., O'Halloran, I.P., Parkin, G.W. and Dunfield, K.E. (2012). Bacterial contamination of tile drainage water and shallow groundwater under different application methods of liquid swine manure. Can. J. Microbiol., 58(5): 668-677.
Samendra, P.S., Masaaki, K., Charles, P.G. and Ian, L.P. (2014). Rapid detection technologies for monitoring microorganisms in water. Biosens J, 3: 109.
Scandura, J.E. and Sobsey, M.D. (1997). Viral and bacterial contamination of groundwater from on-site sewage treatment systems. Water Sci. Technol., 35(11-12): 141-146
SCC (2019). Directory of accredited product, process and service certification bodies. Standards Council of Canada, Ottawa, Ontario. Accessed December 2019 from: www.scc.ca/en/accreditation/product-process-and-service-certification/directory-of-accredited-clients.Schoenen, D. and Kolch, A. (1992). Photoreactivation of E. coli depending on light intensity after UV irradiation. Zentralbl. Hyg., 192: 565-570.
Schuster, C.J., Ellis, A.G., Robertson, W.J., Charron, D.F., Aramini, J.J., Marshall, B.J. and Medeiros, D.T. (2005). Infectious disease outbreaks related to drinking water in Canada, 1974-2001. Can. J. Public Health, 96 (4):254-255.
Schwartz, T., Hoffmann, S. and Obst, U. (1998). Formation and bacterial composition of young, natural biofilms obtained from public bank-filtered drinking water systems. Water Res., 32(9): 2787-2797.
September, S.M., Els, F.A., Venter, S.N. and Brözel, V.S. (2007). Prevalence of bacterial pathogens in biofilms of drinking water distribution systems. J. Water Health, 5(2): 219-227.
Shaban, A.M., Haroun, B.M., Ali, M.A. and Elras, M.A. (2008). Comparison between conventional membrane filter and PCR methods for detection of coliform, E. coli and Salmonella in drinking water. J Appl Sci Res, 4(12): 1769-1776.
Shurtz, K.M., Jones, S.C., Sowby, R.B., Hill, D.S. and Woodbury, D.K. (2017). Hydraulic modeling finds, fixes chlorine residual gaps. Opflow, 43(6): 28-30.
Simpson, H. (2004). Promoting the management and protection of private water wells. J. Toxicol. Environ. Health Part A, 67(20-22): 1679-1704.
Smeets, P., Rietveld, L., Hijnen, W., Medema, G. and Stenström, T. (2006). Ch.4: Efficacy of water treatment processes. In: MICRORISK (microbiological risk assessment: A scientific basis for managing drinking water safety from source to tap), pp. 4.1-4.64.
Snead, M.C., Olivieri, V.P., Kawata, K. and Krusé, C.W. (1980). The effectiveness of chlorine residuals in inactivation of bacteria and viruses introduced by post treatment contamination. Water Res., 14: 403.
Somaratne, N. and Hallas, G. (2015). Review of risk status of groundwater supply wells by tracing the source of coliform contamination. Water (Switzerland), 7(7): 3878-3905.
Sommer, R., Lhotsky, M., Haider, T. and Cabaj, A. (2000). UV inactivation, liquid-holding recovery, and photoreactivation of Escherichia coli O157 and other pathogenic Escherichia coli strains in water. J. Food Protection, 63(8): 1015-1020.
Speight, V.L., Kalsbeek, W.D. and DiGiano, F.A. (2004). Randomized stratified sampling methodology for water quality in distribution systems. J. Water Resour. Plann. Manag., 130: 330-338.
Statistics Canada (2013a). Households and the environment survey (HES) 2011.
Statistics Canada (2013b). Survey of drinking water plants 2011.
Stevens, M., Ashbolt, N. and Cunliffe, D. (2003). Review of coliforms as microbial indicators of drinking water quality - recommendations to change the use of coliforms as microbial indicators of drinking water quality. National Health and Medical Research Council (NHMRC), Biotext Pty Ltd, Biotext Pty Ltd, Canberra, Australia.
Storey, M.V., van der Gaag, B. and Burns, B.P. (2011). Advances in on-line drinking water quality monitoring and early warning systems. Water Res., 45(2): 741-747.
Swistock, B.R., Clemens, S., Sharpe, W.E. and Rummel, S. (2013). Water quality and management of private drinking water wells in Pennsylvania. J. Environ. Health, 75(6): 60-66.
Sworobuk, J.E., Law, C.B. and Bissonnette, G.K. (1987). Assessment of the bacteriological quality of rural groundwater supplies in northern West Virginia. Water Air Soil Pollut., 36(1-2): 163-170.
Szabo, J. and Minamyer, S. (2014). Decontamination of chemical agents from drinking water infrastructure: A literature review and summary. Environ. Int., 72: 119-123.
Tantawiwat, S., Tansuphasiri, U., Wongwit, W., Wongchotigul, V. and Kitayaporn, D. (2005). Development of multiplex PCR for the detection of total coliform bacteria for Escherichia coli and Clostridium perfringens in drinking water. Southeast Asian J. Trop. Med. Public Health, 36(1): 162-169.
Toccalino, P.L. and Hopple, J.A. (2010). Quality of source water from public-supply wells in the United States, 1993-2007. U.S. Geological Survey (USGS), Circular 1346, Washington, DC.
Toranzos, G.A. and McFeters, G.A. (1997). Detection of indicator microorganisms in environmental freshwaters and drinking waters. In: Manual of environmental microbiology. Hurst, C.J., Knudsen, G.R., McInerney, M.J., Stetzenbach, L.D. and Walter, M.V. (eds.). ASM Press, Washington, D.C., pp. 184-194.
Uhlmann, S., Galanis, E., Takaro, T., Mak, S., Gustafson, L., Embree, G., Bellack, N., Corbett, K. and Isaac-Renton, J. (2009). Where's the pump? associating sporadic enteric disease with drinking water using a geographic information system, in British Columbia, Canada, 1996-2005. J. Water Health, 7(4): 692-698.
U.S. EPA (1991). Guidance manual for compliance with the filtration and disinfection requirements for public water systems using surface water sources. U.S. Environmental Protection Agency, Washington, DC.
U.S. EPA (1999). EPA guidance manual: disinfection profiling and benchmarking. U.S. Environmental Protection Agency, Washington, DC (EPA-815-R-99-013).
U.S. EPA (2002). Health risks from microbial growth and biofilms in drinking water distribution systems. Office of Ground Water and Drinking Water, Washington, DC.
U.S. EPA (2003). Ultraviolet disinfection guidance manual (draft). Office of Water, U.S. Environmental Protection Agency, Washington, DC (EPA 815).
U.S. EPA (2006). 40 CFR parts 9, 141 and 142. National primary drinking water regulations: Ground water rule. Final rule. Fed. Regist., 71(216): 65573-65660.
U.S. EPA (2010). Detection of biological suspensions using online detectors in a drinking water distribution system simulator. Office of Research and Development, Cincinnati, OH
U.S. EPA (2013). National primary drinking water standards: Revisions to the total coliform rule. final rule. Fed. Regist., 78(30): 10269.
U.S. EPA (2017). Analytical methods approved for compliance monitoring under the revised total coliform rule. Office of Water, EPA, EPA 821-F-17-004, Washington, DC.
van der Gaag, B. and Volz, J. (2008). Real-time on-line monitoring of contaminants in water: Developing a research strategy from utility experiences and needs. KIWA Water Research, Nieuwegein.
van der Kooij, D. and van der Wielen, P.W.J.J. (2014). Microbial growth in drinking-water supplies: Problems, causes, control and research needs. IWA Publishing, London, UK.
van Der Kooij, D., Veenendaal, H.R. and Scheffer, W.J.H. (2005). Biofilm formation and multiplication of Legionella in a model warm water system with pipes of copper, stainless steel and cross-linked polyethylene. Water Res., 39(13): 2789-2798.
van Lieverloo, J.H.M., Mesman, G.A.M., Bakker, G.L., Baggelaar, P,K., Hamed, A. and Medema, G. (2007). Probability of detecting and quantifying fecal contamination of drinking water by periodically sampling for E. coli: a simulation model study. Water Res., 41: 4299-4308.
Wallender, E.K., Ailes, E.C., Yoder, J.S., Roberts, V.A. and Brunkard, J.M. (2014). Contributing factors to disease outbreaks associated with untreated groundwater. Groundwater, 52(6): 886-897.
Wang, H., Masters, S., Edwards, M.A., Falkinham, J.O.,3rd and Pruden, A. (2014). Effect of disinfectant, water age, and pipe materials on bacterial and eukaryotic community structure in drinking water biofilm. Environ. Sci. Technol., 48(3): 1426-1435.
Wang, H., Bédard, E., Prévost, M., Camper, A.K., Hill, V.R. and Pruden, A. (2017). Methodological approaches for monitoring opportunistic pathogens in premise plumbing: A review. Water Res., 117: 68-86.
Warnecke, M. (2006). Cryptosporidium oocyst interactions with drinking water pipe biofilms. Cooperative Research Center for Water Quality and Treatment, Research report 5, Adelaide, AUS.
White, C., Tancos, M. and Lytle, D.A. (2011). Microbial community profile of a lead service line removed from a drinking water distribution system. Appl. Environ. Microbiol., 77(15): 5557-5561.
WHO (1972). International standards for drinking-water, 3rd edition. World Health Organization, Geneva, Switzerland. Available at: https://apps.who.int/iris/handle/10665/40042
WHO (1976). Surveillance of drinking-water quality. World Health Organization, Geneva, Switzerland (WHO Monograph Series No. 63). Available at: https://apps.who.int/iris/handle/10665/41802
WHO (2004). Guidelines for drinking-water quality, 3rd edition. Vol. 1. Recommendations. World Health Organization, Geneva, Switzerland. Available at: https://www.who.int/water_sanitation_health/publications/gdwq3/en/
WHO (2011). Water safety in buildings. WHO Press. Geneva, Switzerland. Available at: whqlibdoc.who.int/publications/2011/9789241548106_eng.pdf
WHO (2014). Water safety in distribution systems. WHO Document Production Services, Geneva, Switzerland. Available at: https://www.who.int/water_sanitation_health/publications/water-safety-in-distribution-system/en/
WHO (2017). Guidelines for drinking-water quality: Fourth edition incorporating first addendum. World Health Organization, Geneva, Switzerland. Available at : https://www.who.int/water_sanitation_health/publications/drinking-water-quality-guidelines-4-including-1st-addendum/en/
Wingender, J. and Flemming, H. (2004). Contamination potential of drinking water distribution network biofilms. Water Sci. Technol., 49(11-12): 277-286.
Wingender, J. and Flemming, H. (2011). Biofilms in drinking water and their role as reservoir for pathogens. Int. J. Hyg. Environ. Health, 214(6): 417-423.
Worakhunpiset, S. and Tharnpoophasiam, P. (2009). Influence of enrichment broths on multiplex PCR detection of total coliform bacteria, Escherichia coli and Clostridium perfringens, in spiked water samples. Southeast Asian J. Trop. Med. Public Health, 40(4): 795-800.
Yang, J., LeChevallier, M.W., Teunis, P.F.M. and Xu, M. (2011). Managing risks from virus intrusion into water distribution systems due to pressure transients. J. Water Health, 9(2): 291-305.
Yoder, J., Roberts, V., Craun, G.F., Hill, V., Hicks, L.A., Alexander, N.T., Radke, V., Calderon, R.L., Hlavsa, M.C., Beach, M.J. and Roy, S.L. (2008). Surveillance for waterborne disease and outbreaks associated with drinking water and water not intended for drinking--United States, 2005-2006. MMWR Surveill Summ, 57(9): 39-62.
Zacheus, O.M., Iivanainen, E.K., Nissinen, T.K., Lehtola, M.J. and Martikainen, P.J. (2000). Bacterial biofilm formation on polyvinyl chloride, polyethylene and stainless steel exposed to ozonated water. Water Res., 34(1): 63-70.
Zhang, Y., Hong, P.Y., LeChevallier, M.W. and Liu, W.T. (2015). Phenotypic and phylogenetic identification of coliform bacteria obtained using 12 coliform methods approved by the U.S. Environmental Protection Agency. Appl. Environ. Microbiol., 81(17): 6012-6023.
Zhang, Y., Oh, S. and Liu, W.T. (2017). Impact of drinking water treatment and distribution on the microbiome continuum: An ecological disturbance's perspective. Environ. Microbiol., 19(8): 3163-3174.
Zhou, X., Li, H., Sun, L., Mo, Y., Chen, S., Wu, X., Liang, J., Zheng, H., Ke, C., Varma, J.K., Klena, J.D., Chen, Q., Zou, L. and Yang, X. (2012). Epidemiological and molecular analysis of a waterborne outbreak of norovirus GII.4. Epidemiol. Infect., 140(12): 2282-2289.
Zimmer, J.L. and Slawson, R.M. (2002). Potential repair of Escherichia coli DNA following exposure to UV radiation from both medium- and low-pressure UV sources used in drinking water treatment. Appl. Environ. Microbiol., 68(7): 3293-3299.
Appendix A: Decision tree for routine microbiological testing of municipal-scale systems
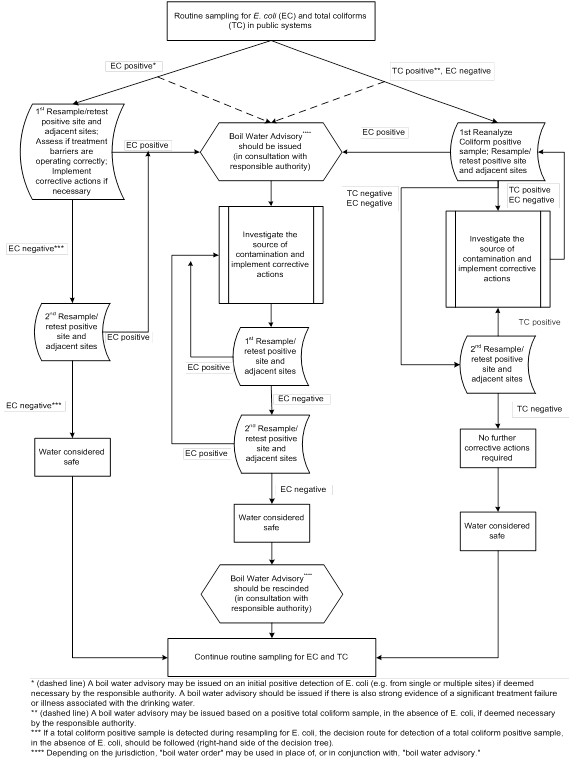
Figure 1 text description
The recommended actions for responding to routine drinking water samples in public systems that are positive for E. coli and/or total coliforms are presented as a decision tree. The decision tree is made up of boxes that are connected to each other using single direction arrows. Each box in the decision tree contains a recommended action. The path taken through the decision tree, and therefore the recommended actions, are dependent on the results from the microbiological sampling. The left hand side of the decision tree presents the actions to be taken when samples are positive for E. coli. The right hand side of the decision tree presents the actions to be taken when samples are positive for total coliforms but negative for E. coli. The middle of the decision tree presents the actions to be taken if a boil water advisory has been issued.
On the left hand side of the decision tree, if E. coli is detected during routine sampling, the recommended action is to issue a boil water advisory in consultation with the responsible authorities. If only a single site is positive for E. coli, the recommended action is the positive site and adjacent sites should undergo a first re-sampling for E. coli and total coliforms (Footnote *). If any re-sampled site is positive for E. coli, the recommended action is to issue a boil water advisory in consultation with the responsible authorities. Depending on the jurisdiction, a boil water advisory may be issued on a single site contamination. If the re-sampled site was negative for E. coli and total coliforms, the recommended action is to carryout a second re-sampling at the positive site and at the adjacent sites. If any of the second re-sampled sites are positive for E. coli, the recommended action is to issue a boil water advisory in consultation with the responsible authorities. If the second re-sampled sites are negative for E. coli and total coliforms, the water is considered safe and the recommended action is to continue routine sampling.
The right hand side of the decision tree should be followed if a total coliform sample is positive in the absence of E. coli. Therefore, if total coliforms are detected during the first or second re-sampling for E. coli, or, if a routine sample is positive for total coliforms, in the absence of E. coli, the recommended action is to re-sample the positive site and adjacent sites for E. coli and total coliforms (Footnote **). If E. coli is detected, the recommended action is to issue a boil water advisory in consultation with the responsible authorities. A boil water advisory may be issued based on a positive total coliform, in the absence of E. coli, if deemed necessary by the responsible authority. If the re-sampled sites are negative for both total coliforms and E. coli, the recommended action is the positive sites and the adjacent sites should undergo a second re-sampling for E. coli and total coliforms. If these sites are negative for E. coli and total coliforms, then no further action is required, the water is considered safe and the recommended action is to continue with routine sampling. If total coliforms are detected in any of the re-sampled sites, in the absence of E. coli, the recommended action is to investigate the source of the contamination, implement corrective actions, and then return to the top of the decision tree, to the box recommending the first re-sampling of the positive sites and adjacent sites (Footnote ***).
In all instances where the decision route leads to issuing a boil water advisory, the centre of the decision tree should be followed (Footnote ****). After issuing the boil water advisory, the recommended actions are to investigate the source of the contamination, implement corrective actions and undergo a first re-sampling of the positive sites and adjacent sites. If E. coli is detected, the arrow directs the decision route back to investigating and implementing corrective actions. If the first re-sampling is negative for E. coli, a second re-sampling at the positive sites and adjacent sites is recommended. If the second re-sampling is positive for E. coli, the decision route is directed back to investigating and implementing corrective actions. If the second re-sampling is negative for E. coli, the water is considered safe and the recommended action is to rescind the boil water advisory in consultation with the responsible authorities, and to then continue with routine sampling.
Footnotes
- Footnote 1
-
A boil water advisory may be issued on an initial positive detection of E. coli (e.g. from single or multiple sites) if deemed necessary by the responsible authority. A boil water advisory should be issued if there is also strong evidence of a significant treatment failure or illness associated with the drinking water.
- Footnote 2
-
A boil water advisory may be issued based on a positive total coliform, in the absence of E. coli, if deemed necessary by the responsible authority.
- Footnote 3
-
If a total coliform positive sample is detected during resampling for E. coli, the decision route for detection of a total coliform positive sample, in the absence of E. coli, should be followed (right-hand side of the decision tree).
- Footnote 4
-
Depending on the jurisdiction, "boil water order" may be used in place of, or in conjunction with, "boil water advisory."
Appendix B: Decision tree for routine microbiological testing of residential-scale systems
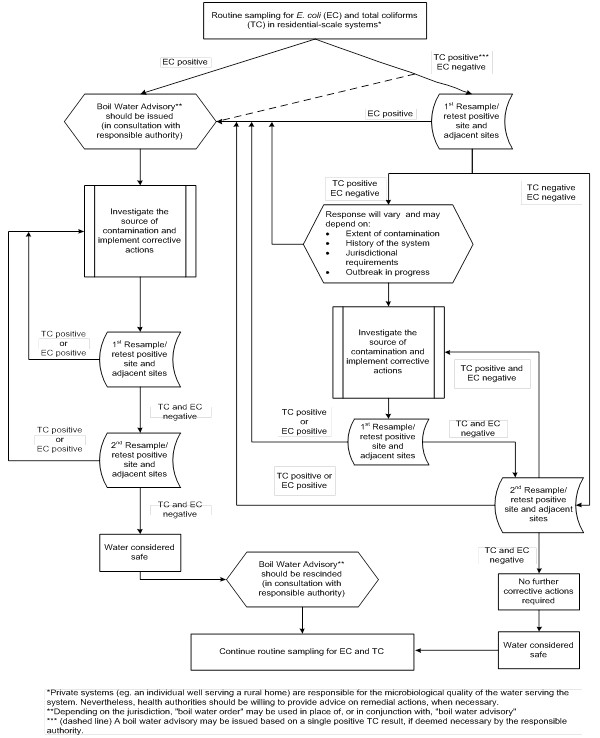
Figure 2 - Text description
The recommended actions for responding to routine drinking water samples in semi-public systems that are positive for E. coli and/or total coliforms are presented as a decision tree (Footnote *). The decision tree is made up of boxes that are connected to each other using single direction arrows. Each box in the decision tree contains a recommended action. The path taken through the decision tree, and therefore the recommended actions that should be taken, are dependent on the results from the microbiological sampling. The left hand side of the decision tree presents the actions to be taken when samples are positive for E. coli and a boil water advisory has been issued (Footnote **). The right hand side of the decision tree presents the actions to be taken when samples are positive for total coliforms but negative for E. coli.
On the left hand side of the decision tree, if E. coli is detected during routine sampling the recommended action is to issue a boil water advisory in consultation with the responsible authorities.
The right hand side of the decision tree should be followed if a total coliform sample is positive in the absence of E. coli. For this decision route, the recommended action is to undergo a first re-sampling of the positive sites and at adjacent sites for E. coli and total coliforms (Footnote ***). If E. coli is detected, the recommended action is to issue a boil water advisory in consultation with the responsible authorities. A boil water advisory may also be issued based on a positive total coliform result in the absence of E. coli, if deemed necessary by the responsible authority. If the first re-sampled sites are negative for both total coliforms and E. coli, the recommended action is the positive sites and the adjacent sites should undergo a second re-sampling for E. coli and total coliforms. If E. coli is detected in the second re-sampling, the recommended action is to issue a boil water advisory in consultation with the responsible authorities. If the second re-sampling sites are negative for E. coli and total coliforms, then no further corrective actions are required, the water is considered safe and the recommended action is to continue with routine sampling. If total coliforms are detected in any of the re-sampled sites, in the absence of E. coli, the recommended action depends on the extent of the contamination, the history of the system, the jurisdictional requirements and if there is an outbreak in progress (Footnote ***). The recommendation can be to either investigate the source of the contamination, implement corrective actions, and carryout a first re-sampling of the positive sites and adjacent sites, or to issue a boil water advisory in consultation with the responsible authority. If the decision route taken was to carryout the first re-sampling, and the sites are negative for both total coliforms and E. coli, the recommended action is to undergo a second re-sampling for E. coli and total coliforms. If these sites are negative for E. coli and total coliforms, than the water is considered safe and the recommended action is to continue with routine sampling. If either the first or the second re-sampling results in a total coliform or an E. coli positive sample, the recommended action is to issue a boil water advisory in consultation with the responsible authority.
In all instances where the decision route leads to issuing a boil water advisory, the recommended action is to then investigate the source of the contamination, implement corrective actions and undergo a first re-sampling of the positive sites and adjacent sites. If E. coli or total coliforms are detected, the arrow directs the decision route back to investigating and implementing corrective actions. If the first re-sampling is negative for E. coli and total coliforms, a second re-sampling at the positive sites and adjacent sites is recommended. If the second re-sampling is positive for E. coli or total coliforms, the decision route is directed back to investigating and implementing corrective actions. If the second re-sampling is negative for E. coli and total coliforms, the water is considered safe and the recommended action is to rescind the boil water advisory in consultation with the responsible authorities, and to then continue with routine sampling.
Footnotes
- Footnote 1
-
Private systems (eg. an individual well serving a rural home) are responsible for the microbiological quality of the water serving the system. Nevertheless, health authorities should be willing to provide advice on remedial actions, when necessary.
- Footnote 2
-
Depending on the jurisdiction, "boil water order" may be used in place of, or in conjunction with, "boil water advisory"
- Footnote 3
-
A boil water advisory may be issued based on a single positive TC result, if deemed necessary by the responsible authority.
Appendix C: List of acronyms
- AGI
- acute gastrointestinal illness
- ANSI
- American National Standards Institute
- AOC
- assimilable organic carbon
- ATP
- adenosine triphosphate
- BDOC
- biodegradable organic carbon
- BGLB
- brilliant green lactose bile broth
- BOM
- biodegradable organic matter
- CT
- concentration of disinfectant × contact time
- DBP
- disinfection by-product
- E. coli
- Escherichia coli
- EU
- European Union
- GUDI
- groundwater under the direct influence of surface water
- HCGI
- highly credible gastrointestinal illness
- ISO
- International Organization for Standardization
- IT
- intensity of UV light × contact time
- MAC
- maximum acceptable concentration
- MF
- membranefiltration
- MTF
- multiple tubefiltration
- NSF
- NSFInternational
- OPPP
- opportunistic premise plumbing pathogen
- P-A
- presence-absence
- PCR
- polymerase chain reaction
- PE
- Polyethylene
- PVC
- Polyvinyl chloride
- QA
- quality assurance
- QC
- quality control
- RO
- reverse osmosis
- RT-PCR
- reverse transcription polymerase chain reaction
- SCC
- Standards Council of Canada
- U.S.
- United States
- U.S. EPA
- United States Environmental Protection Agency
- UV
- ultraviolet
- WHO
- World Health Organization