PMPRB Guidelines
The coming-into-force of the amended Patented Medicines Regulations (“Regulations”) has been further delayed past January 1, 2022. Consequently, the new Guidelines will not be coming into effect on January 1, 2022.
The Board announced the decision resulting from the consultation on the definition of Gap medicines and the timeline for compliance on March 17, 2021.
Table of Contents
- I. Preface
- II. Interpretation
- III. Legal Framework
- IV. Filing Requirements Pertaining to Price Reviews
- V. Price Review Process
- VI. Reassessment
- VII. Investigations
- VIII. Voluntary Compliance Undertaking (VCU)
- IX. Hearing Recommendation
- X. Excessive Price Hearing Process and Remedies
- XI. Failure to File Hearing
- XII. Complaints
- XIII. Appendices
- A. Domestic Therapeutic Class Comparison (dTCC) and International Therapeutic Class Comparison (iTCC)
- B. Reasonable Relationship Test and Comparable Dosage Forms
- C. Pharmacoeconomic Value Assessment
- D. Market Size Adjustment Methodology
- E. Scientific Review Process
- F. Summary of Compliance Timelines
I. Preface
1. The Patented Medicine Prices Review Board (PMPRB) was created in 1987 as the consumer protection “pillar” of a major set of reforms to the Patent Act (“Act”). The PMPRB is a quasi-judicial body with a regulatory mandate to prevent pharmaceutical patentees from charging consumers excessive prices during the statutory monopoly period. Its creation arose out of concern that stronger patent protection for medicines might cause their prices to rise unacceptably and become unaffordable to consumers. As a member of the Health Portfolio, the PMPRB contributes to a modern and sustainable health system by ensuring that Canadians continue to have access to patented medicines at non-excessive prices.
2. These Guidelines, which are issued pursuant to subsection 96(4) of the Act, are intended to provide transparency and predictability to patentees regarding the triage and review process typically engaged in by public servant employees of the PMPRB (“Staff”) in assessing whether a patented medicine appears to be priced excessively in any market in Canada. The Guidelines also provide an overview of the processes that patentees should be aware of regarding their filing obligations under the Patented Medicines Regulations (“Regulations”).
3. If a patented medicine appears to be priced excessively based on these Guidelines and an acceptable Voluntary Compliance Undertaking (“VCU”) has not been submitted by the patentee, the Chairperson may receive a recommendation from Staff that a hearing be held on the matter. If such a hearing is deemed to be in the public interest by the Chairperson, and it is confirmed at a hearing by a panel composed of Board members (“Hearing Panel”) that the patented medicine was priced excessively in any market, an order may be issued to the patentee that the price be reduced and that measures be taken to offset any excess revenues that may have been earned through sales of the patented medicine at an excessive price.
II. Interpretation
4. The Guidelines provide information on the PMPRB’s general approach to the price review process and investigations. They supersede all previous guidance documents, policy communiqués and written or verbal statements of any kind by the PMPRB regarding the administration of the price review process and investigations, including all previous versions of the PMPRB’s Compendium of Guidelines, Policies and Procedures. The Guidelines should be read in conjunction with the Act, the Regulations, the appendices to these Guidelines and other related guidance documents published in the future by the PMPRB from time to time, including the Help section of the online filing tool, which takes the place of the previous Patentee’s Guide to Reporting.
5. In accordance with subsection 96(4) of the Act, these Guidelines are not binding on Staff, the Chairperson, Hearing Panels, or patentees, and are not intended to create any legal rights or presumptions, to restate the law, or to constitute a definitive statement on the interpretation of the legislation related to the PMPRB. In any given case, the approach taken by Staff and the ultimate resolution of issues will depend on the particular circumstances of the matter in question. Final interpretation of the law is the responsibility of the Board (sitting as a Hearing Panel) and is subject to review by the courts.
6. Certain aspects of these Guidelines may be revisited by the PMPRB in light of experience and changing circumstances. Guidelines and policies issued by the PMPRB are developed in an open manner with opportunities for full consultation with interested parties. When any changes to the Guidelines are considered, stakeholders will be consulted by the PMPRB in accordance with the commentary process established under subsection 96(5) of the Act.
7. Every reasonable effort will be made by the PMPRB to assist patentees in understanding the Guidelines and their application. For example, upon request, patentees will be advised by Staff on the appropriate methodologies to be applied in the price review of patented medicines sold in Canada. In addition, upon request and if there is sufficient information satisfying the Board that the price at which a patentee is selling or proposes to sell a patented medicine would not be found to be excessive, a non-binding certificate to that effect may be issued under subsection 98(4) of the Act.
8. The Guidelines do not provide an exhaustive description of all steps that may be taken or all issues that may arise in the context of a price review. In exceptional circumstances or in the event of a hearing, any methods or tests deemed appropriate and consistent with the Act and Regulations may be used by the PMPRB, regardless of whether they are addressed in the Guidelines or otherwise differ from the approach set out therein. In no case will Staff or Board members be bound or limited by these Guidelines.
9. For additional information on these Guidelines or Staff’s general approach to price reviews and investigations, please see the PMPRB’s website or contact Staff at the following:
Patented Medicine Prices Review Board
Box L40
Standard Life Centre
333 Laurier Avenue West
Suite 1400
Ottawa, Ontario
K1P 1C1
Attention: Secretary of the Board
III. Legal Framework
10. The PMPRB was established in 1987 as part of a sweeping set of amendments to the Patent Act brought into effect by Bill C-22Footnote 1. These amendments strengthened patent protection for medicines, both in terms of the scope of patentable subject matter and length of the patent-derived exclusivity period, thereby creating what policy makers believed to be a more favourable investment climate in Canada for pharmaceutical research and development (R&D). As a concession to opponents of these changes who were concerned that stronger patent protection for medicines might cause prices to rise to unacceptable levels and become unaffordable to Canadians, Bill C-22 also created the PMPRB. The PMPRB has a regulatory mandate to ensure that the prices of patented medicines are not excessive. In essence, Bill C-22 sought to strike a balance between the need to recognize and reward pharmaceutical innovation by providing patentees with a period of market exclusivity and ensuring that prices charged during that exclusivity period remain reasonable. The PMPRB’s existence as the only sector-specific price ceiling regulator under the Act reflects a recognition by policy makers that the unfettered ability to set prices for patented medicines is not in the public interest given the unique harm that can ensue if consumers are made to pay excessive prices for them.
11. The PMPRB has a dual mandate: in its regulatory role, it protects consumers by ensuring that the prices of patented medicines are not excessive; in its reporting role, it provides information on pricing trends in the pharmaceutical industry via its Annual Reports. Further to a directive from the Minister of Health under section 90 of the Act, the PMPRB also supports informed and evidence-based health policy by reporting on medicine price, utilization and cost trends under the National Prescription Drug Utilization Information System (NPDUIS) initiative.
12. The PMPRB Board consists of five members appointed by the Governor–in-Council under section 91 of the Act for terms of up to five (5) years, renewable once. The Chairperson of the Board acts as the Chief Executive Officer (CEO) of the PMPRB and has supervision over and direction of its work. The PMPRB employs public servants (i.e., Staff) pursuant to section 94 of the Act to carry out its day-to-day work. The PMPRB’s Executive Director is its senior public servant, Chief Operating Officer (COO) and Chief Financial Officer (CFO), and is responsible for the management of Staff.
13. The PMPRB is established under the Act as an independent, quasi-judicial body. To ensure this independence and autonomy, no express or implicit power is provided under the Act to Health Canada or any other government entity to direct the PMPRB in the exercise of its regulatory function. The PMPRB maintains an arm’s length relationship from the Minister of Health (who is responsible for the sections of the Act pertaining to the PMPRB), the Minister of Innovation, Science and Economic Development (who is responsible for the Act as a whole) and its various stakeholders. Similarly, the PMPRB is structured in a manner that separates the work and functions of Staff, the Chairperson and Board members. Investigation, litigation and reporting functions reside with Staff and are separate from the adjudication functions that are reserved for Board members only.
14. The monitoring of patentees’ compliance with regulatory filing requirements and the administrative price reviews of patented medicines are the responsibility of Staff. When a patented medicine appears to be priced excessively and the issue cannot be resolved through voluntary price reduction and/or measures to offset revenues from sales at that price by the patentee, the matter may be brought by the Executive Director to the Chairperson who will determine whether a hearing is in the public interest. If the decision to hold a hearing is made, a Notice of Hearing is issued, and a Hearing Panel is appointed by the Chairperson to adjudicate the matter. The Chairperson’s decision to issue a Notice of Hearing is a purely administrative act and does not express his or her view of the merits of the underlying matter.
15. The PMPRB reviews the prices of patented medicines sold at arm’s-length by patentees. Sales in Canada may include, but are not limited to, patented medicines subject to a Notice of Compliance (NOC), the Special Access Programme (“SAP”), the List of Drugs for an Urgent Public Health Need, or Clinical Trial Applications. The PMPRB has no authority over prices charged by parties other than patentees, such as prices charged by wholesalers or retailers, or over pharmacists’ professional fees.
16. Under the Act, the PMPRB is given jurisdiction to determine whether a patented medicine is or has been sold by a rights holder (a patentee, former patentee or the person for the time being entitled to the benefit of a certificate of supplementary protection for an invention pertaining to a medicine) at an excessive price in any market in Canada.Footnote 2 The term “patentee” is defined in the Act as a person who is entitled to the benefit of a patent for an invention for a period, including any other person entitled to exercise rights in relation to the patent, such as a holder of an express or implied license.Footnote 3
17. An invention pertains to a medicine if the invention is intended or capable of being used for medicine or for the preparation or production of medicine. The phrase “pertain to a medicine” has a broad meaning. The Federal Court of Appeal has determined that the nature of that connection may be “tenuous”Footnote 4. It may be satisfied, for example, where there is “only be a slender thread of a connection between a patented invention and the medicine sold in Canada”Footnote 5.Footnote 6
18. The term “medicine” is defined in the Act as including a drug (i.e., a substance or a mixture of substances manufactured, sold or represented for use in (i) the diagnosis, treatment, mitigation or prevention of a disease, disorder or abnormal physical state, or its symptoms, in human beings or animals; or (ii) restoring, correcting or modifying organic functions in human beings or animals) and a medicinal ingredient.Footnote 7 Unless otherwise specified, in these Guidelines, a reference to a “medicine” includes all dosage forms and strengths (e.g. all Drug Identification Numbers or “DINs” for medicines that have been assigned a DIN) of the medicine.
19. The PMPRB recognizes that the term “medicinal ingredient” is generally understood to mean the “active pharmaceutical ingredient” (API) used as raw materials during the manufacture of the finished dosage form. Patented medicines under the PMPRB’s jurisdiction include vaccines, topical preparations, anaesthetics and diagnostic products used in vivo, regardless of delivery mechanism (i.e., trans-dermal patch, capsule, injectable, inhaler). However, the PMPRB does not consider medical devices, in vitro diagnostic products and disinfectants that are not used in vivo to be patented medicines for the purpose of price review provisions in the Act.
20. The PMPRB has jurisdiction during the life of an eligible and issued patent including the pre-grant period (from the patent application date). The PMPRB also has jurisdiction for the extended period of protection granted via a certificate of supplementary protection.Footnote 8
21. The PMPRB’s jurisdiction over the price at which a patented medicine is sold in any market in Canada persists after the patent has been dedicated and until the cancellation or surrender of the patent pursuant to the express provisions of the Act or the expiry of the term of the patent. Patent dedication is not expressly recognized in the Act as a mechanism by which patent rights may be terminated before the normal expiry of the patent term.
22. Orders issued by the PMPRB are enforceable in the same manner as orders of the Federal Court or any superior court in Canada and may be enforced by the PMPRB or by the Federal Court. Decisions embodied in orders issued by the PMPRB may be subject to judicial review by the Federal Court in accordance with administrative law principles and the Federal Courts Act.
IV. Filing Requirements Pertaining to Price Reviews
23. Access to timely and accurate information regarding the sale of patented medicines is necessary for the PMPRB to fulfil its regulatory mandate. Therefore, patentees and former patentees are required to submit this information to the PMPRB.
24. The information that must be submitted is set out in section 82 of the Act and in the Regulations. The Help section of the online filing tool, which takes the place of the previous Patentee’s Guide to Reporting, is intended to assist patentees in identifying the content and form of the information required to be submitted and the deadlines for doing so set out in the legislation. Patentees are responsible for compliance with filing obligations. These statutory obligations cannot be waived or amended by Staff.
25. Information that patentees or former patentees may be required to file under the Regulations includes, but is not limited to:
- A notification describing a patentee’s intention to offer a patented medicine for sale in a market in Canada in which such medicine has not previously been sold (i.e., the first sale of the medicine), along with related information (Notification of Intention to Sell a Patented Medicine);
- Prescribed information relating to the identity and characteristics of a patented medicine, such as the product monograph for the medicine or equivalent information, and any DINs assigned for each dosage form and strength of the medicine;
- Prescribed information relating to the price of a patented medicine, such as information concerning the price at which each dosage form and strength of the medicine is or has been sold in any market in Canada or in any of the eleven countries set out in the Regulations (the “PMPRB11”); or
- Prescribed information relating to cost-utility analyses prepared by publicly funded Canadian health technology assessment (HTA) agencies, for which the outcomes are expressed as the cost per quality-adjusted life year (QALY) for each indication that is the subject of the analysis; and
- Prescribed information relating to the estimated maximum use of the patented medicine in Canada for a given time period, by quantity of the medicine in final dosage form.
26. As per section 7 of the Regulations, patentees shall submit required filings by email using the electronic forms on the PMPRB’s website. The forms must bear the electronic signature of an authorized individual who certifies that the information is true and complete.
27. It is the responsibility of each patentee to independently ensure that the information filed with the PMPRB (including domestic and foreign prices) is accurate. Ad hoc audits of patentee filings, including pricing, revenue and patent information, may be conducted from time to time by Staff. In the event of such an audit, patentees may be asked to provide additional supporting materials and/or corrections or confirmation of the information filed.
28. Failure to file required information within the specified period or the filing of erroneous or false information may have significant consequences for patentees or former patentees. An order for certain remedies may be sought by Staff from the Board, including an ex parte order requiring that the missing information be submitted. Alternatively, the matter may lead to summary conviction proceedings under subsection 76.1(1) of the Act. Further, the filing of false information is an indictable offence under section 76 of the Act that, on conviction, can lead to monetary fines or terms of imprisonment.
29. The Act provides for the confidentiality of information submitted to the PMPRB in certain circumstances. Specifically, information or documents provided to the PMPRB in accordance with the provisions dealing with pricing information in sections 80, 81 and 82 of the Act, or in any proceeding relating to excessive prices under section 83, are privileged and cannot be disclosed to the public without authorization of the disclosing party, unless such information has been disclosed at a public hearing under section 83 of the Act or is subject to the exceptions outlined in section 87(2) of the Act.
30. Information provided to the PMPRB may be subject to certain provisions in the Access to Information Act and the Privacy Act.
V. Price Review Process
31. The price review process consists of a series of steps whereby (i) patented medicines are categorized based on their date of introduction and market characteristics and (ii) ceiling prices are identified and used to assess the patented medicines’ prices. Price reviews are normally conducted by Staff using the methods and tests set out in these Guidelines based on the information filed by the patentees and/or obtained by Staff from relevant outside sources such as public formularies.
32. As an initial review step, patented medicines are categorized as one of the following: (1) Grandfathered medicines; (2) Line Extensions of Grandfathered medicines; (3) Gap medicines; and (4) New medicines.
33. Grandfathered medicines are all dosage forms and strengths of medicines for which the patentee was assigned a DIN prior to August 21, 2019 regardless of whether those dosage forms and strengths have been approved for new indications (without a DIN change) after August 21, 2019.
34. Line Extensions are new dosage forms and strengths of Grandfathered medicines to which a DIN was assigned on or after August 21, 2019.
35. Gap medicines are medicines for which a DIN was assigned on or after August 21, 2019 and prior to July 1, 2021 and first sold in Canada prior to July 1, 2021.
36. New medicines are all other dosage forms and strengths of medicines that do not fall into one of the above categories.
37. The price review factors under s. 85(1) of the Act that apply to the above four categories of patented medicines are as follows.
- Grandfathered medicines:
- The prices at which the medicine has been sold in the relevant market
- The prices at which other medicines in the same therapeutic class have been sold in the relevant market
- The prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada
- Changes in the Consumer Price Index (CPI)
- New medicines, Line Extensions and Gap medicines:
- The prices at which the medicine has been sold in the relevant market
- The prices at which other medicines in the same therapeutic class have been sold in the relevant market
- The prices at which the medicine and other medicines in the same therapeutic class have been sold in countries other than Canada
- Changes in the Consumer Price Index (CPI)
- The pharmacoeconomic value in Canada of the medicine
- The size of the market for the medicine in Canada
- The gross domestic product in Canada and the gross domestic product per capita in Canada
38. The specific review processes and tests applicable to New medicines, Grandfathered medicines, Line Extension medicines and Gap medicines are explained in greater detail hereafter.
A. Price Review Process for New Medicines
39. Information filed by patentees and information obtained from other sources is reviewed by the PMPRB to assess whether any patented medicine introduced in Canada appears to be priced excessively. The following diagram illustrates the review process for New medicines:
Schematic of Draft Guidelines
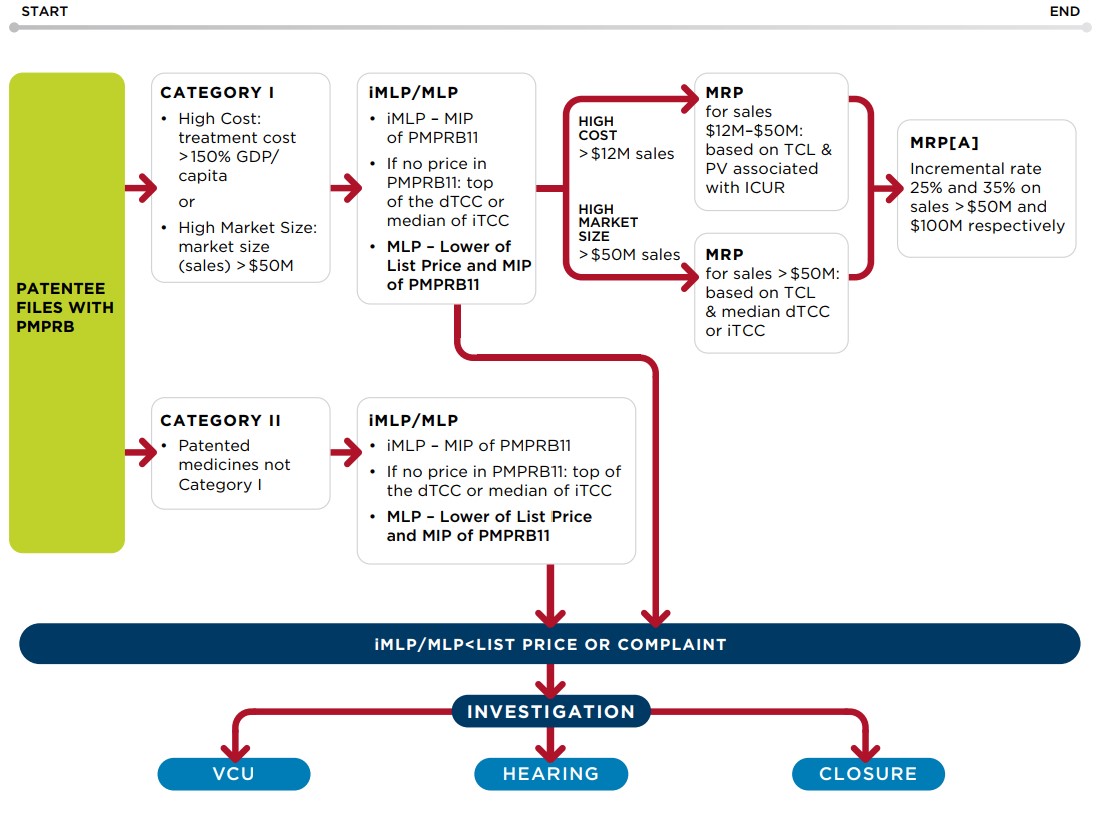
Figure description
This is a flowchart describing generally illustrating the review process for New patented medicines (patented medicines with sales after January 2021):
First, patentees file with the PMPRB. The new medicines categorization is established. The patented medicine is classified as either category I (medicines whose annual treatment costs are greater than 150% of GDP/Capita and/or medicines with a maximum expected market size greater than $50) or Category II (all patented medicines not in Category I).
An iMLP is determined based on the MIP of the available PMPRB11 prices. If there are no prices in the PMPRB11 countries, the iMLP is based on the top of the dTCC or the iTCC. The iMLP is calculated at introduction and maintained until the MLP is set. If the patented medicine’s list price is above the iMLP, an investigation can be opened, which may lead to either a VCU, a hearing, or the closure of the investigation.
The MLP is equal to either the lower of the list price or the MIP once there is data for a minimum of 5 countries or 3 years have elapsed since introduction or the RR if the patented medicine is a line extension. If, after 3 years post-introduction, there are no prices available for the PMPRB11 countries, the MLP is based on the top of the dTCC or the median of the iTCC. The MLP is maintained within a 10% margin of the MIP until the end of PMPRB jurisdiction. If the patented medicine’s list price is greater than the MLP, an investigation can be opened, which may lead to either a VCU, a hearing, or the closure of the investigation.
Category II medicines are only subject to a MLP.
Category I medicines are also subject to a MLP. In addition, Category I medicines are provided with a MRP. However, only the MLP is used to assess whether an investigation should be triggered, and the MRP is not used as an investigation trigger.
The test used for the MRP for category I medicines that have annual costs greater than 150% of GDP/capita are different for different sales.
For sales made where the annual market size is less than $12 million, the MRP is equal to the MLP.
For sales made after the annual market size rises to between $12 million and $50 million, the MRP is equal to a discount percentage based on the TCL and PV value associated with the ICUR (for ICURs between $100K-$200K per QALY) which can be up to a maximum 50% discount off the MLP. If there is to PV value, the maximum discount of 50% off the MLP is applied.
For sales made after the annual market size rises above $50 million, the MRP is subject to an additional market size adjustment which can range between 25% to 35%.
The test used for the MRP for category I medicines that have a maximum expected market size that is greater than $50 million are also different for different sales.
For sales made where the annual market size is less than $50 million, the MRP is equal to the MLP.
For sales made after the annual market size rises above $50 million, the MRP is based on a discount off the MLP which itself is based on the TCL and median dTCC and can be up to a maximum 50% discount, an additional 25%-35% market size adjustment may also apply.
Each of the above steps is described in greater detail hereafter.
Step 1: iMLP
40. At introduction (i.e. upon the first sale in any market in Canada), an interim Maximum List Price ceiling is set (the “iMLP”) for the sale of the medicine. The iMLP is set by the median international publicly available ex-factory list price (“MIP”) for the PMPRB11 countries for which the patentee has provided information during the interim period (defined below). The list priceFootnote 9 of a medicine can be no higher than the iMLP for the interim period, failing which it may be subject to additional review or investigation by Staff.
41. If the patentee has not filed international price information for the PMPRB11 countries after two reporting periods (i.e. the introductory period in which the medicine is sold in Canada and one subsequent period), the iMLP is set by the top of the domestic Therapeutic Class Comparison (“dTCC”). The dTCC will be calculated based on the highest cost of treatment among the comparator medicines, derived by taking into account the lowest price of the comparator medicine(s) (see Appendix A for further details). If there are no domestic therapeutic class comparators, the iMLP may be set by the median of the international Therapeutic Class Comparison (“iTCC”).
42. The iMLP is calculated once and only applies during the interim period, which lasts until the earlier of:
- three (3) years from the date of the introduction of the medicine in Canada; or
- when the patentee has filed international price information for at least five (5) of the PMPRB11 countries.
At the end of the interim period, the MLP will be set (see Step 2), and the iMLP will cease to apply.
43. If, at introduction, the patentee files international price information for at least five (5) of the PMPRB11 countries, the iMLP does not apply and the MLP is set immediately (see section below).
Step 2: MLP
44. Subject to the procedure described above, the iMLP will be replaced by a Maximum List Price (“MLP”).
45. If the patentee has filed international prices by the end of the interim period, the MLP is set by the lower of the list price and the MIP. Otherwise, the MLP is set by the lower of the list price and the top of the dTCC, which is calculated based on the highest cost of treatment across the comparator medicines, derived by taking into account the lowest price of the comparator medicine(s) (see Appendix A for further details).
46. If the patentee has not filed international prices by the end of the interim period, and there are no domestic therapeutic class comparators, the MLP may be set by the median of the iTCC.
47. There may be circumstances where the MLP is lower than the iMLP. In these circumstances, patentees must comply with the MLP within one (1) reporting period of the MLP being set.
48. As explained in greater detail in section VI (“Reassessment”), if in subsequent periods, the MIP exceeds the MLP by more than 10%, the MLP may be adjusted based on the lagged change in the consumer price index (“CPI”)Footnote 10. For medicines with multiple strengths and dosage forms (e.g., where multiple DINs have been assigned), comparison of the MLP against the MIP is only conducted for the strength and dosage form in respect of which the MLP was initially set by the MIP and not for strength and dosage forms in respect of which the MLPs were set by the Reasonable Relationship (“RR”) test (as described in Appendix B). However, if a strength and dosage form’s MLP is adjusted for CPI as per this process, additional MLPs for the strengths and dosage forms set by the RR test in reference to this MLP will be adjusted in accordance with the RR methodology.
49. The list price of a medicine can be no higher than the applicable MLP or highest international price (“HIP”) failing which it may be subject to additional review or investigation by Staff.
a) List Price of the patented medicine in Canada
50. As per the Regulations, patentees must file information about the list price for each dosage form, strength and package size (e.g., for each DIN) of the medicine in each province and territory.
51. As per the Regulations, patentees must file information about the list price for each dosage form, strength and package size (i.e., for each DIN) of the patented medicine in each province and territory.
b) List Prices of the patented medicine in the PMPRB11
52. International price tests are based on the information filed by patentees. Where there are multiple list prices in the same country, the lowest such price will generally be used to set ceilings based on international prices.
b) List Prices of the patented medicine in the PMPRB11
53. The PMPRB’s website provides guidance to patentees on potential price information sources.
54. When comparing prices of a medicine in Canada with prices in the PMPRB11, the local currency is converted into Canadian dollars using exchange rates calculated as the simple average of the thirty-six (36) monthly average noon spot exchange rates for each country (taken to eight (8) decimal places) as published by the Bank of Canada. During a medicine’s introductory period, the thirty-six (36) months ending in the second month of the previous reporting period (i.e., February or August) will be used. Subsequently, the thirty-six (36) months ending in the second month of the reporting period under review will be used.
Step 3: MRP/MRP[A] (Category I new medicines only)
55. New medicines will be classified as either Category I or Category II based on certain criteria described below. In addition to the iMLP/MLP, Category I medicines are subject to a “Maximum Rebated Price” ceiling (“MRP”) that may be further adjusted based on the size of the market (MRP[Adjusted] (“MRP[A]”)). The calculation of both the MRP and the MRP[A] is explained below and in Appendices C and D.
56. The MRP takes into account Therapeutic Criteria Level (TCL) (levels I–IV and the scientific review process as described in Appendix E), pharmacoeconomic value and market size of the medicine (see Appendix C). Although Staff will calculate the MRP or MRP[A] and provide it to patentees for information purposes, an investigation will only be triggered by the patentee’s failure to comply with the iMLP or MLP. However, in the event an investigation is triggered, Staff may consider net priceFootnote 11 and the MRP or MRP[A] in deciding next steps.
a) Classifying a medicine as Category I
57. A New medicine will be classified as Category I if it meets either of the following criteria:
- 12-month treatment cost greater than 150% of GDP per capita (“High Cost medicines”): this value will be based on the introductory period pricing information filed by the patentee, the medicine’s 12-month treatment cost will be calculated in relation to the maximum dosage per course of treatment listed in the product monograph; the maximum number of courses of treatment per 12 months, taking into account the nature of the condition, clinical practices, and other relevant criteria; and the highest Canadian list price. If a list price is not available, the net price will be used.
- Estimated or actual market size (sales) exceeds $50 million annually (“High Market Size medicines”): this value can be based on either (i) an estimate filed under the Regulations; or (ii) the actual revenue filed under the Regulations. Footnote 12
58. All other New medicines will be classified as Category II.
59. Notwithstanding the above, BiosimilarFootnote 13 and GenericFootnote 14 medicines will always be classified as Category II.
b) MRP and MRP[A] calculation
60. The MRP is calculated depending on the relevant criteria that led to the medicine’s categorization as Category I and the availability and content of a cost-utility analysis.
61. For medicines that (1) are High Cost medicines with sales at MLP greater than $12 million per year; and (2) have an available cost-utility analysis, the MRP is calculated as follows:
- The Incremental Cost-Utility Ratio (“ICUR”) measured in cost per quality-adjusted life years (“QALYs”) for each indication of the medicine will be identified from the cost-utility analyses filed by the patentee.
- The ICUR will be compared against the applicable Pharmacoeconomic Value Threshold (“PVT”) and reduction cap, based on its Therapeutic Criteria Level (see Appendix C, “Pharmacoeconomic Value Assessment” and chart reproduced below).
- The price at which the medicine’s ICUR would be equivalent to the PVT will be identified (the “Pharmacoeconomic Price” or “PEP”).
- The MRP will be determined assuming that the first $12 million are realized for quantities sold at the MLP, while the remaining sales up to $50 million are realized for quantities sold at the PEP subject to the reduction cap. The MRP may be further adjusted for market size (MRP[A]) if the medicine realizes annual unit sales such that, if priced at the MLP, revenues would be in excess of $50 million (see Appendix D, “Market Size Adjustment Methodology”). Because this adjustment takes place at predictable sales levels, the patentee will be informed of both its current MRP and of its expected future MRP[A].
Price adjustment based on Therapeutic Criteria Level for MRP calculation Therapeutic Criteria Level
(See Appendix E – The Scientific Review Process)PVT Reduction Cap off MLP Level I $200K/ QALY 20% Level II $150K/ QALY 30% Level III $150K/ QALY 40% Level IV $100K/ QALY 50% Pharmacoeconomic analysis does not report an ICUR Median of dTCC subject to 50% reduction cap No pharmacoeconomic analysis filed 50% of MLP
62. In the absence of an available ICUR from the information filed by the patentee, the MRP will be set by the median of the dTCC, subject to a 50% reduction cap off the MLP and subject to market size adjustments.
63. The MRP or MRP[A] can never be higher than the MLP.
64. For medicines that are High Cost medicines and have an estimated maximum revenue greater than $12 million per year, but do not have a filed pharmacoeconomic analysis, the MRP will be set by 50% of the MLP (see Appendix C).
65. For High Market Size medicines that are not High Cost medicines, the MRP is calculated as follows:
- If the actual market size is less than $50 million, the MRP is set by the MLP.
- If the actual market size is more than $50 million, the MRP is set by the lower of the MLP and the median of the dTCC.
66. The MRP[A] is recalculated annually.
Step 4: Identification of relevant indication
67. For medicines with more than one approved indicationFootnote 15, the indication for which the MRP (and MLP, if applicable) will be assessed will be determined by Staff (the “Relevant Indication”). This could occur at introduction, or as part of a reassessment if additional indications are approved during the life cycle of the medicine (see section VI).
68. For Category I medicines, the Relevant Indication will be the indication triggering the Category I classification. For Category I medicines where more than one, or no indication( s) meet the Category I criteria, and for Category II medicines, the Relevant Indication will be the indication treating the condition with the highest prevalence (i.e., the largest patient population) or estimated use.
Step 5: Compliance review timeline
69. The timing for compliance with the iMLP/MLP ceilings described in this section is as follows:
- iMLP: iMLP: patentees must comply with the iMLP at market entry if it has been set at that time, or within one (1) reporting period of the iMLP being set.
- MLP: patentees must comply with the MLP at market entry if the MLP is set at that time, or within one (1) reporting period of the MLP being set.
B. Price Review Process for Grandfathered, Line Extension and Gap Medicines
70. Grandfathered and Line Extension medicines will be subject to a MLP but not to a iMLP. Gap medicines will be assessed in accordance with the procedures described at paragraphs 40–43.
71. The MLP for Grandfathered and Line Extension medicines is set by the lower of
- the HIP for the PMPRB11 countries for which the patentee has provided information; or
- the medicine’s ceiling (e.g. the “NEAP”) under the Guidelines as they were prior to the issuance of these Guidelines.
72. The HIP and list prices for Grandfathered and Line Extension medicines will be calculated in the same manner as for New medicines under these Guidelines (see section V(A), “Price Review Process for New Medicines”).
73. The MLP for Gap medicines is set by the lower of
- the MIP for the PMPRB11 countries for which the patentee has provided information; or
- the medicine’s ceiling (e.g. the “NEAP” or the “MAPP”) under the Guidelines as they were prior to the issuance of these Guidelines.
74. The list price of a medicine can be no higher than the applicable MLP, failing which it may be subject to additional review or investigation by Staff.
75. For Grandfathered, Line Extension and Gap medicines, if the MLP is set by the medicine’s NEAP and if there is evidence that its calculation was significantly impacted by the reporting of benefits, the patentee may submit a request for the MLP to be set higher. Any such request must include details and supporting documentation of the reported benefits and, where available, a history of the medicine’s list price changes, as well as any other relevant information requested by Staff. If the requisite information supports a reassessment, the MLP will be adjusted to the lower of (i) the applicable international price test for the PMPRB11 countries for which the patentee has provided information, or (ii) the highest compliant list price of the medicine under the Guidelines as they were prior to the issuance of these Guidelines.
76. Patentees must comply with the MLP within one (1) reporting period of the MLP being set for Line Extension medicines and for Grandfathered or Gap medicines.
VI. Reassessment
77. The categories or price ceilings of medicines may be reassessed to ensure that they remain relevant in light of material changes in market conditions.
Reassessment of New and Gap medicines
78. In the case of New medicines, a reassessment may be conducted if any of the following occurs:
- A medicine (Category I or Category II) is approvedFootnote 16 for a new indication;
- A Category II medicine has sales exceeding the market size threshold (see Appendix D), contrary to the initial estimate filed by the patentee;
- A Category I medicine’s cost-utility analysis is updated; or
- If in two consecutive periods, the prevailing MIP is lower than the MLP by more than 10%.
79. A medicine that is approved for a new indication may have its Relevant Indication changed in accordance with the procedures described in section V. A new indication may alter the medicine’s market size, therapeutic class comparators, and cost-effectiveness. As a result, there may be a corresponding increase or decrease in the MRP.
80. A Category II medicine that is approved for a new indication (except for Biosimilars and Generic medicines) may be reassessed as Category I if it triggers the relevant screening criteria. For example, if the medicine’s sales at MLP increase above the annual Market Size Threshold, contrary to the initial market size estimate filed by the patentee.
81. A reassessment from Category II to Category I will result in the medicine being given a MRP and a new MLP.
82. For New and Gap medicines, if the prevailing MIP is lower than the MLP by more than 10% for two consecutive reporting periods, the MLP will be reset by the prevailing MIP. If the medicine has additional dosage forms and strengths with MLPs set by the RR test (see Appendix B), those MLPs will be adjusted accordingly.
Reassessment of Grandfathered and Line Extension medicines
83. In the case of Grandfathered medicines and their Line Extensions, if the prevailing HIP is lower than the MLP for two consecutive reporting periods, the MLP will be reset by the prevailing HIP.
Compliance with reassessed ceilings
84. The patentee will be notified in the event that reassessment criteria have been triggered and will be subsequently notified of the adjusted MLP/MRP. Patentees must comply with the new MLP within one (1) reporting period of the new MLP being set.
VII. Investigations
85. An investigation is an in-depth review of the price of a patented medicine conducted by Staff. As part of an investigation, information provided by the patentee and information that may be obtained from other sources is reviewed by Staff. Investigations are purely administrative in nature and Board members are not involved in this process. If an investigation results in a hearing, the Hearing Panel must undertake an independent de novo review of the price of the patented medicine to determine whether it is excessive under section 83 of the Act. Accordingly, the positions taken by Staff or the patentee(s) during the investigation may differ from those taken during a hearing.
86. The criteria for commencing an investigation have been developed with the intention of making the most efficient use of the PMPRB’s human and financial resources. The fact that the price of a patented medicine is not subject to an investigation does not necessarily mean that its price is not excessive and vice-versa. It only means that the investigation criteria under the Guidelines have not been met in the particular circumstances.
A. Investigation Criteria
87. In general, an investigation will be commenced by Staff when any of the following situations occur:
- the list price of any dosage form or strength of a medicine appears to be above the corresponding applicable iMLP or the MLP by more than 5%; or
- the cumulative potential revenues earned as a result of pricing above the iMLP or the MLP (“potential excess revenues”) appear to exceed $50,000 for the medicine (i.e., across all dosage forms and strengths) in a calendar year; or
- A complaint is received.
88. Notwithstanding the above, in the case of Biosimilars, medicines for veterinary use, over the counter (OTC) medicines, and vaccinesFootnote 17, an investigation will only be commenced by Staff if a complaint is received.
89. In the case of Generic medicines, Staff will only review information relating to the identity of the medicine and/or pricing upon the commencement of an investigation by Staff, which can only occur when all three of the following conditions are met:
- A complaint has been received in respect of the Generic medicine;
- The patentee of the Generic medicine is the only company in Canada which is selling a generic version of the medicine in Canada; and
- The Generic medicine is not the subject of a pricing agreement with the pan-Canadian Pharmaceutical Alliance (pCPA) to which it is compliant. The onus of proving to Staff that a Generic medicine is subject to, and compliant with, a pricing agreement with pCPA will rest with the patentee for that Generic medicine.
90. Any Generic medicine that is the subject of an investigation will be reviewed by Staff in accordance with the applicable Guidelines. With respect to domestic and international price comparisons for Generic medicines, Staff will consider, but will not be limited to, using the domestic and international prices of the brand reference product as comparators. If the reference brand product is no longer being sold in the Canadian or the international market, Staff will consider, but will not be limited to, using the former brand reference product price as a comparator (as adjusted by CPI if applicable).
91. Notwithstanding paragraphs 87 and 88, the price of any patented medicine which appears on the List of Drugs for Exceptional Importation and Sale pursuant to the March 30, 2020 Interim Order Respecting Drugs, Medical Devices and Foods for a Special Dietary Purpose in Relation to COVID-19 or on the list(s) published by Health Canada pursuant to the September 16, 2020 Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19 will not be subject to review or investigation unless a complaint is received from either the federal Minister of Health or any of her provincial or territorial counterparts. This will be case for as long as the Interim Orders are in effect and the medicine remains listed. Absent a pre-existing complaint, upon the expiry or repeal of the Interim Order(s) or the removal of a medicine from the applicable list(s), Staff will review the price of the medicines subject to this measure based on the prevailing international and domestic list prices, and not on the introductory discounted prices in Canada.
92. When the list price of a medicine is higher than the iMLP or MLP under the Guidelines but does not meet the criteria for commencing an investigation, the patentee will be notified and the medicine will be reported in the PMPRB’s Annual Report as “Does Not Trigger Investigation.” In such an instance, no immediate action will be taken by Staff; however, in order to avoid an investigation at a later date, the list price of a medicine can be no higher than the applicable ceiling price and any revenues that may have accrued must be offset, failing which the price may be subject to additional review or investigation by Staff.
B. Investigation Process
93. When an investigation is commenced by Staff, the patentee will be notified, and the medicine will be reported in the PMPRB’s Annual Report as “Under Investigation”. Price reviews and investigations cannot result in a legal determination that the price of a medicine is excessive under the Act. Such a determination can only be made by a Hearing Panel after the patentee or former patentee has been provided with a reasonable opportunity to be heard, as required by section 83 of the Act.
1. Additional review of filings
94. When a medicine comes under investigation, its pricing history from introduction will be reviewed by Staff. All information filed by the patentee is analyzed and clarification may be sought. For example, if a price seems to be in error or unexpected, or if there are discrepancies in the patentee’s filings, the patentee may be asked for an explanation or to provide additional supporting materials. Any relevant materials not filed by the patentee, such as dTCC and iTCC prices, may also be considered by Staff. In addition, an analysis of the appropriateness of the applicable category and tests is conducted by Staff to take into account the facts of the case and the particularities of the relevant markets in which the medicine is sold. For example, Staff may consider whether the actual market size is materially lower than the estimated market size, or whether the medicine is a vaccine, blood product or other product subject to a tendering process.
95. Staff may utilize any of the tests described in the Guidelines and modifications or variations of those tests (e.g., MIP instead of HIP or median as opposed to the top of the dTCC, or comparing the net price to the MRP) depending on what it believes most appropriate to the factual circumstances surrounding the price of the medicine under investigation.
2. Calculation of potential excess revenues
96. In the event that the price of a medicine exceeds the ceilings established under the Guidelines, the patentee will be notified that its price(s) are “outside the thresholds set out in the Guidelines” and the applicable price ceilings will be identified. Cumulative potential excess revenues will also begin to be calculated by Staff based on net prices filed by the patentee regardless of the type of price ceiling or test being used. Because the PMPRB has jurisdiction over the patented medicine in the pre-grant patent period, potential excess revenues related to its sales during that period will be included in the calculations.
97. In some cases, the tests and ceilings used during the investigation may differ from the initial thresholds that led to the triggering of the investigation. In such cases, the investigation ceilings (as opposed to the triggering ceilings) will be used to calculate potential excess revenues.
98. Finally, in the event of a hearing, Staff may argue that different ceilings from those set out in the Guidelines or used during the investigation apply, and/or seek a remedy in the form of excess revenues that differs from the cumulative potential excess revenues calculated during an investigation. In addition, where Staff believes the patentee or former patentee has engaged in a policy of selling the medicine at an excessive price, it may seek an order that the patentee offset up to twice the amount of excess revenues.
3. Investigation outcomes
99. Possible outcomes of an investigation include:
- A Voluntary Compliance Undertaking (“VCU”), as described in section VIII;
- The issuance of a Notice of Hearing if, upon the recommendation of Staff, the Chairperson considers it to be in the public interest; and/or
- The closure of the investigation.
100. The closure of an investigation may follow from a VCU or a determination that further examination of the price of a patented medicine is not warranted at that point in time in view of facts and considerations that come to light during the investigation.
101. The closure of an investigation is an administrative act and does not constitute a legal determination or an admission by the PMPRB that the price of the patented medicine is not excessive. The closure of an investigation does not preclude the possibility of the opening or re-opening of further investigation(s) or the commencement of a hearing in the future.
VIII. Voluntary Compliance Undertaking (VCU)
102. At any time prior to the issuance of a Notice of Hearing, a patentee may submit a VCU to Staff. A VCU is a promise by the patentee to reduce its price(s) and/or offset any potential excess revenues from the sale of a patented medicine that is subject to an investigation. A proposed VCU does not constitute an admission by the patentee that the price of the patented medicine is excessive.
103. VCU negotiations with patentees are conducted by Staff, and it is the PMPRB’s policy that the Chairperson not be involved. If negotiations result in a VCU proposal that Staff believes would be acceptable to the Chairperson, it will be referred to the Chairperson by the Executive Director for his or her consideration. Staff cannot independently determine whether a VCU proposal is acceptable and cannot make any assurances to the patentee regarding the likelihood that the Chairperson will consider it acceptable.
104. The consideration of a VCU is an administrative procedure and does not constitute an admission or determination by the PMPRB that the price submitted by the patentee, or used to calculate a revenue offset, is not excessive. However, the acceptance of a VCU by the Chairperson will result in the closure of an investigation.
105. The PMPRB reports publicly on all VCUs that the Chairperson has accepted. In submitting a signed VCU, a patentee must consent to its publication either in full or redacted form. The reported information can include disclosure of a copy of the VCU or terms included in the VCU. The reported information may appear in the PMPRB’s Annual Report, on the PMPRB’s website, in the PMPRB’s publications such as the NEWSletter, and on social media platforms.
106. Requests for VCU negotiations or proposals “without prejudice” cannot be considered by Staff. VCUs are unilateral promises by patentees and not settlement agreements. However, parts of the discussions between patentees and Staff that relate to the content of the patentee’s filings may be subject to the protections set out in sections 87 and 88 of the Patent Act. In addition, provisions of the Access to Information Act may apply.
107. If a Notice of Hearing has been issued, patentees may pursue settlement through the negotiation of a settlement agreement, which must be approved by the Hearing Panel. Requests for settlement agreements or proposals are considered by Staff on a “without prejudice” basis.
IX. Hearing Recommendation
108. When an investigation into the price of a patented medicine is completed and the matter is not resolved, the Executive Director may submit a report to the Chairperson. The Chairperson may decide to issue a Notice of Hearing if he or she is of the opinion that it is in the public interest. A decision to issue a Notice of Hearing is not adjudicative and no analysis is undertaken by the Chairperson as to whether the facts alleged by Staff are, or will be, proven. Until a matter is brought before a Hearing Panel at a public hearing, no other Board member is informed of the results of Staff’s review or investigation into the price of a patented medicine.
109. The decision of whether the price of a patented medicine is excessive is made by the Hearing Panel alone after the public hearing is held.
X. Excessive Price Hearing Process and Remedies
110. PMPRB hearings are public proceedings. During a hearing, submissions and evidence from the parties are heard by a Hearing Panel consisting of at least two Board members. The Hearing Panel determines whether a patented medicine is being or has been sold at an excessive price in any market in Canada by taking into consideration the available information relating to the factors set out in section 85 of the Act.
111. For more information about hearings, please consult the PMPRB Rules of Practice and Procedure, the published standard set of procedures to be followed by all participants in hearings before the PMPRB. The Rules set out the PMPRB’s procedures in accordance with the requirement under the Act to resolve matters as informally and expeditiously as the circumstances and considerations of fairness permit. Practice directions and further information about previous and ongoing hearings are also publicly available on the PMPRB’s website.
112. Under the Act, the PMPRB is empowered to make remedial orders when it finds, following a hearing, that a patentee (or former patentee) is selling, or has sold, a patented medicine in any market in Canada at an excessive price.Footnote 18
113. In broad terms, the PMPRB has the power to impose two main forms of remedy after a hearing: (i) orders directing the patentee to cause the maximum price at which the patentee sells the patented medicine in a market to be reduced to such level as the PMPRB considers not to be excessive; and (ii) orders directing the patentee to offset the amount of the excess revenues estimated by it to have been derived by the patentee from the sale of the patented medicine at an excessive price by either (a) reducing the price at which the patentee sells the patented medicine; (b) reducing the price at which the patentee sells one other medicine to which a patented invention of the patentee pertains; or (c) paying to Her Majesty in right of Canada an amount specified in the order.
114. If a Hearing Panel finds that the patentee or former patentee has engaged in a policy of selling the patented medicine at an excessive price, it may order the patentee to offset up to twice the amount of excess revenues estimated by it to have been derived from the sale of the patented medicine at an excessive price. The extent and duration of sales of the patented medicine at an excessive price will be considered by the PMPRB in making this finding and order.
XI. Failure to File Hearing
115. When it is the opinion of Staff that a patentee has failed or refused to provide the PMPRB with the pricing, sales, or revenues and like information required by law, the Executive Director may submit a report to the Chairperson. The Chairperson may decide to issue a Notice of Hearing if he or she is of the opinion that it is in the public interest to hold a hearing to determine whether the patentee has, in fact, breached the reporting requirements of the Act and Regulations.
116. As with excess price hearings, a decision to issue a Notice of Hearing is not adjudicative and no analysis is undertaken by the Chairperson as to whether the facts alleged by Staff are, or will be, proven. Until a matter is brought before a Hearing Panel at a public hearing, no other Board member is informed of the results of Staff’s review or investigation into the matter. The decision of whether a patentee has failed to file required information is made by the Hearing Panel alone after the public hearing is held. If, as the result of such a hearing, the Hearing Panel finds that the patentee is in breach of its reporting requirements, it may order the patentee to provide the PMPRB with the required information and documents as per section 81 and/or section 88 of the Act.
117. In addition, as per subsection 76.1(1) of the Act, every person who contravenes or fails to comply with the filing requirements set out in section 80, 81, 82 or 88, or any order made thereunder, is guilty of an offence punishable on summary conviction and liable to a fine or to imprisonment.
XII. Complaints
118. Any individual or group who believes that the price of a patented medicine in any market in Canada is excessive may submit a complaint to the PMPRB. A complaint may be submitted by telephone, in writing, or electronically using the contact information available on the PMRPB’s “How to Make a Complaint” page.
119. Generally, complaints are a trigger for an investigation by Staff into the price of a patented medicine. The complainant is not part of that investigation or of any resulting hearing (unless the complainant applies to become an intervener in the hearing). The complainant is not required to provide any documents or evidence to the PMPRB. Any investigation will be based on materials provided by the patentee or otherwise obtained by Staff.
120. Due to limitations on disclosure set out in sections 87 and 88 of the Patent Act and in the Access to Information Act, the complainant will be only be informed of the outcome of an investigation if the process results in a VCU or a Notice of Hearing.
XIII. Appendices
A. Domestic Therapeutic Class Comparison (dTCC) and International Therapeutic Class Comparison (iTCC)
dTCC TEST
As described in section V of these Guidelines, the domestic Therapeutic Class Comparison (“dTCC”) test is used as part of the price ceiling calculation in certain circumstances. The dTCC test compares a patented medicine’s list price with the list prices of other medicines identified by scientific review for comparison purposes.
For High Market Size medicines, the MRP is set by the lower of the MLP and the median of the dTCC taking into account the applicable reduction caps set out in the table below, and the application of market size adjustment as per Appendix D.
| Therapeutic Criteria Level (See Appendix E – The Scientific Review Process) |
dTCC Reduction Cap |
|---|---|
| Level I | 20% |
| Level II | 30% |
| Level III | 40% |
| Level IV | 50% |
Identification of medicines for comparison purposes
The World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology’s Anatomical Therapeutic Chemical (ATC) Classification System is used in the selection of medicines to be used for comparison purposes.
The medicines used for comparison purposes will typically be those identified under the ATC classification system at the sub-class level above the single chemical substance. This will normally be the fourth sub-class level but could include the next higher subclass or another sub-class. In some instances, it may be appropriate to select from the fifth or single chemical substance level.
A medicine of the same ATC therapeutic class as the patented medicine under review may be omitted if it is unsuitable for comparison. For example, a medicine with a primary indication other than the primary indication of the patented medicine under review may be omitted from the comparison.
All medicines identified for comparison that have the same approved indication or use as the Relevant Indication of the patented medicine under review will be included in the review. This review is based on Staff research and may include additional research by a Drug Information Centre (DIC) or consideration of evidence submitted by patentees.
Where there are multiple sellers of a medicine identified as a comparable medicine, the lowest price of the comparable medicine will be identified. The final therapeutic class comparison price is the highest price across all comparable medicines for the MLP and the median for the MRP.
For a patented medicine that is a new dosage form or strength of the same medicinal ingredient as one or more existing medicines, its comparators will be those existing medicines that are available in the same or comparable dosage form and have the same indication or use. This will apply regardless of whether the dosage regimens are the same or differ materially.
For a product that is a combination of medicines, where each of the medicines of the combination are sold in Canada and have the same indication or use, its comparators will be limited to the component medicines.
Comparable dosage regimens
The comparable dosage regimen used for comparison purposes will normally be the maximum of the usual recommended dosage in the Product Monograph (or similar information) taking into account relevant clinical variables. The most appropriate strength of the medicine will be chosen for a particular dosage regimen. Generally, a dosage regimen based on a course of treatment will be applicable to acute indications, while a per-day regimen (based on maintenance dose) will be applicable to chronic indications.
Price sources
Patentees do not file prices for the patented medicine’s comparators. Public sources will be used for the prices of the medicines used for comparison purposes in order to conduct a dTCC test. Provincial formularies will be the starting point in Staff’s identification of prices. The lowest price for each of the medicines identified for comparison purposes will be used. Any medicine (patented or non-patented) identified for comparison purposes may be excluded from a dTCC test if Staff has reason to believe it is being sold at an excessive price.
dTCC test
Following the identification of medicines for comparison purposes and of their lowest price for each medicine, the cost of comparable courses of treatment for each medicine will be calculated. These costs of treatment will be ordered and the top or the median identified. In the event of an even number of medicines used for comparison purposes, the median will be the simple average of the middle two costs of treatment. This median cost of treatment will then be divided by the constituent units of the comparable course of treatment for the medicine under review to establish a per-unit price.
iTCC test
As described in section V of these Guidelines, the international Therapeutic Class Comparison (“iTCC”) test may be used in certain circumstances. The iTCC test compares a patented medicine’s list price with the list prices of other medicines identified by scientific review for comparison purposes in the eleven (11) comparator countries listed in the Regulations.
Identification of medicines for comparison purposes and comparable dosage regimens
The iTCC test uses the same medicines identified for comparison purposes and comparable dosage regimens as the dTCC test, following the procedures set out above. In cases where no domestic comparators have been identified, Staff may assess whether there are additional medicines approved for the same approved indication or use as the medicine under review in any of the comparator countries.
Price sources
Patentees do not file international prices for the international comparators to the patented medicine. National formularies will be the starting point in Staff’s identification of prices. The lowest price for each of the medicines identified for comparison purposes will be used.
Any medicine (patented or non-patented) identified for comparison purposes may be excluded from an iTCC test if Staff has reason to believe it is being sold at an excessive price.
iTCC test
Following the identification of medicines for comparison purposes and of the lowest price for each medicine, the cost of comparable courses of treatment for each comparator medicine in each comparator country will be calculated. These costs of treatment will be ordered and the median identified for each country. In the event of an even number of comparator medicines used for comparison purposes, the median will be the simple average of the middle two costs of treatment. These medians will be ordered in a “median of the medians” will be identified. This median cost of treatment will then be divided by the constituent units of the comparable course of treatment for the medicine under review to establish a per-unit price. Local currencies will be converted to Canadian dollars using the methodology described in section V of these Guidelines.
B. Reasonable Relationship Test and Comparable Dosage Forms
Reasonable relationship test
The Reasonable Relationship (RR) test may be conducted to determine the MLP of a new additional strength of a patented medicine with other existing strengths, where the new additional strength has the same medicinal ingredient, indication, dosage regimen, and same or comparable dosage form as the existing strength(s). There is no RR test until a MLP is set for the reference strength.
MLP
Where a patented medicine is not or is no longer in the interim period, the first strength that met the criteria for the transition from iMLP to MLP will become the reference strength. If there are multiple strengths that meet this criterion within a reporting period, the reference strength will be selected among these based on scientific considerations. Once the reference strength is identified, the ceiling for the new additional higher strengths will be established based on the proportional relationship between the strengths, subject to a HIP cap for the new additional strength. Specifically, the MLP of the new higher additional strength will be set to be equivalent to the price per standard unit of the reference strength. The MLP for the new additional lower strength will not be set higher than the price of the reference strength provided that this does not result in a price for the new additional strength that is above the HIP. If the list price for the strength that is getting the MLP set by the RR is lower than the calculation, the MLP should be set by the lower of the list price and the RR test.
MRP
The MRP is calculated as a reduction from the MLP. Therefore, the RR test application for a new additional strength of a patented medicine subject to the MRP will be done at the MLP level for the reference strength and the corresponding price reduction percentage will be carried over to the new additional strengths of the patented medicine.
MRP[A]
The MRP[A] is an adjustment based on the MRP as a function of market size and will thus change accordingly.
Comparable dosage forms
The following are considered comparable dosage forms for the purpose of the RR test. Formulations within each group are considered comparable, but dosage forms in a different group are not.
Topical (T)
- Aerosol
- Aerosol (foam)
- Cream
- Disc (extended release)
- Disc
- Dressings
- Gel
- Gel (controlled release)
- Liposomes
- Liquid
- Lotion
- Ointment
- Pad
- Paint
- Paste
- Patch
- Patch (extended release)
- Pencil
- Plaster
- Powder
- Shampoo
- Soap Bar
- Solution
- Sponge
- Spray
- Spray (bag-on-valve)
- Spray (metered dose)
- Stick
- Strip
- Swab
- Tincture
Nasal (N) / Pulmonary (P)
- Aerosol
- Aerosol-metered dose
- Drops
- Gas
- Metered dose preparation
- Powder
- Powder (metered dose)
- Solution
- Solution (extended release)
- Spray
- Spray (metered dose)
- Stick
Oral Solid (S)
- Bar (chewable)
- Caplet
- Capsule
- Effervescent granules
- Effervescent powder
- Effervescent tablet
- Film (soluble)
- Globules
- Granules
- Gum
- Lozenge
- Modified release caplet
- Modified release capsule
- Modified release tablet
- Pellet
- Piece (chewable)
- Powder (extended release)
- Strip
- Tablet
- Tablet (chewable)
- Tablet (oral disintegrating)
- Tablet for suspension
- Wafer
Oral Liquid (L)
- Drops
- Elixir
- Emulsion
- Gel
- Granules for solution
- Granules for suspension
- Granules for suspension (delayed release)
- Granules for suspension (extended release)
- Liquid
- Modified release liquid
- Powder (extended release)
- Powder for solution
- Powder for suspension
- Solution
- Solution (extended release)
- Spray
- Suspension
- Suspension (extended release)
- Syrup
- Syrup (extended release)
- Tea (herbal)
- Tincture
Vaginal (V)
- Cone
- Cream
- Douche
- Foam
- Gel
- Gel (controlled release)
- Implant
- Insert
- Insert (extended release)
- Ovule
- Pellet
- Ring (slow release)
- Sponge
- Suppository
- Suppository (sustained release)
- Tampon
- Vaginal tablet
- Vaginal tablet (effervescent)
Parenteral (J)
- Bolus
- Implant
- Kit
- Liposomes
- Modified release injection
- Pellet (implantable)
- Powder for solution
- Powder for suspension (sustained-release)
- Solution
- Solution (extended release)
- Suspension for emulsion
- Suspension (extended release)
Otic (E) / Opthalmic (Y)
- Drops
- Gel
- Gel (controlled release)
- Implant
- Insert
- Insert (extended release)
- Liquid
- Modified release ocular device
- Ointment
- Powder for solution
- Solution
- Solution (extended release)
- Suspension
Rectal (R)
- Cream
- Enema
- Foam
- Insert
- Ointment
- Ovule
- Stick
- Suppository
- Suppository (sustained release)
- Suspension
- Suspension (extended release)
Dental/Sublingual Buccal (M)
- Emulsion
- Film (soluble)
- Floss
- Gel
- Gel (controlled release)
- Gum
- Lozenge
- Metered-dose pump
- Modified release buccal tablet
- Mouthwash (gargle)
- Paste
- Powder (effervescent)
- Powder for suspension
- Solution
- Solution (extended release)
- Spray – buccal
- Spray – sublingual
- Stick
- Strip
- Sublingual tablet
- Suspension
- Suspension (extended release)
- Swab
- Tablet (orally disintegrating)
- Tablet
- Tooth paste
- Tooth powder
- Wafer
C. Pharmacoeconomic Value Assessment
As described in section V of these Guidelines, a pharmacoeconomic value assessment is used as part of the calculation of the MRP. Under the Regulations, patentees are required to file prescribed information relating to all pharmacoeconomic analyses of a patented medicine prepared by publicly funded Canadian organizations if the pro-rated cost of the treatment for that medicine over a 12-month period is greater than or equal to 50% of GDP per capita at the time of the publication of the analysis.
The typical source of pharmacoeconomic analysis used for this assessment will be the Common Drug Review (CDR) Pharmacoeconomic Reports and pan-Canadian Oncology Drug Review (pCODR) Final Economic Guidance Reports of the Canadian Agency for Drugs and Technologies in Health (CADTH). Analyses developed by the Institut national d’excellence en santé et services sociaux (INESSS) in its Évaluations aux fins d’inscription or the National Advisory Committee on Immunization (NACI) will also be considered.
As part of this assessment, Staff calculations will rely on the base case reanalysis conducted by the public agency, as opposed to the analysis conducted with the base case model submitted by the patentee. This will be a cost-utility analysis model in which health outcomes are expressed as QALYs. The Incremental Cost-Utility Ratio (“ICUR”) measured in cost per quality-adjusted life years (“QALYs”) for each indication of the medicine will be identified from the cost-utility analyses filed by the patentee.
If the agency’s report does not report an ICUR a dTCC will be conducted. In such cases, the MRP will be set by the median of the dTCC, subject to a 50% reduction cap off the MLP and to market size adjustments as discussed in Appendix D. For medicines with multiple indications, the pharmacoeconomic assessment for the Relevant Indication, as determined by the procedure in section V of these Guidelines, will be used.
The ICUR will be compared against the applicable Pharmacoeconomic Value ThresholdFootnote 19 (“PVT”), based on the Therapeutic Criteria Levels provided in the table below. The MRP is based on the PEP (the price at which the medicine’s ICUR would be equivalent to the PVT) but is subject to the maximum percentage reduction cap indicated in the table.
The PMPRB relies on agencies like CADTH, INESSS and NACI to provide estimates of the PEP corresponding to the established PVTs, or information that enables the calculation of the PEP in their reporting.
| Therapeutic Criteria Level (See Appendix E – The Scientific Review Process) |
PVT | Reduction Cap off MLP |
|---|---|---|
| Level I | $200K/ QALY | 20% |
| Level II | $150K/ QALY | 30% |
| Level III | $150K/ QALY | 40% |
| Level IV | $100K/ QALY | 50% |
| Pharmacoeconomic analysis does not report an ICUR | Median of dTCC subject to 50% cap | |
| No pharmacoeconomic analysis filed | 50% of MLP | |
D. Market Size Adjustment Methodology
As described in section V of the Guidelines, a market size adjustment is applied to Category I medicines when sales at MRP[A] exceed $50 million across all dosage forms and strengths of the medicine (e.g., all DINs combined). This adjustment will be applied annually in accordance with the following table:
| Annual sales‡ | MRP | Incremental MRP adjustment factor | MLP adjustment factor |
|---|---|---|---|
| <$12M | MLP | 0% | 1 |
| $12M-50M | Greater of PEP and Cap | ($12M - $12M * (MRP/MLP)) / Sales§ + (MRP/MLP) | |
| $50M-$100M | -25% | ($21.5M - $9M * (MRP/MLP)) / Sales§ + (1 - 25%) * (MRP/MLP) | |
| >$100M | -35% | ($32M - $7.8M * (MRP/MLP)) / Sales§ + (1 - 35%) * (MRP/MLP) |
‡ Annual Sales are calculated at MRP[A].
§ Sales are calculated at MLP.
| Annual sales‡ | MRP | Incremental MRP adjustment factor | MLP adjustment factor |
|---|---|---|---|
| <50M | MLP | 0% | 1 |
| $50M-$100M | Lowest of the MLP and the median of the dTCC | -25% | ($50M - $37.5M * (MRP/MLP)) / Sales§ + (1 - 25%) * (MRP/MLP) |
| >$100M | -35% | ($56.7M - $32.5M * (MRP/MLP)) / Sales§ + (1 - 35%) * (MRP/MLP) |
‡ Annual Sales are calculated at MRP[A].
§ Sales are calculated at MLP.
For example, a medicine requiring an ICUR and realizing between $50 million and $100 million in sales based on units sold at the MRP will have its MRP[A] set as follows:
MRP[A] = MLP * ($21.5M - $9M * (MRP/MLP)) / Sales§ + (1 - 25%) * (MRP/MLP)
MRP[A] = MLP * MLP adjustment factor.
The MLP adjustment factor is calculated for each annual sales range, as per the corresponding formulas. Each MLP adjustment factor is then multiplied by the sales at MLP. The MLP adjustment factor that applies is the one that when multiplied with sales calculated at MLP, it results in an amount that falls within the corresponding annual sales range. The MRP[A] is determined by multiplying the MLP by that adjustment factor.
E. Scientific Review Process
The PMPRB’s scientific review is an evidence-based process that considers clinical, pharmacoeconomic and other relevant information about the patented medicine for the purpose of determining its Therapeutic Criteria Level.
The scientific review of a patented medicine is based on information from a variety of sources such as those set out below:
- Patentee Submission – Patentees may provide Staff with a brief submission document which clearly explains the rationale for the patentee’s proposals relative to the therapeutic criteria, medicines identified for comparison purposes, and comparable dosage regimens. Information on submission procedures is available on the PMPRB online filing tool.
- Research by a Drug Information Centre (DIC) – Staff may use the services of various drug information centres to obtain scientific information, such as clinical trial information, clinical practice guidelines, etc. The basis of the review by the DIC is the product monograph or information similar to that contained in a product monograph if a NOC has not been granted.
- Research by Staff – Staff may also update research and supplement data and evidence from the patentee and DIC using other sources.
- PMPRB Human Drug Advisory Panel (HDAP) – On an ad hoc basis, Staff may consult with the HDAP to provide clinical context pertaining to the scientific information that is being considered by Staff.
Therapeutic Criteria Level I
The patented medicine is the first medicine to be sold in Canada that effectively treats a particular illness or effectively addresses a particular indication in a clinically impactful manner. Clinically impactful improvement includes improvements in quality of life, mortality or significant reductions in disease severity or healthcare utilization, preferably based on high quality evidence using hard clinical endpoints rather than surrogate outcomes. A high QALY gain is normally associated with medicines at this level.
Therapeutic Criteria Level II
The patented medicine provides a considerable improvement in therapeutic effect, relative to other medicines sold in Canada, in a clinically impactful manner. Clinically impactful improvement includes improvements in quality of life, mortality or significant reductions in disease severity or healthcare utilization, preferably based on high quality, active-comparator controlled evidence using hard clinical endpoints rather than surrogate outcomes. High quality network-meta-analysis may also be considered. A high QALY gain is normally associated with medicines at this level.
Therapeutic Criteria Level III
The patented medicine provides moderate absolute improvement in therapeutic effect, relative to other medicines sold in Canada. The medicine may have (a) an increase in clinically relevant efficacy; (b) be associated with a reduction in the incidence or grade of important adverse reactions; or (c) be associated with clinically relevant increased ease of use characteristics (e.g. route of administration, convenience, increased compliance, etc.), however, these improvements may provide limited meaningful clinical impact or may be based on lower quality clinical evidence. Patented medicines at this level are normally associated with moderate incremental QALY gains with a relatively high degree of certainty or a high QALY gains with a relatively low degree of certainty.
Therapeutic Criteria Level IV
The patented medicine provides slight or no improvement relative to other medicines sold in Canada. Alternatively, or in addition, the medicine has very limited (or no) robust clinical evidence available to clearly identify its degree of clinically relevant therapeutic improvement relative to other medicines sold in Canada. Limited or no additional QALY gains or significant uncertainty due to poor quality evidence are associated with medicines at this level.
Identification of Medicines for Comparison Purposes
The World Health Organization (WHO) Collaborating Centre for Drug Statistics Methodology’s Anatomical Therapeutic Chemical (ATC) Classification System may be used in the selection of medicines for comparison purposes. The medicines used for comparison purposes will typically be those identified under the ATC classification system at the sub-class level above the single chemical substance. This will normally be the fourth sub-class level but could include the next higher sub-class or another sub-class. In some instances, it may be appropriate to select from the fifth or single chemical substance level. A medicine of the same ATC therapeutic class as the medicine under review may be omitted if it is unsuitable for comparison. For example, a medicine with a primary indication/use other than the primary indication/use of the patented medicine under review may be omitted from the comparison.
All medicines used for comparison purposes will typically have the same approved indication or use as the patented medicine under review.
If the iTCC includes medicines with the same approved indication or use as the patented medicine under review, that information may be considered.
For a patented medicine that is a new dosage form or strength of the same medicinal ingredient as one or more existing medicines, its comparators will be those existing medicines that are available in the same or comparable dosage form and have the same indication or use. This will apply regardless of whether the dosage regimens are the same or differ materially.
For a product that is a combination of medicines, where each of the medicines of the combination are sold in Canada and have the same indication or use, its comparators will be limited to the component medicines.
Identification of Comparable Dosage Regimens
The comparable dosage regimen used for comparison purposes will normally be the maximum of the usual recommended dose in the product monograph (or similar information) taking into account relevant clinical variables. The most appropriate strength of the medicine will be chosen for a particular dosage regimen. Generally, a dosage regimen based on a course of treatment will be applicable to acute indications, while a per-day regimen (based on maintenance dose) will be applicable to chronic indications.
F. Summary of Compliance Timelines
| Patented Medicine Category | Tests used for the iMLP | Tests used for the MLP (3 years or 5 countries min.) | MRP | Reassessment Triggers | Compliance Assessment |
|---|---|---|---|---|---|
| Grandfathered | N/A | Lower of NEAP and HIP | N/A | If MLP > HIP in two consecutive reporting periods | 1 reporting period |
| Line extension of Grandfathered | N/A | Lower of list and RR or level pricing subject to HIP | N/A | If MLP > HIP in two consecutive reporting periods | 1 reporting period |
| Gap | Lower of list and MIP | Lower of list, NEAP/MAPP and MIP | N/A | If MLP > MIP by 10% in two consecutive reporting periods | 1 reporting period |
| New medicine | MIP | Lower of list and MIP (or dTCC or iTCC if no IP*) | N/A | If MLP > MIP by 10% in two consecutive reporting periods Market size trigger Updated therapeutic/ HTA evidence/New relevant indication | 1 reporting period |
| New dosage form and strength of New medicine | MIP | Lower of list and RR or level pricing subject to HIP | N/A | Same as New medicine for all dosage forms and strengths | 1 reporting period |
*IP: international price
