Archived Evaluation of the Coordination of Antimicrobial Resistance (AMR ) Activities

Download the alternative format
(PDF format, 703 K, 44 pages)
Organization: Public Health Agency of Canada
Published: March 2019
Prepared by
Office of Audit and Evaluation
Health Canada and the Public Health Agency of Canada
March 2019
Table of contents
- Executive summary
- Management Response and Action Plan
- 1.0 Evaluation Purpose
- 2.0 Program Description
- 3.0 Evaluation Description
- 4.0 Findings
- 5.0 Conclusions
- 6.0 Recommendations
- Appendix 1 – Vision, Goals and Expected Outcomes from the Pan-Canadian Framework
- Appendix 2 – Evaluation Description
List of Tables
- Figure 1: Key Events in Canada's Response to AMR since 2014
- Figure 2. Understanding Canadian AMR Strategies
- Table 1: Program Expenditures ($M)
- Table 2: Limitations and Mitigation Strategies
- Figure 3. Understanding the difference between coordination and leadership
- Figure 4. Organizational Approaches Used in Other Countries
- Figure 5. AMR annual expenditures
- Figure 6. Parallels between the AMR and Opioid Responses
List of Acronyms
- AMR
- Antimicrobial resistance
- AMU
- Antimicrobial use
- CARSS
- Canadian Antimicrobial Resistance Surveillance System
- CIPARS
- Canadian Integrated Program for Antimicrobial Resistance Surveillance
- CNISP
- Canadian Nosocomial Infection Surveillance Program
- CIHR
- Canadian Institutes of Health Research
- GRDI
- Genomics Research and Development Initiative
- HESA
- Standing Committee on Health
- OAG
- Office of the Auditor General
- OECD
- Organisation for Economic Co-operation and Development
- PHAC
- Public Health Agency of Canada
- WHO
- World Health Organization
Executive summary
Evaluation Purpose
This evaluation examined the design, delivery and coordination of the Public Health Agency of Canada’s (PHAC) antimicrobial resistance (AMR) activities since 2013-14. This is the first evaluation of PHAC’s AMR activities.
Program Description
Since their introduction in the 1940s, antimicrobialshave revolutionized the treatment of infections. Over the past few decades, however, some microorganisms have become increasingly resistant to available antimicrobials and are creating significant risks to human health, the health care system and the economy. If left unaddressed, AMR will cause serious infections to become untreatable, existing treatments to become more expensive, and procedures like chemotherapy for cancer to become so risky that they may not be readily availableEndnote 1. Estimates show that, if no action is taken, by 2050 AMR could lead to 10 million deaths worldwide every yearEndnote 2 and the annual reduction in global Gross Domestic Product could be as large as the losses resulting from the 2008–09 global financial crisisEndnote 3. In this context, AMR is recognized as one of the most serious global health threats facing the world today.
Canada works to address AMR using a One Health approach, where multiple sectors communicate and work together to achieve better public health outcomes. This approach acknowledges the broad use of antimicrobial agents in multiple sectors, the interconnection between the health of humans, animals, and the environment, as well as the need for collaborative efforts across different sectors, including various federal departments, provincial and territorial governments, industries, health care professionals, and others.
Over the past couple of years, PHAC has been coordinating both federal and pan-Canadian approaches to addressing AMR. In addition, PHAC acts as Canada’s point of contact on various international AMR-related initiatives and undertakes various activities in relation to the four pillars of action identified in Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action including:
- Surveillance, through data collection systems that provide a picture of AMR and antimicrobial use (AMU);
- Stewardship, through activities to raise awareness of AMR among the population and professionals;
- Infection prevention and control, through collaboration with provincial/territorial partners, professional organizations and stakeholders to develop evidence-based guidelines and promote best practices; and,
- Research and innovation including activitiesto gain a greater understanding of factors that contribute to the development of AMR.
Between 2013-14 and 2016-17, PHAC spent approximately $8.5M per year on activities related to surveillance, stewardship, and federal and pan-Canadian coordination. This estimate does not include the cost of international activities, AMR-sensitive activities (i.e., activities that may have an impact on AMR, but have other objectives), vaccinations, and general infection prevention and control.
Conclusions
Over the last four years, PHAC has undertaken a significant amount of work to advance progress and address gaps in relation with its AMR activities. In addition, PHAC has been very active in liaising on a wide range of international initiatives on Canada’s behalf. The development of a Federal Framework on AMR and an associated Action Plan, along with the subsequent release of a pan-Canadian Framework, are recognized by external key informants as major endeavours that PHAC successfully coordinated.
However, overall progress to date to tackle AMR appears to be slow, considering that, in 2011, PHAC committed to establishing a pan-Canadian strategy to address AMR by mid-2015Endnote 4. Since then, PHAC has worked with federal and pan-Canadian partners to develop a federal framework (2014) and a federal Action Plan (2015) outlining the Government of Canada’s strategy to address AMR, as well as a pan-Canadian Framework outlining the context and the foundation to guide a pan-Canadian approach (2017). This pan-Canadian Framework was completed two years after the Office of the Auditor General of Canada (OAG) concluded that PHAC had “not succeeded in achieving consensus on the scope of a pan-Canadian strategy to address antimicrobial resistance”.
In May 2018, the Standing Committee on Health (HESA) released a report calling on the Government of Canada to accelerate the development of the pan-Canadian Action PlanEndnote 5. At the time of this evaluation, a pan-Canadian Action Plan was being developed to guide the implementation of the Framework, and its completion was expected in summer or early fall 2019.
As shown by Country Self-Assessments reported by the World Health Organization (WHO), the Food and Agriculture Organization of the United Nations, and the World Organisation for Animal Health, Canada has to strengthen the operationalization and monitoring of the implementation of an Action Plan, the integration of surveillance systems and the awareness of AMR among all key stakeholders (e.g., general public, doctors, dentists, pharmacists, nurses, medicine sellers)Endnote 6.
The evaluation did not find conclusive evidence that AMR was a priority for the Government of Canada. For example, AMR was not identified in any policy-setting documents, such as the Speech from the Throne or BudgetsFootnote a. AMR was not in the Minister of Health mandate letters, which identified a wide array of priorities, including bringing in tougher regulations to eliminate trans fats and raising awareness on concussion treatment. In addition, it is not clear whether Canada has had a sufficient level of leadership to address AMR. Questions remain on whether it PHAC’s role to be the national leader that will raise the profile of this issue and make it a priority on the government policy agenda, as well as mobilize stakeholders to take coordinated actions.
PHAC has essentially conducted its activities to address AMR in a reactive manner following the OAG’s report in 2015. At the time of this evaluation, PHAC did not yet have the organizational structures, resources, or strategic orientations to guide its own activities in a sustainable manner moving forward, although it was widely recognized that tackling this issue would require efforts for many years to come. It is important to recognize that these challenges are not necessarily consistent across all of PHAC’s AMR activities. For example, surveillance activities have been organized in a more sustainable manner than policy activities related to national and international coordination. It is also important to recognize that this evaluation was conducted at a time when PHAC was revisiting the organization, design, delivery, and the strategic orientations of its AMR activities.
Many external and internal key informants noted that maintaining the status quo represents a risk that PHAC will not be able to deliver on its commitments, due to a lack of adequate resources and lack of commitment from key policy-setting documents (e.g., ministerial mandate letter). This could hurt PHAC’s reputation both domestically and internationally, and these concerns have begun to emerge in the public space. Several news articles published in Canadian media over the last two yearsFootnote b have raised criticisms of PHAC and the Government of Canada’s response, or lack thereof, to AMR as an existing and growing threat.
Ultimately, beyond reputational risks, AMR could be considered as a slow-moving tsunami that carries a significant burden on human health, health care, and the Canadian economy as a whole.
Recommendations
The evaluation evidence discussed in this report led to the identification of the following recommendations. No specific recommendations have been made regarding surveillance, since the recently completed evaluations of PHAC healthcare-associated infection activities, and of food-borne and water-borne enteric illness activities have already made recommendations on improving the scope and dissemination of surveillance informationFootnote c Endnote 7.
Recommendation 1: Clearly articulate PHAC's role, responsibilities and priorities
PHAC should clearly articulate its roles, responsibilities and priorities to address AMR within the federal and pan-Canadian landscape.
The evaluation found that many of PHAC’s AMR activities, especially those related to domestic and international policy and coordination, have been essentially reactive and not driven by strategic orientations. As well, the evaluation found that roles and responsibilities of PHAC, especially in the area of national and pan-Canadian coordination, are not clearly defined. Considering that tackling AMR will require action over the long term and will involve many government and non-government players, it is recommended that PHAC clearly articulate its role, responsibilities and priorities in order to guide its core activities moving forward.
Recommendation 2: Communicating with partners and stakeholders on PHAC’s role, responsibilities, priorities and activities
PHAC should have open and regular communications with its federal and pan-Canadian partners on its role, responsibilities and priorities in addressing AMR, and on current work and next steps to meet federal and pan-Canadian commitments.
The evaluation found a disconnect between partners’ expectations towards PHAC’s role and responsibilities and views of PHAC staff about what this role should be. In this context, it is recommended that PHAC clearly communicate the nature and scope of its role and responsibilities to partners and stakeholders, and manage their expectations accordingly. As well, the evaluation found that the limited engagement PHAC has had over the past year with partners and stakeholders has sparked concerns that PHAC is disengaging from its commitment. Although partners have been re-engaged since the time of data collection for the evaluation, it is recommended that PHAC continue to maintain open and regular communication and engagement with those partners.
Recommendation 3: Organization of AMR activities
PHAC should establish the necessary structures to ensure all of its AMR activities, including policy and surveillance, are coordinated efficiently or integrated, where relevant. It should also continue work to establish an AMR program with clear accountability to accomplish its role in addressing AMR.
PHAC established its AMR policy and coordination activities to be temporary, but was in the process of reorganizing those activities at the time of the evaluation. In doing so, PHAC should continue work to establish an AMR program with clear accountability to accomplish its role in addressing AMR. As well, PHAC should organize and resource all of its activities to ensure that it can continue to address AMR on an ongoing basis. Considering that the evaluation noted areas for improvement with respect to the coordination and integration of some activities and especially of the different surveillance systems, it is recommended that PHAC continue work to establish structures allowing a more efficient coordination of those activities and/or their integration, where relevant.
Recommendation 4: Timely release of the pan-Canadian Action Plan
PHAC should make every effort it can to ensure the timely release of the pan-Canadian Action Plan by summer or early fall of 2019.
At the time of the evaluation, PHAC expected to release the pan-Canadian Action Plan by summer or early fall 2019. However, initial commitments to develop a pan-Canadian strategy were made in 2011, the OAG called on PHAC to take action on that front in 2015, and HESA called on the Government of Canada to accelerate the development of the Action Plan in 2018. In this context, it is recommended that PHAC do everything possible to coordinate the completion of the Action Plan in a timely manner, as per current commitments.
Management Response and Action Plan
| Recommendations | Response | Action Plan | Deliverables | Expected Completion Date | Accountability | Resources |
|---|---|---|---|---|---|---|
| Recommendation as stated in the evaluation report | Identify whether program management agrees, agrees with conditions, or disagrees with the recommendation, and why | Identify what action(s) program management will take to address the recommendation | Identify key deliverables | Identify timeline for implementation of each deliverable | Identify Senior Management and Executive (DG and ADM level) accountable for the implementation of each deliverable | Describe the human and/or financial resources required to complete recommendation, including the source of resources (additional vs. existing budget) |
Recommendation 1 Clearly articulate PHAC's role, responsibilities and priorities: PHAC should clearly articulate its roles, responsibilities and priorities to address AMR within the federal and pan-Canadian landscape. |
Management agrees with the recommendation. | Develop statement on PHAC roles and long-term objectives on AMR. | Presentation to PHAC Executive Committee on PHAC roles and responsibilities for AMR – including for domestic / international leadership, surveillance and stewardship. | October 30, 2019 | VP, IDPCB | Utilise resources from within IDPCB. |
Recommendation 2 Communicating with partners and stakeholders on PHAC’s role, responsibilities, priorities and activities: PHAC should have open and regular communications with its federal and pan-Canadian partners on its role, responsibilities and priorities in addressing AMR, and on current work and next steps to meet federal and pan-Canadian commitments. |
Management agrees with the recommendation. | Develop and share stakeholder engagement plan. | Develop stakeholder engagement plan for human health. |
October 30, 2019 | VP, IDPCB | Utilise resources from within IDPCB. |
| Communicate roles and responsibilities with government and non-government partners and stakeholders. | November 30, 2019 | |||||
Recommendation 3 Organization of AMR activities:PHAC should establish the necessary structures to ensure all of its AMR activities, including policy and surveillance, are coordinated efficiently or integrated, where relevant. It should also continue work to establish an AMR program with clear accountability to accomplish its role in addressing AMR. |
Management agrees with the recommendation. | Examine existing activities and desired results vis-à-vis PHAC roles and responsibilities. | Proposal to PHAC Executive Committee on sustainable options for AMR function. |
December 30, 2019 | VP, IDPCB | Utilise resources from within IDPCB. |
| Sustainable model for AMR policy and coordination, and PHAC core activities (i.e. surveillance). | September 30, 2020 | |||||
Recommendation 4 Timely release of the pan-Canadian Action Plan: PHAC should make every effort it can to ensure the timely release of the pan-Canadian Action Plan by summer or early fall of 2019. |
Management agrees with the recommendation. | Review and revise the critical path for the pan-Canadian Action Plan. | Critical path. |
March 31, 2019 |
VP, IDPCB | Utilize resources from within IDPCB. |
| Action Plan finalized. | September 30, 2019 |
1.0 Evaluation Purpose
This evaluation examined the design, delivery, and coordination of the Public Health Agency of Canada’s (PHAC) antimicrobial resistance (AMR) activities. Specifically, it examined PHAC’s role in addressing AMR, how PHAC’s activities in AMR are coordinated internally and resourced, and what progress was made in implementing AMR activities since 2013-14. This is the first evaluation of PHAC’s AMR activities.
2.0 Program Description
2.1 Context: The Risks of AMR
Introduced in the 1940s, antimicrobials (e.g., antibiotics, antifungal, antivirals, antiparasitics) have revolutionized how modern medicine treats infections in humans, animals, and plants, as well as enabling a more intensive production of food animals to feed an increasing global demand for animal proteinEndnote 8. However, over the past decades, antimicrobials have increasingly become less effective in treating infections worldwideEndnote 9 and some infections are more difficult to treat with existing antimicrobials. The ability of microorganisms (e.g., bacteria, fungi, viruses, parasites) to resist antimicrobials is what we call antimicrobial resistance (AMR).
AMR has been observed in various health care-acquired infections (e.g., C. difficile; methicillin-resistant Staphylococcus aureus) and in other infections, such as gonorrhea and tuberculosis. Over the last few years, AMR rates in Canada have been similar to, or lower than, those of other developed countries, but upward trends were observed for some infections, such as Methicillin-resistant Staphylococcus aureus blood stream infections in pediatric hospitals, and Vancomycin-resistant Enterococcus blood stream infections in adult hospitalsEndnote 10.
The widespread use of antimicrobials in human and veterinary medicine, as well as in agricultural settings, is a major contributor to accelerating the development and spread of AMR. At the same time, treatment options are becoming less available, since the development of a new class of antimicrobials has been extremely limited, with only five new classes of antimicrobials introduced in the last 50 years, as opposed to 14 new classes introduced between 1935 and 1968Endnote 11.
If AMR is left unaddressed, serious infections will become untreatable, illnesses will last longer and become more severe, treatments will become more expensive, and the risk of death will increase. If infections cannot be prevented or treated, procedures such as organ transplants, chemotherapy for cancer, and major surgeries (e.g., caesarean deliveries, hip and knee replacements) may become so risky that they may not be readily availableEndnote 12. Estimates show that if no action is taken, AMR could lead to 10 million deaths worldwide every year by 2050Endnote 13. The economic impacts would also be significant, as the annual reduction in global Gross Domestic Product caused by AMR could be as large as the losses resulting from the 2008–09 global financial crisisEndnote 14. In this context, AMR has been recognized by Canada, international organizations (e.g., the WHO), and other countries as one of the most serious global health threats facing the world today.
2.2 Canada's Response to AMR
Canada, other countries, and international organizations, such as the WHO, the Food and Agriculture Organization of the United Nations, and the World Organisation for Animal Health, have recognized the need to take a coordinated approach to limit the spread of AMR and protect the effectiveness of existing antimicrobials. Actions to address AMR in Canada are pursued using a One Health approach, which acknowledges the interconnection between the health of humans, animals, and the environment, as well as the need for collaborative efforts across different sectors, including various federal departments, provincial and territorial governments, industries, health care professionals, and others working in the AMR field.
Action to address AMR in Canada started in 1997, with the development of a strategy co-sponsored by Health Canada and the Canadian Infectious Disease Society.Footnote d This strategy emphasized action around surveillance, stewardship, and infection prevention and control. It included 27 recommendations, one of which was the creation of the Canadian Committee on Antibiotic Resistance (CCAR) in 1998, which was disbanded in 2009.Endnote 15
Over the past couple of years, PHAC has been coordinating both federal and pan-Canadian strategies to address AMR. In 2011, PHAC committed to establishing a pan-Canadian strategy to address AMR by mid-2015Endnote 16. A first step was taken in 2014, with the release of Antimicrobial Resistance and Use in Canada: A Federal Framework for Action. Coordinated by PHAC, in collaboration with four other federal departments, this Framework outlined the approach to be taken by the Government of Canada.
In 2015, the Office of the Auditor General of Canada (OAG) concluded that PHAC had “not succeeded in achieving consensus on the scope of a pan-Canadian strategy to address antimicrobial resistance”. The report also concluded that PHAC “has not determined how it will address the weaknesses it has identified in its collection, analysis, and dissemination of surveillance information on antimicrobial resistance and antimicrobial use.”Endnote 17
Since the OAG report, PHAC worked with federal departments to release an Action Plan in 2015 that outlined concrete actions to be taken by federal departments to implement the vision described in the 2014 Framework (as illustrated in Figure 1 on the following page). During that same year, PHAC launched the Canadian Antimicrobial Resistance Surveillance System (CARSS) to synthesize and analyze information from PHAC's surveillance systems and laboratory reference services.
Figure 1. Key Events in Canada's Response to AMR since 2014

Figure 1 - Text description
2014
- Publication of the Antimicrobial Resistance and Use in Canada: A Federal Framework for Action
2015
- Office of the Auditor General of Canada (OAG) report on Antimicrobial Resistance
- Release of the Federal Action Plan on Antimicrobial Resistance and Use in Canada: Building on the Federal Framework for Action
- Creation of the Canadian Antimicrobial Resistance Surveillance System (CARSS)
2017
- Release of Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action
2018
- Release of the Progress Report on the 2015 Federal Action Plan on the Antimicrobial Resistance and Use
- Release of the House of Commons' Standing Committee on Health (HESA) report A Study on the Status of Antimicrobial Resistance in Canada and Related Recommendations
As shown in Figure 2, at the pan-Canadian level, PHAC has made progress towards addressing the OAG recommendations by coordinating the development of a pan-Canadian Framework, with input from nine other federal departments and agencies, provincial and territorial governments, academics, non-governmental organizations, industries, and subject matter experts representing human health, animal health, and agriculture sectors at all levels. Released in 2017, Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action outlines the context around AMR in Canada, and provides the foundation to guide the pan-Canadian approach. At the time of this evaluation, a pan-Canadian Action Plan was being developed to guide the implementation of the Framework. Its completion was expected in summer or early fall 2019.
Figure 2. Understanding Canadian AMR Strategies
The federal strategy:
Outlines the approach to be taken by the Government of Canada to reduce the public health risks and impacts of AMR. At the time of the evaluation, eleven departments were involved.
Key documents outlining the strategy:
- Federal Framework (2014)
- Federal Action Plan (2015)
- Progress report to account for progress in implementing the Action Plan (2018)
The pan-Canadian strategy:
Outlines the coordinated approach to be taken by the government of Canada, provinces and territories, and other stakeholders (i.e., academics, non-governmental organizations, industries and subject matter experts) to strengthen Canada's ability to combat the risks of AMR in a coordinated, multisectoral and effective manner.
Key documents outlining the strategy:
- Pan-Canadian Framework (2017)
- Pan-Canadian Action Plan (expected for 2019).
Also, in summer 2018, PHAC released the Progress Report on the 2015 Federal Action Plan on the Antimicrobial Resistance and Use to account for achievements to date in implementing the objectives outlined in the Federal Action Plan.
Finally, in May 2018, the House of Commons Standing Committee on Health (HESA) released a report calling on the Government of Canada to accelerate its efforts to combat AMREndnote 18. The report made ten recommendations, including the need for PHAC to accelerate the development of the pan-Canadian Action Plan, appoint a federal advisor to be a national champion on AMR, and expand surveillance systems. With PHAC’s coordination, both the Minister of Health and the Minister of Agriculture issued a Government of Canada responseEndnote 19 agreeing with the recommendations, or the intent of the recommendations, and emphasizing actions already underway within various federal departments.
2.3 PHAC's AMR Activities
In addition to coordinating the development of federal and pan-Canadian strategies, Canada has recognized the need to act on four distinct pillarsEndnote 20 and PHAC has been carrying out activities within its own areas of responsibility to advance progress across each of those pillars including:
- Surveillance, through data collection systems that provide a picture of AMR and antimicrobial use (AMU);
- Stewardship, through activities to raise awareness of AMR among the population and professionals;
- Infection prevention and control, through collaboration with provincial/territorial partners, professional organizations and stakeholders to develop evidence-based guidelines and promote best practices; and
- Research and innovation including activitiesto gain a greater understanding of factors that contribute to the development of AMR.
PHAC also acts as Canada’s point of contact on various international AMR-related initiatives. PHAC’s involvement in each of those areas is further discussed in section 4.1 of this report.
In terms of organization, there is currently no AMR program at PHAC, but various branches and groups currently address different components of AMR activities. Section 4.2 of the report provides more detail on how activities are organized and coordinated internally.
At the time of writing this report, there was no logic model specific to PHAC AMR activities. However, there was a logic model describing the vision of the pan-Canadian Framework, as well as expected outcomes from actions to be taken under each of the four pillars listed above (see Appendix 1). Overall, the global vision pursued by Canadian partners, including PHAC, is to protect the health of humans, animals, and the environment through comprehensive and coordinated actions to conserve the effectiveness of antimicrobials, now and into the future
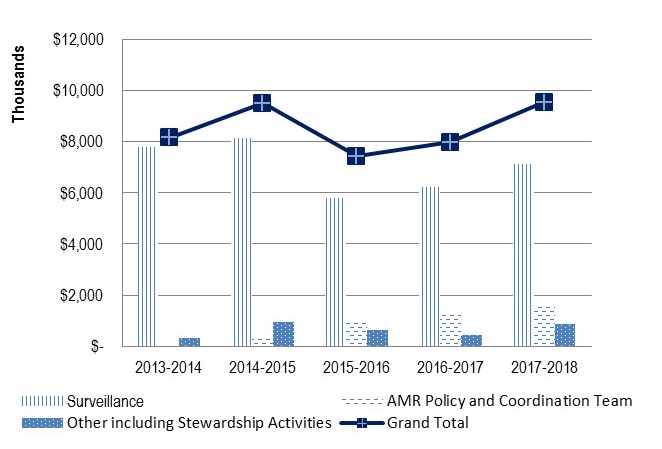
2.4 AMR Expenditures
Between 2013-14 and 2016-17, PHAC spent, on average, $8.5M per year for activities related to surveillance, stewardship, and federal or pan-Canadian coordination (See Table 1 on the following page). This estimate does not include the full costs associated with international activities. Most AMR-related funding is dedicated to various surveillance systemsFootnote e.
| Year | SalariesTable 1 Footnote a | Operations and ManagementTable 1 Footnote b | CapitalTable 1 Footnote c | Total |
|---|---|---|---|---|
| 2013-14 | $4,917 | $2,658 | $226 | $8,138 |
| 2014-15 | $5,783 | $3,345 | $0 | $9,493 |
| 2015-16 | $5,274 | $2,044 | $61 | $7,434 |
| 2016-17 | $5,416 | $2,455 | $24 | $7,977 |
| 2017-18 | $6,833 | $2,546 | $102 | $9,543 |
Source: Cost estimates based on analyses from the Chief Financial Officer and Centres involved in delivering AMR activities |
||||
3.0 Evaluation Description
3.1 Evaluation Scope, Approach, and Design
This evaluation examined the role, objectives, and priorities of PHAC with respect to AMR activities, as well as the design, delivery, and coordination of those activities. This is the first evaluation of PHAC’s AMR-related activities. The evaluation did not examine the impacts of those activities, although some of those impacts were already covered by other program evaluations conducted by PHAC. As well, the evaluation did not examine the AMR activities of other federal government departments, provinces and territories, and stakeholders.
The evaluation included a review of program documents, financial data, and literature on how other countries address AMR. Forty-seven interviews in total were conducted, including 21 with internal staff and 26 with external key informants from both the human and animal sectors. These external informants include representatives from other federal government departments, provincial and territorial governments, professional associations and researchers. They were selected for interviews based on their interaction with PHAC, as part of the development of the federal or pan-Canadian strategies.
The evaluation leveraged information collected under previous evaluations, such as the 2018 evaluations of PHAC’s health care-associated infections activitiesEndnote 21 and of PHAC’s food- and water-borne enteric illness activitiesEndnote 22. See Appendix 2 for more details on the evaluation approach and methodology.
Data was analyzed by triangulating information gathered from the different lines of evidence listed above, with the intention of increasing the reliability and credibility of evaluation findings and conclusions.
3.2 Limitations and Mitigation Strategies
The following table outlines the limitations encountered during the implementation of the data collection methods selected for this evaluation. Also noted are the mitigation strategies put in place to ensure that evaluation findings could be used with confidence in guiding program planning and decision making.
| Limitation | Impact | Mitigation Strategy |
|---|---|---|
| The evaluation provides a snapshot of PHAC’s AMR activities at a particular point in time. Some activities, or the organization of those activities, were evolving at the time of the evaluation. | The state of AMR activities described in this report may have changed since the completion of the evaluation. For example, interviews were completed between August and mid-September 2018 and captured external informants’ perceptions of PHAC’s engagement at that point in time. As shown in internal documentation, those engagements have continued to evolve since the completion of the interviews. | The report acknowledges when findings describe activities or features of the AMR response that were evolving or in the process of being redefined. The evaluation captured as many of the recent developments in the implementation and organization of PHAC AMR activities as possible. |
| The evaluation team has not been able to collect sufficient data to provide meaningful comparison of how organization and design of AMR activities at PHAC compares with those of other countries. | The evaluation only provides limited comparisons of the organization and design of PHAC AMR activities with those of other countries. | Comparisons are included in the report only when enough information is available to allow meaningful discussion. |
4.0 Findings
4.1 PHAC's role in AMR
PHAC’s involvement in surveillance, stewardship, and in infection prevention and control, as well as in being Canada’s liaison on international initiatives appears to be clearly defined and understood. The nature of PHAC’s involvement in providing pan-Canadian coordination and in research and innovation is, however, not clearly defined and does not fully align with partner and stakeholder expectations.
As per the Public Agency of Canada Act, PHAC’s mandate includesEndnote 23:
- Taking public health measures, including measures relating to health protection and promotion, population health assessment, health surveillance, disease and injury prevention, and public health emergency preparedness and response;
- Fostering collaboration within the field of public health and coordinating federal policies and programs in the area of public health;
- Promoting cooperation and consultation in the field of public health with provincial and territorial governments; and
- Fostering cooperation in that field with foreign governments and international organizations, as well as other interested persons or organizations.
Taking action to address AMR across the four identified pillars and providing a national and international coordination function is consistent with this mandate. However, as shown by evaluation evidence, the level of PHAC involvement and how clearly this involvement is defined and understood varies depending on the pillar or area of activity.
- Surveillance: As noted in the Pan-Canadian Framework, as well as in the 2018 progress report on the Federal Framework,Endnote 24 PHAC is carrying out the surveillance of infectious diseases. PHAC has been involved in various national surveillance programs to monitor AMR and AMU. Overall, PHAC’s involvement in conducting AMR surveillance is clearly defined and it aligns with the expectations of key informants.
- Stewardship: Stewardship is an area of shared responsibility with the provinces and territories, who are responsible for public health and health care settings, and with other federal departmentsEndnote 25. Within that landscape, as noted in the federal Action Plan, internal documents, and by many key informants, PHAC takes actions to raise awareness and knowledge of AMR among the general public, and to promote optimal antimicrobial use to human and animal health stakeholders. PHAC carries out these activities in collaboration with provinces and territories and other organizations. Moreover, raising awareness of AMR among the Canadian population is also part of the Chief Public Health Officer’s priorities.Endnote 26
- Infection prevention and control is also an area of shared responsibility. The provinces and territories are primarily responsible for the administration and delivery of health care services. The federal involvement in this area lies in developing and disseminating information and professional practice guidelines for infection prevention and control.Endnote 27 PHAC provides advice and guidance for health care organizations and providers to help prevent and control the spread of infections in health care and community settings. PHAC collaborates with provincial and territorial partners, professional organizations, and stakeholders to develop evidence-based guidelines and tools, and to promote best practices through awareness and dissemination of these products. As noted by key informants and demonstrated by a recent evaluation of PHAC’s health care-associated infections activities, PHAC’s involvement in this area is generally well understood by partners/stakeholders.
- Documents and many internal and external key informants noted that PHAC has assumed a supporting role in research and innovation. As noted in the progress report on the Federal Framework, CIHR is the main federal organization involved in developing knowledge and conducting research and development to create innovative tools and alternative therapies that will prevent and limit the spread of AMR. Internal documents explain that PHAC supports ongoing domestic health research and innovation, while collaborating with international partners to contribute to global research efforts on AMR, AMU, novel therapies, and alternatives to antimicrobials. For example, PHAC and other departments participate in the five-year federal Genomics Research and Development Initiative (GRDI)-AMR project. PHAC leverages whole genome sequencing and genome analysis to gain a greater understanding of the factors that contribute to the development of AMR, and to identify critical exposure pathways by which antimicrobial-resistant bacteria reach humans. PHAC also collaborates with other federal departments to determine vaccine research priorities. A few external key informants noted that PHAC could have a greater involvement in this area by leveraging other departments to incentivize research and innovation, but this view is not shared by internal key informants.
In addition to its involvement under the four pillars, both the progress report on the Federal Framework and internal key informants identified that PHAC has been liaising on international initiatives on Canada’s behalf. For example, as noted in the progress report on the Federal Framework, PHAC has led Canada’s engagement to develop and implement a Global Action Plan on AMR, with the support of other federal departments. PHAC has also continued to support the development of an integrated and global package of activities to combat AMR, as part of its work under the Global Health Security AgendaFootnote f. As explained by a key informant, the purpose of these engagements is to share information, knowledge, and experience, and collaboratively address issues related to AMR.
Figure 3. Understanding the difference between coordination and leadership
Based on information collected for this evaluation, the difference between national coordination and leadership can be understood as follows:
Coordination consists of:
- Coordinating input from partners to achieve a consensus on a coordinated approach to addressing AMR.
Leadership consists of the coordination activities noted above plus:
- Being the voice that draws attention to AMR and its risks in order to make it a priority for different stakeholders; and
- Calling on stakeholders to take action to address AMR.
As well, the evaluation found that both internal and external key informants have a shared understanding that PHAC is assuming a federal and national coordination role consisting of bringing partners together to develop federal and pan-Canadian strategies. However, most external partners reported that PHAC’s involvement in this should go beyond coordination and extends to providing national leadership. Based on interviews with partners/stakeholders and internal key informants, expectations around PHAC’s leadership include the following:
- being the voice in Canada that will raise the profile of AMR, in order to increase the level of priority granted to the file by different levels of governments and stakeholders;
- mobilizing relevant stakeholders to take action using a One Health approach; and
- coordinating and convening partners.
The perception of external informants is not the same as PHAC informants, who generally stated that the Agency’s involvement should be limited to coordinating the development of, and reporting on, the two frameworks and action plans (see Figure 3 below for a definition of the difference between coordination and leadership). A few internal key informants noted that PHAC has limited resources and levers with which to exercise broader leadership functions.
Public documents do not provide a clear answer as to whether PHAC’s role is only about coordination or if it also includes leadership. Several documents, such as the Federal Framework and progress report, clearly state that PHAC is the federal agency in charge of coordinating the development of the federal and pan-Canadian strategies. However, the 2015 Audit of AMR from the Office of the Auditor General identified a leadership role for PHAC and noted that PHAC is “coordinating national responses to public health threats and has identified antimicrobial resistance as such a threat. The Agency provides national leadership on the public health aspects of antimicrobial resistance and antimicrobial use.”Endnote 28
This is supported further by many other public documents, which refer to broader leadership functions. Among these, PHAC’s mandate indicates, “While public health is a shared responsibility, PHAC’s mission is to promote and protect the health of Canadians through leadership, partnership, innovation and action in public health.” The Federal Framework also outlines that PHAC“provides national leadership on the public health aspects of antimicrobial resistance and use, and works with domestic and international partners in areas of surveillance, laboratory analysis, infectious disease outbreaks, awareness, and public health guidance development”. The Agency itself has noted in its 2017-18 Departmental PlanEndnote 29 that it acts “as a catalyst for Canadian action on AMR priorities, including achieving stakeholder consensus on key indicators and targets for reducing antimicrobial resistance, promoting appropriate antimicrobial use, and advancing commitments made in the Action Plan”. Similarly, some internal documents and briefings also indicate that PHAC acts as the federal lead for Canada’s One Health approach.
In addition to the difference in perceptions about PHAC’s role, there appears to be a general lack of leadership in addressing AMR in Canada. For example, witnesses in the HESA hearings noted a clear need for a national voice and greater federal leadership in AMR. Similarly, most HESA recommendations called on PHAC to take action on AMR, including appointing a federal advisor to be the national champion for combatting AMR across Canada. Several key informants, both external and internal, plus key documentation noted that PHAC should be the lead.
4.2 Internal organization
Although AMR-related activities are spread out within PHAC, governance mechanisms put in place have allowed a certain level of internal coordination. However, organizational changes in terms of staff and structures have created challenges to ensuring an effective coordination of those activities.
At the time of the evaluation, there was no AMR program at PHAC. Different branches within PHAC conducted activities in the AMR area, but most of the activities were undertaken by the following centres within the Infectious Disease Prevention and Control Branch:
- The Centre for Communicable Diseases and Infection Control is undertaking:
- Surveillance, in collaboration with the National Microbiology Laboratory, through the Canadian Nosocomial Infection Surveillance Program (CNISP), that monitors AMU and AMR in hospitalized patients, and through CARSS, that synthesizes and analyzes information from PHAC's various surveillance systems and laboratory reference services.
- Stewardship priority projects through collaboration with provinces and territories and external partners (e.g., co-hosting professional learning workshops, continuing education curriculum development or developing priorities for stewardship) and raising awareness of AMR and appropriate antibiotic use.
- Infection prevention and control activities, through the development of national guidelines to prevent the transmission of infections in health care settings.
- National and international AMR policy and coordination activities, through the AMR Policy and Coordination Team (formely known at the AMR Tiger Team). This includes overseeing the implementation of the Federal Framework and Action Plan, leading and coordinating the development of the pan-Canadian Framework and Action Plan, and active international engagements.
- The Centre for Food-borne, Environmental and Zoonotic Infectious Diseases is:
- Conducting AMR and AMU surveillance along the food chain from animals to humans through the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), in collaboration with the National Microbiology Laboratory.
- Contributing to research and innovation through multiple externally-funded projects, including the five-year federal GRDI-AMR project, in partnership with the National Microbiology Laboratory and other departments.
- Providing expertise to international initiatives undertaken by the WHO, Pan American Health Organization and the World Organisation for Animal Health to provide best practices and build capacity for integrated surveillance along the food chain.
- The National Microbiology Laboratory is:
- Providing national public health laboratory leadership, surveillance for infectious disease, including bacterial pathogens and viral and zoonotic diseases that develop resistance, and specialized reference and diagnostic services for provinces and territories. The Laboratory also provides networking platforms (e.g., Canadian Network for Public Health Intelligence, Canadian Public Health Laboratory Network) to improve public health laboratory response capacity across Canada.
- Advancing research and innovation in the study of AMR in infectious diseases. This includes leading GRDI-AMR projects with outputs like pathogen genome data sets, bioinformatics (in silico) tools, and an analysis platform for federal partners.
- The Centre for Immunization and Respiratory Infectious Diseases is involved in AMR as it relates to the role of vaccines and immunization programs in avoiding infections and use of antimicrobials.
In addition to the Infectious Disease Prevention and Control Branch, other organizational areas of PHAC are involved in AMR, including the following:
- The Chief Public Health Officer has identified promoting the sound use of antibiotics and raising awareness of the risk of AMR as one of her six priority areas for action. The Chief Public Health Officer is the federal advisor and national champion on AMR.
- The Office of International Affairs is the single window for international affairs matters for the Health Portfolio. In that capacity, it coordinates PHAC’s international involvement in AMR issues, especially during multilateral discussions where AMR is not the only subject being covered. The Office also supports the AMR Policy and Coordination Team, who is liaising on behalf of Canada on AMR-specific initiatives.
- The Communications and Public Affairs Branch collaborates with the Centre for Communicable Diseases and Infection Control to conduct public awareness activities and communication strategies that raise awareness of AMR.
- The Chief Dental Officer liaises between PHAC and national dental professional and regulatory organizations on matters relating to AMR. At the time of the evaluation, there was no clear indication that the Chief Dental Officer was actively involved in the development or the governance of AMR activities.
- The Health Security and Infrastructure Branch’s regional offices gather strategic intelligence on AMR and AMU, enabling jurisdictional public health capacity, namely through stakeholder engagement and knowledge mobilisation, and enhancing emergency preparedness and response infrastructure.
Governance mechanisms have been put in place to help coordinate and share information across the various branches involved in AMR within PHAC. Those mechanisms include bi-weekly bilateral meetings on AMR between the Director General of Centre for Communicable Diseases and Infection Control, the Director of the AMR Tiger Team (now reorganized as the AMR Policy and Coordination Team) and PHAC’s President, monthly meetings between Vice-Presidents and Director Generals involved in AMR activities, and monthly meetings of Directors involved in AMR. Periodic pan-Canadian meetings were also held between staff in regional offices involved in AMR. Overall, internal key informants believed these governance mechanisms have worked well.
However, views on how well AMR activities are coordinated in practice varied depending on the type of activities. While communication and international activities were perceived as being well coordinated, a few internal key informants identified a need for better coordination across the various areas involved in surveillance. The coordination of those activities was seen as challenging, as different centres are responsible for different surveillance programs that are not specific to AMR and driven by other priorities.
Another area that presents a challenge is the organization and location of the AMR policy and coordination function. This was initially established as a temporary function (i.e., Tiger Team) to develop the federal and pan-Canadian Frameworks and the Action Plan in response.
In September 2017, the AMR Tiger Team was moved from the Vice-President’s Office of the Infectious Disease Prevention and Control Branch to the Centre for Communicable Diseases and Infection Control, and was eventually renamed the AMR Policy and Coordination Team. A few internal key informants wondered if the relocation of the Policy and Coordination Team to within a Centre was ideal, and if it reduced the profile of the function. At the same time, none of these key informants had a clear answer on the ideal location of the team. A few internal key informants noted that work was underway to determine how best to organize the function moving forward. There was also some discussion underway at the time of the evaluation on the creation of a program unit in charge of AMR activities. Figure 4 below presents examples of other countries that have established high-profile functions to tackle AMR. As well, Figure 6 on page 14 further discusses how the AMR activities have been conducted in comparison to the Canadian response to the opioid crisis.
Some internal and external key informants noted that, following the release of the pan-Canadian Framework and with the reorganization of the AMR Policy and Coordination Team, some changes in senior management and staff departures had created challenges. They noted that relationships built with partners tended to disappear with the departure of some staff and that corporate knowledge of the file was also lost.
Figure 4. Organizational Approaches Used in Other Countries
United States
PHAC's model of embedding the policy function within a Centre contrasts with the approach taken by other countries that established a central policy function at the government level. In the United States, the Presidential Advisory Council on Combating Antibiotic-Resistant Bacteria reports directly to the President via the Health Secretary. This Advisory Council regroups human and animal health experts from various fields including agriculture, pharmacy, public health, healthcare, etc. The evaluation has not found evidence of any systematic reporting on AMR activities at that level in Canada.
England
In England, the Department of Health and Social Care, along with the Department for Environment, Food and Rural Affairs, and Public Health England leads a wide-ranging program involving organizations from public and private sectors and helps shape international activities. A cross-government steering group involving a range of partners across the human and animal health, research, industrial, and academic sectors is overseeing the implementation of the AMR strategy. Dame Sally Davies, Chief Medical Officer for England, acts has a champion for AMR and has pushed the file as a priority in England. She has also challenged other countries to be more active on the issue.
At the time of the evaluation, multiple external key informants perceived that Canada did not have a similar national champion for AMR as in England. This was highlighted as a challenge, because AMR is a crosscutting issue where silos exist across government levels. While HESA called on the Government of Canada to appoint a federal advisor to be the national champion for combatting AMR, PHAC has responded that it will continue to rely on the leadership of the Chief Public Health Officer as federal advisor and federal champion.
4.3 Resources
Key informants perceive that the current level of resources allocated to AMR activities are not sufficient and limits activities PHAC can undertake. As well, activities related to CARSS, as well as the policy and coordination function, have only received limited permanent resources over the years.
Between 2013-14 and 2017-18, PHAC spent approximately $8.5M per year for activities related to surveillance, federal, pan-Canadian, and international coordination as well as other activities (e.g., stewardship). This estimate does not include the full costs related to international activities. As shown in Figure 5 below, roughly $7M per year has been spent on surveillance, $0.9M for policy and coordination activities and $0.6M for other various activities.
Overall, the estimated spending of about $8.5M per year represented roughly 1.4% of PHAC’s overall budget. In comparison, PHAC invested roughly 4% of its budget in Immunization and Respiratory Infectious Disease Activities and 7% of its budget in the Federal Initiative to Address HIV/AIDS.
A majority of internal key informants and a few external key informants noted that the current level of funding is not sufficient and limits activities that PHAC can undertake both domestically and internationally. A few key informants also noted that there is a risk that PHAC may not be able to deliver on its commitment due to the lack of resources. In addition, although most of PHAC’s expenditures are dedicated to surveillance work, including CNISP, CIPARS, CARSS and National Microbiology Laboratory activities, many external key informants noted that there is a lack of resources in this specific area, affecting the level of coverage of current surveillance systems and PHAC’s ability to be innovative. In fact, as highlighted in the financial data, surveillance expenditures have decreased over time and this has mostly affected CIPARS and the National Microbiology Laboratory.
While there is a general perception within PHAC that the level of funding to AMR activities is insufficient, it is not fully clear what resources would be needed to accomplish AMR objectives sustainably moving forward. As explained by a few internal key informants, PHAC has never been able to fully develop a business case and proper costing for its AMR activities, and this was identified as an area to address in the future.
Another challenge noted by internal key informants was that CARSS, as well as the policy and coordination function, have only received limited permanent resources over the years. Those activities have been funded mainly through business cases presented over several years to reallocate funding internally on a temporary basis, and with the use of banking days. This could be partly explained by the fact that the AMR Tiger Team, now the Policy and Coordination Team, was initially established as a temporary function and there was no program specifically responsible for AMR at the time of this evaluation. Nonetheless, internal key informants explained that the use of banking days and temporary funds has made it difficult to get buy-in from partners and to recruit staff, as it is not possible to undertake competitions to fill positions that do not officially exist. It has also created difficulties in attracting staff with the appropriate skills and expertise to fill temporary positions.
In this regard, a few internal and external key informants noted that PHAC does not necessarily have the right blend of skills and expertise to conduct AMR activities. Furthermore, they mentioned that staff members with the appropriate technical expertise are not sufficiently involved in discussions, which have tended to be driven by generalists and policy staff.
4.4 Prioritization of AMR
There has not been a sufficient level of priority granted to the AMR file by either PHAC or the Government of Canada.
Canada has made several international commitments to address AMR. For example, in 2015, PHAC provided funding to the World Bank to explore the economic cost of AMR. As well, Canada pledged support for a political declaration made at a high-level meeting of the General Assembly on AMR, wherein the United Nations General Assembly passed a resolution adopting the declaration on October 5, 2016. In 2017, Global Affairs Canada, following discussions with PHAC, made a contribution of $9 million to the WHO in support of a comprehensive global approach to combatting AMR. Also in the fall of 2017, Canada was the Chair of the Global Health Security Agenda AMR Action Package for a one-year period. Although Canada has been active internationally, internal key informants indicated that Canada’s international commitments have been essentially reactive and not necessarily guided by proactive and strategic orientations.
Furthermore, evidence that AMR has been part of the Government of Canada domestic priorities is very limited. AMR was identified as a priority in the 2013 to 2018 PHAC Strategic PlanEndnote 30 and is part of the six priority areas for action identified by the Chief Public Health Officer in June 2018. However, there was no reference to addressing AMR in the Minister of Health’s most recent mandate letter, although it identifies a wide array of priorities, including bringing in tougher regulations to eliminate trans fats and raising awareness of concussion treatment. There has also been no reference to addressing AMR in the various Speeches from the Throne and federal Budgets from the last five years, with the exception of Budget 2015.
Overall, many external and a few internal key informants do not think that AMR is a priority for PHAC or the Government of Canada, and perceive that the current level of prioritization is insufficient, considering the risks that AMR represents for human health and lives, as well as for the Canadian health system and economy.
Of note, some internal key informants explained that PHAC’s action on AMR have been essentially reactive as opposed to driven by strategic orientations. A few of those key informants acknowledged the need to determine the next steps, objectives, and organization of PHAC AMR activities to make them sustainable moving forward.
Figure 6 below provides a comparison of how the prioritisation and organisation of AMR activities compare to the approach taken by the Government of Canada to response to the opioid crisis.Figure 6. Parallels between the AMR and Opioid Response
A few key informants noted that AMR has many similarities to the opioid crisis, as they are both public health issues that have prescription of medical drugs as a contributing factor. They are both complex files that need coordinated action from multiple federal departments, provinces and territories, health organizations, experts, etc. Both the AMR and the opioid responses were organized to address a serious immediate issue that requires long-term action; however, the opioid response addresses an immediate crisis, while AMR aims to prevent a crisis. In both cases, there is a recognized need to define an appropriate response moving forward.
The organizational approach taken by Health Canada to coordinate and lead the federal response to the opioid crisis differs from PHAC's approach to AMR. The opioid response is led by a senior official (i.e., Assistant Deputy Minister) who is also in charge of implementing broader strategies and regulations around controlled substances. Both files are coordinated through governance structures involving senior governmental officials. In the case of opioids, it is clear that Health Canada is providing coordination and leadership while it is not clear whether PHAC is providing leadership on AMR. In both cases, there were concerns raised by partners that their involvement in the governance committees consisted more of receiving information than being engaged in strategic discussions.
While the need to address the problem of AMR has been known and recognized for many years, AMR has not been identified as a key priority of Government of Canada agenda-setting documents in the years covered by this evaluation. By comparison, the Government of Canada has clearly identified the opioid crisis as a priority and resources were clearly committed to addressing the opioid crisis in the 2017 and 2018 federal budgets.
4.5 Progress in Implementing Activities
4.5.1 Surveillance
PHAC has made considerable progress in addressing the need for AMR surveillance information. However, progress still needs to be made with respect to the scope, integration, analysis, and sharing of surveillance data.
Over the past five years, PHAC has continued to strengthen its surveillance systems through multiple initiatives, including the following:
- Launching CARSS to provide integrated information gathered from PHAC's surveillance systems and laboratory reference services and released in an annual report;
- Conducting two pilot surveillance initiatives to address gaps in community settings;
- Identifying AMR surveillance requirements for priority organisms;
- Integrating the AMR-AMU Surveillance Transformation Plan into the governance and coordination of CARSS;
- Piloting the collection of AMR susceptibility data from clinical laboratories to better understand community-associated AMR issues and trends; and
- Working with Health Canada to analyze data on antimicrobial prescriptions collected in Indigenous communities to provide a more comprehensive picture of AMU in Canada.
The work that PHAC conducted in surveillance was generally well perceived by external key informants. In particular, many external key informants complimented CIPARS and mentioned that it is recognized as a world leader in this area.
However, as noted in internal documents and previous program evaluations, there remain several gaps that limit the development of a comprehensive understanding of AMR and AMU in Canada and where or how to target efforts. The main gaps remain around the scope, analysis, integration and dissemination of surveillance information:
- Coverage: As noted in internal documents, current national surveillance systems are more focused on infections than pathogens and provide limited data on resistance. Canada collects limited data on AMR/AMU in small, non-academic hospitals and no data for rural and Northern health care settings.Endnote 31 There are limitations in the coverage of commodities and farmed animals, thus affecting the understanding of trends in AMR across the food chain.Endnote 32Internal documents indicate there is insufficient data and evidence on effective interventions and actions to prevent AMR. In addition, a few internal and external key informants mentioned a need to collect more data on the use and prescription of antimicrobials and on certain pathogens.
- Integration: Internal documents indicate a lack of information on the connections between AMU practices, observed patterns of resistance, and the spread of pathogens. Documents also noted a need to pursue the integration of animal and human health data. A few internal and external key informants mentioned that more needs to be done to better integrate different surveillance programs and coordinate work conducted across Canada (e.g., improving the consistency of case definitions).
- Analysis and presentation: Some internal key informants mentioned that improvements are needed to report a clear story on AMR trends in Canada. A similar concern was also raised by stakeholders interviewed for the evaluation of PHAC food-borne and enteric-borne illness activitiesEndnote 33.
- Dissemination: As documented in recent evaluations, PHAC’s publicly available surveillance data is often not up-to-date and not posted in a timely mannerEndnote 34. An internal key informant explained that if stakeholders cannot access data from PHAC in a timely way, they will turn elsewhere, and it will be difficult for them to maintain confidence in PHAC as a leader in surveillance.
Data from other countries show that the state of surveillance in Canada is comparable to the United States, but lags behind most Western European countries and Australia. At a minimum, those countries have been able to implement national AMR surveillance systems covering antibiotics in hospitals and outpatient clinics, with external quality assurance and a national coordinating centre that produces reports on resistance levelsEndnote 35. That being said, according to internal documents and several internal key informants, at the time of the evaluation, PHAC was continuing its work to strengthen and expand the scope of the AMR and AMU surveillance systems. For example, as part of the policy work conducted to develop the pan-Canadian Action Plan, PHAC conducted a gap analysis to identify where to improve AMR and AMU surveillance.
4.5.2 Stewardship
PHAC has developed a strategy to guide efforts to increase the awareness of AMR and it is expected that the Chief Public Health Officer will contribute to strengthen those efforts moving forward. However, at the time of the evaluation, Canada was behind similar countries in this area.
Over the past five years, PHAC has advanced various stewardship activities to promote prudent use of antimicrobials and raise awareness of AMR. These include various public awareness activities (e.g., annual Antibiotic Awareness Week, use of social media to improve awareness), as well as holding roundtables, consultations, and workshops with human and animal health stakeholders to seek their perspectives on stewardship.
An internal key informant explained that, with the exception of Antibiotic Awareness Week, activities to raise awareness of AMR were not guided by a strategy until 2017. Although there have been public awareness activities throughout each year to promote key accomplishments, the majority of the work has been reactive and issue based. Since then, AMR has become one of the priorities listed in PHAC's External Communication Strategy. An interdepartmental working group has also been established to engage other departments to ensure coordinated communications around AMR.
The objectives of the communication strategy are to increase awareness, knowledge and understanding of AMR, antibiotic use and the risks of AMR. The strategy targets the general public, including some at-risk groups (e.g., seniors), prescribers (i.e., dentists, nurse practitioners, and physicians), and dispensers (i.e., pharmacists).
As stated previously in this report, one of the Chief Public Health Officer of Canada’s priorities is to increase awareness of AMR. She has also been designated as the federal government champion on AMR. The communication strategy intends to leverage her role for various communication initiatives. At the time of the evaluation, the Chief Public Health Officer’s actions on AMR were under development.
As noted in a report released by the Organisation for Economic Co-operation and Development (OECD) in November 2018, Canada is behind most OECD countries in terms of awareness-raising campaignsEndnote 36. Canadian campaigns to date have been small-scale and targeted to some relevant stakeholders while many Western Europe countries and the United States “have focused on national scale government-supported activities implemented to change behaviour regarding AMR in target groups in human health, both public and private sectors, with monitoring undertaken of their awareness and behaviour change over last five years”. Endnote 37
4.5.3 Infection Prevention and Control
PHAC has issued various guidelines on the prevention of AMR-related infections, but evaluation data shows that plans in this area are unclear.
PHAC provides advice and guidance for health care organizations and providers, to prevent and control the spread of infections in health care and community settings. In this regard, PHAC has developed the following guidelines on the prevention and control of AMR-related infections since 2013:
- Routine practices and additional precautions assessment and educational tools / Infectious Disease Prevention and Control(2013);
- Clostridium Difficile Infection - Infection Prevention and Control Guidance for Management in Long-term Care Facilities (2013);
- Infection Prevention and Control Guidance for Management in Acute Care Settings (2013);
- AMR-related guidance for treatment of resistant gonorrhoea (2017); and
- Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings (2017).
A recent evaluation of health care-associated infection activities raised concerns about the timeliness of PHAC guidelines in this area, including AMR-related guidanceEndnote 38. As well, interviews with a few internal key informants revealed a lack of clarity on how key partners and stakeholders would be consulted in the process for developing future guidelines, as well as a lack of awareness of activities underway in this area and associated timelines.
Canada is behind the United States, the United Kingdom, and Australia in terms of developing national infection prevention and control programs, and implementing related guidelinesFootnote g.
4.5.4 Research and Innovation
PHAC has been implementing new technologies to improve the detection of AMR.
PHAC supports ongoing domestic health research and innovation, while collaborating with international partners to contribute to global research efforts on AMR, AMU, novel therapies and antimicrobial alternatives. PHAC participates in the interdepartmental GRDI-AMR, which has devoted $20 million annually across various federal departments and agencies for genomic research, including research to inform policies, practices, and products to mitigate the development of AMR.
Over the past years, PHAC has developed assays informed by new molecular and whole genome sequencing to predict antimicrobial susceptibilities in Neisseria gonorrhoea and has been using new whole genome sequencing and analysis tools to rapidly identify and assess emerging threats (e.g., Mcr-1-inferred colistin resistance in food). PHAC has also collaborated with other departments to publish the federal vaccine research and development priorities for key areas of public health concern.
As noted earlier, some external key informants noted that PHAC could collaborate more with other departments to find ways to incentivize research and innovation.
4.5.5 International coordination
PHAC has carried out a significant number of international engagements on Canada’s behalf. However, PHAC does not have the capacity to support all opportunities for international engagement and does not have a clear vision of where to prioritize efforts.
PHAC has been coordinating most of Canada’s international policy and political international engagements and initiatives, while other federal departments or agencies have been involved in some technical and research collaborations. Overall, the volume of involvement by PHAC has been significant. Among other things, PHAC is involved on Canada’s behalf in the following initiatives, which may also include the participation of other federal departments or agencies:
- Canada was a leading country on the Global Health Security Agenda Antimicrobial Resistance Action Package;
- Canada is a member for the Transatlantic Taskforce on Antimicrobial ResistanceFootnote h; and
- Canada contributes expertise in integrated surveillance along the food chain to WHO and Pan American Health Organization capacity-building activities.
Internal key informants explained that Canada is recognized as a leader for its active international engagements. However, opportunities for international engagements are numerous and according to key informants, PHAC has neither the capacity to support all those engagements, nor a clear direction of where to prioritize efforts.
4.5.6 National coordination activities
PHAC’s coordination of the two Frameworks and the federal Action Plan was seen by external key informants as a significant accomplishment. There are some challenges with the governance mechanisms used to coordinate these efforts, but the main area of concern relates to the level of engagement PHAC had with its partners/stakeholders over the previous year, as well as with the progress made so far with the development of the pan-Canadian Action Plan.
Many external key informants were complimentary of the work accomplished by PHAC to coordinate the development of the two Frameworks and of the federal Action Plan. The development of the pan-Canadian Framework was seen by external key informants as a particularly challenging endeavour, as it involved stakeholders from a multitude of sectors. External key informants noted that PHAC was successful in convening all partners and in consolidating and reconciling different positions.
To coordinate the development of the Frameworks and Action Plan, PHAC has implemented two different governance mechanisms at federal and pan-Canadian levels:
- The federal governance mechanism involves eleven federal departments or agenciesFootnote i and provides overall strategic vision and leadership for addressing AMR domestically, and for Canada’s contribution to the international agenda. The governance structure includes: 1) an Interdepartmental Deputy Head committee, 2) an Interdepartmental Assistant Deputy Minister Committee and, 3) an Interdepartmental Director General Committee.
- At the pan-Canadian level, discussions happen through: 1) a Federal/Territorial/Provincial Deputy Minister Champion Committee chaired by two Deputy Minister Champions on the agriculture side and two on the human side, 2) an AMR Steering Committee grouping senior federal/provincial/territorial representatives, and 3) four task groups (one for each of the surveillance, stewardship, infection prevention and control, and research and innovation pillars), comprised of representatives from federal/provincial/territorial governments, industries, academia, and other stakeholders. This governance structure has links to other national committees on both the health side (i.e., the Public Health Network Council, the Council of Chief Medical Officers of Health, and the Conference of Deputy Ministers of Health) and on the agriculture side (i.e., the Council of Chief Veterinary Officers, Assistant Deputy Minister-level regulatory and policy committees, and the federal, provincial, and territorial ministers of Agriculture).
Views were mixed on how well the federal and pan-Canadian governance mechanisms worked. A few internal and external key informants reported that they worked well, or as well as they could, considering their size and complexity. An internal key informant even mentioned that Canada is seen as a good example in terms of its ability to bring a range of stakeholders together in a governance structure. There are, however, areas for improvement, as some external key informants reported that partners/stakeholders had not been engaged in a meaningful way in the development of the Framework. The committees were often used to share information and provide an opportunity for partners to react to proposals as opposed to fully engaging them in developing those proposals. As well, partners from outside of the Government of Canada reported not having a clear understanding of the roles and responsibilities of the various federal departments involved.
As noted in section 3.2, a limitation on this evaluation was the timing of data collection, which occurred in summer 2018. At that time, a majority of external key informants noted that they had received only limited communication and engagement from PHAC on the next steps for the development of the pan-Canadian Action Plan over the previous twelve months or so. In fact, a review of available meeting agendas and records of decisions shows that, with the exception of the Interdepartmental Director General Committee, which is part of the federal governance mechanism, none of the other federal or pan-Canadian committees met between December 2017 and October 2018. This slowdown in the level of engagement of the various governance committees coincided roughly with the release of the pan-Canadian Framework and the internal reorganization of the AMR Policy and Coordination Team.
A majority of external key informants interviewed raised concerns regarding the length of time taken to develop the Action Plan. They noted that the work accomplished on the Framework put AMR on the agenda of federal, provincial, and territorial governments, industry, and other stakeholders. They also feared that the window of opportunity may have been closing due to the limited engagement they had over the previous months. This was seen as a risk by external key informants since the development of the pan-Canadian Action Plan relied on building and maintaining relationships, and on the willingness of various stakeholders to take a coordinated approach to address AMR.
Although PHAC has had limited interaction with partners/stakeholders since fall of 2017, internal key informants and documents indicated that PHAC continued to advance some foundational work to support the development of the pan-Canadian Action Plan during that period. Among other things, PHAC held a One Health roundtable (March 2018) with stakeholders from the human health, agriculture, and environment sectors to identify potential common areas of action at the pan-Canadian level. PHAC also collaborated with other departments to identify gaps in AMR response and opportunities for action. As well, through its regional offices, PHAC has contributed to stakeholder engagement and knowledge mobilization around AMU and AMR over the last 12 months or so. For example, PHAC funded, and was represented on the scientific committees of, the 2018 Journée Annuelle de Santé Publique (held in Montreal, Quebec), which held a day-long intersectoral conference on AMR.
Internal and governance committee documents also demonstrate that PHAC had started to reconvene with its pan-Canadian partners and stakeholders to advance development of the Action Plan in October 2018, but the evaluation did not assess whether the reengagement was timely enough to preserve the momentum for coordinated action on AMR.
At the time of this report, the expected completion date for the pan-Canadian Action Plan was end of summer or early fall 2019. This is about four years after the initial commitment made in 2011 to establish a pan-Canadian strategy to address AMR by mid-2015, and slightly behind the commitment made in the Government of Canada response to the HESA report to release the Plan by summer 2019.
Of note, as explained by the OECD in a report published in fall 2018, “Canada has national AMR Plan objectives, with no operational plan and monitoring arrangements, lagging behind what most OECD countries are doing in this area”.Endnote 39 Most Western European countries are ahead in the completion of a national Action Plan, with an operational plan and monitoring arrangements. The U.S. is even further ahead, with funding sources identified and a monitoring process in place.
Of note, concerns have started to emerge in the public space, as several new articles published in the Canadian media over the last two yearsFootnote j have raised criticisms about PHAC and the Government of Canada response, or lack of response to AMR as an existing and growing threat.
5.0 Conclusions
When looking at work undertaken across the four pillars, as well as the federal, pan-Canadian, and international coordination activities, it is clear that PHAC has made significant accomplishments to address AMR over the last five years. There is also general agreement among partners and stakeholders that the development of the two Frameworks and the federal Action Plan was a major endeavour that PHAC has successfully coordinated.
However, progress made to date appears to be slow, considering the initial commitments to develop a pan-Canadian strategy by 2015. As well, compared to Western Europe, the United States, and Australia, Canada has to strengthen the operationalization and monitoring of the implementation of an Action Plan, the integration of surveillance systems and the awareness of AMR among all key stakeholders (e.g., general public, doctors, dentists, pharmacists, nurses, medicine sellers).
Evaluation evidence discussed in this report shows that it is not clear whether Canada has had a sufficient level of leadership and prioritization on the AMR file. Questions remain as to whether it is PHAC’s role to be a national leader that will raise the profile of the file and make it a priority on the Government of Canada’s policy agenda, and to mobilize stakeholders to take coordinated action in the area. As well, there remains a lack of clarity in public messaging with respect to what federal and pan-Canadian partners and stakeholders should expect in terms of PHAC’s coordination versus leadership role.
PHAC’s activities to address AMR have essentially been conducted in a reactive manner since the 2015 OAG report. At the time of this evaluation, PHAC did not have the organizational structures, resources, and strategic orientations in place to guide its activities in a sustainable manner moving forward, although it is widely recognized that tackling this issue will require efforts for many years to come. However, it is important to recognize that these challenges are not necessarily consistent across all PHAC AMR activities. For example, surveillance activities have been organized in a more sustainable manner than policy activities around national and international coordination. It is also important to recognize that this evaluation was conducted at a time when PHAC was facing a turning point and was thinking of revisiting the organization, design, delivery, and strategic orientations of its AMR activities.
Many external and internal key informants noted that maintaining the status quo represents a risk that PHAC will not be able to deliver on its commitments, due to a lack of adequate resources and an inability to keep partners and stakeholders mobilized into taking action. This could hurt PHAC’s reputation both domestically and internationally.
Ultimately, beyond reputational risks, AMR is a viable and documented public health threat that carries a significant burden on human health, health care, and the Canadian economy as a whole.
6.0 Recommendations
The evaluation evidence discussed in this report led to the identification of the following recommendations. No specific recommendations have been made regarding surveillance, since the recently completed evaluations of PHAC healthcare-associated infection activities, and of food-borne and water-borne enteric illness activities have already made recommendations on improving the scope and dissemination of surveillance informationFootnote kEndnote 40.
Recommendation 1: Clearly articulate PHAC's role, responsibilities and priorities
PHAC should clearly articulate its roles, responsibilities and priorities to address AMR within the federal and pan-Canadian landscape.
The evaluation found that many of PHAC’s AMR activities, especially those related to domestic and international policy and coordination, have been essentially reactive and not driven by strategic orientations. As well, the evaluation found that roles and responsibilities of PHAC, especially in the area of national and pan-Canadian coordination, are not clearly defined. Considering that tackling AMR will require action over the long term and will involve many government and non-government players, it is recommended that PHAC clearly articulate its role, responsibilities and priorities in order to guide its core activities moving forward.
Recommendation 2: Communicating with partners and stakeholders on PHAC’s role, responsibilities, priorities and activities
PHAC should have open and regular communications with its federal and pan-Canadian partners on its role, responsibilities and priorities in addressing AMR, and on current work and next steps to meet federal and pan-Canadian commitments.
The evaluation found a disconnect between partners’ expectations towards PHAC’s role and responsibilities and views of PHAC staff about what this role should be. In this context, it is recommended that PHAC clearly communicate the nature and scope of its role and responsibilities to partners and stakeholders, and manage their expectations accordingly. As well, the evaluation found that the limited engagement PHAC has had over the past year with partners and stakeholders has sparked concerns that PHAC is disengaging from its commitment. Although partners have been re-engaged since the time of data collection for the evaluation, it is recommended that PHAC continue to maintain open and regular communication and engagement with those partners.
Recommendation 3: Organization of AMR activities
PHAC should establish the necessary structures to ensure all of its AMR activities, including policy and surveillance, are coordinated efficiently or integrated, where relevant. It should also continue work to establish an AMR program with clear accountability to accomplish its role in addressing AMR.
PHAC established its AMR policy and coordination activities to be temporary, but was in the process of reorganizing those activities at the time of the evaluation. In doing so, PHAC should continue work to establish an AMR program with clear accountability to accomplish its role in addressing AMR. As well, PHAC should organize and resource all of its activities to ensure that it can continue to address AMR on an ongoing basis. Considering that the evaluation noted areas for improvement with respect to the coordination and integration of some activities and especially of the different surveillance systems, it is recommended that PHAC continue work to establish structures allowing a more efficient coordination of those activities and/or their integration, where relevant.
Recommendation 4: Timely release of the pan-Canadian Action Plan
PHAC should make every effort it can to ensure the timely release of the pan-Canadian Action Plan by summer or early fall of 2019.
At the time of the evaluation, PHAC expected to release the pan-Canadian Action Plan by summer or early fall 2019. However, initial commitments to develop a pan-Canadian strategy were made in 2011, the OAG called on PHAC to take action on that front in 2015, and HESA called on the Government of Canada to accelerate the development of the Action Plan in 2018. In this context, it is recommended that PHAC do everything possible to coordinate the completion of the Action Plan in a timely manner, as per current commitments.
Appendix 1 – Vision, Goals and Expected Outcomes from the Pan-Canadian Framework
Antimirobial Resistance and Antimicrobial Use
A Pan-Canadian Framework for Action
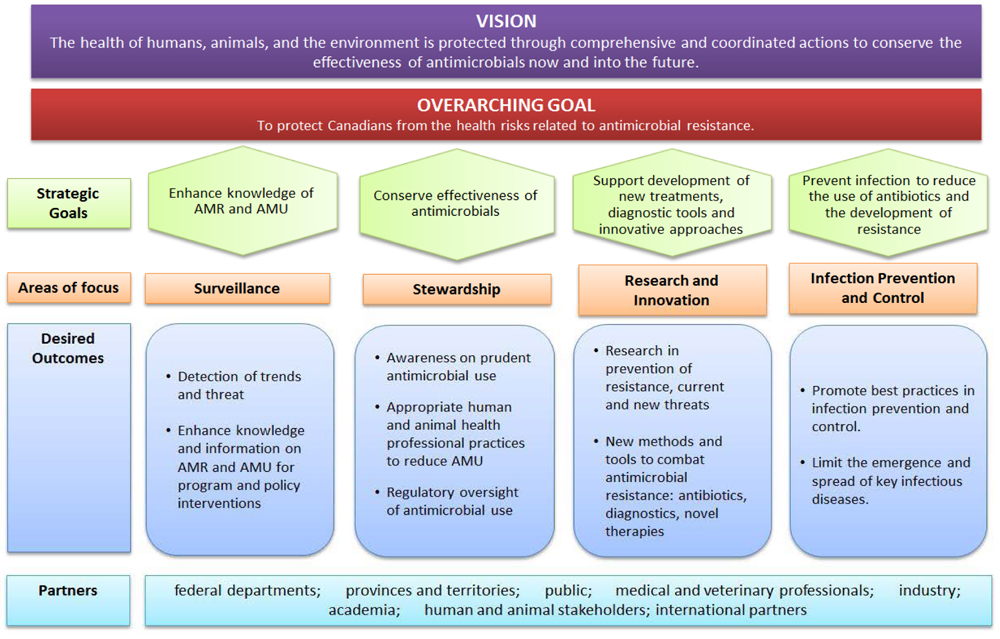
Appendix 1 - Text Equivalent
This figure demonstrates the vision overarching and strategic goals, areas of focus, and desired outcomes from the Pan-Canadian Framework for Action, along with its partners.
The vision of the Framework is:
- The health of humans, animals, and the environment is protected through comprehensive and coordinated actions to conserve the effectiveness of antimicrobials now and into the future.
The overarching goal is:
- To protect Canadians from the health risks related to antimicrobial resistance.
Under the overarching goal are the strategic goals, which are:
- Enhance knowledge of AMR and AMU;
- Conserve effectiveness of antimicrobials;
- Support development of new treatments, diagnostic tools and innovative approaches; and,
- Prevent infection to reduce the use of antibiotics and the development of resistance.
Under each strategic goal is an area of focus, which are, in order:
- Surveillance;
- Stewardship;
- Research and Innovation; and,
- Infection Prevention and Control.
Each area of focus has desired outcomes:
- Surveillance
- Detection of trends and threats
- Enhance knowledge and information on AMR and AMU for program and policy interventions
- Stewardship
- Awareness on prudent antimicrobial use
- Appropriate human and animal health professional practices to reduce AMU
- Regulatory oversight of antimicrobial use
- Research and Innovation
- Research in prevention of resistance, current and new threats
- New methods and tools to combat antimicrobial resistance: antibiotics, diagnostics, novel therapies
- Infection Prevention and Control
- Promote best practices in infection prevention and control
- Limit the emergence and spread of key infectious diseases.
Lastly are the partners:
- Federal departments;
- Provinces and territories;
- Public;
- Medical and veterinary professionals;
- Industry;
- Academia;
- Human and animal stakeholders; and,
- International partners.
Appendix 2 – Evaluation Description
Evaluation scope
The evaluation covered the period from 2013-14 to the end of summer 2018, and included all of PHAC AMR activities. It did not assess AMR activities carried out by other federal departments, nor provinces and territories.
The evaluation examined questions in relation to the objectives and priorities, role, and design, delivery, and coordination of AMR activities, as shown in the Table below.
| Core Issues | Evaluation Questions |
|---|---|
| Issue #1: Objectives and priorities |
|
| Issue #2: Role |
|
| Issue #3: Design and delivery of activities |
|
Data Collection and Analysis Methods
Evaluators collected and analyzed data from multiple sources. Data collection started in June 2018 and ended in December 2018. Data for the evaluation was collected using the following methods:
- Literature review – A search of literature including material from other countries and international organizations with respect to AMR activities was conducted. Documents including national Frameworks, Action Plans, progress reports and information on the Government of Canada’s response to the opioid crisis were also reviewed.
- Program document and file review – Approximately 430 documents, held by the divisions involved in AMR activities, were reviewed to obtain information regarding the coordination of AMR activities at PHAC.
- Financial data review – A review of financial data from 2013-14 to 2016-17 was conducted for AMR expenditures, including operations and management (O&M), salary and other expenditures.
- Key informant interviews – Interviews were conducted with 47 key informants who were, for the most part, in management, executive or expert positions:
- 21 interviews were internal to PHAC. These key informants were selected for their knowledge of, and experience with, PHAC AMR activities; and
- 26 interviews were with external partners/stakeholders including other federal government departments (n=9), provincial and territorial governments (n=7), and other partners/stakeholders (e.g., research and academia, non-for-profit organizations [n=10]). External key informants have been interacting with PHAC as part of the development of the federal or pan-Canadian strategies.
- Media review: News articles on AMR published since February 2017 by Canadian media were examined.
Data was analyzed by triangulating information gathered from the different methods listed above. The use of multiple lines of evidence and triangulation was intended to increase the reliability and credibility of the evaluation findings and conclusions.
Endnotes
- Endnote 1
-
Minister of Health. 2017. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. Retrieved from:https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-Framework-action.html
Return to footnote 1 referrer
- Endnote 2
-
O’Neil. 2016. Tackling Drug Resistant Infections Globally: Final Report and Recommendations (UK Review on Antimicrobial Resistance). Retrieved from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
Return to footnote 2 referrer
- Endnote 3
-
World Bank. 2017. Drug Resistant Infections A Threat to our Economic Future. Retrieved from: http://documents.worldbank.org/curated/en/323311493396993758/pdf/114679-REVISED-v2-Drug-Resistant-Infections-Final-Report.pdf
Return to footnote 3 referrer
- Endnote 4
-
Office of the Auditor General of Canada. 2015. Spring Reports of the Auditor General of Canada. Report 1—Antimicrobial Resistance. Retrieved from: http://www.oag-bvg.gc.ca/internet/English/parl_oag_201504_01_e_40347.html
Return to footnote 4 referrer
- Endnote 5
-
Report of the Standing Committee on Health. 2018. A study on the Status of Antimicrobial Resistance in Canada and Related Recommendations. Retrieved from:https://www.ourcommons.ca/DocumentViewer/en/42-1/HESA/report-16/
Return to footnote 5 referrer
- Endnote 6
-
World Health Organisation, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health. 2018. Global Database for Antimicrobial Resistance Country Self-Assessment. Retrieved from: https://amrcountryprogress.org/
Return to footnote 6 referrer
- Endnote 7
-
Public Health Agency of Canada. 2018. Evaluation of the Public Health Agency of Canada’s Food-borne and Water-borne Enteric Illness Activities 2012-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/report-evaluation-food-borne-water-borne-enteric-illness-activities-2012-2017.html. Public Health Agency of Canada. 2018. Evaluation of Healthcare-Associated Infection Activities at the Public Health Agency of Canada 2012-13 to 2016-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/healthcare-associated-infection-activities-2012-2017.html
Return to footnote 7 referrer
- Endnote 8
-
Minister of Health. 2017. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. Retrieved from: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-Framework-action.html
Return to footnote 8 referrer
- Endnote 9
-
Minister of Health. 2017. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. Retrieved from: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-Framework-action.html
Return to footnote 9 referrer
- Endnote 10
-
Public Health Agency of Canada. 2017. Canadian Antimicrobial Resistance Surveillance System 2017 Report. Retrieved from: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-antimicrobial-resistance-surveillance-system-2017-report-executive-summary.html
Return to footnote 10 referrer
- Endnote 11
-
Minister of Health. 2017. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. Retrieved from: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-Framework-action.html
Return to footnote 11 referrer
- Endnote 12
-
Minister of Health. 2017. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. Retrieved from: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-Framework-action.html
Return to footnote 12 referrer
- Endnote 13
-
O’Neil. 2016. Tackling Drug Resistant Infections Globally: Final Report and Recommendations (UK Review on Antimicrobial Resistance). Retrieved from: https://amr-review.org/sites/default/files/160518_Final%20paper_with%20cover.pdf
Return to footnote 13 referrer
- Endnote 14
-
World Bank. 2017. Drug Resistant Infections A Threat to our Economic Future. Retrieved from: http://documents.worldbank.org/curated/en/323311493396993758/pdf/114679-REVISED-v2-Drug-Resistant-Infections-Final-Report.pdf
Return to footnote 14 referrer
- Endnote 15
-
Conly. John M. Antimicrobial resistance programs in Canada 1995-2010: a critical evaluation. Antimicrobial Resistance and Infection Control, 2012 1:10. Retrieved November 28, 2018: https://aricjournal.biomedcentral.com/track/pdf/10.1186/2047-2994-1-10
Return to footnote 15 referrer
- Endnote 16
-
Office of the Auditor General of Canada. 2015. Spring Reports of the Auditor General of Canada. Report 1—Antimicrobial Resistance. Retrieved from: http://www.oag-bvg.gc.ca/internet/English/parl_oag_201504_01_e_40347.html
Return to footnote 16 referrer
- Endnote 17
-
Office of the Auditor General of Canada. 2015. Spring Reports of the Auditor General of Canada. Report 1—Antimicrobial Resistance. Retrieved from: http://www.oag-bvg.gc.ca/internet/English/parl_oag_201504_01_e_40347.html
Return to footnote 17 referrer
- Endnote 18
-
Report of the Standing Committee on Health. 2018. A study on the Status of Antimicrobial Resistance in Canada and Related Recommendations. Retrieved from: https://www.ourcommons.ca/DocumentViewer/en/42-1/HESA/report-16/
Return to footnote 18 referrer
- Endnote 19
-
Government of Canada. undated. Government response to the sixteenth report of the Standing Committee on Health: A Study on the Status of Antimicrobial Resistance in Canada and Related Recommendations. Retrieved from: https://www.ourcommons.ca/content/Committee/421/HESA/GovResponse/RP10003524/421_HESA_Rpt16_GR/421_HESA_Rpt16_GR-e.pdf
Return to footnote 19 referrer
- Endnote 20
-
Minister of Health. 2017. Tackling Antimicrobial Resistance and Antimicrobial Use: A Pan-Canadian Framework for Action. Retrieved from: https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/tackling-antimicrobial-resistance-use-pan-canadian-Framework-action.html
Return to footnote 20 referrer
- Endnote 21
-
Public Health Agency of Canada. 2018. Evaluation of Healthcare-Associated Infection Activities at the Public Health Agency of Canada 2012-13 to 2016-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/healthcare-associated-infection-activities-2012-2017.html
Return to footnote 21 referrer
- Endnote 22
-
Public Health Agency of Canada. 2018. Evaluation of the Public Health Agency of Canada’s Food-borne and Water-borne Enteric Illness Activities 2012-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/report-evaluation-food-borne-water-borne-enteric-illness-activities-2012-2017.html
Return to footnote 22 referrer
- Endnote 23
-
Department of Justice Canada. 2006. Public Health Agency of Canada Act. Retrieved from: https://laws-lois.justice.gc.ca/eng/acts/P-29.5/page-1.html#docCont
Return to footnote 23 referrer
- Endnote 24
-
Progress Report on the 2015 Federal Action Plan on Antimicrobial Resistance and Use. Retrieved from: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/progress-report-2015-federal-action-plan-antimicrobial-resistance-use.html
Return to footnote 24 referrer
- Endnote 25
-
Government of Canada. 2014. Antimicrobial Resistance and Use in Canada: A Federal Framework for Action. Retrieved from: https://www.canada.ca/content/dam/canada/health-canada/migration/healthy-canadians/alt/pdf/drugs-products-medicaments-produits/buying-using-achat-utilisation/antibiotic-resistance-antibiotique/antimicrobial-Framework-cadre-antimicrobiens-eng.pdf.
Return to footnote 25 referrer
- Endnote 26
-
Government of Canada Response to the recommendations from the Standing Committee on Health’s study on the Status of Antimicrobial Resistance in Canada. Retrieved from: https://www.ourcommons.ca/content/Committee/421/HESA/GovResponse/RP10003524/421_HESA_Rpt16_GR/421_HESA_Rpt16_GR-e.pdf
Return to footnote 26 referrer
- Endnote 27
-
Government of Canada. 2015. Federal Action Plan on Antimicrobial Resistance and Use in Canada – Building on the Federal Framework for Action. Retrieved from: http://healthycanadians.gc.ca/alt/pdf/publications/drugs-products-medicaments-produits/antibiotic-resistance-antibiotique/action-plan-daction-eng.pdf
Return to footnote 27 referrer
- Endnote 28
-
Office of the Auditor General of Canada. 2015. Spring Reports of the Auditor General of Canada. Report 1—Antimicrobial Resistance. Retrieved from:http://www.oag-bvg.gc.ca/internet/English/parl_oag_201504_01_e_40347.html
Return to footnote 28 referrer
- Endnote 29
-
2017–18 Departmental Plan: Public Health Agency of Canada. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/reports-plans-priorities/2017-2018-report-plans-priorities.html
Return to footnote 29 referrer
- Endnote 30
-
Public Health Agency of Canada. 2013. Strategic Horizon 2013-2018. Retrieved from: http://publications.gc.ca/collections/collection_2014/aspc-phac/HP55-1-2013-eng.pdf
Return to footnote 30 referrer
- Endnote 31
-
Public Health Agency of Canada. 2018. Evaluation of Healthcare-Associated Infection Activities at the Public Health Agency of Canada 2012-13 to 2016-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/healthcare-associated-infection-activities-2012-2017.html
Return to footnote 31 referrer
- Endnote 32
-
Public Health Agency of Canada. 2018. Evaluation of the Public Health Agency of Canada’s Food-borne and Water-borne Enteric Illness Activities 2012-17. Retrieved from:https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/report-evaluation-food-borne-water-borne-enteric-illness-activities-2012-2017.html
Return to footnote 32 referrer
- Endnote 33
-
Public Health Agency of Canada. 2018. Evaluation of the Public Health Agency of Canada’s Food-borne and Water-borne Enteric Illness Activities 2012-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/report-evaluation-food-borne-water-borne-enteric-illness-activities-2012-2017.html
Return to footnote 33 referrer
- Endnote 34
-
Public Health Agency of Canada. 2018. Evaluation of the Public Health Agency of Canada’s Food-borne and Water-borne Enteric Illness Activities 2012-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/report-evaluation-food-borne-water-borne-enteric-illness-activities-2012-2017.html. Public Health Agency of Canada. 2018. Evaluation of Healthcare-Associated Infection Activities at the Public Health Agency of Canada 2012-13 to 2016-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/healthcare-associated-infection-activities-2012-2017.html
Return to footnote 34 referrer
- Endnote 35
-
World Health Organisation, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health. 2018. Global Database for Antimicrobial Resistance Country Self-Assessment. Retrieved from: https://amrcountryprogress.org/
Return to footnote 35 referrer
- Endnote 36
-
Organisation for Economic Co-operation and Development (OECD). 2018. Stemming the Superbug Tide in Canada. Retrieved from: http://www.oecd.org/canada/Stemming-the-Superbug-Tide-in-Canada.pdf. based on data from World Health Organisation, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health. 2018. Global Database for Antimicrobial Resistance Country Self-Assessment. Retrieved from: https://amrcountryprogress.org/
Return to footnote 36 referrer
- Endnote 37
-
World Health Organisation, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health. 2018. Global Database for Antimicrobial Resistance Country Self-Assessment. Retrieved from: https://amrcountryprogress.org/
Return to footnote 37 referrer
- Endnote 38
-
Public Health Agency of Canada. 2018. Evaluation of Healthcare-Associated Infection Activities at the Public Health Agency of Canada 2012-13 to 2016-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/healthcare-associated-infection-activities-2012-2017.html
Return to footnote 38 referrer
- Endnote 39
-
Organisation for Economic Co-operation and Development (OECD). 2018. Stemming the Superbug Tide in Canada. Retrieved from: http://www.oecd.org/canada/Stemming-the-Superbug-Tide-in-Canada.pdf based on data from World Health Organisation, Food and Agriculture Organization of the United Nations and World Organisation for Animal Health. 2018. Global Database for Antimicrobial Resistance Country Self-Assessment. Retrieved from: https://amrcountryprogress.org/
Return to footnote 39 referrer
- Endnote 40
-
Public Health Agency of Canada. 2018. Evaluation of the Public Health Agency of Canada's Food-borne and Water-borne Enteric Illness Activities 2012-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/report-evaluation-food-borne-water-borne-enteric-illness-activities-2012-2017.html Public Health Agency of Canada. 2018. Evaluation of Healthcare-Associated Infection Activities at the Public Health Agency of Canada 2012-13 to 2016-17. Retrieved from: https://www.canada.ca/en/public-health/corporate/transparency/corporate-management-reporting/evaluation/healthcare-associated-infection-activities-2012-2017.html
Return to footnote 40 referrer
Footnotes
- Footnote a
-
With the exception of Budget 2015, which announced funding for the Canadian Institutes of Health Research (CIHR) to advance research on AMR.
Return to footnote a referrer
- Footnote b
-
The review covered articles published since February 2017. A total of 55 articles from 23 different Canadian news organizations were reviewed. Several of the articles reviewed were repeated by different news organizations.
Return to footnote b referrer
- Footnote c
-
The evaluation of the health care-associated infection activities recommended exploring options to improve program effectiveness and efficiency by focusing on improving: a) the timeliness of surveillance and guidance knowledge products and b) the coverage of CNISP surveillance data. The evaluation of the food-borne and water-borne enteric illness activities recommended to improve access to upstream surveillance information and ensure the content of upstream information products is adapted to the needs of stakeholders.
Return to footnote c referrer
- Footnote d
-
The Public Health Agency of Canada did not exist at that time, as it was created in 2004.
Return to footnote d referrer
- Footnote e
-
These surveillance systems include the Canadian Nosocomial Infection Surveillance Program (CNISP), the Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) and the Canadian Antimicrobial Resistance Surveillance System (CARSS).
Return to footnote e referrer
- Footnote f
-
Targeted completion is 2019.
Return to footnote f referrer
- Footnote g
-
It is important to acknowledge that both the Government of Canada and the provinces and territories share responsibility for this area.
Return to footnote g referrer
- Footnote h
-
Technical experts from Canada, the European Union, Norway, and the United States collaborate and share best practices to strengthen domestic and global efforts to improve appropriate therapeutic use of antimicrobial drugs in medical and veterinary communities, prevent health care- and community-associated drug-resistant infections, and develop strategies for improving the pipeline of new antimicrobial drugs.
Return to footnote h referrer
- Footnote i
-
The eleven departments and agencies involved are PHAC; Health Canada; the Canadian Food Inspection Agency; CIHR; Agriculture and Agri-Food Canada; Innovation, Science and Economic Development Canada; the National Research Council; Environment and Climate Change Canada; Global Affairs Canada; and Fisheries and Ocean Canada.
Return to footnote i referrer
- Footnote j
-
The review covered articles published since February 2017. A total of 55 articles from 23 different Canadian news organizations were reviewed. Several of the articles reviewed were published in several different news organizations.
Return to footnote j referrer
- Footnote k
-
The evaluation of the healthcare-associated infection activities recommended to explore options to improve program effectiveness and efficiency focusing on improving: a) the timeliness of surveillance and guidance knowledge products and b) the coverage of CNISP surveillance data. The evaluation of the food-borne and water-borne enteric illness activities recommended to improve access to upstream surveillance information and ensure the content of upstream information products is adapted to the needs of stakeholders.
Return to footnote k referrer