Drug Shortages in Canada and their Impact on Public Drug Plans, 2017/18 to 2019/20
Presented at CAPT 2022, October 17-18, 2022
Étienne Gaudette, PhD
Introduction
Background and objective: Drug shortages are an issue of great importance to the full range of Canadians they affect, from patients to healthcare providers and insurers. This presentation highlights key findings of a new report by the Patented Medicine Prices Review Board on Canadian drug shortages.Footnote 1
Approach: The research analyzes data collected on the Drug Shortages Canada website as well as sales information from IQVIA MIDAS® database and the Canadian Institute for Health Information’s National Prescription Drug Utilization Information System database. Using this information, this poster reports on trends in Canadian drug shortages, resolution rates of shortages, and the impact of shortages on public plan beneficiaries.
Data
Drug shortages: Shortage reports were extracted from the Drug Shortages Canada website. Holders of drug market authorizations issued by Health Canada (manufacturers) are required to report when they are not able to meet demand for a product.Footnote 2
Sales data: The IQVIA MIDAS® database (all rights reserved) was used to determine the proportion of drugs sold in Canada impacted by shortages and the market segment of drugs in shortage. MIDAS data reflects the national retail and hospital sectors in Canada and internationally, including payers in all market segments (public, private, and out-of-pocket).
Public drug plans: Monthly beneficiary data from publicly funded drug plans were compiled at the chemical subgroup-level using the National Prescription Drug Utilization Information System (NPDUIS) database, representing approximately 7 million publicly insured active beneficiaries. Public drug plans from British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, New Brunswick, Nova Scotia, Prince Edward Island, and Newfoundland were included in the analysis, as well as the Non-Insured Health Benefits program.
Study period: The study period comprised fiscal years (Apr. to Mar.) 2017/18 to 2019/20 and preceded most of the disruptions from the COVID-19 pandemic.
Definitions
Drug-level shortages consider outcomes at the drug identification number (DIN) level for shortage reports with an “actual” status. This concept was used in Figures 1 to 3.
Chemical subgroup-level shortages occur when shortages with an “actual” status are reported for at least one DIN within a group of drugs defined by the fourth level of the World Health Organization’s Anatomical Therapeutic Chemical classification system (ATC4), which includes drugs with similar chemical components used to treat identical or related indications. This concept was used in Figure 4.
An active beneficiary is an individual with at least one claim accepted for reimbursement by a drug plan or program. The percent change in the number of active beneficiaries shown in Figure 4 was calculated using the ratio of the average monthly number of beneficiaries during the shortage and up to six months prior to the shortage.
Results
Shortages impacted over 1 in 4 drugs sold in Canada annually.
Between April 1, 2017, and March 31, 2020, a total of 8,558 shortage reports were filed by Canadian manufacturers.
In 2019/20, shortages were reported for 29% of all the prescription medicines sold in Canada (Figure 1).
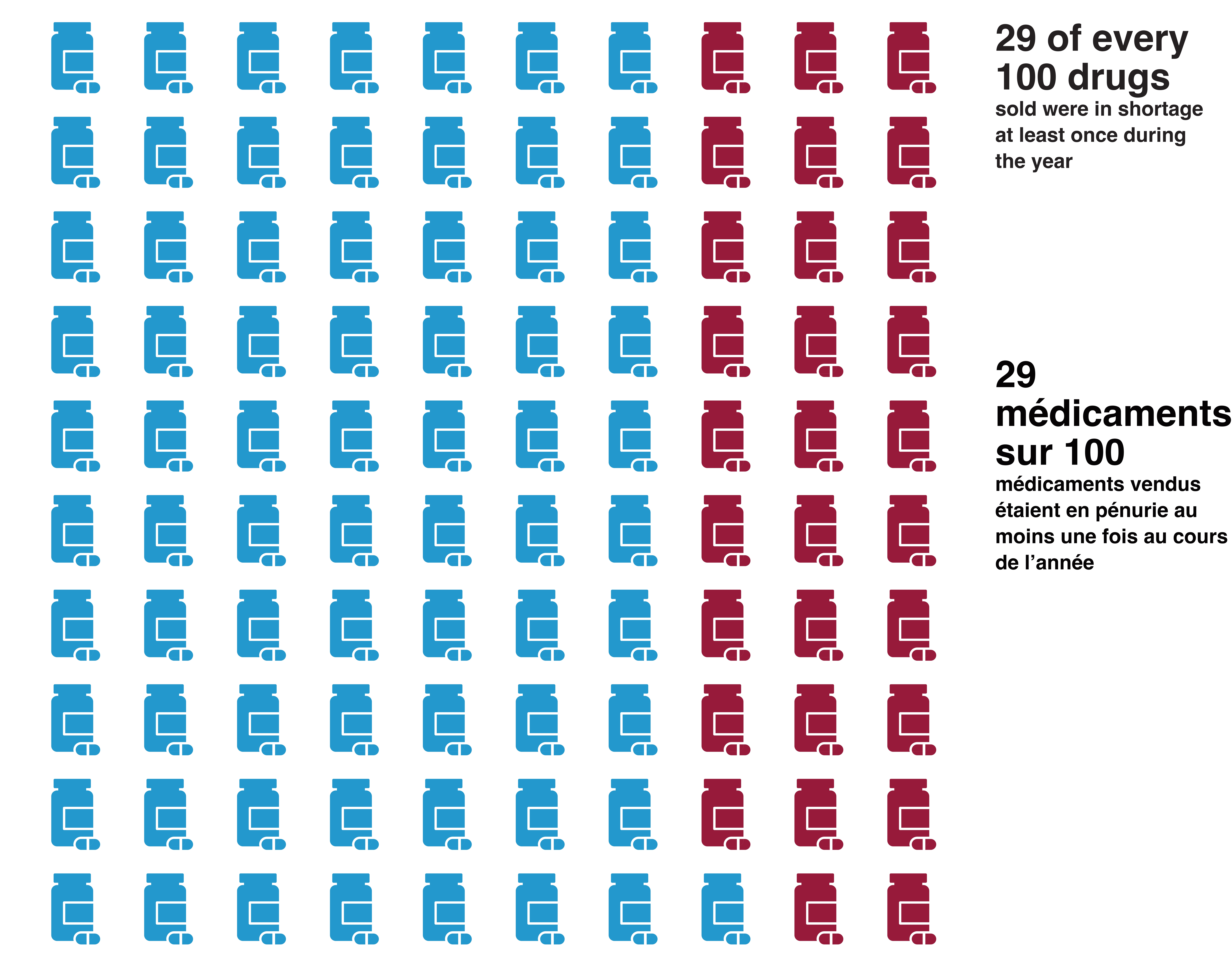
Figure description
One hundred icons of a prescription container are assembled in a ten-by-ten grid, representing all drugs sold in Canada. Seventy-one containers are shown in one colour, representing the percentage of drugs sold in Canada that were not in shortage during the year. Twenty-nine containers are in a different colour, representing the percentage of drugs sold in Canada that were in shortage at least once during the year.
Data sources: www.drugshortagescanada.ca; MIDAS® database, 2019-2020, IQVIA (all rights reserved).
Most shortages impacted drugs with available substitutions.
91% of shortages reports concerned non-patented drugs with multiple manufacturers, which have multiple competing versions of the same medicinal ingredient on the market (Figure 2).
Patented medicines and non-patented medicines with a single manufacturer, which have limited substitutions available for patients, accounted for 7% and 2% of reports, respectively.
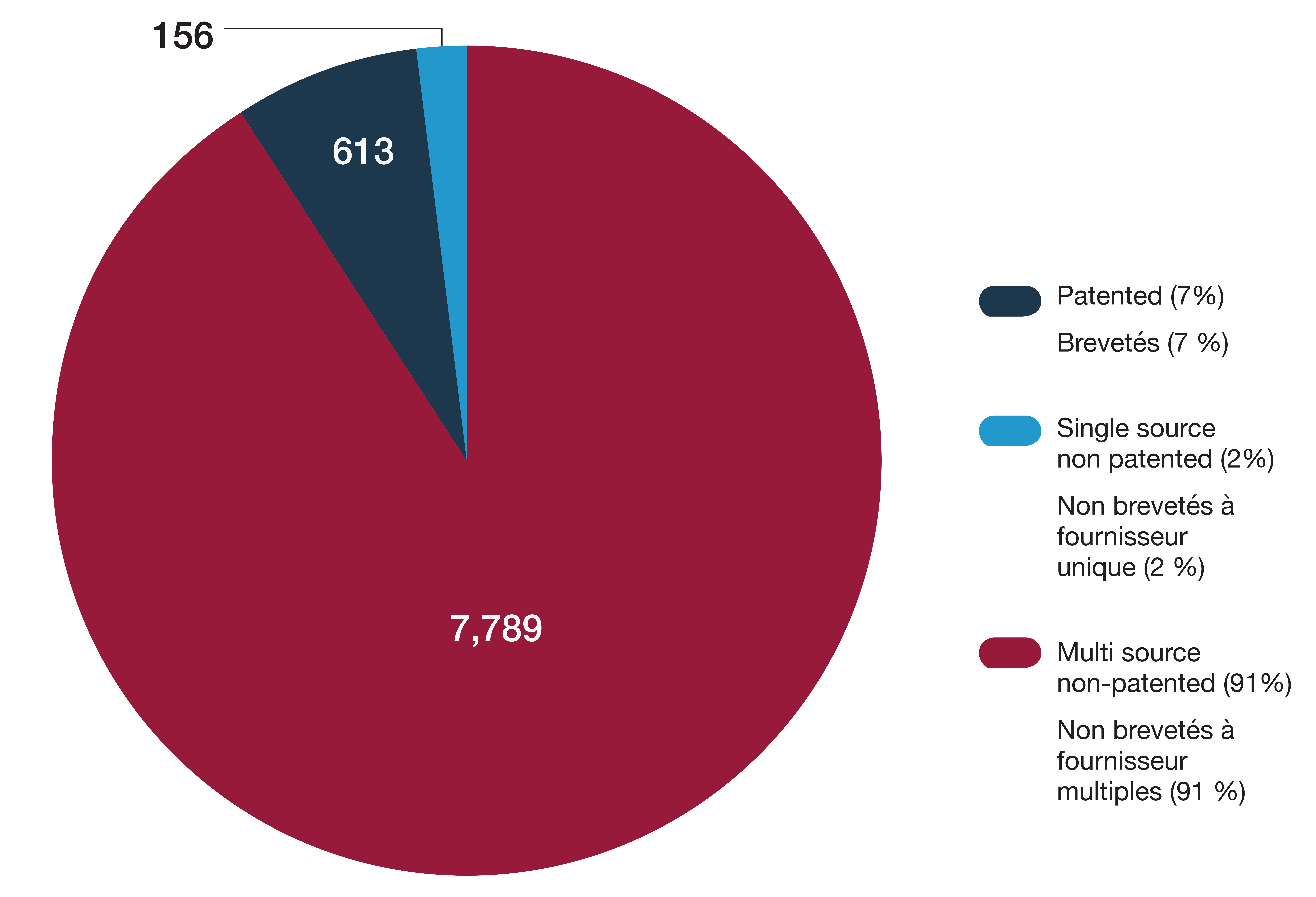
Figure description
A pie chart gives the distribution of drug shortages reports from fiscal years 2017/18 to 2019/20 by three market segments: patented, single-source non-patented, and multi-source non-patented. The distribution is shown in terms of the number of shortages as well as the share of total shortages for the entire period.
| Market segment | Number of shortages | Share of shortages |
|---|---|---|
Patented |
613 |
7% |
Single-source non-patented |
156 |
2% |
Multi-source non-patented |
7,789 |
91% |
Data sources: www.drugshortagescanada.ca; MIDAS® database, 2017-2020, IQVIA (all rights reserved); PMPRB.
Over half of drug shortages reported were resolved within three months.
55% of shortages were updated to “resolved” within 3 months and 74% within 6 months (Figure 3).
Shortages of patented and non-patented medicines with a single manufacturer were resolved faster than those of non-patented medicines with multiple manufacturers.
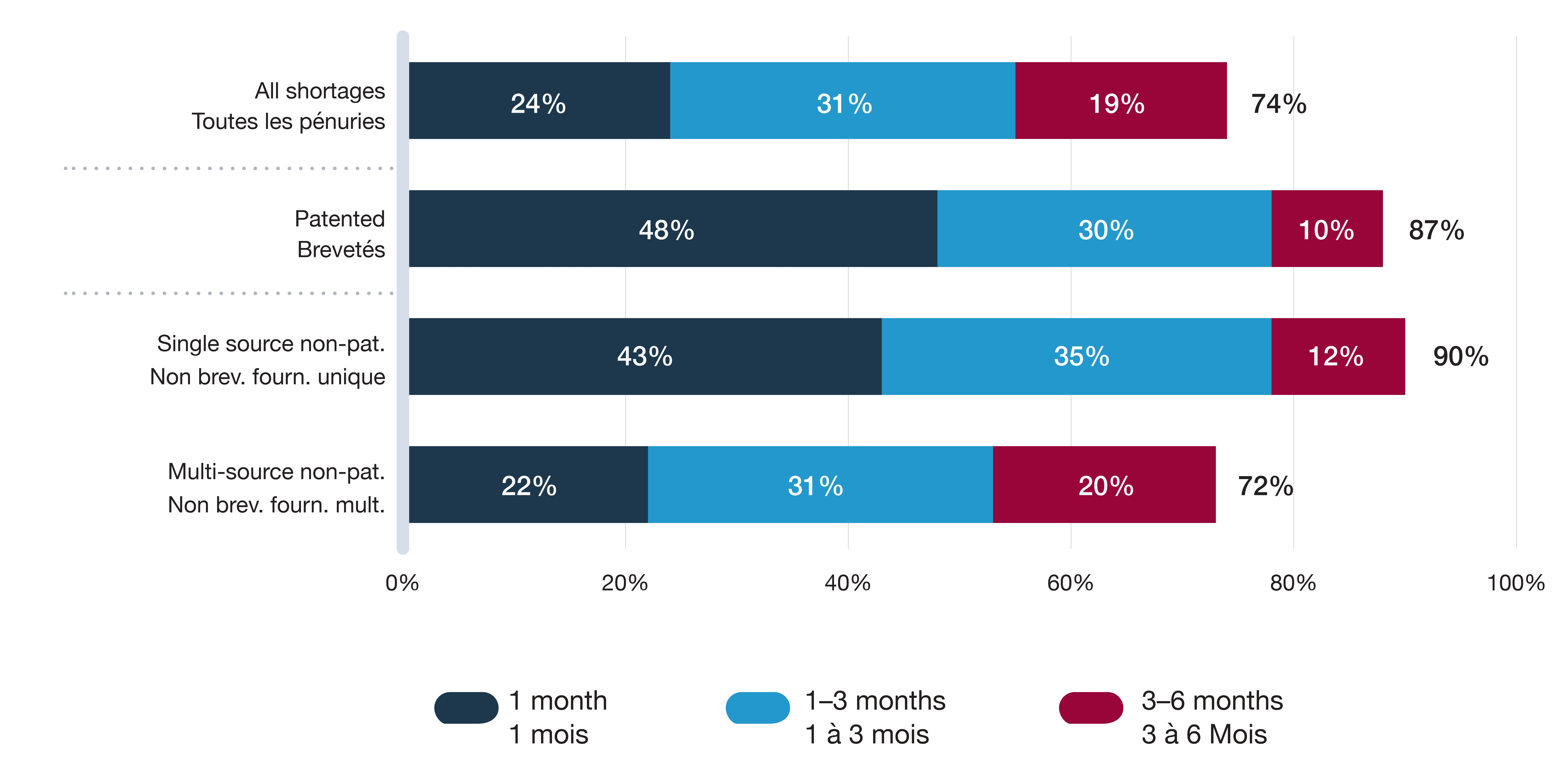
Figure description
A stacked horizontal bar graph shows the distribution of drug shortages reported from fiscal years 2017/18 to 2019/20 by the time to reach the resolved status. They are sorted by three timeframes: one month, one to three months, and three to six months after onset. Results are presented for all shortages for the patented, single-source non-patented, and multi-source non-patented market segments.
| Share resolved within one month of onset | Share resolved between one and three months of onset | Share resolved between three and six months of onset | Total share resolved within six months of onset | |
|---|---|---|---|---|
All shortages |
24% |
31% |
19% |
74% |
Patented |
48% |
30% |
10% |
87% |
Single-source non-patented |
43% |
35% |
12% |
90% |
Multi-source non-patented |
22% |
31% |
20% |
72% |
Data sources: www.drugshortagescanada.ca; MIDAS® database, 2017-2020, IQVIA (all rights reserved); PMPRB.
Fewer than 1 in 10 shortages were associated with a steep decline in the number of beneficiaries who filled prescriptions.
Only 8% of shortages were followed by a >20% decline in public plan beneficiaries who were dispensed a prescription for a drug in the same chemical subgroup (Figure 4).Footnote 3
Shortages associated with a >20% decline primarily occurred in subgroups of drugs with small numbers of beneficiaries and few generic drugs available for substitution.
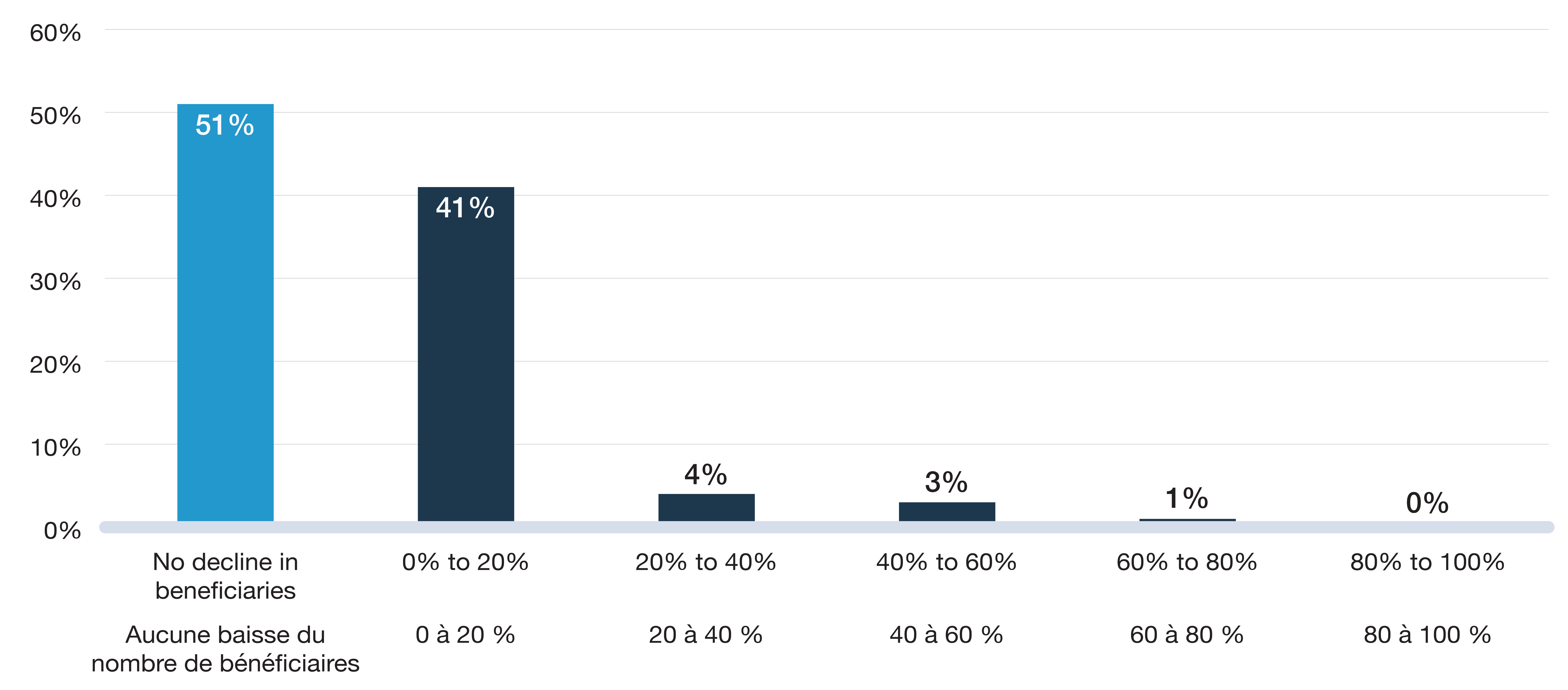
Figure description
A bar graph shows the distribution of drug shortages by the decline in the number of active beneficiaries during the shortage at the chemical subgroup (ATC4) level. The decline in beneficiaries is measured as a percent decrease in the number of beneficiaries relative to the six months prior to the onset of the shortage. Shortages reported between 2017/18 and 2019/20 fiscal years are assessed for this analysis with a total of 532 shortages at the ATC4 level.
| Percent decline in beneficiaries | Share of shortages at the ATC4 level |
|---|---|
No decline in beneficiaries |
51% |
0% to 20% |
41% |
20% to 40% |
4% |
40% to 60% |
3% |
60% to 80% |
1% |
80% to 100% |
0% |
Data sources: www.drugshortagescanada.ca; NPDUIS Database, Canadian Institute for Health Information (CIHI).
Conclusions
While a sizable share of drugs sold in Canada were impacted by shortages between 2017/18 and 2019/20, the vast majority were in respect of non-patented drugs with multiple competing manufacturers, which allow for substitutions in many cases. These findings provide important context but are not intended to downplay the harmful impact that shortages can have on individual patients and the health care system, particularly when substitutions are not possible or when variations in strength and formulation are not interchangeable.
More in-depth analyses of these findings and other aspects of Canadian drug shortages are presented in the new report Drug Shortages in Canada and their Impact on Public Drug Plans, 2017/18 to 2019/20, available online.Footnote 1
Limitations
The chemical subgroup analysis only captured substitutions within the chemical subgroup and did not capture potential valid alternatives from other subgroups. The analyses are observational in nature and variations may be caused by other factors. For example, macroeconomic fluctuations and policy changes during the analysis may have impacted the population eligible for coverage.