Changes in Canadian Guidelines for Conducting Budget Impact Analysis
Introduction and objective
In Canada, budget impact analyses (BIAs) are used to make informed reimbursement decisions. The Patented Medicine Prices Review Board’s (PMPRB) 2020 BIA Guidelines provide a standardized approach and detailed recommendations for developing a BIA for submission to the Canadian Agency for Drugs and Technologies in Health (CADTH) or to one of the participating federal/provincial/territorial (FPT) drug plans.
The 2020 Guidelines supersede those first published by the PMPRB in May 2007. While the 2007 Guidelines were considered to be a primary resource for the preparation of pharmaceutical BIAs in Canada, they no longer reflect current best practices. This update was necessary to keep current with developments in the BIA methods required by Canadian public drug plans, CADTH, and international guidelines.
The Guidelines document provides a detailed explanation of essential factors to consider when developing a BIA, information on which data sources to use and how to use them, and an outline of the reporting format. The major updates to the Guidelines involve added flexibility in selecting the time horizon and new recommendations for the target population assessment, the consideration of compliance and persistence in the cost of treatments, evolving indications, and off-label comparators. Other modifications include a new Excel model, updated methods used in uncertainty analysis, and selecting relevant comparators.
Methods
Systematic Literature Reviews
To inform the changes, the PMPRB undertook a systematic review of national and international BIA guidelines and best practices in collaboration with EVERSANATM (formerly Cornerstone Research Group Inc.). A literature review of BIA guidelines was conducted2, including an examination of international standards of practice for BIAs from several countries, such as Canada1, Australia3, England and Wales4, Belgium5, Ireland6, France7, Poland8, and the Netherlands9, as well as guidelines from the International Society for Pharmacoeconomics and Outcomes Research (ISPOR)10. Provincial drug plan templates for Alberta11, Ontario12, and Manitoba13 were also considered, in addition to the relevant guidance for Quebec14 and CADTH (pCODR)15.
Stakeholder Analysis
Representatives from provincial drug plans, as well as CADTH, pCPA, INESSS, and Health Canada were surveyed to gather input on the main issues to be addressed: gaps and challenges in the former Guidelines, the time horizon, non-drug- and condition-related costs, comparators, and multiple indications. All feedback received was considered when revising the Guidelines. Additionally, updates to the CADTH documents Procedures for the CADTH Common Drug Review and Interim Plasma Protein Product Review and Procedures for the CADTH pan-Canadian Oncology Drug Review were taken into account when making the revisions.
New recommendations
Analytic Framework
- Population
An open population should be used in the target population assessment when applicable (e.g., the rate of mortality and disease progression should be considered). - Scenarios to be compared
Evolving indications should be considered. - Drug costs
The cost of companion diagnostic tests or medical devices that are covered by the drug plan should be reported.
The BIA should address the impact of compliance and persistence with therapy on the cost of treatments if required by the drug plan.
Input and Data Sources
- Selecting relevant comparators
Medicines that are commonly used off-label for the same indication(s) as the new drug and that are listed on the public drug plan formulary may be included in the base-case or the scenario analysis, depending on the drug plan requirements.
Modified recommendations
Analytic Framework
- Perspective
The BIA should be performed from a drug plan perspective and focus on drug-related costs that have a direct impact on the plan budget. Non-drug-related costs are normally excluded from the base-case analysis; however, they may be acknowledged in the BIA or presented in a scenario analysis. In some exceptional cases, healthcare system-related costs may be included in the base-case analysis. - Time horizon
A time horizon consisting of a one-year baseline period and a three-year forecast period is still recommended as a general rule, but with the added flexibility to include additional years or to allow for the cycles selected. - Population
Equal status is given to population-based and claims data-based approaches (Figure 1), with the preferred approach to be determined based on the available data and market specifics. Clearer guidance is provided on how to select the best approach.
It was noted that market growth in the claims data-based approach should be based on the forecasted growth of both claims and claimants, when data on the unique number of claimants is available.
Figure 1. Population-based and claims data-based models
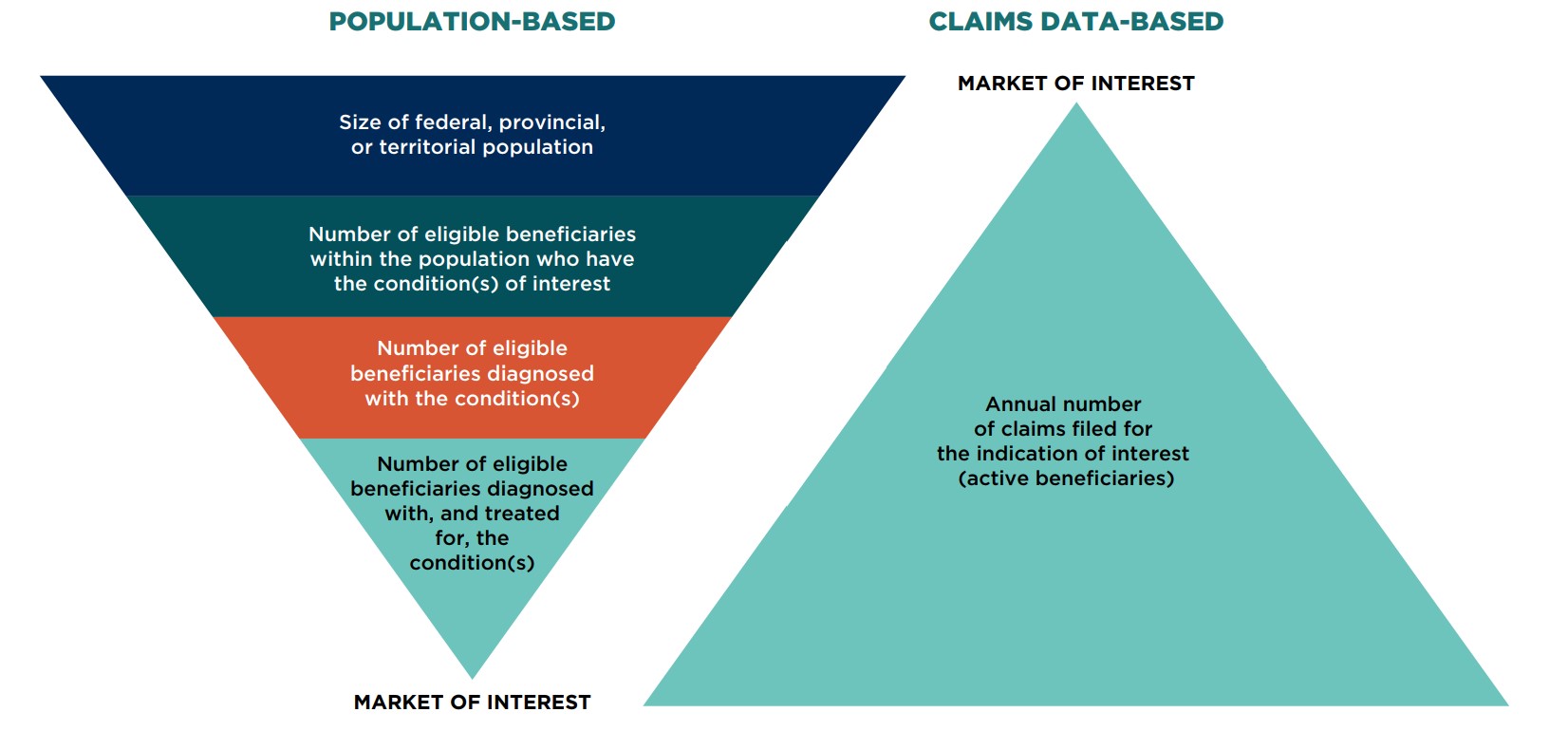
Figure description
Two tiered, pyramid-shaped diagrams demonstrate the process for estimating the market size using a population-based model and a claims data-based model.
Population-based model
The market of interest is determined by first assessing the size of the federal, provincial, or territorial population. Within that population, the scope is narrowed to the number of eligible beneficiaries who have the condition(s) of interest. The number of eligible beneficiaries diagnosed with the condition(s) must then be determined. Finally, from among that group, the market of interest is composed of the number of eligible beneficiaries diagnosed with, and treated for, the condition(s).
Claims data-based model
Using claims data, the market of interest is defined by identifying the annual number of claims filed for the indication of interest (active beneficiaries).
- Drug costs
Although premiums and deductibles are typically not factored into the calculation of costs, they should be considered if required by the drug plan (e.g., for income-based plans with universal coverage). - Characterizing uncertainty
Both deterministic sensitivity and scenario analyses should be provided with the submitted BIA to inform decision makers of the sensitivity of the model to specific assumptions. Probabilistic sensitivity analyses (PSA) may be considered but are not required. Values used in sensitivity and scenario analyses should be supported by citable data sources.
Input and Data Sources
- Selecting relevant comparators
More clarity was added to the approach for considering relevant comparators:- Non-drug alternatives are excluded from the base-case analysis but should be mentioned in the report and can be included in the scenario analysis if they are expected to have a significant impact.
- Non-benefit comparators can be added in the base-case or scenario analysis depending on the likelihood that they will be added to the formulary.
- Anticipated generic entries can be considered in the scenario analysis.
- Estimating drug cost - estimating therapeutic equivalencies
Drug wastage and sharing of units should already be accounted for in the drug cost and do not require additional adjustments. If required, these can be addressed in a scenario analysis. - Estimation of the current size of the market - determining the market size: claims data-based model
Claims-based models should consider any listing restrictions for the new drug, as well as for its competitors.
It is emphasized that internal manufacturer projections without validation and citable documentation cannot be considered as a reliable data source.
Reporting Format
- Report contents
Aggregated and disaggregated budget impact results should be reported for each year.
Conclusion
The enhanced methodology of the PMPRB’s 2020 BIA Guidelines, as well as the increased transparency and consistency afforded by these revisions, will ensure that they continue to be a reliable and comprehensive reference for conducting BIAs in Canada.
The full publication is available on the PMPRB website. A sample BIA Excel model can be obtained directly from the PMPRB at pmprb.npduis-sniump.cepmb@pmprb-cepmb.gc.ca.
About PMPRB - NPDUIS
The National Prescription Drug Utilization Information System (NPDUIS) is a research initiative that operates independently of the regulatory activities of the PMPRB. It publishes a variety of studies that provide decision makers with critical information and intelligence on price, utilization, and cost trends so that Canada’s healthcare system has more comprehensive and accurate information on how medicines are used and on sources of cost pressures.
Reference
- Marshall DA, Douglas PR, Drummond MF, et al. 2008. Guidelines for conducting pharmaceutical budget impact analyses for submission to public drug plans in Canada. Pharmacoeconomics. 26(6):477-95.
- Foroutan N, Tarride JE, Xie F, Levine M. 2018. A methodological review of national and transnational pharmaceutical budget impact analysis guidelines for new drug submissions. Clinicoecon Outcomes Res. 10:821-854. DOI: 10.2147/CEOR.S178825
- Australian Department of Health. 2016. Guidelines for preparing a submission to the Pharmaceutical Benefits Advisory Committee. Version 5.0. Canberra: Commonwealth of Australia. Available: https://pbac.pbs.gov.au/
- NICE. 2020. Resource impact of NICE guidance. London: National Institute for Health and Care Excellence. Available: https://www.nice.org.uk/about/what-we-do/into-practice/resource-impact-assessment [accessed Jan. 27, 2018]
- Neyt M, Cleemput I, Sande SV, Thiry N. 2015. Belgian guidelines for budget impact analyses. Acta Clin Belg. 70(3):175-180. DOI: 10.1179/2295333714Y.0000000118
- HIQA. 2018. Guidelines for the budget impact analysis of health technologies in Ireland. Cork: Health Information and Quality Authority. Available: https://www.hiqa.ie/sites/default/files/2018-01/HIQA_BIA_Guidelines_2018_0.pdf
- Ghabri S, Autin E, Poullié AI, Josselin JM. 2018. The French National Authority for Health (HAS) Guidelines for Conducting Budget Impact Analyses (BIA). Pharmacoeconomics. 36(4):407-417. DOI: 10.1007/s40273-017-0602-5
- The Agency for Health Technology Assessment and Tariff System [Polish]. 2016. Health Technology Assessment Guidelines. Available: https://www.aotm.gov.pl/www/wp-content/uploads/wytyczne_hta/2016/20161104_HTA_Guidelines_AOTMiT.pdf
- Zorginstituut Nederland. 2016. Richtlijn voor het uitvoeren van economische evaluaties in de gezondheidszorg [Guideline for economic evaluations in healthcare]. Diemen: Zorginstituut Nederland. Available: https://www.zorginstituutnederland.nl/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg (in Dutch only)
- Sullivan SD, Mauskoph JA, Augustovski, et al. 2014. Budget impact analysis—Principles of good practice: Report of the ISPOR 2012 Budget Impact Analysis Good Practice II Task Force. Value Health. 17(1):5-14.
- Alberta Health. 2018. Budget impact assessment for the Alberta Drug Benefit List. Version 9. Edmonton: Government of Alberta. Available: https://www.ab.bluecross.ca/dbl/pdfs/bia-form.docx
- Ontario Ministry of Health and Long-Term Care. 2016. Ontario Guidelines for Drug Submission and Evaluation. Toronto: Government of Ontario. Available: http://www.health.gov.on.ca/en/pro/programs/drugs/drug_submissions/guideline_templates.aspx
- Manitoba Health, Seniors and Active Living. 2017. Manitoba Drug Benefits and Interchangeability Formulary. Available: https://www.gov.mb.ca/health/mdbif/sub.html
- INESSS. 2018. Guidance Document for Submitting a Request to INESSS. Québec: l’Institut national d’excellence en santé et en services sociaux. Available: https://www.inesss.qc.ca/en/activities/drug-products/manufacturer-information-centre/registration-application.html
- CADTH. 2020. Procedures for the CADTH pan-Canadian Oncology Drug Review. Ottawa: Canadian Agency for Drugs and Technologies in Health. Feb. 2020. Available: https://www.cadth.ca/sites/default/files/pcodr/pCODR%27s%20Drug%20Review%20Process/pcodr-procedures.pdf