Meds Pipeline Monitor 2023

November 2024
ISSN 2562-3834
Cat. No.: H79-5E-PDF
Contact Information
Patented Medicine Prices Review Board
Standard Life Centre
Box L40
333 Laurier Avenue West
Suite 1400
Ottawa, ON K1P 1C1
Tel.: 1-877-861-2350
TTY 613-288-9654
Email: PMPRB.Information-Renseignements.CEPMB@pmprb-cepmb.gc.ca
Acknowledgements
This report was prepared by the Patented Medicine Prices Review Board (PMPRB) as part of the National Prescription Drug Utilization Information System (NPDUIS) research initiative.
The PMPRB wishes to acknowledge the members of the NPDUIS Advisory Committee for their expert oversight and guidance in the preparation of this report. Please note that the statements and findings for this report do not necessarily reflect those of the members or their organizations.
We gratefully acknowledge Patricia Carruthers-Czyzewski, BScPhm, MSc, Sintera Inc. for providing pharmaceutical expertise and for her contribution to the scientific analysis.
Appreciation goes to Allison Carey for leading this project, and to Brian O’Shea and Kevin Pothier for their oversight in the development of the report. The PMPRB also wishes to acknowledge the editorial contributions of Shirin Paynter.
Disclaimer
The NPDUIS research initiative operates independently of the regulatory activities of the Board of the PMPRB. The research priorities, data, statements, and opinions expressed or reflected in NPDUIS reports do not represent the position of the PMPRB with respect to any regulatory matter. NPDUIS reports do not contain information that is confidential or privileged under sections 87 and 88 of the Patent Act, and the mention of a medicine in an NPDUIS report is not and should not be understood as an admission or denial that the medicine is subject to filings under sections 80, 81, or 82 of the Patent Act or that its price is or is not excessive under section 85 of the Patent Act.
Although this information is based in part on data obtained under license from GlobalData and the MIDAS® Database proprietary to IQVIA Solutions Canada Inc. and/or its affiliates (“IQVIA”), the statements, findings, conclusions, views, and opinions expressed in this report are exclusively those of the PMPRB and are not attributable to either GlobalData or IQVIA.
Suggested Citation
Patented Medicine Prices Review Board. (2024). Meds Pipeline Monitor, 2023. Ottawa: PMPRB.
Executive Summary
Meds Pipeline Monitor (MPM) is a horizon scanning report that features a selection of new medicines undergoing clinical evaluation or in pre-registration that may have an impact on future clinical practice and drug spending in Canada.
This edition expands the review for the selected medicine candidates in Phase III clinical trials or pre-registration to include information on other drugs in Phase II that share the same mechanism of action or indication. Having insight into other drugs under investigation (i.e., in Phase II) may provide additional information on the potential place in therapy for these pipeline candidates. Medicines in Phase III clinical trials or pre-registration are selected as candidates for the ‘new medicines’ list if they have the potential to address an unmet therapeutic need, offer a novel mechanism of action or therapeutic benefit over existing therapies, or treat a serious condition. The medicines in Phase II are also examined to identify other drugs that are in earlier phases of the pipeline that contain the same indication or mechanism of action as the selected medicine candidates.
The report collects data from two main sources: GlobalData’s Healthcare database, which identifies medicines currently undergoing clinical evaluation, and Health Canada’s Drug and Health Product Submissions Under Review Lists, which provide information on new medicines under review in Canada.
Highlights of the Meds Pipeline Monitor 2023
- As of April 2024, the pipeline contained over 12,000 new medicines in various stages of clinical development, compared to just over 9,000 the year before. The number of drugs in the pipeline is increasing by an average of 19% per year since 2019.
- Oncology continues to dominate the therapeutic mix in 2023, with cancer treatments representing one third (33%) of medicines in all phases of clinical trials. Treatments for infectious diseases and central nervous system diseases held the second and third largest share of the pipeline, at 13% and 12%, respectively.
- On average, 20% of medicines in Phase III clinical trials and pre-registration in 2023 had an early orphan designation approved through the U.S. FDA or the EMA, which is roughly a 33% decrease from previous years.
- Twenty new medicines were selected for the 2023 new medicines list (Table 4) based on their potential to impact the Canadian healthcare system. Fourteen of the medicines listed in this year’s report (Tables 4 through 7) have forecasted global annual revenues over US $1 billion by 2029.
- Of the 51 new and retained medicines listed in the previous edition (MPM 2022), 15 received market authorization, 23 were retained on this year’s list as they continued to satisfy the selection criteria, and 13 were removed as their clinical trials were discontinued or they no longer meet the selection criteria.
- Six new medicines under review by Health Canada were selected for this report as they have a novel mechanism of action or have demonstrated improved efficacy and/or safety in clinical trials.
List of Terms
For the purpose of this report, the following terms and associated definitions apply.
- Cell therapy
- The transplantation of human cells to replace or repair damaged tissue and/or cells.
- Clinical efficacy
- The maximum response achievable from a medicine in research settings and the capacity for sufficient therapeutic effect in clinical settings.Footnote i
- Gene therapy
- A technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene, as defined by Health Canada.Footnote ii
- Market authorization
- The process of approval for a medicine to be marketed in a given country. In Canada, market approval is granted following a substantive scientific evaluation of a product's safety, efficacy, and quality, as required by the Food and Drugs Act and Regulations.Footnote iii
- Medicinal ingredient
- A chemical or biological substance responsible for the claimed pharmacologic effect of a drug product. Sometimes referred to as a molecule, active substance, or active ingredient.Footnote iv
- Medicine
- A broad term encompassing both the final drug product and medicinal ingredient(s); this encompasses chemically manufactured active substances and biologics, including gene therapies. Medicines are reported at the medicinal ingredient level and can refer to a single ingredient or a unique combination of ingredients.
- New medicine
- A medicinal ingredient that has not previously received market authorization by a regulator.Footnote iv
- Orphan medicine
- A medicine used to treat a rare disease. For the purposes of this study, orphan medicines are defined as having an orphan designation granted by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA) for the relevant indication.
- Patent evergreening
- The acquisition of additional patents for minor modifications to an existing pharmaceutical product in order to extend the patent life of the medicine (e.g., new delivery systems, new dosages, new uses, new combinations or new forms).Footnote v
- Phase I
- These trials test an experimental medicine on a small group of people for the first time. The purpose is to look at the medicine's safety, determine a safe dosage range, and monitor if there are any side effects.
- Phase II
- In this phase, the medicine is given to a larger group of people (usually 100 or more) to gather data on how well the medicine works to treat a disease or condition, check its safety on a wider range of people, and determine the best dose.Footnote vi
- Phase III
-
These controlled or uncontrolled trials are conducted after preliminary evidence suggesting efficacy of the medicine has been demonstrated. They are intended to gather additional and confirmatory information about the clinical efficacy and safety of the medicine under the proposed conditions of use.Footnote ii Phase III trials are usually randomized with double-blind testing in several hundred to several thousand patients.
- Pre-registration
- A medicine is in the pre-registration phase once all the necessary clinical trials have been completed and it is waiting for registration or approval for use by a governing body.Footnote vii
Phases of clinical trials
Introduction
This edition of the Meds Pipeline Monitor (MPM) features a selection of new medicines in Phase III clinical trials or pre-registration that have the potential to impact clinical practice and drug spending in Canada.
The methodology, which is detailed in the next section, uses a specific set of criteria to identify a list of new medicines in the pipeline from the GlobalData Healthcare database, as well as a list of medicines currently under review from Health Canada’s Drug and Health Product Submissions Under Review (SUR) Lists. The new medicines listed in this report are selected based on a scientific review of the literature and clinical trial outcomes to determine if the medicine may impact the Canadian healthcare system by: addressing an unmet therapeutic need; offering a novel mechanism of action or therapeutic benefit over existing therapies; or treating a serious condition. Medicines reported in previous editions of the MPM are also reviewed and updated in this report. This report also provides an update on the medicines in last year’s edition that have since received market authorization by either the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), or Health Canada. Likewise, the new medicines featured in this report will be monitored in future editions of the MPMto identify candidates that successfully enter the market.
To provide context for the selection of medicines, the MPM includes a snapshot of the number of drugs in each clinical phase of the pipeline year over year (2019-2023), and a breakdown of the various therapeutic areas for each phase of clinical development.
Meds Pipeline Monitor is a companion publication to Meds Entry Watch, which analyzes the market launch patterns of newly approved medicines in Canada and internationally. Together, these two PMPRB reports monitor the market continuum of late-stage pipeline medicines and new approvals, providing decision makers, researchers, patients, clinicians, and other stakeholders with information on the emerging medicines and evolving cost pressures.
Methodology
Snapshot of the Pipeline
The snapshot of the 2023 pipeline identifies the composition of medicines in various phases of clinical development. For the purpose of this analysis, a full list of pipeline medicines was retrieved from GlobalData’s Healthcare database in April 2024 and the selected medicine candidates for this year’s report have been validated as of August 30, 2024.
New medicinal ingredients are identified as those with no prior approvals through the US Food and Drug Administration (FDA), the European Medicines Agency (EMA), or Health Canada. The distribution of new medicines by therapeutic area corresponds to the indication under evaluation, as reported by GlobalData. Note that a single new medicine may be undergoing multiple clinical studies for separate indications.
Meds Pipeline Monitor
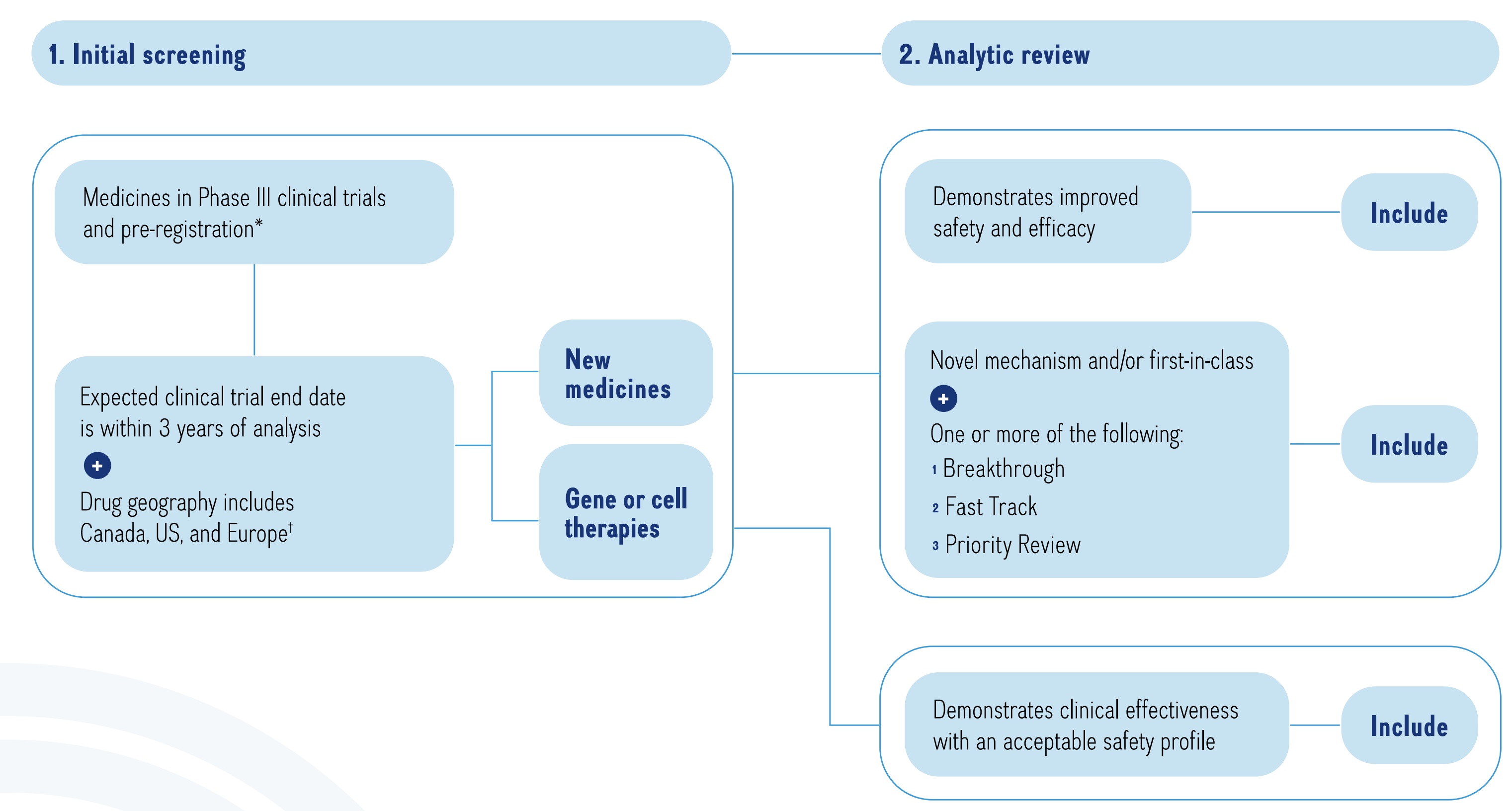
The MPM selects new medicines in Phase III clinical trials or pre-registration in Canada, the United States, and Europe. Many of the pipeline candidates are first-in-class or represent novel mechanisms for treatment in a specific therapeutic area. For this reason, this report includes additional review on other drugs undergoing Phase II clinical evaluation that share the same indication or mechanism of action in earlier stages of the pipeline (i.e., Phase II). Pipeline medicines are selected for inclusion using a two-stage process (Figure 1). The initial screening stage selects medicines in the late phases of clinical evaluation, while the analytic review stage involves a more rigorous appraisal of each potential candidate to identify medicines that may have a significant clinical and budgetary impact.
* In pre-registration with the US Food and Drug Administration (FDA).
† Has Phase III clinical trials in Canada, the United States, or geographic Europe (excluding Russia and Türkiye).
Figure description
This is a flowchart describing the process used to select the listed medicines. The chart consists of two steps:
1. Initial Screening
This step begins with all medicines in Phase III clinical trials or pre-registration with the US Food and Drug Administration. Of these medicines, the next step includes only those with expected clinical trial end dates within three years of the analysis and drug geography including Canada, the US, and Europe. To qualify for the drug geography, a medicine must have Phase III clinical trials in Canada, the US, and/or geographic Europe (excluding Russia and Türkiye).
2. Analytic Review
The analytic review step of the process is divided into two parts: one path for new medicines and the other for gene therapies.
New medicines must meet at least one of the following requirements to be included in the list:
- Demonstrates improved safety and efficacy
- Novel mechanism and/or first-in-class, with the addition of one or more of Breakthrough, Fast Track, and Priority Review designations
Gene and cell therapies must demonstrate clinical effectiveness with an acceptable safety profile to be included in the list.
Stage 1. Initial screening
GlobalData’s Healthcare database is used to identify a list of medicines undergoing Phase III clinical trials or in pre-registration. These medicines serve as the basis for the initial screening stage.
The drug geography, defined as the geographical region or country in which the medicine is either marketed or in pipeline development, is restricted to Canada and other countries with similar regulatory and approval processes: the US and geographic Europe (excluding Russia and Türkiye). Only new medicinal ingredients that have adequate data that supports increased efficacy and safety from clinical trials are considered as candidates for inclusion.
Medicines approved or sold in Canada, the US, or Europe for any other indication or in any other strength or formulation are excluded during the selection process, as are medicines whose clinical trials are inactive, suspended, withdrawn, or terminated.
Stage 2: Analytic screening
Selection criteria
Following the initial screening, the second stage of the process considers a number of selection criteria to determine the final list of pipeline candidates. These criteria are detailed in Table 1.
Earlier phases of the pipeline (i.e., Phase II) are also examined to determine if there are other medicines with the same indication or mechanism of action as the selected candidates in Phase III and pre-registration. This provides additional information on the number of novel, first-in-class medicines that are undergoing clinical evaluation in Phase II that may influence the therapeutic significance of the selected candidates in Phase III and pre-registration.
Table 1. Selection criteria for the Meds Pipeline Monitor
| Selection criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
Novel mechanism / First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class In addition, the medicine must fall into one or more of the three following FDA designations for expedited development and review:
|
Additional descriptive information
A profile of each successful pipeline candidate is provided, including the indication and mechanism of action, as well as a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified. Table 2 provides a detailed description of these key attributes.
Table 2. Key attributes of new medicines selected for the Meds Pipeline Monitor
| Attribute | Relevance | Data sources |
|---|---|---|
| Phase III clinical trials in Canada | Medicines tested in Canada are likely to be of interest to Canadians |
GlobalData Healthcare; Health Canada Clinical Trials Database; Health Canada Drug and Health Product Submissions Under Review; National Institutes of Health (NIH) Clinical Trial Registry |
| Rare or orphan designation | Medicines used to treat rare diseases or conditions that generally have high treatment costs and may result in substantial spending | GlobalData Healthcare |
| Biologic medicine | These complex molecules produced by living organisms are expected to have high costs, resulting in substantial spending | |
| Add-on therapy | Medicines designed to be used in conjunction with existing medicines may increase the treatment cost and contribute to higher spending | |
| Potential evergreening | Modified forms of the same product in order to extend the patent life. (e.g., new delivery systems, new dosages, new uses, new combinations or new forms) |
The profile also provides details of potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
The indications and therapeutic areas of the featured medicines correspond to their Phase III clinical trial or pre-registration stage. A single clinical trial may assess multiple indications within the same therapeutic area. These medicines may also have additional indications at various phases of clinical evaluation that are not mentioned in this report. The scientific description and key attributes provided are focused on the specified indication(s) for the selected medicines.
Medicines reported for a given year are reassessed for each following edition of the MPM. They may be retained on the MPM list if they continue to meet the selection criteria. Medicines for which clinical trials have been discontinued or for which the selection criteria is no longer met are not reported in subsequent editions.
Spotlight on Canada
Health Canada’s Drug and Health Product Submissions Under Review (SUR) Lists are assessed using a modified approach to the selection criteria to establish a list of medicines that may have the potential to impact Canadian drug spending or clinical practice.
Medicines listed in the SUR include new drug submissions containing medicinal ingredients that have not been approved in Canada for any indication, in any strength or form. Unlike the selection of medicines identified in the pipeline lists, these medicines may have previously received market authorization through the U.S. FDA or the EMA.
Selection Criteria
Following this initial screening, the medicine must demonstrate at least one of three selection criteria to qualify for inclusion in the report. These criteria are listed in Table 3.
Table 3. Selection criteria for the list of medicines currently under review by Health Canada
| Selection Criteria |
|---|
| Improved safety and efficacy shown in clinical trials: a medicine that demonstrates increased safety, new outcome measures, or increased life expectancy or quality of life |
| Novel mechanism or First-in-class: a medicine that uses a new mechanism of biochemical interaction to produce a medical effect, or a medicine that is the first in its therapeutic class |
| Gene or cell therapy: a technique for the treatment of genetic disease in which a gene that is absent or defective is replaced by a healthy gene; or the transplantation of human cells to replace or repair damaged tissue and/or cells |
Additional descriptive information
The profile of each medicine under review includes the key attributes listed in Table 2, as well as the indication and mechanism of action, and a summary of the applicable published outcomes from clinical trials. Specific attributes that may influence the potential uptake or cost of each medicine are also identified, as well as potential cost implications, if available, which includes the forecasted global revenues reported by GlobalData.
Although FDA designations for expedited development or review are not a selection criteria for this list, relevant Breakthrough, Fast Track, and Priority Review designations are indicated where available. For a description of these designations, see Table 1.
Indications and therapeutic areas correspond to the information provided by GlobalData. The scientific description and key attributes provided are focused on the specified indication(s) for the selected medicine. For medicines under review for multiple indications, the primary indication is used.
Data Sources
The GlobalData Healthcare database is the primary data source for the identification of pipeline medicines and their corresponding clinical information. GlobalData Healthcare tracks medicines from pre-clinical discovery, through clinical trials, to market launch and subsequent sales. The database is a comprehensive resource of medicines under various stages of clinical development. Search capabilities allow for controlled selection of specific attributes, including but not limited to the following: phase of clinical development, therapeutic area, molecule type, indication, drug geography, mechanism of action, and regulatory designations.
Health Canada’s Drug and Health Product Submissions Under Review (SUR) Lists are used to determine the featured selection of new medicines currently undergoing review by Health Canada. The SUR is a publicly available set of lists that identify pharmaceutical and biologic drug submissions containing new medicinal ingredients not previously approved in Canada that have been accepted for review. This applies to submissions accepted on or after April 1, 2015.
As this selection is restricted to new medicines, additional sources of information are cross-referenced to confirm that the candidates have not previously been approved or sold. These include recorded sales data from the IQVIA MIDAS® Database (all rights reserved); regulatory approval records from the National Institutes of Health (NIH), U.S. FDA, the EMA, and Health Canada; and information in Health Canada’s Clinical Trials database and ClinicalTrials.org.
Limitations
This analysis captures a snapshot of the pipeline over a specific time period. Although it is assumed to be representative of the composition of medicines over the entire year, the pipeline is fairly dynamic and the share of medicines in any particular therapeutic area will vary.
This assessment is restricted to medicines under development for market in Canada and other countries with similar regulatory and approval processes: the US and Europe (excluding Russia and Türkiye). Medicines that have not yet received market authorization in these countries were considered as potential pipeline candidates, even if they have been approved elsewhere in the world.
Some of the selected medicines may be undergoing clinical trials for additional indications; this analysis only reports on indications in the late stages of development—that is, in Phase III clinical trials or pre-registration with the U.S. FDA—that satisfy the selection criteria set out in the methodology.
For each selected pipeline medicine, the primary manufacturer(s) and trade name, if available, are given along with the indication. In some cases, additional manufacturers, including subsidiaries, may also be involved in the development of the medicine with the primary companies, or other manufacturers may be developing the same medicine for other indications.
Although this report attempts to identify the most important pipeline medicines, the selection is not exhaustive and some medicines that are not included in this selection may have a significant impact on future clinical practice and drug spending in Canada.
Unless otherwise specified, the featured lists capture the composition of the pipeline as of April 2024 and are validated as of the end of August 2024. Due to the unpredictability and fast-moving nature of pipeline medicines entering the market, some of the medicines listed in this edition may have been approved or marketed in Canada, the US, or Europe following this date. Pipeline medicines that have not been included in this report due to the timing of the selection may presently meet the selection criteria; these, along with the rest of the drug pipeline, will be considered for the next edition of the report.
Snapshot of the 2023 Pipeline
The number of new pharmaceutical developments in the pipeline is increasing year over year. In 2023, over 12,000 new medicines were undergoing clinical evaluation, which has been increasing by an average of 19% per year since 2019.
Figure 2 provides a snapshot of the pipeline including the number of new medicinal ingredients in each phase of clinical development over the last 5 years.
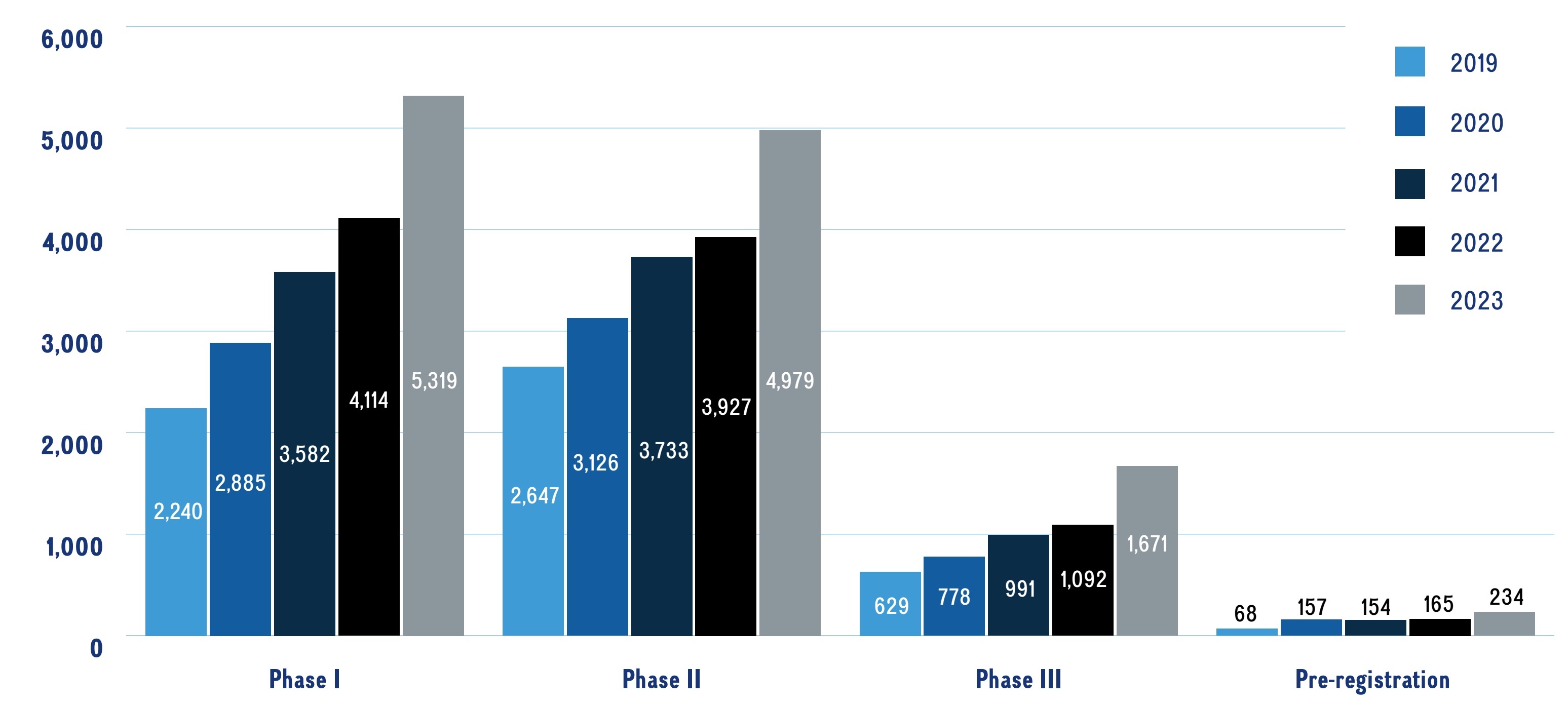
Figure description
A bar graph shows the total number of new medicines in each phase of the pipeline by their highest phase of development from 2019 to 2023. Totals are given for each year and phase.
| Phase I | Phase II | Phase III | Pre-registration | |
|---|---|---|---|---|
2019 |
2,240 |
2,647 |
629 |
68 |
2020 |
2,885 |
3,126 |
778 |
157 |
2021 |
3,582 |
3,733 |
991 |
154 |
2022 |
4,114 |
3,927 |
1,092 |
165 |
2023 |
5,319 |
4,979 |
1,671 |
234 |
Data source: GlobalData Healthcare database (accessed April 2024); IQVIA MIDAS© Database.
Figure 3a illustrates the distribution of new medicines by therapeutic area from Phase I through to pre-registration. Although the findings show that pipeline medicines represented a wide range of therapeutic areas in 2023, cancer treatments dominated the therapeutic mix in each phase of the pipeline, accounting for one third (33%) of medicines in all phases of clinical evaluation. Other important pipeline therapies include those for infectious diseases (13%) and central nervous system therapies (12%).

Figure description
A stacked bar graph gives the distribution of new medicines by therapeutic rea from Phase I through to pre-registration.
| Therapeutic Area | Phase I | Phase II | Phase III | Pre-registration | All Phases |
|---|---|---|---|---|---|
Oncology |
38% |
33% |
20% |
24% |
33% |
Infectious disease |
12% |
13% |
18% |
8% |
13% |
Central Nervous System |
12% |
13% |
12% |
10% |
12% |
Metabolic disorders |
6% |
5% |
8% |
10% |
6% |
Immunology |
6% |
5% |
5% |
7% |
5% |
Gastrointestinal |
5% |
5% |
3% |
4% |
5% |
Cardiovascular |
5% |
5% |
9% |
6% |
5% |
Respiratory |
4% |
5% |
4% |
2% |
4% |
Musculoskeletal disorders |
2% |
3% |
4% |
4% |
3% |
Dermatology |
2% |
5% |
4% |
3% |
4% |
Ophthalmology |
2% |
3% |
6% |
4% |
3% |
Genetic disorders |
1% |
2% |
1% |
2% |
1% |
Hematological disorders |
1% |
2% |
3% |
5% |
2% |
Other |
4% |
2% |
4% |
10% |
3% |
Data source: GlobalData Healthcare database (accessed April 2024).
Figure 3b illustrates the top indications and number of medicines undergoing Phase II, Phase III or pre-registration in the major therapeutic areas in the pipeline in 2023.
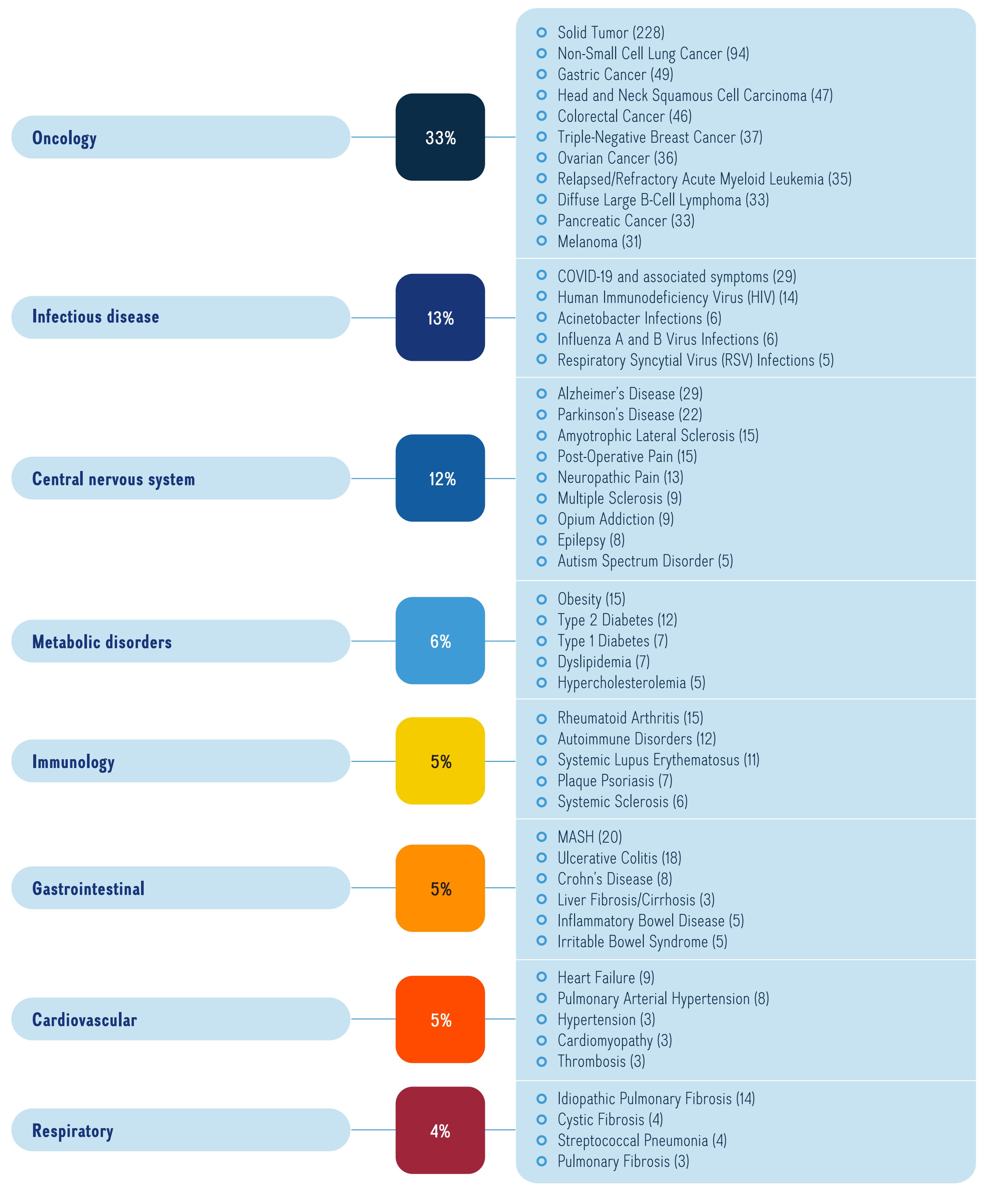
Figure description
A horizontal bar graph indicates the top indications by number of medicines in all phases of the pipeline.
| Therapeutic Area | All Phases | |
|---|---|---|
Oncology |
33% |
Solid Tumor (228) Non-Small Cell Lung Cancer (94) Gastric Cancer (49) Head and Neck Squamous Cell Carcinoma (47) Colorectal Cancer (46) Triple-Negative Breast Cancer (37) Ovarian Cancer (36) Relapsed/Refractory Acute Myeloid Leukemia (35) Pancreatic Cancer (33) Diffuse Large B-Cell Lymphoma (33) Melanoma (31) |
Infectious disease |
13% |
COVID-19 and associated symptoms (29) Human Immunodeficiency Virus (HIV) (14) Acinetobacter Infections (6) Influenza A and B Virus Infections (6) Respiratory Syncytial Virus (RSV) Infections (5) |
Central Nervous System |
12% |
Alzheimer’s Disease (29) Parkinson’s Disease (22) Post-Operative Pain (15) Amyotrophic Lateral Sclerosis (15) Neuropathic Pain (13) Multiple Sclerosis (9) Opium Addiction (9) Epilepsy (8) Autism Spectrum Disorder (5) |
Metabolic disorders |
6% |
Obesity (15) Type 2 Diabetes (12) Type 1 Diabetes (7) Dyslipidemia (7) Hypercholesterolemia (5) |
Immunology |
5% |
Rheumatoid Arthritis (15) Autoimmune Disorders (12) Systemic Lupus Erythematosus (11) Plaque Psoriasis (7) Systemic Sclerosis (6) |
Gastrointestinal |
5% |
MASH (20) Ulcerative Colitis (18) Crohn’s Disease (8) Inflammatory Bowel Disease (5) Irritable Bowel Syndrome (5) Liver Fibrosis/Cirrhosis (3) |
Cardiovascular |
5% |
Heart Failure (9) Pulmonary Arterial Hypertension (8) Hypertension (3) Thrombosis (3) Cardiomyopathy (3) |
Respiratory |
4% |
Idiopathic Pulmonary Fibrosis (14) Cystic Fibrosis (4) Streptococcal Pneumonia (4) Pulmonary Fibrosis (3) |
Orphan medicines, as designated by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA), accounted for a notable proportion of medicines in the 2023 pipeline. Figure 4 provides the shares of orphan designated medicines for all phases in the pipeline from 2020-2023. Orphan designated medicines make up a greater share in the later stages of the pipeline, increasing from 6% in Phase I to 22% in pre-registration in 2023.
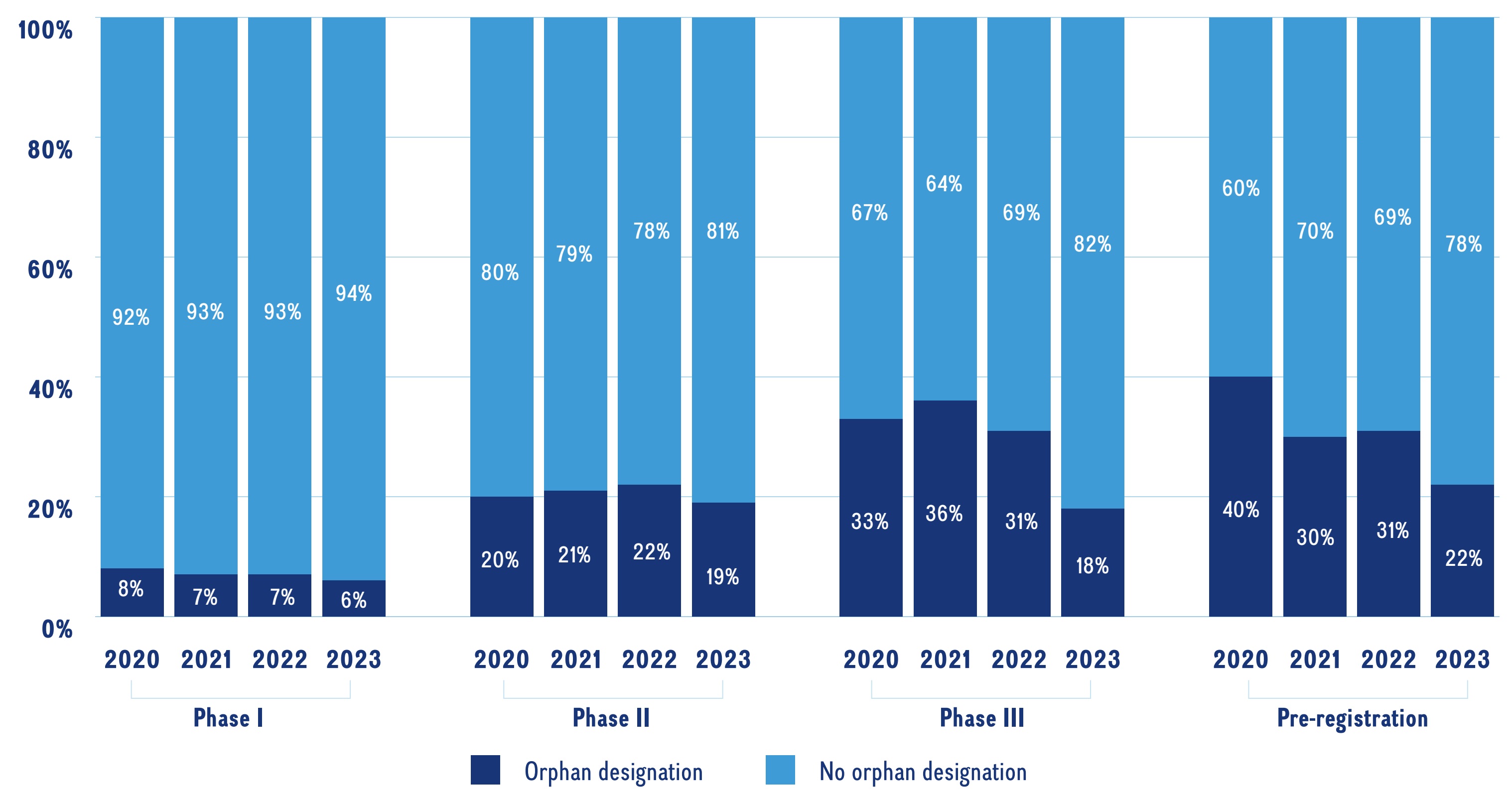
Figure description
A stacked bar graph gives the proportion of orphan designated medicines to non-orphan designated medicines in the pipeline by phase of development from 2020 to 2023.
| Phase of Development | 2020 | 2021 | 2022 | 2023 | |
|---|---|---|---|---|---|
Orphan designation |
Phase I |
8% |
7% |
7% |
6% |
Phase II |
20% |
21% |
22% |
19% |
|
Phase III |
33% |
36% |
31% |
18% |
|
Pre-registration |
40% |
30% |
31% |
22% |
|
No orphan designation |
Phase I |
92% |
93% |
93% |
94% |
Phase II |
80% |
79% |
78% |
81% |
|
Phase III |
67% |
64% |
69% |
82% |
|
Pre-registration |
60% |
70% |
69% |
78% |
Note: Includes all pipeline medicines with a highest development stage of Phase I to pre-registration that are being developed for market in Canada, the United States, or geographic Europe (excluding Russia and Türkiye). Orphan medicines were defined as pipeline medicines that have been granted an orphan designation by the US Food and Drug Administration (FDA) or the European Medicines Agency (EMA).
Data source: GlobalData Healthcare database (accessed April 2024).
Meds Pipeline Monitor 2023
The following tables include selected new medicine candidates for 2023 (Table 4), retained medicines from previous editions of the Meds Pipeline Monitor (Table 5), and medicines from previous editions that have gained market authorization (Table 6).
Medicines in Phase III clinical trials or pre-registration are considered as candidates for the Meds Pipeline Monitor (MPM) if they have the potential to impact future clinical practice and drug spending in Canada (e.g., address an unmet therapeutic need, offer a novel mechanism of action or therapeutic benefit over existing therapies, or treat a serious condition).
Screening new medicine candidates
Of the 1,905 pipeline medicines in Phase III and pre-registration in 2023, twenty (20) new medicines were selected for inclusion in the new medicines list (Table 4). Many of the pipeline candidates are first-in-class or represent novel mechanisms for the treatment of specific therapeutic areas. Having insight into other drugs under investigation (i.e., in Phase II) may provide additional information on the potential place in therapy of these pipeline candidates. The medicines in Phase II were examined to identify other drugs in the pipeline that have the same indication or mechanism of action as those listed in the 2023 new medicines list. The description for each new medicine listed in the 2023 new medicines list includes a statement indicating if there are any other drugs in Phase II development with the same indication or mechanism of action. Appendix A (Table A2) provides some further insights into the other drugs identified in Phase II for the indications targeted by the pipeline candidates. It is important to keep in mind that not all drugs in Phase II development will progress to Phase III. According to an industry analysis, Phase II clinical programs experience the lowest success rate of the development phases, with only 28.9% of developmental candidates advancing to Phase III.1
Of the new medicines featured in previous reports, 23 were retained as recent evidence continues to support promising clinical benefit and satisfies the selection criteria (Table 5). Fifteen of the 2022 pipeline medicines have received market authorization in the US, Europe, or Canada as of August 30, 2024 (Table 6), while 13 were removed from the list as their clinical trials were discontinued or they no longer fulfill the selection criteria.
Screening biosimilars
The availability of biosimilars could significantly impact costs in a wide range of therapeutic areas. Appendix A (Table A1) provides a list of the identified biosimilars in Phase III clinical trials and indicates whether a biosimilar currently exists for the originator biologic.
Table 4. Selected new medicines for 2023
Cardiovascular
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Aficamten Cytokinetics Inc.
|
Hypertrophic cardiomyopathy |
Clinical trials
Forecasted revenue
|
Nerinetide NoNO Inc.
|
Acute ischemic stroke |
Clinical trials
Forecasted revenue
|
Pelacarsen sodium Novartis AG
|
Cardiovascular disease; Hyperlipidemia |
Clinical trials
Forecasted revenue
|
Central Nervous System
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Fosigotifator (ABBVCLS-7262) Calico Life Sciences LLC
|
Amyotrophic lateral sclerosis (ALS) |
Clinical trials
Forecasted revenue
|
Iclepertin Boehringer Ingelheim International GmbH
|
Cognitive impairment associated with schizophrenia (CIAS) |
Clinical trials
Forecasted revenue
|
Resiniferatoxin Grunenthal GmbH
|
Osteoarthritis pain |
Clinical trials
Forecasted revenue
|
Xanomeline-trospium (KarXT) Karuna Therapeutics Inc.
|
Schizophrenia; Psychosis |
Clinical trials
Forecasted revenue
|
Dermatology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Prademagene zamikeracel Abeona Therapeutics Inc.
|
Epidermolysis bullosa |
Clinical trials
Forecasted revenue
|
Gastrointestinal Disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Efruxifermin Akero Therapeutics Inc.
|
Metabolic dysfunction-associated steatohepatitis (MASH) |
Clinical trials
Forecasted revenue
|
Obefazimod Abivax SA
|
Ulcerative colitis |
Clinical trials
Forecasted revenue
|
Genetic disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Fazirsiran sodium Arrowhead Pharmaceuticals Inc.
|
Alpha-1 antitrypsin deficiency (A1AD) |
Clinical trials
Forecasted revenue
|
Genito Urinary System And Sex Hormones
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Inaxaplin (VX19-147) Vertex Pharmaceuticals Inc.
|
Focal segmental glomerulosclerosis (FSGS); Chronic kidney disease (chronic renal failure) |
Clinical trials
Forecasted revenue
|
Metabolic disorders
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
RGX-121 RegenxBio Inc.
|
Mucopolysaccharidosis II (MPS II) (Hunter syndrome) |
Clinical trials
Forecasted revenue
|
Oncology
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
Datopotamab deruxtecan Daiichi Sankyo Co Ltd.
|
Breast cancer, both HR-positive/HER2-negative and triple-negative |
Clinical trials
Forecasted revenue
|
Gemcitabine (GemRIS) Johnson & Johnson
|
Non-muscle invasive bladder cancer (NMIBC) (superficial bladder cancer); Muscle invasive bladder cancer (MIBC) |
Clinical trials
Forecasted revenue
|
Patidegib hydrochloride Sol-Gel Technologies Ltd.
|
Gorlin syndrome (basal cell nevus syndrome/nevoid basal cell carcinoma syndrome) |
Clinical trials
Forecasted revenue
|
Revumenib citrate (SNDX-5613) Syndax Pharmaceuticals Inc.
|
Acute lymphocytic leukemia (ALL); Acute lympho-blastic leukemia; Refractory acute myeloid leukemia; Relapsed acute myeloid leukemia |
Clinical trials
Forecasted revenue
|
Vorasidenib citrate Les Laboratoires Servier SAS
|
Astrocytoma; Low-grade glioma; Oligodendroglioma |
Clinical trials
Forecasted revenue
|
Respiratory
| Medicine (Trade name) Company | Indication(s) | Description and Key Attributes |
|---|---|---|
AD-109 (atomoxetine + R-oxybutynin) Apnimed, Inc.
|
Obstructive sleep apnea (OSA) |
Clinical trials
Forecasted revenue
|
Brensocatib Insmed Inc.
|
Bronchiectasis |
Clinical trials
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q2-2024, and are given in US dollars.
Data source: GlobalData Healthcare database.
Table 5. Update on pipeline medicines retained from the 2022 Meds Pipeline Monitor
Cardiovascular
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Abelacimab Anthos Therapeutics Inc.
|
Deep vein thrombosis (DVT); Pulmonary embolism; Atrial fibrillation |
Clinical trials
Forecasted revenue
|
Etripamil Milestone Pharmaceuticals Inc.
|
Supraventricular tachycardia |
Clinical trials
Forecasted revenue
|
Obicetrapib NewAmsterdam Pharma Company
|
Dyslipidemia; Heterozygous familial hypercholesterolemia (HeFH); Atherosclerosis |
Clinical trials
Forecasted revenue
|
Central Nervous System
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Soticlestat Takeda Pharmaceutical Co Ltd.
|
Lennox-Gastaut syndrome; Dravet syndrome (severe myoclonic epilepsy of infancy) |
Clinical trials
Forecasted revenue
|
Latozinemab (previously AL-001) Alector Inc.
|
Frontotemporal dementia (FTD) |
Clinical trials
Forecasted revenue
|
Valiltramiprosate (previously ALZ-801) Alzheon Inc.
|
Alzheimer's disease (AD) |
Clinical trials
Forecasted revenue
|
Midomafetamine (MDMA) Lykos Therapeutics (formerly Multidisciplinary Association for Psychedelic Studies Public Benefit Corporation)
|
Post-traumatic stress disorder (PTSD) |
Clinical trials
Forecasted revenue
|
ND-0612 (levodopa/carbidopa for subcutaneous infusion) Neuroderm, a Mitsubishi Tanabe Pharma Corp subsidiary
|
Parkinson's disease (PD) |
Clinical trials
Forecasted revenue
|
Gastrointestinal disorders
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Seladelpar lysine CymaBay Therapeutics Inc.
|
Primary biliary cholangitis (primary biliary cirrhosis) |
Clinical trials
Forecasted revenue
|
Genetic disorders
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
REC-2282 Recursion Pharmaceuticals Inc.
|
Neurofibromatosis type II (NF2) |
Clinical trials
Forecasted revenue
|
Genito urinary system and sex hormones
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Gepotidacin mesylate GlaxoSmithKline plc
|
Cystitis; Urinary tract infections (UTI) |
Clinical trials
Forecasted revenue
|
Hematological disorders
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Bentracimab SFJ Pharmaceuticals Inc.
|
Bleeding and clotting disorders |
Clinical trials
Forecasted revenue
|
Fitusiran Sanofi
|
Hemophilia A; Hemophilia B |
Clinical trials
Forecasted revenue
|
Hormonal disorders
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Palopegteriparatide Ascendis Pharma AS
|
Hypoparathyroidism |
Clinical trials
Forecasted revenue
|
Immunological disorders
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Garadacimab CSL Ltd.
|
Hereditary angioedema (HAE) (C1 esterase inhibitor [C1-INH] deficiency) |
Clinical trials
Forecasted revenue
|
Infectious diseases
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Zoliflodacin Innoviva Inc.
|
Uncomplicated cervical and urethral gonorrhea |
Clinical trials
Forecasted revenue
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Birtamimab Prothena Corp plc
|
Primary systemic amyloidosis |
Clinical trials
Forecasted revenue
|
Oncology
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Bemarituzumab Amgen Inc.
|
Adenocarcinoma of the gastroesophageal junction; Gastric cancer; Bladder cancer; Gastroesophageal (GE) junction carcinomas |
Clinical trials
Forecasted revenue
|
Navitoclax dihydrochloride AbbVie Inc.
|
Myelofibrosis |
Clinical trials
Forecasted revenue
|
Rusfertide acetate Protagonist Therapeutics Inc.
|
Polycythemia vera (PV) |
Clinical trials
Forecasted revenue
|
SGX-301 Hypericin sodium (synthetic hypericin) Soligenix Inc.
|
Cutaneous T-cell lymphoma (CTCL) |
Clinical trials
Forecasted revenue
|
Zolbetuximab Astellas Pharma Inc.
|
Adenocarcinoma of the gastroesophageal junction; Gastric cancer |
Clinical trials
Forecasted revenue
|
Ophthalmology
| Medicine (Trade name) Company | Indication(s) | Update |
|---|---|---|
Lenadogene nolparvovec GenSight Biologics SA
|
Leber’s hereditary optic neuropathy (Leber optic atrophy) |
Clinical trials
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q2-2024, and are given in US dollars.
Data source: GlobalData Healthcare database.
Table 6. Pipeline medicines from the 2022 Meds Pipeline Monitor that have gained market authorization
Cardiovascular
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Aprocitentan (Tryvio) Idorsia Pharmaceutical Ltd.
|
Resistant hypertension |
Approval
Forecasted revenue
|
Sotatercept (Winrevair) Acceleron Pharma Inc.
|
Pulmonary arterial hypertension (PAH) |
Approval
Forecasted revenue
|
Dermatology
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Beremagene geperpavec (Vyjuvek) Krystal Biotech Inc.
|
Epidermolysis bullosa |
Approval
Forecasted revenue
|
Gastrointestinal disorders
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
RBX-2660 (Rebyota) Ferring Pharmaceuticals Inc.
|
Clostridium difficile infections (C. difficile associated disease) |
Approval Approved by the U.S. FDA (Rebyota; November 30, 2023).195 Forecasted revenue
|
Resmetirom (Rezdiffra) Madrigal Pharmaceuticals Inc.
|
Metabolic dysfunction-associated steatohepatitis (MASH) |
Approval
Forecasted revenue
|
Genetic disorders
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Delandistrogene moxeparvovec (Elevidys) Sarepta Therapeutics Inc.
|
Duchenne muscular dystrophy |
Approval
Forecasted revenue
|
Hematological disorders
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Danicopan (Voydeya) Alexion Pharmaceuticals Inc.
|
Paroxysmal nocturnal hemoglobinuria (PNH) |
Approval
Forecasted revenue
|
Fidanacogene (Beqvez) Pfizer Inc.
|
Hemophilia B (factor IX deficiency) |
Approval
Forecasted revenue
|
Immunological disorders
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Omidubicel (Omisirge) Gamida Cell Ltd.
|
Hematopoietic stem cell transplantation |
Approval
Forecasted revenue
|
Metabolic Disorders
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Donislecel (Lantidra) CellTrans Inc.
|
Type 1 diabetes (juvenile diabetes) |
Approval
Forecasted revenue
|
Insulin icodec (Awiqli) Novo Nordisk AS
|
Type 1 diabetes (juvenile diabetes); Type 2 diabetes |
Approval
Forecasted revenue
|
Pegunigalsidase alfa (Elfabrio) Chiesi Farmaceutici SpA
|
Fabry disease (FD) |
Approval
Forecasted revenue
|
Oncology
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Imetelstat sodium (Rytelo) Geron Corp
|
Myelodysplastic syndrome; Post-essential thrombocythemia myelofibrosis (post-ET MF); Post-polycythemia vera myelofibrosis (PPV-MF) |
Approval
Forecasted revenue
|
Ophthalmology
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Avacincaptad pegol sodium (Izervay) Astellas Pharma Inc.
|
Geographic atrophy (GA) |
Approval
Forecasted revenue
|
Women’s health
| Medicine (Trade name) Company | Indication(s) | Approval Status and Key Attributes |
|---|---|---|
Fezolinetant (Veozah) Astellas Pharma Inc.
|
Vasomotor symptoms of menopause (hot flashes) |
Approval
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q2-2024, and are given in US dollars.
Data source: GlobalData Healthcare database.
Spotlight on Canada
This section includes a list of select medicines currently under review by Health Canada that may have a significant impact on future clinical practice and drug spending. Medicines included on this list may be new to Canada but have been approved in other jurisdictions.
Table 7 highlights six new medicines currently on Health Canada’s Drug and Health Product SUR Lists that have a novel mechanism of action or have demonstrated improved safety and efficacy in clinical trials. Of the five medicines reported in the 2022 edition, all but one have received market authorization from Health Canada. The central nervous system drug masitinib mesylate, indicated for amyotrophic lateral sclerosis (ALS), was issued a Notice of Deficiency (2024-02), and the submission was withdrawn from Health Canada.
The SUR Lists are publicly available sources that identify pharmaceutical and biologic drug submissions with new medicinal ingredients that have been accepted for review in Canada.
Table 7. Selected new medicines currently under review by Health Canada, 2023
Immunological disorders
| Medicine (Trade name) Company | Anticipated Indication(s)† | Description and Key Attributes |
|---|---|---|
Leniolisib Joenja (U.S.) Pharming Technologies BV
|
Activated phosphoinositide 3-kinase delta syndrome (APDS) |
Clinical trials
Forecasted revenue
|
Tapinarof Vtama (U.S.) Dermavant Sciences GmbH
|
Plaque psoriasis |
Clinical trials
Forecasted revenue
|
Oncology
| Medicine (Trade name) Company | Anticipated Indication(s)† | Description and Key Attributes |
|---|---|---|
Avapritinib Ayvakit (U.S.) Blueprint Medicines Corporation
|
Gastrointestinal stromal tumor (GIST) |
Clinical trials
Forecasted revenue
|
Ivosidenib Tibsovo (U.S.) Servier Canada Inc.
|
Acute myeloid leukemia (AML) |
Clinical trials
Forecasted revenue
|
Momelotinib dihydrochloride monohydrate Ojjaara (U.S.) Omjjara (EMA) GlaxoSmithKline Inc.
|
Myelofibrosis (MF) |
Clinical trials
Forecasted revenue
|
Ophthalmology
| Medicine (Trade name) Company | Anticipated Indication(s)† | Description and Key Attributes |
|---|---|---|
Perfluorohexyl-octane Miebo (U.S.) Bausch & Lomb Inc.
|
Keratoconjunctivitis sicca (dry eye) |
Clinical trials
Forecasted revenue
|
* Consensus forecasts for global revenue data were collected from GlobalData, Q2-2024, and are given in US dollars.
† Health Canada’s Drug and Health Product Submissions Under Review Lists provide the therapeutic area for the medicine under review but do not specify the indication. The indication listed in Table 7 is based on the information about the medicine in the literature and/or approvals in other jurisdictions. When there is an aligned review, in some cases the indication was confirmed by the CDA Reimbursement Review report.
Data source: GlobalData Healthcare database.
Appendix A
Table A1: Biosimilars in Phase III or preregistration (based on data extract from 2024-04-03)
| Medicine | Reference product in Canada | Other biosimilars marketed in Canada at this time (Y/N) and biosimilars under review by HC | Companies developing a biosimilar | Indication |
|---|---|---|---|---|
Aflibercept |
Eylea (Bayer Inc.) |
N Under HC review (as of Apr 3):
|
Alteogen Inc. Celltrion Inc. Formycon AG Sam Chun Dang Pharm Co Ltd. |
Wet (neovascular/exudative) macular degeneration |
Amgen Inc. |
Macular edema |
|||
Celltrion Inc. |
Age related macular degeneration |
|||
Celltrion Inc. |
Choroidal neovascularization |
|||
Celltrion Inc. |
Cystoid macular edema; Diabetic macular edema |
|||
Bevacizumab |
Avastin (Hoffmann-La Roche Limited) |
Y |
Prestige BioPharma Ltd. |
Non-small cell lung cancer |
Denosumab |
Prolia/Xgeva (Amgen Canada Inc.) |
N Under HC review (as of Apr 3):
|
Alvotech SA Biocon Ltd. Celltrion Inc. Eden Biologics Inc. Fresenius Kabi SwissBioSim GmbH Gedeon Richter plc I'rom Group Co Ltd. (Japan) Shanghai Henlius Biotech Inc. |
Post menopausal osteoporosis |
Biocon Ltd. |
Bone fracture |
|||
Biocon Ltd. |
Bone metastasis |
|||
Eculizumab |
Soliris (Alexion Pharma GmbH) |
N Under HC review (as of Apr 3):
|
Paroxysmal nocturnal hemoglobinuria |
|
Filgrastim |
Neulasta (Amgen Canada Inc.) |
Y Under HC review (as of Apr 3):
|
Chemotherapy induced neutropenia |
|
Ocrelizumab |
Ocrevus (Hoffmann-La Roche Limited) |
N |
Celltrion Inc. |
Relapsing remitting multiple sclerosis (RRMS) |
Omalizumab |
Xolair (Novartis Pharmaceuticals Canada Inc.) |
N Under HC review (as of Apr 3):
|
Celltrion Inc. |
Allergic asthma |
Celltrion Inc. |
Chronic urticaria or hives |
|||
Celltrion Inc. |
Nasal polyps (nasal polyposis); Rhinosinusitis |
|||
Celltrion Inc. |
Food allergy |
|||
Pegfilgrastim |
Neulasta (Amgen Canada Inc.) |
Y Under HC review (as of Apr 3):
|
Curateq Biologics Pvt Ltd. |
Chemotherapy induced neutropenia |
Pertuzumab |
Perjeta (Hoffmann-La Roche Limited) |
N |
Shanghai Henlius Biotech Inc. |
Human epidermal growth factor receptor 2 positive breast cancer (HER2+ breast cancer) |
Ranibizumab |
Lucentis (Novartis Pharmaceuticals Canada Inc.) |
Y |
Lupin Ltd. |
Wet (neovascular/exudative) macular degeneration |
Rituximab |
Rituxan (Hoffmann-La Roche Limited) |
Y Under HC review (as of Apr 3):
|
Non-Hodgkin’s lymphoma; chronic lymphocytic leukemia; rheumatoid arthritis; granulomatosis with polyangiitis and microscopic polyangiitis |
|
Tocilizumab |
Actemra (Hoffmann-La Roche Limited) |
N Under HC review (as of Apr 3):
|
Celltrion Inc. Mochida Pharmaceutical Co Ltd. |
Rheumatoid arthritis |
Trastuzumab |
Herceptin (Hoffmann-La Roche Limited) |
Y Under HC review (as of Apr 3):
|
Prestige BioPharma Ltd. |
Human epidermal growth factor receptor 2 positive breast cancer (HER2+ breast cancer) |
Prestige BioPharma Ltd. |
Adenocarcinoma of the gastroesophageal junction |
|||
Prestige BioPharma Ltd. |
Gastric cancer |
|||
Ustekinumab |
Stelara (Janssen Inc.) |
N Under HC review (as of Apr 3):
|
Biocon Ltd. Samsung Bioepis Co Ltd. |
Psoriatic arthritis |
Biocon Ltd. Formycon AG Samsung Bioepis Co Ltd. STgen Bio Co Ltd. |
Plaque psoriasis (psoriasis vulgaris) |
|||
Biocon Ltd. Samsung Bioepis Co Ltd. |
Crohn's disease (regional enteritis) |
|||
Samsung Bioepis Co Ltd. |
Ulcerative colitis |
Table A2: Drugs in Phase II for indications targeted by pipeline candidates, 2023
| Pipeline candidate | Indication | Drugs in Phase II and mechanism of action (MoA)* |
|---|---|---|
ABBVCLS-7262 |
Amyotrophic lateral sclerosis (ALS) |
Drugs in Phase II for this indication and their MoA :
There are no other drugs targeting this indication with the same MoA as ABBVCLS-7262 (a eukaryotic translation initiation factor 2 subunit beta activator) in Phase II development at this time.
|
AD109 (atomoxetine + R-oxybutynin) |
Obstructive sleep apnea (OSA) |
There are no other drugs targeting this specific indication in Phase II development at this time. |
Aficamten |
Hypertrophic cardiomyopathy |
There are no other drugs targeting this specific indication in Phase II development at this time. |
Brensocatib |
Bronchiectasis |
Drugs in Phase II for this indication and their MoA :
There are no other drugs targeting this indication with the same MoA as brensocatib (a dipeptidyl peptidase 1 inhibitor) in Phase II development at this time. |
Datopotamab deruxtecan |
Breast cancer, both HR-positive/HER2-negative and triple-negative |
Drugs in Phase II for this indication and their MoA :
There are no other drugs for all types of breast cancer targeted by datopotamab (a TROP2-targeted antibody-drug conjugate) in Phase II development at this time. |
Efruxifermin |
Metabolic dysfunction-associated steatohepatitis (MASH) |
Drugs in Phase II for this indication and their MoA :
There is one other drug (NN-9499) targeting this indication with the same MoA as efruxifermin (a fibroblast growth factor receptor agonist) in Phase II development at this time. |
Ensifentrine |
Chronic obstructive pulmonary disease |
Drugs in Phase II for this indication and their MoA :
There are no other drugs targeting this indication with the same MoA as ensifentrine (a PDE 3 and 4 inhibitor) in Phase II development at this time. |
Fazirsiran |
Alpha-1 antitrypsin deficiency (A1AD) |
Drugs in Phase II for this indication and their MoA :
There are other drugs (belcesiran, INBRX-101) targeting this indication with the same MoA as fazirsiran (an alpha 1 antitrypsin Inhibitor) in Phase II development at this time. |
Gemcitabine (GemRIS, TAR-200) |
Non-muscle invasive bladder cancer (NMIBC) (superficial bladder cancer); Muscle invasive bladder cancer (MIBC) |
Drugs in Phase II for these indications and their MoA :
There is no other drug targeting both indications with the same MoA as gemcitabine (a ribonucleoside diphosphate reductase large subunit inhibitor) in Phase II development at this time. |
Iclepertin |
Kidney transplant rejection |
Drugs in Phase II for this indication and their MoA :
There is no other drug targeting this indication with the same MoA as iclepertin (a sodium and chloride dependent glycine transporter 1 inhibitor) in Phase II development at this time. |
Inaxaplin |
Focal segmental glomerulosclerosis |
Drugs in Phase II for this indication and their MoA :
There is no other drug targeting this indication with the same MoA as inaxaplin (an apolipoprotein L1 inhibitor) in Phase II development at this time. |
Nerinetide
|
Acute ischemic stroke |
Drugs in Phase II for this indication and their MoA :
There is no other drug targeting this indication with the same MoA as nerinetide (a neuroprotective eicosapeptide) in Phase II development at this time. |
Obefazimod |
Ulcerative colitis |
Drugs in Phase II for this indication and their MoA :
There is no other drug targeting this indication with the same MoA as obefazimod (a eukaryotic translation initiation factor 4E inhibitor/ interleukin 22 receptor agonist) in Phase II development at this time. |
Patidegib |
Gorlin syndrome (basal cell nevus syndrome/nevoid basal cell carcinoma syndrome) |
Drugs in Phase II for this indication and their MoA :
There is no other drug targeting this indication with the same MoA as patidegib (a hedgehog signaling pathway blocker) in Phase II development at this time. |
Pelacarsen sodium |
Cardiovascular disease; Hyperlipidemia |
Drugs in Phase II for this indication and their MoA :
There is no other drug targeting the hyperlipidemia indication with the same MoA as pelacarsen (an apolipoprotein A inhibitor) in Phase II development at this time. |
Prademagene zamikeracel |
Epidermolysis bullosa |
Drugs in Phase II for this indication and their MoA :
There is one drug (PTR-01) targeting this indication with the same MoA as prademagene zamikeracel (a collagen type 7 replacement) in Phase II development at this time. |
Resiniferatoxin |
Osteoarthritis pain |
Drugs in Phase II for this indication and their MoA :
There is one other drug (CGS-2005) targeting this indication with the same MoA as resiniferatoxin (a transient receptor potential cation channel subfamily v member 1 activator) in Phase II development at this time. |
Revumenib |
Acute lymphocytic leukemia (ALL); Acute lympho-blastic leukemia; Refractory acute myeloid leukemia; Relapsed acute myeloid leukemia |
Drugs in Phase II for these indications and their MoA :
There are other drugs (DS-1594b, DSP-5336, ziftomenib) targeting all indications with the same MoA as revumenib (a menin-mixed lineage leukemia inhibitor) in Phase II development at this time. |
RGX-121 |
Mucopolysaccharidosis II (MPS II) (Hurler syndrome) |
Drugs in Phase II for this indication and their MoA :
There is one other drug (RGX-111) targeting this indication with the same MoA as RGX-121 (an iduronate 2 sulfatase activator) in Phase II development at this time. |
Xanomeline-trospium (KarXT) |
Schizophrenia; Psychosis |
Drugs in Phase II for this indication and their MoA :
There are other drugs (ANAVEX-371, NBI-1117568, RL-007) targeting the schizophrenia indication with the same MoA as xanomeline-trospium (a muscarinic acetylcholine receptor agonist) in Phase II development at this time. |
Abbreviations:
AMP: adenosine monophosphate; GABA: gamma-aminobutyric acid ; GLP: glucagon like peptide; GSK: glycogen synthase kinase; PDE: phosphodiesterase; PG: prostaglandin; PPAR: peroxisome proliferator activated receptor; TNF: tumour necrosis factor.
* Data source: GlobalData’s Healthcare database (accessed April 2024).
References
- Clinical Development Success Rates and Contributing Factors 2011–2020. https://go.bio.org/rs/490-EHZ-999/images/ClinicalDevelopmentSuccessRates2011_2020.pdf (accessed October 21, 2024).
- Sebastian SA, Padda I, Lehr EJ, Johal G. Aficamten: A Breakthrough Therapy for Symptomatic Obstructive Hypertrophic Cardiomyopathy. Am J Cardiovasc Drugs. 2023 Sep;23(5):519-532. doi: 10.1007/s40256-023-00599-0. Epub 2023 Aug 1.
- O’Riordan M. 2024. SEQUOIA-HCM: Aficamten Boosts Exercise Capacity in Obstructive HCM. 13 May 2024. https://www.tctmd.com/news/sequoia-hcm-aficamten-boosts-exercise-capacity-obstructive-hcm#:~:text=Effect%20on%20LVEF%3A%20No%20Worrisome%20Signs&text=To%20TCTMD%2C%20Maron%20explained%20that,wider%20therapeutic%20window%20than%20mavacamten.
- A Phase 3, Multi-Center, Randomized, Double-blind, Placebo-controlled Trial to Evaluate the Efficacy and Safety of CK-3773274 in Adults With Symptomatic Hypertrophic Cardiomyopathy and Left Ventricular Outflow Tract Obstruction. ClinicalTrials.gov:NCT05186818 (completed). https://clinicaltrials.gov/study/NCT05186818?intr=Aficamten%20&aggFilters=phase:3&rank=5
- A Phase 3, Multi-Center, Randomized, Double-Blind Trial to Evaluate the Efficacy and Safety of Aficamten Compared to Placebo in Adults With Symptomatic Non-Obstructive Hypertrophic Cardiomyopathy. ClinicalTrials.gov: NCT06081894 (recruiting). https://clinicaltrials.gov/study/NCT06081894?intr=Aficamten%20&aggFilters=phase:3&rank=3
- A Phase 3, Multi-center, Randomized, Double-blind Trial to Evaluate the Efficacy and Safety of Aficamten Compared to Metoprolol in Adults With Symptomatic Obstructive Hypertrophic Cardiomyopathy. ClinicalTrials.gov: NCT05767346 (recruiting). https://clinicaltrials.gov/study/NCT05767346?intr=Aficamten%20&aggFilters=phase:3&rank=4
- An Open-Label Study of Aficamten for Chinese Patients With Symptomatic Obstructive Hypertrophic Cardiomyopathy. ClinicalTrials.gov: NCT06116968 (recruiting). https://clinicaltrials.gov/study/NCT06116968?intr=Aficamten%20&aggFilters=phase:3&rank=1
- A Phase 2/3 Multicenter, Randomized, Double-Blind, Placebo-Controlled and Open-Label Extension Trial to Evaluate the Efficacy and Safety of Aficamten in a Pediatric Population With Symptomatic Obstructive Hypertrophic Cardiomyopathy. ClinicalTrials.gov: NCT06412666 (not yet recruiting). https://clinicaltrials.gov/study/NCT06412666?intr=Aficamten%20&aggFilters=phase:3&rank=2
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Dammavalam V, Lin S, Nessa S, Daksla N, Stefanowski K, et al. Neuroprotection during Thrombectomy for Acute Ischemic Stroke: A Review of Future Therapies. Int J Mol Sci. 2024 Jan 10;25(2):891. doi: 10.3390/ijms25020891.
- A Multicentre, Randomized, Double-blinded, Placebo-controlled, Parallel Group, Single-dose Design to Determine the Efficacy and Safety of Nerinetide in Participants With Acute Ischemic Stroke Undergoing Endovascular Thrombectomy Excluding Thrombolysis. ClinicalTrials.gov: NCT04462536 (completed). https://clinicaltrials.gov/study/NCT04462536?intr=Nerinetide&aggFilters=phase:3&rank=1
- A Multicenter, Randomized, Double-Blind, Placebo-Controlled Study to Determine the Efficacy and Safety of Intravenous NA-1 Initiated by Paramedics in the Field for Acute Cerebral Ischemia Within Three Hours of Symptom Onset. ClinicalTrials.gov: NCT02315443 (completed). https://clinicaltrials.gov/study/NCT02315443?intr=Nerinetide&aggFilters=phase:3&rank=2
- A Multicentre, Randomized, Double-blinded, Placebo-controlled, Parallel Group, Single-dose Design to Determine the Efficacy and Safety of Intravenous NA-1 in Subjects With Acute Ischemic Stroke Undergoing Endovascular Thrombectomy. ClinicalTrials.gov: NCT02930018 (completed). https://clinicaltrials.gov/study/NCT02930018?intr=Nerinetide&aggFilters=phase:3&rank=3
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Kosmas CE, Bousvarou MD, Papakonstantinou EJ, Tsamoulis D, Koulopoulos A, et al. Novel Pharmacological Therapies for the Management of Hyperlipoproteinemia(a). Int J Mol Sci. 2023 Sep 3;24(17):13622. doi: 10.3390/ijms241713622.
- A Single Arm, Open Label Extension (OLE), Multicenter Study to Evaluate Long-term Safety and Tolerability of Pelacarsen (TQJ230) in Patients With Cardiovascular Disease Who Have Successfully Completed the Apheresis Parent Study. ClinicalTrials.gov: NCT05900141 (recruiting). https://clinicaltrials.gov/study/NCT05900141?intr=pelacarsen%20&aggFilters=phase:3&rank=1
- A Randomized Double-blind, Placebo-controlled, Multicenter Study to Evaluate the Efficacy, Safety and Tolerability of Pelacarsen (TQJ230) in US Black/African American & Hispanic Patient Populations With Elevated Lp(a) and Established Atherosclerotic Cardiovascular Disease. ClinicalTrials.gov: NCT06267560 (not yet recruiting). https://clinicaltrials.gov/study/NCT06267560?intr=pelacarsen%20&aggFilters=phase:3&rank=2
- A Randomized, Double-blind, Placebo-controlled, Multicenter Trial Assessing the Reduction of the Rate of Lipoprotein Apheresis After Treatment With Pelacarsen (TQJ230) Compared to Placebo in Patients With Hyperlipoproteinemia(a) and Established Cardiovascular Disease Undergoing Weekly Lipoprotein Apheresis in Germany. ClinicalTrials.gov: NCT05305664 (active, not recruiting). https://clinicaltrials.gov/study/NCT05305664?intr=pelacarsen%20&aggFilters=phase:3&rank=3
- A Randomized Double-blind, Placebo-controlled, Multicenter Trial Assessing the Impact of Lipoprotein (a) Lowering With Pelacarsen (TQJ230) on Major Cardiovascular Events in Patients With Established Cardiovascular Disease. ClinicalTrials.gov: NCT04023552 (active, not recruiting). https://clinicaltrials.gov/study/NCT04023552?intr=pelacarsen%20&aggFilters=phase:3&rank=4
- Calico/AbbVie. ABBV-CLS-7262 for ALS. HEALEY ALS Platform Trial Regimen F. 2023. https://www.massgeneral.org/assets/mgh/pdf/neurology/als/regimenf_calicosabbv-cls-7262drugsciencewebinar_2023.pdf
- HEALEY ALS Platform Trial - Regimen F ABBV-CLS-7262. ClinicalTrials.gov: NCT05740813 (enrolling by invitation). https://clinicaltrials.gov/study/NCT05740813?intr=NCT05740813&rank=1
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Rosenbrock H, Desch M, Wunderlich G(2). Development of the novel GlyT1 inhibitor, iclepertin (BI 425809), for the treatment of cognitive impairment associated with schizophrenia. Eur Arch Psychiatry Clin Neurosci. 2023 Oct;273(7):1557-1566. doi: 10.1007/s00406-023-01576-z. Epub 2023 Mar 27.
- Dudzik P, Lustyk K, Pytka K. Beyond dopamine: Novel strategies for schizophrenia treatment. Med Res Rev. 2024 Apr 23. doi: 10.1002/med.22042. Online ahead of print.
- An Open Label, Single Arm, Extension Trial to Examine Long-term Safety of Iclepertin Once Daily in Patients With Schizophrenia Who Have Completed Previous Iclepertin Phase III Trials (CONNEX-X). ClinicalTrials.gov: NCT05211947 (recruiting). https://clinicaltrials.gov/study/NCT05211947?intr=Iclepertin&aggFilters=phase:3&rank=1
- A Phase III Randomized, Double-blinded, Placebo-controlled Parallel Group Trial to Examine the Efficacy and Safety of Iclepertin Once Daily Over 26 Week Treatment Period in Patients With Schizophrenia (CONNEX-2). ClinicalTrials.gov: NCT04846881 (active, not recruiting). https://clinicaltrials.gov/study/NCT04846881?intr=Iclepertin&aggFilters=phase:3&rank=2
- A Phase III Randomized, Double-blind, Placebo-controlled Parallel Group Trial to Examine the Efficacy and Safety of Iclepertin Once Daily Over 26 Week Treatment Period in Patients With Schizophrenia (CONNEX-1). ClinicalTrials.gov: NCT04846868 (active, not recruiting). https://clinicaltrials.gov/study/NCT04846868?intr=Iclepertin&aggFilters=phase:3&rank=3
- A Phase III Randomized, Double-blind, Placebo-controlled Parallel Group Trial to Examine the Efficacy and Safety of Iclepertin Once Daily Over 26 Week Treatment Period in Patients With Schizophrenia (CONNEX-3). ClinicalTrials.gov: NCT04860830 (active, not recruiting). https://clinicaltrials.gov/study/NCT04860830?intr=Iclepertin&aggFilters=phase:3&rank=4
- A Phase 3, Multi-Center, Double-Blind, Randomized, Placebo-Controlled Trial to Evaluate the Efficacy and Safety of Reldesemtiv in Patients With Amyotrophic Lateral Sclerosis (ALS). NCT04944784 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04944784?term=Reldesemtiv&phase=2&draw=2&rank=1
- A Phase 3, Open-Label Extension of COURAGE-ALS (CY 5031). NCT05442775 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05442775?term=Reldesemtiv&phase=2&draw=2&rank=2
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Szallasi A. Resiniferatoxin: Nature's Precision Medicine to Silence TRPV1-Positive Afferents Int J Mol Sci. 2023 Oct 10;24(20):15042. doi: 10.3390/ijms242015042.
- Grünenthal Press release. 2023. Grünenthal`s resinferatoxin receives Breakthrough Therapy Designation from U.S. FDA for pain associated with osteoarthritis of the knee. 22 May 2023. https://www.grunenthal.com/en/press-room/press-releases/2023/pr-fda-breakthrough-therapy-designation-resiniferatoxin
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Kaul I, Sawchak S, Correll CU, Kakar R, Breier A, et al. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial.Lancet. 2024 Jan 13;403(10422):160-170. doi: 10.1016/S0140-6736(23)02190-6. Epub 2023 Dec 14.
- Psychology Today. 2024. Why This Potential New Medication for Schizophrenia Is Important. 6 February 2024. https://www.psychologytoday.com/ca/blog/demystifying-psychiatry/202402/why-this-potential-new-medication-for-schizophrenia-is
- A Phase 3, Randomized, Double-blind, Parallel-group, Placebo-controlled, Multicenter Study to Evaluate the Efficacy and Safety of KarXT in Acutely Psychotic Hospitalized Adults With DSM-5 Schizophrenia. ClinicalTrials.gov: NCT04659161 (completed). https://clinicaltrials.gov/study/NCT04659161?intr=KarXT&aggFilters=phase:3&page=1&rank=7
- An Open-label Extension Study to Assess the Long-term Safety, Tolerability, and Efficacy of KarXT in Subjects With DSM-5 Schizophrenia. ClinicalTrials.gov: NCT04659174 (completed). https://clinicaltrials.gov/study/NCT04659174?intr=KarXT&aggFilters=phase:3&page=1&rank=8
- A Phase 3, Randomized, Double-blind, Parallel-group, Placebo-controlled, Multicenter Study to Evaluate the Efficacy and Safety of KarXT in Acutely Psychotic Hospitalized Adults With DSM-5 Schizophrenia. ClinicalTrials.gov: NCT04738123 (completed). https://clinicaltrials.gov/study/NCT04738123?intr=KarXT&aggFilters=phase:3&page=2&rank=11
- An Open-label Extension Study to Assess the Long-term Safety and Tolerability of Adjunctive KarXT in Subjects With Inadequately Controlled Symptoms of Schizophrenia. ClinicalTrials.gov: NCT05304767 (enrolling by invitation). https://clinicaltrials.gov/study/NCT05304767?intr=KarXT&aggFilters=phase:3&page=1&rank=4
- A Phase 3, Randomized, Double-blind, Placebo-controlled Study to Evaluate the Safety and Efficacy of Adjunctive KarXT in Subjects With Inadequately Controlled Symptoms of Schizophrenia. ClinicalTrials.gov: NCT05145413 (recruiting). https://clinicaltrials.gov/study/NCT05145413?intr=KarXT&aggFilters=phase:3&page=1&rank=5
- An Open-label Study to Assess the Long-term Safety, Tolerability, and Efficacy of KarXT in De Novo Subjects With DSM-5 Schizophrenia. ClinicalTrials.gov: NCT04820309 (active, not recruiting). https://clinicaltrials.gov/study/NCT04820309?intr=KarXT&aggFilters=phase:3&page=1&rank=9
- A Phase 3, Multicenter, Two-part Study With a 5-week Double-blind Part (Randomized, Parallel-group, Placebo-controlled) Followed by a 12-week Open-label Extension Part, to Evaluate the Efficacy and Safety of KarXT in Acutely Psychotic Hospitalized Chinese Adult Subjects With DSM-5 Schizophrenia. ClinicalTrials.gov: NCT05919823 (recruiting). https://clinicaltrials.gov/study/NCT05919823?intr=KarXT&aggFilters=phase:3&page=1&rank=10
- A Multi-center, Open-label Study to Assess the Effectiveness, Long-term Safety, Tolerability, and Durability of Effect of KarXT in Patients With DSM-5 Diagnosis of Schizophrenia. ClinicalTrials.gov: NCT05643170 (terminated). https://clinicaltrials.gov/study/NCT05643170?intr=KarXT&aggFilters=phase:3&page=1&rank=6
- A Phase 3 Global, Multicenter, Open-Label Extension Study to Assess the Long-Term Safety and Tolerability of KarXT in Subjects With Psychosis Associated With Alzheimer's Disease. ClinicalTrials.gov: NCT05980949 (enrolling by invitation). https://clinicaltrials.gov/study/NCT05980949?intr=KarXT&aggFilters=phase:3&page=1&rank=1
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled Relapse Prevention Study to Evaluate the Safety and Efficacy of KarXT for the Treatment of Psychosis Associated With Alzheimer's Disease. ClinicalTrials.gov: NCT05511363 (recruiting). https://clinicaltrials.gov/study/NCT05511363?intr=KarXT&aggFilters=phase:3&page=1&rank=2
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Safety and Efficacy of KarXT for the Treatment of Psychosis Associated With Alzheimer's Disease. ClinicalTrials.gov: NCT06126224 (recruiting). https://clinicaltrials.gov/study/NCT06126224?intr=KarXT&aggFilters=phase:3&page=1&rank=3
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Abeona Therapeutics Inc. Press Release, 2024. Abeona Therapeutics Provides Regulatory Update on Pz-cel, 22 April 2024. https://investors.abeonatherapeutics.com/press-releases/detail/276/abeona-therapeutics-provides-regulatory-update-on-pz-cel
- VIITAL: A Phase 3 Study of EB-101 for the Treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB). ClinicalTrials.gov: NCT04227106 (completed). https://clinicaltrials.gov/study/NCT04227106?intr=EB-101&aggFilters=phase:3&rank=1
- Ernst D. 2024. FDA’s Decision on Recessive Dystrophic Epidermolysis Bullosa Treatment Delayed. 24 April 2024. https://www.empr.com/home/news/drugs-in-the-pipeline/fdas-decision-on-recessive-dystrophic-epidermolysis-bullosa-treatment-delayed/
- Abeona Therapeutics Inc. Press Release, 2024. Abeona Therapeutics Provides Regulatory Update on Pz-cel, 22 April 2024. https://investors.abeonatherapeutics.com/press-releases/detail/276/abeona-therapeutics-provides-regulatory-update-on-pz-cel
- A Phase 3b Study for the Treatment of Recessive Dystrophic Epidermolysis Bullosa (RDEB) in New and Previously EB-101 Treated Patients. ClinicalTrials.gov: NCT05725018 (recruiting). https://clinicaltrials.gov/study/NCT05725018?intr=EB-101&aggFilters=phase:3&rank=2
- Abeona Therapeutics® Reports Second Quarter 2024 Financial Results and Concludes Type A Meeting with FDA to Align on Upcoming Pz-cel BLA Resubmission. Press Release 2024. https://investors.abeonatherapeutics.com/press-releases/detail/285/abeona-therapeutics-reports-second-quarter-2024-financial
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Harrison SA, Ruane PJ, Freilich BL, Neff G, Patil R, et al. Efruxifermin in non-alcoholic steatohepatitis: a randomized, double-blind, placebo-controlled, phase 2a trial. Nat Med. 2021 Jul;27(7):1262-1271. doi: 10.1038/s41591-021-01425-3. Epub 2021 Jul 8.
- Harrison SA, Frias JP, Neff G, Abrams GA, Lucas KJ, et al; HARMONY Study Group. Safety and efficacy of once-weekly efruxifermin versus placebo in non-alcoholic steatohepatitis (HARMONY): a multicentre, randomised, double-blind, placebo-controlled, phase 2b trial.Lancet Gastroenterol Hepatol. 2023 Dec;8(12):1080-1093. doi: 10.1016/S2468-1253(23)00272-8. Epub 2023 Oct 3.
- Harrison SA, Frias JP, Lucas KJ, Reiss G, Neff G, et al. Safety and Efficacy of Efruxifermin in Combination With a GLP-1 Receptor Agonist in Patients With NASH/MASH and Type 2 Diabetes in a Randomized Phase 2 Study. Clin Gastroenterol Hepatol. 2024 Mar 4:S1542-3565(24)00226-X. doi: 10.1016/j.cgh.2024.02.022. Online ahead of print.
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of Efruxifermin in Subjects With Non-Cirrhotic Nonalcoholic Steatohepatitis (NASH)/Metabolic Dysfunction-Associated Steatohepatitis (MASH) and Fibrosis. ClinicalTrials.gov: NCT06215716 (recruiting). https://clinicaltrials.gov/study/NCT06215716?intr=Efruxifermin&aggFilters=phase:3&rank=1
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study Evaluating the Safety and Efficacy of Efruxifermin in Subjects With Non-invasively Diagnosed Nonalcoholic Steatohepatitis (NASH)/Metabolic Dysfunction-Associated Steatohepatitis (MASH) and Nonalcoholic Fatty Liver Disease (NAFLD)/Metabolic Dysfunction-Associated Steatotic Liver Disease (MASLD). ClinicalTrials.gov: NCT06161571 (recruiting). https://clinicaltrials.gov/study/NCT06161571?intr=Efruxifermin&aggFilters=phase:3&rank=2
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Vermeire S, Solitano V, Peyrin-Biroulet L, Tilg H, Danese S, et al. Obefazimod: A First-in-class Drug for the Treatment of Ulcerative Colitis. J Crohns Colitis. 2023 Nov 8;17(10):1689-1697. doi: 10.1093/ecco-jcc/jjad067.
- A Randomized, Double-blind, Multicenter Phase III Study to Evaluate the Long-term Efficacy and Safety of ABX464 25 mg or 50 mg Once Daily as a Maintenance Therapy in Subjects With Moderately to Severely Active Ulcerative Colitis. ClinicalTrials.gov: NCT05535946 (recruiting). https://clinicaltrials.gov/study/NCT05535946?intr=obefazimod&aggFilters=phase:3&rank=1
- A Randomized, Double-blind, Placebo-controlled, Multicenter Phase III Study to Evaluate the Efficacy and Safety of ABX464 Once Daily for Induction Treatment in Subjects With Moderately to Severely Active Ulcerative Colitis. ClinicalTrials.gov: NCT05507216 (recruiting). https://clinicaltrials.gov/study/NCT05507216?intr=obefazimod&aggFilters=phase:3&rank=2
- A Randomized, Double-blind, Placebo-controlled, Multicenter Phase III Study to Evaluate the Efficacy and Safety of ABX464 Once Daily for Induction Treatment in Subjects With Moderately to Severely Active Ulcerative Colitis. ClinicalTrials.gov: NCT05507203 (recruiting). https://clinicaltrials.gov/study/NCT05507203?intr=obefazimod&aggFilters=phase:3&rank=3
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- A Phase 3, Open-Label Extension Study to Evaluate the Long-Term Safety and Efficacy of Fazirsiran in Participants With Alpha-1 Antitrypsin Deficiency-Associated Liver Disease. ClinicalTrials.gov: NCT05899673 (enrolling by invitation). https://clinicaltrials.gov/study/NCT05899673?intr=Fazirsiran%20&aggFilters=phase:3&rank=1
- A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study to Evaluate the Safety and Efficacy of Fazirsiran in the Treatment of Alpha-1 Antitrypsin Deficiency-Associated Liver Disease With METAVIR Stage F1 Fibrosis. ClinicalTrials.gov: NCT06165341 (recruiting). https://clinicaltrials.gov/study/NCT06165341?intr=Fazirsiran%20&aggFilters=phase:3&rank=2
- A Randomized, Double-blind, Placebo-Controlled, Phase 3 Study to Evaluate the Efficacy and Safety of Fazirsiran in the Treatment of Alpha-1 Antitrypsin Deficiency-Associated Liver Disease With METAVIR Stage F2 to F4 Fibrosis. ClinicalTrials.gov: NCT05677971 (recruiting). https://clinicaltrials.gov/study/NCT05677971?intr=Fazirsiran%20&aggFilters=phase:3&rank=3
- Egbuna O, Zimmerman B, Manos G, Fortier A, Chirieac MC, et al; VX19-147-101 Study Group. Inaxaplin for Proteinuric Kidney Disease in Persons with Two APOL1 Variants. N Engl J Med. 2023 Mar 16;388(11):969-979. doi: 10.1056/NEJMoa2202396.
- Gbadegesin R, Lane B. Inaxaplin for the treatment of APOL1-associated kidney disease. Nat Rev Nephrol. 2023 Aug;19(8):479-480. doi: 10.1038/s41581-023-00721-0.
- A Phase 2/3 Adaptive, Double-blind, Placebo-Controlled Study to Evaluate the Efficacy and Safety of VX-147 in Adult and Pediatric Subjects With APOL1-mediated Proteinuric Kidney Disease. ClinicalTrials.gov: NCT05312879 (recruiting). https://clinicaltrials.gov/study/NCT05312879?intr=VX-147&aggFilters=phase:3&rank=1
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- REGENXBIO Inc. Press Release. 2024. REGENXBIO Announces Pivotal Trial of RGX-121 for the Treatment of MPS II Achieves Primary Endpoint, February 2024. https://www.prnewswire.com/news-releases/regenxbio-announces-pivotal-trial-of-rgx-121-for-the-treatment-of-mps-ii-achieves-primary-endpoint-302056283.html
- A Phase 1/2/3 Multicenter, Open-Label Study to Evaluate the Efficacy, Safety, Tolerability, and Pharmacodynamics of RGX-121 in Pediatric Subjects With MPS II (Hunter Syndrome). ClinicalTrials.gov: NCT03566043 (active, not recruiting). https://clinicaltrials.gov/study/NCT03566043?intr=RGX-121&aggFilters=phase:3&rank=1
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- European Society for Medical Oncology (ESMO). News, 2024. Datopotamab Deruxtecan Shows Promising Activity in Heavily Pretreated Advanced HR-positive/HER2-negative and Triple-Negative Breast Cancers. 07 May 2024. https://www.esmo.org/oncology-news/datopotamab-deruxtecan-shows-promising-activity-in-heavily-pretreated-advanced-hr-positive-her2-negative-and-triple-negative-breast-cancers
- AstraZeneca/Daiichi Sankyo. Press Release, 2024. Datopotamab deruxtecan Biologics License Application accepted in the US for patients with previously treated metastatic HR-positive, HER2-negative breast cancer. 2 April 2024. https://www.astrazeneca.com/media-centre/press-releases/2024/fda-accepts-dato-dxd-bla-for-breast-cancer.html
- AstraZeneca. Press Release, 2024. Datopotamab deruxtecan Biologics License Application accepted in the US for patients with previously treated metastatic HR-positive, HER2-negative breast cancer. 2 April 2024. https://www.astrazeneca.com/media-centre/press-releases/2024/fda-accepts-dato-dxd-bla-for-breast-cancer.html
- European Society for Medical Oncology (ESMO). News, 2024. Datopotamab Deruxtecan Shows Promising Activity in Heavily Pretreated Advanced HR-positive/HER2-negative and Triple-Negative Breast Cancers. 07 May 2024. https://www.esmo.org/oncology-news/datopotamab-deruxtecan-shows-promising-activity-in-heavily-pretreated-advanced-hr-positive-her2-negative-and-triple-negative-breast-cancers
- A Phase 3, Open-label, Randomised Study of Datopotamab Deruxtecan (Dato-DXd) Versus Investigator's Choice of Chemotherapy in Patients Who Are Not Candidates for PD-1/PD-L1 Inhibitor Therapy in First-line Locally Recurrent Inoperable or Metastatic Triple-negative Breast Cancer (TROPION Breast02). ClinicalTrials.gov: NCT05374512 (recruiting). https://clinicaltrials.gov/study/NCT05374512?intr=Datopotamab%20&aggFilters=phase:3&rank=6
- A Phase III, Open-label, Randomised Study of Datopotamab Deruxtecan (Dato-DXd) With or Without Durvalumab Compared With Investigator's Choice of Chemotherapy (Paclitaxel, Nab-paclitaxel or Gemcitabine + Carboplatin) in Combination With Pembrolizumab in Patients With PD-L1 Positive Locally Recurrent Inoperable or Metastatic Triple-negative Breast Cancer (TROPION-Breast05). ClinicalTrials.gov: NCT06103864 (recruiting). https://clinicaltrials.gov/study/NCT06103864?intr=Datopotamab%20&aggFilters=phase:3&rank=8
- A Phase-3, Open-Label, Randomized Study of Dato-DXd Versus Investigator's Choice of Chemotherapy (ICC) in Participants With Inoperable or Metastatic HR-Positive, HER2-Negative Breast Cancer Who Have Been Treated With One or Two Prior Lines of Systemic Chemotherapy (TROPION-Breast01). ClinicalTrials.gov: NCT05104866 (active, not recruiting). https://clinicaltrials.gov/study/NCT05104866?intr=Datopotamab%20&aggFilters=phase:3&rank=9
- A Phase 3 Open-label, Randomised Study of Datopotamab Deruxtecan (DatoDXd) With or Without Durvalumab Versus Investigator's Choice of Therapy in Patients With Stage I-III Triple-negative Breast Cancer Who Have Residual Invasive Disease in the Breast and/or Axillary Lymph Nodes at Surgical Resection Following Neoadjuvant Systemic Therapy (TROPION-Breast03). ClinicalTrials.gov: NCT05629585 (recruiting). https://clinicaltrials.gov/study/NCT05629585?intr=Datopotamab%20&aggFilters=phase:3&rank=10
- A Phase III, Open-label, Randomised Study of Neoadjuvant Datopotamab Deruxtecan (Dato-DXd) Plus Durvalumab Followed by Adjuvant Durvalumab With or Without Chemotherapy Versus Neoadjuvant Pembrolizumab Plus Chemotherapy Followed by Adjuvant Pembrolizumab With or Without Chemotherapy for the Treatment of Adult Patients With Previously Untreated Triple-Negative or Hormone Receptor-low/HER2-negative Breast Cancer (D926QC00001; TROPION-Breast04). ClinicalTrials.gov: NCT06112379 (recruiting). https://clinicaltrials.gov/study/NCT06112379?intr=Datopotamab%20&aggFilters=phase:3&page=2&rank=11
- AstraZeneca/Daiichi Sankyo. Press Release, 2024. Datopotamab deruxtecan Biologics License Application accepted in the US for patients with previously treated metastatic HR-positive, HER2-negative breast cancer. 2 April 2024. https://www.astrazeneca.com/media-centre/press-releases/2024/fda-accepts-dato-dxd-bla-for-breast-cancer.html
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Broderick JM. 2023. TAR-200 Produces High CR Rates, Tolerability in BCG-unresponsive NMIBC. 1 May 2023. https://www.onclive.com/view/tar-200-produces-high-cr-rates-tolerability-in-bcg-unresponsive-nmibc
- Johnson & Johnson. News release, 2024. TAR-200 monotherapy shows greater than 80% complete response rate in patients with high-risk non–muscle-invasive bladder cancer.4 May 2024. https://www.prnewswire.com/news-releases/tar-200-monotherapy-shows-greater-than-80-complete-response-rate-in-patients-with-high-risk-nonmuscle-invasive-bladder-cancer-302135628.html
- A Phase 3, Open-Label, Multi-Center, Randomized Study Evaluating the Efficacy and Safety of TAR-200 in Combination With Cetrelimab or TAR-200 Alone Versus Intravesical Bacillus Calmette-Guérin (BCG) in Participants With BCG-naïve High-Risk Non-Muscle Invasive Bladder Cancer. ClinicalTrials.gov: NCT05714202 (recruiting). https://clinicaltrials.gov/study/NCT05714202?intr=TAR-200&aggFilters=phase:3&rank=1
- A Phase 3, Randomized, Open-label, Multi-center Study Evaluating the Efficacy and Safety of TAR-200 Versus Investigator's Choice of Intravesical Chemotherapy in Participants Who Received Bacillus Calmette-Guérin (BCG) and Recurred With High-risk Non-muscle-invasive Bladder Cancer (HR-NMIBC) and Who Are Ineligible for or Elected Not to Undergo Radical Cystectomy. ClinicalTrials.gov: NCT06211764 (recruiting). https://clinicaltrials.gov/study/NCT06211764?intr=TAR-200&aggFilters=phase:3&rank=2
- A Phase 3, Multi-center, Randomized Study Evaluating Efficacy of TAR-200 in Combination With Cetrelimab Versus Concurrent Chemoradiotherapy in Participants With Muscle-Invasive Urothelial Carcinoma (MIBC) of the Bladder Who Are Not Receiving Radical Cystectomy. ClinicalTrials.gov: NCT04658862 (recruiting). https://clinicaltrials.gov/study/NCT04658862?intr=TAR-200&aggFilters=phase:3&rank=3
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Cosio T, Di Prete M, Di Raimondo C, Garofalo V, Lozzi F, et al. Patidegib in Dermatology: A Current Review. Int J Mol Sci. 2021 Oct 3;22(19):10725. doi: 10.3390/ijms221910725.
- A Multicenter, Randomized, Double-blind, Vehicle-controlled, Phase 3 Efficacy and Safety Study of Patidegib Topical Gel, 2%, for the Reduction of Disease Burden of Persistently Developing Basal Cell Carcinomas (BCCs) in Subjects With Basal Cell Nevus Syndrome. ClinicalTrials.gov: NCT03703310 (completed). https://clinicaltrials.gov/study/NCT03703310?intr=patidegib%20&aggFilters=phase:3&rank=2
- A Multicenter, Randomized, Double Blind, Vehicle-controlled, Phase 3 Efficacy and Safety Study of Patidegib Gel 2% for the Reduction of Disease Burden of Persistently Developing Basal Cell Carcinomas (BCCs) in Subjects With Gorlin Syndrome. ClinicalTrials.gov: NCT06050122 (recruiting). https://clinicaltrials.gov/study/NCT06050122?intr=patidegib%20&aggFilters=phase:3&rank=1
- A Phase 3, Multicenter, Open-Label Extension Study of Patidegib Topical Gel, 2% in Subjects With Gorlin Syndrome (Basal Cell Nevus Syndrome). ClinicalTrials.gov: NCT04308395 (terminated). https://clinicaltrials.gov/study/NCT04308395?intr=patidegib%20&aggFilters=phase:3&rank=3
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Sava J. 2022. FDA Grants Breakthrough Therapy Designation to SNDX-5613 for R/R KMT2Ar Acute Leukemia. 6 December 2022. https://www.targetedonc.com/view/fda-grants-breakthrough-therapy-designation-to-sndx-5613-for-r-r-kmt2ar-acute-leukemia
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Mellinghoff IK, van den Bent MJ, Blumenthal DT, Touat M, Peters KB, et al.; INDIGO Trial Investigators. Vorasidenib in IDH1- or IDH2-Mutant Low-Grade Glioma. N Engl J Med. 2023 Aug 17;389(7):589-601. doi: 10.1056/NEJMoa2304194. Epub 2023 Jun 4.
- Corcept Therapeutics to Start Phase 3 Trial of Relacorilant Plus Nab-Paclitaxel in Patients With Platinum-Resistant Ovarian Cancer. June 2, 2022. https://www.globenewswire.com/en/news-release/2022/06/06/2456669/0/en/Corcept-Therapeutics-to-Start-Phase-3-Trial-of-Relacorilant-Plus-Nab-Paclitaxel-in-Patients-With-Platinum-Resistant-Ovarian-Cancer.html
- A Phase 3, Multicenter, Randomized, Double-blind, Placebo-Controlled Study of AG-881 in Subjects With Residual or Recurrent Grade 2 Glioma With an IDH1 or IDH2 Mutation. ClinicalTrials.gov: NCT04164901 (active, not recruiting). https://clinicaltrials.gov/study/NCT04164901?intr=Vorasidenib%20&aggFilters=phase:3&rank=1
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Apnimed, Inc. Press Release, 2023. Apnimed Presented Positive Phase 2b Results on AD109, an Investigational Oral Drug for Obstructive Sleep Apnea, for the First Time at ATS 2023. May 21, 2023. https://apnimed.com/apnimed-presented-positive-phase-2b-results-on-ad109-an-investigational-oral-drug-for-obstructive-sleep-apnea-for-the-first-time-at-ats-2023/
- Rosenberg R, Abaluck B, Thein S. Combination of atomoxetine with the novel antimuscarinic aroxybutynin improves mild to moderate OSA. J Clin Sleep Med. 2022 Dec 1;18(12):2837-2844. doi: 10.5664/jcsm.10250.
- Schweitzer PK, Taranto-Montemurro L, Ojile JM, Thein SG, Drake CL, et al. The Combination of Aroxybutynin and Atomoxetine in the Treatment of Obstructive Sleep Apnea (MARIPOSA): A Randomized Controlled Trial.Am J Respir Crit Care Med. 2023 Dec 15;208(12):1316-1327. doi: 10.1164/rccm.202306-1036OC.
- Apnimed, Inc. Press Release, 2023. Apnimed Announces First Patient Dosed in LunAIRo, the First Phase 3 Clinical Study of AD109, a Potential Nighttime Oral Treatment for Obstructive Sleep Apnea. September 21, 2023. https://apnimed.com/apnimed-apnimed-announces-first-patient-dosed-in-lunairo-the-first-phase-3-clinical-study-of-ad109-a-potential-nighttime-oral-treatment-for-obstructive-sleep-apnea/
- Phase 3 Randomized Double-Blind Placebo-Controlled 1-year Parallel-Arm Study to Compare a Fixed Dose Combination of Aroxybutynin/Atomoxetine (AD109) to Placebo in Obstructive Sleep Apnea (LunAIRo Study). ClinicalTrials.gov: NCT05811247 (active, not recruiting). https://www.clinicaltrials.gov/study/NCT05811247?intr=AD109&aggFilters=phase:3&rank=1
- Phase 3 Randomized Double-Blind Placebo-Controlled 6-month Parallel-Arm Study to Compare a Fixed Dose Combination of Aroxybutynin/Atomoxetine (AD109) to Placebo in Obstructive Sleep Apnea (SynAIRgy Study). ClinicalTrials.gov: NCT05813275 (recruiting). https://www.clinicaltrials.gov/study/NCT05813275?intr=AD109&aggFilters=phase:3&rank=2
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Gunsolley JC, Chalmers JD, Sibila O, Fernandez C, Scannapieco FA. Periodontal Effects of the Reversible Dipeptidyl Peptidase 1 Inhibitor Brensocatib in Bronchiectasis. JDR Clin Trans Res. 2023 Sep 25:23800844231196884. doi: 10.1177/23800844231196884. Online ahead of print.
- Chalmers JD, Haworth CS, Metersky ML, Loebinger MR, Blasi F, et al; WILLOW Investigators. Phase 2 Trial of the DPP-1 Inhibitor Brensocatib in Bronchiectasis. N Engl J Med. 2020 Nov 26;383(22):2127-2137. doi: 10.1056/NEJMoa2021713. Epub 2020 Sep 7.
- A Phase 3, Randomized, Double-Blind, Placebo-Controlled Study to Assess the Efficacy, Safety, and Tolerability of Brensocatib Administered Once Daily for 52 Weeks in Subjects With Non-Cystic Fibrosis Bronchiectasis - The ASPEN Study. ClinicalTrials.gov: NCT04594369 (active, not recruiting). https://clinicaltrials.gov/study/NCT04594369?intr=Brensocatib&aggFilters=phase:3&rank=1
- A Randomised Double-blind Placebo-controlled Trial of Brensocatib (INS1007) in Patients With Severe COVID-19. ClinicalTrials.gov: NCT04817332 (completed). https://clinicaltrials.gov/study/NCT04817332?intr=Brensocatib&aggFilters=phase:3&rank=2
- GlobalData’s Healthcare database (accessed April 2024; Phase II list).
- Anthos Therapeutics. Press Release, 2024. First-Ever Cost-Effectiveness Analysis of a Factor XI Inhibitor Demonstrates that Abelacimab, if Approved, Could Offer Significant Cost Savings as Compared to a Current Standard of Care Anticoagulant. 6 May 2024. https://anthostherapeutics.com/press-release/anthos_2024-08-15_release-16/
- A Multicenter, Randomized, Open-label, Blinded Endpoint Evaluation, Phase 3 Study Comparing the Effect of Abelacimab Relative to Apixaban on Venous Thromboembolism (VTE) Recurrence and Bleeding in Patients With Cancer Associated VTE. ClinicalTrials.gov: NCT05171049 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05171049?term=Abelacimab&draw=2&rank=2
- A Multicenter, Randomized, Open-label, Blinded Endpoint Evaluation, Phase 3 Study Comparing the Effect of Abelacimab vs. Dalteparin on Venous Thromboembolism (VTE) Recurrence and Bleeding in Patients With GI/GU Associated VTE. ClinicalTrials.gov: NCT05171075 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05171075?term=Abelacimab&draw=2&rank=3
- A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled, Parallel-group Study to evaLuate the effIcacy and Safety of abeLacimab in High-risk Patients With Atrial Fibrillation Who Have Been Deemed Unsuitable for Oral antiCoagulation (LILAC-TIMI 76). ClinicalTrials.gov: NCT05712200 (recruiting). https://clinicaltrials.gov/study/NCT05712200?intr=Abelacimab&aggFilters=phase:3&rank=1
- Stambler BS, Camm AJ, Alings M, Dorian P, Heidbuchel H, et al; RAPID Investigators. Self-administered intranasal etripamil using a symptom-prompted, repeat-dose regimen for atrioventricular-nodal-dependent supraventricular tachycardia (RAPID): a multicentre, randomised trial. Lancet. 2023 Jul 8;402(10396):118-128. doi: 10.1016/S0140-6736(23)00776-6. Epub 2023 Jun 15.
- Multi-Centre, Randomized, Double-Blind, Placebo-Controlled, Efficacy, and Safety Study of Etripamil Nasal Spray for the Termination of Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia. NODE 301 Trial. ClinicalTrials.gov: NCT03464019 (completed). https://clinicaltrials.gov/ct2/show/NCT03464019?term=Etripamil&phase=2&draw=2&rank=5
- The NODE-303 Study: Multi-Centre, Multi-National,Open Label, Safety Study of Etripamil Nasal Spray for Patients With Paroxysmal Supraventricular Tachycardia. ClinicalTrials.gov: NCT04072835 (completed). https://clinicaltrials.gov/ct2/show/NCT04072835?term=Etripamil&phase=2&draw=2&rank=1
- Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Efficacy and Safety Study of Etripamil Nasal Spray Self-Administration for the Termination of Spontaneous Episodes of Paroxysmal Supraventricular Tachycardia in Chinese Patients. ClinicalTrials.gov: NCT05410860 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05410860?term=Etripamil&phase=2&draw=2&rank=3
- An Open Label Extension Study of Etripamil Nasal Spray in Patients With Paroxysmal Supraventricular Tachycardia. ClinicalTrials.gov: NCT04952610 (Enrolling by invitation). https://clinicaltrials.gov/ct2/show/NCT04952610?term=Etripamil&phase=2&draw=2&rank=2
- Iapoce C. 2023. Etripamil Nasal Spray Application Submitted to FDA for PSVT Treatment. 24 October 24, 2023. https://www.hcplive.com/view/etripamil-nasal-spray-application-submitted-fda-psvt-treatment
- Rehman WU, Yarkoni M, Ilyas MA, Athar F, Javaid M, et al. Cholesteryl Ester Transfer Protein Inhibitors and Cardiovascular Outcomes: A Systematic Review and Meta-Analysis. J Cardiovasc Dev Dis. 2024 May 16;11(5):152. doi: 10.3390/jcdd11050152.
- A Placebo-Controlled, Double-Blind, Randomized, Phase 3 Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With a History of HeFH Who Are Not Adequately Controlled by Their Lipid Modifying Therapies. ClinicalTrials.gov: NCT05425745 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT05425745?term=Obicetrapib&phase=2&draw=2&rank=1
- A Placebo-Controlled, Double-Blind, Randomized Phase 3 Study to Evaluate the Effect of 10mg Obicetrapib in Participants With HeFH and/or ASCVD Who Are Not Adequately Controlled by Their Lipid Modifying Therapies. ClinicalTrials.gov: NCT05142722 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT05142722?term=Obicetrapib&phase=2&draw=2&rank=2
- Placebo Controlled, Double Blind, Randomized Cardiovascular Outcome Study to Evaluate the Effect of 10 mg Obicetrapib in Participants With ASCVD Not Adequately Controlled Despite Maximally Tolerated Lipid Modifying Therapies. ClinicalTrials.gov: NCT05202509 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05202509?term=Obicetrapib&phase=2&draw=2&rank=3
- A Placebo-Controlled, Double-Blind, Randomized, Phase 3 Study to Evaluate the Effect of Obicetrapib 10 mg and Ezetimibe 10 mg Fixed Dose Combination Daily on Top of Maximally Tolerated Lipid-Modifying Therapy in Participants With Heterozygous Familial Hypercholesterolemia (HeFH) and/or Atherosclerotic Cardiovascular Disease (ASCVD) or Multiple ASCVD Risk Factors. ClinicalTrials.gov: NCT06005597 (recruiting). https://clinicaltrials.gov/study/NCT06005597?intr=Obicetrapib&aggFilters=phase:3&rank=4
- A Placebo-controlled, Double-blind, Randomized, Phase 3 Study to Evaluate the Effect of Obicetrapib/Ezetimibe Fixed Dose Combination Daily on Coronary Plaque Characteristics in Participants With Atherosclerotic Cardiovascular Disease on Coronary CT Angiography (REMBRANDT Trial). ClinicalTrials.gov: NCT06305559 (not yet recruiting). https://clinicaltrials.gov/study/NCT06305559?intr=Obicetrapib&aggFilters=phase:3&rank=5
- A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy, Safety, and Tolerability of Soticlestat as Adjunctive Therapy in Pediatric and Young Adult Subjects With Dravet Syndrome (DS). ClinicalTrials.gov: NCT04940624 (completed). https://clinicaltrials.gov/ct2/show/NCT04940624?term=Soticlestat&phase=2&draw=2&rank=2
- A Multicenter, Randomized, Double-Blind, Placebo-Controlled, Parallel Group Study to Evaluate the Efficacy, Safety, and Tolerability of Soticlestat as Adjunctive Therapy in Pediatric and Adult Subjects With Lennox-Gastaut Syndrome (LGS). ClinicalTrials.gov: NCT04938427 (completed). https://clinicaltrials.gov/ct2/show/NCT04938427?term=Soticlestat&phase=2&draw=2&rank=3
- A Phase 3, Prospective, Open-Label, Multisite, Extension of Phase 3 Studies To Assess the Long-Term Safety and Tolerability of Soticlestat as Adjunctive Therapy in Subjects With Dravet Syndrome or Lennox-Gastaut Syndrome (ENDYMION 2). ClinicalTrials.gov: NCT05163314 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05163314?term=Soticlestat&phase=2&draw=2&rank=1
- An Open-label, Nonrandomized, Phase 3 Study to Evaluate the Efficacy and Safety of Soticlestat in Participants With Dravet Syndrome or Lennox-Gastaut Syndrome Who Have Been Exposed to Fenfluramine. ClinicalTrials.gov: NCT06422377 (recruiting). https://clinicaltrials.gov/study/NCT06422377?intr=Soticlestat&aggFilters=phase:3&rank=1
- Meglio M. 2023. Latozinemab Shows No Impact on Disease Progression in C9orf72 Frontotemporal Dementia Despite increasing Progranulin Expression. 1 November, 2023. https://www.neurologylive.com/view/latozinemab-shows-no-impact-disease-progression-c9orf72-frontotemporal-dementia-increasing-progranulin-expression
- A Phase 3, Multicenter, Randomized, Double Blind, Placebo Controlled Study to Evaluate the Efficacy and Safety of AL001 in Individuals at Risk for or With Frontotemporal Dementia Due to Heterozygous Mutations in the Progranulin Gene. ClinicalTrials.gov: NCT04374136 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04374136?term=AL001&phase=2&draw=2&rank=1
- A Continuation Study of Latozinemab in Participants With Neurodegenerative Disease. ClinicalTrials.gov: NCT06111014 (enrolling by invitation). https://www.clinicaltrials.gov/study/NCT06111014?intr=AL001&aggFilters=phase:3&rank=2
- Alector, Inc. News Release. 2024. FDA Grants Latozinemab Breakthrough Therapy Designation for Frontotemporal Dementia Due to a Progranulin Gene Mutation (FTD-GRN). 7 February, 2024. https://investors.alector.com/news-releases/news-release-details/fda-grants-latozinemab-breakthrough-therapy-designation
- A Phase 3, Multicenter, Randomized, Double-blind, Placebo-controlled Study of the Efficacy, Safety and Biomarker Effects of ALZ-801 in Subjects With Early Alzheimer's Disease and APOE4/4 Genotype. ClinicalTrials.gov: NCT04770220 (completed). https://clinicaltrials.gov/ct2/show/NCT04770220?term=NCT04770220&draw=2&rank=1
- Long-term Extension of a Phase 3, Multicenter, Randomized, Double-Blind, Placebo- Controlled Study of the Efficacy, Safety, and Biomarker Effects of ALZ-801 in Subjects With Early Alzheimer's Disease and APOE4/4 Genotype. ClinicalTrials.gov: NCT06304883 (enrolling by invitation). https://clinicaltrials.gov/study/NCT06304883?intr=ALZ-801&aggFilters=phase:3&rank=1
- A Multi-Site Open-Label Safety Extension Study of Manualized MDMA-Assisted Psychotherapy for the Treatment of Participants With Posttraumatic Stress Disorder. ClinicalTrials.gov: NCT04714359 (completed). https://clinicaltrials.gov/ct2/show/NCT04714359?term=NCT04714359&draw=2&rank=1.
- MDMA-assisted Massed Prolonged Exposure for PTSD. ClinicalTrials.gov: NCT06117306 (not yet recruiting). https://clinicaltrials.gov/study/NCT06117306?intr=Midomafetamine%20&aggFilters=phase:3&rank=4
- IISPT2: Examining 3,4-methylenedioxymethamphetamine (MDMA) Effects on Psychological, Relational and Hyperarousal-Related Neural Reactivity Mechanisms in Veterans With PTSD and MI. ClinicalTrials.gov: NCT06394284 (recruiting). https://clinicaltrials.gov/study/NCT06394284?intr=Midomafetamine%20&aggFilters=phase:3&rank=5
- Shinozuka K, Tabaac BJ, Arenas A, Beutler BD, Cherian K, et al. Psychedelic Therapy: A Primer for Primary Care Clinicians-3,4-Methylenedioxy-methamphetamine (MDMA). Am J Ther. 2024 Mar-Apr 01;31(2):e141-e154. doi: 10.1097/MJT.0000000000001722.
- Lykos Therapeutics News Release. 2024. Lykos Therapeutics Announces FDA Acceptance and Priority Review of New Drug Application for MDMA-Assisted Therapy for PTSD. 9 February, 2024. https://www.prnewswire.com/news-releases/lykos-therapeutics-announces-fda-acceptance-and-priority-review-of-new-drug-application-for-mdma-assisted-therapy-for-ptsd-302058901.html
- Formulary Watch. 2024. FDA Sets Date for Advisory Committee Meeting for Midomafetamine for PTSD. 8 May, 2024. https://www.formularywatch.com/view/fda-sets-date-for-advisory-committee-meeting-for-midomafetamine-for-ptsd
- NeuroDerm Ltd. News Release. 2023. NeuroDerm Announces Highly Positive Results from the Pivotal Phase III BouNDless Trial Evaluating ND0612 in Parkinson's Disease Patients with Motor Fluctuations. 9 January 2023. https://www.prnewswire.com/news-releases/neuroderm-announces-highly-positive-results-from-the-pivotal-phase-iii-boundless-trial-evaluating-nd0612-in-parkinsons-disease-patients-with-motor-fluctuations-301715848.html
- Espay AJ, Stocchi F, Pahwa R, Albanese A, Ellenbogen A, et al; BouNDless Study Group. Safety and efficacy of continuous subcutaneous levodopa-carbidopa infusion (ND0612) for Parkinson's disease with motor fluctuations (BouNDless): a phase 3, randomised, double-blind, double-dummy, multicentre trial. Lancet Neurol. 2024 May;23(5):465-476. doi: 10.1016/S1474-4422(24)00052-8. Epub 2024 Mar 15.
- A Multicenter, Randomized, Active-controlled, Double-blind, Double-dummy, Parallel Group Clinical Trial, Investigating the Efficacy, Safety, and Tolerability of Continuous Subcutaneous ND0612 Infusion in Comparison to Oral IR-LD/CD in Subjects With Parkinson's Disease Experiencing Motor Fluctuations (BouNDless). ClinicalTrials.gov: NCT04006210 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04006210?term=NCT04006210&draw=2&rank=1.
- Hirschfield GM, Bowlus CL, Mayo MJ, Kremer AE, Vierling JM, et al; RESPONSE Study Group. A Phase 3 Trial of Seladelpar in Primary Biliary Cholangitis. N Engl J Med. 2024 Feb 29;390(9):783-794. doi: 10.1056/NEJMoa2312100. Epub 2024 Feb 21.
- A 52-week, Placebo-controlled, Randomized, Phase 3 Study to Evaluate the Safety and Efficacy of Seladelpar in Subjects With Primary Biliary Cholangitis (PBC) and an Inadequate Response to or an Intolerance to Ursodeoxycholic Acid (UDCA). ClinicalTrials.gov: NCT03602560 (completed). https://clinicaltrials.gov/ct2/show/NCT03602560?term=Seladelpar&phase=2&draw=2&rank=2
- RESPONSE: A Placebo-controlled, Randomized, Phase 3 Study to Evaluate the Efficacy and Safety of Seladelpar in Patients With Primary Biliary Cholangitis (PBC) and an Inadequate Response to or an Intolerance to Ursodeoxycholic Acid (UDCA). ClinicalTrials.gov: NCT04620733 (completed). https://clinicaltrials.gov/ct2/show/NCT04620733?term=Seladelpar&phase=2&draw=2&rank=3
- ASSURE: An Open Label Long-Term Study to Evaluate the Safety and Tolerability of Seladelpar in Subjects With Primary Biliary Cholangitis (PBC). ClinicalTrials.gov: NCT03301506 (recruiting). https://clinicaltrials.gov/ct2/show/NCT03301506?term=Seladelpar&phase=2&draw=2&rank=1
- CymaBay Therapeutics, Inc. News Release, 2024. CymaBay Announces FDA Acceptance of NDA and Priority Review for Seladelpar for the Treatment of Primary Biliary Cholangitis. 12 February 2024. https://www.prnewswire.com/news-releases/cymabay-announces-fda-acceptance-of-nda-and-priority-review-for-seladelpar-for-the-treatment-of-primary-biliary-cholangitis-302059522.html
- A Parallel-group, Two-staged, Phase 2/3, Randomized, Multicenter Study to Evaluate the Efficacy and Safety of REC-2282 in Participants With Progressive NF2 Mutated Meningiomas. ClinicalTrials.gov: NCT05130866 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05130866?term=REC-2282&draw=2&rank=1
- GSK to submit drug application for new antibiotic to U.S. FDA. November 4, 2022. https://www.pmlive.com/pharma_news/gsk_to_submit_drug_application_for_new_antibiotic_to_us_fda_1480238
- The Pharmaceutical Journal. 2024. First-in-class antibiotic provides effective treatment for UTIs, trial results suggest. 14 February 2024. https://pharmaceutical-journal.com/article/news/first-in-class-antibiotic-provides-effective-treatment-for-utis-trial-results-suggest
- SERB Pharmaceuticals. News Release, 2024. FDA has accepted a BLA for bentracimab, the first and only ticagrelor reversal agent, for filing and priority review. August 2, 2024. https://finance.yahoo.com/news/fda-accepted-bla-bentracimab-first-123500872.html
- Young G, Srivastava A, Kavakli K, Ross C, Sathar J, et al. Efficacy and safety of fitusiran prophylaxis in people with haemophilia A or haemophilia B with inhibitors (ATLAS-INH): a multicentre, open-label, randomised phase 3 trial. Lancet. 2023 Apr 29;401(10386):1427-1437. doi: 10.1016/S0140-6736(23)00284-2. Epub 2023 Mar 29.
- Srivastava A, Rangarajan S, Kavakli K, Klamroth R, Kenet G, et al. Fitusiran prophylaxis in people with severe haemophilia A or haemophilia B without inhibitors (ATLAS-A/B): a multicentre, open-label, randomised, phase 3 trial. Lancet Haematol. 2023 May;10(5):e322-e332. doi: 10.1016/S2352-3026(23)00037-6. Epub 2023 Mar 29.
- An Open-label, Long-term Safety and Efficacy Study of Fitusiran in Patients With Hemophilia A or B, With or Without Inhibitory Antibodies to Factor VIII or IX. ClinicalTrials.gov: NCT03754790 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT03754790?term=Fitusiran&phase=2&draw=2&rank=2
- ATLAS-PPX: an Open-label, Multinational, Switching Study to Describe the Efficacy and Safety of Fitusiran Prophylaxis in Patients With Hemophilia A and B Previously Receiving Factor or Bypassing Agent Prophylaxis. ClinicalTrials.gov: NCT03549871 (completed). https://clinicaltrials.gov/study/NCT03549871?intr=fitusiran&aggFilters=phase:3&rank=5
- ATLAS-PEDS: An Open-label, Multinational Study of Fitusiran Prophylaxis in Male Pediatric Subjects Aged 1 to Less Than 12 Years With Hemophilia A or B. ClinicalTrials.gov: NCT03974113 (active not recruiting). https://clinicaltrials.gov/study/NCT03974113?intr=fitusiran&aggFilters=phase:3&rank=1
- Drugs.com. 2024. Ascendis Pharma Announces Extension of U.S. Food and Drug Administration Review Period for TransCon PTH for Adults with Hypoparathyroidism. 14 May 2024. https://www.drugs.com/nda/transcon_pth_240514.html
- Craig TJ, Reshef A, Li HH, Jacobs JS, Bernstein JA, et al. Efficacy and safety of garadacimab, a factor XIIa inhibitor for hereditary angioedema prevention (VANGUARD): a global, multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2023 Apr 1;401(10382):1079-1090. doi: 10.1016/S0140-6736(23)00350-1. Epub 2023 Feb 28.
- A Multicenter, Double-blind, Randomized, Placebo-controlled, Parallel-arm Study to Investigate the Efficacy and Safety of Subcutaneous Administration of CSL312 (Garadacimab) in the Prophylactic Treatment of Hereditary Angioedema. ClinicalTrials.gov: NCT04656418 (completed). https://clinicaltrials.gov/ct2/show/NCT04656418?term=Garadacimab&phase=2&draw=2&rank=1
- An Open-label Study to Evaluate the Long-term Safety and Efficacy of CSL312 (Garadacimab) in the Prophylactic Treatment of Hereditary Angioedema. ClinicalTrials.gov: NCT04739059 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04739059?term=Garadacimab&phase=2&draw=2&rank=2
- CSL News Release. 2023. CSL's Garadacimab, a First-in-Class Factor XIIa Inhibitor, Receives FDA and EMA Filing Acceptance. 14 December 2023. https://www.prnewswire.com/news-releases/csls-garadacimab-a-first-in-class-factor-xiia-inhibitor-receives-fda-and-ema-filing-acceptance-302016048.html#xd_co_f=NzVlYzk3NGEtZjE5OS00OGNmLTllYmQtOGE3Mzk1MTI4YzAw~
- Health Canada. Drug and Health Product Submissions Under Review (SUR): New drug submissions under review. https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review/new-drug-submissions-under-review.html
- Global Antibiotic Research & Development Partnership (GARDP) News. 2023. Positive results announced in largest pivotal phase 3 trial of a first-in-class oral antibiotic to treat uncomplicated gonorrhoea. 1 November 2023. https://gardp.org/positive-results-announced-in-largest-pivotal-phase-3-trial-of-a-first-in-class-oral-antibiotic-to-treat-uncomplicated-gonorrhoea/
- Gertz MA, Cohen AD, Comenzo RL, Kastritis E, Landau HJ, et al. Birtamimab plus standard of care in light-chain amyloidosis: the phase 3 randomized placebo-controlled VITAL trial. Blood. 2023 Oct 5;142(14):1208-1218. doi: 10.1182/blood.2022019406.
- A Phase 3, Randomized, Multicenter, Double-Blind, Placebo-Controlled, Efficacy and Safety Study of Birtamimab Plus Standard of Care vs. Placebo Plus Standard of Care in Mayo Stage IV Subjects With Light Chain (AL) Amyloidosis. ClinicalTrials.gov: NCT04973137 (recruiting). https://clinicaltrials.gov/ct2/show/NCT04973137?term=NCT04973137&draw=2&rank=1.
- Wainberg ZA, Kang YK, Lee KW, Qin S, Yamaguchi K, et al. Bemarituzumab as first-line treatment for locally advanced or metastatic gastric/gastroesophageal junction adenocarcinoma: final analysis of the randomized phase 2 FIGHT trial.Gastric Cancer. 2024 May;27(3):558-570. doi: 10.1007/s10120-024-01466-w. Epub 2024 Feb 3.
- A Phase 1b/3 Study of Bemarituzumab Plus Chemotherapy and Nivolumab Versus Chemotherapy and Nivolumab Alone in Subjects With Previously Untreated Advanced Gastric and Gastroesophageal Junction Cancer With FGFR2b Overexpression. ClinicalTrials.gov: NCT05111626 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05111626?term=Bemarituzumab&phase=2&draw=2&rank=1
- A Randomized, Multi-center, Double-blind, Placebo-controlled Phase 3 Study of Bemarituzumab Plus Chemotherapy Versus Placebo Plus Chemotherapy in Subjects With Previously Untreated Advanced Gastric or Gastroesophageal Junction Cancer With FGFR2b Overexpression. ClinicalTrials.gov: NCT05052801 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05052801?term=Bemarituzumab&phase=2&draw=2&rank=2
- A Randomized, Double-Blind, Placebo-Controlled, Phase 3 Study Of Navitoclax In Combination With Ruxolitinib Versus Ruxolitinib In Subjects With Myelofibrosis (TRANSFORM-1). ClinicalTrials.gov: NCT04472598 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04472598?term=Navitoclax&phase=2&draw=2&rank=1
- A Randomized, Open-Label, Phase 3 Study Evaluating Efficacy and Safety of Navitoclax in Combination With Ruxolitinib Versus Best Available Therapy in Subjects With Relapsed/Refractory Myelofibrosis (TRANSFORM-2). ClinicalTrials.gov: NCT04468984 (active, not recruiting). https://clinicaltrials.gov/ct2/show/NCT04468984?term=Navitoclax&phase=2&draw=2&rank=2
- Kremyanskaya M(1), Kuykendall AT(1), Pemmaraju N(1), Ritchie EK(1), Gotlib J(1), et al; REVIVE Trial Investigators. Rusfertide, a Hepcidin Mimetic, for Control of Erythrocytosis in Polycythemia Vera. N Engl J Med. 2024 Feb 22;390(8):723-735. doi: 10.1056/NEJMoa2308809.
- A Phase 3 Study of the Hepcidin Mimetic Rusfertide (PTG-300) in Patients With Polycythemia Vera. ClinicalTrials.gov: NCT05210790 (recruiting). https://clinicaltrials.gov/ct2/show/NCT05210790?term=Rusfertide&draw=2&rank=1
- Soligenix, Inc. News Release 2023. Soligenix Receives Refusal to File Letter from U.S. FDA for HyBryte™ New Drug Application in the Treatment of Cutaneous T-Cell Lymphoma. 14 February 2023. https://ir.soligenix.com/2023-02-14-Soligenix-Receives-Refusal-to-File-Letter-from-U-S-FDA-for-HyBryte-TM-New-Drug-Application-in-the-Treatment-of-Cutaneous-T-Cell-Lymphoma
- Soligenix, Inc. News Release. 2023. Soligenix Announces Scheduling of Type A Meeting with the U.S. FDA to Review Proposed Study Design for a Second Phase 3 Study Evaluating HyBryte™ in the Treatment of Cutaneous T-Cell Lymphoma, 11 May 2023. https://ir.soligenix.com/2023-05-11-Soligenix-Announces-Scheduling-of-Type-A-Meeting-with-the-U-S-FDA-to-Review-Proposed-Study-Design-for-a-Second-Phase-3-Study-Evaluating-HyBryte-TM-in-the-Treatment-of-Cutaneous-T-Cell-Lymphoma
- Soligenix, Inc. News Release. 2023. HyBryte™ Expanded Treatment Trial in Cutaneous T-Cell Lymphoma Opens Enrollment. 10 August 2023. https://ir.soligenix.com/2023-08-10-HyBryte-TM-Expanded-Treatment-Trial-in-Cutaneous-T-Cell-Lymphoma-Opens-Enrollment
- Astellas Pharma Inc. News. 2024. Astellas’ VYLOY™ (zolbetuximab) Approved in Japan for Treatment of Gastric Cancer. 26 March 2024. https://www.astellas.com/en/news/29026
- Conroy R. 2024. FDA Issues CRL for Zolbetuximab in Advanced CLDN18.2+ Gastric Cancer. 9 January 2024. https://www.cancernetwork.com/view/fda-issues-crl-for-zolbetuximab-in-advanced-cldn18-2-gastric-cancer
- Carelli V(1)(2), Newman NJ(3)(4)(5), Yu-Wai-Man P(6)(7)(8)(9), Biousse V(3)(4)(5),; the LHON Study Group. Indirect Comparison of Lenadogene Nolparvovec Gene Therapy Versus Natural History in Patients with Leber Hereditary Optic Neuropathy Carrying the m.11778G>A MT-ND4 Mutation. Ophthalmol Ther. 2023 Feb;12(1):401-429. doi: 10.1007/s40123-022-00611-x. Epub 2022 Nov 30.
- Long-term Follow-up of ND4 LHON Subjects Treated With GS010 Ocular Gene Therapy in the RESCUE or REVERSE Phase III Clinical Trials (RESTORE). ClinicalTrials.gov: NCT03406104 (completed). https://clinicaltrials.gov/study/NCT03406104?intr=Lenadogene%20nolparvovec%20&aggFilters=phase:3&rank=1
- Randomized, Double-Masked, Sham-Controlled Clinical Trial to Evaluate the Efficacy of a Single Intravitreal Injection of GS010 in Subjects Affected for More Than 6 Months and To 12 Months by LHON Due to the G11778A Mutation in the ND4 Gene. ClinicalTrials.gov: NCT02652780 (completed). https://clinicaltrials.gov/study/NCT02652780?intr=Lenadogene%20nolparvovec%20&aggFilters=phase:3&rank=3
- A Randomized, Double-Masked, Sham-Controlled Clinical Trial to Evaluate the Efficacy of a Single Intravitreal Injection of GS010 in Subjects Affected for 6 Months or Less by LHON Due to the G11778A Mutation in the Mitochondrial ND4 Gene. ClinicalTrials.gov: NCT02652767 (completed). https://clinicaltrials.gov/study/NCT02652767?intr=Lenadogene%20nolparvovec%20&aggFilters=phase:3&rank=4
- Efficacy and Safety of Bilateral Intravitreal Injection of GS010: A Randomized, Double-Masked, Placebo-Controlled Trial in Subjects Affected With G11778A ND4 Leber Hereditary Optic Neuropathy for Up to One Year. ClinicalTrials.gov: NCT03293524 (active, not recruiting). https://clinicaltrials.gov/study/NCT03293524?intr=Lenadogene%20nolparvovec%20&aggFilters=phase:3&rank=2
- Tryvio (aprocitentan). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/217686Orig1s000Correctedltr.pdf
- Winrevair (sotatercept). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/761363Orig1s000ltr.pdf
- Vyjuvek (beremagene geperpavec). FDA Letter of approval: https://www.fda.gov/media/168356/download?attachment
- REBYOTA (fecal microbiota, live-jslm). FDA Letter of approval: https://www.fda.gov/media/163586/download
- Rezdiffra (resmetirom). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/217785Orig1s000ltr.pdf
- FDA News Release. 2023. FDA Approves First Gene Therapy for Treatment of Certain Patients with Duchenne Muscular Dystrophy. 22 June 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-first-gene-therapy-treatment-certain-patients-duchenne-muscular-dystrophy
- Voydeya (danicopan). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/218037Orig1s000ltr.pdf
- Health Canada. Drug and Health Product Submission under Review (SUR): New drug submissions under review. https://www.canada.ca/en/health-canada/services/drug-health-product-review-approval/submissions-under-review/new-drug-submissions-under-review.html
- BEQVEZ (fidanacogene elaparvovec). FDA Letter of approval. https://www.fda.gov/media/178139/download?attachment
- FDA News Release. 2023. FDA approves omidubicel to reduce time to neutrophil recovery and infection in patients with hematologic malignancies. 17 April 2023. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-omidubicel-reduce-time-neutrophil-recovery-and-infection-patients-hematologic
- Lantidra (donislecel). FDA Letter of approval: https://www.fda.gov/media/169921/download?attachment
- AWIQLI (insulin icodec). Health Canada. Drug Product Database online query. Available at: https://health-products.canada.ca/dpd-bdpp/?lang=eng
- Elfabrio (pegunigalsidase alfa). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/761161Orig1s000ltr.pdf
- Elfabrio (pegunigalsidase alfa). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/elfabrio
- RYTELO (imetelstat sodium). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2024/217779Orig1s000ltr.pdf
- Izervay (avacincaptad pegol sodium). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/217225Orig1s000ltr.pdf
- Veozah (fezolinetant). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/216578Orig1s000ltr.pdf
- Veozah (fezolinetant). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/veoza
- De SK. Leniolisib: a novel treatment for activated phosphoinositide-3 kinase delta syndrome. Front Pharmacol. 2024 Feb 12;15:1337436. doi: 10.3389/fphar.2024.1337436. eCollection 2024.
- Joenja (leniolisib). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/217759Orig1s000ltr.pdf
- Rao VK, Kulm E, Šedivá A, Plebani A, Schuetz C, et al. Interim analysis: Open-label extension study of leniolisib for patients with APDS. J Allergy Clin Immunol. 2024 Jan;153(1):265-274.e9. doi: 10.1016/j.jaci.2023.09.032. Epub 2023 Oct 4.
- GlobalData’s Healthcare database (accessed April 3, 2024; Phase II list).
- Bobonich M, Gorelick J, Aldredge L, Bruno MJ, DiRuggiero D, et al. Tapinarof, a Novel, First-in-Class, Topical Therapeutic Aryl Hydrocarbon Receptor Agonist for the Management of Psoriasis. J Drugs Dermatol. 2023 Aug 1;22(8):779-784. doi: 10.36849/jdd.7317.
- VTAMA (tapinarof ). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2022/215272Orig1s000ltr.pdf
- Armstrong AW, McConaha JL. Tapinarof cream 1% once daily for the treatment of adults with mild to severe plaque psoriasis: A novel topical therapy targeting the aryl hydrocarbon receptor. J Manag Care Spec Pharm. 2023 Dec;29(12-a Suppl):S1-S13. doi: 10.18553/jmcp.2023.29.12-a.s1.
- FDA approves avapritinib for advanced systemic Mastocytosis. June 16, 2021. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-avapritinib-advanced-systemic-mastocytosis
- AYVAKIT (avapritinib). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/ayvakyt
- Gotlib J, Castells M, Elberink HO, Siebenhaar F, Hartmann K, et al. Avapritinib versus Placebo in Indolent Systemic Mastocytosis. NEJM Evid. 2023 Jun;2(6):EVIDoa2200339. doi: 10.1056/EVIDoa2200339. Epub 2023 May 23.
- GlobalData’s Healthcare database (accessed April 3, 2024; Phase II list).
- Bruzzese A, Labanca C, Martino EA, Mendicino F, et al. Ivosidenib in acute myeloid leukemia. Expert Opin Pharmacother. 2023 Sep-Dec;24(18):2093-2100. doi: 10.1080/14656566.2023.2272659. Epub 2024 Jan 5.
- FDA approves ivosidenib in combination with azacitidine for newly diagnosed acute myeloid leukemia. August 1, 2022. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-ivosidenib-combination-azacitidine-newly-diagnosed-acute-myeloid-leukemia
- TIBSOVO (ivosidenib). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/tibsovo
- Bruzzese A, Labanca C, Martino EA, Mendicino F, et al. Ivosidenib in acute myeloid leukemia. Expert Opin Pharmacother. 2023 Sep-Dec;24(18):2093-2100. doi: 10.1080/14656566.2023.2272659. Epub 2024 Jan 5.
- Helwick C. Study Examines Real-World Outcomes With Ivosidenib vs Venetoclax in Acute Myeloid Leukemia. 2023. https://ascopost.com/issues/digital-supplement-ash-meeting-highlights-2023/study-examines-real-world-outcomes-with-ivosidenib-vs-venetoclax-in-acute-myeloid-leukemia/
- GSK plc. Ojjaara (momelotinib) approved in the US as the first and only treatment indicated for myelofibrosis patients with anaemia. September 15, 2023. https://www.gsk.com/en-gb/media/press-releases/ojjaara-momelotinib-approved-in-the-us-as-the-first-and-only-treatment-indicated-for-myelofibrosis-patients-with-anaemia/
- OJJAARA (momelotinib). European Medicines Agency. https://www.ema.europa.eu/en/medicines/human/EPAR/omjjara
- Bruzzese A, Martino EA, Labanca C, Mendicino F, Lucia E, et al. Momelotinib in myelofibrosis. Expert Opin Pharmacother. 2024 Apr;25(5):521-528. doi: 10.1080/14656566.2024.2343780. Epub 2024 Apr 18.
- GSK. Press Release, 2024. GSK Canada’s submission for momelotinib for the treatment of myelofibrosis accepted for review by Health Canada. January 30, 2024. https://ca.gsk.com/en-ca/media/press-releases/gsk-canada-s-submission-for-momelotinib-for-the-treatment-of-myelofibrosis-accepted-for-review-by-health-canada/
- Gupta V, Oh S, Devos T, Dubruille V, Catalano J, et al. Momelotinib vs. ruxolitinib in myelofibrosis patient subgroups by baseline hemoglobin levels in the SIMPLIFY-1 trial. Leuk Lymphoma. 2024 Mar 19:1-13. doi: 10.1080/10428194.2024.2328800. Online ahead of print.
- Chifotides HT, Bose P, Verstovsek S. Momelotinib: an emerging treatment for myelofibrosis patients with anemia. J Hematol Oncol. 2022 Jan 19;15(1):7. doi: 10.1186/s13045-021-01157-4.
- MIEBO (perfluorohexyloctane ). FDA Letter of approval: https://www.accessdata.fda.gov/drugsatfda_docs/appletter/2023/216675Orig1s000ltr.pdf
- Sheppard JD, Evans DG, Protzko EE. A review of the first anti-evaporative prescription treatment for dry eye disease: perfluorohexyloctane ophthalmic solution.Am J Manag Care. 2023 Nov;29(14 Suppl):S251-S259. doi: 10.37765/ajmc.2023.89464.