Patented Medicine Prices Review Board 2025-26 Departmental Plan
On this page
- From the Acting Chairperson
- Plans to deliver on core responsibilities and internal services
- Planned spending and human resources
- Corporate information
- Supplementary information tables
- Federal tax expenditures
- Definitions
From the Acting Chairperson

Anie Perrault
Acting Chairperson, PMPRB
The PMPRB is an independent, quasi-judicial body with the mandate to monitor the price at which Rights Holders sell their patented medicines in Canada to ensure that this price is not excessive. The PMPRB is also responsible for reporting annually to Parliament through the Minister of Health on its activities for the previous year, pricing trends of all patented medicines, and research and development expenditures reported to the PMPRB by pharmaceutical Rights Holders.
The PMPRB Board is currently working to advance new Guidelines that address July 2022 amendments to the Patented Medicine Regulations. This multi-year project resulted in draft Guidelines at the end of 2024, which will form the foundation for a 2025-26 release of final Guidelines. Rights Holders have been filing pricing information based on the amendments since 2022.
The PMPRB is committed to developing Guidelines that promote administrative efficiency and provide transparency and predictability to Rights Holders on the procedures followed by Staff to identify potential hearing candidates. To meet this goal, we will be working internally to update database mechanisms and find efficiencies and improvements in our price review and hearings procedures. We will also be focusing on clear and transparent communication throughout this process to ensure that interested parties are informed of the changes to the Guidelines and supported in the transition as they come into effect.
The PMPRB’s reporting function continues to support decision making on drug spending and innovation in Canada by providing the Minister of Health with high-quality analytic information on domestic and international trends. The reporting schedule for 2025-26 will introduce studies on private drug plans as well as generic and biosimilar medicines, which contribute to a larger portrait of Canada’s pharmaceutical market.
Internally, the PMPRB has targeted goals to develop and deliver on our Inclusion, Diversity, Equity, and Accessibility (IDEA) priorities in the coming year. We are also debuting a revised Departmental Results Framework for 2025-26, as reflected in the following sections of this report. The updates take into account recent clarifications to the PMPRB’s jurisdiction and focus results reporting on the heart of the PMPRB’s mandate and scope of influence.
Collectively, these priorities represent an active and promising year of service at the PMPRB. The PMPRB’s Chairperson, Mr. Thomas Digby, stepped down from his role on March 6, 2025, to pursue other opportunities. We would like to thank him for his leadership and guidance, which brought stability to the organization. As Vice-Chairperson, I have assumed the duties of Acting Chairperson until a permanent replacement for Mr. Digby is appointed by the Governor in Council. As we turn to a new chapter for our organization, I look forward to maintaining the momentum we have built together in a shared effort to realizing the PMPRB’s important mandate.
Anie Perrault
Acting Chairperson, PMPRB
Plans to deliver on core responsibilities and internal services
Core responsibilities and internal services:
Core responsibility 1: Monitor Patented Medicine Prices
In this section
Description
The PMPRB monitors the prices of patented medicines and initiates public hearings to determine whether a price is excessive. It also monitors and provides information on trends in pharmaceutical sales and pricing and on research and development spending by Rights Holders through the issuance of its Annual Report.
Quality of life impacts
Canada’s Quality of Life Framework uses 84 indicators to measure what matters most to Canadians and to help drive evidence-based budgeting and decision-making at the federal level. The PMPRB’s core responsibility, though limited, overlaps indirectly with several of these indicators, including household finances indicators listed under the framework’s domain of “Prosperity” and the indicator “Cost-related non-adherence to prescription medication” under the domain of “Health”.
As many prescription medicines do not have less expensive generic versions available, monitoring the prices of patented medicines and intervening where the price appears to be excessive can contribute to the affordability of medicines for Canadian patients, which in turn can minimize cost-related non-adherence to these prescriptions. This is particularly relevant through the framework’s “Sustainability and Resilience lens”, which is aimed at building resilience and maintaining the longevity of policies and quality of life in the years to come.
Indicators, results and targets
This section presents details on the department’s indicators, the actual results from the three most recently reported fiscal years, the targets and target dates approved in 2025-26 for Monitor Patented Medicine Prices. Details are presented by departmental result.
Table 1: Timely and effective monitoring of patented medicine price information provided by Rights Holders
Table 1 provides a summary of the target and actual results for each indicator associated with the results under Monitor Patented Medicine Prices.
| Departmental Result Indicators | Actual Results* | Target | Date to achieve target |
|---|---|---|---|
Percentage of Rights Holder filings monitored within the calendar year |
2021-22: N/A |
95% |
December 2027 |
Percentage of Hearings completed within 24 months of the issuance of a Notice of Hearing |
2021-22: N/A |
90% |
March 2028 |
Number of months after the relevant calendar year that the PMPRB’s Annual Report is submitted to the Minister of Health |
2021-22: N/A |
Fewer than 12 months |
March 2026 |
* As all three Departmental Results Indicators are new as of 2025-26, data is not available for historical results. Baseline results have been indicated for 2023-24, where possible.
Additional information on the detailed results and performance information for the PMPRB’s program inventory is available on GC InfoBase.
Plans to achieve results
The following section describes the planned results for Monitor Patented Medicine Prices in 2025-26.
Timely and effective monitoring of patented medicine price information provided by Rights Holders
Results we plan to achieve
- Following the development of draft Guidelines in 2024-25, the PMPRB will be engaged in completing and implementing final Guidelines, which are intended to take effect in January 2026. The Board intends these Guidelines to achieve two main objectives: to enhance the Board’s administrative efficiency, and to provide transparency and predictability to Rights Holders on the procedures followed by Staff to identify potential hearing candidates.
- To this end, the PMPRB will be examining options to streamline and improve the hearings process. Staff will also be updating the PMPRB’s internal database. These efforts will support a smooth transition to new Guidelines.
- The PMPRB is committed to clear and effective communication through the rollout of the new Guidelines and all relevant processes. Following the issuance of the new Guidelines, information sessions will be offered to support those impacted by the transition. Updates on progress in the Guidelines development process are shared through the PMPRB’s social media, on X and LinkedIn, and in the PMPRB NEWSletter.
- The PMPRB will continue to deliver high-quality reporting on the pharmaceutical market to the Minister of Health in the coming year. Along with regular reports and series, publications planned for 2025-26 include new analytic reports on generic and biosimilar medicines. These reports are generated by the PMPRB’s National Prescription Drug Utilization Information System (NPDUIS) initiative group at the request of the Minister of Health through s.90 of the Patent Act. The library of reports is available to access on the PMPRB’s website.
Key risks
The PMPRB has identified and is addressing key risks to the achievement of this core responsibility in 2025-26.
The PMPRB continues to monitor changes in the geopolitical and economic context in which it operates. As the market for pharmaceuticals is complex, international, and interdependent, changes in trading relationships could have an impact on the prices of medicines in Canada. The PMPRB actively monitors the prices of medicines based on factors laid out in the Patent Act and associated Regulations and is empowered to take action in the event of a sudden rise in prices.
The Guidelines development process has been a lengthy undertaking characterized by numerous consultations with interested parties from across the country. In the meantime, the PMPRB is monitoring patented medicine prices under its Interim Guidance, which was last amended in September 2023. As the Interim Guidance was intended as a temporary measure to bridge the gap in which active Guidelines are not available, the final transition to new Guidelines may create a greater pressure on PMPRB Staff.
The PMPRB has limited capacity to hold hearings and issues Guidelines as a mechanism to narrow down the number of medicine prices that are subject to a hearing. To mitigate the effects of this transition, the PMPRB is preparing internally with a collaborative review of internal processes. This review is aimed at efficient, effective, and well-documented procedures for all aspects of the price monitoring process, from data collection to potential hearings.
Along with standard communications with the public, the PMPRB intends to provide information sessions to promote a well-rounded understanding of the new process and to support those affected by this change. This also includes completing the transition of PMPRB’s public-facing website to Canada.ca, which will make key information more accessible to readers.
Planned resources to achieve results
Table 2: Planned resources to achieve results for Monitor Patented Medicine Prices
Table 2 provides a summary of the planned spending and full-time equivalents required to achieve results.
| Resource | Planned |
|---|---|
Spending |
$14,628,709 |
Full-time equivalents |
58 |
Complete financial and human resources information for the PMPRB’s program inventory is available on GC InfoBase.
Related government priorities
Gender-based analysis plus
Sex and gender differences, race, ethnicity, age, and mental or physical disability are all factors in the accessibility, affordability, and appropriate use of prescription medicines and medical devices. Differences in sex and gender+ roles, income and utilization of health care services can affect access to medicines and health insurance, as well as prescribing patterns and medicine use, and may have important repercussions for health and well-being.
Since the price of an individual patented medicine does not vary for the sex or gender+ of the user, the PMPRB’s price monitoring process cannot take explicit account of the diversity of user groups or their economic situation. The PMPRB’s mandate to ensure non-excessive patented medicine prices can benefit all populations indirectly through health system reinvestments and improved access to better care. However, the benefits are greater for those who pay out of pocket for their medicine, who do not have adequate drug coverage, who have a greater need for prescription medications, or whose socioeconomic status makes it more difficult to afford their medication.
An intersectional study published by Statistics Canada in 2024 found that prescription drug insurance coverage varied for men and women across factors such as marital status, immigrant status, racialized group, sexual orientation, household income, number of chronic conditions, and employment-related factors. The findings showed:
- lower levels of employer-sponsored drug insurance coverage among both recent and established immigrant men and women;
- lower levels of employer-sponsored drug insurance coverage among almost all racialized groups;
- lower proportions employer-sponsored drug insurance plan among bisexual men and women than heterosexual men and women; and
- more women than men skipping filling prescription drugs because of cost.
Through the Pharmaceutical Trends program, the PMPRB is leveraging data from the National Prescription Drug Utilization Information System (NPDUIS) database hosted by the Canadian Institute for Health Information (CIHI) to report on analytic research topics informed by GBA Plus.
As part of this initiative, the 8th edition of the PMPRB’s Meds Entry Watch report included an analysis age and sex differences in patients using semaglutide, based on public drug plan claims for 2022. Semaglutide was the top-selling medicine for public drug plans between 2017 and 2022 and is primarily indicated for the treatment of type 2 diabetes. In 2022, female claimants were slightly more likely to use semaglutide, making up 56% of claims. Age was also a factor in utilization for both sexes, with claimants aged 65 and older more likely to be male. These trends may be explained in part by off-label use and the prevalence of diabetes by age across sexes, and they may shift going forward as the approved use semaglutide expands to include weight management without diabetes.
In 2025-26, demographic-driven analysis will be conducted on drug plan spending and drug usage. These reports are being produced by the NPDUIS initiative group of the PMPRB as a result of a request from the Minister of Health under s. 90 of the Patent Act.
United Nations 2030 Agenda for Sustainable Development and the UN Sustainable Development Goals
Information on the PMPRB’s contributions to Canada’s Federal Implementation Plan on the 2030 Agenda and the Federal Sustainable Development Strategy can be found in the PMPRB’s 2023–2027 Departmental Sustainable Development Strategy.
Program inventory
Monitor Patented Medicine Prices is supported by the following programs:
- Patented Medicine Price Monitoring Program
- Pharmaceutical Trends Program
Additional information related to the program inventory for Monitor Patented Medicine Prices is available on the Results page on GC InfoBase.
Summary of changes to reporting framework since last year
Amendments to the Patented Medicine Regulations in July 2022 acknowledged important clarifications to the PMPRB’s mandate that gave rise to a review of the PMPRB’s reporting framework for 2025-26.
Importantly, the PMPRB does not have the authority to set, mandate, or regulate prices for patented medicines and can only intervene to order that the price of a medicine be reduced in instances where it has found the price to be excessive after a full hearing on the merits. The PMPRB can also only address excessive pricing at the ex-factory price level and cannot consider indirect rebates or discounts or issues such as affordability, payor budget constraints, or cost-effectiveness of medicines.
As such, the following changes have been made to the PMPRB’s reporting framework for this year:
- The PMPRB’s Core Responsibility was revised from “Regulate” to “Monitor” Patented Medicine Prices and the description was also amended accordingly. These changes ensure that the PMPRB’s Core Responsibility reflects the PMPRB’s mandate and jurisdiction, as presented in the Patent Act.
- The PMPRB’s Departmental Result was revised to focus in on the PMPRB’s limited but important field of influence.
- All Departmental Results Indicators were replaced with measures that better support the revised Departmental Result and are more relevant to the PMPRB’s current operational context.
Internal services
In this section
Description
Internal services are the services that are provided within a department so that it can meet its corporate obligations and deliver its programs. There are 8 categories of internal services at the PMPRB:
- management and oversight services
- human resources management services
- financial management services
- information management services
- information technology services
- real property management services
- materiel management services
- acquisition management services
Note that the PMPRB includes communications services and legal services under its core responsibility.
Plans to achieve results
This section presents details on how the department plans to achieve results and meet targets for internal services.
In 2025-26, work will continue on the PMPRB’s Inclusion, Diversity, Equity, and Accessibility (IDEA) workplan, including efforts to identify hiring targets to meet or exceed workforce availability and representation, particularly in management. This work will include the development and broadening of the PMPRB’s mentorship and sponsorship programs in collaboration with partner departments. The PMPRB is also engaged in modernizing its codes of conduct for Staff and Board members.
The PMPRB will embark on an internal information management project related to retention and disposition of documents in 2025-26. This project will complement the recent reconstruction of its internal information management system.
Planned resources to achieve results
Table 3: Planned resources to achieve results for internal services this year
Table 3 provides a summary of the planned spending and full-time equivalents required to achieve results.
| Resource | Planned |
|---|---|
Spending |
$3,500,223 |
Full-time equivalents |
23 |
Complete financial and human resources information for the PMPRB’s program inventory is available on GC InfoBase.
Planning for contracts awarded to Indigenous businesses
Government of Canada departments are to meet a target of awarding at least 5% of the total value of contracts to Indigenous businesses each year. This commitment is to be fully implemented by the end of 2024-25.
In compliance with Indigenous Services Canada requirements, the PMPRB has implemented a strategy to ensure procurement from Indigenous businesses and suppliers meets or exceed a minimum of 5% of the value of all contracts. To meet this target, Indigenous suppliers have been sourced for information technology (IT) equipment such as tablets and monitors and IT services, and are a priority for contracted graphic design and web development services.
Procurement from Indigenous suppliers has been incorporated into the budget planning and monitoring cycle, as well as in the Operational Plan cycle, to ensure that the minimum 5% target is met or exceeded in coming years.
Table 4: Percentage of contracts planned and awarded to Indigenous businesses
Table 4 presents the current, actual results with forecasted and planned results for the total percentage of contracts the department awarded to Indigenous businesses.
| 5% Reporting Field | 2023-24 Actual Result | 2024-25 Forecasted Result | 2025-26 Planned Result |
|---|---|---|---|
Total percentage of contracts with Indigenous businesses |
17.9% |
5.3% |
7.6% |
Planned spending and human resources
This section provides an overview of the PMPRB’s planned spending and human resources for the next three fiscal years and compares planned spending for 2025-26 with actual spending from previous years.
Spending
This section presents an overview of the department's planned expenditures from 2022-23 to 2027-28.
Budgetary performance summary
Table 5: Three-year spending summary for core responsibilities and internal services (dollars)
Table 5 presents how much money the PMPRB spent over the past three years to carry out its core responsibilities and for internal services. Amounts for the current fiscal year are forecasted based on spending to date.
| Core responsibilities and Internal services | 2022-23 Actual Expenditures | 2023-24 Actual Expenditures | 2024-25 Forecast Spending |
|---|---|---|---|
Monitor Patented Medicine Prices |
8,687,581 |
10,223,542 |
9,274,519 |
Subtotal(s) |
8,687,581 |
10,223,542 |
9,274,519 |
Internal services |
3,333,947 |
3,820,603 |
3,930,716 |
Total(s) |
12,021,528 |
14,044,145 |
13,205,235 |
Analysis of the past three years of spending
Actual spending increased between 2022-23 and 2023-24 as a result of compensation adjustments from collective agreements signed in 2023-24, an increase in the number of full-time equivalents (FTEs), and a corresponding rise in Employee Benefits Payments (EBP). Spending on information technology equipment, health science consultants, and language training also contributed to this difference.
The forecast spending for 2024-25 is based on actual spending and anticipated spending to year end, which does not anticipate full use of the Special Purpose Allotment (SPA) for hearings. As the PMPRB is currently conducting price reviews under Interim Guidance, spending on hearings has been lower than normal. At the time of preparing this report, forecast spending of the SPA amounted to approximately $128,000 of the $4.5 million allotted. This accounts for the variance in 2024-25 forecast spending and 2025-26 planned spending shown in Table 6.
More financial information from previous years is available on the Finances section of GC Infobase.
Table 6: Planned three-year spending on core responsibilities and internal services (dollars)
Table 6 presents how much money the PMPRB plans to spend over the next three years to carry out its core responsibilities and for internal services.
| Core responsibilities and Internal services | 2025-26 Planned Spending | 2026-27 Planned Spending | 2027-28 Planned Spending |
|---|---|---|---|
Monitor Patented Medicine Prices |
14,628,709 |
14,664,786 |
14,664,786 |
Subtotal |
14,628,709 |
14,664,786 |
14,664,786 |
Internal services |
3,500,223 |
3,507,506 |
3,507,506 |
Total |
18,128,932 |
18,152,292 |
18,152,292 |
Analysis of the next three years of spending
For purposes of forecasting planned spending, it is necessary to assume that the entire SPA funding will be spent because these expenditures are dependent on the number of hearings, and the length and complexity of the hearings held, which are difficult to predict. Any unspent amount is returned to the Consolidated Revenue Fund.
More detailed financial information on planned spending is available on the Finances section of GC Infobase.
Funding
This section provides an overview of the department's voted and statutory funding for its core responsibilities and for internal services. For further information on funding authorities, consult the Government of Canada budgets and expenditures.
Graph 1: Approved funding (statutory and voted) over a six-year period
Graph 1 summarizes the department's approved voted and statutory funding from 2022-23 to 2027-28.
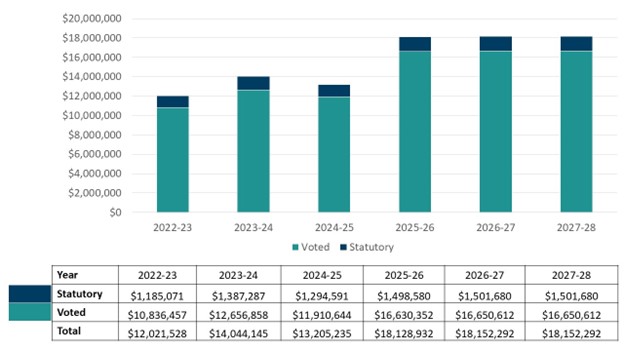
Text description of graph 1
A bar graph shows the distribution of the PMPRB’s total approved funding, including actual and planned spending, by statutory and voted for six fiscal years, from 2022-23 to 2027-28.
| Fiscal year | Total | Voted | Statutory |
|---|---|---|---|
2022-23 |
$12,021,528 |
$10,836,457 |
$1,185,071 |
2023-24 |
$14,044,145 |
$12,656,858 |
$1,387,287 |
2024-25 |
$13,205,235 |
$11,910,644 |
$1,294,591 |
2025-26 |
$18,128,932 |
$16,630,352 |
$1,498,580 |
2026-27 |
$18,152,292 |
$16,650,612 |
$1,501,680 |
2027-28 |
$18,152,292 |
$16,650,612 |
$1,501,680 |
Analysis of statutory and voted funding over a six-year period
It is necessary to assume that SPA funding will be required in its entirety for planned spending in future years. Forecast spending on the SPA in 2024-25 is only $128,000, with a projected lapse of $4.3 million. This accounts for the difference between total forecast spending in 2024-25 and planned spending for 2025-26.
Actual spending increased between 2022-23 and 2023-24 as a result of compensation adjustments from collective agreements signed in 2023-24, an increase in the number of full-time equivalents (FTEs), and a corresponding rise in Employee Benefits Payments (EBP).
For further information on the PMPRB’s departmental appropriations, consult the 2025-26 Main Estimates.
Future-oriented condensed statement of operations
The future-oriented condensed statement of operations provides an overview of the PMPRB’s operations for 2024-25 to 2025-26.
Table 7: Future-oriented condensed statement of operations for the year ended March 31, 2026 (dollars)
Table 7 summarizes the expenses and revenues which net to the cost of operations before government funding and transfers for 2024-25 to 2025-26. The forecast and planned amounts in this statement of operations were prepared on an accrual basis. The forecast and planned amounts presented in other sections of the Departmental Plan were prepared on an expenditure basis. Amounts may therefore differ.
| Financial information | 2024-25 Forecast results | 2025-26 Planned results | Difference (Planned results minus forecasted) |
|---|---|---|---|
Total expenses |
15,377,387 |
19,955,798 |
4,578,411 |
Total revenues |
66 |
- |
(66) |
Net cost of operations before government funding and transfers |
15,377,321 |
19,955,798 |
4,578,477 |
Analysis of forecasted and planned results
The PMPRB is projecting $20.0 million in expenses based on 2025-26 Main Estimates and accrual information. This amount does not include future supplementary estimates. It represents an increase of $4.6 million from 2024-25 forecast results.
The increase is primarily attributable to the $4.5 million planned in 2025-26 for the SPA. It is necessary to assume that SPA funding will be required in its entirety for planned spending in future years. However, forecast spending on the SPA in 2024-25 is only $0.7 million.
The 2025-26 planned expenses by core responsibility are as follows:
- Regulate Patented Medicine Prices $16.0 million; and,
- Internal services $4.0 million.
PMPRB receives most of its funding through annual Parliamentary appropriations.
A more detailed Future-Oriented Statement of Operations and associated Notes for 2025-26, including a reconciliation of the net cost of operations with the requested authorities, is available on the PMPRB’s website.
Human resources
This section presents an overview of the department’s actual and planned human resources from 2022-23 to 2027-28.
Table 8: Actual human resources for core responsibilities and internal services
Table 8 shows a summary of human resources, in full-time equivalents, for the PMPRB’s core responsibilities and for its internal services for the previous three fiscal years. Human resources for the current fiscal year are forecasted based on year to date.
| Core responsibilities and internal services | 2022-23 Actual full-time equivalents | 2023-24 Actual full-time equivalents | 2024-25 Forecasted full-time equivalents |
|---|---|---|---|
Monitor Patented Medicine Prices |
55 |
58 |
55 |
Subtotal |
55 |
58 |
55 |
Internal services |
22 |
24 |
25 |
Total |
77 |
82 |
80 |
Analysis of human resources over the last three years
Actual FTEs increased from 2022-23 to 2023-24 as a result of backfilling staff departures and fulfilling postponed staffing actions. FTE levels are expected to stabilize for 2024-25 onward.
Table 9: Human resources planning summary for core responsibilities and internal services
Table 9 shows information on human resources, in full-time equivalents, for each of the PMPRB’s core responsibilities and for its internal services planned for the next three years.
| Core responsibilities and internal services | 2025-26 Planned full-time equivalents | 2026-27 Planned full-time equivalents | 2027-28 Planned full-time equivalents |
|---|---|---|---|
Monitor Patented Medicine Prices |
58 |
58 |
58 |
Subtotal |
58 |
58 |
58 |
Internal services |
23 |
23 |
23 |
Total |
81 |
81 |
81 |
Analysis of human resources for the next three years
Planned FTE levels are expected to be consistent with current levels through 2027-28.
Corporate information
Departmental profile
Appropriate minister(s): The Honourable Marjorie Michel
Institutional head: Anie Perrault, Acting Chairperson
Ministerial portfolio: Health
Enabling instrument(s): Patent Act and Patented Medicines Regulations
Year of incorporation / commencement: 1987
Other: The Minister of Health is responsible for the pharmaceutical provisions of the Patent Act set out in sections 79 to 103. Although the PMPRB is part of the Health Portfolio, because of its quasi-judicial responsibilities the PMPRB carries out its mandate at arm’s length from the Minister.
The PMPRB also operates independently of
- Health Canada, which approves medicines for marketing in Canada based on their safety, efficacy, and quality;
- Canada’s Drug Agency, which performs health technology assessment and assembles expert committees to make recommendations on which medicines should qualify for reimbursement under publicly funded drug programs;
- the Institut national d’excellence en santé et en services sociaux (INESSS), which evaluates medicines to make recommendations on reimbursement by public plans in Quebec;
- the pan-Canadian Pharmaceutical Alliance (pCPA), which negotiates the list prices on behalf of the publicly funded drug programs across Canada; and
- federal, provincial, and territorial public drug plans and private drug plans, which approve the listing of medicines on their respective formularies for reimbursement purposes.
Departmental contact information
Mailing address:
The Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario
K1P 1C1
Telephone: 1-877-861-2350
TTY: 613-288-9654
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Website(s): https://www.canada.ca/en/patented-medicine-prices-review.html
Supplementary information tables
The following supplementary information tables are available on the PMPRB’s website:
Information on the PMPRB’s Departmental Sustainable Development Strategy can be found on the PMPRB’s website.
Federal tax expenditures
The PMPRB’s Departmental Plan does not include information on tax expenditures.
The tax system can be used to achieve public policy objectives through the application of special measures such as low tax rates, exemptions, deductions, deferrals and credits. The Department of Finance Canada publishes cost estimates and projections for these measures each year in the Report on Federal Tax Expenditures.
This report also provides detailed background information on tax expenditures, including descriptions, objectives, historical information and references to related federal spending programs as well as evaluations and GBA Plus of tax expenditures.
Definitions
List of terms
appropriation (crédit)
Any authority of Parliament to pay money out of the Consolidated Revenue Fund.
budgetary expenditures (dépenses budgétaires)
Operating and capital expenditures; transfer payments to other levels of government, departments or individuals; and payments to Crown corporations.
core responsibility (responsabilité essentielle)
An enduring function or role performed by a department. The intentions of the department with respect to a core responsibility are reflected in one or more related departmental results that the department seeks to contribute to or influence.
Departmental Plan (plan ministériel)
A report on the plans and expected performance of an appropriated department over a 3year period. Departmental Plans are usually tabled in Parliament each spring.
departmental result (résultat ministériel)
A consequence or outcome that a department seeks to achieve. A departmental result is often outside departments’ immediate control, but it should be influenced by program-level outcomes.
departmental result indicator (indicateur de résultat ministériel)
A quantitative measure of progress on a departmental result.
departmental results framework (cadre ministériel des résultats)
A framework that connects the department’s core responsibilities to its departmental results and departmental result indicators.
Departmental Results Report (rapport sur les résultats ministériels)
A report on a department’s actual accomplishments against the plans, priorities and expected results set out in the corresponding Departmental Plan.
full-time equivalent (équivalent temps plein)
A measure of the extent to which an employee represents a full person-year charge against a departmental budget. For a particular position, the full-time equivalent figure is the ratio of number of hours the person actually works divided by the standard number of hours set out in the person’s collective agreement.
gender-based analysis plus (GBA Plus) (analyse comparative entre les sexes plus [ACS Plus])
Is an analytical tool used to support the development of responsive and inclusive policies, programs, and other initiatives. GBA Plus is a process for understanding who is impacted by the issue or opportunity being addressed by the initiative; identifying how the initiative could be tailored to meet diverse needs of the people most impacted; and anticipating and mitigating any barriers to accessing or benefitting from the initiative. GBA Plus is an intersectional analysis that goes beyond biological (sex) and socio-cultural (gender) differences to consider other factors, such as age, disability, education, ethnicity, economic status, geography (including rurality), language, race, religion, and sexual orientation.
Using GBA Plus involves taking a gender- and diversity-sensitive approach to our work. Considering all intersecting identity factors as part of GBA Plus, not only sex and gender, is a Government of Canada commitment.
government priorities (priorités gouvernementales)
For the purpose of the 2025-26 Departmental Plan, government priorities are the high-level themes outlining the government’s agenda in the most recent Speech from the Throne.
horizontal initiative (initiative horizontale)
An initiative where two or more federal departments are given funding to pursue a shared outcome, often linked to a government priority.
Indigenous business (entreprise autochtones)
For the purpose of the Directive on the Management of Procurement Appendix E: Mandatory Procedures for Contracts Awarded to Indigenous Businesses and the Government of Canada’s commitment that a mandatory minimum target of 5% of the total value of contracts is awarded to Indigenous businesses, a department that meets the definition and requirements as defined by the Indigenous Business Directory.
interested parties (parties intéressées)
For the purposes of the PMPRB, any group or individual who is impacted by or takes interest in matters under the PMPRB’s jurisdiction. In the context of consultations, interested parties might include pharmaceutical Rights Holders, patient and health groups, public and private payers, distributors, and researchers and academics, among others.
non‑budgetary expenditures (dépenses non budgétaires)
Non-budgetary authorities that comprise assets and liabilities transactions for loans, investments and advances, or specified purpose accounts, that have been established under specific statutes or under non-statutory authorities in the Estimates and elsewhere. Non-budgetary transactions are those expenditures and receipts related to the government's financial claims on, and obligations to, outside parties. These consist of transactions in loans, investments and advances; in cash and accounts receivable; in public money received or collected for specified purposes; and in all other assets and liabilities. Other assets and liabilities, not specifically defined in G to P authority codes are to be recorded to an R authority code, which is the residual authority code for all other assets and liabilities.
performance (rendement)
What a department did with its resources to achieve its results, how well those results compare to what the department intended to achieve, and how well lessons learned have been identified.
performance indicator (indicateur de rendement)
A qualitative or quantitative means of measuring an output or outcome, with the intention of gauging the performance of a department, program, policy or initiative respecting expected results.
plan (plan)
The articulation of strategic choices, which provides information on how a department intends to achieve its priorities and associated results. Generally, a plan will explain the logic behind the strategies chosen and tend to focus on actions that lead to the expected result.
planned spending (dépenses prévues)
For Departmental Plans and Departmental Results Reports, planned spending refers to those amounts presented in Main Estimates.
A department is expected to be aware of the authorities that it has sought and received. The determination of planned spending is a departmental responsibility, and departments must be able to defend the expenditure and accrual numbers presented in their Departmental Plans and Departmental Results Reports.
program (programme)
Individual or groups of services, activities or combinations thereof that are managed together within the department and focus on a specific set of outputs, outcomes or service levels.
program inventory (répertoire des programmes)
Identifies all the department’s programs and describes how resources are organized to contribute to the department’s core responsibilities and results.
result (résultat)
A consequence attributed, in part, to a department, policy, program or initiative. Results are not within the control of a single department, policy, program or initiative; instead they are within the area of the department’s influence.
statutory expenditures (dépenses législatives)
Expenditures that Parliament has approved through legislation other than appropriation acts. The legislation sets out the purpose of the expenditures and the terms and conditions under which they may be made.
target (cible)
A measurable performance or success level that a department, program or initiative plans to achieve within a specified time period. Targets can be either quantitative or qualitative.
voted expenditures (dépenses votées)
Expenditures that Parliament approves annually through an appropriation act. The vote wording becomes the governing conditions under which these expenditures may be made.