Federal/Provincial/Territorial Public Health Response Plan for Biological Events
October 31, 2017
Table of Contents
- Administration and Amendments
- Executive Summary
- 1. Introduction
- 2. Context for the Plan
- 3. Concept of Operations (CONOPS)
- 4. F/P/T Governance
- Appendix A – Glossary of Terms and List of Acronyms
- Appendix B – Plan Development: Guiding Principles
- Appendix C – Main F/P/T Roles and Responsibilities
- Appendix D – Special Advisory Committee (SAC) Roles and Responsibilities under the F/P/T Public Health Response Plan
- Appendix E – F/P/T SAC Secretariat Roles and Responsibilities under the F/P/T Public Health Response Plan
- Appendix F – Technical Advisory Committee (TAC) Terms of Reference
- Appendix G – Logistics Advisory Committee (LAC) Terms of Reference
- Appendix H – Anticipated Products and Pathways for a Coordinated F/P/T Response
- Appendix I – Existing Committees, Working Groups and other Expert Resources
- Appendix J Sample F/P/T Business Cycle
- Appendix K – Task Groups: Generic Terms of Reference
- Appendix L –Relationship of the F/P/T Public Health Response Plan to other F/P/T Coordinating Instruments
- Appendix M – Task Group Members
Administration and Amendments
This document was prepared for the Federal/Provincial/Territorial (F/P/T) Public Health Network Council (PHNC) as an overarching governance framework to guide F/P/T public health responses to biological events. It was developed by an expert task groupFootnote 1 comprised of experts in public health and emergency management, as identified by members of the Public Health Infrastructure Steering Committee (PHI-SC) and the Communicable and Infectious Disease Steering Committee (CID-SC). It was approved by PHN on October 17, 2017.
The Public Health Agency of Canada (PHAC), Centre for Emergency Preparedness and Response (CEPR) maintains the Federal, Provincial, Territorial Public Health Response Plan for Biological Events as an evergreen document on behalf of the PHNC.
The need to update the plan will be reviewed every three years at a minimum by PHI-SC and any changes will be tracked and noted as amendments in the plan. In addition, the need for revision will also be guided by after action reviews following the response to a real or simulated events requiring implementation of this plan, in whole or in part. The revision process will be coordinated on behalf of PHNC by the PHI-SC in consultation with CID-SC and led by CEPR. A time-limited joint task group may be established to conduct this work which may include recommendations for the development of new event-specific Annexes as required, to further support implementation of this plan.
Minor amendments will be approved by PHI-SC and CID-SC. Major revision, significantly altering the governance structure may require review and approval by PHNC.
Inquiries or comments on the Federal, Provincial/Territorial Public Health Response Plan for Biological Events should be directed to:
Director
Office of Situational Awareness and Operations
Centre for Emergency Preparedness and Response
Public Health Agency of Canada
100 Colonnade Road
A.L. 6201A
Ottawa, ON K1A 0K9
Email: HPOC_COPS@phac-aspc.gc.ca
Note to Readers
Henceforth, first occurrences in the text of terms that are listed in the Glossary are formatted in bold. Titles of plans, supporting documents and response levels are formatted in italics.
Executive Summary
This plan has been developed as a response plan for the Federal/Provincial/Territorial (F/P/T) health sector in order to facilitate formal coordination of F/P/T responses to public health events that are biological in nature and of a severity, scope or significance to require a high level F/P/T response. Informed by lessons learned from past F/P/T public health responses and best practices of current F/P/T structures (i.e., the Public Health Network structure and Special Advisory Committees), this plan focuses on the implementation of F/P/T responses led by senior-level public health decision-makers at the federal, provincial and territorial level in order to facilitate an efficient, timely, evidence-informed and consistent approach across jurisdictions to event-specific response activities. Improving effective engagement amongst public health, health care delivery and health emergency management authorities during a coordinated F/P/T response is a key objective of this plan. It is intended to serve as an F/P/T resource for F/P/T public health and emergency management authorities; specifically those who are involved in public health response preparedness and implementation. In order to further support coordination of public health events at a national level, this plan aims to build on the strengths of existing F/P/T tools and mechanisms while providing a single, overarching user-friendly response plan that is scalable and flexible enough to be utilized in full or in part for a range of F/P/T public health responses.
The concept of operations of the plan indicates how notification of public health events that potentially require a coordinated F/P/T response should be made to the Public Health Agency of Canada (PHAC), and how response needs are assessed to determine the appropriate level of F/P/T response coordination required. Four response levels that range from routine to emergency response are included to facilitate scaling of response activities as needed. The plan includes the details of a governance structure intended to be activated for those events in which a coordinated F/P/T response (i.e., led by senior-level decision makers) is deemed necessary and/or beneficial. The governance structure aims to: streamline response processes to a public health event; facilitate clarity on roles, responsibilities and approval processes; facilitate a high degree of situational awareness; and centralize risk management and task delegation. It incorporates three main streams: a Technical stream, a Logistics stream and a Communications stream. These streams are led by advisory committees/working groups and have been included in order to facilitate clarity regarding roles for issue management, response support, product development (e.g., recommendations, guidance, protocols), policy review and approval processes. "Cross stream" support and coordination will be essential to an efficient, informed and transparent response and therefore mechanisms for achieving this are also included.
Coordinated F/P/T responses will be conducted with each activated committee/group in the governance structure fulfilling the roles and responsibilities and decision-making processes as described in Section 4 of this plan and according to their respective terms of reference (included in corresponding appendices). Specifically, the Special Advisory Committee (SAC) will be the main approval/decision-making body for the duration of a coordinated F/P/T response under this plan, with governance structure products going to the Conference of Deputy Ministers of Health (CDMH) as required.
Public health emergencies involving multiple jurisdictions in Canada are relatively rare events. This plan is not exclusively an emergency response plan and therefore is expected to also be utilized for events not meeting the threshold of a public health emergency (i.e., for events requiring or that would benefit from enhanced F/P/T coordination); thus facilitating familiarity and opportunities to modify and improve this plan based on response experience.
This document is not intended to replace existing F/P/T health sector arrangements but rather is intended to complement and interact with the existing suite of plans and protocols currently in use by the health sector by providing an overarching governance framework with which the existing protocols will interact and/or align. Changes to those existing plans and protocols will be made following approval of this plan in order to clarify these linkages.
1. Introduction
Preface/Background
This document is a response plan for the Federal/Provincial/Territorial (F/P/T) health sector in order to facilitate formal coordination of F/P/T responses to public health events that are biological in nature. It is not intended to replace existing F/P/T health sector arrangements but rather is intended to complement and when applicable, be used in conjunction with the existing suite of plans and protocols currently in use by the health sector by providing an overarching governance framework that can be used to respond to a spectrum of public health events caused by biological agents. It is also expected that this plan will serve as the governance framework under which future and existing hazard-specific F/P/T health sector plans, protocols and guidance will be situated.
As required by legislation, all jurisdictions in Canada have plans that set out the steps to be taken in the event of an emergency or disaster. These plans identify linkages and channels of communication to other ministries, programs and agencies of the Government and contribute to a coordinated, system-wide approach to emergency management that can be applied if necessary in a whole of government response. In addition, the F/P/T health sector has in place well established hazard-specific tools that are routinely used to effectively plan for and manage public health events, including the Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector (CPIP) and Food-borne Illness Outbreak Response Protocol (FIORP) and others. In order to further support coordination of public health events at a national level, this plan aims to build on the strengths of these existing tools and mechanisms while providing a single, overarching user-friendly response plan and F/P/T governance structure that is scalable and flexible enough to be utilized in full or in part for a range of F/P/T public health responses to biological events. For a further description of the interface and relationship between this plan and other key plans at the F/P/T level, see Appendix L: Relationship of the F/P/T Public Health Response Plan to other F/P/T Coordinating Instruments.
Aim
The aim of this plan is to outline how F/P/T responses to public health events caused by biological agents will be conducted and coordinated. This response plan will provide clarity with respect to: considerations for F/P/T responses; response objectives and corresponding activities; governance mechanisms that support F/P/T response efforts and deliverables; and roles, responsibilities and accountabilities within those governance mechanisms.
This plan is intended to serve as a resource for F/P/T public health and emergency management authorities; specifically those that are involved in public health response preparedness and implementation. Those working in particular public health program areas can focus on hazard-specific preparedness activities (e.g., the CPIP) and response protocols (e.g., FIORP), knowing that if transition to a high level coordinated F/P/T response is needed this plan exists and would be used to provide that function.
Scope
The focus of this plan is on public health events that are biological in nature and require a public health response at both the P/T and federal levels. While the focus of this plan is public health, it should be emphasized that any public health event will be health system-wide and will require coordination between public health and health care delivery and other sectors. Details regarding response coordination with the respective health care systems of the provinces and territories are outside the scope of this plan.
Further, as a response plan, issues regarding mitigation, preparedness and recovery are also beyond the scope of this document. Activities relating to mitigation, preparedness are dealt with through the activities of existing committees and task groups within the Public Health Network that are actively engaged in health emergency management. However, should there be a need for enhanced F/P/T coordination in the recovery of a public health event (e.g., continued psychosocial response to a bioterrorism event or pandemic), consideration may be given to leveraging the governance components of this plan to support recovery activities.
Biological agents are the cause of biological events and include bacteria, viruses, fungi, other microorganisms and their associated toxins. They have the ability to adversely affect human health in a variety of ways, ranging from relatively mild, allergic reactions to serious medical conditions and death. These organisms are widespread in the natural environment; they are found in water, soil, plants, and animals.
Biological events can be naturally occurring disease outbreaks at national and international levels, accidental exposure to pathogens (disease causing agent) in the context of biomedical diagnostics and research, significant shortages of drugs and biologics or intentional use of pathogens or biotoxin (poisonous substance produced by a living organism) against humans, plants, or animals for harmful purposes. The scope of this plan is intended for the situations where the principle issue is human health and includes biological agents found in the environment, or diagnosed in animals, that have the potential for transmission to humans (zoonosis).
The following are examples of the range of scenarios where this plan may be applicable. It may be applied for a biological public health event in a single P/T with the potential for spread/involvement to another P/T, to multijurisdictional outbreaks that require coordination with federal and P/T partners (e.g., large and complex foodborne outbreak requiring significant coordination at a senior level beyond the scope of the FIORP), to shortages of medical countermeasures (e.g., vaccine shortage), to public health emergencies in Canada (e.g., H1N1 pandemic influenza). The management of large-scale public health events with international implications in which federal coordination is necessary (e.g., ebola, zika) are also within the scope of this plan. Biological events that are restricted to animal, plant, or food health or safety are outside the scope of this plan.
It is recognized that public health events that are intentional in nature (e.g., bioterrorism) will require a law enforcement/security response in addition to a public health response. While the elements of the public health response to an intentional event may not significantly differ from those described in this plan (and therefore this plan may be utilized for the public health consequence management), the linkages to the law enforcement/security response are not within the scope of this plan. It is expected however, that the governance structure for a biological event where the intent is malicious, would be similar to that as described in this plan.
Following endorsement, training and use (i.e., proof of concept), this plan will become a model for development of an all hazard F/P/T governance for the health sector that can be applied if required for F/P/T coordinated responses to other events such as natural disasters or Chemical, Biological, Radiological/Nuclear, Explosive (CBRNE) events.
Objectives
The specific objectives of this plan include:
- defining a flexible F/P/T governance mechanism that can be used consistently for a coordinated response to all biological public health events that would benefit from high level F/P/T collaboration;
- identifying escalation considerations and response levels for a scalable response, and
- improving effective engagement amongst public health, health care delivery and health emergency management authorities during a coordinated F/P/T response.
Through the achievement of these objectives it is expected that, at the time of a response, notification processes and inter-jurisdictional information-sharing will be enhanced; public and professional communication expectations will be addressed; and advanced planning and decision-making between and amongst multiple jurisdictions will be facilitated.
2. Context for the Plan
Risk Environment
This plan has been developed at a time when public health risks have been relatively well defined and assessed, and risk mitigation activities are ongoing. However, it is recognized that many risk drivers are so broad and expansive that even coordinated public health interventions are unlikely to mitigate those risks. Some of the risk drivers associated with emerging infectious disease are: globalization of people and animals, climate change, changes in land use, movement/displacement of people, population density and urbanization, and changes in farming practices and antibiotic use. Many of these risks are manifesting outside of Canada but have a real or potential impact on the health of the public in Canada. It is in this risk environment that health authorities in Canada must be prepared to respond to biological hazards.
Previous and ongoing public health responses have addressed everything from epidemics of novel respiratory pathogens (e.g., Severe Acute Respiratory Syndrome - SARS) and pandemics (e.g., H1N1 influenza), to emerging infections (e.g., west nile virus, lyme disease) and international or travel-related public health threats (e.g., ebola, zika).
Throughout 2013-14, the Council of Chief Medical Officers of Health (CCMOH) was involved in the response to a number of significant public health events including infectious disease: (H7N9; MERS-CoV; H5N1; H1N1, seasonal influenza), food-borne illness: (E coli O157:J7 (XL Foods, Inc.), and vaccine supply issues: (2014 influenza vaccine shortage). The CCMOH subsequently identified inconsistencies in the management of these events and requested the development of a plan for response to public health events of national concern to ensure consistency, timeliness and scalability of F/P/T response activities.
It is within the context of experiences from past public health events that the guiding principles used for the development of this plan and anticipated response activities associated with this plan were derived. Specifically, lessons learned from an intensive review of the governance structure utilized during the F/P/T response to the H1N1 influenza pandemic in 2008-9 identified the need for a nimble, flexible governance that can be applied consistently, in whole or in part, to a range of public health scenarios and the need to clarify roles and responsibilities as well as decision-making and approval processes at various levels.Footnote 2
Guiding Principles
The guiding principles used for the development of this plan and anticipated response activities were based on lessons learned or identified from previous public health responses and best practices. They include:
- Efficiency
- Timeliness
- Transparency
- Commitment
- Engagement
- Representativeness
- Health Equity
- Flexibility
- Effectiveness, and
- Ethical and Evidence-Informed Decision-Making.
More details regarding these principles are located in Appendix B: Plan Development Guiding Principles.
The contents of this plan and in particular the governance structure and concept of operations, aim to facilitate the following of these principles in order to appropriately operationalize best practices (such as the activation of the Special Advisory Committee) and other learnings from previous public health responses.
During a response there will be a need for a consistent, coordinated approach that is both scalable and flexible. Throughout the response it may be necessary to modify guidance, protocols, or recommendations in order to adapt the response to the evolving circumstances. Ideally, any significant changes will be made in conjunction with an articulated change in response objectives (e.g., preventing introduction into Canada vs. preventing spread of illness within Canada). It is recognized that at any one point during the response the objectives of the response may vary from jurisdiction to jurisdiction within Canada depending on the local impact of the public health event and risk assessments; however, F/P/T governments should aim to work collaboratively to facilitate a common set of F/P/T public health response objectives to every extent possible, recognizing roles and responsibilities differ, the impact of the event will likely be different in each jurisdiction and F/P/T health care systems function differently.
Public Health and Emergency Management Roles
Public Health authorities conduct and manage responses to public health events via:Footnote 3
- monitoring and surveillance activities,
- risk assessment,
- public health measures (e.g., public education, case and contact management, trace-back/trace-forward, travel/border measures, vector control, mitigation of risk from animals, etc.),
- laboratory networks,
- connections with a clinical research network and other health care delivery partners,
- vaccine (and other medical countermeasures) programs,
- the provision of specific health services and evidence-informed recommendations,
- engagement with key stakeholders (e.g., occupational health authorities, health care institutions, law enforcement), and
- risk communications.
Emergency Management authorities facilitate and support coordination of responses to public health events by:
- using a platform and tools for planning and coordination of integrated response activities,
- addressing issues regarding mutual assistance/aid (e.g., via the Operational Framework for Mutual Aid Surge Requests [OFMAR]),
- providing logistical guidance and support, and
- expediting and facilitating the sharing of information and other resources across the health sector and with other relevant sectors domestically and internationally.
The response activities implemented and coordination required will vary depending on the type of public health event and response objectives (which may change over the course of the response). Therefore this plan includes references to potential response activities in conjunction with response objectives, a governance structure that is flexible and scalable, and a concept of operations that facilitates awareness of the entire response process.
F/P/T Authorities/Roles and Responsibilities
The main roles, responsibilities and authorities of the federal Health Portfolio and the provincial and territorial public health authorities during a public health response to a biological hazard are listed in Appendix C: Main F/P/T Roles and Responsibilities. A coordinated F/P/T response requires collaborative and inter-operable infrastructures, response capacities and harmonized activities. During a public health response,the role of theF/P/T governments will be to work collaboratively to establish an overall agreed upon strategy that articulates, why, what and how. The 'what' are interventions that can be implemented as needed across Canada and that correspond to response needs and objectives, recognizing that some or all jurisdictions may implement them dependant on the roles and responsibilities of the jurisdiction and circumstances of the event. These interventions may include: developing/modifying protocols for surveillance and laboratory testing, providing recommendations for public health measures and the use of medical countermeasures, identifying research needs and developing and implementing an F/P/T communication strategy that allows P/T governments to develop harmonized communication plans and stakeholder engagement strategies.Footnote 4
If a coordinated F/P/T response is implemented under this plan, the federal Health Portfolio will facilitate the coordination of the response through the Health Portfolio Operations Centre (HPOC) including participation on the F/PT governance structure committees/groups as described in this document and through its support of the F/P/T Special Advisory Committee Secretariat. See Section 4 F/PT Governance for more specific information on the HPOC's role.
3. Concept of Operations (CONOPS)
The following figure depicts the main steps in the concept of operations of this plan.
Figure 1. Concept of Operations
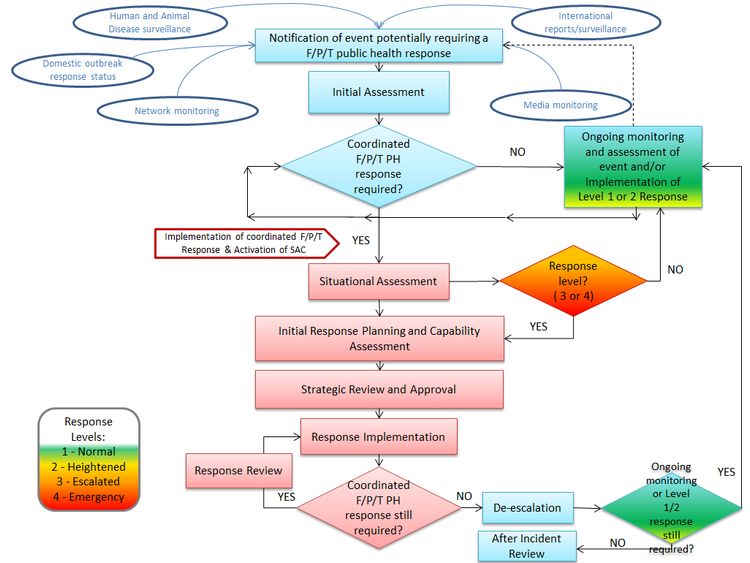
Figure 1: Concept of Operations - Text Description
The graphic illustrates the sequence of events that are expected to take place in order to activate the governance structure of the plan. The first step is an initial notification of an event potentially requiring an F/P/T public health response under the plan
The examples of potential sources of the notification are:
- Human and animal disease surveillance
- Domestic outbreak response status
- Network monitoring
- International reports and surveillance
- Media monitoring
The next step in the process is the initial assessment. At this step there is an assessment as to whether there is a coordinated F/P/T public health response required.
If it is 'not' required, ongoing monitoring and assessment of the event and/ or implementation of a Level 1 or 2 response continues.
If it is required, implementation of a coordinated F/P/T response takes place and the Special Advisory Committee (SAC) is activated.
The next step is a situational assessment. At this step there is a decision as to whether or not the event warrants an escalation of the response to Level 3 or 4. If the decision is 'no', ongoing monitoring and assessment of the event/and or implementation of the Level 1 or 2 response resumes.
If the decision is 'yes', the response moves on to the initial response planning and capability assessment phase.
This phase is followed by:
- Strategic review and approval
- Response implementation
At the response implementation phase there is a response review conducted and a decision is made as to whether a coordinated F/P/T public health response is required.
If the answer is 'yes', the response implementation phase continues.
If the answer is 'no', de-escalation is initiated and a decision is made on whether ongoing monitoring or a Level 1 or 2 response is still required. If the answer is 'yes', ongoing monitoring and assessment of the event and/or implementation of Level 1 or 2 response continues.
If the answer is 'no', the After Incident Review is initiated.
The governance structure for a coordinated F/P/T response (described in Section 4) contains three response streams that can be activated under this plan. They include a Technical Advisory Committee (TAC), a Logistics Advisory Committee (LAC), and a Communications group. These committees/groups report to the Special Advisory Committee (SAC), which in turn is supported by an F/P/T SAC Secretariat. The F/P/T SAC Secretariat facilitates and manages the intersection between the SAC and the three response streams in coordination with the HPOC. The governance structure for this plan is intended to be activated for a coordinated F/P/T response which for the purposes of this plan is considered a Level 3 - Escalated or Level 4 - Emergency response. Descriptions of the response levels are found in Figure 2.
3.1 Notification
Consistent with the scope of this plan, an F/P/T coordinated response could be necessary due to the presence of unusual, unexpected or serious illness, or the identification of a risk of unusual, unexpected or serious illness, within or outside of Canada.
It is expected that these public health events will be detected through a variety of sources including: Canadian human and animal disease surveillance activities; monitoring networks (e.g., laboratory, health security); national and international reports/surveillance (e.g., International Health Regulations [IHR] notifications and the Global Public Health Intelligence Network [GPHIN]).
The PHAC should be notified of all public health events that potentially require a coordinated F/P/T response so that an assessment can be completed and an appropriate response level can be determined in a timely manner. All notifications that have the potential to be a Public Health Emergency of International Concern (PHEIC) should be made according to the timelines required under the IHRFootnote 5.
Notification of these public health events occurring within Canada should be made by the affected jurisdiction/department to the federal HPOC Watch Office
by phone (1-800-545-7661 or 613-952-7940)
or
through the single window email: HPOC_COPS@phac-aspc.gc.ca.
Notification of public health events, identified by the PHAC, that are occurring outside of Canada will be assessed, managed and communicated according to existing operational protocols.
If an event is being monitored or a Level 1 or 2 response is ongoing (see Figure 2 for description of response levels), for example, a response to a food-borne illness outbreak using the FIORP, then the 'notification' may be that the circumstances (e.g., health impact, scope and/or risk) have changed enough to warrant consideration of escalating response efforts to include additional senior management coordination at an F/P/T level.
3.2 Initial Assessment
The HPOC Watch Office will immediately refer all notifications of public health events that they receive to the appropriate PHAC program area for follow-up and assessment coordination. Outside of regular business hours the HPOC Watch Office will refer the notification to the PHAC Medical Officer On-Call for action as he/she deems necessary (including determining whether an initial assessment needs to occur on an urgent basis or whether it can be referred to the PHAC program area for follow-up on the next business day).
The initial assessment will include a rapid risk assessment and a situational analysis which will largely be dependent on the information available from the source of the notification. If the notification is coming from a province or territory, a representative with appropriate authority and expertise from that jurisdiction (and possibly other affected provinces and territories) will be engaged in the process. In the situation when a response is already occurring, for example under the FIORP, then the leads from that response (e.g., Outbreak Investigation Coordinating Committee [OICC] members) would be engaged in this initial assessment process. If the notification is coming from an international source, the PHAC program area will determine the participants to be engaged for the initial assessment process; this may include experts external to the PHAC. Sharing of public health information throughout the response is expected to occur as per the Multilateral Information Sharing Agreement (MLISA) or requirements under IHR obligations.
The purpose of the initial assessment is to determine what actions and/or resources are needed in order to respond to the public health event and specifically whether those actions would benefit from a coordinated F/P/T response in order to mitigate the health impact or risk to Canadians. Not all notifications will require this type of high-level coordination to respond and many will be managed through routine practices; an assessment of response needs will determine the next steps.
If there is not enough information at the time of the initial assessment to determine if a coordinated F/P/T response should be recommended this decision can be deferred until more information is available at which time a follow-up assessment can be completed. It is recognized that initial assessments could occur more than once for the same event in the form of a repeat or follow-up assessments.
A special meeting of the Council of Chief Medical Officers of Health (CCMOH) may be held for information sharing and to discuss ongoing monitoring of the situation if a coordinated F/P/T response is not deemed necessary at the time of the initial assessment and what will occur in the event the health impact or risk of the event changes significantly. Ongoing assessment is expected to occur as required until a potential event is concluded.
If one or more of the following needs are identified during the initial assessment, a coordinated F/P/T response may be recommended.
- Federal surge capacity or centralized planning because multiple jurisdictions are affected or have been put at risk by the public health event (e.g., a vaccine supply issue, an event requiring rapid advanced planning/preparedness) and requirements for coordination exceed routine/existing capacities
- New or revised guidance documents, recommendations or activities for the public health response (e.g., if an outbreak due to an unknown or new pathogen with high potential for human to human transmission occurs, and/ or a disease is new to Canada and no established program currently exists)
- Collated Canadian incidence data on daily/urgent basis (e.g., for IHR reporting requirements)
- Analysis of epidemiological data from multiple jurisdictions to inform the response
- Bulk purchasing of medical countermeasures (MCM) or equipment
- Consistent use across multiple jurisdictions of limited resources (e.g., MCM)
- Consistent approach to border screening, contact identification and follow-up, and/or public and professional communications (e.g., due to a Public Health Emergency of International Concern (PHEIC) occurring outside of Canada)
It is recognized that expectations or demands at a political level may necessitate activation of this plan's governance irrespective of the criteria above.
Implementation
The findings of the initial assessment may include a recommendation to implement a coordinated F/P/T response. If the public health event is occurring in Canada, the reporting and affected jurisdictions will be involved in making and endorsing the recommendation. The recommendation and rationale will be presented by the PHAC program area on behalf of the group completing the initial assessment, to the co-chairs of the PHNC, the chair of the Council of Chief Medical Officers of Health (CCMOH) and the Deputy Minister Liaison (or their respective designates) who together will make the decision to implement a coordinated F/P/T response. If the decision is to implement a coordinated F/P/T response then a SAC and F/P/T SAC Secretariat will be established at this time.
The HPOC and the F/P/T SAC Secretariat, as appropriate will communicate the decision, via email, to implement the coordinated F/P/T response and a SAC to all of the provinces and territories and implicated federal departments and to the Conference of Deputy Ministers of Health (CDMH) via the Deputy Minister Liaison. The F/P/T SAC Secretariat, with the support of the HPOC will also make the arrangements to convene a situational awareness teleconference with the new SAC and any additional key stakeholders and or external experts as soon as feasible with consideration given to the urgency of the situation.
If implementation of a coordinated F/P/T response is not deemed necessary then ongoing monitoring and assessment of the public health event and the response to the public health event will continue through routine processes/protocols and the rest of this concept of operations would not be implemented as described below.
3.3 Situational Assessment
An initial situational awareness teleconference will be scheduled and organized with invitations being distributed by the HPOC. The Chief Public Health Officer (CPHO) or a designate, will chair the teleconference. Participants on the call will include the newly established SAC members, any additional P/T representatives (e.g., P/T program area managers, P/T Health Emergency Managers and P/T Emergency Operations Centre [EOC] representatives), federal HP representatives (e.g., PHAC regional representatives, Health Canada [HC] representatives), and possibly external liaisons including representatives from Public Safety Canada/Government Operations Centre (GOC), Royal Canadian Mounted Police (RCMP)/law enforcement, federal populations and potentially non-governmental organizations (NGOs). The P/T participants will be determined by the individual provinces and territories. The purpose of this first teleconference will be to:
- debrief all participants on the details of the public health event and rapid risk assessment results,
- to determine what F/P/T response level is appropriate to meet the immediate F/P/T response needs,
- to ensure familiarity and accessibility of this plan,
- to identify what parts of the governance structure to activate, and
- to begin identification of individuals who will participate in committees under the governance structure.
During this teleconference a time and date will be set for the next teleconference during which the initial response planning, capability assessment and business cycle will be discussed.
F/P/T Response Level
In order to operationalize this scalable plan, F/P/T response levels are included to illustrate the considerations and potential scenarios corresponding to the different response levels; these are identified in Figure 2 F/P/T Response Levels. The need for a particular F/P/T response level (as indicated in the considerations for implementation) may be identified by a province or territory (e.g., CMOH), a group containing federal and P/T representatives and/or external experts (e.g., an OICC), the PHAC or HC. The information to illustrate this need will be collected during the notification and initial assessment and will be utilized during the situational assessment teleconference. The SAC will decide whether a Level 3 - Escalated or Level 4 - Emergency response level is required during the first situational assessment teleconference. Throughout the response the needs will be reassessed and the response level may change accordingly.
The majority of this concept of operations is intended to focus on the response to public health events in which a coordinated F/P/T response is needed or would be beneficial; specifically when the initial assessment indicates that a Level 3 - Escalated or Level 4 - Emergency response level is required.
The main difference between response levels is the level of activity required by HPOC and F/P/T SAC Secretariat to support the F/P/T governance structure as well as the components of the governance structure that are required to be activated. For example, a Level 3 response may be facilitated largely by coordination provided by the F/P/T SAC Secretariat, which would lead in issue triage, situational awareness, response planning, and task delegation in close consultation with the SAC co-chairs. The HPOC Incident Management System (IMS) structure may only be activated for minimal support. Level 4 responses may require full activation of the three response streams as well as the activation of additional task groups to carry out the required response functions and the full activation of the HPOC IMS.
A coordinated F/P/T response does not necessarily mean that each province or territory is engaged in the response to the same degree or that each province or territory is experiencing cases or equal risk of cases occurring. For example, during the response to the SARS outbreak the provinces with active disease transmission were responding at a pace and level of activity much greater than those that did not have cases. However there was a need for a coordinated F/P/T response during the SARS outbreak in order to:
- support the heavily affected provinces;
- facilitate consistent surveillance;
- coordinate public health and infection control practices across the country;
- provide informed and consistent technical and public messaging.
| Level | Considerations | Example scenario(s) | Main Objective for the F/P/T response | Governance Structure Activation |
|---|---|---|---|---|
| 1-Routine | Need for information sharing, regarding a public health event, between affected jurisdiction and other federal, provincial, territorial or international authorities (e.g. WHO) | An outbreak of measles within a single jurisdiction. | Information Sharing | n/a - use routine channels/standing committees |
| 2-Heightened | Need for a routine public health response (i.e., outbreak response, response to an exposure or risk assessment) involving one or more jurisdictions. | A foodborne outbreak is occurring in multiple jurisdictions. | Outbreak Control | n/a - Potential use of Governance Structure concepts may be applied Implement response structure per regular coordinating instruments (e.g., FIORP OICC) |
| 3-Escalated | A coordinated F/P/T response is required for a public health event that: | no data | no data |
Partial HPOC Event Manager and partial HPOC IMS as required SAC and SAC Secretariat need for full P/T representation will be determined based on event (may only be affected areas), need for a TAC and LAC will also be determined based on response needs and tempo. Communications Task/subgroups and support teams as needed |
| a) is occurring in multiple jurisdictions within Canada and is unusual in its progression or severity requiring additional response support to manage either the pace or extended duration of the event. | A foodborne outbreak in Canada is resulting in unusual illness or requires additional response support. | Outbreak Control | ||
| b) is occurring outside of Canada and is being caused by an unusual or unknown pathogen and/or has been declared a Public Health Emergency of International Concern | Zika epidemic in the Americas | Outbreak Prevention | ||
| c) has potential implications for the Canadian health care system | New outbreak due to a highly antibiotic resistant bacterium. Large scale vaccine supply issue with potential singificant public health implications. | Risk Mitigation | ||
| d) will potentially require the provision of aid in the form of human resources (i.e., mobilizations) or medical counter measures held by Canada | Ebola outbreak in Africa | Support / Aid | ||
| 4-Emergency | A coordinated F/P/T response is required for: | no data | no data | Full SAC SAC Secretariat TAC, LAC full HPOC IMS Communications Task/subgroups and support teams as needed |
| a) an event in Canada that is causing significant illness and has the potential for rapid spread | MERS is being transmitted from person to person in Canada. | Outbreak Control | ||
| b) a risk in Canada that has the potential for causing significant illness and/or could spread internationally from Canada | A medical counter measure produced in Canada is contaminated or causing unexpected serious adverse events. | Risk Mitigation / Exposure Control | ||
| c) a PHEIC declaration outside of Canada that could cause significant illness within Canada. | A novel influenza virus is spreading efficiently between humans. | Outbreak Prevention | ||
| Note: The need to consider implementing a coordinated F/P/T response may be identified by a P/T (e.g. CMOH), an OICC (i.e., established for an ongoing response), PHAC or HC and information to support this need would be collection as part of the notification and initial assessment. | ||||
Note: The F/P/T response levels are not intended to represent a progression of activity for a single public health event (i.e., from Level 1 to Level 4). Some public health events may require an immediate Level 4 response.
3.4 Initial Response Planning and Capability Assessment
Once the situational assessment is completed and the F/P/T response level has been determined as Level 3 or 4, initial response planning will commence.
The HPOC will:
- mobilize an HPOC Incident Management System (IMS) structure that is appropriate for the public health event,
- draft a F/P/T business cycle proposal,
- prepare a status report regarding federal resources, assets (e.g., MCM, National Emergency Strategic Stockpile [NESS]) and capabilities (e.g., mobilizations) that may be needed for the response,
- summarize any requests for assistance/aid that have been received (including international requests),
- distribute terms of reference (TOR) documents for components of the governance structure that may be activated to the P/Ts and put out a request for identification of participants in the governance structure,
- develop a proposal for the initial main response objective and incident action plan for the first operational period.
AffectedFootnote 6 provinces and territories in Canada (if applicable) will:
- send to the HPOC single window email account (HPOC_COPS@phac-aspc.gc.ca) an email indicating that it is an affected jurisdiction, a list of potential participants in the governance structure groups that may be activated under the established SAC (and preferred role), any immediate response issues/needs and any updates regarding the public health event in the jurisdiction,
- assess their own capabilities and identify any areas where support is needed.
Un-affected provinces and territories in Canada (if applicable) will:
- send to the HPOC single window email account (HPOC_COPS@phac-aspc.gc.ca) an email indicating that it is an un-affected jurisdiction, a list of potential participants that would like to be considered for a role in the governance structure groups that may be activated under the established SAC (and preferred role), any immediate response issues/needs and any updates regarding the public health event in the jurisdiction,
- assess their own readiness to respond, including capabilities and identify any areas where support would potentially be needed.
3.5 Strategic Review and Approval
At the next situational awareness call the initial overall response goals, F/P/T incident action plan, operational period and business cycle will be discussed, edited as necessary by PHAC staff and submitted for approval to the SAC by the F/P/T SAC Secretariat. The SAC will then confirm the need for and activate the committees of the governance structure as necessary, after which those groups will respectively identify the need for task groups that will report to them during the response. The F/P/T SAC Secretariat will provide support to the SAC as required throughout the response.
3.6 Response Implementation
The coordinated F/P/T response will be focused on fulfilling the response needs (see highlighted box in Section 3.2) identified during the initial assessment. It is recognized that each affected jurisdiction and PHAC will also be implementing their own response in particular to manage operational issues and other response activities that do not require F/P/T coordination and that will be achieved through their respective established procedures and response structures (e.g., IMS). This response plan is meant to complement these jurisdictional responses. To facilitate the flexibility needed for the scope of this plan it will be up to each jurisdiction to identify who will represent them within the governance structure in this plan.
The coordinated F/P/T response will proceed with each activated group in the governance structure fulfilling the roles and responsibilities and decision-making processes as described in Section 4 of this plan and according to their respective terms of reference (see corresponding appendices). Specifically, the SAC will be the main approval/decision-making body for the duration of the Level 3 or 4 response, with governance structure products going to the CDMH as required. The F/P/T SAC Secretariat will be the conduit through which F/P/T issues and/or response needs will be received, triaged and disseminated for action within the governance structure. The response will be consistent with established roles and responsibilities of F/P/T governments (see Appendix C), and with the public health and emergency management roles and responsibilities (see Section 2) respectively. Although consistent application of products developed and approved through the governance structure in a coordinated F/P/T response is desirable, it is recognized that use of these products in each province and territory may be limited by competent authorities or legislation.
Business Cycle
The F/P/T business cycle will depend on the pace of the event (i.e., how quickly the situation and risks are changing) and demands of the response. The demands of the response will be determined by:
- information sharing/debriefing requirements and expectations,
- the number and complexity of issues raised that require an F/P/T response,
- the number and type of products/actions that need to be generated and approved through the governance structure,
- the communications response requirements, and
- the status/availability of resources (e.g., MCM, human).
The business cycle will include the situational awareness and planning teleconferences, deadlines for submission of data for inclusion in various products and reports (e.g., situation reports, collated Canadian incidence data), and product release times. The schedule will to the extent possible take into consideration the different time zones in Canada and the possibility that some individuals will be participating on multiple committees in addition to responding to their own jurisdictional response requirements. All participants on committees within the governance structure are encouraged to delegate roles and responsibilities and identify a designated alternate to act as back up when necessary. This is particularly important if the health event is expected to require a prolonged response (e.g., for pandemic influenza). A sample business cycle is located in Appendix J: Sample F/P/T Business Cycle.
Management by Objectives
Management by objectives is one of several emergency management principles that have been tested and proven effective over time in settings ranging from business and industry to government agencies. This term describes a top-down management activity that involves: establishing the event objectives, selecting the appropriate strategy(s) to achieve the objectives and implementing the strategy in order to achieve overarching goals.Footnote 7 Utilizing this management by objectives approach facilitates clarity regarding the rationale for F/P/T response actions, recommendations and products. It also enables the federal government, provinces and territories to identify why their response may differ from the F/P/T response; specifically if their jurisdiction is experiencing a different level of impact of the public health event and therefore has different goals and objectives (e.g., a province or territory with no cases may retain the outbreak prevention goal while other provinces and/or territories have moved to outbreak control). F/P/T response is the sum of collective responses in all jurisdictions.
Response Goals and Objectives
Many response objectives, along with corresponding response needs or strategies, can be anticipated ahead of time or are already documented in hazard-specific plans and preparedness guidance like the FIORP or CPIP. The overall response goal for the coordinated F/P/T response should be articulated and confirmed during planning meetings and the need to change the goal, for example from outbreak prevention to outbreak control, should be identified based on ongoing situational and risk assessments conducted as part of the Technical Advisory Committee (TAC) activities. Example goals, objectives and corresponding potential strategies are presented in Tables 2 below. Similarly, many of the governance structure products that might be needed to address the response objectives can be identified in advance (see Appendix H: Anticipated Products and Pathways for a Coordinated F/P/T Response).
| Overall Response Goals | Specific corresponding objectives | Potential Strategies | Corresponding Governance Structure Response Functions |
|---|---|---|---|
| Outbreak Prevention | Prevent/delay introduction of cases and potential pathogen exposure sources | provision of advice to travellers leaving Canada for affected areas, screening for symptomatic people at international POE, increase immunity in Canadian population through use of MCM, implement import restrictions on potential pathogen exposure sources (e.g., food products), active surveillance for illness on incoming international vessels/conveyances (e.g., require declaration of "no illness" on board) arriving from affected areas, quarantine | Public health measures, MCM (Procurement), Surveillance, Policy analysis & integration |
| rapid case identification | sensitive case definition, availability of rapid laboratory diagnositics | Surveillance, Laboratory | |
| protect responders and health care providers | educate responders/HCW re: transmission routes and infection control practices, prophylactic use of MCM, provision/ensure access of PPE | Public health measures, IPC, MCM (Procurement), Occupational health, Deployable resources, Policy analysis & integration |
| Overall Response Goals | Specific corresponding objectives | Potential Strategies | Corresponding Governance Structure Response Functions |
|---|---|---|---|
| Outbreak Control | rapid case identification | sensitive case definition, availability of rapid laboratory diagnositics | Surveillance, Laboratory |
| protect responders and health care providers | educate responders/HCW re: transmission routes and infection control practices, prophylactic use of MCM, provision/ensure access of PPE | Public health measures, IPC, MCM (Procurement), Occupational health, Deployable resources, Policy analysis & integration | |
| minimize spread | case isolation, rapid contact identification and management, decontamination at source, vector control, mitigate risk from animal sources/exposures | Public health measures, ICP, Occupational health, Health care delivery engagement, Policy analysis & integration | |
| characterize the epidemiology of the outbreak | standardized detailed case data collection form (e.g., first 100 cases), epidemiological analysis of data, comparision with data from other affected areas (i.e., international) | Surveillance, Laboratory, Technical expert engagement, Research | |
| prevent community spread | public education re: social distancing, infection control (hand washing) practices, quarantine of contacts, delay/cancel large public gatherings | Public health measures, ICP, Occupational health, Emergency communications support and coordination, Policy analysis & integration | |
| prevent hospital or nosocomial spread/ transmission | Development of national infection prevention and control guidance for healthcare settings | Infection prevention and control and occupational health for healthcare settings | |
| minimize social disruption | implement risk communication strategy, provide guidance for workers and workplaces, identify risk factors as opposed to linking risk to geographic areas | Strategic communication/product development, Information dissemination, Emergency risk communications support and coordination, Occupational health, Public health measures, Policy analysis & integration | |
| mitigate clinical severity | ensure access to early treatment, facilitate provision of clinical care guidance, ensure adequate supply of medical equipment (e.g., ventilators) | MCM (Procurement), Deployable resources, Health care delivery engagement, Technical expert engagement, Policy analysis & integration |
| Overall Response Goals | Specific corresponding objectives | Potential Strategies | Corresponding Governance Structure Response Functions |
|---|---|---|---|
| Risk Mitigation | protect responders and health care providers | educate responders/HCW re: transmission routes and infection control practices, prophylactic use of MCM, provision/ensure access of PPE | Public health measures, IPC, MCM (Procurement), Occupational health, Deployable resources, Policy analysis & integration |
| minimize spread | case isolation, rapid contact identification and management, decontamination at source, vector control, mitigate risk from animal sources/exposures | Public health measures, ICP, Occupational health, Health care delivery engagement, Policy analysis & integration |
| Overall Response Goals | Specific corresponding objectives | Potential Strategies | Corresponding Governance Structure Response Functions |
|---|---|---|---|
| Mitigate Impact/social disruption | mitigate clinical severity | ensure access to early treatment, facilitate provision of clinical care guidance, ensure adequate supply of medical equipment (e.g., ventilators) | MCM (Procurement), Deployable resources, Health care delivery engagement, Technical expert engagement, Policy analysis & integration |
| reduce health care system impact | provide guidance for: in home care of cases/contacts/exposed individuals, triage call centres, use of surge/alternate sites, cohorting within facilities | Public health measures, Health care delivery engagement, Policy analysis & integration | |
| minimize public anxiety | implement risk communcation strategy, provide timely information to public and media, use risk assessments and technical informaton to support communication product development, identify spokespersons, describe public health actions taken and why | Strategic communication/product development, Information dissemination, Emergency risk communications support and coordination, Communciation surveillance, Public health measures, Risk assessment, Policy analysis & integration |
Response Review
It will be necessary as the response progresses to review the response actions that have been implemented and whether the response goals and objectives have been achieved. If a coordinated F/P/T response is still required then the SAC will determine if it is necessary to modify the response, in terms of actions and response level (e.g., change from Level 3 to 4 or vice versa). If the SAC recommends that a coordinated F/P/T response is no longer necessary, de-escalation planning will commence.
3.7 De-escalation
The coordination of de-escalation planning under this plan will be led by the F/P/T SAC Secretariat and HPOC Planning Group. This process will be informed by situational and risk assessments (e.g., risk assessment indicates that the overall likelihood of infection/exposure for Canadians is now 'low' and the impact for most of those infected/exposed will be 'low'). Surveillance information, the number of outstanding F/P/T response products and activities, and possibly information derived from modelling will form the basis for these assessments.
A de-escalation plan will be developed by the HPOC Planning Group in consultation with the relevant provinces and territories and will be forwarded to the SAC for approval by the F/P/T SAC Secretariat. It will include the criteria for de-escalating the response to a Level 2 – Heightened or Level 1- Routine F/P/T response level thereby de-activating the coordinated F/P/T response and the implemented F/P/T governance structure. The criteria should be specific to the event but may include the following concepts:
- Incidents associated with the response are at a level of activity that can be managed through a routine or heightened response.
- Normal day-to-day operations of the HPOC can handle any requests from the World Health Organization (WHO) or its partners.
- Requests for international support have declined or been addressed.
- The governance structure has decreased in size due to decreased demand for coordinated F/P/T products or issue management.
- Planning meetings and situational awareness meetings are occurring less frequently – e.g., on a weekly or ad hoc basis.
- Situation reports and epidemiology/laboratory reports are now being produced weekly rather than daily by the HPOC and the National Microbiology Laboratory (NML).
- Communications has reported a decrease in media and public interest in the situation. Demand for media requests, ministerial correspondence, web correspondence, and social media overall has steadily declined.
The decision to de-escalate the response from Level 3 – Escalated or Level 4 – Emergency response and resume routine F/P/T operations will be made by the co-chairs of the PHNC, the chair of the CCMOH and the Deputy Minister Liaison as per the SAC terms of reference. This decision to de-escalate will be communicated to all stakeholders via situational awareness calls and by emails issued by the HPOC and F/P/T Secretariat.
Recovery activities will be initiated by F/P/T jurisdictions as needed and may be initiated prior to the de-escalation of this plan's governance. The process of returning to 'normal operations' (vs normal conditions) is defined as de-escalation and is separate from recovery.
Recovery consists of activities aimed at restoring normal conditions after an emergency. Public health recovery from an emergency may range from hours to years depending on many factors such as persistence of the hazard, the magnitude of the event, the size and vulnerability of the affected populations and jurisdictions coping capabilities. While recovery activities are outside the scope of this plan, it is recognized activities such as scientific and analytical advice, risk assessment and guidance to physicians are examples of support that may require on-going F/P/T coordinated activities and use this plan in order to help address the long-term health effects.
3.8 After Incident Review
Following de-escalation of this plan and return to a routine F/P/T response level or shift to ongoing recovery not requiring use of this plan, an after incident review will be initiated by the PHAC through the F/P/T SAC Secretariat. Participants in the response may be asked to participate in review and/or lessons learned types of exercises. Review of the contents and utility of this plan should also occur with an aim to identifying the need for revisions to the plan, supporting tool development and training.
4. F/P/T Governance
4.1 Structure
The governance structure below in Figure 2 aims to: streamline response processes to a public health event; clarify roles, responsibilities and approval processes; facilitate a high degree of situational awareness; and centralize risk management and task delegation. It has been developed based on best practices and lessons learned from prior large-scale public health responses. The general response functions identified in the structure are being presented for illustrative purposes and represent an example of a fully activated structure. At the time of implementation this structure will be customized to suit the specific event and scale of the required response. See appendix A for list of acronyms used in this figure.
Figure 2. Governance Structure for a Coordinated F/P/T Response
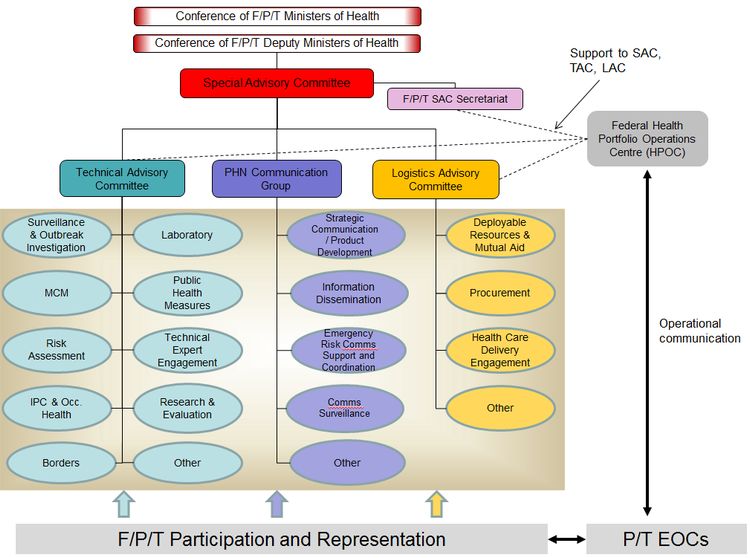
Figure 2: Governance Structure for a Coordinated F/P/T Response - Text Description
Overview: The governance structure is made up of a Special Advisory Committee (SAC) and three main response streams (technical, logistical and communications) each led by Advisory Committees/Working Groups. The governance structure, through the SAC, reports to and is accountable to the Conference of F/P/T Deputy Ministers of Health (CDMH) which reports to the Conference of F/P/T Ministers of Health.
The SAC is composed of the members of the Pan-Canadian Public Health Network Council and the Council of Chief Medical Officers of Health (CCMOH) and is supported by an F/P/T SAC Secretariat.
The F/P/T SAC Secretariat supports the SAC and the Technical and Logistics Advisory Committees of the governance by assuming multiple coordination functions.
The 3 Advisory/Working Groups which report to SAC are the Technical Advisory Committee, the PHN Communications Group and the Logistics Advisory Committee.
Each of these Advisory/Working groups have specific Task Groups that report to them and that can be activated in an event as needed. The Task Groups are listed below.
The Technical Advisory Committee has the following Task Groups reporting to it:
- Surveillance and Outbreak Investigation
- Medical Countermeasures
- Risk Assessment
- Infection Prevention and Control and Occupational Health
- Border Services
- Laboratory
- Public health measures
- Technical Expert Engagement
- Research and Evaluation
- Other
The Public Health Network Communication Group has the following Task Groups reporting to it:
- Strategic Communication/Product Development
- Information Dissemination
- Emergency Risk Communications Support and Coordination
- Communications Surveillance
- Other
The Logistics Advisory Committee has the following Task Groups Reporting to it:
- Deployable Resources and Mutual Aid
- Procurement
- Health Care Delivery Engagement
- Other
The governance structure is supported by the participation of representatives from federal, provincial and territorial emergency operations centres.
The Health Portfolio Operations Centre (HPOC) provides overall support to the governance structure through support to the FPT SAC Secretariat, and representation on the TAC and LAC.
The HPOC further supports emergency operations by operational communication with the operations centres of P/T Ministries of health.
Note: In Figure 2, reporting relationships are depicted by solid lines and support relationships are depicted by dashed lines.
4.2 Governance Structure Groups - Roles, Responsibilities and Decision-Making
It is strongly recommended that the descriptions described below are read in conjunction with the corresponding terms of reference attached as appendices to this plan.
Special Advisory Committee
The Special Advisory Committee (SAC) has a mandate to provide advice to the F/P/T Conference of Deputy Ministers of Health (CDMH) pertaining to the coordination, public health policy and technical content on matters related to response to a significant public health event. If it is determined that a coordinated F/P/T response is required (following initial assessment of the situation as per the concept of operations described in Section 3 of this document), a SAC will be activated.
Products may be developed within the governance structure of this plan which may include, but are not limited to: recommendations, guidance documents, protocols, and communication products. SAC will be the main forum for F/P/T approval/endorsement of such products however, for purely technical products (e.g., surveillance case definitions, laboratory testing protocols), the SAC may choose to delegate approval to the Technical Advisory Committee (TAC). If necessary, the SAC will involve the CDMH or the Deputy Minister Liaison in decision-making either by an expression of interest by the CDMH or when the scale of the public health event and subsequent resource implications for the response activities indicate. With the support of the F/P/T SAC Secretariat, the SAC co-chairs will provide SAC endorsed products to the CMDH as required.
The SAC is chaired by the co-chairs of the Public Health Network Council (PHNC). For details regarding the SAC composition and activities related to this plan can be found in Appendix D: Special Advisory Committee Roles and Responsibilities under the FPT Public Health Response Plan.
F/P/T governments recognize that each jurisdiction will decide whether or not to implement the recommendations or products of the SAC, and will do so according to the needs of the jurisdiction and/or its legislative framework.
FPT Special Advisory Committee Secretariat (FPT SAC Secretariat)
The F/P/T SAC Secretariat will support the SAC by assuming multiple coordination functions any time when the SAC is activated. Under this plan, a SAC is activated for a coordinated F/P/T response which for the purposes of this plan is a Level 4 - Emergency responseand a Level 3 – Escalated response. In this governance structure, the F/P/T SAC Secretariat manages the intersection between the SAC and the three response streams of the governance: the Technical Advisory Committee (TAC), the PHN Communications Group, and the Logistics Advisory Committee (LAC) and will provide cross-stream support through the planning and tracking of tasks.
Membership
The F/P/T SAC Secretariat will be composed of F/P/T senior level policy staff. Members may come from the current Public Health Network Secretariat. These individuals routinely provide policy support to PHNC members and have extensive experience in providing strong context, content and process knowledge of policy and program areas in public health. In addition, their day-to-day roles and responsibilities include policy analysis and options, linking with technical expertise, supporting communications, information sharing and knowledge exchange and product and tool development. Furthermore, they are often linked in with inter-governmental relations officials within the provinces and territories and at the federal level. Each jurisdiction will have the opportunity to identify an appropriate policy representative to serve on the F/P/T SAC secretariat.
Key responsibilities
The F/P/T SAC Secretariat, with the support of the HPOC Incident Management System (IMS) structure, will be responsible for rapid centralized analysis of issues and F/P/T response needs, prioritization and distribution of tasks, with the aim of improving efficiency, increasing situational awareness, and facilitating engagement of external resources. This will be achieved through liaison with the SAC co-chairs and through planning meetings convened with the HPOC Planning Group and co-chairs of the TAC, LAC and PHN Communications Group.
Specifically the F/P/T SAC Secretariat, with direction from SAC co-chairs, will identify what type of product/action is required, task this to the appropriate group(s) within the governance structure and monitor progress. The F/P/T SAC Secretariat will identify and prioritize response issues and F/P/T response needs in consultation with the SAC co-chairs and co-chairs of the TAC, LAC and PHN Communications Group and will provide a mechanism for P/Ts, other groups within the governance structure, and other stakeholders to bring their issues forward to the SAC.
It is expected that the F/P/T SAC Secretariat will routinely liaise with the co-chairs of the SAC, TAC, LAC and PHN Communications Group and with the HPOC Event Manager as required throughout the response. The F/P/T SAC Secretariat with the support of the HPOC, will convene situational awareness and planning meetings as described below.
Applying Strategic Policy Lens and Product Integration
Another key activity of the F/P/T SAC Secretariat is to apply a strategic policyFootnote 8 lens to all products (developed by groups within the governance structure) that are being brought forward to SAC for discussion, approval and/or endorsement. It is also responsible to integrate products developed by different streams within the governance structure as requested by SAC.
Product integration would include combining content developed by the multiple streams into one document in which case the F/P/T SAC Secretariat will be formatting the final combined product and adding any summaries or strategic policy analysis but will not make any fundamental changes to the previously approved/endorsed content. An example of when this would occur is if SAC requested a single product that includes technical recommendations, logistical issues and a communication response (or any combination of work from two streams) – such as a vaccine response strategy. This process is intended to help expedite and support evidence-informed decision-making by the SAC.
The F/P/T SAC Secretariat will be the contact point for P/Ts, other groups within the governance structure, and other stakeholders to bring their issues forward to the SAC (note: focus should be on F/P/T issues). The HPOC IMS Planning Group may support this process through maintenance of activity trackers or other tools. The mechanism for bringing issues to the F/P/T Secretariat will be via emailFootnote 9 to the HPOC.
Where practical, the results of planning and situational awareness meetings will be captured in meeting minutes/record of decisions taken by HPOC IMS staff but also in situation reports and incident action plans developed by the HPOC IMS Planning Group.
Note: The F/P/T SAC Secretariat is not responsible for approving documents (aside from verifying the F/P/T incident action plan).
The F/P/T SAC Secretariat will:
- be the coordination point for receiving issues that may require an F/P/T response action and discussion with SAC;
- in consultation with SAC co-chairs and through planning meetings with HPOC, TAC, LAC and PHN Communications Group co-chairs, identify what type of product/action is required and which group within the governance structure will be the lead on developing the product or completing the action;
- track progress towards completion of product/action;
- convene situational awareness calls and hold planning meetings as needed or requested by SAC co-chairs;
- policy analysis and option development;
- product and strategic policy integration:
- directly supports federal and P/T SAC co-chairs and SAC members and Deputy Minister Liaison at SAC meetings teleconferences or CDMH meetings;
- Links the SAC with federal and P/T inter-governmental relations officials.
Convening Planning Meetings
The F/P/T SAC Secretariat, with coordination support from the HPOC IMS will convene planning meetings whose frequency will depend on the pace of the evolving public health event. HPOC IMS staff will be present for agenda support and coordination and minutes. These meetings will be conducted by teleconference and participants will include: co-chairs from the activated committees within the governance structure (TAC, PHN Communications Group and LAC) and HPOC IMS Planning Group representatives for incident action plan development. This limited participation (compared to situational awareness meetings) is expected to facilitate responsiveness and efficiency.
The purpose of these meetings will be to receive and anticipate F/P/T needs or issues which will then be triaged by the group and prioritized for action. The group as a whole will identify what products/actions are required and the F/P/T SAC Secretariat will delegate tasks to appropriate groups (i.e. the TAC, the LAC, Public Health Network Communications Group, or PHAC). This delegation of tasks will determine the required approval process for the products. The meeting participants will also set expected timelines for each product/action and these will be documented in the F/P/T incident action plan and reported on at the situational awareness meetings. The F/P/T incident action plan is developed by HPOC Planning Group and will be forwarded to the SAC via the F/P/T SAC Secretariat for approval. The incident action plan will document the objectives of the F/P/T response. Planning meetings will be used to review these objectives on an ongoing basis and to determine when to recommend to SAC changing them in order to direct response activities appropriately.
Convening Situational Awareness meetings
The F/P/T SAC Secretariat, with the coordination support of the HPOC will convene meetings for situational awareness as needed based on the pace of the evolving public health event or at the request of SAC co-chairs. These meetings will be conducted by teleconference and participants will include a broader audience than planning meetings: SAC co-chairs and members, co-chairs from the activated committees within the governance structure as well as activated task group leads, CCMOH members (i.e., those not already engaged), P/T EOC representatives, PHAC regional representatives, HPOC and HPOC Event Manager, NML IMS Operations and Planning Chiefs, federal population representatives and potentially NGOs (if involved/affected by the response). The P/T participants will be determined by the individual provinces and territories. The Director General, Centre for Emergency Preparedness and Response (CEPR) or the appointed HPOC Event Manager will chair the meetings.
The purpose of these meetings will be to receive epidemiological situation updates from the P/T representatives, international updates (as indicated) from the HPOC IMS, and to confirm the F/P/T response objectives and objectives of individual jurisdictions if they differ from the F/P/T response objectives. This meeting will also be an opportunity to receive update on F/P/T incident action plan progress (e.g. approvals made by SAC or by the CDMH). The meeting will also be the forum for the provision of updates on research findings and communication products and tactics.
Response Streams
The governance structure includes three main streams: a Technical stream, a Logistics stream and a Communications stream. These streams are led by advisory committees/groups and have been included in the governance structure in order to facilitate clarity regarding roles for issue management, response support, product development (e.g., recommendations, guidance, protocols), and approval/endorsement processes. "Cross stream" support and coordination will be essential to an efficient, informed and transparent response and will be delivered by the F/P/T SAC Secretariat in coordination with the HPOC.
It is anticipated that most of the response issues will be addressed by products that require technical, operational, logistical, communication and policy input (e.g., identification of priority groups for receipt of a medical countermeasure). In these instances the content developed by each stream will be reviewed from a program policy perspective prior to being approved by the respective advisory committee (i.e., Technical Advisory Committee or Logistics Advisory Committee) or endorsed by the Public Health Network Communications GroupFootnote 10. Products approved or endorsed at this level will then be sent to the F/P/T SAC Secretariat in order to prepare them for discussion at the SAC, this will be achieved through product integration and addition of a strategic policy analysis if required. Many of the products that might be needed as part of a coordinated F/P/T response can be identified in advance. Appendix H: Anticipated Products and Pathways for a Coordinated F/P/T Response includes a list of examples of potential products, along with development and approval pathways, in order to stimulate thinking regarding potential requirements and how the governance structure would function at the time of a response.
Technical Advisory Committee
The Technical stream will be led by an F/P/T committee, the Technical Advisory Committee (TAC) and as such will be expected to approve technical products prior to them going to the SAC. Any program policy implications associated with a technical product should be considered and addressed at the TAC level prior to it going to the SAC for approval. Inclusion of a senior level F/P/T program policy representative on the TAC is intended to facilitate this process through the provision of advice and support. The response functions depicted on the technical side of the governance structure will largely be focused on the characteristics of the public health event and what needs to be done from a technical perspective to achieve the response objectives. The TAC will be co-chaired by the F/P/T co-chairs of the Communicable Infectious Disease Steering Committee (CID-SC) or their designates. Further details regarding the TAC composition and activities are located in Appendix F: Technical Advisory Committee Terms of Reference.
Under the TAC, task groups will be established to address technical response functions (e.g., surveillance, laboratory, medical countermeasures) and to provide technical input into other governance structure products such as communication products. However, whenever possible pre-existing groups will be engaged prior to establishing a new task group. A generic terms of reference for these task groups can be found in Appendix K: Task Groups Generic Terms of Reference, and a list of existing groups that may be enlisted or leveraged to function as a technical task group during a response is in Appendix I: Existing Committees, Working Groups and other Expert Resources.
Upon request these task groups would develop products such as epidemiological reports, guidance on public health measures, and recommendations on the type of MCM (e.g., medications or vaccines) to be used. The TAC would inform the task groups when their expected products are to include a policy perspective so that incorporation of this content could be included early in the development of the product. The TAC would also oversee the engagement of external experts and liaisons for example to address issues outside the scope of public health practice such as clinical care guidelines. The approval authority for purely technical products (that do not have significant resource implications) could be delegated by the SAC to the TAC. It is expected that relatively few products will fall into this category.
Public Health Network Communications Group
The Public Health Network Communication Group (PHN CG) is an existing group that is used to support consistent and coordinated public communications across jurisdictions during public health issues of national significance. The PHN CG can also be used to provide communications advice and support to the PHNC. During a response this group will support the SAC as required. The group is chaired by both a P/T and federal representative and is comprised of F/P/T communicators responsible for public health files in their respective jurisdictions. The PHN CG has developed a protocolFootnote 11 that aims to ensure early notification, coordinated and pro-active communications and real-time evaluation between and amongst affected jurisdictions with respect to emerging public health issues. It is expected that when the governance structure for a coordinated F/P/T response is activated as part of this plan, the communication related response functions will be coordinated through the PHN CG for the duration of the response to enable P/T governments can align their communication strategies.
Logistics Advisory Committee
The Logistics stream will be led by an F/P/T committee, the Logistics Advisory Committee (LAC), and as such will be expected to approve logistical products prior to them going to the SAC. Any policy implications associated with a logistical product should be considered and addressed at the LAC level prior to it going to the SAC for approval. Inclusion of a senior level F/P/T program policy representative on the LAC is intended to facilitate this process.
The LAC will be largely focused on how the response activities will be implemented in order to achieve the response objectives. The LAC will be co-chaired by the F/P/T co-chairs of the Public Health Infrastructure Steering Committee (PHI-SC) or their designates. Further details regarding the LAC composition and activities can be found in Appendix G: Logistics Advisory Committee Terms of Reference. Under the LAC, task groups will be established (or pre-existing groups may be engaged) to address the logistical response functions (e.g., policy support, mutual aid, deployable resources) and to provide logistical input into other governance structure products such as communication products. A generic terms of reference for these task groups can be found in Appendix K: Task Groups Generic Terms of Reference. A list of existing groups that may be enlisted or leveraged to function as an operational or logistical task group during a response can be found in Appendix I: Existing Committees, Working Groups and other Expert Resources. Upon request these task groups would develop products such as funding agreements, aid agreements and recommendations regarding resource acquisition and utilization. The LAC would inform the task groups when their expected products are to include a policy perspective so that incorporation of this content could be included early in the development of the product. The LAC would also oversee the engagement of health care delivery stakeholders as required for the response.
Health Portfolio Operations Centre (HPOC)
The HPOC serves as the Health Portfolio 'single window' for the coordination of response activities to significant public health events of national interest within the Health Portfolio's mandate, and acts as the point of contact for providing emergency management governance support and operational communications. HPOC supports and facilitates emergency operations by expediting and facilitating the sharing of information and supporting F/P/T response activities and communication in coordination with the operations centres of the PHAC regions, National Microbiology Laboratory (NML) and P/T ministries of health. The HP employs an emergency response structure modeled after the Incident Management System (IMS). For the purposes of this plan, the fully escalated HPOC IMS is not described here however the HPOC is included in Figure 2 to illustrate how it will support the governance structure.
Within this plan's governance structure, the HPOC:
- may provide support to both the Technical and Logistics streams by supplying a Technical Support Team and Logistics Support Team which will provide administrative and other coordination support to the TAC and LAC as required.
- is represented in the governance by the by the HPOC Operations Chief who is a member of the TAC and the HPOC Logistics Chief who is a member on the LAC.
- the HPOC Planning Chief, in coordination with the F/P/T SAC Secretariat, facilitates planning meetings which are used to receive and anticipate F/P/T needs or issues which will then be triaged and prioritized for action.
- the HPOC Planning Group is responsible for development of the F/P/T incident action plan.
Further, the federal members of the F/P/T SAC Secretariat are members of the Policy Group within the HPOC IMS and will provide situational awareness to the HPOC Event Manger and HPOC IMS regarding the activities of the governance streams. It is expected the HPOC Event Manager and other HPOC IMS representatives will be requested to participate in SAC meetings by the CPHO or other PHAC official.
Additional overall support functions of the HPOC including single window, business cycle, situation reports and liaison response functions, etc., will be carried out per the HPOC's normal response processes and as required to support the event and the HPOC IMS will be activated accordingly by PHAC senior management. For additional details on activities of the HPOC under this plan refer to Section 3.4: Initial Response Planning and Capability Assessment.
Appendix A – Glossary of Terms and List of Acronyms
For the purpose of this plan the following terms are defined as indicated below. The definitions come from various sources including to but not limited to the CPIP, MLISA, IHR, Federal Emergency Response Plan (FERP), F/P/T MOUs and Government of Canada Emergency Management Vocabulary among others.
- affected
- Is meant to encompass the occurrence of an impact (e.g., illness) and/or the presence of risk. Provinces and territories will determine whether they are affected or not; this may change over time and also will be influenced by the degree of perceived risk
- all hazards
- Referring to the entire spectrum of hazards, whether they be natural or human-induced
- all hazard emergency response
- An emergency management approach that recognizes that the actions required to mitigate the effects of emergencies are essentially the same, irrespective of the nature of the incident, thereby permitting an optimization of planning, response and support resources.
- biological
- Relating to living organisms
- biological agent
- Biological agents are living organisms that include bacteria, viruses, fungi, other microorganisms and their associated toxins. They have the ability to adversely affect human health in a variety of ways, ranging from relatively mild, allergic reactions to serious medical conditions and death. These organisms are widespread in the natural environment; they are found in water, soil, plants, and animals. Anthrax and ebola are examples of biological agents
- CBRNE events
- A potential, perceived or actual act with chemical, biological, radiological, nuclear or explosive materials that are, or are suspected to be, used in a deliberate or intentional way to cause harm
- centralized planning
- Response requirement assessment, objective setting and implementation direction that occurs in an F/P/T forum with leadership from senior-level decision makers
- concept of operations
- A concise description of how an organization(s) is to operate in order to achieve specific goals
- consequence management
- The coordination and implementation of measures and activities undertaken to alleviate the harm, loss, hardship and suffering caused by an emergency
- coordinated F/P/T response
- Led by senior-level public health decision-makers at the federal, provincial and territorial level, those actions identified and planned in an F/P/T forum to facilitate a consistent approach across jurisdictions to event-specific response activities. Does not need to be multi-jurisdictional - may be implemented for a biological public health event in a single P/T with the potential for spread to, or involvement of another P/T (eg. mutual aid). Considered activation of response Level 3 or Level 4 of this plan
- disease
- An illness or medical condition, irrespective of origin or source, that presents or could present significant harm to humans
- emergency
- A present or imminent event that requires prompt coordination of actions concerning persons or property to protect the health safety or welfare of people, or to limit damage to property or the environment
- emergency management
- The management of emergencies concerning all hazards, including all activities and risk management measures related to prevention and mitigation, preparedness, response and recovery
- epidemic
- Outbreak of infection that spreads rapidly and affects many individuals in a given area or population at the same time
- epidemiology
- The study of the distribution and determinants of health-related states or events in specified populations, and the application of this study to control of health problems
- event
- Manifestation of a disease or an occurrence that creates a potential for disease
- federal populations
- Those populations for which the federal government either provides health care benefits, goods and/or services or reimburses the cost of providing health care benefits
- F/P/T governance structure
- A coordination structure and common set of roles and responsibilities for federal, provincial and territorial governments within the health sector
- F/P/T response
- Those activities jointly taken by public health authorities representing the federal government and a province or territory in response to a public health event.Footnote 12
- hazard
- A potentially damaging physical event, phenomenon or human activity that may cause the loss of life or injury, property damage, social and economic disruption or environmental degradation
- hazard-specific
- Strategies for managing a specific hazard. Hazard-specific annexes explain the procedures that are unique to a hazard type and may be short or long depending on the details needed to explain the actions, roles, and responsibilities. The information in these annexes is not repeated elsewhere in the plan.
- Health Portfolio
- The Health Portfolio supports the federal Minister of Health in maintaining and improving the health of Canadians. It includes Health Canada, the Public Health Agency of Canada, the Canadian Food Inspection Agency, the Canadian Institutes of Health Research, and the Patented Medicine Prices Review Board. For the purposes of this plan, the term as used throughout this document refers to the Public Health Agency of Canada and Health Canada
- incident action plan
- At an operational level, formally documents response goals/ objectives and an overall response strategy - contains general tactics to achieve goals/objectives while providing important information on event and response parameters
- incident management system
- A standardized on-scene emergency-management concept specifically designed to allow its user(s) to adopt an integrated organizational structure equal to the complexity and demands of single or multiple incidents, without being hindered by jurisdictional boundaries
- infectious disease
- A disease that is caused by an infectious agent or biological toxin
- initial assessment
- Assessments that occur before the need for a coordinated F/P/T response identified under this plan. It is recognized that initial assessments therefore could occur more than once for the same event in the form of a repeat or follow-up assessment.
- lessons learned
- A lesson identified for which validated remedial action may be implemented, resulting in a tangible improvement in performance or capability
- medical counter measures
- Refers vaccines, antimicrobials, therapeutics, and diagnostics that address the public health and medical consequences of chemical, biological, radiological, and nuclear events; pandemic influenza; and emerging infectious diseases
- mitigation/prevention
- Involves actions intended to reduce the risk of emergencies and/or their impacts. These actions can take various forms, for example, surveillance for the early identification of risks, or the use of regulations to ensure the safety of consumer products
- pandemic
- An epidemic occurring worldwide, crossing international boundaries and usually affecting a large number of people
- pathogen
- Any disease-producing microorganism or material
- preparedness
- Is a phase of emergency management consisting of making decisions and taking measures before an emergency, in order to be ready to effectively respond and recover. Emergency planning (including business continuity planning), training and exercises, the stockpiling of prophylaxis drugs and having in place MOUs with external stakeholders to ensure that services, facilities and equipment are secured in an emergency are considered preparedness activities
- Public health
- An organized activity of society to promote, protect, improve, and when necessary, restore the health of individuals, specified groups, or the entire population. It is a combination of sciences, skills, and values that function through collective societal activities and involve programs, services, and institutions aimed at protecting and improving the health of all people
- public health emergency
- Means an extraordinary, unexpected, or unusual event which is determined by application of the criteria in Annex B of the MOU on the Sharing of Information During a Public Health Emergency (which was derived and adapted from Annex 2 of the IHRs)
- public health emergency of international concern
- Defined in the International Health Regulations (2005) as "an extraordinary event which is determined, as provided in these Regulations: to constitute a public health risk to other States through the international spread of disease; and to potentially require a coordinated international response". This definition implies a situation that: is serious, unusual or unexpected; carries implications for public health beyond the affected State's national border; and may require immediate international action.
- public health event that is biological in nature
- A manifestation of illness in a population or a significant occurrence that creates the risk of illness in a population due to a biological agent for which a public health response would be required to mitigate the associated impact or risk
- public health information
- Any information, including aggregate information, sub-aggregate information, record level information, identifiable information and any other information, that is governed by the MLISA
- Public health measure
- Non-pharmaceutical interventions that can be taken by individuals and communities to help prevent, control or mitigate infectious disease. Public health measures range from actions taken by individuals (e.g., hand hygiene, self-isolation) to actions taken in community settings and workplaces (e.g., increased cleaning of common surfaces) to those that require extensive community preparation (e.g., pro-active school closures)
- Public health risk
- A likelihood of an event that may affect adversely the health of human populations
- recovery
- Is a phase of emergency management consisting of activities aimed at restoring normal conditions after an emergency
- response
- Actions taken during or immediately before or after an emergency to manage its consequences and minimize suffering and loss
- response plan
- A risk-based plan developed and maintained to respond to an emergency. This type of plan includes operational plans which are generally geared to tasks and actions and provide the detail required for a coordinated response to specific hazards identified through a risk assessment process and tactical plans which generally focus on managing personnel, equipment, and resources that play a direct role in the on-site response to an emergency
- risk
- The combination of the likelihood and the consequence of a specified hazard being realized; refers to the vulnerability, proximity or exposure to hazards, which affects the likelihood of adverse impact
- risk assessment
- A component of risk management meant to provide evidence-informed information and analysis for making informed decisions on how to treat particular risks and select between options
- scalable
- The equal applicability of this plan for F/P/T response to public health events which can be applied in whole or in part, (as much as needed), depending on increasing/decreasing scope and intensity of the event
- situation report
- A report that provides current information about an emergency, immediate and future response actions, an analysis of the impact of the emergency and issues identification
- surveillance
- The routine, systematic and ongoing collection, collation and analysis of public health information for public health purposes
- whole of government response
- An approach that aligns the efforts of all jurisdictions so that all potential resources can be applied to minimizing the event's negative health, social and economic impacts. Public Safety Canada is responsible for coordinating the whole of government response when the federal government is involved in the response to an emergency. Within the provinces and territories a similar function is performed by the appropriate ministry or emergency measures organization
List of Acronyms
- ADM
- Assistant Deputy Minister
- AMMI
- Association of Medical Microbiology and Infectious Disease Canada
- CATMAT
- Committee to Advise on Tropical Medicine and Travel
- CBRNE
- Chemical, Biological, Radiological/Nuclear, Explosive
- CCDIC
- Centre for Communicable Diseases and Infection Control
- CCMOH
- Council of Chief Medical Officers of Health
- CDMH
- Conference of Deputy Ministers of Health
- CEPR
- Centre for Emergency Preparedness and Response
- CFIA
- Canadian Food Inspection Agency
- CIHR
- Canadian Institutes of Health Research
- CIRID
- Centre for Immunization and Respiratory Infectious Diseases
- CPHLN
- Canadian Public Health Laboratory Network
- OICC
- Outbreak Investigation Coordinating Committee
- CID-SC
- Communicable and Infectious Disease Steering Committee
- CONOPS
- Concept of Operations
- CPHO
- Chief Public Health Officer
- CPIP
- Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector
- EOC
- Emergency Operations Centre
- EVD
- Ebola virus disease
- FERP
- Federal Emergency Response Plan
- FIORP
- Food-borne Illness Outbreak Response Protocol
- F/P/T
- Federal/Provincial/Territorial
- F/P/T DM
- Federal/Provincial/Territorial Deputy Minister
- GOC
- Government Operations Centre
- GPHIN
- Global Public Health Intelligence Network
- HC
- Health Canada
- HCW
- Health Care Workers
- HP
- Health Portfolio
- HPOC
- Health Portfolio Operations Centre (federal)
- HSIB
- Health Security Infrastructure Branch
- IDPCB
- Infectious Diseases Prevention and Control Branch
- IHR
- International Health Regulations
- IPC
- Infection Prevention and Control
- IMS
- Incident Management System
- IPC
- Infection Prevention and Control
- LAC
- Logistics Advisory Committee
- MCM
- medical countermeasures
- MERS
- Middle East Respiratory Syndrome
- MLISA
- Multilateral Information Sharing Agreement
- MOU
- Memorandum of Understanding
- NACI
- National Advisory Committee on Immunization
- NESS
- National Emergency Strategic Stockpile
- NGO
- Non-governmental Organization
- NML
- National Microbiology Laboratory
- Occ Health
- Occupational Health
- OFMAR
- Operational Framework for Mutual Aid Surge Requests for Health Care Professionals
- PH
- public health
- PHAC
- Public Health Agency of Canada
- PHEIC
- Public Health Emergency of International Concern
- PHI-SC
- Public Health Infrastructure Steering Committee
- PHN
- Public Health Network
- PHNC
- Public Health Network Council
- PHN CG
- Public Health Network Communications Group
- P/T
- Provincial/Territorial
- POE
- Ports of Entry
- RCMP
- Royal Canadian Mounted Police
- SAC
- Special Advisory Committee
- SARS
- Severe Acute Respiratory Syndrome
- TAC
- Technical Advisory Committee
- TOR
- Terms of Reference
- WHO
- World Health Organization
Appendix B – Plan Development: Guiding Principles
The guiding principles for the development of this plan and anticipated response activities associated with this plan were derived from a review of best practices and lessons learned documents; specifically an intensive review of the governance structure utilized during the F/P/T response to the H1N1 influenza pandemic in 2008-9. The latter, involved a structured survey of responders engaged at various levels within the H1N1 governance structure. A total of 38 individuals from the federal, provincial, territorial and regional levels provided responses which informed the following list of guiding principles.
Efficiency – the response should be as efficient as possible. This could be achieved by utilizing emergency management principles such as issue triage and management by objectives. For example, the response must include clear requests for appropriate deliverables, delegation of tasks and responsibilities, and strong adherence to meeting management principles, in order to ensure every responder's time is used efficiently. In addition, where there are expert groups or standing committees that regularly meet (i.e., when a response is not underway); these groups could be leveraged during a response. There also needs to be awareness of what other groups are doing, issues that "overlap", and expectations for products (including timelines, focus and target audience) in order for the response to proceed efficiently.
Timeliness – During a response it is important that deliverables are produced, approved and distributed in a timely manner in order to facilitate optimal use and maintain credibility and public confidence. It has been observed that health professionals and the public will seek and use information developed outside of Canada for other populations if Canadian response actions are not timely.
Transparency – Participants in the response need to be able to see how their deliverables (including but not limited to recommendations) are being received, assessed and possibly amended by decision-makers in order to have confidence in the value of their work. For example, if recommendations are not accepted or deliverables are amended, the rationale for these decisions should be provided as feedback to the originators of the work.
Commitment – There needs to be a commitment to functioning differently during an emergency response (i.e., not just doing the same thing faster), specifically by embracing emergency management principles. Commitments regarding surge capacity and/or mutual aid also need to be considered and respected.
Engagement – In addition to increasing awareness of the roles and responsibilities of various groups within the response governance structure, improving linkages/engagement between working groups, with external to government subject matter experts, and amongst public health, health care delivery and emergency management authorities, will serve to improve the overall goal and objectives of this response plan.
Representativeness – All provinces, territories and federalFootnote 13 authorities will be involved in the decision-making process for issues that have significant resource or policy implications for their jurisdiction/population-served. Similarly all provinces, territories and federal authorities will have the opportunity to participate in the approval process for deliverables (e.g., recommendations, protocols) that are expected to be followed/utilized in their respective jurisdictions/populations during the public health response.
Note: some deliverables (e.g., research summaries, guidance documents, treatment guidelines) may be developed by PHAC or external to government subject matter experts for use by the provinces and territories as they deem appropriate.
Additional guiding principles derived from a review of best practices include the following:
Health Equity – Response activities should be implemented in a manner that facilitates health equity. Health equity means that all people can reach their full health potential and should not be disadvantaged from attaining it because of their race, ethnicity, religion, gender, age, social class, socioeconomic status or other socially determined circumstance. The World Health Organization (WHO) defines social determinants of health as the circumstances in which people are born, develop, live and age and the systems put in place to deal with illness.
Flexibility – During a response actions taken should be tailored to the situation and subject to change as new information becomes available. F/P/T governments are expected to work collaboratively to facilitate a consistent response to F/P/T public health response objectives; however it is recognized that at any one point during the response the objectives of the response may vary from jurisdiction to jurisdiction within Canada depending on the local impact of the public health event and risk assessments. Flexibility is required in order to adapt the response to the evolving public health event.
Effectiveness – The potential effectiveness of a response action needs to be considered prior to implementation. Effectiveness is considered to be the extent to which a specific intervention, procedure, regimen, or service, when deployed in the field, does what it is intended to do for a defined populationFootnote 14.
Ethical decision-making – ethical principles and societal values should be explicit and embedded in all decision-making, including the processes used to reach decisions. It is especially important to ensure that all actions respect ethical guidelines tailored to the concerns of public health, while respecting the rights of individuals as much as possibleFootnote 15.
Evidence-informed decision-making – Decisions should be based on the best available evidence to the extent possible. It is recognized that other factors also enter into decision-making, such as legal and institutional constraints, values, costs and availability of resources.
Appendix C – Main F/P/T Roles and Responsibilities
During an F/P/T public health response the federal government's responsibilities may include but are not limited to:
- facilitating the coordination of the overall F/P/T response;
- supporting the development of technical guidance, technical and policy recommendations, protocols, and other products that may be required to facilitate a consistent F/P/T response;
- acting as the national focal point for the WHO on all IHR (2005) matters and managing all international aspects of the response to a public health event caused by a biological agent (e.g., technical discussions, aid requests);
- seeing that risk assessments are prepared and communicated, as required;
- facilitating access to surge capacity (from federal programs, if needed) with regards to employees and resources (including mobilizing medical supplies in the National Emergency Strategic Stockpile), to support P/T responses as required;
- facilitating the acquisition of extra medical supplies through Procurement Services and Purchasing Canada and other federal agencies as appropriate;
- providing travel health notices and other health-related information relevant to international travel;
- exercising powers under the Quarantine Act to protect public health by taking comprehensive measures to help prevent the introduction and spread of communicable diseases in Canada. Such measures may include, but are not limited to, the screening, examining and detaining of arriving and departing international travellers, conveyances (e.g., airplanes and cruise ships) and their goods and cargo;
- providing regulatory authorization to market medical countermeasures (i.e., medications and vaccines);
- acting as the focal point for vaccine manufacturers and international regulatory collaboration;
- providing regulatory authorization to conduct clinical trials;
- negotiating with manufacturers and establishing contracts for the F/P/T purchase of medical countermeasures and/or medical equipment (e.g., ventilators);
- conducting national monitoring of adverse reactions to medications and vaccines;
- providing medications and/or vaccines to federal populations not covered by arrangements for P/T provision; and
- provision of health services, medications, supplies and equipment for specified federal populationsFootnote 16/employees who normally access federally operated health care services.
During an F/P/T public health response provincial/territorial governments' responsibilities may include but are not limited to:
- providing health care services to individuals within their jurisdictions, including federal populations while leveraging agreements that are in place. Contributing to the development, review and approval of technical guidance, technical and policy recommendations, protocols, and other products that may be required to facilitate a consistent F/P/T response;
- communications response and messaging within their jurisdictions with the understanding that messaging should be coordinated for consistency of information and response through the PHN CG;
- conducting surveillance and reporting data to the federal level as required under the International Health Regulations and as agreed upon for the duration of the public health event response;
- providing medications and/or vaccines to recommended populations;
- sharing information regarding distribution and use of medications and vaccines in their respective jurisdictions;
- monitoring and reporting adverse vaccine reactions;
- developing plans to increase surge capacity;
- developing and maintaining memoranda of understanding and protocols, as needed, to facilitate interprovincial/territorial movement of patients and licensed health care professionals during a response and other aspects of mutual aid;
- developing, as necessary, a strategy for collecting and monitoring data on health care service use;
- ensuring the provision of medications, supplies and equipment required for provision of health care services; and
- working collaboratively to establish protocols and guidelines for prioritizing health care services during times of high service demand and staff or supply shortages in their respective jurisdictions.
During an F/P/T public health response both federal and provincial/territorial governments' responsibilities may include but are not limited to:Footnote 17
- promptly implementing surveillance standards and protocols;
- implementing standardized laboratory standards and protocols;
- establishing and supporting F/P/T policies and recommendations on the use of medical countermeasures (MCM), specifically medications and/or vaccines;
- collaborating on strategies to mitigate the consequences of potential insufficient or delayed MCM;
- developing and implementing public health guidance according to response objectives;
- facilitating development and dissemination of clinical care guidance as needed;
- ensuring the specific needs of federal populations are reflected in the overall F/P/T response;
- implementing/adapting an F/P/T communication response that considers the linguistic, literacy and cultural diversity of Canada and allows for the alignment of messaging by F/P/T jurisdictions where appropriate;
- communicating and engaging with the general public, media and stakeholder groups regarding F/P/T plans and response activities;
- establishing/implementing protocols for timely sharing of information, including but not limited to surveillance information, jurisdictional communications, strategies and messaging, and response interventions and impacts; and
- identifying and addressing rapid research response priorities and leveraging existing research undertakings.
Appendix D – Special Advisory Committee (SAC) Roles and Responsibilities under the F/P/T Public Health Response Plan
Role
When activated, the Special Advisory Committee (SAC) has a time-limited mandate to provide advice to the F/P/T Conference of Deputy Ministers of Health (CDMH) pertaining to the coordination, public health policy and technical content on matters related to response to a significant public health event. The SAC co-chairs will provide regular updates to the CDMH as required based on the urgency of the issue (at co-chairs' discretion).
Within the context of the F/P/T Public Health Response Plan, products may be developed within the governance structure that include, but are not limited to: recommendations, guidance documents, protocols, and communication products. SAC will be the main forum for F/P/T approval/endorsement of such products, however, for purely technical products (e.g., surveillance case definitions, laboratory testing protocols) the SAC may choose to delegate approval to the Technical Advisory Committee (TAC). If necessary, the SAC will involve the CDMH or the Deputy Minister Liaison in decision-making either by an expression of interest by the CDMH or when the scale of the public health event and subsequent resource implications for the response activities indicate.
Membership and Participation
The Special Advisory Committee will consist of the members of the Pan-Canadian Public Health Network Council (PHNC) and the Council of Chief Medical Officers of Health (CCMOH). Members will identify a designated alternate with decision-making authority to act as a back-up when necessary.
The Special Advisory Committee will be chaired by the co-chairs of the PHNC. The designated alternate co-chairs will be the chair of the CCMOH and the Vice President of the Public Health Agency of Canada (PHAC) Health Security Infrastructure Branch (HSIB).
Jurisdictions are encouraged to include their respective emergency management representatives, and any other relevant P/T health system representatives on SAC meeting teleconferences.
In the context of the F/P/T Public Health Response Plan for Biological Events, members will include representatives from each province and territory and federal population (e.g., First Nations) affected by the public health event; representatives from un-affected areas (if applicable) have the option of providing a representative to this group. This committee will be supported by the F/P/T SAC Secretariat for response planning and tasking and for strategic policy and product integration support.
Responsibilities
The Special Advisory Committee is responsible for advising on the coordination of F/P/T preparedness and response planning across the health sector and has the responsibility to make recommendations related to technical, logistical and communication issues in public health. Products that will be reviewed by the CDMH will be brought forward by the SAC co-chairs with the assistance of the F/P/T SAC Secretariat.
The Special Advisory Committee will also coordinate with other sectors related to response. Members of the Special Advisory Committee will act as liaisons to the health care sector within their respective jurisdictions and then provide jurisdictional views to the Special Advisory Committee to ensure that the full continuum of the health sector is considered when undertaking preparedness and response planning.
Activities
The SAC will:
- activate and "sunset" the Technical Advisory Committee (TAC), Logistics Advisory Committee (LAC) and Public Health Network Communication Group within the context of the governance structure as needed for the response;
- engage other government departments and NGOs as needed;
- conduct SAC meetings;
- review and discuss governance structure products/proposed actions (including strategic policy content) and either approve/endorse governance structure products/actions, and forward to CDMH for decision as necessary, or, provide feedback to the F/P/T SAC Secretariat and the product developers for further revisions.
SAC meetings
SAC meetings will occur as needed based on the pace of the evolving public health event and the need to review, discuss and approve/endorse governance structure products. These meetings will be conducted by teleconference and participants will include: SAC members, liaisons and invited external representatives (based on agenda). The focus of these meetings will be the review and approval/endorsement of governance structure products or the provision of feedback to the F/P/T SAC Secretariat and product developers for further revisions.
The meeting will also be a forum for the provision of jurisdictional views to ensure that the full continuum of the health sector is considered when undertaking response activities.
Products
SAC will produce (via advisory committees and task groups active in the governance structure) approved/endorsed technical, logistical and communication oriented documents, including recommendations/advice for the CDMH when required. The results of SAC meetings will be captured in minutes of meetings including a record of decision developed by the F/P/T SAC Secretariat.
Quorum and Decisions
Quorum for SAC meetings shall be attendance by a simple majority of members (or their respective designated alternates).
Decision-making will be made by consensus where consensus is defined as agreement that all can "live with" the decision. Majority opinion will be taken as the decision and/or recommendation to the CDMH. Approval or endorsement decisions will be in the context of the F/P/T response and response objectives. F/P/T governments recognize that each jurisdiction can decide whether or not to implement approved products according to its individual needs, response objectives, and/or legislative frameworks. Minority dissenting opinions will be noted and communicated to the CDMH.
Activation and Deactivation
In the context of the F/P/T Public Health Response Plan, the SAC will be activated to facilitate the strategic/policy response to F/P/T public health events that are of a scope and/or urgency to warrant a Level 3 – Escalated or Level 4 – Emergency response. The co-chairs of the PHNC, the chair of the CCMOH and the Deputy Minister Liaison will decide when to activate and deactivate the SAC based on assessments, situational analysis and requests received from P/T public health authorities and recommendations made by the F/P/T SAC Secretariat.
Appendix E – F/P/T SAC Secretariat Roles and Responsibilities under the F/P/T Public Health Response Plan
Role
The F/P/T SAC Secretariat will support the SAC by assuming multiple coordination functions any time when the SAC is activated. Under this plan, a SAC is activated for a coordinated F/P/T response which for the purposes of this plan is a Level 4 - Emergency responseand a Level 3 – Escalated response. In this governance structure, the F/P/T SAC Secretariat manages the intersection between the SAC and the three response streams of the governance: the Technical Advisory Committee (TAC), the PHN Communications Group, and the Logistics Advisory Committee (LAC) and will provide cross-stream support through the planning and tracking of tasks.
Key responsibilities
The F/P/T SAC Secretariat, with the support of the HPOC Incident Management System (IMS) structure, will be responsible for rapid centralized analysis of issues and F/P/T response needs, prioritization and distribution of tasks, with the aim of improving efficiency, increasing situational awareness, and facilitating engagement of external resources. This will be achieved through liaison with the SAC co-chairs and through planning meetings convened with the HPOC Planning Group and co-chairs of the TAC, LAC and PHN Communications Group.
Specifically the F/P/T SAC Secretariat, with direction from SAC co-chairs, will identify what type of product/action is required, task this to the appropriate group(s) within the governance structure and monitor progress. The F/P/T SAC Secretariat will identify and prioritize response issues and F/P/T response needs in consultation with the SAC co-chairs and co-chairs of the TAC, LAC and PHN Communications Group and will provide a mechanism for P/Ts, other groups within the governance structure, and other stakeholders to bring their issues forward to the SAC.
It is expected that the F/P/T SAC Secretariat will routinely liaise with the co-chairs of the SAC, TAC, LAC and PHN Communications Group and with the HPOC Event Manager as required throughout the response. The F/P/T SAC Secretariat with the support of the HPOC, will convene situational awareness and planning meetings as described below.
Applying Strategic Policy Lens and Product Integration
Another key activity of the F/P/T SAC Secretariat is to apply a strategic policyFootnote 18 lens to all products (developed by groups within the governance structure) that are being brought forward to SAC for discussion, approval and/or endorsement. It is also responsible to integrate products developed by different streams within the governance structure as requested by SAC.
Product integration would include combining content developed by the multiple streams into one document in which case the F/P/T SAC Secretariat will be formatting the final combined product and adding any summaries or strategic policy analysis but will not make any fundamental changes to the previously approved/endorsed content. An example of when this would occur is if SAC requested a single product that includes technical recommendations, logistical issues and a communication response (or any combination of work from two streams) – such as a vaccine response strategy. This process is intended to help expedite and support evidence-informed decision-making by the SAC.
The F/P/T SAC Secretariat will be the contact point for P/Ts, other groups within the governance structure, and other stakeholders to bring their issues forward to the SAC (note: focus should be on F/P/T issues). The HPOC IMS Planning Group may support this process through maintenance of activity trackers or other tools. The mechanism for bringing issues to the F/P/T Secretariat will be via emailFootnote 19 to the HPOC.
Where practical, the results of planning and situational awareness meetings will be captured in meeting minutes/record of decisions taken by HPOC IMS staff but also in situation reports and incident action plans developed by the HPOC IMS Planning Group.
Note: The F/P/T SAC Secretariat is not responsible for approving documents (aside from verifying the F/P/T incident action plan).
Activities
The F/P/T SAC Secretariat will:
- be the coordination point for receiving issues that may require an F/P/T response action and discussion with SAC;
- in consultation with SAC co-chairs and through planning meetings with HPOC, TAC, LAC and PHN Communications Group co-chairs, identify what type of product/action is required and which group within the governance structure will be the lead on developing the product or completing the action;
- track progress towards completion of product/action;
- convene situational awareness calls and hold planning meetings as needed or requested by SAC co-chairs;
- policy analysis and option development;
- product and strategic policy integration:
- directly supports federal and P/T SAC co-chairs and SAC members and Deputy Minister Liaison at SAC meetings teleconferences or CDMH meetings;
- Links the SAC with federal and P/T inter-governmental relations officials.
Convening Planning Meetings
The F/P/T SAC Secretariat, with coordination support from the HPOC IMS will convene planning meetings whose frequency will depend on the pace of the evolving public health event. HPOC IMS staff will be present for agenda support and coordination and minutes. These meetings will be conducted by teleconference and participants will include: co-chairs from the activated committees within the governance structure (TAC, PHN Communications Group and LAC) and HPOC IMS Planning Group representatives for incident action plan development. This limited participation (compared to situational awareness meetings) is expected to facilitate responsiveness and efficiency.
The purpose of these meetings will be to receive and anticipate F/P/T needs or issues which will then be triaged by the group and prioritized for action. The group as a whole will identify what products/actions are required and the F/P/T SAC Secretariat will delegate tasks to appropriate groups (i.e. the TAC, the LAC, Public Health Network Communications Group, or PHAC). This delegation of tasks will determine the required approval process for the products. The meeting participants will also set expected timelines for each product/action and these will be documented in the F/P/T incident action plan and reported on at the situational awareness meetings. The F/P/T incident action plan is developed by HPOC Planning Group and will be forwarded to the SAC via the F/P/T SAC Secretariat for approval. The incident action plan will document the objectives of the F/P/T response. Planning meetings will be used to review these objectives on an ongoing basis and to determine when to recommend to SAC changing them in order to direct response activities appropriately.
Convening Situational Awareness Meetings
The F/P/T SAC Secretariat, with the coordination support of the HPOC will convene meetings for situational awareness as needed based on the pace of the evolving public health event or at the request of SAC co-chairs. These meetings will be conducted by teleconference and participants will include a broader audience than planning meetings: SAC co-chairs and members, co-chairs from the activated committees within the governance structure as well as activated task group leads, CCMOH members (i.e., those not already engaged), P/T EOC representatives, PHAC regional representatives, HPOC and HPOC Event Manager, NML IMS Operations and Planning Chiefs, federal population representatives and potentially NGOs (if involved/affected by the response). The P/T participants will be determined by the individual provinces and territories. The Director General, Centre for Emergency Preparedness and Response (CEPR) or the appointed HPOC Event Manager will chair the meetings.
The purpose of these meetings will be to receive epidemiological situation updates from the P/T representatives, international updates (as indicated) from the HPOC IMS, and to confirm the F/P/T response objectives and objectives of individual jurisdictions if they differ from the F/P/T response objectives. This meeting will also be an opportunity to receive update on F/P/T incident action plan progress (e.g. approvals made by SAC or by the CDMH). The meeting will also be the forum for the provision of updates on research findings and communication products and tactics.
Quorum and Decisions
Decision-making will be made by consensus.
Activation and Deactivation
The F/P/T SAC Secretariat will be formed as part of activation of a SAC in order to facilitate the response to F/P/T public health events that are of a scope and/or urgency to warrant a Level 4 – Emergency response or Level 3 – Escalated response under this plan.
Appendix F – Technical Advisory Committee (TAC) Terms of Reference
Role
The Technical Advisory Committee (TAC) will direct requests for products/actions (e.g., recommendations, guidelines, protocols) received from the F/P/T SAC Secretariat to the appropriate technical task groups. The TAC will provide a forum for vetting/sharing of technical information amongst task groups, and will be responsible for the inclusion of a program policy analysis as needed for technical products. The TAC will approve/endorse technical products/actions that will go to the Special Advisory Committee (SAC) for approval/decision via the F/P/T SAC Secretariat. The SAC may choose to delegate the approval of purely technical products to the TAC.
Membership
The TAC will be co-chaired by the F/P/T co-chairs of the Communicable Infectious Disease Steering Committee (CID-SC) or their designates. Members will include technical representatives from each P/T and federal population (e.g., First Nations) affected by the event; representatives from un-affected areas (if applicable) have the option of providing a representative to this group. All F/P/T representatives (and their designated alternates) should have decision-making authority for their respective jurisdiction on technical issues; these representatives will be identified by the provinces and territories. It is recognized any P/T may have limited ability to participate in TAC due to resource demands in its own jurisdiction. An F/P/T policy representative will also be on this committee in order to provide advice and support on program policy issues. Additional (non-voting) members will include: the HPOC IMS Operations Chief, and technical task group leads. Members will identify a designated alternate to act as a back-up when necessary. Liaisons (e.g., SAC liaison, F/P/T SAC Secretariat liaison, liaison from the Conference of Deputy Ministers of Health [CDMH]) and external experts may attend TAC meetings but would also not be voting members of the group.
This committee will be supported by a Technical Support Team (supplied by PHAC through the HPOC) if required. Cross-stream coordination with the Logistics and Communications streams will be supplied by the F/P/T SAC Secretariat and HPOC.
Activities
The TAC will:
- Activate and "sunset" technical task groups as needed
- Refer tasks (i.e., requests for products from the F/P/T SAC Secretariat) to appropriate task groups
- Engage NGO or research community (or P/T agencies) as needed
- Conduct TAC meetings
- Consider the policy implications of TAC recommendations and include policy content in products as necessary
- Approve/endorse technical products/actions (e.g., guidance documents, recommendations, protocols) that are being sent to F/P/T SAC Secretariat for preparation/strategic policy analysis before going to SAC for approval/decision
TAC meetings
TAC meetings will occur as needed based on the pace of the evolving event and the need to review technical products or proposed actions. These meetings will be conducted by teleconference and participants will include: TAC members, liaisons and invited external experts (based on agenda).
These meetings will provide a forum for technical task groups to receive direction from TAC, seek input and provide updates on products under development, share technical information amongst task groups (e.g., case definitions, epidemiological characteristics) – to ensure consistency between task group products, and to provide products for TAC approval/endorsement. The meeting will also be a forum for the provision of updates on research findings (that would subsequently be shared at situational awareness meetings), modelling work and risk assessments. Any technical issues that need to be actioned should also be raised at this meeting so that the TAC chair can bring them to the attention of F/P/T SAC Secretariat as needed.
The results of TAC meetings will be captured in records of decisions by the Technical Support Team provided by HPOC.
Products
TAC will produce (via task groups) approved technical documents, including recommendations for the SAC and CDMH. All products will go to SAC by way of the F/P/T SAC Secretariat who will integrate content from other streams and/or do a strategic policy analysis as needed, prior to sending the products to SAC.
Quorum and Decisions
Quorum for TAC meetings shall be attendance by a simple majority of voting members (or their respective designates).
Decision-making will be made by consensus where consensus is defined as agreement that all can "live with" the decision. Approval or endorsement decisions will be in the context of the F/P/T response and response objectives. F/P/T governments recognize that each jurisdiction can decide whether or not to implement TAC products according to its individual needs, response objectives, and/or legislative frameworks. Minority dissenting opinions will be noted and communicated to the F/P/T SAC Secretariat.
Activation and Deactivation
The TAC will be activated by SAC to facilitate the technical response to F/P/T public health events that are of a scope and/or urgency to warrant a Level 3 – Escalated or Level 4 – Emergency response. The SAC will decide when to activate and deactivate the TAC based on situational analysis and requests received from P/T public health authorities and recommendations made by the F/P/T SAC Secretariat.
Appendix G – Logistics Advisory Committee (LAC) Terms of Reference
Role
The Logistics Advisory Committee (LAC) will direct requests for products/actions (e.g., recommendations, guidelines, protocols) received from the F/P/T SAC Secretariat to the appropriate logistics task groups. The LAC will provide a forum for vetting/sharing of information amongst the logistical task groups, and will be responsible for the inclusion of a program policy analysis as needed for logistical products. The LAC will approve/endorse products/actions that will go to the Special Advisory Committee (SAC) for approval/decision via the F/P/T SAC Secretariat.
Membership
The LAC will be co-chaired by the F/P/T co-chairs of the Public Health Infrastructure Steering Committee (PHI-SC) or their designates. Members will include representatives from each P/T and federal population (e.g., First Nations) affected by the public health event; representatives from un-affected areas (if applicable) have the option of providing a representative to this group. All F/P/T representatives (and their designated alternates) should have decision-making authority for their respective jurisdiction on logistical and policy issues; these representatives will be identified by the provinces and territories. It is recognized any P/T may have limited ability to participate in LAC due to resource demands in its own jurisdiction. A policy representative will also be on this committee in order to provide advice and support on program policy issues. Additional (non-voting) members will include: the HPOC IMS Logistics Chief, and logistical task group leads. Members will identify a designated alternate to act as a back-up when necessary. Liaisons (e.g., SAC liaison, F/P/T SAC Secretariat liaison, liaison from the Conference of Deputy Ministers of Health [CDMH]) and external experts may attend LAC meetings but would also not be voting members of the group.
This committee will be supported by a Logistical Support Team (supplied by PHAC through the HPOC). Cross-stream coordination with the Technical and Communications streams will be supplied by the F/P/T SAC Secretariat and HPOC.
Activities
The LAC will:
- Activate and "sunset" logistical task groups as needed
- Refer tasks (i.e., requests for products from the F/P/T SAC Secretariat) to appropriate task groups
- Engage health care delivery sector or NGOs as needed
- Conduct LAC meetings
- Consider the policy implications of LAC recommendations and include policy content in products as necessary
- Approve/endorse operational/logistical products and/or actions (e.g., deployment plans, policy guidance, recommendations, protocols) that are being sent to the F/P/T SAC Secretariat for preparation/strategic policy analysis before going to SAC for approval/decision.
LAC meetings
LAC meetings will occur as needed based on the pace of the evolving event and the need to review logistical products or proposed actions. These meetings will be conducted by teleconference and participants will include: LAC members, liaisons and invited external experts (based on agenda).
These meetings will provide a forum for logistical task groups to receive direction from LAC, seek input and provide updates on products under development, share information amongst task groups (e.g., applicable policies, MOUs, mutual aid agreements) – to ensure consistency between task group products, and to provide products for LAC approval/endorsement. Any logistical or policy issues that need to be actioned should also be raised at this meeting so that the LAC chair can bring them to the attention of F/P/T SAC Secretariat as needed.
The results of LAC meetings will be captured in records of decisions by the Logistical Support Team provided by HPOC.
Products
LAC will produce (via task groups) approved logistical documents, including recommendations for the SAC and CDMH. All products will go to SAC by way of the F/P/T SAC Secretariat who will integrate content from other streams and/or do a strategic policy analysis as needed, prior to sending the products to SAC.
Quorum and Decisions
Quorum for LAC meetings shall be attendance by a simple majority of voting members (or their respective designated alternates).
Decision-making will be made by consensus where consensus is defined as agreement that all can "live with" the decision. Approval or endorsement decisions will be in the context of the F/P/T response and response objectives. F/P/T governments recognize that each jurisdiction can decide whether or not to implement LAC products according to its individual needs, response objectives, and/or legislative frameworks. Minority dissenting opinions will be noted and communicated to the F/P/T SAC Secretariat.
Activation and Deactivation
The LAC will be activated by SAC to facilitate the response to F/P/T public health events that are of a scope and/or urgency to warrant a Level 3 – Escalated or Level 4 – Emergency response. The SAC will decide when to activate and deactivate the LAC based on situational analysis and requests received from P/T public health authorities and recommendations made by the F/P/T SAC Secretariat.
Appendix H - Anticipated Products and Pathways for a Coordinated F/P/T Response
Below is a table that includes examples of products, specifically recommendations and guidance documents, which might be needed during a coordinated F/P/T response. This is not intended to be a complete list but is provided to illustrate how products might be developed, reviewed and approved within an F/P/T governance structure.
Although the target audience for products developed in this F/P/T forum may be front-line workers, the provinces and territories would determine the use and distribution of these products within their respective jurisdictions. Each jurisdiction can decide whether or not to implement F/P/T governance structure products according to its individual needs, response objectives, and/or legislative frameworks.
Acronyms in table:
- CATMAT
- Committee to Advise on Tropical Medicine and Travel
- CFIA
- Canadian Food Inspection Agency
- CIHR
- Canadian Institutes of Health Research
- F/P/T DM
- Federal/Provincial/Territorial Deputy Minister
- HC
- Health Canada
- HPOC
- Health Portfolio Operations Centre
- IMS
- Incident Management System
- LAC
- Logistics Advisory Committee (part of F/P/T governance structure)
- MCM
- Medical Countermeasures
- NACI
- National Advisory Committee on Immunization
- NGO
- Non-governmental Organization
- PH
- Public Health
- PHN CG
- Public Health Network Communications Group
- SAC
- Special Advisory Committee (part of F/P/T governance structure)
- TAC
- Technical Advisory Committee (part of F/P/T governance structure)
| Type of product | Examples | Target Audience or Expected Users *examples only: not all inclusive list | Lead Authors or developers | Intermediate approvals or endorsements | Product Integration and/or Strategic policy analysis | Final Approvals or Decision makers |
|---|---|---|---|---|---|---|
| Technical recommendation or guidance | Laboratory tests, procedures, protocols and standards | Public Health Laboratory Network members, Hospital and private laboratories | CPHLN or TAC task group | CPHLN or TAC | SAC Secretariat | TACTable 3 footnote */SAC |
| Research priorities | Academia, PT public health agencies, Health care providers, CIHR | TAC task group | TAC | SAC Secretariat | TACTable 3 footnote */SAC | |
| Methods for disinfection or decontamination (setting dependent) (e.g., airline industry) | Infection prevention and control professionals, Emergency responders, Occupational health and safety, industry, PT health authorities | TAC task group | TAC | SAC Secretariat | TACTable 3 footnote */SAC | |
| Methods for disinfection or decontamination for healthcare settings | Infection prevention and control professionals, Emergency responders, Occupational health and safety, PT health authorities, PT professional regulatory bodies | TAC and Infection Prevention and Control Expert Working Group | TAC and Infection Prevention and Control Expert Working Group | SAC Secretariat | TACTable 3 footnote */SAC | |
| Case and contact definitions to be used for Canadian surveillance | PT health authorities | TAC task group or HPOC IMS Operations group on Surveillance | TAC | SAC Secretariat | TACTable 3 footnote */SAC | |
| Type, dose and indications for use of MCM | Health care providers, PT health authorities, PT professional regulatory bodies, Occupational health and safety | TAC task group or clinical care authority | possibly NACI or CATMAT for endorsment or directly to TAC | SAC Secretariat | TACTable 3 footnote */SAC | |
| Combined recommendation or guidance (i.e., technical, operational, logistical, communications etc.) | Priority groups for use of limited MCM | PT health authorities, Health care providers, PT professional regulatory bodies | TAC task group for technical portion of recommendation, LAC task group for strategic operational portion. | TAC and LAC | SAC Secretariat | SAC or Conference of FPT DM of Health |
| Type of MCM and/or equipment to purchase or stockpile (e.g., medications, ventilators, masks) and how much to purchase | Hospital and/or PH purchasing authorities, PT health care authorities, P/T professional regulatory bodies | TAC task group for technical portion of recommendation, LAC task group for inclusion of how this should be done. | TAC and LAC | SAC Secretariat | SAC or Conference of FPT DM of Health | |
| Areas/individuals to be legally obliged to take a public health action (e.g., self-isolation, quarantine farm/zone, recall product?) | PT health authorities, other government departments (e.g., CFIA) | TAC task group or existing authorities (e.g., CFIA, HC) | TAC and LAC | SAC Secretariat | SAC or Conference of FPT DM of Health | |
| When to change F/P/T response objective(s) | PT health authorities, F/P/T Communications, F/P/T governance structure participants | TAC task group | TAC and LAC SAC Secretariat HPOC IMS Planning Group HPOC IMS Event Manager |
SAC Secretariat | SAC | |
| Content of public communications (e.g., regarding risk and precautions to take) | F/P/T communications, PT health authorities, public | Communications with input from TAC | PHN CG | SAC Secretariat | SAC or Conference of FPT DM of Health | |
| Logistical protocol, recommendation or guidance | Funding mechanism – who pays for what? | F/P/T governments | LAC task group | LAC | SAC Secretariat | SAC or Conference of FPT DM of Health |
| Use of designated sites | F/P/T governments | LAC task group | LAC | SAC Secretariat | SAC or Conference of FPT DM of Health | |
| Use of surge workers | PT health authorities, NGOs | LAC task group | LAC | SAC Secretariat | SAC or Conference of FPT DM of Health | |
| How and when to engage external resources (e.g., NGO's) | PT health authorities, NGOs | LAC task group | LAC | SAC Secretariat | SAC or Conference of FPT DM of Health | |
|
Acronyms:
|
||||||
Appendix I – Committees, Working Groups and Expert Resources
During a response it may be necessary to activate task groups within the governance structure of this plan to provide products corresponding to the response functions identified in the governance structure (see Figure 2 in the main plan). Ideally existing groups that have expertise and experience working together would be engaged prior to the creation of new or ad hoc task groups. To facilitate identification of these groups the following list has been compiled and linked to the response function they would likely fulfill. Note: this is not intended as an all-inclusive list and is expected to be expanded upon on an ongoing basis. It is also recognized that work will be ongoing to further refine linkages to these groups so that they can be leveraged in a public health event necessitating use of this plan.
| Group | Type | TOR includes PH event response? | Expected Response Function (in F/P/T governance structure) | Currently active? | Contact |
|---|---|---|---|---|---|
| Vaccine Supply Working Group | F/P/T | Y | Procurement (recommendations) | Y | CIRID PHAC |
| National Advisory Committee on Immunization (NACI) | Expert | Y | Technical advice re vaccine prioritization, dosage, | Y | CIRID PHAC |
| Canadian Pandemic Influenza Plan Task Group | Expert | N | Overall technical advice | Y | CIRID PHAC |
| Surveillance Expert Working Group | F/P/T | Y | Surveillance Advice | Y | CIRID PHAC |
| FPT Public Health Emergency Management Task Group / Health Emergency Management Directors | P/T | Y | Operations/Logistics support (e.g., Health Care Delivery engagement) | Y | CEPR PHAC |
| Infection Prevention and Control Expert Working Group | Expert | Y | IPC advice for healthcare settings and occupational health advice related to healthcare workers | Y | CCDIC PHAC |
| The Committee to Advise on Tropical Medicine and Travel (CATMAT) | Expert | Y | Technical advice on travel medicine and tropical disease | Y | IDPC PHAC |
| Canadian Public Health Laboratory Network (CPHLN) | F/P/T | Y | Rapid and coordinated nationwide laboratory response to emerging and re-emerging communicable diseases | Y | NML PHAC |
Appendix J – Sample F/P/T Business Cycle
This is an example of how an F/P/T Business Cycle might look. It includes both meetings and deadlines for submission of information for various products. The frequency of the meetings will depend on the pace of the evolving event. The appendices regarding term of reference for SAC, F/P/T SAC Secretariat, TAC and LAC include more details regarding meeting expectations. The frequency of production of an F/P/T incident action plan is indicated as "weekly" in this example business cycle – this will be determined by F/P/T SAC Secretariat in consultation with HPOC Planning Group on an ongoing basis.
| Lead | Activity | Time (ET) | Participants |
|---|---|---|---|
| SAC SecretariatTable 5 footnote 1 | Submission deadline for issues to be addressed at planning meeting (including draft weekly F/P/T Incident Action Plan –Mondays only) | 10:30 | P/Ts, HPOC IMS |
| F/P/T SAC SecretariatTable 5 footnote 2 | Planning Meeting | 11:00 | F/P/T SAC Secretariat, HPOC support (for agenda coordination and minutes), Co-chairs of the activated response stream committees (TAC, LAC, PHN Communications) and federal HPOC IMS Planning Group representatives (for weekly Incident Action Plan development). |
| F/P/T SAC SecretariatTable 5 footnote 2 | Situational Awareness Meeting | 12:00 | F/P/T SAC Secretariat, SAC Co-chairs and SAC members, CCMOH members, P/T Health Emergency Managers, P/T EOC representatives, PHAC regional representatives, federal HPOC including Event Manager and IMS Operations and Planning Chiefs, NML Ops and Planning, Co-chairs of activated response stream committees and Task group leads, and liaisons. The P/T participants will be determined by the individual P/Ts. |
| TACTable 5 footnote 3 | Technical Advisory Committee Meeting | 13:30 | TAC members, TAC technical and policy support people, active technical task group representatives, liaisons, invited external experts (based on agenda) |
| LACTable 5 footnote 3 | Logistics Advisory Committee Meeting | 13:30 | LAC members, LAC logistical and policy support people, active task group representatives, liaisons, invited external experts (based on agenda) |
| HPOCTable 5 footnote 1 | Submission deadline for daily situational awareness data (e.g. case counts) | 14:00 | P/Ts, Federal population representatives |
| SACTable 5 footnote 4 | Special Advisory Committee Meeting | 14:30 | SAC members, F/P/T SAC Secretariat representatives, liaisons, invited external representatives (based on agenda). |
| HPOC IMSTable 5 footnote 1 | Release of Sit Rep, Release of F/P/T SAC Secretariat approved weekly Incident Action Plan | 15:00 | Distribution lists to be determined |
| F/P/T CommunicationsTable 5 footnote 1 | Public release of information as required | As required | F/P/T Communications representatives |
|
Please contact HPOC at 1-800-545-7661 or 613-952-7940 to set up non-scheduled meetings
|
|||
Appendix K – Task Groups: Generic Terms of Reference
Role
Task groups will be activated by the Technical Advisory Committee (TAC) or the Logistics Advisory Committee (LAC) to respond to requests from the SAC for the development of technical or logistical products including but not limited to: guidance documents, research summaries, reports, proposals, protocols or recommendations. When possible, existing groups will be utilized in order to maximize efficiency and/or continuity of response activities (see Appendix I: Existing Committees, Working Groups and other Expert Resources).
These task groups will be supported by the HPOC IMS and corresponding PHAC leads in addition to a communications resource person (which could be federal or P/T).
Membership
The task groups will be co-chaired by one federal and one P/T or technical expert lead. Members will include: multidisciplinary experts, P/T representatives that provide general P/T perspective in addition to their own expertise (i.e., does not need to include P/T representatives from all jurisdictions), federal experts, and when appropriate federal population representatives (e.g., First Nations).
Activities
The task groups will:
- convene, consult and develop requested response to issue (e.g., guidance document, proposal, recommendation);
- present their products to TAC or LAC for discussion, input, cross task group awareness and approval/endorsement;
- collect comments and guide any revisions regarding draft documents;
- engage NGOs, research communities, and other stakeholders (e.g., in the area of health care delivery) as needed; and
- coordinate with external expert organizations (e.g., Association of Medical Microbiology and Infectious Disease [AMMI]) to foster linkages between public health technical response products and products outside of the scope of the public health response (e.g., clinical care guidelines).
Task group meetings
Task group meetings will occur as needed based on the pace of the evolving event and the need to develop and discuss draft products. These meetings will be conducted by teleconference with task group members, liaisons and invited external experts (based on agenda). The results of task group meetings are captured in records of decisions.
Products
The technical task groups will produce draft documents for TAC approval/endorsement. The group will produce draft documents for LAC approval/endorsement.
Quorum and Decisions
Quorum for task group meetings shall be attendance by a simple majority of members (or their respective designated alternates).
Decision-making will be made by consensus where consensus is defined as agreement that all can "live with" the content and wording of the products developed by the group. Decisions to approve the content of the draft products will be in the context of the F/P/T response and response objectives. F/P/T governments recognize that each jurisdiction can decide to implement endorsed products according to its individual needs, response objectives, and/or legislative frameworks. Minority dissenting opinions will be noted and communicated to the TAC or LAC as appropriate, or to F/P/T SAC Secretariat when product approval is being sought.
Activation and Deactivation
Each task group will be activated and deactivated by the TAC or LAC as needed in order to facilitate the technical and logistical response to an F/P/T public health event that is of a scope and/or urgency to warrant a Level 3 – Escalated or Level 4 – Emergency response. The SAC will decide when to activate and deactivate the governance structure (including TAC and LAC) based on situational analysis and requests received from P/T public health authorities and recommendations made by the F/P/T SAC Secretariat.
Appendix L: Relationship of the F/P/T Public Health Response Plan to other F/P/T Coordinating Instruments
Figure 3. Relationship between F/P/T Plans
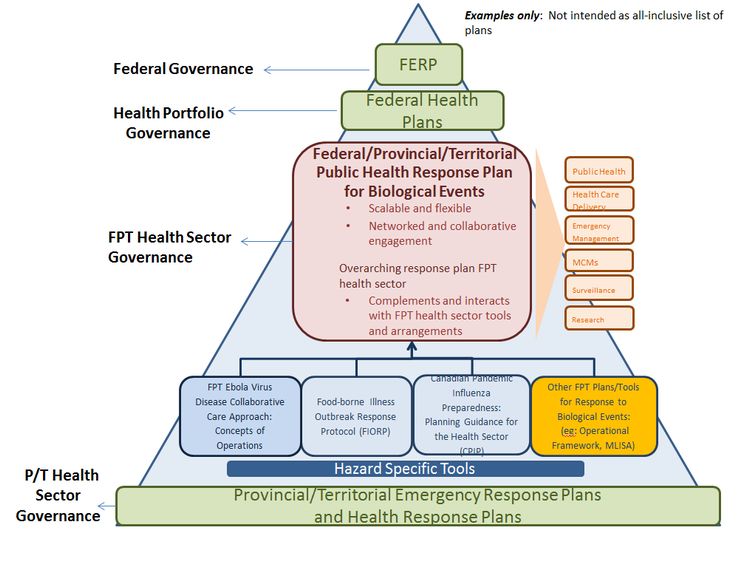
Figure 3: Relationship between F/P/T Plans - Text Description
This graphic illustrates a hierarchy of emergency response plans at all levels of government.
The capstone of the pyramid is the Federal Emergency Response Plan (FERP) as it describes the federal governance structure all federal departments are to use in all hazard emergency response.
Under the FERP are Federal Health Plans. This is to illustrate how Federal Health Plans such as the Health Portfolio Emergency Response Plan need to be aligned with the FERP. They also outline the governance structure the Health Portfolio uses for emergencies.
The next tier below of the pyramid is the Federal, Provincial Territorial Public Health Response Plan for Biological Events. This plan describes the F/P/T Health Sector's governance structure. Below are activities that will be facilitated by this response plan:
- Public health
- Health Care delivery
- Emergency Management
- Medical Countermeasures
- Surveillance
- Research
At the next tier of the pyramid, and supporting the FPT Public Health Response Plan for Biological Events, are various F/P/T plans and protocols used by the F/P/T Health Sector for various hazards:
Such as:
- FPT Ebola Virus Disease Collaborative Care approach: Concept of Operations
- The Foodborne Illness Outbreak Response Protocol (FIORP)
- The Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector (CPIP)
- Other FPT Plans/Tools for Response to Biological Events
At the next level below of the pyramid are Hazard Specific Tools which are meant to illustrate additional plans for specific hazards not included in the graphic which is not an all-inclusive list of plans
At the base of the pyramid are Provincial/Territorial Emergency Response Plans and Health Response Plans. As required by legislation all jurisdictions in Canada and Ministries of Health maintain emergency response plans. These plans describe provincial/territorial health sector governance.
Legislation
Legislation establishes the legal basis and framework for managing emergencies in Canada in both the health and public safety/national security realms. It defines the authority and responsibilities that allow governments to take the necessary steps to protect the health, safety and welfare of people and the environment during emergencies. Legislation requires all federal, provincial and territorial governments in Canada to have comprehensive emergency management plans respecting preparation for, response to, and recovery from emergencies.
Federal and P/T Response Plans
At the federal level, all departmental response plansFootnote 20 must be aligned with Public Safety Canada's Federal Emergency Response Plan (FERP), the Government of Canada's 'all hazards' response plan, which describes the general roles and responsibilities of federal institutions in an emergency, including a description of the 'whole of government' governance framework.Footnote 21 The FERP provides the federal framework for responding to emergencies that require a centralized Government of Canada approach. The FERP applies to domestic emergencies and to international emergencies with a domestic impact. It can be considered the "capstone" to all federal departments' response plans and as such, Health Portfolio response plans and Health Portfolio governance are aligned with concepts of the FERP.
Similarly, provinces and territories maintain their respective response plans which describe emergency response governance, linkages and channels of communication between ministries, programs and agencies of government, non-governmental organizations and the private sector. All P/Ts have their own governance and response structures for coordinating the response to emergencies impacting the health sector.
FPT Public Health Response Plan for Biological Events
The F/P/T Public Health Response Plan for Biological Events seeks to bridge the gap between P/T public health response plans and federal health response plans by providing a single, common overarching governance framework for the F/P/T health sector that can be applied, in full or in part, during a significant public health event requiring a coordinated F/P/T response. Though tailored to biological events, it is expected that this plan could be applied to a range of hazards requiring a response from senior public health decision-makers.
Further, there are various well established F/P/T health sector agreements specific to public health response and emergency management that are used to facilitate inter-jurisdictional coordination and response capacities for public health events. The F/P/T Public Health Response Plan for Biological Events is intended to complement and where appropriate be used in conjunction with these existing mechanisms in order to assist in harmonizing F/P/T response efforts.
For example:
- Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector (CPIP):Footnote 22 Provides planning guidance for the health sector for pan-Canadian preparedness and response. It consists of a main body to provide strategic guidance and a framework for pandemic preparedness and response; as well as supporting technical annexes to provide specific operational advice and technical guidance. The CPIP will be the operational planFootnote 23 used to guide a pandemic and the F/P/T Public Health Response Plan for Biological Events will provide the governance structure.
- Food-borne Illness Outbreak Response Protocol (FIORP):Footnote 24 Sets out the key guiding principles and operating procedures for the identification and response to multi-jurisdictional food-borne illness outbreaks in order to enhance collaboration and coordination among partners, establish clear lines of communication, and improve the efficiency and effectiveness of response. The FIORP operates under a governance structure that can be aligned and integrated with the F/P/T Public Health Response Plan for Biological Events as required.
- The F/P/T Ebola Virus Disease Collaborative Care Concept of Operations is also a hazard-specific mechanism situated under this plan. The EVD Concept of Operations specifically focuses on the transport, treatment and care of EVD patients in Canada as supported by a Collaborative Treatment Centre Network. Beyond this collaborative approach, the broader response to an ebola case and/or outbreak in Canada (e.g., public communications, border measures, etc.) is as governed through the activation of the F/P/T Public Health Response Plan for Biological Events.
- The Operational Framework for Mutual Aid Surge Requests for Health Care Professionals (OFMAR): is another example of an F/P/T tool that at a more granular or tactical level, can support the public health response to a biological event by providing a mechanism to implement the F/P/T MOU on the Provision of Mutual Aid in Relation to Health Resources During an Emergency Affecting the Health of the PublicFootnote 25, and thus is seen as supporting the overarching objectives of the F/P/T Public Health Response Plan for Biological Events.
- Multi-lateral Information Sharing Agreement (MLISA): Provides the legal authority for sharing of information on communicable disease and public health events between signatories. Signatories currently include all provinces and territories, and the Public Health Agency (PHAC) and Health Canada (HC). MLISA Annexes will further provide procedures to agree on the specifics of information to be shared and the methods for that sharing. Public health information sharing under the F/P/T Public Health Response Plan for Biological Events will be guided by the MLISA or other agreements such as the IHR.
Appendix M – Task Group Members
The F/P/T Public Health Response Plan for Biological Events was developed by a task group established in the summer of 2016 with a one year mandate from the Public Health Network Council (PHNC) to provide expert opinion on emergency management and technical and science-based recommendations from a public health perspective. Members of the task group were nominated by the Public Health Infrastructure Steering Committee (PHI-SC) and Communicable and Infectious Diseases Steering Committee (CID-SC) based on their unique expertise in health emergency management, public health and communicable/infectious disease (human and animal; respiratory, food-borne), and laboratory sciences, among others.
Thomas Appleyard
Manager: Training, Exercises & Awareness (A)
Emergency Management Branch
Population and Public Health Division
Ministry of Health and Long-Term Care
Toronto, (Ontario)
Tamela Carroll
Senior Program Advisor
New Brunswick Department of Health
Office of the Chief Medical Officer of Health Communicable Disease Branch
Fredericton, (New Brunswick)
Jean-François Duperré
A/Executive Director
Center for Emergency Preparedness and Response
Public Health Agency of Canada
Ottawa, (Ontario)
Kelly Folz
Manager
Intergovernmental Affairs
Intergovernmental and Stakeholder Policy Division
Public Health Agency of Canada
Ottawa, (Ontario)
Mélanie Goulette Nadon
Senior Communications Advisor
Public Health Strategic Communications Directorate
Communications and Public Affairs Branch
Health Canada
Ottawa, (Ontario)
Dr. Karen Grimsrud
Chief Medical Officer of Health
Office of the Chief Medical Officer of Health
Edmonton, (Alberta)
Steve Guercio
Executive Director
National Microbiology Laboratory
Infectious Disease Prevention and Control Branch
Public Health Agency of Canada
Winnipeg, (Manitoba)
Alternate
Dr. Cindi Corbett
Director, Bacterial Pathogens Division
National Microbiology Laboratory
Infectious Disease Prevention and Control Branch
Public Health Agency of Canada
Winnipeg, (Manitoba)
Erin Henry
Director
Communicable Disease Control Division
Office of Population and Public Health
Population Health and Primary Care Directorate
First Nations and Inuit Health Branch
Health Canada
Ottawa, (Ontario)
Alternate
Fanie Lalonde
National Program Manager
Communicable Disease Emergencies/Infection Prevention & Control Program
Office of Population & Public Health
Population Health and Primary Care Directorate
First Nations and Inuit Health Branch
Health Canada
Ottawa, (Ontario)
Althea House
Manager Seasonal and Pandemic Influenza
Centre for Immunization and Respiratory Infectious Diseases
Infectious Disease Prevention and Control Branch
Public Health Agency of Canada
Ottawa, (Ontario)
John Lavery (Co-Chair)
Executive Director
Health Emergency Management British Columbia
2nd Floor-1770 West 7th Avenue
Vancouver, (British Columbia)
Alternate
Kathryn Forge
Director, Emergency Management Unit
Health Emergency Management British Columbia
Jason Letto
Manager
Health Emergency Management Program
Department of Health and Community Services
Government of Newfoundland and Labrador
St. John's, (Newfoundland)
Garnet Matchett
Director of Operations
Health Emergency Management Unit
Saskatchewan Ministry of Health
Regina, (Saskatchewan)
Robin McNeill
Sr. Emergency Management Planner
Office of Situational Awareness and Operations
Centre for Emergency Preparedness and Response
Health Security and Infrastructure Branch
Public Health Agency of Canada
Ottawa, (Ontario)
Dr. Howard Njoo (Co-Chair)
Deputy Chief Public Health Officer
Office of the Chief Public Health Officer
Public Health Agency of Canada
Ottawa, (Ontario)
Gary O'Toole
Director, Public Health
Nova Scotia Health Authority, Northern Zone
Colchester East Hants Health Centre
Truro, (Nova Scotia)
Dr. Katarina Pintar
Manager, Policy Integration Division
Centre for Food-Borne, Environmental and Zoonotic Infectious Diseases
Infectious Disease Prevention and Control Branch
Public Health Agency of Canada
Ottawa, (Ontario)
Dr. Barry N. Pakes
Deputy Chief Medical Officer of Health (acting)
Department of Health, Government of Nunavut
PO Box 1000, Station 1000
Iqaluit, (Nunavut)
Dr. Michel Savard
Médecin conseil
Direction générale de santé publique
Ministère de la Santé et des Services sociaux
Montréal, (Québec)
Jill Sciberras
Nursing Advisor
Centre for Emergency Preparedness and Response
Public Health Agency of Canada
Toronto, (Ontario)
Dr. Saqib Shahab
Government of Saskatchewan
Chief Medical Health Officer
Ministry of Health, Population Health Branch
Regina, (Saskatchewan)
Mariyam Syed
Emergency Management Analyst
Public Health and Compliance Division
Health Protection Branch
Alberta Ministry of Health
Edmonton, (Alberta)
Alternate
Kimberley Nelson
Manager Emergency Management Unit
Public Health and Compliance Division
Health Protection Branch
Alberta Ministry of Health
Edmonton, (Alberta)
John Topping
Director
Office of Situational Awareness and Operations
Centre for Emergency Preparedness and Response
Public Health Agency of Canada
Ottawa, (Ontario)
Dr. Elise Weiss
Acting Chief Provincial Public Health Officer
Manitoba Health, Seniors and Active Living
Government of Manitoba
4004-300 Carlton Street
Winnipeg, (Manitoba)
Judith Wood Bayne
Regional Director – Atlantic Region
Health Promotion and Chronic Disease Prevention Branch
Public Health Agency of Canada
Halifax, (Nova Scotia)
Alternate
Lise Gauthier
Regional Coordinator Emergency Management Unit– Québec
Health Promotion and Chronic Disease Prevention Branch
Public Health Agency of Canada
Montreal, (Québec)
Footnotes
- Footnote 1
-
See Appendix M for task group membership
- Footnote 2
- Footnote 3
-
Not all examples are applicable in Québec or are the responsibility of public health authorities in in Québec where the concept of public health is distinguished from the public health system.
- Footnote 4
-
In Québec, public health is responsible for medical countermeasures for immunization and prevention activities, not for treatment or medication.
- Footnote 5
-
The IHR requires that all urgent events of international concern (i.e., events with serious public health impact and/or unusual or unexpected nature with high potential for spread) be assessed at the national level and reported to the World Health Organization (WHO) within 48 hours of notification. Annex 2 of the IHR provides a decision instrument designed to assist with the assessment and notification of events that may constitute a Public Health Emergency of International Concern.
- Footnote 6
-
The term "affected" is meant to encompass the occurrence of an impact (e.g., illness) and/or the presence of risk. Provinces and territories will determine whether they are affected or not; this may change over time and also will be influenced by the degree of perceived risk.
- Footnote 7
-
Source: Justice Institute of British Columbia, Incident Command System Orientation Student Manual, January 2006.
- Footnote 8
-
Strategic policy concerns broad issues of national and international importance. This could include border screening, explaining Canada's response posture to international partners and dealing with the trade implications of a public health event.
- Footnote 9
-
This process will be incorporated into the Business Cycle.
- Footnote 10
-
Communications products are endorsed not approved by the PHN CG.
- Footnote 11
-
Federal, Provincial, Territorial (FPT) Public Communications Protocol on Emerging Public Health Issues and Events, January 21, 2015.
- Footnote 12
-
It is recognized that during an emergency, action may be taken by one level of government while at the same time other action may be taken jointly. For example, hospital or public hygiene guidelines may be jointly developed but implementation may be done by each level of government or by the federal government on behalf of affected jurisdictions.
- Footnote 13
-
This reference is intended to refer to those federal authorities that are responsible for the delivery of health care services to specific populations (e.g., on-reserve First Nations communities, federal prison population).
- Footnote 14
-
Source: Last, J.M. A Dictionary of Epidemiology, 2nd edition, 1988
- Footnote 15
-
Source: http://www.phac-aspc.gc.ca/cpip-pclcpi/assets/pdf/report-rapport-2015-eng.pdf
- Footnote 16
-
Federal populations include the following: First Nations on-reserve, inclusive of First Nations who have assumed responsibility for health services under a transfer agreement; patients at hospitals operated by Veterans Affairs Canada for services that are not already insured by the province; active members of Canadian Forces; federal offenders or inmates of federal penitentiaries; refugee claimants, protected persons, detainees under the Immigration and Refugee Protection Act, rejected refugee claimants, and other specified populations; and Canada-based staff at missions abroad.
- Footnote 17
-
Provinces and territories are responsible for the use and distribution of these products in their territories. Each level of government may decide whether or not to implement the products of the F/P/T governance structure in accordance with its individual needs, objectives, or legislative frameworks.
- Footnote 18
-
Strategic policy concerns broad issues of national and international importance. This could include border screening, explaining Canada's response posture to international partners and dealing with the trade implications of a public health event.
- Footnote 19
-
This process will be incorporated into the Business Cycle.
- Footnote 20
-
Response plans describe actions to be taken immediately before, during or immediately after an incident to manage its consequences.
- Footnote 21
- Footnote 22
- Footnote 23
-
Operational plans are generally geared to tasks and actions and provide the detail required for a coordinated response to specific hazards identified through a risk assessment process.
- Footnote 24
- Footnote 25
Page details
- Date modified: