Meds Pipeline Monitor 2022
Contact Information
Patented Medicine Prices Review Board
Standard Life Centre
Box L40
333 Laurier Avenue West
Suite 1400
Ottawa, ON K1P 1C1
Tel.: 1-877-861-2350
TTY 613-288-9654
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Acknowledgements
This report was prepared by the Patented Medicine Prices Review Board (PMPRB) as part of the National Prescription Drug Utilization Information System (NPDUIS) initiative.
The PMPRB wishes to acknowledge the members of the NPDUIS Advisory Committee for their expert oversight and guidance in the preparation of this report. Please note that the statements and findings for this report do not necessarily reflect those of the members or their organizations.
We gratefully acknowledge Patricia Carruthers-Czyzewski, BScPhm, MSc, Sintera Inc. for providing pharmaceutical expertise and for her contribution to the scientific analysis.
Appreciation goes to Allison Carey for leading this project, and Tanya Potashnik and Kevin Pothier for their oversight in the development of the report. The PMPRB also wishes to acknowledge the contribution of the editorial staff, Shirin Paynter.
Disclaimer
NPDUIS operates independently of the regulatory activities of the Board of the PMPRB. The research priorities, data, statements, and opinions expressed or reflected in NPDUIS reports do not represent the position of the PMPRB with respect to any regulatory matter. NPDUIS reports do not contain information that is confidential or privileged under sections 87 and 88 of the Patent Act, and the mention of a medicine in an NPDUIS report is not and should not be understood as an admission or denial that the medicine is subject to filings under sections 80, 81, or 82 of the Patent Act or that its price is or is not excessive under section 85 of the Patent Act.
Although this information is based in part on data obtained under license from GlobalData and the MIDAS® Database proprietary to IQVIA Solutions Canada Inc. and/or its affiliates (“IQVIA”), the statements, findings, conclusions, views, and opinions expressed in this report are exclusively those of the PMPRB and are not attributable to either GlobalData or IQVIA.
Suggested Citation
Patented Medicine Prices Review Board. (2023). Meds Pipeline Monitor, 2022. Ottawa: PMPRB.
Executive Summary
Meds Pipeline Monitor (MPM) is a horizon scanning report that features a selection of new medicines undergoing clinical evaluation or in pre-registration that may have an impact on future clinical practice and drug spending in Canada.
This edition expands the review for the selected medicine candidates in Phase III clinical trials or pre-registration to include information on other drugs in Phase II that share the same mechanism of action or indication. Having insight into other drugs under investigation (i.e., in Phase II) may provide additional information on the potential place in therapy for these pipeline candidates. Medicines in Phase III clinical trials or pre-registration are selected as candidates for the ‘new medicines’ list if they have the potential to address an unmet therapeutic need, offer a novel mechanism of action or therapeutic benefit over existing therapies, or treat a serious condition. The medicines in Phase II are also examined to identify other drugs that are in earlier phases of the pipeline that contain the same indication or mechanism of action as the selected medicine candidates.
The report collects data from two main sources: GlobalData’s Healthcare database, which identifies medicines currently undergoing clinical evaluation, and Health Canada’s Drug and Health Product Submissions Under Review Lists, which provide information on new medicines under review in Canada.
Highlights of the Meds Pipeline Monitor 2022
- In 2022, the pipeline contained over 9,000 new medicines in various stages of clinical development, compared to just under 8,500 the year before. The number of drugs in the pipeline is increasing by an average of 11% per year since 2018.
- Oncology continues to dominate the therapeutic mix in 2022, with cancer treatments representing almost one third (30%) of medicines in all phases of clinical trials. Treatments for infectious diseases held the second largest share of the pipeline, at 15%, due to the increased number of medicines for the treatment of COVID-19.
- Nearly one third (31%) of medicines in Phase III clinical trials or pre-registration had an early orphan designation approved through the US FDA or the EMA, which is consistent with the increasing trend in the prevalence of orphan-designated medicines entering the pharmaceutical market.
- Trends in the 2022 pipeline include a growing number of novel gene and cell therapies that are expanding to larger patient groups (e.g., Duchenne muscular dystrophy). The biosimilars pipeline is also expanding to therapeutic areas including asthma (omalizumab), bone health (denosumab), and myocardial infarction (tenecteplase).
- Twenty-eight new medicines including five new gene and cell therapies were selected for the 2022 MPM new medicines list based on their potential to impact the Canadian healthcare system. Nine of the medicines listed in this year’s report have forecasted global annual revenues of over US $1 billion by 2028, one of which was approved by Health Canada in February 2023.
- Of the 42 new and retained medicines listed in the previous edition (MPM 2021), six received market authorization, 25 were retained on this year’s list as they continued to satisfy the selection criteria, and 11 were removed as their clinical trials were discontinued or they no longer meet the selection criteria.
- As of September 2022, 550 vaccines and therapies were undergoing clinical evaluation globally for the prevention and treatment of COVID-19. Health Canada is reviewing 14 new and supplemental drug submissions for the prevention and treatment of COVID-19.
List of Terms
For the purpose of this report, the following terms and associated definitions apply.
- Cell therapy
- The transplantation of human cells to replace or repair damaged tissue and/or cells.
- Clinical efficacy
- The maximum response achievable from a medicine in research settings and the capacity for sufficient therapeutic effect in clinical settings.Footnote i
- Gene therapy
- A technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene, as defined by Health Canada.Footnote ii
- Market authorization
- The process of approval for a medicine to be marketed in a given country. In Canada, market approval is granted following a substantive scientific evaluation of a product's safety, efficacy, and quality, as required by the Food and Drugs Act and Regulations.Footnote iii
- Medicinal ingredient
- A chemical or biological substance responsible for the claimed pharmacologic effect of a drug product. Sometimes referred to as a molecule, active substance, or active ingredient.Footnote iv
- Medicine
- A broad term encompassing both the final drug product and medicinal ingredient(s); this encompasses chemically manufactured active substances and biologics, including gene therapies. Medicines are reported at the medicinal ingredient level and can refer to a single ingredient or a unique combination of ingredients.
- Patent evergreening
- The acquisition of additional patents for minor modifications to an existing pharmaceutical product in order to extend the patent life of the medicine (e.g., new delivery systems, new dosages, new uses, new combinations or new forms).Footnote v
- New medicine
- A medicinal ingredient that has not previously received market authorization by a regulator.Footnote iv
- Orphan medicine
- A medicine used to treat a rare disease. For the purposes of this study, orphan medicines are defined as having an orphan designation granted by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for the relevant indication.
- Phase I
- These trials test an experimental medicine on a small group of people for the first time. The purpose is to look at the medicine's safety, determine a safe dosage range, and monitor if there are any side effects.
- Phase II
- In this phase, the medicine is given to a larger group of people (usually 100 or more) to gather data on how well the medicine works to treat a disease or condition, check its safety on a wider range of people, and determine the best dose.Footnote vi
- Phase III
-
These controlled or uncontrolled trials are conducted after preliminary evidence suggesting efficacy of the medicine has been demonstrated. They are intended to gather additional and confirmatory information about the clinical efficacy and safety of the medicine under the proposed conditions of use.Footnote ii Phase III trials are usually randomized with double-blind testing in several hundred to several thousand patients.
- Pre-registration
- A medicine is in the pre-registration phase once all the necessary clinical trials have been completed and it is waiting for registration or approval for use by a governing body.Footnote vi
Phases of clinical trials
Introduction
This edition of the Meds Pipeline Monitor (MPM) features a selection of new medicines in Phase III clinical trials or pre-registration that have the potential to impact clinical practice and drug spending in Canada.
The methodology, which is detailed in the next section, uses a specific set of criteria to identify a list of new medicines in the pipeline from the GlobalData Healthcare database, as well as a list of medicines currently under review from Health Canada’s Drug and Health Product Submissions Under Review (SUR) Lists. The new medicines listed in this report are selected based on a scientific review of the literature and clinical trial outcomes to determine if the medicine may impact the Canadian healthcare system by: addressing an unmet therapeutic need; offering a novel mechanism of action or therapeutic benefit over existing therapies; or treating a serious condition. Medicines reported in previous editions of the MPM are also reviewed and updated in this report. This report also provides an update on the medicines in last year’s edition that have since received market authorization by either the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), or Health Canada. Likewise, the new medicines featured in this report will be monitored in future editions of the MPMto identify candidates that successfully enter the market.
To provide context for the selection of medicines, the MPM includes a snapshot of the number of drugs in each clinical phase of the pipeline year over year (2018-2022), and a breakdown of the various therapeutic areas for each phase of clinical development. This edition of the report also highlights select vaccines and other medicines undergoing clinical trials for the prevention and treatment of COVID-19, in global markets as well as in Canada. The medicines assessed for this portion of the analysis include new therapies as well as previously marketed treatments for other indications that have been repurposed for the treatment of COVID-19.
Meds Pipeline Monitor is a companion publication to Meds Entry Watch, which analyzes the market launch patterns of newly approved medicines in Canada and internationally. Together, these two PMPRB reports monitor the market continuum of late-stage pipeline medicines and new approvals, providing decision makers, researchers, patients, clinicians, and other stakeholders with information on the emerging medicines and evolving cost pressures.
Methodology
Snapshot of the Pipeline
The snapshot of the 2022 pipeline identifies the composition of medicines in various phases of clinical development. For the purpose of this analysis, a full list of pipeline medicines was retrieved from GlobalData’s Healthcare database in September 2022 and the selected medicine candidates for this year’s report have been validated as of March 30, 2023.
New medicinal ingredients are identified as those with no prior approvals through the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), or Health Canada. The distribution of new medicines by therapeutic area corresponds to the indication under evaluation, as reported by GlobalData. Note that a single new medicine may be undergoing multiple clinical studies for separate indications.
Meds Pipeline Monitor
The MPM selects new medicines in Phase III clinical trials or pre-registration in Canada, the United States, and Europe. Many of the pipeline candidates are first-in-class or represent novel mechanisms for treatment in a specific therapeutic area. For this reason, this report includes additional review on other drugs undergoing Phase II clinical evaluation that share the same indication or mechanism of action. Pipeline medicines are selected for inclusion using a two-stage process (Figure 1). The initial screening stage selects medicines in the late phases of clinical evaluation, while the analytic review stage involves a more rigorous appraisal of each potential candidate to identify medicines that may have a significant clinical and budgetary impact.

* In pre-registration with the US Food and Drug Administration (FDA).
† Has Phase III clinical trials in Canada, the United States, or geographic Europe (excluding Russia and Turkey).
Figure description
This is a flowchart describing the process used to select the listed medicines. The chart consists of two steps:
1. Initial Screening
This step begins with all medicines in Phase III clinical trials or pre-registration with the US Food and Drug Administration. Of these medicines, the next step includes only those with expected clinical trial end dates within three years of the analysis and drug geography including Canada, the US, and Europe. To qualify for the drug geography, a medicine must have Phase III clinical trials in Canada, the US, and/or geographic Europe (excluding Russia and Turkey).
2. Analytic Review
The analytic review step of the process is divided into two parts: one path for new medicines and the other for gene therapies.
New medicines must meet at least one of the following requirements to be included in the list:
- Demonstrates improved safety and efficacy
- Novel mechanism and/or first-in-class, with the addition of one or more of Breakthrough, Fast Track, and Priority Review designations
Gene therapies must demonstrate clinical effectiveness with an acceptable safety profile to be included in the list.
Stage 1. Initial screening
GlobalData’s Healthcare database is used to identify a list of medicines undergoing Phase III clinical trials or in pre-registration. These medicines serve as the basis for the initial screening stage.
The drug geography, defined as the geographical region or country in which the medicine is either marketed or in pipeline development, is restricted to Canada and other countries with similar regulatory and approval processes: the US and geographic Europe (excluding Russia and Turkey). Only new medicinal ingredients that have adequate data that supports increased efficacy and safety from clinical trials are considered as candidates for inclusion.
Medicines approved or sold in Canada, the US, or Europe for any other indication or in any other strength or formulation are excluded during the selection process, as are medicines whose clinical trials are inactive, suspended, withdrawn, or terminated.
Stage 2: Analytic screening
Selection criteria
Following the initial screening, the second stage of the process considers a number of selection criteria to determine the final list of pipeline candidates. These criteria are detailed in Table 1.
Earlier phases of the pipeline (i.e., Phase II) are also examined to determine if there are other medicines with the same indication or mechanism of action as the selected candidates in Phase III and pre-registration. This provides additional information on the number of novel, first-in-class medicines that are undergoing clinical evaluation in Phase II that may influence the therapeutic significance of the selected candidates in Phase III and pre-registration.
Table 1. Selection criteria for the MPM
| Selection criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
Novel mechanism / First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class In addition, the medicine must fall into one or more of the three following FDA designations for expedited development and review:
|
Additional descriptive information
A profile of each successful pipeline candidate is provided, including the indication and mechanism of action, as well as a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified. Table 2 provides a detailed description of these key attributes.
Table 2. Key attributes of new medicines selected for the MPM
| Attribute | Relevance | Data sources |
|---|---|---|
| Clinical trials in Canada | Medicines tested in Canada are likely to be of interest to Canadians |
GlobalData Healthcare; Health Canada Clinical Trials Database; Health Canada Drug and Health Product Submissions Under Review; National Institutes of Health (NIH) Clinical Trial Registry |
| Rare or orphan designation | Medicines used to treat rare diseases or conditions that generally have high treatment costs and may result in substantial spending | GlobalData Healthcare |
| Biologic medicine | These complex molecules produced by living organisms are expected to have high costs, resulting in substantial spending | |
| Add-on therapy | Medicines designed to be used in conjunction with existing medicines may increase the treatment cost and contribute to higher spending | |
| Potential evergreening | Modified forms of the same product in order to extend the patent life. (e.g., new delivery systems, new dosages, new uses, new combinations or new forms) |
The profile also provides details of potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
The indications and therapeutic areas of the featured medicines correspond to their Phase III clinical trial or pre-registration stage. A single clinical trial may assess multiple indications within the same therapeutic area. These medicines may also have additional indications at various phases of clinical evaluation that are not mentioned in this report. The scientific description and key attributes provided are focused on the specified indication(s) for the selected medicines.
Medicines reported for a given year are reassessed for each following edition of the MPM. They may be retained on the MPM list if they continue to meet the selection criteria. Medicines for which clinical trials have been discontinued or for which the selection criteria is no longer met are not reported in subsequent editions.
Spotlight on Canada
Health Canada’s Drug and Health Product Submissions Under Review (SUR) Lists are assessed using a modified approach to the selection criteria to establish a list of medicines that may have the potential to impact Canadian drug spending or clinical practice.
Medicines listed in the SUR include new drug submissions containing medicinal ingredients that have not been approved in Canada for any indication, in any strength or form. Unlike the selection of medicines identified in the pipeline lists, these medicines may have previously received market authorization through the US FDA or the EMA.
Selection Criteria
Following this initial screening, the medicine must demonstrate at least one of three selection criteria to qualify for inclusion in the report. These criteria are listed in Table 3.
Table 3. Selection criteria for the list of medicines currently under review by Health Canada
| Selection Criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
| Novel mechanism or First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class |
| Gene or cell therapy: a technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene; or the transplantation of human cells to replace or repair damaged tissue and/or cells |
Additional descriptive information
The profile of each medicine under review includes the key attributes listed in Table 2, as well as the indication and mechanism of action, and a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified, as well as potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
Although FDA designations for expedited development or review are not a selection criteria for this list, relevant Breakthrough, Fast Track, and Priority Review designations are indicated where available. For a description of these designations, see Table 1.
Indications and therapeutic areas correspond to the information provided by GlobalData. The scientific description and key attributes provided are focused on the specified indication(s) for the selected medicine. For medicines under review for multiple indications, the primary indication is used.
Emerging COVID-19 Therapies
Vaccines and medicines under development globally with an indication for COVID-19 were extracted for this section of the report, based on a development stage of Phase I, II, and III clinical trials or pre-registration. All such medicines were assessed for this analysis, both new and existing. New medicines were identified as those that have not yet been marketed for any indication, while existing medicines include previously marketed therapies undergoing evaluation for new indications related to the prevention or treatment of COVID-19.
This section also highlights the COVID-19 medicines that have been approved as well as the medicines that are currently undergoing an expedited review process with Health Canada.
Data Sources
The GlobalData Healthcare database is the primary data source for the identification of pipeline medicines and their corresponding clinical information. GlobalData Healthcare tracks medicines from pre-clinical discovery, through clinical trials, to market launch and subsequent sales. The database is a comprehensive resource of medicines under various stages of clinical development. Search capabilities allow for controlled selection of specific attributes, including but not limited to the following: phase of clinical development, therapeutic area, molecule type, indication, drug geography, mechanism of action, and regulatory designations.
Health Canada’s Drug and Health Product Submissions Under Review (SUR) Lists are used to determine the featured selection of new medicines currently undergoing review by Health Canada. The SUR is a publicly available set of lists that identify pharmaceutical and biologic drug submissions containing new medicinal ingredients not previously approved in Canada that have been accepted for review. This applies to submissions accepted on or after April 1, 2015.
As this selection is restricted to new medicines, additional sources of information are cross-referenced to confirm that the candidates have not previously been approved or sold. These include recorded sales data from the IQVIA MIDAS® Database (all rights reserved); regulatory approval records from the National Institutes of Health (NIH), US FDA, the EMA, and Health Canada; and information in Health Canada’s Clinical Trials database and ClinicalTrials.org.
Limitations
This analysis captures a snapshot of the pipeline over a specific time period. Although it is assumed to be representative of the composition of medicines over the entire year, the pipeline is fairly dynamic and the share of medicines in any particular therapeutic area will vary.
This assessment is restricted to medicines under development for market in Canada and other countries with similar regulatory and approval processes: the US and Europe (excluding Russia and Turkey). Medicines that have not yet received market authorization in these countries were considered as potential pipeline candidates, even if they have been approved elsewhere in the world.
Some of the selected medicines may be undergoing clinical trials for additional indications; this analysis only reports on indications in the late stages of development—that is, in Phase III clinical trials or pre-registration with the US FDA—that satisfy the selection criteria set out in the methodology.
For each selected pipeline medicine, the primary manufacturer(s) and trade name, if available, are given along with the indication. In some cases, additional manufacturers, including subsidiaries, may also be involved in the development of the medicine with the primary companies, or other manufacturers may be developing the same medicine for other indications.
Although this report attempts to identify the most important pipeline medicines, the selection is not exhaustive and some medicines that are not included in this selection may have a significant impact on future clinical practice and drug spending in Canada.
Unless otherwise specified, the featured lists capture the composition of the pipeline as of September 2022 and are validated as of the end of March 2023. Due to the unpredictability and fast-moving nature of pipeline medicines entering the market, some of the medicines listed in this edition may have been approved or marketed in Canada, the US, or Europe following this date. Pipeline medicines that have not been included in this report due to the timing of the selection may presently meet the selection criteria; these, along with the rest of the drug pipeline, will be considered for the next edition of the report.
Snapshot of the 2022 Pipeline
The number of new pharmaceutical developments in the pipeline is increasing year over year. In 2022, over 9,000 new medicines were undergoing clinical evaluation, which has been increasing by an average of 11% per year since 2018.
Figure 2 provides a snapshot of the pipeline including the number of new medicinal ingredients in each phase of clinical development over the last 5 years.
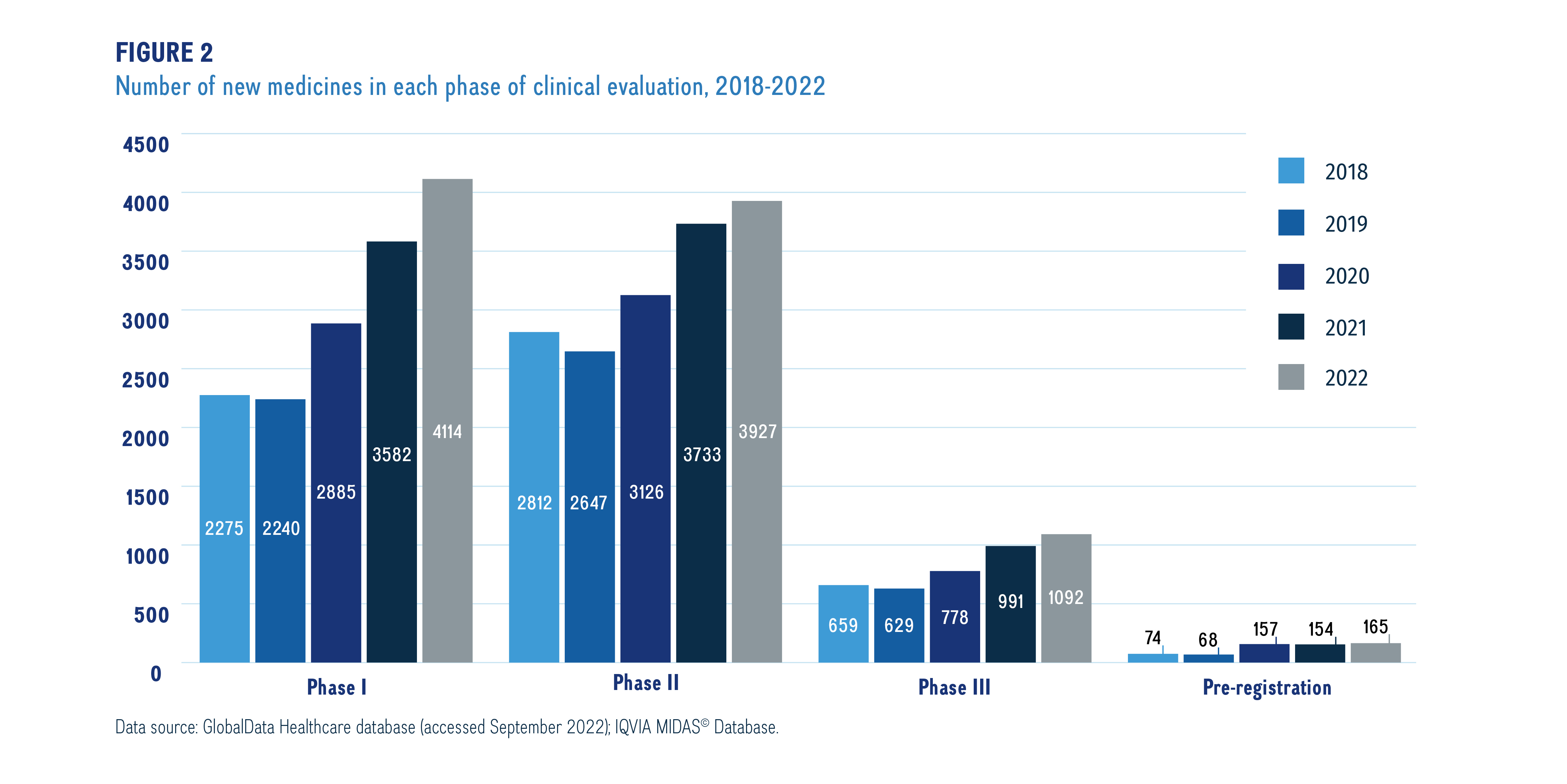
Figure description
This bar graph illustrates a snapshot of the total number of new medicines in each phase of the pipeline by their highest phase of development from 2018 to 2022. Totals are given for each year and phase.
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
2018 |
2,275 |
2,812 |
659 |
74 |
2019 |
2,240 |
2,647 |
629 |
68 |
2020 |
2,885 |
3,126 |
778 |
157 |
2021 |
3,582 |
3,733 |
991 |
154 |
2022 |
4,114 |
3,927 |
1,092 |
165 |
Data source: GlobalData Healthcare database (accessed September 2022); IQVIA MIDAS© Database.
Figure 3a illustrates the distribution of new medicines by therapeutic area from Phase I through to pre-registration. Although the findings show that pipeline medicines represented a wide range of therapeutic areas in 2022, cancer treatments dominated the therapeutic mix in each phase of the pipeline, accounting for nearly one third (30%) of medicines in all phases of clinical evaluation. Other important pipeline therapies include those for infectious diseases (such as COVID-19) and central nervous system therapies.
Figure 3b illustrates the top indications and number of medicines undergoing Phase II, Phase III or pre-registration in the major therapeutic areas in the pipeline in 2022
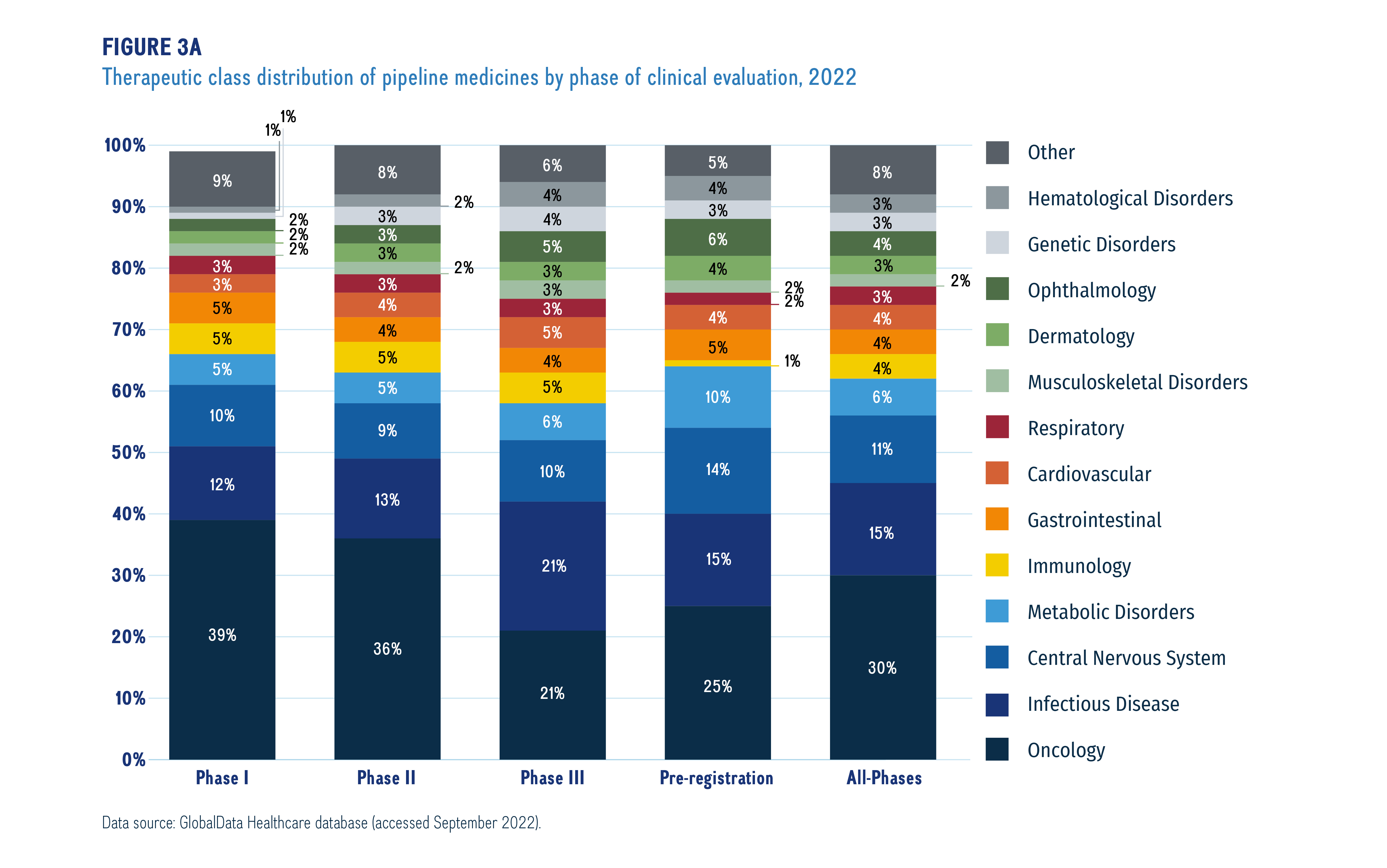
Figure description
A stacked bar graph gives the distribution of new medicines by therapeutic rea from Phase I through to pre-registration.
| Therapeutic Area | Phase I | Phase II | Phase III | Pre-registration | All Phases |
|---|---|---|---|---|---|
Oncology |
39% |
36% |
21% |
25% |
30% |
Infectious disease |
12% |
13% |
21% |
15% |
15% |
Central Nervous System |
10% |
9% |
10% |
14% |
11% |
Metabolic disorders |
5% |
5% |
6% |
10% |
6% |
Immunology |
5% |
5% |
5% |
1% |
4% |
Gastrointestinal |
5% |
4% |
4% |
5% |
4% |
Cardiovascular |
3% |
4% |
5% |
4% |
4% |
Respiratory |
3% |
3% |
3% |
2% |
3% |
Musculoskeletal disorders |
2% |
2% |
3% |
2% |
2% |
Dermatology |
2% |
3% |
3% |
4% |
3% |
Ophthalmology |
2% |
3% |
5% |
6% |
4% |
Genetic disorders |
1% |
3% |
4% |
3% |
3% |
Hematological disorders |
1% |
2% |
4% |
4% |
3% |
Other |
9% |
8% |
6% |
5% |
8% |
Data source: GlobalData Healthcare database (accessed September 2022).
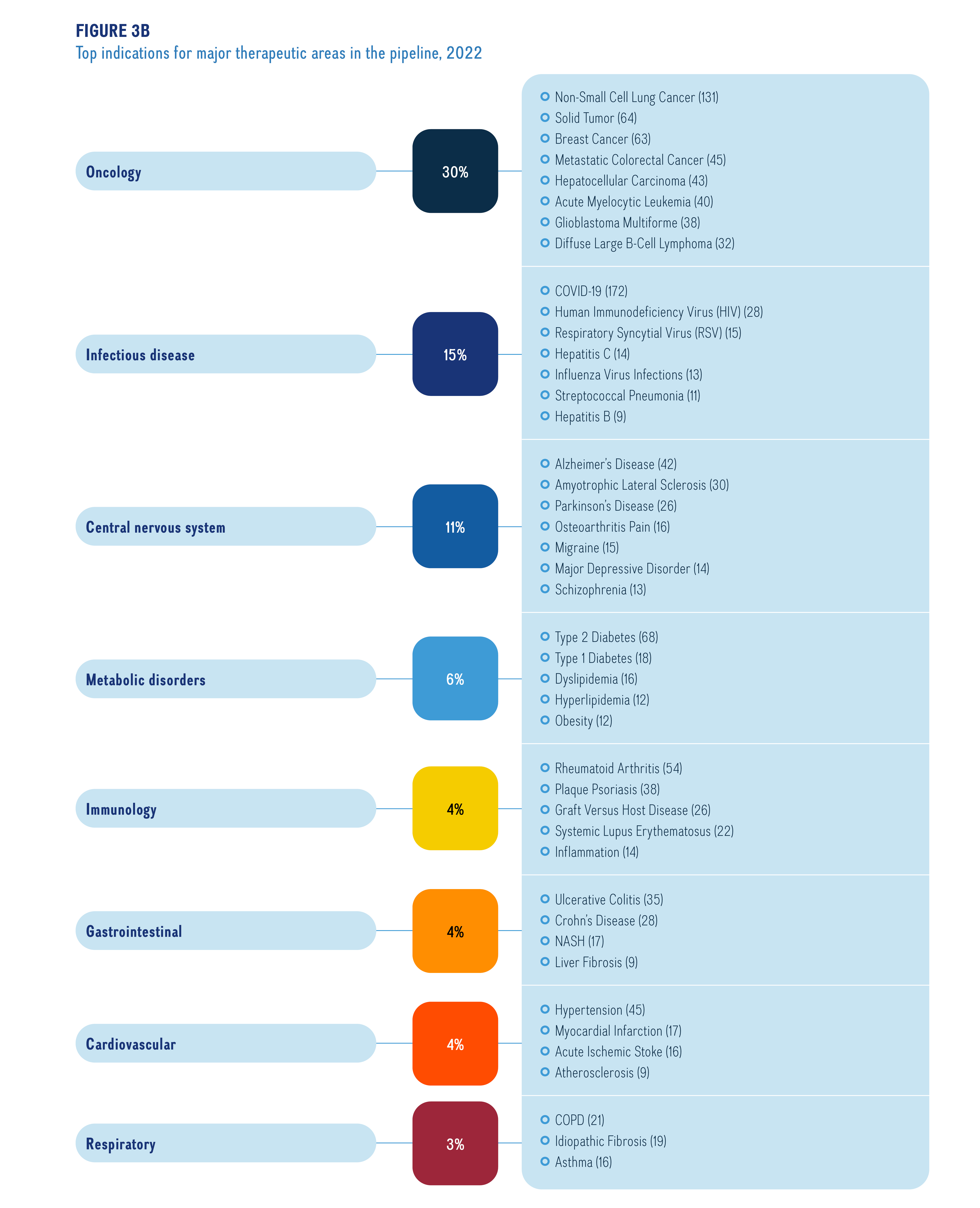
Figure description
A horizontal bar graph indicates the top indications by number of medicines in all phases of the pipeline.
| Therapeutic Area | All Phases | |
|---|---|---|
Oncology |
30% |
Non-Small Cell Lung Cancer (131) Solid Tumor (64) Breast Cancer (63) Metastatic Colorectal Cancer (45) Hepatocellular Carcinoma (43) Acute Myelocytic Leukemia (40) Glioblastoma Multiforme (38) Diffuse Large B-Cell Lymphoma (32) |
Infectious disease |
15% |
COVID-19 (172) Human Immunodeficiency Virus (HIV) (28) Respiratory Syncytial Virus (RSV) (15) Hepatitis C (14) Influenza Virus Infections (13) Streptococcal Pneumonia (11) Hepatitis B (9) |
Central Nervous System |
11% |
Alzheimer’s Disease (42) Amyotrophic Lateral Sclerosis (30) Parkinson’s Disease (26) Osteoarthritis Pain (16) Migraine (15) Major Depressive Disorder (14) Schizophrenia (13) |
Metabolic disorders |
6% |
Type 2 Diabetes (68) Type 1 Diabetes (18) Dyslipidemia (16) Hyperlipidemia (12) Obesity (12) |
Immunology |
4% |
Rheumatoid Arthritis (54) Plaque Psoriasis (38) Graft Versus Host Disease (26) Systemic Lupus Erythematosus (22) Inflammation (14) |
Gastrointestinal |
4% |
Ulcerative Colitis (35) Crohn’s Disease (28) NASH (17) Liver Fibrosis (9) |
Cardiovascular |
4% |
Hypertension (45) Myocardial Infarction (17) Acute Ischemic Stoke (16) Atherosclerosis (9) |
Respiratory |
3% |
COPD (21) Idiopathic Fibrosis (19) Asthma (16) |
Orphan medicines, as designated by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA), accounted for a notable proportion of medicines in the 2022 pipeline. Figure 4 provides the shares of orphan designated medicines for all phases in the pipeline from 2020-2022. Orphan designated medicines make up a greater share in the later stages of the pipeline, increasing from 7% in Phase I to 31% in pre-registration in 2022. This has been a consistent trend since 2020.
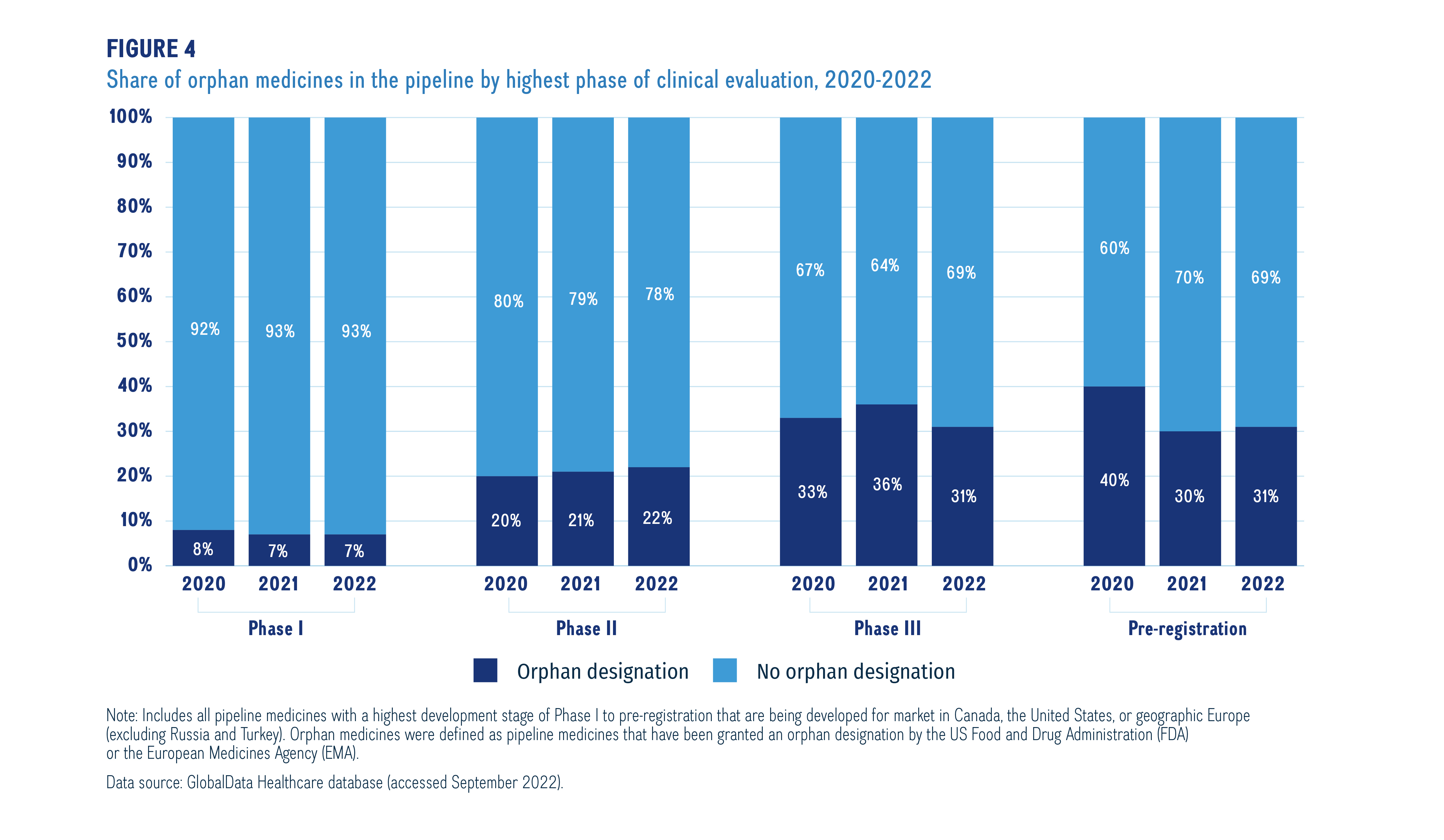
Figure description
A stacked bar graph gives the proportion of orphan designated medicines to non-orphan designated medicines in the pipeline by phase of development from 2020 to 2022.
| Phase of Development | 2020 | 2021 | 2022 | |
|---|---|---|---|---|
Orphan designation |
Phase I |
8% |
7% |
7% |
Phase II |
20% |
21% |
22% |
|
Phase III |
33% |
36% |
31% |
|
Pre-registration |
40% |
30% |
31% |
|
No Orphan designation |
Phase I |
92% |
93% |
93% |
Phase II |
80% |
79% |
78% |
|
Phase III |
67% |
64% |
69% |
|
Pre-registration |
60% |
70% |
69% |
Note: Includes all pipeline medicines with a highest development stage of Phase I to pre-registration that are being developed for market in Canada, the United States, or geographic Europe (excluding Russia and Turkey). Orphan medicines were defined as pipeline medicines that have been granted an orphan designation by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
Data source: GlobalData Healthcare database (accessed September 2022).
Trend Watch 2022
The drug development landscape has been evolving over the last few years, from an earlier emphasis on blockbuster drugs developed in-house by large pharmaceutical companies, to niche markets and “personalized medicine”Footnote viii including various orphan drugs for rare disease, expanding therapeutic areas in gene and cell therapies, and the growing biosimilars pipeline.
Orphan drug approvals
Approvals for specialty medicines continue to increase, with orphan designated medicines accounting for 58% (29) of approved medicines in 2020 in Canada.Footnote ix This upward trend is ongoing: nearly half (49%) of the novel specialty medicines in the pipeline are indicated for orphan conditions. When including orphan cancer drugs, an estimated 80% of specialty drug development in 2022 was for orphan conditions.Footnote xi Aside from cancer drugs, another leading class of pipeline specialty drugs are novel anti-inflammatory therapeutics, including tumor necrosis factor inhibitors and targeted synthetic disease-modifying antirheumatic drugs (DMARDs).Footnote xi
Gene and cell therapies
The gene and cell therapy pipeline is growing rapidly, with more than 600 gene and cell therapies in various phases of development in 2022.Footnote x According to market analysis forecasts from GlobalData, gene therapy will be a $25 billion per year market by 2034.Footnote xi Among the many therapies undergoing clinical evaluation are novel therapies for Duchenne muscular dystrophy (6 drugs in Phase III and one in pre-registration), epidermolysis bullosa (3 drugs in preclinical evaluation, one in Phase II and one in pre-registration), and β thalassemia/sickle cell disease (10 drugs in preclinical evaluation and discovery).Footnote xii
Biosimilars pipeline
Biosimilars can provide patients and doctors with more affordable treatment options, which have the potential to provide savings and contribute to drug coverage sustainability. As of March 2023, 51 biosimilars of 16 innovator reference products have been approved in Canada. By comparison, there have been 40 approvals in the United States and 69 biosimilars approved by the European Medicines Agency.Footnote xiii The global pipeline is growing with over 140 biosimilars in Phase III and pre-registration, including new therapeutic areas such as growth hormone, infertility, bone health, and immunosuppressants. As of March 2023, Health Canada is reviewing 8 biosimilars, two of which (eculizumab and ustekinumab) are the first biosimilar submissions for the reference product.
Meds Pipeline Monitor 2022
The following tables include: selected new medicine candidates for 2022 (Table 4), retained medicines from previous editions of the Meds Pipeline Monitor (Table 5), and medicines from previous editions that have gained market authorization (Table 6).
Medicines in Phase III clinical trials or pre-registration are considered as candidates for the Meds Pipeline Monitor (MPM) if they have the potential to impact future clinical practice and drug spending in Canada (e.g., address an unmet therapeutic need, offer a novel mechanism of action or therapeutic benefit over existing therapies, or treat a serious condition).
Screening new medicine candidates
Of the 1,257 pipeline medicines in Phase III and pre-registration in 2022, twenty-eight (28) new medicines were selected for inclusion in the new medicines list (Table 4). Many of the pipeline candidates are first-in-class or represent novel mechanisms for the treatment of specific therapeutic areas. Having insight into other drugs under clinical evaluation (i.e., in Phase II) may provide additional information on the potential place in therapy of these pipeline candidates. The medicines in Phase II were examined to identify other drugs in the pipeline that have the same indication or mechanism of action as those listed in the 2022 new medicines list. The description for each new medicine listed in the 2022 new medicines list includes a statement indicating if there are any other drugs in Phase II development with the same indication or mechanism of action. Appendix A (Table A3) provides some further insights into the other drugs identified in Phase II for the indications targeted by the pipeline candidates. It is important to keep in mind that not all drugs in Phase II development will progress to Phase III. According to an industry analysis, Phase II clinical programs experience the lowest success rate of the development phases, with only 30.7% of developmental candidates advancing to Phase III. 1
Of the new medicines featured in previous reports, 25 were retained as recent evidence continues to support promising clinical benefit and satisfies the selection criteria (Table 5). Six of the 2021 pipeline medicines have received market authorization in the US, Europe, or Canada as of March 30, 2023 (Table 6), while 11 were removed from the list as their clinical trials were discontinued or they no longer fulfill the selection criteria.
Screening biosimilars
Biosimilars differ from pipeline candidates in that their efficacy and safety is similar to originator biologics. However, their introduction can substantially impact drug spending in specific therapeutic areas. Of the 21 biosimilars identified in Phase III trials in 2022, 14 (67%) would be, if approved, the first biosimilars marketed for the originator biologic. Examples include: cetuximab (for specific cancers), denosumab (for post-menopausal osteoporosis), eculizumab (for paroxysmal nocturnal hemoglobinuria), ocrelizumab (for relapsing remitting multiple sclerosis), omalizumab (for urticaria), romiplostim (for idiopathic thrombocytopenic purpura), tenecteplase (for myocardial infarction), and ustekinumab (for plaque psoriasis).
The availability of these biosimilars could significantly impact costs in a wide range of therapeutic areas. Of note, as of March 30, 2023, a biosimilar for eculizumab and ustekinumab are under review by Health Canada.2 Appendix A (Table A1) provides a list of the identified biosimilars in Phase III clinical trials and indicates whether a biosimilar currently exists for the originator biologic.
Table 4. Selected new medicines for 2022
Cardiovascular
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Abelacimab Anthos Therapeutics Inc
|
Deep vein thrombosis (DVT); Pulmonary embolism; Atrial fibrillation
|
Clinical trials
Forecasted revenue
|
Aprocitentan Idorsia Pharmaceutical Ltd
|
Resistant hypertension
|
Clinical trials
Forecasted revenue
|
Etripamil Milestone Pharmaceuticals Inc Canada
|
Paroxysmal supraventricular tachycardia (PSVT)
|
Clinical trials
Forecasted revenue
|
Obicetrapib NewAmsterdam Pharma BV
|
Dyslipidemia; Heterozygous familial hypercholesterolemia (heFH); Atherosclerosis
|
Clinical trials
Forecasted revenue
|
Sotatercept Acceleron Pharma Inc
|
Pulmonary arterial hypertension (PAH) |
Clinical trials
Forecasted revenue
|
Central Nervous System
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Evobrutinib Merck KGaA
|
Relapsing multiple sclerosis (RMS); Secondary progressive multiple sclerosis (SPMS) |
Clinical trials
Forecasted revenue
|
Reldesemtiv Cytokinetics Inc
|
Amyotrophic lateral sclerosis (ALS) |
Clinical trials
Forecasted revenue
|
Soticlestat Takeda Pharmaceutical Co Ltd
|
Lennox-Gastaut syndrome; Dravet syndrome (severe myoclonic epilepsy of infancy) |
Clinical trials
Forecasted revenue
|
Ulotaront (SEP-363856) Sunovion Pharmaceuticals Inc
|
Schizophrenia |
Clinical trials
Forecasted revenue
|
Dermatology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Beremagene geperpavec Krystal Biotech Inc
|
Epidermolysis bullosa
|
Clinical trials
Forecasted revenue
|
Gastrointestinal Disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Resmetirom Madrigal Pharmaceuticals Inc
|
Non-alcoholic steatohepatitis (NASH); Non-alcoholic fatty liver disease (NAFLD) |
Clinical trials
Forecasted revenue
|
Seladelpar lysine CymaBay Therapeutics Inc
|
Primary biliary cholangitis (primary biliary cirrhosis) |
Clinical trials
Forecasted revenue
|
Genetic disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Delandistrogene moxeparvovec Sarepta Therapeutics Inc
|
Duchenne muscular dystrophy
|
Clinical trials
Forecasted revenue
|
REC-2282 Recursion Pharmaceuticals Inc
|
Neurofibromatosis type II (NF2)-mutated meningiomas |
Clinical trials
Forecasted revenue
|
Immunological disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Garadacimab CSL Ltd
|
Hereditary angioedema (HAE) (C1 esterase inhibitor [C1-INH] deficiency |
Clinical trials
Forecasted revenue
|
Omidubicel Gamida Cell Ltd
|
Hematopoietic stem cell transplantation |
Clinical trials
Forecasted revenue
|
Infectious Diseases
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Posoleucel AlloVir Inc
|
Herpesviridae infections |
Clinical trials
Forecasted revenue
|
Zoliflodacin Entasis Therapeutics Holdings Inc
|
Uncomplicated cervical and urethral gonorrhea |
Clinical trials
Forecasted revenue
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Insulin human, oral (ORMD-0801) Oramed Pharmaceuticals Inc
|
Type 2 Diabetes
|
Clinical trials
Forecasted revenue
|
Insulin icodec Novo Nordisk AS
|
Type 1 diabetes (juvenile diabetes); Type 2 diabetes
|
Clinical trials
Forecasted revenue
|
Oncology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Bemarituzumab Amgen Inc
|
Adenocarcinoma of the gastroesophageal junction; Gastric cancer; Bladder cancer; Gastroesophageal (GE) junction carcinomas |
Clinical trials
Forecasted revenue
|
Iberdomide hydrochloride Bristol-Myers Squibb Co
|
Refractory multiple myeloma; Relapsed multiple myeloma |
Clinical trials
Forecasted revenue
|
Imetelstat sodium Geron Corp
|
Myelodysplastic syndrome; Post-essential thrombocythemia myelofibrosis (post-ET MF); Post-polycythemia vera myelofibrosis (PPV-MF) |
Clinical trials
Forecasted revenue
|
Navitoclax dihydrochloride AbbVie Inc
|
Myelofibrosis |
Clinical trials
Forecasted revenue
|
Relacorilant Corcept Therapeutics Inc
|
Epithelial ovarian cancer |
Clinical trials
Forecasted revenue
|
Rusfertide acetate Protagonist Therapeutics Inc
|
Polycythemia vera (PV)
|
Clinical trials
Forecasted revenue
|
Zolbetuximab Astellas Pharma Inc
|
Adenocarcinoma of the gastroesophageal junction; Gastric cancer
|
Clinical trials
Forecasted revenue
|
Ophthalmology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Lenadogene nolparvovec GenSight Biologics SA
|
Leber’s hereditary optic neuropathy (Leber optic atrophy)
|
Clinical trials
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2022, and are given in US dollars.
Data source: GlobalData Healthcare database.
Table 5. Update on pipeline medicines retained from the 2021 Meds Pipeline Monitor
Cardiovascular
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Apabetalone Resverlogix Corp.
|
Coronary artery disease (CAD) (ischemic heart disease) |
Clinical trials
Forecasted revenue
|
CSL112 CSL Ltd
|
Acute coronary syndrome (ACS) |
Clinical trials
Forecasted revenue
|
Central Nervous System
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Latozinemab Alector Inc
|
Frontotemporal dementia (FTD) |
Clinical trials
Forecasted revenue
|
Valiltramiprosate Alzheon Inc
|
Alzheimer's disease (AD) |
Clinical trials
Forecasted revenue
|
Midomafetamine (MDMA) Multidisciplinary Association for Psychedelic Sudies
|
Post-traumatic stress disorder (PTSD) |
Clinical trials
Forecasted revenue
|
ND-0612 Mitsubishi Tanabe Pharma Corp
|
Parkinson's disease (PD) |
Clinical trials
Forecasted revenue
|
Gastrointestinal disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Brazikumab AstraZeneca Plc
|
Crohn's disease (regional enteritis) |
Clinical trials
Forecasted revenue
|
RBX-2660 Rebiotix Inc
|
Clostridium difficile infections (C. difficile associated disease) |
Clinical trials
Forecasted revenue
|
Genito urinary system and sex hormones
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Gepotidacin mesylate GlaxoSmithKline Plc
|
Cystitis; Urinary tract infections (UTI) |
Clinical trials
Forecasted revenue
|
Hematological disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Bentracimab PhaseBio Pharmaceuticals Inc
|
Bleeding and clotting disorders |
Clinical trials
Forecasted revenue
|
Danicopan Alexion Pharmaceuticals Inc
|
Paroxysmal nocturnal hemoglobinuria (PNH) |
Clinical trials
Forecasted revenue
|
Fidanacogene Pfizer Inc
|
Hemophilia B |
Clinical trials
Forecasted revenue
|
Fitusiran Sanofi
|
Hemophilia A; |
Clinical trials
Forecasted revenue
|
Hormonal disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Palopegteriparatide (TransCon PTH) Ascendis Pharma AS
|
Hypoparathyroidism |
Clinical trials
Forecasted revenue
|
Infectious diseases
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
V-7 Immunitor Inc
|
Tuberculosis (TB) |
Clinical trials
Forecasted revenue
|
Male health
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Fexapotide triflutate Nymox Pharmaceutical Corp
|
Benign prostatic hyperplasia (BPH) |
Clinical trials
Forecasted revenue
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Birtamimab Prothena Corp Plc
|
Primary systemic amyloidosis |
Clinical trials
Forecasted revenue
|
Donislecel (Lantidra) CellTrans Inc
|
Type 1 diabetes (T1D; juvenile diabetes) |
Clinical trials
Forecasted revenue
|
Pegunigalsidase alfa Chiesi Farmaceutici SpA
|
Fabry disease |
Clinical trials
Forecasted revenue Forecasted annual global revenue unknown. |
Oncology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Arfolitixorin Isofol Medical AB
|
Metastatic colorectal cancer |
Clinical trials
Forecasted revenue
|
SGX-301 (HyBryte) Soligenix Inc
|
Cutaneous T-cell lymphoma (CTCL) |
Clinical trials
Forecasted revenue
|
Ipatasertib Genentech, Inc
|
Metastatic |
Clinical trials
Forecasted revenue
|
Motixafortide (BL-8040; Aphexda) BioLineRx Ltd
|
Multiple myeloma (Kahler disease) |
Clinical trials
Forecasted revenue
|
Ophthalmology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Avacincaptad pegol sodium (Zimura) Iveric Bio Inc
|
Geographic atrophy (GA) |
Clinical trials
Forecasted revenue
|
Women’s health
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Fezolinetant Astellas Pharma Inc
|
Vasomotor symptoms of menopause (hot flashes) |
Clinical trials
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2022, and are given in US dollars.
Data source: GlobalData Healthcare database.
Table 6. Pipeline medicines from the 2021 Meds Pipeline Monitor that have gained market authorization
Central Nervous System
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Lecanemab (Leqembi) Eisai Co Ltd
|
Alzheimer's disease (AD) |
Approval
Forecasted revenue
|
Ublituximab (Briumvi) TG Therapeutics, Inc.; LFB S.A.
|
Relapsing multiple sclerosis (RMS) |
Approval
Forecasted revenue
|
Hematological disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Etranacogene dezaparvovec (Hemgenix) CSL Ltd
|
Hemophilia B (factor IX deficiency) |
Approval
Forecasted revenue
|
Infectious Disease
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Oteseconazole (Vivjoa) Mycovia Pharmaceuticals Inc.
|
Recurrent vulvovaginal candidiasis (RVVC) |
Approval
Forecasted revenue
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Teplizumab (Tzield) Provention Bio Inc and Sanofi
|
Type 1 diabetes |
Approval
Forecasted revenue
|
Oncology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Elacestrant (Orserdu) Stemline Therapeutics Inc.
|
Human epidermal growth factor receptor 2 negative breast cancer (HER2- Breast Cancer); Metastatic breast cancer |
Approval
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2022, and are given in US dollars.
Data source: GlobalData Healthcare database.
Spotlight on Canada
This section includes a list of select medicines currently under review by Health Canada that may have a significant impact on future clinical practice and drug spending. Medicines included on this list may be new to Canada but have been approved in other jurisdictions.
Health Canada’s Drug and Health Product Submissions Under Review (SUR) Lists include biosimilars. Although they do not have improved safety and efficacy, their availability could have a potential impact on drug spending. The biosimilars under review, as of September 2022 are: aflibercept, bevacizumab, eculizumab, enoxaparin, pegfilgrastim, and trastuzumab. To date, there are no biosimilars for aflibercept or eculizumab. Their availability could have a potential impact on the costs set aside in drug budgets for the use of these therapies. Appendix A (Table A2) lists the biosimilars currently under review with Health Canada.
Table 7 highlights five new medicines currently on Health Canada’s Drug and Health Product SUR Lists that have a novel mechanism of action or have demonstrated improved safety and efficacy in clinical trials. Of the four medicines reported in the 2021 edition, all have since received market authorization from Health Canada.
The SUR Lists are publicly available sources that identify pharmaceutical and biologic drug submissions with new medicinal ingredients that have been accepted for review in Canada.
Table 7. Selected new medicines currently under review by Health Canada, 2022
Central Nervous System
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Masitinib mesylate AB Science S.A.
|
Amyotrophic lateral sclerosis (ALS) |
Clinical trials
Forecasted revenue
|
Hematological disorders
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Concizumab Novo Nordisk Canada Inc
|
Haemophilia B |
Clinical trials
Forecasted revenue
|
Immunology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Spesolimab Boehringer Ingelheim (Canada) Ltd Ltee
|
Generalized pustular psoriasis (GPP) |
Clinical trials
Forecasted revenue
|
Oncology
| Medicine (Trade name) Company | Indication(s)* | Description and Key Attributes |
|---|---|---|
Ciltacabtagene autoleucel (Carvykti) Janssen Inc
|
Relapsed or refractory multiple myeloma |
Clinical trials
Forecasted revenue
|
Glofitamab Hoffmann-La Roche Limited
|
Diffuse large B-cell lymphoma |
Clinical trials
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q4-2022, and are given in US dollars.
† Health Canada’s Drug and Health Product Submissions Under Review Lists provide the therapeutic area for the medicine under review but do not specify the indication. The indication listed in Table 7 is based on the information about the medicine in the literature and/or approvals in other jurisdictions. When there is an aligned review, in some cases the indication was confirmed by the CADTH Reimbursement Review report.
Data source: GlobalData Healthcare database.
Emerging COVID-19 Therapies
This section of the Meds Pipeline Monitor includes an overview of new and existing pipeline medicines that are under clinical evaluation for indications related to the prevention and treatment of COVID-19. An analysis of global markets provides information on COVID-19 medicines in all phases of clinical trials and pre-registration.
Global markets
The COVID-19 drug pipeline has developed at unprecedented rates over the past 3 years. Published information confirming the safety and efficacy of the various treatments for COVID-19 is continuously evolving.
In addition to the wide variety of vaccines under development, many novel and repurposed medicines are currently being evaluated in clinical trials for their potential benefits in the treatment of COVID-19. These include antivirals, monoclonal antibodies, synthetic peptides and cell therapies.273
A breakdown of the COVID-19 pipeline vaccines and treatments by phase of clinical evaluation is shown in Figure 5. For this snapshot, data was extracted for medicines indicated for the treatment of COVID-19 with a development stage of Phase I, II, III, or pre-registration. These medicines are presented in three categories: vaccines, which are used to prevent infection of the novel coronavirus; treatments (new medicines), which are new medicines used for the prevention or reduction of some of the complications associated with COVID-19 (e.g., pneumonia or respiratory complications and hyperinflammation); and treatments (existing medicines), which are previously marketed medicines that have been repurposed to treat COVID-19 or its symptoms.
Brief Insights
The pipeline for COVID-19 medicines continues to grow, with clinical investigations of novel and existing drugs. The following are some of the most significant advancements in 2022:
- In 2022, over 400 novel medicines were undergoing clinical evaluation for the treatment and prevention of COVID-19. This is a 10% increase from last year.
- Advances in vaccine technology have allowed for rapid updates and approvals for existing vaccines to protect against new strains of COVID-19.
- Bivalent vaccines that target the BA.4 and BA.5 subvariants of Omicron have been approved in many countries worldwide.
- Nasal vaccines are a growing alternative approach to preventing the infection of COVID-19. In 2022, there were 20 nasal vaccines in various phases of the pipeline. Currently there are two nasal or inhaled vaccines approved in China and India.
- More effective treatment options in the pipeline include oral antivirals and new monoclonal antibodies that have shown positive results in clinical trials.
- Stem cell therapy and stem cell-derived organoid models are a growing new treatment option and research method for COVID-19. As of February 2023, there were 53 cell therapies undergoing clinical evaluation in various phases of the pipeline.
Source: GlobalData Healthcare Database (February, 2023); Health Canada (February, 2023).
Figure 5 illustrates the number of clinical trials for COVID-19 treatments and vaccines by latest development phase as of September 2022. The majority of vaccines in the pipeline (97%) are new medicines intended to prevent COVID-19 infection. Antivirals are the most common therapy used to treat COVID-19 symptoms, with 74% new medicines in the pipeline. Other treatment options include monoclonal antibodies, cell therapies and synthetic peptides, that have a larger percentage of redirected and repurposed medicines in the pipeline.
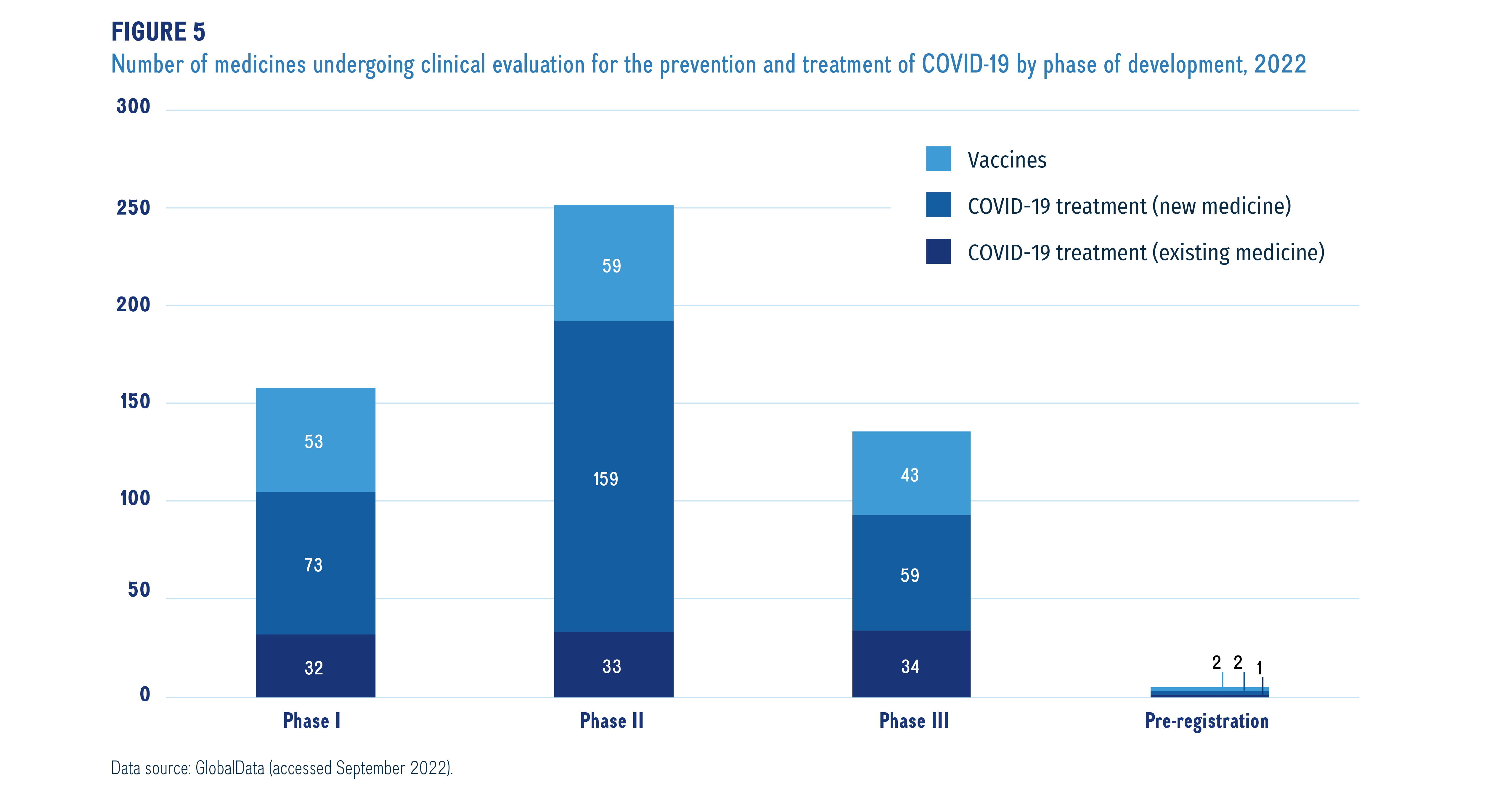
Figure description
This stacked bar graph illustrates the number of COVID-19 treatments and vaccines in the pipeline in 2022. Totals are given for vaccines and treatments, with separate totals for new treatments and repurposed or redirected treatments.
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
Vaccine |
53 |
59 |
43 |
2 |
New treatment |
73 |
159 |
59 |
1 |
Repurposed / Redirected treatment |
32 |
33 |
34 |
2 |
Figure 6 breaks down the COVID-19 vaccines by mechanism of action and highest development phase.Footnote xiv Vaccines are categorized into various vaccine types based on their mechanism of action; for example, while live attenuated vaccines target the whole virus, protein subunit and recombinant vector vaccines target one specific part of the virus.
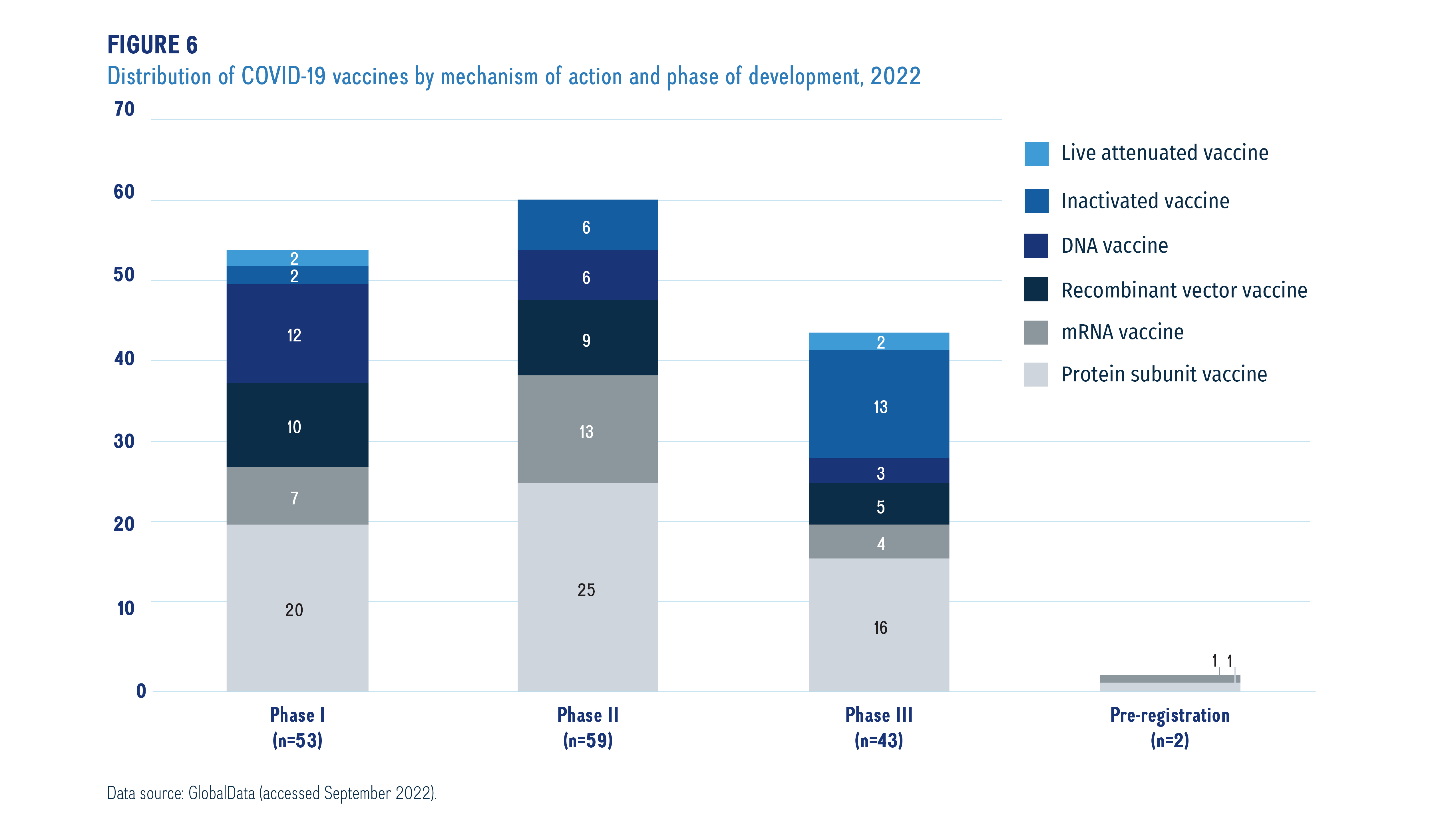
Data source: GlobalData (accessed September 2021).
Figure description
A stacked bar graph gives the distribution of coronavirus vaccines in each phase of clinical evaluation by vaccine type, as of September 2022.
| Protein Subunit Vaccine | Inactivated Vaccine | mRNA Vaccine | Recombinant Vector Vaccine | DNA Vaccine | Live Attenuated Vaccine | Total | |
|---|---|---|---|---|---|---|---|
Phase I |
20 |
2 |
7 |
10 |
12 |
2 |
53 |
Phase II |
25 |
6 |
13 |
9 |
6 |
0 |
59 |
Phase III |
16 |
13 |
4 |
5 |
3 |
2 |
43 |
Pre-registration |
1 |
0 |
1 |
0 |
0 |
0 |
2 |
Canada
COVID-19 continues to impact Canadians, with approximately 182,000 hospitalizations and over 52,000 deaths as of March 2023. Footnote xv Over 85% of Canadians have completed their primary series of vaccinations (two doses). Footnote xvi Health Canada’s most recently approved vaccines are bivalent vaccines that target two different strains of the virus. The updated Moderna Spikevax and Pfizer-BioNTech Comirnaty vaccines target the original SARS-CoV-2 virus as well as the Omicron BA.4 and BA.5 subvariants, which were known to be resistant to previous versions of the vaccines. The vaccines are produced using the same methods as previous COVID-19 vaccines, except that they contain two mRNA components instead of one, which allows them to target more than one strain of the virus.
Health Canada is prioritizing reviews of all COVID-19 vaccine submissions. As of March 2023, Health Canada has approved 6 vaccines, including two mRNA vaccines (Comirnaty and Spikevax), two viral vector-based vaccines (Jcovden and Vaxzevria), one plant-based vaccine (Covifenz), and one protein-based vaccine (Nuvaxovid). Table 8 provides the number of medicines approved by Health Canada for the prevention and treatment of COVID-19, while Table 9 gives the number of COVID-19 medicines under review with Health Canada as of March 2023.
Table 8. COVID-19 treatments and vaccines approved by Health Canada, 2022
| Therapeutic area | Applicant | Medicinal ingredient(s) | Outcome of application | Date of decision/outcome |
|---|---|---|---|---|
Antivirals for systemic use |
Veklury |
Remdesivir (solution for injection) |
Approved under: Food and Drug Regulations; Notice of Compliance issued under the NOC/c Guidance |
27-Jul-20 |
Expanded indication: Food and Drug Regulations; authorized |
22-Apr-22 |
|||
Antivirals for systemic use |
Paxlovid |
Nirmatrelvir and ritonavir (tablets for oral administration) |
Approved under: Food and Drug Regulations; authorized with terms and conditions |
17-Jan-22 |
Immune sera and immunoglobulins |
Evusheld |
Cilgavimab and tixagevimab solution for injection |
Approved under: Food and Drug Regulations; authorized with terms and conditions |
14-Apr-22 |
Expanded indication: Food and Drug Regulations; authorized |
18-Oct-22 |
|||
Immune sera and immunoglobulins |
Casirivimab and imdevimab |
Casirivimab and imdevimab (solution for injection) |
Approved under: Interim order;* authorized with terms and conditions |
09-Jun-21 |
Immune sera and immunoglobulins |
Bamlanivimab |
Bamlanivimab (solution for injection) |
Approved under: Interim order;* authorized with terms and conditions |
20-Nov-20 |
Immune sera and immunoglobulins |
Sotrovimab |
Sotrovimab (solution for injection) |
Approved under: Interim order;* authorized with terms and conditions |
30-Jul-21 |
Immune sera and immunoglobulins |
Actemra |
Tocilizumab solution for injection |
Expanded indication: Food and Drug Regulations; authorized |
13-Oct-22 |
Vaccines |
Covifenz |
Virus-like particles of SARS-CoV-2 spike protein |
Approved under: Food and Drug Regulations; authorized with terms and conditions |
24-Feb-22 |
Vaccines |
Nuvaxovid |
SARS-CoV-2 recombinant spike protein |
Approved under: Adolescent dose (ages 12-17 years) |
06-Dec-22 |
First booster dose |
17-Nov-22 |
|||
Food and Drug Regulations; authorized with terms and conditions |
17-Feb-22 |
|||
Vaccines |
Vaxzevria |
ChAdOx1-S [recombinant] |
Approved under: |
19-Nov-21 |
| Interim order* |
26-Feb-21 |
|||
Vaccines |
Comirnaty |
Tozinameran [mRNA vaccine, BNT162b2] (suspension for injection) |
Approved under: |
09-Dec-22 |
Bivalent booster (ages 12 years and over) |
07-Oct-22 |
|||
Pediatric indication (ages 6 months-5 years) |
09-Sep-22 |
|||
| First booster dose (ages 5-11 years) |
19-Aug-22 |
|||
First booster dose (ages 16-17 years) |
01-Jun-22 |
|||
Food and Drug Regulations; pediatric indication (ages 5-11) |
19-Nov-21 |
|||
Food and Drug Regulations; first booster dose |
09-Nov-21 |
|||
| Food and Drug Regulations; authorized with terms and conditions |
16-Sept-21 |
|||
Interim order;* pediatric indication (ages 12-15) |
05-May-21 |
|||
| Interim order* |
09-Dec-20 |
|||
Vaccines |
Spikevax |
Elasomeran |
Approved under: First booster dose (ages 12-17 years) |
12-Jan-23 |
| Bivalent booster (ages 18 years and over) |
03-Nov-22 |
|||
Bivalent booster (ages 18 years and over) |
01-Sep-22 |
|||
Pediatric indication (ages 6 months-5 years) |
14-Jul-22 |
|||
Pediatric indication (ages 6-11 years) |
17-Mar-22 |
|||
First booster dose |
12-Nov-21 |
|||
Food and Drug Regulations; authorized with terms and conditions |
16-Sept-21 |
|||
Interim order;* pediatric indication (ages 12-17) |
27-Aug-21 |
|||
Interim order* |
23-Dec-20 |
|||
Vaccines |
Jcovden |
AD26.COV2.S [recombinant] |
Approved under: First booster dose |
11-May-22 |
Food and Drug Regulations; authorized with terms and conditions |
23-Nov-21 |
|||
| Interim order* |
05-Mar-21 |
|||
Vaccines |
Covishield |
ChAdOx1-S (recombinant) |
Approved under: Interim order;* authorized with terms and conditions |
26-Feb-2021 (expired 16-Sept-21) |
* The Interim Order Respecting the Importation, Sale and Advertising of Drugs for Use in Relation to COVID-19, approved on May 23, 2020, introduced an alternate pathway to facilitate clinical trials for potential COVID-19 drugs and medicinal devices, while upholding strong patient safety requirements and validity of trial data.
Data source: Drug and vaccine authorizations for COVID-19: List of authorized drugs, vaccines and expanded indications (accessed March 2023):
https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/list-drugs.html
Table 9. COVID-19 treatments and vaccines under review by Health Canada, as of March 2023
| Therapeutic area | Applicant | Medicinal ingredient(s) | Date submission accepted |
|---|---|---|---|
Vaccines |
Pfizer Canada ULC/ BioNTech SE |
Tozinameran |
Feb-22 |
Vaccines - Booster dose |
AstraZeneca Canada Inc. |
[ChAdOx1-S; [recombinant] |
Dec-21 |
Vaccines - Booster dose |
Janssen Inc. |
Ad26.COV2.S |
Dec-21 |
Vaccines - Pediatric dose (ages 6-11) |
ModernaTX, Inc. |
Elasomeran |
Nov-21 |
Vaccines |
Medicago Inc. |
Coronavirus-like particle [CoVLP] |
Aug-21 |
Vaccines |
Novavax Inc. |
NVX-CoV2373 |
Aug-21 |
Vaccines |
Sanofi Pasteur Ltd |
SARS-CoV-2 prefusion spike delta TM protein [recombinant] |
Jul-21 |
Vaccines |
Vaccigen Ltd |
Whole virion inactivated coronavirus |
Jul-21 |
Immune sera and immunoglobulins |
AstraZeneca Canada Inc. |
Cilgavimab, tixagevimab |
Nov-21 |
Immune sera and immunoglobulins |
Celltrion HealthCare Co. Ltd |
Regdanvimab |
May-21 |
Immune sera and immunoglobulins |
Eli Lilly Canada Inc. |
Bamlanivimab* |
Jun-21 |
Immune sera and immunoglobulins |
Hoffmann-La Roche Ltd |
Casirivimab, imdevimab* |
Sept-21 |
Immune sera and immunoglobulins |
GlaxoSmithKline Inc. |
Sotrovimab* |
Oct-21 |
Immune sera and immunoglobulins |
Eli Lilly Canada Inc. |
Etesevimab |
Sept-21 |
Immunosuppressants |
Eli Lilly Canada Inc |
Baricitinib |
Sep-21 |
Antivirals for systemic use |
Gilead Sciences Canada Inc |
Remdesivir |
Apr-21 |
Antivirals for systemic use |
Dr Reddys Laboratories Ltd |
Favipiravir |
Sept-21 |
Antivirals for systemic use |
Merck Canada Inc |
Molnupiravir |
Aug-21 |
| Ceased reviews | |||
Immune sera and immunoglobulins |
CytoDyn Inc. |
Leronlimab |
Mar-21 (Expired) |
Antigout preparations |
Pendopharm Division of Pharmascience Inc |
Colchicine |
Jan-21 |
Other nervous system drugs |
Sanotize Research & Development Corp. |
Nitric oxide |
Jun-21 |
* The applicant has filed a new drug submission under the Food and Drug Regulations to transition this product from the interim order. The product continues to be approved for sale in Canada during this transition period.
Data source: Drug and vaccine authorizations for COVID-19: List of applications received, Health Canada (accessed March 2023): https://www.canada.ca/en/health-canada/services/drugs-health-products/covid19-industry/drugs-vaccines-treatments/authorization/applications.html
Appendix A
Table A1: Biosimilars in Phase III (based on data extract from 2022-09)
| Medicine | Reference product (Manufacturer) | Other biosimilars (Y/N) | Manufacturers developing biosimilars | Indication(s) |
|---|---|---|---|---|
Adalimumab
|
Humira (Abbvie Corp)
|
Y |
Enzene Biosciences Ltd |
Ankylosing spondylitis (Bekhterev's disease) |
Wuhan Institute of Biological Products Co Ltd |
Plaque psoriasis (psoriasis vulgaris) |
|||
Aflibercept
|
Eylea (Bayer Inc)
|
N |
Amgen Inc Alteogen Inc Alvotech SA Celltrion Inc Formycon AG Hexal AG Johnson & Johnson Sam Chun Dang Pharm Co Ltd Samsung Bioepis Co Ltd |
Wet (neovascular/exudative) macular degeneration |
Bevacizumab |
Avastin (Hoffmann-La Roche Limited) |
Y |
Aurobindo Pharma Ltd Prestige BioPharma Ltd SinoCelltech Group Ltd
Centrion† |
Non-small cell lung cancer
N/A |
Cetuximab |
Erbitux |
N |
Ampo Biotechnology Inc Cinnagen Co Enzene Biosciences Ltd R-Pharm |
Head and neck cancer squamous cell carcinoma; Lip cancer; Locally recurrent or locoregional solid malignancies; Oral cavity (mouth) cancer; Pharyngeal neoplasm; Metastatic colorectal cancer |
Darbepoetin alfa |
Aranesp (Amgen Canada Inc) |
N |
Biocad
|
Anemia in chronic kidney disease (renal anemia) |
Denosumab
|
Prolia / Xgeva (Amgen Canada Inc) |
N |
Mabwell Shanghai Bioscience Co Ltd |
Diabetes |
Celltrion Inc Eden Biologics Inc Fresenius Kabi SwissBioSim GmbH Gedeon Richter Plc Mabxience Holding SL Samsung Bioepis Co Ltd Sandoz International GmbH Teva Pharmaceutical Industries Ltd |
Postmenopausal osteoporosis |
|||
Sandoz International GmbH |
Bone metastasis; Giant cell tumour of bone |
|||
Eculizumab |
Soliris (Alexion Pharma GmbH) |
N |
Amgen Inc† Samsung Bioepis Co Ltd |
Paroxysmal nocturnal hemoglobinuria |
Enoxaparin |
Lovenox (Sanofi-Aventis Canada Inc) |
Y |
Baxter Corporation† Fresenius Kabi Canada Ltd† |
N/A |
Etanercept |
Enbrel (Immunex Corporation) |
Y |
Mycenax Biotech Inc
|
Rheumatoid arthritis |
Follitropin alfa |
Gonal-F / Pergoveris (EMD Serono, a division of EMD Inc., Canada) |
N |
Amega Biotech |
Female infertility |
Golimumab |
Simponi (Janssen Inc) |
N |
Bio-Thera Solutions Ltd
|
Psoriatic arthritis |
Liraglutide |
Victoza / Saxenda (Novo Nordisk Canada Inc) |
N |
Shanghai Fosun Pharmaceutical (Group) Co Ltd |
Obesity |
Ocrelizumab
|
Ocrevus (Hoffmann-La Roche Limited) |
N |
Cinnagen Co |
Relapsing remitting multiple sclerosis (RRMS) |
Omalizumab |
Xolair (Novartis Pharmaceuticals Canada Inc)
|
N |
Synermore Biologics Co Ltd |
Allergic asthma |
Celltrion Inc Synermore Biologics Co Ltd Teva Pharmaceutical Industries Ltd |
Chronic urticaria or hives |
|||
Synermore Biologics Co Ltd |
Nasal polyps (nasal polyposis); Rhinosinusitis |
|||
Pegfilgrastim |
Neulasta (Amgen Canada Inc) |
Y |
Lupin Pharma Canada Limited† Nora Pharma Inc† |
Febrile neutropenia |
Pertuzumab |
Perjeta (Hoffmann-La Roche Limited) |
N |
Zydus Lifesciences Ltd |
Human epidermal growth factor receptor 2-positive breast cancer (HER2+ breast cancer) |
Ranibizumab |
Lucentis (Novartis Pharmaceuticals Canada Inc)
|
Y* |
Aurobindo Pharma Ltd Enzene Biosciences Ltd Generium Jecho Biopharmaceuticals Co Ltd Lupin Ltd PharmaResearch BIO Co Ltd |
Wet (neovascular/exudative) macular degeneration |
Romiplostim |
Nplate (Amgen Canada Inc) |
N |
Generium
|
Idiopathic thrombocytopenic purpura (immune thrombocytopenic purpura) |
Tenecteplase |
TNKase (Hoffmann-La Roche Limited) |
N |
Hetero Drugs Ltd
|
Myocardial infarction |
Trastuzumab |
Herceptin (Hoffmann-La Roche Limited) |
Y |
Aprogen Inc Hetero Drugs Ltd Tanvex BioPharma Inc Prestige BioPharma Ltd. (HC)† |
Human epidermal growth factor receptor 2-positive breast cancer (HER2+ Breast Cancer) |
Ustekinumab |
Stelara (Janssen Inc) |
N |
DM Bio Ltd Samsung Bioepis Co Ltd |
Psoriatic arthritis |
Amgen Inc Alvotech SA Bio-Thera Solutions Ltd Celltrion Inc DM Bio Ltd Formycon AG Samsung Bioepis Co Ltd |
Plaque psoriasis (psoriasis vulgaris) |
|||
DM Bio Ltd |
Crohn's disease (regional enteritis); Ulcerative colitis |
|||
JiangSu Qyuns Therapeutics Co Ltd |
Inflammatory bowel disease |
* Approved but not marketed as of December 5, 2022.
† Biosimilar currently under review by Health Canada.
Table A2: New drug submissions under review by Health Canada (February 2023)
| Medicinal ingredient | Therapeutic area | Manufacturer | Date submission accepted for review |
|---|---|---|---|
Aflibercept |
Ophthalmologicals |
BGP Pharma ULC |
2022-05 |
Bevacizumab |
Antineoplastic agents |
Celltrion Healthcare Co Ltd |
|
Eculizumab |
Immunosuppressants |
Amgen Canada Inc |
2022-07 |
Enoxaparin sodium |
Antithrombotic agents |
Baxter Corporation |
2022-09 |
Pegfilgrastim |
Immunostimulants |
Lupin Pharma Canada Limited |
2022-05 |
Pegfilgrastim |
Immunostimulants |
Nora Pharma Inc |
|
Ranibizumab |
Ophthalmologicals |
Teva Canada Limited |
2022-12 |
Trastuzumab |
Antineoplastic agents |
Prestige BioPharma Ltd. |
2021-08 |
Table A3: Drugs in Phase II for indications targeted by pipeline candidates
| Pipeline candidate | Indication(s) | Drugs in Phase II and mechanism of action (MOA)* |
|---|---|---|
Abelacimab |
Deep vein thrombosis (DVT); Pulmonary embolism |
There are no other drugs for this indication with the same MOA as abelacimab (a dual Factor XI and Factor Xia inhibitor) in Phase II development at this time. |
Aprocitentan and Firibastat |
Resistant hypertension |
There are no other drugs for this indication with the same MOA as aprocitentan (a dual endothelin-receptor antagonist) or firibistat (a centrally-acting, aminopeptidase A inhibitor) in Phase II development at this time. |
Bemarituzumab and Zolbetuximab |
Adenocarcinoma of the gastroesophageal junction; Gastric cancer; bladder cancer; Gastroesophageal (GE) junction carcinomas |
There is another drug (MAX-4) with a similar MOA to bemarituzumab (a fibroblast growth factor receptor inhibitor) and others (BNT-141, LM-302) with a similar MOA to zolbetuximab (a claudin-18 inhibitor) in Phase II development at this time. |
Ciltacabtagene autoleucel* and Iberdomide hydrochloride |
Refractory multiple myeloma; Relapsed multiple myeloma |
There are other drugs (CFT-7455, mezigdomide) for this indication with a similar MOA to Iberdomide (a protein cereblon activator) and other gene therapies like ciltacabtagene in Phase II development at this time. |
Concizumab* |
Haemophilia B |
There are no other drugs for this indication with the same MOA as concizumab (a tissue factor pathway inhibitor) in Phase II development at this time. |
Evobrutinib |
Relapsing multiple sclerosis (RMS); Secondary progressive multiple sclerosis (SPMS) |
There are no other drugs for this indication with the same MOA as evobrutinib (a selective, CNS penetrant immunomodulator that irreversibly blocks BTK) in Phase II development at this time. |
FCR001 |
Kidney transplant rejection |
There are other cell therapies like FCR001 for this indication in Phase II development at this time. |
Firibastat |
Refer to information under “aprocitentan” |
|
Garadacimab |
Hereditary angioedema (HAE) (C1 esterase inhibitor [C1-INH] deficiency |
There are no other drugs for this indication with the same MOA as garadacimab (an immunoglobulin G4 monoclonal antibody that interferes with FXIIa-mediated coagulation) in Phase II development at this time. |
Glofitamab |
B-cell lymphomas |
There are no other drugs for this indication with the same MOA as glofitamab (a CD20xCD3 T-cell engaging bispecific antibody) in Phase II development at this time. |
Iberdomide hydrochloride |
Refer to information under “ciltacabtagene” |
|
Imetelstat and Navitoclax
|
Myelodysplastic dyndrome; Post-rssential thrombocythemia myelofibrosis (post-ET MF); Post-polycythemia vera myelofibrosis (PPV-MF) |
There are no other drugs for this indication with the same MOA as imetelstat (a telomerase inhibitor) or navitoclax (an antagonist of a subset of the B-cell leukemia 2 family of proteins) in Phase II development at this time. |
Masitinib* and Reldesemtiv |
Amyotrophic Lateral Sclerosis (ALS) |
There are no other drugs for this indication with the same MOA as masitinib (a selective tyrosine kinase inhibitor) or reldesemtiv (a fast skeletal muscle troponin activator) or in Phase II development at this time. |
Navitoclax dihydrochloride |
Refer to information under “imetelstat” |
|
Obicetrapib
|
Dyslipidemia; Heterozygous familial hypercholesterolemia (heFH); Atherosclerosis |
There are no other drugs for this indication with the same MOA as obicetrapib (a CETP inhibitor) in Phase II development at this time. |
Pegcetacoplan* |
Paroxysmal Nocturnal Hemoglobinuria |
There are no other drugs for this indication with the same MOA as pegcetacoplan (a complement C3 and fragment C3b inhibitor) in Phase II development at this time. |
Relacorilant |
Epithelial ovarian cancer |
There are no other drugs for this indication with the same MOA as relacorilant (a glucocorticoid receptor antagonist) in phase ii development at this time. |
Reldesemtiv |
Refer to information under “masitinib” |
|
REC-2282 |
Neurofibromatoses Type II (NF2) |
Drugs in Phase II for this indication and their MOA:
There is no other drug for this indication with the same MOA as REC-2282 (a pan-histone deacetylase inhibitor) in Phase II development at this time. |
Resmetirom |
Non-alcoholic steatohepatitis (NASH); Non-alcoholic fatty liver disease (NAFLD) |
There are other drugs (ASC-41, TERN-501, VK-2809) for this indication with a similar MOA to resmetirom (a thyroid hormone receptor beta agonist) in Phase II development at this time. |
Rusfertide acetate |
Polycythemia vera |
There are no other drugs for this indication in Phase II development at this time. |
Seladelpar |
Primary biliary cholangitis (primary biliary cirrhosis) |
There are no other drugs for this indication with the same MOA as seladelpar (a PPAR-delta agonist) in Phase II development at this time. |
Setmelanotide |
Obesity |
There are no other drugs for this indication with the same MOA as setmelanotide (a melanocortin-4 receptor agonist) in Phase II development at this time. |
Soticlestat |
Lennox-Gastaut syndrome; Dravet syndrome (severe myoclonic epilepsy of infancy) |
There are no other drugs for this indication with the same MOA as soticlestat (a cholesterol 24-hydroxylase inhibitor) in Phase II development at this time. |
Sotatercept |
Pulmonary arterial hypertension (PAH) |
There are no other drugs for this indication with the same MOA as sotatercept (an activin receptor type IIA-Fc fusion protein) in Phase II development at this time. |
Spesolimab* |
Generalized pustular psoriasis (GPP) |
There are no other drugs for this indication in Phase II development at this time. |
Tremelimumab |
Hepatocellular carcinoma |
There are other drugs (ADG-126, BMS-986249, ONC-392, PSB-205, zalifrelimab) for this indication with a similar MOA to tremelimumab (a cytotoxic T lymphocyte protein 4 inhibitor) in Phase II development at this time. |
Ulotaront |
Schizophrenia |
There is one other drug (ralmitaront) for this indication with a similar MOA to ulotaront (a TAAR1 agonist) in Phase II development at this time. |
Zolbetuximab |
Refer to information under “bemarituzumab” |
|
Zoliflodacin |
Uncomplicated cervical and urethral gonorrhea |
There are no other drugs for this indication in Phase II development at this time. |
*Under review by Health Canada.
Abbreviations: ACE: angiotensin converting enzyme; ASA: acetylsalicylic acid; BTK: Bruton's tyrosine kinase; CETP: cholesteryl ester transfer protein; CNS: central nervous system; JAK: Janus Kinase; MOA: mechanism of action; PPAR: peroxisomal proliferator-activated receptor; TAAR1: trace amine associated receptor 1.
Data source: GlobalData Healthcare database (accessed September 2022).
References
- Clinical Development Success Rates 2006-2015. https://www.bio.org/sites/default/files/legacy/bioorg/docs/Clinical%20Development%20Success%20Rates%202006-2015%20-%20BIO,%20Biomedtracker,%20Amplion%202016.pdf (accessed January 13, 2023).
- Drug and Health Product Submissions Under Review (SUR). https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review/new-drug-submissions-under-review.html
- Anthos Therapeutics Announces that Abelacimab Has Received FDA Fast Track Designation for the Prevention of Stroke and Systemic Embolism in Patients with Atrial Fibrillation. September 8, 2022. https://www.globenewswire.com/news-release/2022/09/08/2512472/0/en/Anthos-Therapeutics-Announces-that-Abelacimab-Has-Received-FDA-Fast-Track-Designation-for-the-Prevention-of-Stroke-and-Systemic-Embolism-in-Patients-with-Atrial-Fibrillation.html
- Verhamme P, Yi BA, Segers A, Salter J, Bloomfield D, et al. Abelacimab for Prevention of Venous Thromboembolism. N Engl J Med. 2021 Aug 12;385(7):609-617. doi: 10.1056/NEJMoa2105872. Epub 2021 Jul 19.
- Li T, Liu J, Wu W. Factor XI, a potential target for anticoagulation therapy for venous thromboembolism. Front Cardiovasc Med. 2022 Oct 31;9:975767. doi: 10.3389/fcvm.2022.975767. eCollection 2022.
- Gómez-Outes A, Suárez-Gea ML, Pérez-Cabeza AI, García-Pinilla JM. Pharmacotherapy for stroke prevention in nonvalvular atrial fibrillation: current strategies and future directions. Expert Opin Pharmacother. 2022 Nov 17. doi: 10.1080/14656566.2022.2149323. Online ahead of print.
- A Multicenter, Randomized, Open-label, Blinded Endpoint Evaluation, Phase 3 Study Comparing the Effect of Abelacimab Relative to Apixaban on Venous Thromboembolism (VTE) Recurrence and Bleeding in Patients With Cancer Associated VTE. NCT05171049 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05171049?term=Abelacimab&draw=2&rank=2
- A Multicenter, Randomized, Open-label, Blinded Endpoint Evaluation, Phase 3 Study Comparing the Effect of Abelacimab vs. Dalteparin on Venous Thromboembolism (VTE) Recurrence and Bleeding in Patients With GI/GU Associated VTE. NCT05171075 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05171075?term=Abelacimab&draw=2&rank=3
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- McCoy EK, Lisenby KM. Aprocitentan (a Dual Endothelin-Receptor Antagonist) for Treatment-Resistant Hypertension. J Cardiovasc Pharmacol. 2021 Jun 1;77(6):699-706. doi: 10.1097/FJC.0000000000001023.
- Schlaich MP, Bellet M, Weber MA, Danaietash P, Bakris GL, et al; PRECISION investigators. Dual endothelin antagonist aprocitentan for resistant hypertension (PRECISION): a multicentre, blinded, randomised, parallel-group, phase 3 trial. Lancet. 2022 Nov 4:S0140-6736(22)02034-7. doi: 10.1016/S0140-6736(22)02034-7. Online ahead of print.
- Late-Breaking Data from Pivotal Phase 3 PRECISION Study Demonstrates Significant and Sustained Effect of Aprocitentan on Lowering Blood Pressure for Patients with Difficult-to-Control Hypertension. November 7, 2022. https://www.jnj.com/late-breaking-data-from-pivotal-phase-3-precision-study-demonstrates-significant-and-sustained-effect-of-aprocitentan-on-lowering-blood-pressure-for-patients-with-difficult-to-control-hypertension#:~:text=Specifically%2C%20after%204%20weeks%2C%20aprocitentan,6.8%2C%20%2D0.8%3B%20p%20%3D
- Höcht C, Allo MA, Polizio AH, Morettón MA, et al. New and developing pharmacotherapies for hypertension. Expert Rev Cardiovasc Ther. 2022 Aug;20(8):647-666. doi: 10.1080/14779072.2022.2105204. Epub 2022 Aug 3.
- Idorsia submits a New Drug Application to the US FDA for aprocitentan for the treatment of patients with difficult-to-control hypertension. December 20, 2022. https://www.biospace.com/article/releases/idorsia-submits-a-new-drug-application-to-the-us-fda-for-aprocitentan-for-the-treatment-of-patients-with-difficult-to-control-hypertension/
- Multi-center, Blinded, Randomized Study With Aprocitentan in Subjects With Uncontrolled Blood Pressure and Chronic Kidney Disease Stage 3 or 4. NCT04162366 (Withdrawn [A business decision was made to not initiate this study]). https://clinicaltrials.gov/ct2/show/NCT04162366?term=Aprocitentan&phase=2&draw=2&rank=1
- Multi-center, Blinded, Randomized, Parallel-group, Phase 3 Study With Aprocitentan in Subjects With Resistant Hypertension (RHT). NCT03541174 (completed). https://clinicaltrials.gov/ct2/show/NCT03541174?term=Aprocitentan&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Weintraub S, Frishman WH. A Novel Calcium Channel Blocker: Etripamil: What is the Future of Intranasal Drug Delivery in the Treatment of Cardiac Arrhythmias? Cardiol Rev. 2021 Sep-Oct 01;29(5):253-258. doi: 10.1097/CRD.0000000000000362.
- An Open Label Extension Study of Etripamil Nasal Spray in Patients With Paroxysmal Supraventricular Tachycardia. NCT04952610 (Enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT04952610?term=Etripamil&phase=2&draw=2&rank=2
- RAPID: Positive Top-line Results for Etripamil Nasal Spray in Paroxysmal SVT. October 17, 2022. https://www.tctmd.com/news/rapid-positive-top-line-results-etripamil-nasal-spray-paroxysmal-svt
- Kashou AH, Noseworthy PA. Etripamil nasal spray: an investigational agent for the rapid termination of paroxysmal supraventricular tachycardia (SVT). Expert Opin Investig Drugs. 2020 Jan;29(1):1-4. doi: 10.1080/13543784.2020.1703180. Epub 2019 Dec 12.
- Multi-Centre, Open-Label, Safety Study of Etripamil Nasal Spray in Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia The NODE-302 Trial (Extension of NODE-301). NCT03635996 (completed). https://clinicaltrials.gov/ct2/show/NCT03635996?term=Etripamil&phase=2&draw=2&rank=4
- The NODE-303 Study: Multi-Centre, Multi-National,Open Label, Safety Study of Etripamil Nasal Spray for Patients With Paroxysmal Supraventricular Tachycardia. NCT04072835 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04072835?term=Etripamil&phase=2&draw=2&rank=1
- Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Efficacy and Safety Study of Etripamil Nasal Spray Self-Administration for the Termination of Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia in Chinese Patients. NCT05410860 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05410860?term=Etripamil&phase=2&draw=2&rank=3
- Multi-Centre, Randomized, Double-Blind, Placebo-Controlled, Efficacy, and Safety Study of Etripamil Nasal Spray for the Termination of Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia. NODE 301 Trial. NCT03464019 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03464019?term=Etripamil&phase=2&draw=2&rank=5
- An Open Label Extension Study of Etripamil Nasal Spray in Patients With Paroxysmal Supraventricular Tachycardia. NCT04952610 (Enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT04952610?term=Etripamil&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Nurmohamed NS, Ditmarsch M, Kastelein JJP. Cholesteryl ester transfer protein inhibitors: from high-density lipoprotein cholesterol to low-density lipoprotein cholesterol lowering agents? Cardiovasc Res. 2022 Nov 10;118(14):2919-2931. doi: 10.1093/cvr/cvab350.
- Obicetrapib decreases LDL, increases HDL in phase 2 trial. September 2, 2022. https://www.healio.com/news/cardiology/20220909/obicetrapib-decreases-ldl-increases-hdl-in-phase-2-trial
- Obicetrapib decreases LDL, increases HDL in phase 2 trial. September 2, 2022. https://www.healio.com/news/cardiology/20220909/obicetrapib-decreases-ldl-increases-hdl-in-phase-2-trial
- A Placebo-Controlled, Double-Blind, Randomized, Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With a History of HeFH Who Are Not Adequately Controlled by Their Lipid Modifying Therapies. NCT05425745 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05425745?term=Obicetrapib&phase=2&draw=2&rank=1
- A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10mg Obicetrapib in Participants With HeFH and/or ASCVD Who Are Not Adequately Controlled by Their Lipid Modifying Therapies. NCT05142722 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05142722?term=Obicetrapib&phase=2&draw=2&rank=2
- Placebo Controlled, Double Blind, Randomized Cardiovascular Outcome Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With ASCVD Not Adequately Controlled Despite Maximally Tolerated Lipid Modifying Therapies. NCT05202509 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05202509?term=Obicetrapib&phase=2&draw=2&rank=3
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Rayroux C, Lador F, Soccal PM, Plojoux J, Adler D. [Pulmonology 2021: year in review] [Article in French; Abstract available in French from the publisher] Rev Med Suisse. 2022 Jan 19;18(764-5):64-68. doi: 10.53738/REVMED.2022.18.764-65.64.
- Merck Announces Positive Top-line Results from Pivotal Phase 3 STELLAR Trial Evaluating Sotatercept for the Treatment of Adults with Pulmonary Arterial Hypertension (PAH). October 10, 2022. https://www.merck.com/news/merck-announces-positive-top-line-results-from-pivotal-phase-3-stellar-trial-evaluating-sotatercept-for-the-treatment-of-adults-with-pulmonary-arterial-hypertension-pah/
- Hoeper MM, Badesch DB, Ghofrani HA, Gibbs JSR, Gomberg-Maitland M,et al. Phase 3 Trial of Sotatercept for Treatment of Pulmonary Arterial Hypertension. N Engl J Med. 2023 Mar 6. doi: 10.1056/NEJMoa2213558. Online ahead of print.
- Sotatercept- the breakthrough PAH has been waiting for? November 10, 2022. https://www.clinicaltrialsarena.com/comment/sotatercept-breakthrough-pah/
- An Open-label Long-term Follow-up Study to Evaluate the Effects of Sotatercept When Added to Background Pulmonary Arterial Hypertension (PAH) Therapy for the Treatment of PAH. NCT04796337 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04796337?term=Sotatercept&phase=2&draw=2&rank=1
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Evaluate Sotatercept When Added to Maximum Tolerated Background Therapy in Participants With Pulmonary Arterial Hypertension (PAH) World Health Organization (WHO) Functional Class (FC) III or FC IV at High Risk Mortality. NCT04896008 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04896008?term=Sotatercept&phase=2&draw=2&rank=2
- A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Evaluate Sotatercept When Added to Background Pulmonary Arterial Hypertension (PAH) Therapy in Newly Diagnosed Intermediate- and High-risk PAH Patients. NCT04811092 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04811092?term=Sotatercept&phase=2&draw=2&rank=3
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Compare the Efficacy and Safety of Sotatercept Versus Placebo When Added to Background Pulmonary Arterial Hypertension (PAH) Therapy for the Treatment of PAH. NCT04576988 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04576988?term=Sotatercept&phase=2&draw=2&rank=4
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- von Hundelshausen P, Siess W. Bleeding by Bruton Tyrosine Kinase-Inhibitors: Dependency on Drug Type and Disease. Cancers (Basel). 2021 Mar 4;13(5):1103. doi: 10.3390/cancers13051103.
- Schneider R, Oh J. Bruton's Tyrosine Kinase Inhibition in Multiple Sclerosis. Curr Neurol Neurosci Rep. 2022 Nov;22(11):721-734. doi: 10.1007/s11910-022-01229-z. Epub 2022 Oct 27.
- Merck KGaA, Darmstadt, Germany Highlights New Data for Evobrutinib, First BTKi to Demonstrate Sustained Clinical Benefit for People with RMS through Three and a Half Years of Treatment. October 26, 2022. https://www.emdgroup.com/en/news/evobrutinib-26-10-2022.html
- A Phase III, Multicenter, Randomized, Parallel Group, Double Blind, Double Dummy, Active Controlled Study of Evobrutinib Compared With an Interferon Beta 1a (Avonex®), in Participants With RMS to Evaluate Efficacy and Safety. NCT04032171 (terminated early and comparator changed). https://clinicaltrials.gov/ct2/show/NCT04032171?term=Evobrutinib&phase=2&draw=2&rank=1
- A Phase III, Multicenter, Randomized, Parallel Group, Double Blind, Double Dummy, Active Controlled Study of Evobrutinib Compared With an Interferon Beta 1a (Avonex®), in Participants With Relapsing Multiple Sclerosis to Evaluate Efficacy and Safety. NCT04032158 (terminated early and comparator changed). https://clinicaltrials.gov/ct2/show/NCT04032158?term=Evobrutinib&phase=2&draw=2&rank=2
- A Phase III, Multicenter, Randomized, Parallel Group, Double Blind, Double Dummy, Active Controlled Study of Evobrutinib Compared With Teriflunomide, in Participants With Relapsing Multiple Sclerosis to Evaluate Efficacy and Safety (evolutionRMS 2). NCT04338061 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04338061?term=Evobrutinib&phase=2&draw=2&rank=3
- A Phase III, Multicenter, Randomized, Parallel Group, Double Blind, Double Dummy, Active Controlled Study of Evobrutinib Compared With Teriflunomide, in Participants With Relapsing Multiple Sclerosis to Evaluate Efficacy and Safety (evolutionRMS 1). NCT04338022 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04338022?term=Evobrutinib&phase=2&draw=2&rank=4
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Phase 3 Trial of Reldesemtiv in ALS Announced. August 11, 2021. https://www.neurologylive.com/view/phase-3-trial-reldesemtiv-als-announced
- Reldesemtiv Earns FDA’s Orphan Drug Designation for ALS Treatment. December 19, 2019. https://alsnewstoday.com/news/reldesemtiv-earns-fdas-orphan-drug-designation-for-als-treatment/
- Cytokinetics Updates Phase 3 COURAGE-ALS Trial of Reldesemtiv. December 14, 2021. https://alsnewstoday.com/news/moderate-to-fast-progressing-als-courage-als-trial-patients-reldesemtiv-cytokinetics/
- Shefner JM, Andrews JA, Genge A, Jackson C, Lechtzin N, et al. A Phase 2, Double-Blind, Randomized, Dose-Ranging Trial Of Reldesemtiv In Patients With ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2021 May;22(3-4):287-299. doi: 10.1080/21678421.2020.1822410. Epub 2020 Sep 24.
- Cytokinetics Updates Phase 3 COURAGE-ALS Trial of Reldesemtiv. December 14, 2021. https://alsnewstoday.com/news/moderate-to-fast-progressing-als-courage-als-trial-patients-reldesemtiv-cytokinetics/
- Shefner JM, Andrews JA, Genge A, Jackson C, Lechtzin N, et al. A Phase 2, Double-Blind, Randomized, Dose-Ranging Trial Of Reldesemtiv In Patients With ALS. Amyotroph Lateral Scler Frontotemporal Degener. 2021 May;22(3-4):287-299. doi: 10.1080/21678421.2020.1822410. Epub 2020 Sep 24.
- Rudnicki SA, Andrews JA, Genge A, Jackson C, Lechtzin N, et al; FORTITUDE-ALS STUDY GROUP. Prescription and acceptance of durable medical equipment in FORTITUDE-ALS, a study of reldesemtiv in ALS: post hoc analyses of a randomized, double-blind, placebo-controlled clinical trial. Amyotroph Lateral Scler Frontotemporal Degener. 2022 May;23(3-4):263-270. doi: 10.1080/21678421.2021.1946083. Epub 2021 Jul 5.
- A Phase 3, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Reldesemtiv in Patients With Amyotrophic Lateral Sclerosis (ALS). NCT04944784 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04944784?term=Reldesemtiv&phase=2&draw=2&rank=1
- A Phase 3, Open-Label Extension of COURAGE-ALS (CY 5031). NCT05442775 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05442775?term=Reldesemtiv&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Soticlestat, A New Potential Treatment for Epilepsy It Inhibits Cholesterol Catabolism. July 7, 2022. https://journals.lww.com/neurotodayonline/Fulltext/2022/07070/Soticlestat,_A_New_Potential_Treatment_for.9.aspx
- Nishi T, Metcalf CS, Fujimoto S, Hasegawa S, Miyamoto M, et al. Anticonvulsive properties of soticlestat, a novel cholesterol 24-hydroxylase inhibitor. Epilepsia. 2022 Jun;63(6):1580-1590. doi: 10.1111/epi.17232. Epub 2022 Mar 30.
- Hahn CD, Jiang Y, Villanueva V, Zolnowska M, Arkilo D, et al. A phase 2, randomized, double-blind, placebo-controlled study to evaluate the efficacy and safety of soticlestat as adjunctive therapy in pediatric patients with Dravet syndrome or Lennox-Gastaut syndrome (ELEKTRA). Epilepsia. 2022 Oct;63(10):2671-2683. doi: 10.1111/epi.17367. Epub 2022 Aug 4.
- A Phase 3, Prospective, Open-Label, Multisite, Extension of Phase 3 Studies To Assess the Long-Term Safety and Tolerability of Soticlestat as Adjunctive Therapy in Subjects With Dravet Syndrome or Lennox-Gastaut Syndrome (ENDYMION 2). NCT05163314 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05163314?term=Soticlestat&phase=2&draw=2&rank=1
- A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy, Safety, and Tolerability of Soticlestat as Adjunctive Therapy in Pediatric and Young Adult Subjects With Dravet Syndrome (DS). NCT04940624 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04940624?term=Soticlestat&phase=2&draw=2&rank=2
- A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy, Safety, and Tolerability of Soticlestat as Adjunctive Therapy in Pediatric and Adult Subjects With Lennox-Gastaut Syndrome (LGS). NCT04938427 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04938427?term=Soticlestat&phase=2&draw=2&rank=3
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Koblan KS, Kent J, Hopkins SC, Krystal JH, Cheng H, et al. A Non-D2-Receptor-Binding Drug for the Treatment of Schizophrenia. N Engl J Med. 2020 Apr 16;382(16):1497-1506. doi: 10.1056/NEJMoa1911772.
- Kane JM. A New Treatment Paradigm: Targeting Trace Amine-Associated Receptor 1 (TAAR1) in Schizophrenia. J Clin Psychopharmacol. 2022 Sep-Oct 01;42(5 Suppl 1):S1-S13. doi: 10.1097/JCP.0000000000001596.
- Synan C, Bowen C, Heal DJ, Froger-Colléaux C, Beardsley PM, et al. Ulotaront, a novel TAAR1 agonist with 5-HT1A agonist activity, lacks abuse liability and attenuates cocaine cue-induced relapse in rats. Drug Alcohol Depend. 2022 Feb 1;231:109261. doi: 10.1016/j.drugalcdep.2021.109261. Epub 2021 Dec 31.
- A 52-week, Open-label Study to Evaluate the Long-term Safety and Tolerability of SEP-363856 in Patients With Schizophrenia in Japan. NCT05359081 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05359081?term=SEP-363856&phase=2&draw=2&rank=1
- An 8-Week, Open-Label Study Evaluating the Effectiveness, Safety and Tolerability of SEP-363856 in Subjects With Schizophrenia Switched From Typical or Atypical Antipsychotic Agents. NCT05628103 (not yet recruiting). https://clinicaltrials.gov/ct2/show/NCT05628103?term=SEP-363856&phase=2&draw=2&rank=2
- An Open-label Extension Study to Assess the Safety and Tolerability of SEP-363856 in Subjects With Schizophrenia. NCT04109950 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04109950?term=SEP-363856&phase=2&draw=2&rank=3
- A Randomized, Double-blind, Parallel-group, Placebo-controlled, Fixed-dose, Multicenter Study to Evaluate the Efficacy and Safety of SEP-363856 in Acutely Psychotic Subjects With Schizophrenia. NCT04092686 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04092686?term=SEP-363856&phase=2&draw=2&rank=4
- A Randomized, Double-blind, Parallel-group, Placebo-controlled, Fixed-dose, Multicenter Study to Evaluate the Efficacy and Safety of SEP-363856 in Acutely Psychotic Subjects With Schizophrenia. NCT04072354 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04072354?term=SEP-363856&phase=2&draw=2&rank=5
- A Randomized, Double-blind, Active Comparator-Controlled Study to Evaluate the Long-term Safety and Tolerability of SEP-363856 in Subjects With Schizophrenia. NCT04115319 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04115319?term=SEP-363856&phase=2&draw=2&rank=6
- A Randomized, Double-blind, Parallel-group, Placebo Controlled, Fixed-dose, Multicenter Study to Evaluate the Efficacy and Safety of SEP 363856 in Acutely Psychotic Patients With Schizophrenia, Followed by an Open-label Extension Phase. NCT04825860 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04825860?term=SEP-363856&phase=2&draw=2&rank=8
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Vyjuvek (Beremagene Geperpavec). Last updated April 1, 2022. https://epidermolysisbullosanews.com/kb103/
- A Phase III Double Blinded, Placebo-Controlled, Efficacy and Safety Study of Beremagene Geperpavec (B-VEC, Previously "KB103") for the Treatment of Dystrophic Epidermolysis Bullosa (DEB). NCT04491604 (completed). https://clinicaltrials.gov/ct2/show/NCT04491604?term=B-VEC&cond=Dystrophic+Epidermolysis+Bullosa&draw=2&rank=1
- Guide SV, Gonzalez ME, Bağcı IS, Agostini B, Chen H, et al. Trial of Beremagene Geperpavec (B-VEC) for Dystrophic Epidermolysis Bullosa. N Engl J Med. 2022 Dec 15;387(24):2211-2219. doi: 10.1056/NEJMoa2206663.
- Vyjuvek (Beremagene Geperpavec). Last updated April 1, 2022. https://epidermolysisbullosanews.com/kb103/
- Efficacy of Beremagene Geperpavec in Patients With Dystrophic Epidermolysis Bullosa. December 29, 2022. https://www.practiceupdate.com/content/efficacy-of-beremagene-geperpavec-in-patients-with-dystrophic-epidermolysis-bullosa/146343
- Open Label Treatment of Beremagene Geperpavec (B-VEC). NCT04917874 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04917874?term=NCT04917874&draw=2&rank=1
- Fraile JM, Palliyil S, Barelle C, Porter AJ, Kovaleva M. Non-Alcoholic Steatohepatitis (NASH) - A Review of a Crowded Clinical Landscape, Driven by a Complex Disease. Drug Des Devel Ther. 2021 Sep 22;15:3997-4009. doi: 10.2147/DDDT.S315724. eCollection 2021.
- Younossi ZM, Stepanova M, Taub RA, Barbone JM, Harrison SA. Hepatic Fat Reduction Due to Resmetirom in Patients With Nonalcoholic Steatohepatitis Is Associated With Improvement of Quality of Life. Clin Gastroenterol Hepatol. 2022 Jun;20(6):1354-1361.e7. doi: 10.1016/j.cgh.2021.07.039. Epub 2021 Jul 27.
- Positive Topline Phase 3 MAESTRO-NAFLD-1 Data Demonstrate Resmetirom was Safe, Well-Tolerated and Provided Statistically Significant Improvements in Key Measures of Liver and Cardiovascular Health. January 31, 2022. https://ir.madrigalpharma.com/news-releases/news-release-details/positive-topline-phase-3-maestro-nafld-1-data-demonstrate
- A 52-Week, Phase 3, Open-Label Extension Study, With a Single-blind Lead-in, to Evaluate Safety and Biomarkers of Resmetirom (MGL-3196) in Patients With Non-alcoholic Fatty Liver Disease (NAFLD), MAESTRO-NAFLD-Open-Label-Extension (MAESTRO-NAFLD-OLE). NCT04951219 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04951219?term=Resmetirom&phase=2&draw=2&rank=1
- A 52-Week, Phase 3 Study to Evaluate Safety and Biomarkers of Resmetirom (MGL-3196) in Patients With Non-alcoholic Fatty Liver Disease (NAFLD) (MAESTRO-NAFLD-1). NCT04197479 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04197479?term=Resmetirom&phase=2&draw=2&rank=2
- A Randomized Double-blind Placebo-controlled Phase 3 Study to Evaluate the Effect of Resmetirom on Liver-related Outcomes in Patients With Well-compensated (Child-Pugh A) Non-alcoholic Steatohepatitis (NASH) Cirrhosis (MAESTRO-NASH OUTCOMES). NCT05500222 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05500222?term=Resmetirom&phase=2&draw=2&rank=3
- A Phase 3, Multinational, Double-Blind, Randomized, Placebo-Controlled Study of MGL-3196 (Resmetirom) in Patients With NASH and Fibrosis to Resolve NASH and Reduce Progression to Cirrhosis and/or Hepatic Decompensation. NCT03900429 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03900429?term=Resmetirom&phase=2&draw=2&rank=4
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Kremer AE, Mayo MJ, Hirschfield G, Levy C, Bowlus CL, et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022 Jan;42(1):112-123. doi: 10.1111/liv.15039. Epub 2021 Aug 26.
- Kremer AE, Mayo MJ, Hirschfield G, Levy C, Bowlus CL, et al. Seladelpar improved measures of pruritus, sleep, and fatigue and decreased serum bile acids in patients with primary biliary cholangitis. Liver Int. 2022 Jan;42(1):112-123. doi: 10.1111/liv.15039. Epub 2021 Aug 26.
- Bowlus CL, Galambos MR, Aspinall RJ, Hirschfield GM, Jones DEJ, et al. A phase II, randomized, open-label, 52-week study of seladelpar in patients with primary biliary cholangitis. J Hepatol. 2022 Aug;77(2):353-364. doi: 10.1016/j.jhep.2022.02.033. Epub 2022 Mar 30.
- Bowlus CL, Galambos MR, Aspinall RJ, Hirschfield GM, Jones DEJ, et al. A phase II, randomized, open-label, 52-week study of seladelpar in patients with primary biliary cholangitis. J Hepatol. 2022 Aug;77(2):353-364. doi: 10.1016/j.jhep.2022.02.033. Epub 2022 Mar 30.
- A 52-week, Placebo-controlled, Randomized, Phase 3 Study to Evaluate the Safety and Efficacy of Seladelpar in Subjects With Primary Biliary Cholangitis (PBC) and an Inadequate Response to or an Intolerance to Ursodeoxycholic Acid (UDCA). NCT03602560 (completed). https://clinicaltrials.gov/ct2/show/NCT03602560?term=Seladelpar&phase=2&draw=2&rank=2
- ASSURE: An Open Label Long-Term Study to Evaluate the Safety and Tolerability of Seladelpar in Subjects With Primary Biliary Cholangitis (PBC). NCT03301506 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03301506?term=Seladelpar&phase=2&draw=2&rank=1
- RESPONSE: A Placebo-controlled, Randomized, Phase 3 Study to Evaluate the Efficacy and Safety of Seladelpar in Patients With Primary Biliary Cholangitis (PBC) and an Inadequate Response to or an Intolerance to Ursodeoxycholic Acid (UDCA). NCT04620733 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04620733?term=Seladelpar&phase=2&draw=2&rank=3
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- As Delandistrogene moxeparvovec moves closer to clinical approval, what is the likelihood that the drug will be approved? January 3, 2023. https://www.pharmaceutical-technology.com/data-analysis/delandistrogene-moxeparvovec-what-is-the-likelihood-that-drug-will-be-approved/
- Sarepta Therapeutics Announces That U.S. FDA has Accepted for Filing and Granted Priority Review for the Biologics License Application for SRP-9001, Sarepta’s Gene Therapy for the Treatment of Ambulant Individuals with Duchenne Muscular Dystrophy. November 28, 2022. https://investorrelations.sarepta.com/news-releases/news-release-details/sarepta-therapeutics-announces-us-fda-has-accepted-filing-and
- A Phase 3 Multinational, Randomized, Double-Blind, Placebo-Controlled Systemic Gene Delivery Study to Evaluate the Safety and Efficacy of SRP-9001 in Subjects With Duchenne Muscular Dystrophy (EMBARK). NCT05096221 (active not recruiting). https://clinicaltrials.gov/ct2/show/NCT05096221?term=delandistrogene&draw=2&rank=4
- Recursion Announces Initiation of Phase 2/3 Trial for the Treatment of NF2-Mutated Meningiomas at Children's Tumor Foundation NF Conference. June 20, 2022. https://www.prnewswire.com/news-releases/recursion-announces-initiation-of-phase-23-trial-for-the-treatment-of-nf2-mutated-meningiomas-at-childrens-tumor-foundation-nf-conference-301570906.html
- Recursion Announces Initiation of Phase 2/3 Trial for the Treatment of NF2-Mutated Meningiomas at Children's Tumor Foundation NF Conference. June 20, 2022. https://www.prnewswire.com/news-releases/recursion-announces-initiation-of-phase-23-trial-for-the-treatment-of-nf2-mutated-meningiomas-at-childrens-tumor-foundation-nf-conference-301570906.html
- Recursion Announces Initiation of Phase 2/3 Trial for the Treatment of NF2-Mutated Meningiomas at Children's Tumor Foundation NF Conference. June 20, 2022. https://www.prnewswire.com/news-releases/recursion-announces-initiation-of-phase-23-trial-for-the-treatment-of-nf2-mutated-meningiomas-at-childrens-tumor-foundation-nf-conference-301570906.html
- A Parallel-group, Two-staged, Phase 2/3, Randomized, Multicenter Study to Evaluate the Efficacy and Safety of REC-2282 in Participants With Progressive NF2 Mutated Meningiomas. NCT05130866 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05130866?term=REC-2282&draw=2&rank=1
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Craig TJ, Reshef A, Li HH, Jacobs JS, Bernstein JA, et al. Efficacy and safety of garadacimab, a factor XIIa inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial.Lancet. 2023 Feb 28:S0140-6736(23)00350-1. doi: 10.1016/S0140-6736(23)00350-1. Online ahead of print.
- CSL Announces Positive Top-Line Phase 3 Results for Garadacimab as Preventive Treatment in Patients with Hereditary Angioedema (HAE). August 17, 2022. https://www.csl.com/news/2022/20220817-csl-announces-positive-results-for-garadacimab#:~:text=About%20HAE%20and%20Garadacimab&text=Garadacimab%20is%20a%20novel%20Factor,form%20of%20bradykinin%2Dmediated%20angioedema.
- A Multicenter, Double-blind, Randomized, Placebo-controlled, Parallel-arm Study to Investigate the Efficacy and Safety of Subcutaneous Administration of CSL312 (Garadacimab) in the Prophylactic Treatment of Hereditary Angioedema. NCT04656418 (completed). https://clinicaltrials.gov/ct2/show/NCT04656418?term=Garadacimab&phase=2&draw=2&rank=1
- An Open-label Study to Evaluate the Long-term Safety and Efficacy of CSL312 (Garadacimab) in the Prophylactic Treatment of Hereditary Angioedema. NCT04739059 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04739059?term=Garadacimab&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Horwitz ME, Stiff PJ, Cutler C, Brunstein C, Hanna R, et al. Omidubicel vs standard myeloablative umbilical cord blood transplantation: results of a phase 3 randomized study. Blood. 2021 Oct 21;138(16):1429-1440. doi: 10.1182/blood.2021011719.
- Horwitz ME, Stiff PJ, Cutler C, Brunstein C, Hanna R, et al. Omidubicel vs standard myeloablative umbilical cord blood transplantation: results of a phase 3 randomized study. Blood. 2021 Oct 21;138(16):1429-1440. doi: 10.1182/blood.2021011719.
- Lin C, Sajeev G, Stiff PJ, Brunstein CG, Cutler C, et al. Health-Related Quality of Life Following Allogeneic Hematopoietic Cell Transplantation with Omidubicel versus Umbilical Cord Blood. Transplant Cell Ther. 2023 Jan;29(1):52.e1-52.e9. doi: 10.1016/j.jtct.2022.09.018. Epub 2022 Sep 28.
- Gamida Cell Provides Regulatory Update on Omidubicel. November 21, 2022. https://www.businesswire.com/news/home/20221121005927/en/Gamida-Cell-Provides-Regulatory-Update-on-Omidubicel#:~:text=Omidubicel%20is%20an%20investigational%20stem,or%20any%20other%20health%20authority.
- Horwitz ME, Stiff PJ, Cutler C, Brunstein C, Hanna R, et al. Omidubicel vs standard myeloablative umbilical cord blood transplantation: results of a phase 3 randomized study. Blood. 2021 Oct 21;138(16):1429-1440. doi: 10.1182/blood.2021011719.
- Posoleucel (Viralym-M, ALVR105): A multi-virus specific T cell therapy (VST) targeting five devastating viral pathogens. https://www.allovir.com/products/alvr105
- AlloVir Announces Positive Final Results in Phase 2 Posoleucel Multi-Virus Prevention Study In Oral Presentation at the 64th ASH Annual Meeting and Exposition. December 10, 2022. https://www.businesswire.com/news/home/20221210005012/en/AlloVir-Announces-Positive-Final-Results-in-Phase-2-Posoleucel-Multi-Virus-Prevention-Study-In-Oral-Presentation-at-the-64th-ASH-Annual-Meeting-and-Exposition
- Phase 3, Randomized, Double-Blind, Placebo-Controlled Trial, With Cross-Over, of Posoleucel (ALVR105) for the Treatment of Adenovirus Infection in Pediatric and Adult Participants Receiving Standard of Care Following Allogeneic Hematopoietic Cell Transplantation. NCT05179057 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05179057?term=posoleucel&phase=2&draw=2&rank=1
- Phase 2/3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of ALVR105 (Viralym-M) Compared to Placebo for the Prevention of AdV, BKV, CMV, EBV, HHV-6, and JCV Infection and/or Disease, in High-Risk Patients After Allogeneic Hematopoietic Cell Transplant. NCT05305040 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05305040?term=posoleucel&phase=2&draw=2&rank=3
- Phase 2/3, Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Safety and Efficacy of ALVR105 (Viralym-M) Compared to Placebo for the Prevention of AdV, BKV, CMV, EBV, HHV-6, and JCV Infection and/or Disease, in High-Risk Patients After Allogeneic Hematopoietic Cell Transplant. NCT04693637 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04693637?term=posoleucel&phase=2&draw=2&rank=4
- Newman LM, Kankam M, Nakamura A, Conrad T, Mueller J, et al. Thorough QT Study To Evaluate the Effect of Zoliflodacin, a Novel Therapeutic for Gonorrhea, on Cardiac Repolarization in Healthy Adults. Antimicrob Agents Chemother. 2021 Nov 17;65(12):e0129221. doi: 10.1128/AAC.01292-21. Epub 2021 Oct 4.
- Bradford PA, Miller AA, O'Donnell J, Mueller JP. Zoliflodacin: An Oral Spiropyrimidinetrione Antibiotic for the Treatment of Neisseria gonorrheae, Including Multi-Drug-Resistant Isolates. ACS Infect Dis. 2020 Jun 12;6(6):1332-1345. doi: 10.1021/acsinfecdis.0c00021.Epub 2020 May 12.
- A Multi-center, Randomized, Open-label, Non Inferiority Trial to Evaluate the Efficacy and Safety of a Single, Oral Dose of Zoliflodacin Compared to a Combination of a Single Intramuscular Dose of Ceftriaxone and a Single Oral Dose of Azithromycin in the Treatment of Patients With Uncomplicated Gonorrhoea. NCT03959527 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03959527?term=Zoliflodacin&phase=2&draw=2&rank=1
- A Double-Blinded, Placebo-controlled, Phase 3 Study to Evaluate the Efficacy and Safety of ORMD-0801 in Uncontrolled Type 2 DM Subjects on Diet Control Alone, Metformin Monotherapy, or Two or Three Oral Glucose-lowering Agents. NCT04817215 (not yet recruiting). https://clinicaltrials.gov/ct2/show/NCT04817215?term=ORMD-0801&phase=2&draw=2&rank=1
- A Double-Blind, Placebo-controlled, Multi-center Randomized, Phase 3 Study to Evaluate the Efficacy and Safety of ORMD-0801 in T2DM Subjects With Inadequate Glycemic Control on Diet Control Only or on Diet Control and Metformin Monotherapy. NCT04754334 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04754334?term=ORMD-0801&phase=2&draw=2&rank=2
- A Double-Blinded, Placebo-controlled, Double Dummy, Multi-center Randomized, Phase 3 Study to Evaluate the Efficacy and Safety of ORMD-0801 in Subjects With T2DM With Inadequate Glycemic Control on 1, 2 or 3 Oral Glucose-lowering Agents. NCT04606576 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04606576?term=ORMD-0801&phase=2&draw=2&rank=3
- Kjeldsen TB, Hubálek F, Hjørringgaard CU, Tagmose TM, Nishimura E, et al. Molecular Engineering of Insulin Icodec, the First Acylated Insulin Analog for Once-Weekly A dministration in Humans. J Med Chem. 2021 Jul 8;64(13):8942-8950. doi: 10.1021/acs.jmedchem.1c00257. Epub 2021 May 4.
- Rosenstock J, Bajaj HS, Janež A, Silver R, Begtrup K, et al; NN1436-4383 Investigators. Once-Weekly Insulin for Type 2 Diabetes without Previous Insulin Treatment. N Engl J Med. 2020 Nov 26;383(22):2107-2116. doi: 10.1056/NEJMoa2022474. Epub 2020 Sep 22.
- Rosenstock J, Bajaj HS, Janež A, Silver R, Begtrup K, et al; NN1436-4383 Investigators. Once-Weekly Insulin for Type 2 Diabetes without Previous Insulin Treatment. N Engl J Med. 2020 Nov 26;383(22):2107-2116. doi: 10.1056/NEJMoa2022474. Epub 2020 Sep 22.
- A 26-week Trial Comparing the Effect and Safety of Once Weekly Insulin Icodec and Once Daily Insulin Degludec, Both With or Without Non-insulin Anti-diabetic Drugs, in Subjects With Type 2 Diabetes Treated With Basal Insulin. NCT04770532 (completed). https://clinicaltrials.gov/ct2/show/NCT04770532?term=Insulin+icodec&phase=2&draw=2&rank=1
- Effectiveness and Safety of Once Weekly Insulin Icodec Used With DoseGuide Versus Once Daily Basal Insulin Analogues in an Insulin naïve Type 2 Diabetes Population in a Clinical Practice Setting. NCT04760626 (completed). https://clinicaltrials.gov/ct2/show/NCT04760626?term=Insulin+icodec&phase=2&draw=2&rank=5
- A 26-week Trial Comparing the Effect and Safety of Once Weekly Insulin Icodec and Once Daily Insulin Glargine 100 Units/mL, Both in Combination With Bolus Insulin With or Without Non-insulin Anti-diabetic Drugs, in Subjects With Type 2 Diabetes on a Basal-bolus Regimen. NCT04880850 (completed). https://clinicaltrials.gov/ct2/show/NCT04880850?term=Insulin+icodec&phase=2&draw=2&rank=6
- A 26-week Double Blinded, Multiregional, Trial Comparing the Effect and Safety of Once Weekly Insulin Icodec and Once Daily Insulin Degludec 100 Units/mL, Both in Combination With Non-insulin Anti-diabetic Drugs, in Insulin naïve Subjects With Type 2 Diabetes. NCT04795531 (completed). https://clinicaltrials.gov/ct2/show/NCT04795531?term=Insulin+icodec&phase=2&draw=2&rank=7
- A 78-week Trial Comparing the Effect and Safety of Once Weekly Insulin Icodec and Once Daily Insulin Glargine 100 Units/mL, Both in Combination With Non-insulin Anti-diabetic Treatment, in Insulin naïve Subjects With Type 2 Diabetes. NCT04460885 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04460885?term=Insulin+icodec&phase=2&draw=2&rank=2
- Efficacy and Safety of Once Weekly Insulin Icodec Compared to Once Daily Insulin Degludec 100 Units/mL, Both in Combination With Insulin Aspart, in Adults With Type 1 Diabetes. A 26-week, Randomised, Multicentre, Open-label, Active-controlled, Parallel Group, Two Armed, Treat-to-target Trial Investigating the Effect on Glycaemic Control and Safety of Treatment With Once Weekly Insulin Icodec Compared to Once Daily Insulin Degludec, Both in Combination With Insulin Aspart in Adults With Type 1 Diabetes, With a 26-week Extension Investigating Long Term Safety. NCT04848480 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04848480?term=Insulin+icodec&phase=2&draw=2&rank=3
- A 52 Week Study Comparing the Efficacy and Safety of Once Weekly IcoSema and Once Weekly Insulin Icodec, Both Treatment Arms With or Without Oral Anti Diabetic Drugs, in Participants With Type 2 Diabetes Inadequately Controlled With Daily Basal Insulin. NCT05352815 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05352815?term=Insulin+icodec&phase=2&draw=2&rank=4
- A 52 Week Study Comparing the Efficacy and Safety of Once Weekly IcoSema and Daily Insulin Glargine 100 Units/mL Combined With Insulin Aspart, Both Treatment Arms With or Without Oral Anti Diabetic Drugs, in Participants With Type 2 Diabetes Inadequately Controlled With Daily Basal Insulin. COMBINE 3. NCT05013229 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05013229?term=Insulin+icodec&phase=2&draw=2&rank=8
- A 52 Week Study Comparing the Efficacy and Safety of Once Weekly IcoSema and Once Weekly Semaglutide, Both Treatment Arms With or Without Oral Anti Diabetic Drugs, in Participants With Type 2 Diabetes Inadequately Controlled With a GLP 1 Receptor Agonist. COMBINE 2. NCT05259033 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05259033?term=Insulin+icodec&phase=2&draw=2&rank=9
- [No authors listed]. FGFR Inhibitor Stymies Gastric Cancer. Cancer Discov. 2021 May;11(5):OF3. doi: 10.1158/2159-8290.CD-NB2021-0312. Epub 2021 Feb 11.
- A Phase 1b/3 Study of Bemarituzumab Plus Chemotherapy and Nivolumab Versus Chemotherapy and Nivolumab Alone in Subjects With Previously Untreated Advanced Gastric and Gastroesophageal Junction Cancer With FGFR2b Overexpression. NCT05111626 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05111626?term=Bemarituzumab&phase=2&draw=2&rank=1
- A Randomized, Multi-center, Double-blind, Placebo-controlled Phase 3 Study of Bemarituzumab Plus Chemotherapy Versus Placebo Plus Chemotherapy in Subjects With Previously Untreated Advanced Gastric or Gastroesophageal Junction Cancer With FGFR2b Overexpression. NCT05052801 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05052801?term=Bemarituzumab&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Lonial S, Popat R, Hulin C, Jagannath S, Oriol A, et al. Iberdomide plus dexamethasone in heavily pretreated late-line relapsed or refractory multiple myeloma (CC-220-MM-001): a multicentre, multicohort, open-label, phase 1/2 trial. Lancet Haematol. 2022 Nov;9(11):e822-e832. doi: 10.1016/S2352-3026(22)00290-3. Epub 2022 Oct 6.
- A Phase 3, Two-Stage, Randomized, Multicenter, Open-label Study Comparing Iberdomide, Daratumumab and Dexamethasone (IberDd) Versus Daratumumab, Bortezomib, and Dexamethasone (DVd) in Subjects With Relapsed or Refractory Multiple Myeloma (RRMM). NCT04975997 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04975997?term=Iberdomide&phase=2&draw=2&rank=1
- GEM21menos65. A Phase III Trial for NDMM Patients Who Are Candidates for ASCT Comparing Extended VRD Plus Early Rescue Intervention vs Isatuximab-VRD vs Isatuximab-V-Iberdomide-D. NCT05558319 (not yet recruiting). https://clinicaltrials.gov/ct2/show/NCT05558319?term=Iberdomide&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Mascarenhas J, Harrison CN, Kiladjian JJ, Komrokji RS, Koschmieder S, et al. Imetelstat in intermediate-2 or high-risk myelofibrosis refractory to JAK inhibitor: IMpactMF phase III study design. Future Oncol. 2022 Jul;18(22):2393-2402. doi: 10.2217/fon-2022-0235. Epub 2022 May 5.
- Steensma DP, Fenaux P, Van Eygen K, Raza A, Santini V,et al. Imetelstat Achieves Meaningful and Durable Transfusion Independence in High Transfusion-Burden Patients With Lower-Risk Myelodysplastic Syndromes in a Phase II Study. J Clin Oncol. 2021 Jan 1;39(1):48-56. doi: 10.1200/JCO.20.01895. Epub 2020 Oct 27.
- A Randomized Open-Label, Phase 3 Study to Evaluate Imetelstat (GRN163L) Versus Best Available Therapy (BAT) in Patients With Intermediate-2 or High-risk Myelofibrosis (MF) Relapsed / Refractory (R/R) to Janus Kinase (JAK) Inhibitor. NCT04576156 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04576156?term=Imetelstat&phase=2&draw=2&rank=1
- A Study to Evaluate Imetelstat (GRN163L) in Transfusion-Dependent Subjects With IPSS Low or Intermediate-1 Risk Myelodysplastic Syndrome (MDS) That is Relapsed/Refractory to Erythropoiesis-Stimulating Agent (ESA) Treatment. NCT02598661 (recruiting). https://clinicaltrials.gov/ct2/show/NCT02598661?term=Imetelstat&phase=2&draw=2&rank=4
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Pandravada S, Sandler S. The Role of Navitoclax in Myelofibrosis. Cureus. 2021 Sep 14;13(9):e17976. doi: 10.7759/cureus.17976. eCollection 2021 Sep.
- Harrison CN, Garcia JS, Somervaille TCP, Foran JM, Verstovsek S, et al. Addition of Navitoclax to Ongoing Ruxolitinib Therapy for Patients With Myelofibrosis With Progression or Suboptimal Response: Phase II Safety and Efficacy. J Clin Oncol. 2022 May 20;40(15):1671-1680. doi: 10.1200/JCO.21.02188. Epub 2022 Feb 18.
- A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study Of Navitoclax In Combination With Ruxolitinib Versus Ruxolitinib In Subjects With Myelofibrosis (TRANSFORM-1). NCT04472598 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04472598?term=Navitoclax&phase=2&draw=2&rank=1
- A Randomized, Open-Label, Phase 3 Study Evaluating Efficacy and Safety of Navitoclax in Combination With Ruxolitinib Versus Best Available Therapy in Subjects With Relapsed/Refractory Myelofibrosis (TRANSFORM-2). NCT04468984 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04468984?term=Navitoclax&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Corcept Therapeutics to Start Phase 3 Trial of Relacorilant Plus Nab-Paclitaxel in Patients With Platinum-Resistant Ovarian Cancer. June 2, 2022. https://www.globenewswire.com/en/news-release/2022/06/06/2456669/0/en/Corcept-Therapeutics-to-Start-Phase-3-Trial-of-Relacorilant-Plus-Nab-Paclitaxel-in-Patients-With-Platinum-Resistant-Ovarian-Cancer.html
- Corcept Therapeutics to Start Phase 3 Trial of Relacorilant Plus Nab-Paclitaxel in Patients With Platinum-Resistant Ovarian Cancer. June 2, 2022. https://www.globenewswire.com/en/news-release/2022/06/06/2456669/0/en/Corcept-Therapeutics-to-Start-Phase-3-Trial-of-Relacorilant-Plus-Nab-Paclitaxel-in-Patients-With-Platinum-Resistant-Ovarian-Cancer.html
- Corcept Therapeutics to Start Phase 3 Trial of Relacorilant Plus Nab-Paclitaxel in Patients With Platinum-Resistant Ovarian Cancer. June 2, 2022. https://www.globenewswire.com/en/news-release/2022/06/06/2456669/0/en/Corcept-Therapeutics-to-Start-Phase-3-Trial-of-Relacorilant-Plus-Nab-Paclitaxel-in-Patients-With-Platinum-Resistant-Ovarian-Cancer.html
- A Phase 3 Study of Relacorilant in Combination With Nab-Paclitaxel Versus Nab-Paclitaxel Monotherapy in Advanced, Platinum-Resistant, High-Grade Epithelial Ovarian, Primary Peritoneal, or Fallopian-Tube Cancer (ROSELLA). NCT05257408 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05257408?term=Relacorilant&phase=2&draw=2&rank=4
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Rusfertide May Eliminate the Need for Phlebotomies in Polycythemia Vera. September 29, 2022. https://www.targetedonc.com/view/rusfertide-may-eliminate-the-need-for-phlebotomies-in-polycythemia-vera
- Protagonist Therapeutics to Present Updated Phase 2 Rusfertide Clinical Results in Polycythemia Vera (PV) at ASCO 2022. May 26, 2022. https://www.prnewswire.com/news-releases/protagonist-therapeutics-to-present-updated-phase-2-rusfertide-clinical-results-in-polycythemia-vera-pv-at-asco-2022-301556326.html
- Pemmaraju N, Kuykendall A, Kremyanskaya M, Ginzburg Y, Ritchie E, Gotlib J, et al. MPN-469 Rusfertide (PTG-300) Treatment Interruption Reverses Hematological Gains and Upon Reinitiation, Restoration of Clinical Benefit Observed in Patients With Polycythemia Vera. Clin Lymphoma Myeloma Leuk. 2022 Oct;22 Suppl 2:S338-S339. doi: 10.1016/S2152-2650(22)01462-8.
- Protagonist Therapeutics to Present Updated Phase 2 Rusfertide Clinical Results in Polycythemia Vera (PV) at ASCO 2022. May 26, 2022. https://www.prnewswire.com/news-releases/protagonist-therapeutics-to-present-updated-phase-2-rusfertide-clinical-results-in-polycythemia-vera-pv-at-asco-2022-301556326.html
- Rusfertide Therapy for Polycythemia Vera Leads to Sustained Hematocrit Control. Mid-July 2022. https://ashpublications.org/ashclinicalnews/news/6294/rusfertide-therapy-for-polycythemia-vera-leads-to
- A Phase 3 Study of the Hepcidin Mimetic Rusfertide (PTG-300) in Patients With Polycythemia Vera. NCT05210790 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05210790?term=Rusfertide&draw=2&rank=1
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Kyuno D, Takasawa A, Takasawa K, Ono Y, Aoyama T, et al. Claudin-18.2 as a therapeutic target in cancers: cumulative findings from basic research and clinical trials. Tissue Barriers. 2022 Jan 2;10(1):1967080. doi: 10.1080/21688370.2021.1967080. Epub 2021 Sep 5.
- Palmieri LJ, Soubeyran I, Pernot S. [Oesogastric cancer - new therapeutic targets]. [Article in French] Bull Cancer. 2022 Nov 9:S0007-4551(22)00340-X. doi: 10.1016/j.bulcan.2022.08.005. Online ahead of print.
- Zolbetuximab With mFOLFOX6 Meets Primary Endpoint in SPOTLIGHT Trial. January 19, 2023. https://dailynews.ascopubs.org/do/zolbetuximab-mfolfox6-meets-primary-endpoint-spotlight-trial
- A Phase 3, Global, Multi-Center, Double-Blind, Randomized, Efficacy Study of Zolbetuximab (IMAB362) Plus CAPOX Compared With Placebo Plus CAPOX as First-line Treatment of Subjects With Claudin (CLDN) 18.2-Positive, HER2-Negative, Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma. NCT03653507 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03653507?term=Zolbetuximab&phase=2&draw=2&rank=1
- A Phase 3, Global, Multi-Center, Double-Blind, Randomized, Efficacy Study of Zolbetuximab (IMAB362) Plus mFOLFOX6 Compared With Placebo Plus mFOLFOX6 as First-line Treatment of Subjects With Claudin (CLDN)18.2-Positive, HER2-Negative, Locally Advanced Unresectable or Metastatic Gastric or Gastroesophageal Junction (GEJ) Adenocarcinoma. NCT03504397 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03504397?term=Zolbetuximab&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Vignal-Clermont C(1)(2), Girmens JF(2)(3), Audo I(2)(3)(4), Said SM(2)(3)(4), Errera MH(2)(3)(5), et al. Safety of Intravitreal Gene Therapy for Treatment of Subjects with Leber Hereditary Optic Neuropathy due to Mutations in the Mitochondrial ND4 Gene: The REVEAL Study.BioDrugs. 2021 Mar;35(2):201-214. doi: 10.1007/s40259-021-00468-9. Epub 2021 Feb 10.
- A Randomized, Double-Masked, Sham-Controlled Clinical Trial to Evaluate the Efficacy of a Single Intravitreal Injection of GS010 in Subjects Affected for 6 Months or Less by LHON Due to the G11778A Mutation in the Mitochondrial ND4 Gene. NCT02652767 (completed). https://clinicaltrials.gov/ct2/show/NCT02652767?cond=NCT02652767&draw=2&rank=1
- Randomized, Double-Masked, Sham-Controlled Clinical Trial to Evaluate the Efficacy of a Single Intravitreal Injection of GS010 in Subjects Affected for More Than 6 Months and To 12 Months by LHON Due to the G11778A Mutation in the ND4 Gene. NCT02652780 (completed). https://clinicaltrials.gov/ct2/show/NCT02652780
- Long-term Follow-up of ND4 LHON Subjects Treated With GS010 Ocular Gene Therapy in the RESCUE or REVERSE Phase III Clinical Trials (RESTORE). NCT03406104 completed). https://clinicaltrials.gov/ct2/show/NCT03406104?term=NCT03406104&draw=2&rank=1
- Carelli V, Newman NJ, Yu-Wai-Man P, Biousse V, Moster ML, et al; the LHON Study Group. Indirect Comparison of Lenadogene Nolparvovec Gene Therapy Versus Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A MT-ND4 Mutation.Ophthalmol Ther. 2023 Feb;12(1):401-429. doi: 10.1007/s40123-022-00611-x. Epub 2022 Nov 30.
- Biousse V, Newman NJ, Yu-Wai-Man P, Carelli V, Moster ML, et al; LHON Study Group. Long-Term Follow-Up After Unilateral Intravitreal Gene Therapy for Leber Hereditary Optic Neuropathy: The RESTORE Study. J Neuroophthalmol. 2021 Sep 1;41(3):309-315. doi: 10.1097/WNO.0000000000001367.
- Vignal-ClermoḤ C, Yu-Wai-Man P, Newman NJ, Carelli V, Moster ML, et al; LHON Study Group. Safety of lenadogene nolparvovec gene therapy over 5 years in 189 patients with Leber hereditary optic neuropathy. Am J Ophthalmol. 2022 Dec 7:S0002-9394(22)00464-0. doi: 10.1016/j.ajo.2022.11.026. Online ahead of print.
- BRIEF—FDA setback for GenSight’s Lumevoq. January 19, 2022. https://www.thepharmaletter.com/in-brief/brief-fda-setback-for-gensight-s-lumevoq
- Toth PP, Schwartz GG, Nicholls SJ, Khan A, Szarek M, et al. Reduction in the risk of major adverse cardiovascular events with the BET protein inhibitor apabetalone in patients with recent acute coronary syndrome, type 2 diabetes, and moderate to high likelihood of non-alcoholic fatty liver disease. Am J Prev Cardiol. 2022 Aug 8;11:100372. doi: 10.1016/j.ajpc.2022.100372. eCollection 2022 Sep.
- A Phase 3, Multicenter, Double-blind, Randomized, Placebo-controlled, Parallel-group Study to Investigate the Efficacy and Safety of CSL112 in Subjects With Acute Coronary Syndrome. NCT03473223 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03473223?term=NCT03473223&draw=2&rank=1
- https://www.dicardiology.com/content/memorialcare-heart-and-vascular-institute-participating-global-study-csl112-patients-acute. March 8, 2022.
- A Phase 3, Multicenter, Randomized, Double Blind, Placebo Controlled Study to Evaluate the Efficacy and Safety of AL001 in Individuals at Risk for or With Frontotemporal Dementia Due to Heterozygous Mutations in the Progranulin Gene. NCT04374136 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04374136?term=AL001&phase=2&draw=2&rank=1
- A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study of the Efficacy, Safety and Biomarker Effects of ALZ-801 in Subjects With Early Alzheimer's Disease and APOE4/4 Genotype.NCT04770220 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04770220?term=NCT04770220&draw=2&rank=1
- Smith KW, Sicignano D), Hernandez AV, White CM. MDMA-Assisted Psychotherapy for Treatment of Posttraumatic Stress Disorder: A Systematic Review With Meta-Analysis. J Clin Pharmacol. 2022 Apr;62(4):463-471. doi: 10.1002/jcph.1995. Epub 2021 Nov 28.
- A Randomized, Double-Blind, Placebo-Controlled, Multi-Site Phase 3 Study of the Efficacy and Safety of Manualized MDMA-Assisted Psychotherapy for the Treatment of Posttraumatic Stress Disorder of Moderate or Greater Severity. NCT04077437 (completed). https://clinicaltrials.gov/ct2/show/NCT04077437?term=NCT04077437&draw=2&rank=1.
- A Multi-Site Open-Label Safety Extension Study of Manualized MDMA-Assisted Psychotherapy for the Treatment of Participants With Posttraumatic Stress Disorder. NCT04714359 (enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT04714359?term=NCT04714359&draw=2&rank=1.
- A Multicenter, Randomized, Active-controlled, Double-blind, Double-dummy, Parallel Group Clinical Trial, Investigating the Efficacy, Safety, and Tolerability of Continuous Subcutaneous ND0612 Infusion in Comparison to Oral IR-LD/CD in Subjects With Parkinson's Disease Experiencing Motor Fluctuations (BouNDless). NCT04006210 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04006210?term=NCT04006210&draw=2&rank=1.
- An Open-label, Long-term Extension Study of Brazikumab in Participants With Moderately to Severely Active Crohn's Disease (INTREPID OLE). NCT03961815 (enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT03961815?term=NCT03961815&draw=2&rank=1.
- A 52-Week, Multicenter, Randomized, Double-blind, Placebo and Active-Controlled, Operationally Seamless Phase 2b/3, Parallel-group Study to Assess the Efficacy and Safety of Brazikumab in Participants With Moderately to Severely Active Crohn's Disease (INTREPID Lead-In). NCT03759288 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03759288?term=NCT03759288&draw=2&rank=1.
- A Phase 3 Prospective, Randomized, Double-blinded, Placebo-controlled Clinical Study to Evaluate the Efficacy and Safety of RBX2660 (Microbiota Suspension) for the Prevention of Clostridium Difficile InfectionNCT03244644 (completed). https://clinicaltrials.gov/ct2/show/NCT03244644?term=NCT03244644&draw=2&rank=1
- Khanna S, Assi M, Lee C, Yoho D, Louie T, et al. Efficacy and Safety of RBX2660 in PUNCH CD3, a Phase III, Randomized, Double-Blind, Placebo-Controlled Trial with a Bayesian Primary Analysis for the Prevention of Recurrent Clostridioides difficile Infection. Drugs. 2022 Oct 26:1-12. doi: 10.1007/s40265-022-01797-x. Online ahead of print.
- Ferring and Rebiotix Present Landmark Phase 3 Data Demonstrating Superior Efficacy of Investigational RBX2660 Versus Placebo to Reduce Recurrence of C. difficile Infection. May 21, 2021. https://www.rebiotix.com/efficacy-rbx2660-vs-placebo-reduce-recurrence-c-difficile-infection/
- A Phase 3 Open-Label Clinical Study to Evaluate the Safety and Tolerability of Rebiotix RBX2660 (Microbiota Suspension) in Subjects With Recurrent Clostridium Difficile Infection. NCT03931941 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03931941?term=NCT03931941&draw=2&rank=1.
- A Phase III, Randomized, Multicenter, Parallel-Group, Double-Blind, Double-Dummy Study in Adolescent and Adult Female Participants Comparing the Efficacy and Safety of Gepotidacin to Nitrofurantoin in the Treatment of Uncomplicated Urinary Tract Infection (Acute Cystitis). NCT04020341 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04020341?term=NCT04020341&draw=2&rank=1.
- A Phase III, Randomized, Multicenter, Parallel-Group, Double-Blind, Double-Dummy Study in Adolescent and Adult Female Participants Comparing the Efficacy and Safety of Gepotidacin to Nitrofurantoin in the Treatment of Uncomplicated Urinary Tract Infection (Acute Cystitis). NCT04187144 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04187144?term=NCT04187144&draw=2&rank=1.
- EAGLE-2 and EAGLE-3 phase III trials for gepotidacin stopped early for efficacy following pre-planned interim analysis by Independent Data Monitoring Committee Issued: London UK. November 3, 2022. https://www.gsk.com/en-gb/media/press-releases/gsk-announces-phase-iii-trials-for-gepotidacin/
- New antibiotic appears to be effective against urinary tract infections, drug company says. November 3, 2022. https://www.cnn.com/2022/11/03/health/new-uti-antibiotic-gepotidacin/index.html
- GSK to submit drug application for new antibiotic to US FDA. November 4, 2022. https://www.pmlive.com/pharma_news/gsk_to_submit_drug_application_for_new_antibiotic_to_us_fda_1480238
- Bhatt DL, Pollack, Jr. CV, Mazer D, Angiolillo DJ, Steg G, et al; for the REVERSE-IT Investigators. Bentracimab for Ticagrelor Reversal in Patients Undergoing Urgent Surgery. NEJM Evid 2021; 1 (3) DOI:https://doi.org/10.1056/EVIDoa2100047. https://evidence.nejm.org/doi/full/10.1056/EVIDoa2100047
- PhaseBio Announces Successful Pre-BLA Meeting with U.S. FDA for Bentracimab. May 16, 2022. https://www.businesswire.com/news/home/20220516005342/en/PhaseBio-Announces-Successful-Pre-BLA-Meeting-with-U.S.-FDA-for-Bentracimab
- Danicopan (ALXN2040) add-on to Ultomiris or Soliris met primary endpoint in ALPHA Phase III trial for patients with paroxysmal nocturnal haemoglobinuria who experience clinically significant extravascular haemolysis. September 16, 2022. https://www.astrazeneca.com/media-centre/press-releases/2022/danicopan-phase-iii-trial-met-primary-endpoint.html
- A Phase 3 Study of Danicopan (ALXN2040) as Add-on Therapy to a C5 Inhibitor (Eculizumab or Ravulizumab) in Patients With Paroxysmal Nocturnal Hemoglobinuria Who Have Clinically Evident Extravascular Hemolysis (EVH). NCT04469465 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04469465?term=NCT04469465&draw=2&rank=1.
- Gene Therapy for Hemophilia Is on the Brink of FDA Approval. November 17, 2022. https://www.managedhealthcareexecutive.com/view/gene-therapy-for-hemophilia-is-on-the-brink-of-fda-approval
- Press Release: Fitusiran prophylaxis reduced bleeds by 61% in people with hemophilia A or B, with or without inhibitors, compared to prior factor or bypassing agent prophylaxis. July 10, 2022. https://www.sanofi.com/en/media-room/press-releases/2022/2022-07-10-13-45-00-2476896
- Press Release: Fitusiran prophylaxis reduced bleeds by 61% in people with hemophilia A or B, with or without inhibitors, compared to prior factor or bypassing agent prophylaxis. July 10, 2022. https://www.sanofi.com/en/media-room/press-releases/2022/2022-07-10-13-45-00-2476896
- An Open-label, Long-term Safety and Efficacy Study of Fitusiran in Patients With Hemophilia A or B, With or Without Inhibitory Antibodies to Factor VIII or IX. NCT03754790 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03754790?term=Fitusiran&phase=2&draw=2&rank=2
- Khan AA, Rubin MR, Schwarz P, Vokes T, Shoback DM, et al. Efficacy and Safety of Parathyroid Hormone Replacement With TransCon PTH in Hypoparathyroidism: 26-Week Results From the Phase 3 PaTHway Trial. J Bone Miner Res. 2022 Oct 21. doi: 10.1002/jbmr.4726. Online ahead of print.
- TransCon PTH Gets Priority Review for Hypoparathyroidism. November 2, 2022. https://www.empr.com/home/news/drugs-in-the-pipeline/transcon-pth-gets-priority-review-for-hypoparathyroidism/
- Tuberculosis Pipeline Landscape Analysis of 38+ Companies by DelveInsight. May 4, 2022. https://www.globenewswire.com/en/news-release/2022/05/04/2436001/0/en/Tuberculosis-Pipeline-Landscape-Analysis-of-38-Companies-by-DelveInsight.html
- Nymox Announces Submission of New Drug Application (NDA) to the FDA for Fexapotide Triflutate. March 03, 2022. https://www.drugs.com/nda/fexapotide_triflutate_220303.html
- Prothena to Present Data on Survival Benefit Observed in Completed Phase 3 Study of Drug Candidate Birtamimab in Patients with Mayo Stage IV AL Amyloidosis at the ASH 2022 Meeting. November 3, 2022. https://www.businesswire.com/news/home/20221103005351/en/Prothena-to-Present-Data-on-Survival-Benefit-Observed-in-Completed-Phase-3-Study-of-Drug-Candidate-Birtamimab-in-Patients-with-Mayo-Stage-IV-AL-Amyloidosis-at-the-ASH-2022-Meeting
- A Phase 3, Randomized, Multicenter, Double-Blind, Placebo-Controlled, Efficacy and Safety Study of Birtamimab Plus Standard of Care vs. Placebo Plus Standard of Care in Mayo Stage IV Subjects With Light Chain (AL) Amyloidosis. NCT04973137 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04973137?term=NCT04973137&draw=2&rank=1.
- CellTrans’ Lantidra™ (donislecel) approaches a decision from the FDA. June 15, 2021. https://www.primetherapeutics.com/news/celltrans-lantidra-donislecel-approaches-a-decision-from-the-fda/
- FDA Meeting Likely to Lead to New Request for PRX-102 Approval. October 22, 2021. https://fabrydiseasenews.com/news/new-fda-approval-request-prx-102-likely-protalix-reports/
- Protalix Resubmits To FDA The BLA For Pegunigalsidase Alfa For Treatment Of Fabry Disease. November 14, 2022. https://www.nasdaq.com/articles/protalix-resubmits-to-fda-the-bla-for-pegunigalsidase-alfa-for-treatment-of-fabry-disease
- Protalix BioTherapeutics and Chiesi Global Rare Diseases Announce the Submission of a Marketing Authorization Application to the European Medicines Agency for PRX-102 for the Treatment of Fabry Disease. February 24, 2022. https://www.prnewswire.com/il/news-releases/protalix-biotherapeutics-and-chiesi-global-rare-diseases-announce-the-submission-of-a-marketing-authorization-application-to-the-european-medicines-agency-for-prx-102-for-the-treatment-of-fabry-disease-301491083.html
- A Randomized, Multicenter, Parallel-group, Phase III Study to Compare the Efficacy of Arfolitixorin Versus Leucovorin in Combination With 5 Fluorouracil, Oxaliplatin, and Bevacizumab in Patients With Advanced Colorectal Cancer. NCT03750786 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03750786?term=NCT03750786&draw=2&rank=1.
- A Phase 3 Multicenter, Randomized, Double-Blind, Placebo Controlled Study to Determine the Efficacy of Topical SGX301 (Synthetic Hypericin) and Fluorescent Bulb-Light Irradiation for the Treatment of Cutaneous T-Cell Lymphoma. NCT02448381 (completed). https://clinicaltrials.gov/ct2/show/NCT02448381?term=NCT02448381&draw=2&rank=1.
- Kim EJ, Mangold AR, DeSimone JA, Wong HK, Seminario-Vidal L, et al. Efficacy and Safety of Topical Hypericin Photodynamic Therapy for Early-Stage Cutaneous T-Cell Lymphoma (Mycosis Fungoides): The FLASH Phase 3 Randomized Clinical Trial. JAMA Dermatol. 2022 Sep 1;158(9):1031-1039. doi: 10.1001/jamadermatol.2022.2749.
- FDA Declines to File New Drug Application for SGX301 in T-Cell Lymphoma. February 20, 2023. https://www.cancernetwork.com/view/fda-declines-to-file-new-drug-application-for-sgx301-in-t-cell-lymphoma
- A Phase III, Randomized, Double-Blind, Placebo-Controlled, Multicenter Trial Testing Ipatasertib Plus Abiraterone Plus Prednisone/Prednisolone, Relative to Placebo Plus Abiraterone Plus Prednisone/Prednisolone in Adult Male Patients With Asymptomatic or Mildly Symptomatic, Previously Untreated, Metastatic Castrate-Resistant Prostate Cancer. NCT03072238 (active, but not recruiting). https://clinicaltrials.gov/ct2/show/NCT03072238?term=NCT03072238&draw=2&rank=1
- BioLineRx Announces U.S. FDA Acceptance of New Drug Application for Aphexda (motixafortide) in Stem Cell Mobilization. November 2022. https://www.drugs.com/nda/aphexda_221110.html
- BioLineRx Announces U.S. FDA Acceptance of New Drug Application for Aphexda (motixafortide) in Stem Cell Mobilization. November 2022. https://www.drugs.com/nda/aphexda_221110.html
- A Phase 3 Multicenter, Randomized, Double Masked, Sham- Controlled Clinical Trial to Assess the Safety and Efficacy of Intravitreal Administration of Zimura (Complement C5 Inhibitor) in Patients With Geographic Atrophy Secondary to Age-Related Macular Degeneration.. NCT04435366 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04435366?term=NCT04435366&draw=2&rank=1.
- FDA accepts NDA for avacincaptad pegol for the treatment of geographic atrophy. February 17, 2023. https://www.modernretina.com/view/fda-approves-nda-for-avacincaptad-pegol-for-the-treatment-of-geographic-atrophy
- U.S. FDA Accepts Astellas' New Drug Application for Fezolinetant. Aug 18, 2022. https://www.prnewswire.com/news-releases/us-fda-accepts-astellas-new-drug-application-for-fezolinetant-301608116.html
- Astellas Announces Topline 12-week Results from Phase 3 Study of Fezolinetant for the Nonhormonal Treatment of Vasomotor Symptoms in Women in Asia. Mar 15, 2022. https://www.astellas.com/en/news/25421
- Astellas has menopause accelerated approval ambition thwarted by FDA at last minute. February 20, 2023. https://www.fiercebiotech.com/biotech/astellas-after-using-prv-has-menopause-accelerated-approval-ambition-thwarted-fda-last
- Leqembi (lecanemab-irmb). FDA Letter or approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/761269Orig1s000ltr.pdf
- Briumvi (ublituximab-xiiy). FDA Letter or approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/761238Orig1s000ltr.pdf
- FDA Approves First Gene Therapy to Treat Adults with Hemophilia B. November 22, 2022. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treat-adults-hemophilia-b
- Vivjoa (oteseconazole). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215888Orig1s000ltr.pdf
- Tzield (teplizumab-mzwv) injection. FDA Letter or approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/761183Orig1s000ltr.pdf
- Orserdu (elacestrant). FDA Letter or approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/217639Orig1s000correctedltr.pdf
- Mora JS(1), Bradley WG(2), Chaverri D(3), Hernández-Barral M(3), Mascias J(3), et al. Long-term survival analysis of masitinib in amyotrophic lateral sclerosis. Ther Adv Neurol Disord. 2021 Jul 19;14:17562864211030365. doi: 10.1177/17562864211030365. eCollection 2021.
- AB Science today announced that it has filed an application for conditional Marketing Authorization to the European Medicines Agency (EMA) for Alsitek (masitinib) in the treatment of amyotrophic lateral sclerosis (ALS). August 24, 2022. https://www.ab-science.com/ab-science-announces-that-it-has-filed-an-application-for-conditional-marketing-authorization-to-ema-for-masitinib-in-the-treatment-of-als/
- Alsitek (masitinib). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/alsitek
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Pasca S. Concizumab as a Subcutaneous Prophylactic Treatment Option for Patients with Hemophilia A or B: A Review of the Evidence and Patient's Perspectives.J Blood Med. 2022 Apr 16;13:191-199. doi: 10.2147/JBM.S242219. eCollection 2022.
- Novo Nordisk A/S: Phase 3 data for concizumab show 86% reduction in treated bleeds in haemophilia A or B with inhibitors. July 10, 2022. https://www.globenewswire.com/news-release/2022/07/10/2476898/0/en/Novo-Nordisk-A-S-Phase-3-data-for-concizumab-show-86-reduction-in-treated-bleeds-in-haemophilia-A-or-B-with-inhibitors.html
- Jiménez-Yuste V, Angchaisuksiri P, Castaman G, et al. Concizumab prophylaxis in patients with haemophilia A or B with inhibitors: Efficacy and safety results from the 32-week primary analysis of the phase 3 explorer7 trial. ISTH 2022 abstracts. Abstract number: LB 01.2 .https://abstracts.isth.org/?advanced=1&title=Concizumab+prophylaxis+in+patients+with+haemophilia+A+or+B+with+inhibitors&theauthor=&affiliation=&meetingid=&s=
- Novo Nordisk A/S: Phase 3 data for concizumab show 86% reduction in treated bleeds in haemophilia A or B with inhibitors. July 10, 2022. https://www.globenewswire.com/news-release/2022/07/10/2476898/0/en/Novo-Nordisk-A-S-Phase-3-data-for-concizumab-show-86-reduction-in-treated-bleeds-in-haemophilia-A-or-B-with-inhibitors.html
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Trial of Spesolimab for Generalized Pustular Psoriasis. December 22, 2021. https://www.mountsinai.org/about/newsroom/2021/trial-of-spesolimab-for-generalized-pustular-psoriasis
- FDA approves the first treatment option for generalized pustular psoriasis flares in adults. September 1, 2022. https://www.boehringer-ingelheim.us/press-release/fda-approves-first-treatment-option-generalized-pustular-psoriasis-flares-adults
- Menter A, Van Voorhees AS, Hsu S. Pustular Psoriasis: A Narrative Review of Recent Developments in Pathophysiology and Therapeutic Options. Dermatol Ther (Heidelb). 2021 Dec;11(6):1917-1929. doi: 10.1007/s13555-021-00612-x. Epub 2021 Oct 9.
- Maçães CO, Lé AM, Torres T. Generalized pustular psoriasis: the new era of treatment with IL-36 receptor inhibitors.J Dermatolog Treat. 2022 Nov;33(7):2911-2918. doi: 10.1080/09546634.2022.2089335. Epub 2022 Jun 21.
- FDA approves the first treatment option for generalized pustular psoriasis flares in adults. September 1, 2022. https://www.boehringer-ingelheim.us/press-release/fda-approves-first-treatment-option-generalized-pustular-psoriasis-flares-adults
- Spevigo (spesolimab-sbzo) intravenous injection. FDA Letter of approval. https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/761244Orig1s000ltr.pdf
- Spevigo (spesolimab). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/summaries-opinion/spevigo
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Chekol Abebe E, Yibeltal Shiferaw M, Tadele Admasu F, Asmamaw Dejenie T. Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. 2022 Sep 2;13:991092. doi: 10.3389/fimmu.2022.991092. eCollection 2022.
- Carvykti. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/carvykti
- Chekol Abebe E, Yibeltal Shiferaw M, Tadele Admasu F, Asmamaw Dejenie T. Ciltacabtagene autoleucel: The second anti-BCMA CAR T-cell therapeutic armamentarium of relapsed or refractory multiple myeloma. Front Immunol. 2022 Sep 2;13:991092. doi: 10.3389/fimmu.2022.991092. eCollection 2022.
- Weisel K, Martin T, Krishnan A, Jagannath S, Londhe A, et al. Comparative Efficacy of Ciltacabtagene Autoleucel in CARTITUDE-1 vs Physician's Choice of Therapy in the Long-Term Follow-Up of POLLUX, CASTOR, and EQUULEUS Clinical Trials for the Treatment of Patients with Relapsed or Refractory Multiple Myeloma. Clin Drug Investig. 2022 Jan;42(1):29-41. doi: 10.1007/s40261-021-01100-y. Epub 2021 Nov 25.
- FDA approves ciltacabtagene autoleucel for relapsed or refractory multiple myeloma. February 28, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ciltacabtagene-autoleucel-relapsed-or-refractory-multiple-myeloma
- Carvykti. European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/carvykti
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Minson A, Dickinson M. Glofitamab CD20-TCB bispecific antibody. Leuk Lymphoma. 2021 Dec;62(13):3098-3108. doi: 10.1080/10428194.2021.1953016. Epub 2021 Jul 15.
- Fixed-Duration Glofitamab Elicits Early, Durable Complete Remissions in Relapsed/Refractory LBCL. Sep 2, 2022. https://www.onclive.com/view/fixed-duration-glofitamab-elicits-early-durable-complete-remissions-in-relapsed-refractory-lbcl
- New Pivotal Data Demonstrate Clinical Benefit of Genentech’s Glofitamab, a Potential First-in-Class Bispecific Antibody for People with Aggressive Lymphoma. May 26, 2022. https://www.gene.com/media/press-releases/14954/2022-05-26/new-pivotal-data-demonstrate-clinical-be
- Minson A, Dickinson M. Glofitamab CD20-TCB bispecific antibody. Leuk Lymphoma. 2021 Dec;62(13):3098-3108. doi: 10.1080/10428194.2021.1953016. Epub 2021 Jul 15.
- GlobalData’s Healthcare database (accessed September 2022; preregistration list)
- GlobalData’s Healthcare database (accessed September 2022; Phase II list).
- Samaddar A, Grover M, Nag VL. 2020. Pathophysiology and Potential Therapeutic Candidates for COVID-19: A Poorly Understood Arena. Front Pharmacol. 11:585888. doi: 10.3389/fphar.2020.585888.
