Chapter 2 of the Canadian Tuberculosis Standards: Transmission and pathogenesis of tuberculosis
On this page
- Authors and affiliations
- Key points
- Transmission
- Pathogenesis
- Disclosure statement
- Funding
- References
Authors and affiliations
Richard Long; Tuberculosis Program Evaluation and Research Unit, Department of Medicine, Faculty of Medicine and Dentistry, University of Alberta, Edmonton, Alberta, Canada
Maziar Divangahi; Meakins-Christie Laboratories, Research Institute of the McGill University Health Centre, Montréal, Québec, Canada
Kevin Schwartzman; McGill International Tuberculosis Centre, Montréal, Québec, Canada; Department of Medicine, McGill University, Montréal, Québec, Canada
Key points
- With few exceptions, infection with Mycobacterium tuberculosis (M. tuberculosis) is acquired by inhalation of small droplet nuclei (1-5 microns in diameter) that contain just a few mycobacteria and that are capable of reaching the alveoli.
- The probability of transmission increases with the following:
- Bacterial burden (smear positivity) in the source patient
- Cavitary or upper lung-zone disease on chest radiograph in the source patient
- Laryngeal disease in the source patient
- Amount and severity of cough in the source patient
- Duration of exposure of the contact
- Proximity of the contact to the source patient
- Crowding and poor room ventilation
- Delays in diagnosis and/or effective treatment of the source patient
- The most effective way to reduce transmission is to promptly diagnose and treat patients with active pulmonary tuberculosis (TB).
- Through innate immune mechanisms, alveolar macrophages eradicate the bacteria in some individuals; in others, the bacteria are able to replicate and establish TB infection. Bacterial factors and host genetic factors that promote or limit acquisition of infection are not well understood.
- Between TB infection and active symptomatic pulmonary tuberculosis are 2 recently described intermediate states: incipient TB, a state that is likely to progress to active disease but does not cause detectable abnormalities; and subclinical TB, a state of disease due to viable M. tuberculosis that does not cause clinical TB-related symptoms, but does cause other abnormalities that can be detected using existing radiologic or microbiologic assays.
1. Transmission
1.1 Introduction
M. tuberculosis is a bacterium that is communicable from one human to another mainly by the aerosol route and rarely by other means such as ingestion or percutaneous inoculation (e.g., through laboratory or hospital accident), or via solid organ or hematopoietic stem cell transplantation.Footnote 1 The reservoir for M. tuberculosis is humans. Bovine TB, which in the past was caused by ingestion of milk heavily infected by Mycobacterium bovis that then penetrated the mucosa of the oropharynx or the gastrointestinal tract, has been much reduced globally and almost completely eliminated in Canada as a result of the pasteurization of milk and tuberculin testing of cattle.
The droplets in aerosols have an extremely slow settling rate (0.5 mm per second or less), which permits their transport by air currents, duct systems or elevator shafts for significant distances from the source case. Large particles settle quickly and are either not inhaled by contacts or, if inhaled, are trapped in the mucus of the upper airway. Only the droplet nuclei in the size range 1 to 5 microns reach the terminal air spaces or alveoli; each is understood to contain only a few bacteria.Footnote 2Footnote 3
The likelihood of a transmission event will depend on the number of infectious droplet nuclei per volume of air (infectious particle density) and the length of time that the uninfected individual spends breathing that air.
1.2. Patient, pathogen and environmental determinants of transmission
Several patient, pathogen and environmental factors determine whether transmission occurs (see Table 1).
| Patient | Pathogen | Environment |
|---|---|---|
|
|
|
Abbreviations: CXR, chest radiograph. |
||
| Ref # | Year of survey | Location | Contacts | Number and % infected contacts by bacteriologic status of index case | General population % positive PPDFootnote a | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | Total no. | S+C+ | S-C+ | S-C- | |||||||
| N | %+ | N | %+ | N | %+ | ||||||
| 8 | 1949-56 | England | 0-14 | 545 | 262 | 63 | 126 | 21 | 157 | 18 | 13% |
| 9 | 1950-53 | England | 0-14 | 823 | 374 | 65 | 228 | 27 | 221 | 18 | 22% |
| 10 | 1963-64 | Holland | All ages | 858Footnote b | 391 | 20 | 467 | 1 | NA | NA | <1% |
| 11 | 1966-71 | Canada-Whites | 0-19 | 2406 | 1210 | 38 | 655 | 12 | 541 | 10 | 2% |
| Canada-Indigenous | 0-19 | 1168 | 592 | 45 | 377 | 31 | 199 | 27 | NA | ||
| 12 | 1967-69 | Rotterdam | 0-14 | 134 | 40 | 50 | 43 | 5 | 51 | 8 | 1% |
| 13 | 1969 | USA | All ages | 130 | 88 | 44 | 14 | 21 | 28 | 14 | NA |
| 14 | 1971-74 | USA | All ages | 761 | 504 | 46 | 257 | 28 | NA | NA | NA |
| 15 | 1975-77 | USA | All ages | 541 | 368 | 40 | 173 | 27 | NA | NA | NA |
Notes: Abbreviations: Footnotes:
|
|||||||||||
1.2.1. Patient (source) factors
With rare exceptions, transmission requires that a TB patient be able to generate an infectious aerosol. Therefore, transmission is predominantly from adolescent or adult patients with adult-type pulmonary TB — defined as upper lung-zone disease, with or without cavitation, but with no discernable adenopathy, on chest radiograph. Younger children can, on occasion, be infectious,Footnote 4 but as a general rule they have few bacilli in their lung lesions, often do not produce sputum and therefore are rarely in a position to transmit.Footnote 5 The ability of pulmonary TB patients to transmit can vary, depending upon a number of factors, listed in the following section. These factors affect contagiousness regardless of the patient's human immunodeficiency virus (HIV) serostatus, although HIV-coinfected TB patients are less infectious than HIV-uninfected TB patients when they have severe immunosuppression.Footnote 6
Sputum smear status
The most infectious pulmonary TB patients are those with smear-positive/culture- positive pulmonary TB, followed by those with smear- negative/culture-positive pulmonary TB, with the least infectious being those with smear-negative/culture- negative pulmonary TB.Footnote 7Footnote 8Footnote 9Footnote 10Footnote 11Footnote 12Footnote 13Footnote 14Footnote 15 (See Table 2 for a summary of the epidemiologic studies on the risk of infection in household [close] contacts grouped according to the bacteriologic status of the source patients.) Sputum that is smear-positive contains 5,000 or more bacteria per milliliter.Footnote 16 Patients with smear-positive bronchoalveolar lavage fluid are considered just as infectious as those with smear-positive sputum.Footnote 16 Those with smear-positive induced sputum are usually considered just as infectious as smear-positive spontaneously expectorated sputum, though there are currently no data that prove this assertion. Using molecular epidemiologic tools alone, the relative transmission rate of smear-negative compared with smear-positive patients has been determined to be 0.17-0.22 or roughly one-fifth the likelihood of transmission.Footnote 17Footnote 18Footnote 19 Using molecular epidemiologic tools combined with conventional epidemiologic, spatial temporal and genomic data, the relative transmission rate between these groups has been determined to be 0.10, or roughly one-tenth the likelihood of transmission.Footnote 20 Higher sputum smear grades are associated with the highest relative risk of transmission.Footnote 21 In addition to the greater infectiousness of smear-positive patients, the risk of disease after infection from a smear-positive case is greater than after a smear-negative case, presumably because of a higher risk of reinfection or a higher infecting dose (see the following section). A positive polymerase chain reaction (PCR) is also a risk factor for transmission.Footnote 21 In the future, semiquantitative results from the real-time PCR method, Xpert MTB/RIF assay, may replace smear microscopy as an indicator of infectiousness.Footnote 22Footnote 23
Disease type on plain chest radiograph
Pulmonary TB patients with cavitation on chest radiograph are considered to be more infectious than pulmonary TB patients without cavitation, after smear status has been taken into account.Footnote 24Footnote 25Footnote 26 Smear-positive pulmonary TB patients with lung cavitation have higher semiquantitative smear results than those without cavitation.Footnote 27Footnote 28 Smear-positive pulmonary TB patients with "typical" chest radiographic findings (upper lung-zone disease, with or without cavitation, and no discernable intrathoracic adenopathy) are more infectious than smear-positive pulmonary TB patients with "atypical" chest radiographic findings (all others).Footnote 29
Laryngeal disease
Patients with laryngeal TB are more infectious than those with pulmonary TB.Footnote 30 Most patients with laryngeal TB also have far advanced pulmonary disease.Footnote 31
Symptomatology
In general, normal breathing produces few infectious particles, a bout of coughing or 5 minutes of speaking in a normal tone produce many more, and a sneeze produces the most.Footnote 32Footnote 33 The likelihood that household contacts will be infected increases with the frequency of cough in the source case.Footnote 13Footnote 34 When the aerial infectivity of the droplets from smear-positive patients was evaluated by artificially atomizing sputum and exposing guinea pigs to a standard dose, there was marked variability in the infectivity of aerosolized sputum, perhaps explaining the extraordinary heterogeneity of infectiousness among patients with smear-positive pulmonary TB.Footnote 35Footnote 36Footnote 37 Thus, although patients may appear to have an equal number of bacteria in their sputum, the physical and chemical properties of their sputum, and/or cough characteristics or behavior may determine whether they produce a large or small number of droplet nuclei. Smoking can increase the risk of transmission, presumably via its effect on one or more of the aforementioned mechanisms.Footnote 38 Allergy or viral upper respiratory tract infection may also increase aerosol formation, but these are not well studied.Footnote 39
Delayed diagnosis
The number of contacts and the duration of exposure of each contact may increase as time to diagnosis increases. The longer the duration of symptoms in the source case, the greater the risk of transmission.Footnote 21
Treatment
Effective treatment, appropriate to drug susceptibility test results, rapidly reduces cough frequency and sputum bacterial countsFootnote 13Footnote 40 (see Chapter 5: Treatment of tuberculosis disease and Appendix B: De-isolation review and recommendations). Given the frequency of drug resistance, the determination that treatment is effective in reducing the infectiousness of a given patient should reflect objective clinical, radiographic and/or microbiologic improvement, and not simply time elapsed since treatment initiation.
1.2.2. Patient (recipient) factors
On the one hand, immunocompetent persons who have been infected in the past have considerable protection against reinfection, estimated to be about 80% (see the following section on Pathogenesis). Immunocompromised persons, on the other hand, may become reinfected despite having been infected and adequately treated in the past. Observational studies suggest that Bacille Calmette-Guerin (BCG) vaccination in infancy offers some protection against infection with M. tuberculosis as detected by an interferon-gamma release assay (IGRA).Footnote 41Footnote 42Footnote 43Footnote 44
1.2.3. Pathogen factors
Data are emerging to suggest that one or more virulence properties of M. tuberculosis may affect its ability to be transmitted.Footnote 45 For example, one strain may be better suited than another to overcoming the innate resistance of the host. Although drug-resistant strains have shown reduced virulence in animal models,Footnote 46 clinical evidence of their transmissibility is compellingFootnote 47Footnote 48Footnote 49Footnote 50 and for practical purposes they should be considered just as transmissible as drug-susceptible strains.
Beijing/W strains have been reported to be hypervirulent, but indices of transmission have been found to be no greater in patients with these strains than in those with other strains.Footnote 51
1.2.4. Environmental factors
With rare exceptions, outdoor exposures are unlikely to result in transmission.Footnote 52 Almost all transmission is understood to occur indoors. Factors in indoor transmission include the following items.
Air circulation and ventilation
Given a defined number of bacteria expelled into the air, the volume of air into which the bacteria are expelled determines the probability that a susceptible individual breathing that air will become infected. A high concentration of viable bacteria in the inhaled air of the contact is favored by small spaces, poor ventilation or recirculation of air, as well as little sunlight (ultraviolet rays). Ventilation dilutes the concentration of infectious droplet nuclei (see Chapter 14: Prevention and control of tuberculosis transmission in healthcare settings).
Proximity to the source patient
Proximity to the source patient is also a determinant of transmission. Related to this is overcrowding: if, as a result of there being many people in a room, an individual is forced into close proximity with an infectious case, their risk of infection is likely to increase. And, of course, the number of persons exposed is increased.
Duration of exposure
In general, hours of exposure to a patient with infectious TB is an important predictor of TB infection.Footnote 53 The duration of exposure associated with transmission is usually prolonged (days, months or even years), although reports have confirmed that, on rare occasions, close indoor exposures as short as a few minutes may be sufficient to infect a contact.Footnote 54
1.3. Measures to prevent transmission
The highest priority should be given to the early diagnosis, prompt isolation of and prompt provision of effective treatment to the source patient. The insidious development of symptoms in most patients with TB commonly results in a delay of weeks or months before the patient presents for diagnosis. At that point, any further delay caused by the physician, nurse or system allows unnecessary transmission to occur. Maintaining an appropriate awareness of TB among health care providers is thus critical to reducing transmission, by enabling them to initiate early diagnosis and treatment.Footnote 55 Administrative and engineering controls that aim to reduce exposure in healthcare and other congregate settings complement — but cannot replace — prompt diagnosis and appropriate therapy. Methods once thought to be important in preventing the transmission of TB, such as disposing of personal items such as cloths or bedding, sterilizing fomites, using caps and gowns, boiling dishes and washing walls, are unnecessary, because they have no bearing on airborne transmission. Most bacteria that lodge on inanimate objects die quickly through the action of drying, heat or sunlight.Footnote 2Footnote 3
2. Pathogenesis
The pathogenesis and transmission of TB are inter-related. M. tuberculosis is almost exclusively a human pathogen and how it interacts with the human host determines its survival. From the perspective of the bacterium, a successful host-pathogen interaction is one that results in ongoing pathogen transmission.Footnote 56
2.1. Evolution of initial infection and host response (classical description)
At the time of initial infection, the distribution of inhaled droplet nuclei in the lung is determined by the pattern of regional ventilation. It thus tends to follow the most direct path to the periphery and to favor the middle and lower lung zones, which receive most of the ventilation.Footnote 57 In immunocompetent hosts, it is theorized that alveolar macrophages ingest the M. tuberculosis organisms. Whether or not those macrophages destroy the bacteria depends on the degree to which they are nonspecifically activated, on host genetic factors and on resistance mechanisms in the bacteria.Footnote 58 If bacteria are successfully cleared, then immunological tests like the tuberculin skin test (TST) and IGRA will remain negative.
When innate macrophage microbicidal activity is inadequate to destroy the initial few bacteria of the droplet nucleus, they replicate logarithmically, doubling every 24 hours until the macrophage bursts to release its bacterial progeny.Footnote 59 New macrophages attracted to the site engulf these bacilli, and the cycle continues. The bacilli spread from the initial lesion via the lymphatic and/or circulatory systems to other parts of the body. This spread may, in fact, be critical to the induction of cellular immunity (see the following section). It is also during this stage of the infection that seeding of the lung apices occurs, which is so critical to the later development of adult-type (infectious) pulmonary TB.Footnote 59 After a period lasting from 3 to 8 weeks, the host develops specific immunity (cell-mediated immunity and delayed-type hypersensitivity) to the bacilli. This is when individuals first show positive results on the TST or IGRA. M. tuberculosis-specific lymphocytes then migrate to the site of infection, surrounding and activating macrophages localized to the site. As the cellular infiltration continues, the center of the cell mass, or granuloma, becomes caseous and necrotic. Later, radiographically demonstrable fibrocalcific residua of the initial infection can be identified, including a calcified granuloma in the lung alone or in combination with a calcified granulomatous focus in a draining lymph node, called a Ranke complex.Footnote 60 Infection and immune conversion are usually asymptomatic; any symptoms that do occur are self-limited. In a small proportion of those infected, erythema nodosum (a cutaneous immunologic response to an extracutaneous TB infection) or phlyctenular conjunctivitis (a hypersensitivity reaction) may develop.
2.2. Early disease progression (primary TB)
A proportion of those who are recently infected are unable to contain the infection, despite the stimulation of cell-mediated immunity, and there is progression to disease in a matter of months. Such early disease progression is a function of age and immunologic response; thus, disease is especially likely to occur in children 0-4 years of age and the immunocompromised.Footnote 61Footnote 62Footnote 63Footnote 64 Local progression in the lung, or lymphohematogenous spread resulting in disseminated (miliary) disease and/or central nervous system disease, may occur as early as 2-to-6 months after infection in infants and severely immunocompromised hosts.Footnote 61Footnote 64 Uncomplicated and asymptomatic lymph node disease (hilar or mediastinal lymphadenopathy without airway involvement) may also occur in the first 2-to-6 months of infection, although there is debate about whether this should be called active disease (see Chapter 9: Pediatric tuberculosis).Footnote 61Footnote 65
At 4-12 months after infection, early disease manifestations include complicated lymph node disease (airway compression, expansile caseating pneumonia, infiltration of adjacent anatomic structures), pleural disease (most commonly a lymphocyte-predominant exudative effusion) and peripheral lymphadenitis (usually in the cervical lymph nodes).Footnote 61 In immunocompetent children and adolescents, early disease is more likely to manifest as intrathoracic adenopathy and in adults as a unilateral pleural effusion. In severely immunocompromised people of any age (e.g., those with advanced HIV or AIDS), early disease may manifest as intrathoracic adenopathy.Footnote 66Footnote 67 Newly infected children who are 10 years of age or older (pubertal) or adolescents, may develop adult-type pulmonary disease (see below) or other types of extrapulmonary TB (for example bone and joint TB) within the first 8-24 months of infection.Footnote 61Footnote 68
For purposes of disease reporting, most but not all patients with a diagnosis of TB made within 18-24 months of infection should be considered to have "primary" disease. Those newly infected persons in whom TB does not develop in this time period have three possible outcomes: they may remain infected indefinitely and never develop disease, they may naturally clear their infection over time or they may progress to active TB disease at a later date, beyond the first 18-24 months. The concept of disease tolerance provides further insight into the aforementioned host-pathogen interactions.
2.3. Disease tolerance
It is now increasingly understood that host defense strategies against infectious diseases comprise both host resistance and disease tolerance. Host resistance is the ability of the host to prevent invasion or to eliminate the pathogen,Footnote 69 while disease tolerance is defined as limiting the tissue damage caused by the pathogen and/or the immune response. Since the discovery of M. tuberculosis more than a century ago, great progress has been made in defining mechanisms of host resistance to this respiratory pathogen. By contrast, our understanding of natural immunity in the 90 to 95% of infected individuals who remain disease-free is extremely limited.
The inability of both the innate and adaptive immune system to eliminate the bacteria forces the host to develop a cellular barrier, referred to as a granuloma, around infected cells. Granuloma formation appears to be the point at which host immunity "switches" from resistance to tolerance. Indeed, studies have elegantly demonstrated that intercellular communication is organized in the granuloma such that pro-inflammatory signaling occurs at the core to control M. tuberculosis growth, while anti-inflammatory signaling at the periphery acts to limit tissue damage.Footnote 70Footnote 71 Thus, the spatial compartmentalization of pro- and anti-inflammatory signaling is critical in granuloma function to prevent M. tuberculosis dissemination.
2.4. TB infection
In the classical concept of tuberculosis infection (TBI), M. tuberculosis bacteria are believed to survive for years at the site of the original infection in the lung and draining lymph nodes and in the small granulomas or solid caseous material of lympho-hematogenously seeded foci. Presumably, local conditions, an intact cell-mediated immunity or the presence of inhibitors result in conditions unfavorable to replication. Recent mapping of the complete genome sequence of the bacterium demonstrates that the organism has the potential to synthesize enzymes involved in anaerobic metabolism.Footnote 72 Although rapid death and autolysis occur after abrupt depletion of oxygen, the organism can shift into a state of dormancy if exposed to gradual reductions in oxygen tension.Footnote 73Footnote 74 Therefore, although M. tuberculosis thrives in an aerobic environment, it possesses the genetic and biochemical capability to survive anaerobically in experimentally oxygen-depleted media. Granuloma formation, with its oxygen-depleted environment, is a defining characteristic of TB. It is this stage of infection that is termed TBI and is usually identified by a positive TST or IGRA in the absence of active disease (see Chapter 4: Diagnosis of tuberculosis infection).
2.5. Reinfection
The elegant studies of R. G. Ferguson in the first half of the 20th century strongly suggest that it takes up to 18 months after the initial infection for cell-mediated immunity to fully mature.Footnote 75Footnote 76 During this period, each successive exposure and infection appears to carry its own inherent risk of disease; the cumulative risk thus becomes a function of the number of infections. This may explain why disease is so much more common in newly infected close contacts of smear-positive cases than it is in newly infected close contacts of smear-negative cases; the former has a greater likelihood than the latter of repeated exposure and reinfection.Footnote 11Footnote 77Footnote 78 More recent studies have also reported a higher risk of disease with greater intensity of exposure.Footnote 79Footnote 80
A meta-analysis of 23 cohorts from the pre-antibiotic era — largely health care workers — estimated that subsequent reinfection (after the first 18 months) of immunocompetent hosts carries a much lower risk of progression to TB disease, estimated to be 21% of the risk of an initial infection progressing to disease.Footnote 81 It remains unknown whether prior infection without development of overt disease is simply a marker for people who are less susceptible to disease development, or whether it truly induces immunity that is better able to prevent progression after reinfection.
In Canada, repeated exposure is rare in most settings, such that active TB generally reflects an initial infection — recent or remote — rather than reinfection.Footnote 82 However, there is clear evidence for the important role of reinfection causing TB morbidity in high-incidence, high-transmission settings. This has been most consistently documented among persons living with HIV who are not receiving anti-retroviral therapy, among whom high rates of recurrent TB disease have been observed long after microbiologic cure of an initial disease episode.Footnote 83Footnote 84 DNA fingerprinting has confirmed that many recurrent episodes relate to new infecting strains rather than to late relapse.
Strong supporting evidence also comes from clinical trials among persons living with HIV in high-transmission settings. These demonstrated high rates of TB disease after completion of preventive therapy, attributable to reinfection after treatment.Footnote 85
Reinfection can also lead to repeated illness in HIV-negative persons who were cured after an initial episode of TB disease, if they are in settings with extremely high TB incidence and transmission.Footnote 85 This may be relevant in a few, very specific Canadian settings (e.g., isolated communities with extensive outbreaks). Some persons in those settings with documented prior treatment for TB disease experienced recurrent disease that was shown by whole genome sequencing to reflect reinfection.Footnote 86Footnote 87 These observations can lead to consideration, on a case-by-case basis, of retreatment of TB infection after new exposure to highly infectious source patients (see Chapter 6: Tuberculosis preventive treatment in adults and Chapter 11: Tuberculosis contact investigation and outbreak management).
2.6. Reactivation TB
In Canada, most TB is understood to be "reactivation" TB (i.e., occurring in adolescents or adults). It usually presents as adult-type pulmonary disease (upper lung-zone fibrocavitary disease — previously referred to as postprimary TB — beginning in small foci that are the result of remote lympho-hematogenous spread), although it may also present as extrapulmonary TB. As mentioned earlier, adult-type pulmonary TB may on occasion be a manifestation of primary TB or a reinfection. In any population group, reactivation of TBI, leading to reactivation TB, is much more likely to occur in people who are immunocompromised.
Patients with adult-type pulmonary TB are much more likely to show lung cavitation (created when caseous material liquefies) that erodes into the bronchi.Footnote 88 Within the unique extracellular environment of cavities, host defenses are ineffectual, and bacteria multiply in large numbers. Because cavities are open to, and discharge their contents into, nearby bronchi, these same bacteria are directly communicable to the outside air when the patient coughs.Footnote 89 From the perspective of public health and the organism's ability to survive as a species, adult-type pulmonary TB is the most important form of TB disease.
Persons with a history of untreated or inadequately treated pulmonary TB or a "high-risk" lung scar (upper lung-zone fibronodular abnormality) on chest radiograph are thought to have a higher bacillary burden, even though "dormant," than those without such a history/radiograph and to be at increased risk of reactivation TB.Footnote 90Footnote 91 This scenario is commonly seen among immigrants referred to public health authorities for medical surveillance.
2.7. Extrapulmonary TB
The pathogenesis of extrapulmonary TB has been attributed to lympho-hematogenous spread at the time of initial primary lung infection, later dissemination from reactivated pulmonary TB or contiguous spread from adjacent organs. Abdominal disease may also result from direct infection through ingestion of infected sputum or contaminated milk (M. bovis). Extrapulmonary TB or combined pulmonary and extrapulmonary TB is more common in those who are severely immunocompromised. Among people coinfected with HIV and TB, the prevalence of extrapulmonary TB increases as the CD4 count decreases (see Chapter 7: Extra-pulmonary tuberculosis).Footnote 66Footnote 67
2.8. Evolution of initial infection and host response: Developing a more nuanced description
Recently, Behr et al. posit a more nuanced understanding of tuberculosis infection.Footnote 92 In an analysis of studies spanning 5 decades, they concluded that the majority of TB-immunoreactive individuals have cleared their infection while retaining immunologic memory of it.Footnote 93 As a result, such patients would not benefit from preventive therapy. Unfortunately, there is no currently available test to identify patients who still harbor viable M. tuberculosis and so would benefit from tuberculosis preventive treatment (see also Chapter 4: Diagnosis of tuberculosis infection and Chapter 6: Tuberculosis preventive treatment in adults).
Added to this conceptualization of infection is a more nuanced understanding of disease with 2 additional states of infection: incipient TB (an intermediate state that is likely to progress to active disease but does not cause detectable abnormalities) and a subclinical state of active TB due to viable M. tuberculosis that does not cause clinical TB-related symptoms, but causes other abnormalities that can be detected using radiologic and microbiologic assays.Footnote 94Footnote 95 These newly described states are conceivably determined by the host's immunological response and the virulence of the pathogen, with the capacity of the host to shift between states. In the future, biomarkers may permit the early diagnosis and treatment of these intermediate states. This more nuanced pathogenesis of infection and disease is summarized in Figure 1.
Figure 1 uses the following abbreviations: MTB, Mycobacterium tuberculosis; TST, tuberculin skin test; IGRA, interferon-gamma release assay.
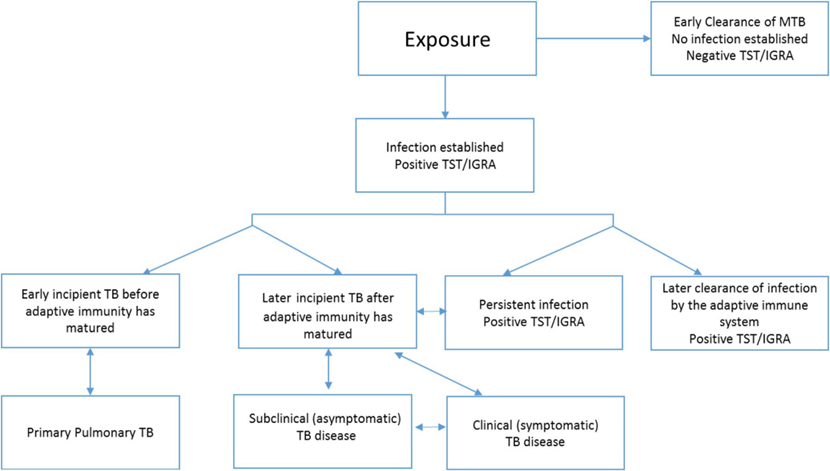
Figure 1: Text description
Following exposure to a person with infectious TB, a contact can either achieve early clearance of MTB in which case there is no infection established and the contact will have a negative result on a TST/IGRA test, or, infection will be established and the contact will have a positive TST/IGRA test. If infection is established, one of 4 disease pathways will follow:
- Early incipient TB before adaptive immunity has matured, which may lead to primary pulmonary TB. This may in turn revert back to early incipient TB.
- Later incipient TB after adaptive immunity has matured, which may lead to subclinical (asymptomatic) disease, persistent infection with positive TST/IGRA, or clinical symptomatic TB disease. These three stages are potentially bidirectional, and therefore an individual may move between the stages of having later incipient TB, subclinical asymptomatic TB disease and clinical symptomatic TB disease.
- Persistent infection with positive TST/IGRA, which may progress to later incipient TB after adaptive immunity has matured. If this occurs, the person with established infection could then progress down the previously described pathway.
- Later clearance of infection by the adaptive immune system with positive TST/IGRA.
2.9. Risk factors for progression from infection to disease
The risk of progression from TBI to active TB is largely dependent on the immune competency of the host. Age and sex appear to directly affect the immunologic response and the risk of disease: morbidity is greater among young children (<5 years of age), especially infants; young adults, especially females; and older adults, especially males. In high-burden countries, the population-attributable fraction of undernutrition for TB is 27% according to the WHO.Footnote 96Footnote 97 In Canada, inadequate diet has been associated with acquiring M. tuberculosis infection in an Inuit community.Footnote 97 In a study from Peru, biosocial household factors contributed to the risk of TB among contacts.Footnote 98 The seasonality of TB (with the highest incidence in spring and early summer) has been attributed to reduced sunlight and vitamin D deficiency during the winter months in some studies but not in others.Footnote 99Footnote 100Footnote 101 Ethnic differences have been offered as factors determining host immune response, with some support.Footnote 102 A growing body of evidence suggests that host genetic factors are important in determining susceptibility to TB as well.Footnote 103Footnote 104Footnote 105 Most important from a clinical perspective are the many medical conditions that are well-known to affect host immunologic response and increase the risk of progression from TBI to active TB disease. These are reviewed in detail in Chapter 4: Diagnosis of tuberculosis infection.
2.10. Future research: Trained immunity
Until recently, virtually all the efforts to develop a TB vaccine have been focused on conventional T cell-mediated immunity. However, there is no direct correlation between increased T-cell responses and protection against TB. Thus, it is not surprising that the results from clinical trials of T cell-based vaccine approaches have been disappointing.Footnote 106Footnote 107 These studies collectively challenge the current dogma that conventional T cells are predominantly engaged in host resistance against TB, but rather indicate the critical role of T cells in disease tolerance and containment of infection.Footnote 108
Contrary to focusing on the adaptive immune response, epidemiological data show that among close household contacts of highly infectious TB patients, up to 50% of exposed individuals do not convert their TST response from negative to positive, suggesting many of these individuals are intrinsically resistant to infection by M. tuberculosis,Footnote 109Footnote 110 These studies support the idea that perhaps the best window of opportunity to eradicate M. tuberculosis is during the early phase of infection, when the bacteria are still in the airway and have not initiated adaptive immunity and granuloma formation. These studies indicate that developing a vaccine targeting innate immunity may prevent TB.Footnote 111 However, designing such a vaccine will require a better understanding of innate immunity, especially the memory capacity of innate cells. Simple organisms such as plants and invertebrates, which only possess innate immune defenses, have demonstrated immunological memory (i.e., the primary exposure to a pathogen resulted in more efficient immunity to a subsequent challenge with the same pathogen).Footnote 112 Similarly, innate immune cells in vertebrates can generate a memory-like response (termed trained immunity), which is more efficient in preventing subsequent infection by a broad spectrum of pathogens and that is largely driven by epigenetic modifications.Footnote 113Footnote 114Footnote 115 Therefore, identifying the key determinants of trained immunity and their protective function will lead to new targets and vaccine strategies against M. tuberculosis.
Disclosure statement
The Canadian Thoracic Society (CTS) TB Standards editors and authors declared potential conflicts of interest at the time of appointment and these were updated throughout the process in accordance with the CTS Conflict of Interest Disclosure Policy. Individual member conflict of interest statements are posted on the CTS website.
Funding
The 8th edition Canadian Tuberculosis Standards are jointly funded by the CTS and the Public Health Agency of Canada, edited by the CTS and published by the CTS in collaboration with the Association of Medical Microbiology and Infectious Disease (AMMI) Canada. However, it is important to note that the clinical recommendations in the Standards are those of the CTS. The CTS TB Standards editors and authors are accountable to the CTS Respiratory Guidelines Committee (CRGC) and the CTS Board of Directors. The CTS TB Standards editors and authors are functionally and editorially independent from any funding sources and did not receive any direct funding from external sources.
The CTS receives unrestricted grants which are combined into a central operating account to facilitate the knowledge translation activities of the CTS Assemblies and its guideline and standards panels. No corporate funders played any role in the collection, review, analysis or interpretation of the scientific literature or in any decisions regarding the recommendations presented in this document.
References
- Footnote 1
-
Bumbacea D, Arend SM, Eyuboglu F, et al. The risk of tuberculosis in transplant candidates and recipients: a TBNET consensus statement. Eur Respir J. 2012;40(4):990–1013. doi:10.1183/09031936.00000712.
- Footnote 2
-
Allen E. Tuberculosis and other mycobacterial infections of the lung. In: Thurlbeck W, Churg A, eds. Pathology of the Lung 2ed. New York: Thieme Medical Publishers; 1995. 229–302.
- Footnote 3
-
Iseman M. How is tuberculosis transmitted? In: Iseman MD, ed. A Clinician's Guide to Tuberculosis. New York, NY: Lippincott Williams and Wilkins; 2000:51–62.
- Footnote 4
-
Curtis AB, Ridzon R, Vogel R, et al. Extensive transmission of Mycobacterium tuberculosis from a child. N Engl J Med. 1999;341(20):1491–1495. doi:10.1056/NEJM199911113412002.
- Footnote 5
-
Starke JR. Transmission of mycobacterium tuberculosis to and from children and adolescents. Seminars in Pediatric Infectious Diseases. 2001;12(2):115–123. doi:10.1053/spid.2001.22785.
- Footnote 6
-
Martinez L, Woldu H, Chen C, et al. Transmission Dynamics in Tuberculosis Patients With Human Immunodeficiency Virus: A Systematic Review and Meta-analysis of 32 Observational Studies. Clin Infect Dis. 2021;73(9):e3446–e3455. doi:10.1093/cid/ciaa1146.
- Footnote 7
-
Menzies D. Issues in the Management of Contacts of Patients with Active Pulmonary Tuberculosis. Can J Public Health. 1997;88(3): 197–201. doi:10.1007/BF03403887
- Footnote 8
-
Rouillon A, Perdrizet S, Parrot R. Transmission of tubercle bacilli: The effects of chemotherapy. Tubercle. 1976;57(4):275–299. doi:10.1016/S0041-3879(76)80006-2.
- Footnote 9
-
Van Zwanenberg D. The influence of the number of bacilli on the development of tuberculous disease in children. Am Rev Respir Dis. 1960;82:31–44. doi:10.1164/arrd.1960.82.1.31.
- Footnote 10
-
Shaw JB, Wynn-Williams N. Infectivity of pulmonary tuberculosis in relation to sputum status. Am Rev Tuberc. 1954;69(5):724–732. doi:10.1164/art.1954.69.5.724.
- Footnote 11
-
Grzybowski S, Barnett GD, Styblo K. Contacts of cases of active pulmonary tuberculosis. Bull Int Union Tuberc. 1975;50(1):90–106.
- Footnote 12
-
van Geuns HA, Meijer J, Styblo K. Results of contact examination in Rotterdam, 1967-1969. Bull Int Union Tuberc. 1975;50(1):107–121.
- Footnote 13
-
Loudon RG, Spohn SK. Cough frequency and infectivity in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1969;99(1):109–111. doi:10.1164/arrd.1969.99.1.109.
- Footnote 14
-
Rose CE, Zerbe GO, Lantz SO, Bailey WC. Establishing priority during investigation of tuberculosis contacts. Am Rev Respir Dis. 1979;119(4):603–609. doi:10.1164/arrd.1979.119.4.603.
- Footnote 15
-
Snider DE, Kelly GD, Cauthen GM, Thompson NJ, Kilburn JO. Infection and disease among contacts of tuberculosis cases with drug-resistant and drug-susceptible bacilli. Am Rev Respir Dis. 1985;132(1):125–132. doi:10.1164/arrd.1985.132.1.125.
- Footnote 16
-
Yeager H, Lacy J, Smith LR, LeMaistre CA. Quantitative studies of mycobacterial populations in sputum and saliva. Am Rev Respir Dis. 1967;95(6):998–1004. doi:10.1164/arrd.1967.95.6.998.
- Footnote 17
-
Behr MA, Warren SA, Salamon H, et al. Transmission of Mycobacterium tuberculosis from patients smear-negative for acid-fast bacilli. Lancet (London, England). 1999;353(9151):444–449. doi:10.1016/S0140-6736(98)03406-0.
- Footnote 18
-
Hernández-Garduño E, Cook V, Kunimoto D, Elwood RK, Black WA, FitzGerald JM. Transmission of tuberculosis from smear negative patients: a molecular epidemiology study. Thorax. 2004;59(4):286–290. doi:10.1136/thx.2003.011759.
- Footnote 19
-
Tostmann A, Kik SV, Kalisvaart NA, et al. Tuberculosis transmission by patients with smear-negative pulmonary tuberculosis in a large cohort in the Netherlands. Clin Infect Dis: An Official Publication of the Infectious Diseases Society of America. 2008;47(9):1135–1142. doi:10.1086/591974.
- Footnote 20
-
Asadi L, Croxen M, Heffernan C, et al. How Much Do Smear-Negative Patients Really Contribute to Tuberculosis Transmissions? Re-Examining an Old Question with New Tools. EClinical Medicine. 2022;43:101250.doi:10.1016/j.eclinm.2021.101250.
- Footnote 21
-
Lohmann EM, Koster BFPJ, Le Cessie S, Kamst-van Agterveld MP, van Soolingen D, Arend SM. Grading of a positive sputum smear and the risk of Mycobacterium tuberculosis transmission. Int J Tuberc Lung Dis. 2012;16(11):1477–1484. doi:10.5588/ijtld.12.0129.
- Footnote 22
-
Lee HS, Kee SJ, Shin JH, et al. Xpert MTB/RIF Assay as a Substitute for Smear Microscopy in an Intermediate-Burden Setting. Am J Respir Crit Care Med. 2019;199(6):784–794. doi:10.1164/rccm.201804-0654OC.
- Footnote 23
-
Van Deun A, Tahseen S, Affolabi D, et al. Sputum smear microscopy in the Xpert® MTB/RIF era. Int J Tuberc Lung Dis: The Official Journal of the International Union against Tuberculosis and Lung Disease. 2019;23(1):12–18. doi:10.5588/ijtld.18.0553.
- Footnote 24
-
Catanzaro A. Nosocomial tuberculosis. Am Rev Respir Dis. 1982;125(5):559–562. doi:10.1164/arrd.1982.125.5.559.
- Footnote 25
-
Bailey WC, Gerald LB, Kimerling ME, et al. Predictive model to identify positive tuberculosis skin test results during contact investigations. JAMA. 2002;287(8):996–1002. doi:10.1001/jama.287.8.996.
- Footnote 26
-
Marks SM, Taylor Z, Qualls NL, Shrestha-Kuwahara RJ, Wilce MA, Nguyen CH. Outcomes of contact investigations of infectious tuberculosis patients. Am J Respir Crit Care Med. 2000;162(6):2033–2038. doi:10.1164/ajrccm.162.6.2004022.
- Footnote 27
-
Palaci M, Dietze R, Hadad DJ, et al. Cavitary disease and quantitative sputum bacillary load in cases of pulmonary tuberculosis. J Clin Microbiol. 2007;45(12):4064–4066. doi:10.1128/JCM.01780-07.
- Footnote 28
-
Heffernan C, Barrie J, Doroshenko A, et al. Prompt recognition of infectious pulmonary tuberculosis is critical to achieving elimination goals: a retrospective cohort study. BMJ Open Resp Res. 2020;7(1):e000521. doi:10.1136/bmjresp-2019-000521.
- Footnote 29
-
Lau A, Barrie J, Winter C, Elamy AH, Tyrrell G, Long R. Chest Radiographic Patterns and the Transmission of Tuberculosis: Implications for Automated Systems. PLoS One. 2016;11(4):e0154032. doi:10.1371/journal.pone.0154032.
- Footnote 30
-
Muecke C, Isler M, Menzies D, Allard R, Tannenbaum TN, Brassard P. The use of environmental factors as adjuncts to traditional tuberculosis contact investigation. Int J Tuberc Lung Dis: The Official Journal of the International Union against Tuberculosis and Lung Disease. 2006;10(5):530–535.
- Footnote 31
-
Rieder HL. The infectiousness of laryngeal tuberculosis: appropriate public health action based on false premises [Counterpoint]. Int J Tuberc Lung Dis. 2009;13(1):4–5.
- Footnote 32
-
Loudon RG, Roberts RM. Singing and the Dissemination of Tuberculosis. Am Rev Respir Dis. 1968;98(2):297–300. doi:10.1164/arrd.1968.98.2.297.
- Footnote 33
-
Loudon RG, Roberts RM. Droplet expulsion from the respiratory tract. Am Rev Respir Dis. 1967;95(3):435–442. doi:10.1164/arrd.1967.95.3.435.
- Footnote 34
-
Turner RD, Birring SS, Darmalingam M, et al. Daily cough frequency in tuberculosis and association with household infection. Int j Tuberc Lung Dis. 2018;22(8):863–870. doi:10.5588/ijtld.17.0652.
- Footnote 35
-
Riley RL, Mills CC, Nyka W, et al. Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. 1959. Am J Epidemiol. 1995;142(1):3–14. doi:10.1093/oxfordjournals.aje.a117542.
- Footnote 36
-
Sultan L, Nyka W, Mills C, O'grady F, Wells W, Riley RL. Tuberculosis disseminators. A study of the variability of aerial infectivity of tuberculous patients. Am Rev Respir Dis. 1960;82:358–369. doi:10.1164/arrd.1960.82.3.358.
- Footnote 37
-
Riley RL, Mills CC, O'grady F, Sultan LU, Wittstadt F, Shivpuri DN. Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis. 1962;85:511–525. doi:10.1164/arrd.1962.85.4.511.
- Footnote 38
-
Lin H-H, Ezzati M, Murray M. Tobacco smoke, indoor air pollution and tuberculosis: a systematic review and meta-analysis. PLoS Med. 2007;4(1):e20. doi:10.1371/journal.pmed.0040020.
- Footnote 39
-
Stein RA. Super-spreaders in infectious diseases. Int J Infect Dis. 2011;15(8):e510-3. doi:10.1016/j.ijid.2010.06.020.
- Footnote 40
-
Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121(6):939–949. doi:10.1164/arrd.1980.121.6.939.
- Footnote 41
-
Soysal A, Millington KA, Bakir M, et al. Effect of BCG vaccination on risk of Mycobacterium tuberculosis infection in children with household tuberculosis contact: a prospective community-based study. Lancet (London, England). 2005;366(9495):1443–1451. doi:10.1016/S0140-6736(05)67534-4.
- Footnote 42
-
Eisenhut M, Paranjothy S, Abubakar I, et al. BCG vaccination reduces risk of infection with Mycobacterium tuberculosis as detected by gamma interferon release assay. Vaccine. 2009;27(44):6116–6120. doi:10.1016/j.vaccine.2009.08.031.
- Footnote 43
-
Eriksen J, Chow JY, Mellis V, et al. Protective effect of BCG vaccination in a nursery outbreak in 2009: time to reconsider the vaccination threshold? Thorax. 2010;65(12):1067–1071. doi:10.1136/thx.2010.140186.
- Footnote 44
-
Basu Roy R, Sotgiu G, Altet-Gómez N, et al. Identifying predictors of interferon-γ release assay results in pediatric latent tuberculosis: a protective role of bacillus Calmette-Guerin?: apTB-NET collaborative study. Am J Respir Crit Care Med. 2012;186(4):378–384. doi:10.1164/rccm.201201-0026OC.
- Footnote 45
-
Albanna AS, Reed MB, Kotar KV, et al. Reduced transmissibility of East African Indian strains of Mycobacterium tuberculosis. PloS One. 2011;6(9):e25075. doi:10.1371/journal.pone.0025075.
- Footnote 46
-
Middlebrook G, Cohn ML. Some observations on the pathogenicity of isoniazid-resistant variants of tubercle bacilli. Science (New York, NY). 1953;118(3063):297–299. doi:10.1126/science.118.3063.297.
- Footnote 47
-
Schaaf HS, Marais BJ, Hesseling AC, Gie RP, Beyers N, Donald PR. Childhood drug-resistant tuberculosis in the Western Cape Province of South Africa. Acta Paediatrica (Oslo, Norway: 1992). 2006;95(5):523–528. doi:10.1080/08035250600675741.
- Footnote 48
-
Moss AR, Alland D, Telzak E, et al. A city-wide outbreak of a multiple-drug-resistant strain of Mycobacterium tuberculosis in New York. Int J Tuberc Lung Dis: The Official Journal of the International Union against Tuberculosis and Lung Disease. 1997;1(2):115–121.
- Footnote 49
-
Drobniewski F, Balabanova Y, Nikolayevsky V, et al. Drug-resistant tuberculosis, clinical virulence, and the dominance of the Beijing strain family in Russia. JAMA. 2005;293(22):2726–2731. doi:10.1001/jama.293.22.2726.
- Footnote 50
-
Gandhi NR, Moll A, Sturm AW, et al. Extensively drug-resistant tuberculosis as a cause of death in patients co-infected with tuberculosis and HIV in a rural area of South Africa. Lancet (London, England). 2006;368(9547):1575–1580. doi:10.1016/S0140-6736(06)69573-1.
- Footnote 51
-
Langlois-Klassen D, Senthilselvan A, Chui L, et al. Transmission of Mycobacterium tuberculosis Beijing Strains, Alberta, Canada, 1991–2007. Emerg Infect Dis. 2013;19(5):701–711. doi:10.3201/eid1905.121578.
- Footnote 52
-
Rea E, Leung T. A cluster of tuberculosis cases linked to smoking: An under-recognized challenge for tuberculosis elimination. Can Commun Dis Rep. 2018;44(3-4):86–90. doi:10.14745/ccdr.v44i34a03.
- Footnote 53
-
Reichler MR, Khan A, Yuan Y, Tuberculosis Epidemiologic Studies Consortium Task Order 2 Team, et al. Duration of Exposure Among Close Contacts of Patients With Infectious Tuberculosis and Risk of Latent Tuberculosis Infection. Clin Infect Dis: An Official Publication of the Infectious Diseases Society of America. 2020;71(7):1627–1634. doi:10.1093/cid/ciz1044.
- Footnote 54
-
Templeton GL, Illing LA, Young L, Cave D, Stead WW, Bates JH. The risk for transmission of Mycobacterium tuberculosis at the bedside and during autopsy. Ann Intern Med. 1995;122(12):922–925. doi:10.7326/0003-4819-122-12-199506150-00005.
- Footnote 55
-
Heffernan C, Rowe BH, Long R. Engaging frontline providers: an important key to eliminating tuberculosis in Canada, and other high-income countries. Can J Public Health. 2021;112(5):872–876. doi:10.17269/s41997-021-00556-x.
- Footnote 56
-
Wells WF. Airborne Contagion and Air Hygiene an Ecological Study of Droplet Infections. Cambridge : Harvard University Press (for The Commonwealth Fund), Mass., U.S.A. London : Geoffrey Cumberlege, Oxford University Press; 1955.
- Footnote 57
-
Murray JF. Bill Dock and the location of pulmonary tuberculosis: how bed rest might have helped consumption. Am J Respir Crit Care Med. 2003;168(9):1029–1033. doi:10.1164/rccm.200307-1016OE.
- Footnote 58
-
Woolwine SC, Bishai WR. Overview of the pathogenesis of tuberculosis from a cellular and molecular perspective. In: Raviglione M, ed. Reichman and Hershfield's Tuberculosis. 3 ed. New York: Informal Healthcare; 2006. 101–116.
- Footnote 59
-
Behr MA, Waters WR. Is tuberculosis a lymphatic disease with a pulmonary portal? Lancet Infect Dis. 2014;14(3):250–255. doi:10.1016/S1473-3099(13)70253-6.
- Footnote 60
-
Collins J, Ej S. Chest Radiology. Lippincott, Williams and Wilkins; 2008.
- Footnote 61
-
Perez-Velez CM, Marais BJ. Tuberculosis in children. N Engl J Med. 2012;367(4):348–361. doi:10.1056/NEJMra1008049.
- Footnote 62
-
Martinez L, Cords O, Horsburgh CR, et al. The risk of tuberculosis in children after close exposure: a systematic review and individual-participant meta-analysis. Lancet (London, England). 2020;395(10228):973–984. doi:10.1016/S0140-6736(20)30166-5.
- Footnote 63
-
Gupta RK, Calderwood CJ, Yavlinsky A, et al. Discovery and validation of a personalized risk predictor for incident tuberculosis in low transmission settings. Nat Med. 2020;26(12):1941–1949. doi:10.1038/s41591-020-1076-0.
- Footnote 64
-
Daley CL, Small PM, Schecter GF, et al. An outbreak of tuberculosis with accelerated progression among persons infected with the human immunodeficiency virus. An analysis using restriction-fragment-length polymorphisms. N Engl J Med. 1992;326(4):231–235. doi:10.1056/NEJM199201233260404.
- Footnote 65
-
Marais BJ, Gie RP, Schaaf HS, et al. The natural history of childhood intra-thoracic tuberculosis: a critical review of literature from the pre-chemotherapy era. Int J Tuberc Lung Dis: The Official Journal of the International Union against Tuberculosis and Lung Disease. 2004;8(4):392–402.
- Footnote 66
-
Post FA, Wood R, Pillay GP. Pulmonary tuberculosis in HIV infection: radiographic appearance is related to CD4+T-lymphocyte count. Tuber Lung Dis: The Official Journal of the International Union against Tuberculosis and Lung Disease. 1995;76(6):518–521. doi:10.1016/0962-8479(95)90527-8.
- Footnote 67
-
Burman WJ, Jones BE. Clinical and radiographic features of HIV-related tuberculosis. Seminars in Respiratory Infections. 2003;18(4):263–271. doi:10.1053/S0882-0546(03)00072-0.
- Footnote 68
-
Stead WW, Kerby GR, Schlueter DP, Jordahl CW. The clinical spectrum of primary tuberculosis in adults. Confusion with reinfection in the pathogenesis of chronic tuberculosis. Ann Intern Med. 1968;68(4):731–745. doi:10.7326/0003-4819-68-4-731.
- Footnote 69
-
Ayres JS, Schneider DS. Tolerance of Infections. Annu Rev Immunol. 2012;30(1):271–294. doi:10.1146/annurev-immunol-020711-075030.
- Footnote 70
-
Cadena AM, Fortune SM, Flynn JL. Heterogeneity in tuberculosis. Nat Rev Immunol. Nov 2017;17(11):691–702. doi:10.1038/nri.2017.69.
- Footnote 71
-
Divangahi M. Are tolerance and training required to end TB? Nat Rev Immunol. 2018;18(11):661–663. doi:10.1038/s41577-018-0070-y.
- Footnote 72
-
Wilson RJ, Pillay DG, Sturm AW. Mycobacterium tuberculosis is not an obligate aerobe. J Infect. 1999;38(3):197–198. doi:10.1016/S0163-4453(99)90253-0.
- Footnote 73
-
Wayne LG, Diaz GA. Autolysis and Secondary Growth of Mycobacterium tuberculosis in Submerged Culture. J Bacteriol. 1967;93(4):1374–1381.
- Footnote 74
-
Wayne LG, Lin KY. Glyoxylate metabolism and adaptation of Mycobacterium tuberculosis to survival under anaerobic conditions. Infect Immun. 1982;37(3):1042–1049. doi:10.1128/iai.37.3.1042-1049.1982.
- Footnote 75
-
Ferguson RG. Studies in Tuberculosis. Toronto, ON: University of Toronto Press; 1955.
- Footnote 76
-
Long R, Lau A. Ferguson's groundbreaking studies influenced our understanding of tuberculosis reinfection. Where to next? Int J Tuberc Lung Dis. 2016;20(10):1285–1287. doi:10.5588/ijtld.16.0447.
- Footnote 77
-
Houk VH, Kent DC, Baker JH, Sorensen K, Hanzel GD. The Byrd study. In-depth analysis of a micro-outbreak of tuberculosis in a closed environment. Arch Environ Health. 1968;16(1):4–6. doi:10.1080/00039896.1968.10665007.
- Footnote 78
-
Houk VN, Baker JH, Sorensen K, Kent DC. The epidemiology of tuberculosis infection in a closed environment. Arch Environ Health. 1968;16(1):26–35. doi:10.1080/00039896.1968.10665011.
- Footnote 79
-
Lee RS, Proulx J-F, Menzies D, Behr MA. Progression to tuberculosis disease increases with multiple exposures. Eur Respir J. 2016;48(6):1682–1689. doi:10.1183/13993003.00893-2016.
- Footnote 80
-
Acuña-Villaorduña C, Jones-López EC, Fregona G, et al. Intensity of exposure to pulmonary tuberculosis determines risk of tuberculosis infection and disease. Eur Respir J. 2018;51(1):1701578. doi:10.1183/13993003.01578-2017.
- Footnote 81
-
Andrews JR, Noubary F, Walensky RP, Cerda R, Losina E, Horsburgh CR. Risk of progression to active tuberculosis following reinfection with Mycobacterium tuberculosis. Clin Infect Dis: An Official Publication of the Infectious Diseases Society of America. 2012;54(6):784–791. doi:10.1093/cid/cir951.
- Footnote 82
-
Jasmer RM, Bozeman L, Schwartzman K, et al. Recurrent tuberculosis in the United States and Canada: relapse or reinfection? Am J Respir Crit Care Med. 2004;170(12):1360–1366. doi:10.1164/rccm.200408-1081OC.
- Footnote 83
-
Narayanan S, Swaminathan S, Supply P, et al. Impact of HIV Infection on the Recurrence of Tuberculosis in South India. J Infect Dis. 2010;201(5):691–703. doi:10.1086/650528.
- Footnote 84
-
Crampin AC, Mwaungulu JN, Mwaungulu FD, et al. Recurrent TB: relapse or reinfection? The effect of HIV in a general population cohort in Malawi. AIDS (London, England). 2010;24(3):417–426. doi:10.1097/QAD.0b013e32832f51cf.
- Footnote 85
-
Den Boon S, Matteelli A, Ford N, Getahun H. Continuous isoniazid for the treatment of latent tuberculosis infection in people living with HIV: a systematic review and meta-analysis. AIDS (London, England). 2016;30(5):797–801. doi:10.1097/QAD.0000000000000985.
- Footnote 86
-
Lee RS, Radomski N, Proulx J-F, et al. Reemergence and amplification of tuberculosis in the Canadian arctic. J Infect Dis. 2015;211(12):1905–1914. doi:10.1093/infdis/jiv011.
- Footnote 87
-
Khan FA, Fox GJ, Lee RS, et al. Housing and tuberculosis in an Inuit village in northern Quebec: a case-control study. CMAJ Open. 2016;4(3):E496–E506. doi:10.9778/cmajo.20160049.
- Footnote 88
-
Dannenberg AM, Sugimoto M. Liquefaction of Caseous Foci in Tuberculosis. Am Rev Respir Dis. 1976;113(3):257–259. doi:10.1164/arrd.1976.113.3.257.
- Footnote 89
-
Long R, Maycher B, Dhar A, Manfreda J, Hershfield E, Anthonisen N. Pulmonary tuberculosis treated with directly observed therapy: serial changes in lung structure and function. Chest. 1998;113(4):933–943. doi:10.1378/chest.113.4.933.
- Footnote 90
-
Chan IHY, Kaushik N, Dobler CC. Post-migration follow-up of migrants identified to be at increased risk of developing tuberculosis at pre-migration screening: a systematic review and meta-analysis. Lancet Infect Dis. 2017;17(7):770–779. doi:10.1016/S1473-3099(17)30194-9.
- Footnote 91
-
Long R, Asadi L, Heffernan C, et al. Is there a fundamental flaw in Canada's post-arrival immigrant surveillance system for tuberculosis? PLoS One. 2019;14(3):e0212706. doi:10.1371/journal.pone.0212706.
- Footnote 92
-
Behr MA, Kaufmann E, Duffin J, Edelstein PH, Ramakrishnan L. Latent Tuberculosis: Two Centuries of Confusion. Am J Respir Crit Care Med. 2021;204(2):142–148. doi:10.1164/rccm.202011-4239PP.
- Footnote 93
-
Behr MA, Edelstein PH, Ramakrishnan L. Revisiting the timetable of tuberculosis. BMJ. 2018;362:k2738. doi:10.1136/bmj.k2738.
- Footnote 94
-
Drain PK, Bajema KL, Dowdy D, et al. Incipient and Subclinical Tuberculosis: a Clinical Review of Early Stages and Progression of Infection. Clin Microbiol Rev. 2018;31(4):1–24. doi:10.1128/CMR.00021-18.
- Footnote 95
-
Kendall EA, Shrestha S, Dowdy DW. The Epidemiological Importance of Subclinical Tuberculosis. A Critical Reappraisal. Am J Respir Crit Care Med. 2021;203(2):168–174. doi:10.1164/rccm.202006-2394PP.
- Footnote 96
-
Lönnroth K, Castro KG, Chakaya JM, et al. Tuberculosis control and elimination 2010-50: cure, care, and social development. Lancet (London, England). 2010;375(9728):1814–1829. doi:10.1016/S0140-6736(10)60483-7.
- Footnote 97
-
Fox GJ, Lee RS, Lucas M, et al. Inadequate Diet Is Associated with Acquiring Mycobacterium tuberculosis Infection in an Inuit Community. A Case-Control Study. Ann Am Thorac Soc.2015;12(8):1153–1162. doi:10.1513/AnnalsATS.201503-156OC.
- Footnote 98
-
Saunders MJ, Wingfield T, Datta S, et al. A household-level score to predict the risk of tuberculosis among contacts of patients with tuberculosis: a derivation and external validation prospective cohort study. Lancet Infect Dis. 2020;20(1):110–122. doi:10.1016/S1473-3099(19)30423-2.
- Footnote 99
-
Martineau AR, Nhamoyebonde S, Oni T, et al. Reciprocal seasonal variation in vitamin D status and tuberculosis notifications in Cape Town, South Africa. PNAS. 2011;108(47):19013–19017. doi:10.1073/pnas.1111825108.
- Footnote 100
-
Fares A. Seasonality of tuberculosis. J Glob Infect Dis. 2011;3(1):46–55. doi:10.4103/0974-777X.77296.
- Footnote 101
-
Willis MD, Winston CA, Heilig CM, Cain KP, Walter ND, Mac Kenzie WR. Seasonality of tuberculosis in the United States, 1993-2008. Clin Infect Dis: An Official Publication of the Infectious Diseases Society of America. 2012;54(11):1553–1560. doi:10.1093/cid/cis235.
- Footnote 102
-
Stead WW, Senner JW, Reddick WT, Lofgren JP. Racial differences in susceptibility to infection by Mycobacterium tuberculosis. N Engl J Med. 1990;322(7):422–427. doi:10.1056/NEJM199002153220702.
- Footnote 103
-
Greenwood CM, Fujiwara TM, Boothroyd LJ, et al. Linkage of tuberculosis to chromosome 2q35 loci, including NRAMP1, in a large aboriginal Canadian family. Am J Hum Genet. 2000;67(2):405–416. doi:10.1086/303012.
- Footnote 104
-
Bellamy R. Susceptibility to mycobacterial infections: the importance of host genetics. Genes & Immunity. 2003;4(1):4–11. doi:10.1038/sj.gene.6363915.
- Footnote 105
-
Pan H, Yan B-S, Rojas M, et al. Ipr1 gene mediates innate immunity to tuberculosis. Nature. 2005;434(7034):767–772. doi:10.1038/nature03419.
- Footnote 106
-
Tameris MD, Hatherill M, Landry BS, et al. Safety and efficacy of MVA85A, a new tuberculosis vaccine, in infants previously vaccinated with BCG: a randomised, placebo-controlled phase 2b trial. Lancet (London, England). 2013;381(9871):1021–1028. doi:10.1016/S0140-6736(13)60177-4.
- Footnote 107
-
Nemes E, Geldenhuys H, Rozot V, et al. Prevention of M. tuberculosis Infection with H4:IC31 Vaccine or BCG Revaccination. N Engl J Med. 2018;379(2):138–149. doi:10.1056/NEJMoa1714021.
- Footnote 108
-
Divangahi M, Khan N, Kaufmann E. Beyond Killing Mycobacterium tuberculosis: Disease Tolerance. Front Immunol. 2018;9:2976. doi:10.3389/fimmu.2018.02976.
- Footnote 109
-
Cobat A, Gallant CJ, Simkin L, et al. Two loci control tuberculin skin test reactivity in an area hyperendemic for tuberculosis. J Exp Med. 2009;206(12):2583–2591. doi:10.1084/jem.20090892.
- Footnote 110
-
Morrison J, Pai M, Hopewell PC. Tuberculosis and latent tuberculosis infection in close contacts of people with pulmonary tuberculosis in low-income and middle-income countries: a systematic review and meta-analysis. Lancet Infect Dis. 2008;8(6):359–368. doi:10.1016/S1473-3099(08)70071-9.
- Footnote 111
-
Divangahi M, Behr MA. Cracking the Vaccine Code in Tuberculosis. Am J Respir Crit Care Med. 2018;197(4):427–432. doi:10.1164/rccm.201707-1489PP.
- Footnote 112
-
Netea MG, Dominguez-Andres J, Barreiro LB, et al. Defining trained immunity and its role in health and disease. Nat Rev Immunol. 2020;20(6):375–388. doi:10.1038/s41577-020-0285-6.
- Footnote 113
-
Divangahi M, Aaby P, Khader SA, et al. Trained immunity, tolerance, priming and differentiation: distinct immunological processes. Nat Immunol. 2021;22(1):2–6. doi:10.1038/s41590-020-00845-6.
- Footnote 114
-
Kaufmann E, Sanz J, Dunn JL, et al. BCG Educates Hematopoietic Stem Cells to Generate Protective Innate Immunity against Tuberculosis. Cell. 2018;172(1-2):176–190.e19. doi:10.1016/j.cell.2017.12.031.
- Footnote 115
-
Khan N, Downey J, Sanz J, et al. Tuberculosis Reprograms Hematopoietic Stem Cells to Limit Myelopoiesis and Impair Trained Immunity. Cell. 2020;183(3):752–770.e22. doi:10.1016/j.cell.2020.09.062.