Prevention and control for health care settings: Canadian Pandemic Influenza Preparedness: Planning Guidance for the Health Sector
For readers interested in the PDF version, the document is available for downloading or viewing:
Prevention and Control of Influenza during a Pandemic for All Healthcare Settings
- E-Links have been inserted to direct the reader to the appropriate Section of the Annex.
- This Annex has been developed for use by healthcare workers including but not limited to those working in infectious diseases, risk management, infection prevention and control, occupational health, occupational hygiene and/or emergency response.
- The recommendations in this Annex must be interpreted by trained personnel prior to implementation in specific situations, organizations or healthcare settings.
- A major assumption underlying this Annex was that a pandemic virus would cause severe disease in patients.
- Should mild disease occur as in pH1N1, recommendations related to PPE may change.
- As with all guidance documents related to pandemic influenza, healthcare organizations and personnel should be mindful of specifc provincial/territorial legislation and policies that may influence or supercede the application of some of the recommendations in this Annex.
- NOTE: This version of Annex F is being published after the pH1N1 Pandemic (H1N1). The assumptions about influenza epidemiology in this document are generalized to include all potential influenza pandemics and are not specific to pH1N1. To view the PHAC Guidance Documents related to pH1N1 go to: Guidance - H1N1 Flu Virus. These Guidance Documents have been adapted from this Annex based on emerging of the epidemiologic evidence and the impact of pH1N1 infection.
- In Canada, public health is a shared responsibility of the Federal, Provincial and Territorial governments.
- These guidelines have been endorsed by the Public Health Network Council.
- Federal, Provincial and Territorial governments reserve the right to implement these guidelines as deemed appropriate in their own jurisdictions.
- Federal, Provincial and Territorial regulations and policies may differ from the recommendations and guidance contained within the CPIP and its associated Annexes.
Table of Contents
- I. Executive Summary
- II. List of Abbreviations
- III. Glossary of Terms
- IV. Introduction: Prevention and Control of Pandemic Influenza in Healthcare Settings
- V. Foundations for a Pandemic Influenza IPC/OH Plan for all Healthcare Settings
- 1. Public Health Assumptions
- 2. Infection Prevention and Control Assumptions used in Annex F
- 3. Occupational Health Assumptions
- 4. Principles of Influenza Exposure and Transmission in Healthcare
- 4.1. Basic Principles of Infectious Disease Epidemiology
- 4.2. Epidemiological Triangle Applied to Pandemic Influenza
- 4.3. Pandemic Influenza: Modes of Exposure and Transmission
- 4.4. Modes of Exposure to Pandemic influenza
- 4.4.1. Pandemic Influenza: Contact Exposure and transmission
- 4.4.2. Pandemic Influenza: Droplet Exposure and Transmission
- 4.4.3. Pandemic Influenza: Airborne Exposure and Transmission
- 4.4.4. Pandemic Influenza: Aerosol-Generating Medical Procedures
- 4.4.5. Pandemic Influenza: Continuum of droplet and airborne exposures
- 5. Literature Reviews that Examine the Modes of Transmission of Influenza
- 5.1. Transmission of Influenza A in Human Beings by Brankston et al.
- 5.2. Expert Panel on Influenza and Personal Protective Respiratory Equipment by the Council of Canadian Academies
- 5.3. Interventions for the Interruption and Reduction of Respiratory Viruses by Jefferson et al.
- 5.4. Synthesis of Assumptions Used in this Annex to Describe the Risks of Pandemic Influenza Virus Transmission in Healthcare
- 6. Hierarchy of Controls in the Inter-pandemic and Pandemic Periods
- 6.1. Background on the Hierarchy of Controls
- 6.2. Application of the Hierarchy of Controls in the Inter-pandemic and Pandemic Periods
- 6.2.1. Organizational Risk Assessment
- 6.2.2. Administrative Controls: Fitness-for-work
- 6.2.3. Administrative Controls: Work Evaluation
- 6.2.4. Administrative Controls: An Active Respiratory Protection Program
- 6.2.5. Administrative Controls: Routine Practices and Additional Precautions
- 6.2.6. Administrative Controls - Pandemic Influenza Precautions
- 6.3. Use of Personal Protective Equipment to Ensure Availability of Supplies During an Influenza Pandemic
- 7. Risk Assessment – A Method to Prevent/Minimize Pandemic Influenza Exposure and/or Transmission in Healthcare Settings
- VI. Planning for an Influenza Pandemic – Using the Organizational Risk Assessment to develop the Pandemic Influenza Infection Prevention and Control and Occupational Health Plan
- 1. Performing an Inter-Pandemic ORA to Evaluate Existing IPC/OH Programs
- 2. Establishing a Pandemic Influenza IPC/OH Plan for the Management of Pandemic Influenza in All Healthcare Organizations
- 3. Planning for the Identification and Management of HCWs with ILI Symptoms
- 4. Planning and Providing Pandemic Influenza Education and Skills Training for HCWs in All Healthcare Organizations
- VII. Pandemic Period: Recommendations to prevent the spread of pandemic influenza in existing healthcare settings
- 1. Pandemic Period Recommendations for Acute Care Settings
- 1.1. Implementation of the Pandemic Influenza IPC/OH Plan for the Acute Care Setting
- 1.2. Acquisition of Up To Date Information on this Pandemic Influenza Viral Strain
- 1.3. Implementation of Pandemic Influenza Precautions in Acute Care Settings
- 1.4. Triage and Assessment
- 1.5. Admission Process
- 1.5.1. Open Separate Influenza and Non-Influenza Inpatient Care Areas
- 1.5.2. Use of Single Rooms
- 1.5.3. Use of Airborne Infection Isolation Rooms
- 1.5.4. Urgent AGMPS
- 1.5.5. Placement of Patients WITHOUT ILI symptoms
- 1.5.6. Placement of Patients WITH ILI symptoms
- 1.5.7. Placement of Patients WITH ILI symptoms and Another Medical Condition
- 1.5.8. Placement of Patients Immune to Influenza
- 1.6. Transfer/Transport of Patients with ILI symptoms Within (i.e., Intra-Facility) and Between (i.e., Inter-Facility) Healthcare Settings
- 1.7. Visitor Responsibilities and Restrictions
- 1.8. Pandemic Period: Work Assignments during the Pandemic Period
- 1.9. Pandemic Period: Pandemic Influenza Education and Skills Training for Healthcare Workers in Acute Care Settings
- 1.10. Influenza Outbreak Detection and Management for Acute Care Settings
- 1.10.1. Detection of a New Influenza Case Among Cohorts of Non-Influenza Patients
- 1.10.2. Separate Patients Who Develop ILI symptoms From Non Influenza Patients
- 1.10.3. Outbreak Declaration
- 1.10.4. Isolation of New Influenza Patients
- 1.10.5. Contact Tracing of Roommates
- 1.10.6. Limit Transfers of Exposed Patients
- 1.10.7. Outbreak Visitor Restrictions
- 1.10.8. Declaring the End of an Influenza Outbreak
- 2. Pandemic Period Recommendations for Infection Prevention and Control Activities in Long-Term Care ( LTC) Settings
- 2.1. Implementation of the Long-Term Care Organization's Pandemic Influenza IPC/OH Plan
- 2.2. Influenza Monitoring for all LTC Residents
- 2.3. Acceleration of Pandemic Influenza Education and Skills Training for HCWs in LTC Organizations
- 2.4. Implement Pandemic Influenza Precautions
- 2.5. Implementation of General Source Control Plans
- 2.6. Activities Outside the Long-Term Care Facility
- 2.7. Admission Area for New Admissions
- 2.8. Influenza Isolation Area
- 2.9. Resident Care Areas
- 2.10. Visitor Restrictions and Exemptions
- 2.11. LTC HCWs : Fitness-for-Work
- 2.12. Pandemic Influenza Outbreak Detection and Response in LTC Facilities
- 3. Ambulatory Care Clinics and Settings
- 4. Community Settings with Infirmaries
- 5. Home Care Settings Where Care or Service is Provided by Regulated and Unregulated HCWs
- 1. Pandemic Period Recommendations for Acute Care Settings
- Appendix A - Influenza Self Assessment Tool
- Appendix B - Recommended Steps for Putting on and Taking off PPE
- Appendix C - Check List - Organizational Risk Assessment for Pandemic Influenza
- Appendix D - Point of Care Risk Assessment Tool for Pandemic Influenza
- Reference List
I. Executive Summary
This Annex has been developed to provide infection prevention and control (IPC) and occupational health (OH) guidance for the planning and management of pandemic influenza for all healthcare organizations, including existing and temporary healthcare settings.
IPC guidance for seasonal influenza (i.e., influenza occurring in the inter-pandemic period) is addressed in other Public Health Agency of Canada (PHAC) documents including Guidelines for Prevention and Control of Occupational Infection in Health Care Footnote 1 and Routine Practices and Additional PrecautionsFootnote 2, Footnote 3, and Guidelines for the Prevention of Health Care-Associated Pneumonia Footnote 4.
This Annex is also part of the Public Health Network Council's comprehensive plan for the management of pandemic influenza in Canada. As far as possible, the recommendations in this Annex complement and support recommendations found in the rest of the Canadian Pandemic Influenza Plan Footnote 5 (see The Canadian Pandemic Influenza Plan for the Health Sector). The recommendations outlined are based on current available scientific evidence and assumptions about the pandemic influenza virus and the potential impact of the pandemic on the Canadian healthcare system. All evidence and assumptions presented in this document are subject to review and change as new information becomes available. As with all guidance documents related to pandemic influenza, healthcare organizations and personnel should be mindful of specific provincial/territorial legislation and policies that may influence or supersede the application of some of the recommendations in this Annex.
The Public Health Agency of Canada's Infection Prevention and Control Program developed this guideline with expert advice from a working group. The Guideline Working Group was comprised of members representing infection prevention and control, occupational health and public health. The multidisciplinary Guideline Working Group reflected a balanced representation of the regions of Canada.
The following individuals formed the Guideline Working Group:
- Dr. Mary Vearncombe, Co-chair, Hospital Epidemiologist, Sunnybrook and Women's College, Toronto, Ontario
- Dr. George Astrakianakis, Co-chair, Senior Occupational Hygienist, BC, Occupational Health and Safety Agency for Healthcare, Vancouver, British Columbia
- Dr. Elizabeth Bryce, Medical Microbiologist, Vancouver Hospital, Vancouver, British Columbia
- Dr. Joanne Langley, Professor of Pediatrics, IWK Health Centre, Halifax, Nova Scotia
- Dr. Virginia Roth, Director, Infection Control, Ottawa Hospital – General Campus, Ottawa, Ontario
- Brenda Dyck, Program Director, IP&C Program, Winnipeg Regional Health Authority, Winnipeg, Manitoba
- Dr. Bonnie Henry, Physician Epidemiologist, BC Centre for Disease Control , Vancouver, British Columbia
- Dr. Bryce Larke, Yukon Medical Health Officer, Whitehorse, Yukon
- Geoffrey Clark, Senior Occupational Hygienist, Worker and Employer Services, WorkSafeBC, Vancouver, British Columbia
- Gene Shematek, President, GMS & Associates, Ltd., GMS & Associates, Ltd., Calgary, Alberta
- Dr. Arthur J. Davies, National Medical Advisor, WHPSP, Health Canada, Saskatoon, Saskatchewan
- Quinn Danyluk, Occupational Hygienist, Fraser Health Authority, New Wesminster, British Columbia
- Mary-Louise Graham, Chief, Biocontainment Division, Public Health Agency of Canada, Ottawa, Ontario
- Dr. Maureen Cividino, Occupational Physician, St. Joseph's Healthcare Centre, Hamilton, Ontario
The Public Health Agency of Canada's team for this guideline included:
- Luna Bengio, Director, Blood Safety Surveillance and Health Care Associated Infections, Centre for Communicable Diseases and Infection Control
- Frederic Bergeron, Nurse Consultant
- Judy Foley, Literature Database Officer
- Louise Marasco, Editing and Quality Control Officer
- Laurie O'Neil, Nurse Consultant
- Shirley Paton, Senior Technical Advisor
- Carole Scott, Publishing Officer/Literature Database
The Pandemic Influenza Precautions recommended (see Glossary) are based on an understanding of the following:
- The principles of infectious disease transmission as applied to influenza;
- The expected modes of exposure to pandemic influenza in healthcare organizations;
- The anticipated population groups at risk of developing influenza;
- The anticipated population groups at risk of severe complications should they develop influenza;
- Assumptions about infection prevention and control and occupational health programs in healthcare organizations;
- A synthesis of recent literature reviews related to influenza transmission;
- Published PHAC documents including published infection prevention and control guidelines, PHAC IPC documents presently under revision; and
- Experience with the pandemic H1N1 influenza virus outbreak in Canada in the spring of 2009.
The recommendations throughout this document are based on the expectation that healthcare organizations have both comprehensive infection prevention and control (IPC) programs Footnote 2, Footnote 3 and effective occupational health (OH) programsFootnote 1. The recommendations are also dependent on the use of Organizational and Point of Care Risk Assessments to allow the evaluation and implementation of safe, effective and timely care of patients with symptoms of suspected influenza and/or influenza-like illness (ILI). The use of such assessments is intended to prevent or minimize pandemic influenza exposure and/or transmission in all healthcare organizations. A timely response to the emergence of an influenza pandemic is only possible when an organization and its personnel are experienced with effective infection prevention and control and occupational health protocols and practices.
As a result of the above assumptions, the following concepts are emphasized throughout this document:
- Organizational Risk Assessments (ORAs), undertaken during the inter-pandemic period, to identify engineering, administrative and personal protective equipment (PPE) controls that will best protect patients, healthcare workers (HCWs) and visitors in the healthcare setting from pandemic influenza viruses;
- Point of Care Risk Assessments (PCRAs), carried out by HCWs prior to the initiation of patient care to determine the appropriate PPE, isolation and cohorting strategies for a given patient, during a given intervention, in a specific room, area or facility;
- Respiratory Protection Programs to ensure that HCWs who may need to wear a respirator (including N95 respirators) are trained, fit-tested and prepared;
- Healthcare organizations providing the tools and training that HCWs require to carry out patient care safely;
- HCWs practicing in a manner that protects themselves and their patients from exposure to infectious agents;
- Comprehensive education and training activities for HCWs undertaken to prepare for a thoughtful, seamless, professional pandemic influenza response;
- A wide range of "source control" policies should be implemented including, but not limited, to a two metre spatial separation between infected sources (e.g., patients) and uninfected hosts (e.g., other patients), admission screening, screening of visitors (either active or passive screening), expanded respiratory and hand hygiene programs (to include not only HCWs, but patients and visitors as well) and the routine use of masks for patients with ILI symptoms; and
- Systematic administrative practices (policies, procedures, patient care practices) should be implemented to enable rapid identification and segregation of patients, HCWs and visitors with ILI symptoms. These may include, but are not limited to, systems for HCWs influenza self assessment and fitness-for-work regimes, establishment of two metre boundaries between patients with ILI symptoms and patients without influenza symptoms, and redirection of patients, and visitors with ILI symptoms to identified areas for complete assessment and management.
Sections I, II, III, and IV provides the Executive Summary, List of Abbreviations, Glossary and Introduction for this Annex.
Section V describes the foundational assumptions and scientific interpretations from the fields of public health, infection prevention and control and occupational health that underpin the discussions and recommendations that follow.
Section VI provides recommendations for comprehensive IPC and OH planning activities to prepare healthcare organizations for the emergence of an influenza pandemic in their community. This section contains specific recommendations for preparing and performing effective inter-pandemic ORAs, developing a pandemic influenza IPC/OH plan, management of HCWs with ILI symptoms and topics and methods for education and skills training of all HCWs.
Section VII contains specific recommendations to prevent the spread of the pandemic influenza virus when providing patient care in all healthcare settings including acute care, long-term care, ambulatory care, community care and professional home care.
The Appendices contain an Influenza Self Assessment Tool, directions for safely putting on and taking off personal protective equipment, a checklist for performing an ORA and a tool for conducting a PCRA.
II. List of Abbreviations
- ABHR(s)
- Alcohol-based hand rub(s)
- AGMP(s)
- Aerosol-generating medical procedure(s)
- AP
- Additional Precautions
- CPIP
- Canadian Pandemic Influenza Plan for the Health Sector
- HAI(s)
- Healthcare-associated infection(s)
- HCW(s)
- Healthcare Worker(s)
- HVAC
- Heating, ventilation and air conditioning
- ILI
- Influenza-like illness
- IPC
- Infection prevention and control
- OH
- Occupational Health
- OHS
- Occupational Health and Safety
- ORA(s)
- Organizational Risk Assessment(s)
- PAPR(s)
- Powered Air Purifying Respirator(s)
- PCRA(s)
- Point of Care Risk Assessment(s)
- pH1N1
- InfluenzaA/California/2009 (H1N1)
- PHAC
- Public Health Agency of Canada
- PPE
- Personal Protective Equipment
- RP
- Routine Practices
- RPAP
- Routine Practices and Additional Precautions
- RPP
- Respiratory Protection Program
- WHO
- World Health Organization
III. Glossary of terms
- Additional Precautions
Extra measures, when Routine PracticesFootnote 2, Footnote 3 alone may not interrupt transmission of an infectious agent.
- Are used in addition to Routine Practices (not in place of).
- Initiated both on condition/clinical presentation (syndrome) and on specific etiology (diagnosis).
- Pandemic Influenza Precautions is one form of "Additional Precautions" protocols.
- Administrative controls
One element in the Hierarchy of ControlsFootnote 6 ,7 . Administrative Controls include but are not limited to: policies and procedures for hand hygiene; training; immunization of patients, HCWs; and, outbreak management and for care of patients with infection. Also see Hierarchy of Controls.
- Aerosols Solid or liquid particles suspended in the air, whose motion is governed principally by particle size, which ranges from 10µm -100µm Footnote 8. (See Aerosol-generating medical procedure.)
Note: Particles less than 10µm (i.e., droplet nuclei) can also be found in aerosols, however, their motion is controlled by other physical parameters.
- Aerosol-generating medical procedure (AGMP)
Any procedure carried out on a patient that can induce the production of aerosols as a result of manipulation of a person's airway Footnote 9.
- Airborne exposure
Exposure to aerosols capable of being inhaled.
- Airborne transmission
Transmission of microorganisms via inhalation of aerosols that results in an infection in a susceptible host.
- Alcohol-based hand rub (ABHR)
An alcohol-containing (60-90%) preparation (liquid, gel or foam) designed for application to the hands to kill or reduce the growth of microorganisms. Such preparations contain one or more types of alcohol with emollients and other active ingredients (see the PHAC Infection Prevention and Control Guidelines Hand Hygiene Practices in Health CareFootnote 10, Footnote 11.
- Cleaning
The physical removal of foreign material (e.g., dust, soil, organic material such as blood, secretions, excretions and microorganisms); cleaning physically removes rather than kills microorganisms. It is accomplished with water, detergents and mechanical actionFootnote 11 .
- Cohort
Physically separating (e.g., in a separate room or ward) two or more patients exposed to, or infected with, the same microorganism from other patients who have not been exposed to, or infected with, that microorganism Footnote 12.
- Cohort staffing
The practice of assigning specific personnel to care only for patients known to be exposed to, or infected with, the same microorganism. Such personnel would not participate in the care of patients who had not been exposed to, or infected with, that microorganismFootnote 12.
- Compliant Patient
Able and willing to wear a mask and/or cover a cough when requested by healthcare workers.
- Contact transmission (direct or indirect)
Direct contact occurs when the transfer of microorganisms results from direct physical contact between an infected or colonized individual and a susceptible host (body surface to body surface)Footnote 2, Footnote 3.
Indirect contact involves the passive transfer of microorganisms to a susceptible host via an intermediate object, (e.g., contaminated hands that are not cleaned between care of patients, contaminated instruments that are not cleaned between patients/uses or other inanimate objects in the patient's immediate environment)Footnote 2, Footnote 3.
- Critical items
Instruments and devices that enter sterile tissues, including the vascular system. Reprocessing critical items, such as surgical equipment or intravascular devices, involves meticulous cleaning followed by sterilizationFootnote 11.
- Decontamination
The removal of microorganisms to leave an item safe for further handlingFootnote 11.
- Disinfection
The inactivation of disease-producing microorganisms, with the exception of bacterial spores. Hospital-grade disinfectants are used on inanimate objects and require a drug identification number (DIN) for sale in CanadaFootnote 11.
- Droplet
Solid or liquid particles suspended in the air, whose motion is governed principally by gravity and whose particle size is greater than 10µm. During an influenza pandemic, droplets will be generated primarily as the result of an infected source coughing or sneezingFootnote 2, Footnote 3.
- Droplet nucleus
A droplet nucleus is the airborne particle resulting from a potentially infectious (microorganism-bearing) droplet from which most of the liquid has evaporated, allowing the particle to remain suspended in airFootnote 13, Footnote 14.
Note: Droplet nuclei can also be found in aerosols, however, their motion is controlled by physical parameters including gravity and air currents.
- Droplet transmission
Transmission that occurs when the droplets that contain microorganisms are propelled a short distance (within 2 metres) through the air and are deposited on the mucous membranes of another person, leading to infection of the susceptible host. Droplets can also contaminate surfaces and contribute to contact transmission (see also contact transmission)Footnote 2, Footnote 3.
- Engineering controls
Measures that eliminate or reduce a hazard at the source. One element of the Hierarchy of ControlsFootnote 6, Footnote 7 that includes measures that reduce exposure to a hazard by applying methods of minimization, isolation or ventilation (e.g., negative pressure rooms). Also see Hierarchy of Controls.
- Exposure
Contact with a microorganism or an infectious disease in a manner such that transmission may occurFootnote 14.
- Fit testing
The use of a qualitative or a quantitative method to evaluate the fit of a specific manufacturer, model and size of respirator on an individual (CSAZ94.4-02 Selection, Use and Care of Respirators) Footnote 15. See also Seal check.
- Fit-for-work
The phrases "fit-for-work", "unfit-for-work" and "fit-for-work with restrictions" are terms used to describe a worker's ability to remain at or return to work following an infectionFootnote 6.
- Forceful Cough
The rapid release of air from the lungs that can be heard while the diaphragm and other muscles involved in breathing press against the lungs, the glottis suddenly opens, producing an explosive outflow of air at high speeds.
- Hand hygiene
A comprehensive term that applies either to hand washing, hand antisepsis and to actions taken to maintain healthy hands and fingernailsFootnote 10, Footnote 11.
- Hazard
A term to describe a condition that has the potential to cause harm. Work-related hazards faced by HCWs are classified in categories: biologic and infectious, chemical, environmental, mechanical, physical, violence and psychosocial Footnote 16.
- Healthcare-Associated Infections
Infections that are transmitted within a healthcare setting (also referred to as nosocomial) during the provision of health care.
- Healthcare Facility
Include but are not limited to acute care hospitals, emergency departments, rehabilitation hospitals, mental health hospitals, and long-term-care (LTC) facilities.
- Healthcare Worker
Individuals who provide health care or support services such as nurses, physicians, dentists, nurse practitioners, paramedics and sometimes emergency first responders, allied health professionals, temporary workers from agencies, unregulated healthcare providers, students, volunteers and workers who provide support services (e.g., food, laundry, housekeeping).
This term encompasses the following individuals in the healthcare setting: healthcare workers including professionals (e.g., nurses, physicians); volunteers; trainees; retirees; temporary workers from agencies; other employees who provide healthcare services; and, workers who provide support services (e.g., food, laundry, housekeeping).
- The organizational entity that is responsible for establishing and maintaining health care services provided by healthcare workers in one or more healthcare settings throughout the healthcare continuum (pre-hospital, acute care, long-term care, ambulatory care (including physicians'offices), community clinic care and professional home care).
- Healthcare Setting
Any location where health care is provided, including emergency care, prehospital care, healthcare facility , LTC, home care, ambulatory care and facilities and locations in the community where care is provided, (e.g., infirmaries in schools, residential or correctional facilities).
Note: Definitions of settings overlap, as some settings provide a variety of care, e.g., chronic care or ambulatory care provided in acute care, complex care provided in LTC, etc.
- Herd immunity
Resistance to the spread of infectious disease in a group because susceptible members are few, making transmission from an infected member unlikelyFootnote 14.
- Hierarchy of Controls
There are three levels/tiers of IPC and OH controls to prevent injury and illness in the workplace: engineering controls, administrative controls and personal protective equipmentFootnote 6, Footnote 7.
- High-level disinfection
The level of disinfection recommended when processing semi-critical items. High-level disinfection processes destroy vegetative bacteria, mycobacteria, fungi and enveloped (lipid) and non-enveloped (non-lipid) viruses but not necessarily bacterial spores. Items must be thoroughly cleaned prior to high-level disinfectionFootnote 11.
- High-risk groups
Individuals at high risk of influenza-related complications including the very young, very old, chronically ill and pregnant women, as outlined in the National Advisory Committee on Immunization's current advisoryFootnote 17, Footnote 18. However, the specific identity of high-risk groups will be determined as the epidemiology of the pandemic influenza virus is known.
- Infection
Situation in which microorganisms are able to multiply within the body and cause a response from the host's immune defences. Infection may or may not lead to clinical diseaseFootnote 14.
- Infection Control Program
An organization wide set of protocols and practices which aim to prevent and limit the spread of infectious agents within a healthcare setting.
- Infectious agent
Terminology used to describe a microorganism or a pathogen capable of causing disease (infection) in a source or a host.
- Infectious waste
The portion of biomedical waste that is capable of producing infectious diseaseFootnote 11.
- Infirmary
An overnight facility where health care is provided by healthcare workers.
- Influenza (Clinical, Confirmed)
An acute, primarily respiratory infection caused by the influenza virus. It is responsible for severe and potentially fatal clinical illness of epidemic and pandemic proportionsFootnote 14.
Clinical case of influenza: when influenza is circulating in the community, the presence of fever of acute onset is a good predictor of influenza. The positive predictive value increases when fever is higher than 38°C and when the onset of the clinical illness is acute (less than 48 hours after the prodrome). Other symptoms, such as sore throat, cough, rhinorrhea, malaise, rigors or chills, myalgia and headache, although non-specific, may also be present Footnote 19.
Confirmed case of influenza: those with laboratory confirmation (i.e., virus isolation from respiratory tract secretions, identification of viral antigens or nucleic acid in the respiratory tract, or a significant rise in levels of serum antibodies) with symptoms and an epidemiological link to a laboratory-confirmed caseFootnote 19.
See Annex G of the CPIP (The Canadian Pandemic Influenza Plan for the Health Sector - Annex G) for further details on pediatric clinical presentation.
- Influenza-Like Illness (ILI)
A constellation of symptoms which may be exhibited by individuals prior to the confirmation of Influenza.
- Inter-pandemic period
The interval between the last pandemic and the onset of the Pandemic Alert Period. During this period no new virus subtypes have been detected in humans although an influenza virus subtype that has caused human infection may be present in animalsFootnote 5.
- Low-level disinfection
The level of disinfection recommended when processing non-critical items or some environmental surfaces. Low-level disinfectants kill most vegetative bacteria and some fungi as well as enveloped (lipid) viruses (e.g., influenza). Low-level disinfectants do not kill mycobacteria or bacterial spores. Low-level disinfectants–detergents are used to clean environmental surfacesFootnote 11.
- Mask
A barrier to prevent droplets from an infected source from contaminating the skin and mucous membranes of the nose and mouth of the wearer, or to trap droplets expelled by the wearer, depending on the intended use. The mask should be durable enough so that it will function effectively for the duration of the given activity. The term "mask" in this document refers to surgical or procedure masks, not to respirators.
- Microorganisms
See Infectious agent.
- Mode of transmission
Mechanism by which an infectious agent is spread (e.g., via contact, through droplets or aerosols)Footnote 10, Footnote 11.
- N95 Respirator
A disposable, (Note: most respirators used for health care purposes are disposable filtering face pieces covering mouth, nose and chin) particulate respirator. Airborne particles are captured from the air on the filter media by interception, inertial impaction, diffusion and electrostatic attraction. The filter is certified to capture at least 95% of particles at a diameter of 0.3 microns; the most penetrating particle size. Particles of smaller and larger size are collected with greater efficiency. The'N' indicates a respirator that is not oil-resistant or oil-proof. N95 respirators are certified by the National Institute for Occupational Health and Safety (NIOSH –organization based in the United States) and must be so stamped on each respiratorFootnote 20 (see also Respirator).
- Non-critical items
Items that touch only intact skin but not mucous membranes. Reprocessing of non-critical items involves cleaning followed by low-level disinfectionFootnote 11.
- Nosocomial Infection
See healthcare-associated infections.
- Occupational Health
For the purposes of this document, this phrase refers to the disciplines of Occupational health medicine and nursing, Occupational Hygiene and Occupational Health and Safety.
- Occupational Health and Safety
"Occupational Health and Safety" is a legal term that is defined in legislation, regulation and/or workplace (e.g., union) contracts that impact a variety of disciplines concerned with protecting the safety, health and welfare of people engaged in work or employment. The use of the phrase "Occupational Health and Safety" invariably refers back to legislation and or regulation that influence workplace safety practices. The definition and therefore the content encompassed by "OHS" legislation varies significantly between and within jurisdictions in Canada.
- Organizational Risk Assessment (ORA)
The activity whereby a healthcare organization identifies:
- a hazard
- the likelihood and consequence of exposure to the hazard and
- the likely means of exposure to the hazard
- the likelihood of exposure in all work areas in a facility/office/practice settings; and then
- evaluates available engineering, administrative and PPE controls needed to minimize the risk of the hazard.
- Outbreak case definitions
Community Case: A patient/resident who does not have any ILI symptoms on admission but that subsequently develops influenza symptoms less than 72 hours after admission will be called a "community case".
Nosocomial Case: A patient or resident that develops ILI symptoms more than 72 hours after admission. Note: all subsequent cases linked to a community case occurring in a healthcare setting will be called a "Nosocomial case".
- Pandemic Influenza
During "normal" influenza epidemics, an average of 10% to 25% of the population becomes ill resulting in an average of 4,000 deaths and 20,000 hospitalizations. During severe influenza A epidemics, 30% to 50% of the population may become ill resulting in 6,000 to 8,000 deaths and 30,000 to 40,000 hospitalizationsFootnote 5.
During a pandemic, historic data shows that over 70% of a population may become infected with the novel virus and the age-specific morbidity and mortality may be quite different from annual epidemics. During the 1918–1919 pandemic, young adults had the highest mortality rates, with nearly half of the influenza-related deaths occurring among persons 20 to 40 years of age. During the 1957–1958 and 1968–1969 pandemics in the United States, persons over 65 years of age accounted for 36% and 48% of influenza-related deaths respectively.
- Pandemic influenza IPC/OH plan
A comprehensive integrated IPC/OH plan to prevent the transmission of the pandemic influenza virus in existing and temporary healthcare settings.
- Pandemic influenza precautions
One form of Additional Precautions recommended to prevent and control the spread of a pandemic influenza in healthcare settings.
- Pandemic period
The interval characterized by increased and sustained transmission in the general population of a new influenza virus subtype which is spreading efficiently between humansFootnote 5.
- Pandemic Wave
The time period that the pandemic influenza virus is the predominant influenza strain, circulating within a community. The pandemic influenza virus is likely to cause more than one wave of illness as the pandemic spreads through a regionFootnote 5.
- Parent organization
The organization responsible for the planning of a temporary healthcare setting (e.g., use of tents, school gymnasiums), operational only when an influenza pandemic has been declared and the need for new assessment or caregiving space arises.
- Patient
For the purposes of this document, the term «patient» will include those receiving health care, including patients, residents or clients.
- Personal protective equipment (PPE)
One element in the Hierarchy of ControlsFootnote 6-8. Personal protective equipment consists of gowns, gloves, masks, facial protection (i.e., masks and eye protection, face shields or masks with visor attachment) or respirators that can be used by HCWs to provide a barrier that will prevent potential exposure to infectious microorganisms.
- Point of care
Refers to place where a patient or resident receives health care from healthcare workers. Point of care incorporates three main elements being present at the same time: the patient, the HCW and an interaction that could result in transmission of an infectious agent.
- Point of Care Risk Assessment (PCRA)
A PCRA is an activity whereby HCWs (in any healthcare setting across the continuum of care):
- Evaluate the likelihood of exposure to an infectious agent
- for a specific interaction
- with a specific patient
- in a specific environment (e.g., single room, hallway)
- under available conditions (e.g., no designated hand washing sink)
- Choose the appropriate actions/PPE needed to minimize the risk of exposure for the specific patient, other patients in the environment, HCWs, visitors, contractors etc.
- Evaluate the likelihood of exposure to an infectious agent
- Respirator
A device to protect the user from inhaling a hazardous atmosphereFootnote 15.
The most common respirator used in health care is a N95 half-face piece filtering respirator. It is a personal protective device that fits tightly around the nose and mouth of the wearer, and is used to reduce the risk of inhaling hazardous airborne particles and aerosols, including dust particles and infectious agentsFootnote 20. See also N95 Respirator, Respiratory Protection Program, Fit testing, Seal check.
- Respiratory hygiene (also referred to as Respiratory Etiquette)
A combination of measures designed to minimize the transmission of respiratory pathogens via droplet or airborne routes in healthcare settingsFootnote 3.
Respiratory hygiene includes covering the mouth and nose with a sleeve during coughing or sneezing; using tissues to contain respiratory secretions during coughing or sneezing with prompt disposal into a hands-free receptacle; wearing a mask when coughing or sneezing to contain droplets and decrease contamination of the surrounding environment; turning the head away from others when coughing or sneezing; and maintaining spatial separation of two metres between themselves and others without symptoms of influenza.
- Routine Practices
A comprehensive set of IPC measures, that have been developed for use in the routine care of all patients at all times in all healthcare settings. Routine Practices aim to minimize or prevent HAIs in all individuals in the healthcare setting including patients, HCWs, visitors, contractors, etc.Footnote 2, Footnote 3.
- Routine Practices and Additional Precautions
See the definition for Additional Precautions and the definition for Routine PracticesFootnote 2, Footnote 3.
- Seal check
A procedure the wearer performs each time a respirator is worn and is performed immediately after putting on the respirator to ensure that there is a good facial seal. Seal check has been called "fit check" in other IPC documents (Appendix A of CSAZ94.4-02 Selection, Use and Care of Respirators)Footnote 15. (See also Fit Test).
- Semi-critical items
Items that come in contact with non-intact skin or mucous membranes but ordinarily do not penetrate them (e.g., endotracheal tubes, endoscopes). Reprocessing semi-critical items involves meticulous cleaning followed by high-level disinfectionFootnote 11.
- Source
The person that may contain an infectious agent/microorganism that can be passed to a susceptible host Footnote 21.
- Sterilization
The destruction of all forms of microbial life including bacteria, viruses, spores and fungiFootnote 14.
- Susceptible host
An individual not possessing sufficient resistance against a particular infectious agent to prevent contracting an infection or disease when exposed to the agent (synonymous with non-immune)Footnote 21.
- Temporary healthcare settings
These sites are healthcare sites not currently established or, if established, they usually offer a different type and/or level of care. The functions of a temporary site may vary depending on the needs of the community but should focus on monitoring, care and support of influenza patients. Temporary healthcare settings are pre-determined for operation before an influenza pandemic and become operational only when an influenza pandemic is declared by the World Health Organization (WHO) or Canadian public health officials. Further information on the pandemic phases can be found in Section 2 of the Canadian Pandemic Influenza Plan for the Health Sector.
- Transmission
The process whereby an infectious agent passes from a source to cause infection in a susceptible hostFootnote 14.
- Virulence
Virulence refers to the ability of the infectious agent to cause severe disease (e.g., Ebola: high; rhinovirus: low)Footnote 14 .
IV. Introduction: Prevention and Control of Pandemic Influenza in Healthcare Settings
Influenza occurs in healthcare settings across Canada each fall and winter and is referred to as "seasonal influenza". All healthcare organizations delivering patient care including prehospital care providers, emergency departments, doctor's offices, intensive care units and long-term care facilities prepare for the arrival of the season's first cases of influenza. This preparation requires a concerted effort from many disciplines and jurisdictions inside and outside the healthcare organization's boundaries. Comprehensive Infection Prevention and Control and Occupational Health programs are important in providing healthcare organizations with effective processes and activities to prevent or minimize transmission of influenza within their organizations.
Periodically, influenza may cause worldwide epidemics, or pandemics, with high rates of illness and death. An influenza pandemic can occur at any time with the potential to cause serious illness, death, and extensive social and economic disruption throughout the world. Experts agree that influenza pandemics are inevitable, however, the timing and severity of any pandemic is unpredictable and occurs with little warning. As well, pandemics may differ in their severity and attack rates. Historically, pandemics infected large numbers of the population with high mortality rates. The 2009 pH1N1 however, was relatively mild in comparisonFootnote 22, Footnote 23. The transmissibility, attack rates, severity indicators, high risk groups and mortality may differ as a result of the epidemiology that emerges with the pandemic. Contingency planning that addresses a continuum from mild to severe disease is recommended to minimize the potentially devastating effects of an influenza pandemic.
The Canadian Pandemic Influenza Plan (CPIP) (see The Canadian Pandemic Influenza Plan for the Health Sector) assumes that any influenza pandemic will first emerge outside of Canada; however, because of the volume and speed of global air travel, the virus will be present in Canada within weeks of its emergence in another part of the world. The pandemic virus may arrive in Canada at any time of year (i.e., potentially outside of the usual influenza season in Canada). The first peak of illness (i.e., beginning of the pandemic wave) in Canada could occur within weeks after the virus arrives in Canada. The first peak in mortality is expected to be approximately one month after the peak in illness.
The pandemic wave may sweep across Canada in one to two months affecting multiple locations simultaneously; the influenza pandemic may occur in two or more waves lasting six to eight weeks in any locality. Overall, the pandemic may last 12 to 18 months and more than one wave may occur within a 12 month period.
Canadian healthcare organizations will likely be impacted by an influenza pandemic. It is therefore essential for these organizations, in coordination with regional and provincial/ territorial governments, to ensure that they can manage an influx of patients with influenza while maintaining the level of patient care required for all other patients.
HCWs, like others in the community, will be exposed to the pandemic strain as they go about their daily activities (e.g., grocery shopping, attending school meetings, group sports) and may become ill and/or unable to come to work Footnote 24.
Healthcare organizations should engage in comprehensive planning to:
- Develop procedures to effectively segregate and streamline patient assessments in order to minimize influenza exposure;
- Determine cohorting strategies for patients with and without influenza, both to minimize influenza exposures and maximize patient care efficiencies (Note: When setting up patient cohorts it will be important to consider whether specific HCWs will be cohorted (i.e., assigned to work only with the patient cohort); and
- Ensure that aerosol-generating medical procedures (AGMPs) are carried out using a process and in an environment that minimizes the exposure risk for HCWs, ensuring that non-infected patients, visitors and others in all healthcare settings are not unnecessarily exposed to the influenza virus.
Canada's annual experience with seasonal influenza outbreaks and of the healthcare organizational challenges associated with limited surge capacity demonstrates the need to carefully and comprehensively prepare for the impact of a major influenza outbreak. A major influenza outbreak may have a substantial impact on the ability of any healthcare organization to keep everyone within its boundaries safe, whether they are providing or receiving healthcare services. Recent experience with pH1N1 influenza virus has highlighted the urgent need for influenza pandemic planning in all healthcare settings including in physicians' offices and other ambulatory care settings.
The materials presented in this Annex have been developed to provide healthcare organizations and HCWs with the information they need to plan for and execute IPC and OH processes intended to prevent exposure to and transmission of pandemic influenza during the provision of health care.
V. Foundations for a Pandemic Influenza IPC/OH Plan for all Healthcare Settings
1. Public Health Assumptions
The following public health assumptions regarding pandemic influenza that are relevant to IPC and OH planning, originate from the Canadian Pandemic Influenza Plan (CPIP) for the Health Sector (see Key Planning Assumptions), December 2006Footnote 5 and have been adapted to include the epidemiology of the pH1N1 influenza virus.
It is important to note that assumptions about the epidemiology and impact of pandemic influenza viruses may change as knowledge emerges about a specific pandemic influenza virus. The level of Pandemic Influenza Precautions required may need to be adapted (e.g., initially, precautions may need to be initiated at a higher level and then relaxed as information becomes available).
- The incubation period, period of communicability and method of transmission for the novel strain are assumed to be consistent with other known influenza strains, as follows:
- Incubation period: one to three days (this may vary depending on the viral strain).
- Period of communicability: 24 hours beforeFootnote 25 and up to seven days after symptom onset (usually up to three to five days in immunocompetent adults, up to seven days in young children; the period of communicability may be increased in immunocompromised adults and children).
- Transmission of infection by asymptomatic individuals is possible but likely to be more efficient when symptoms, such as coughing or sneezing, are present and viral shedding is high (i.e., early in the symptomatic period).
- The novel influenza virus may be transmitted efficiently from person-to-person.
- As a pandemic wave passes through a community, it is likely that most cases of influenza will be caused by the pandemic strainFootnote 5.
- The initial clinical presentation should be consistent with that of known influenza strains.
- Sub-clinical infections may occur.
- The pandemic strain may cause more than one wave of illness.
- It is unlikely that an effective vaccine will be available at the start of pandemic influenza activity in Canada. An effective vaccine may be available for a second wave of the pandemic through the community.
- Mass immunization campaigns may occur when sufficient quantities of the vaccine containing the pandemic influenza strain are available increasing the demand for human resources.
- Pandemic influenza vaccine may be a good match to the circulating pandemic influenza virus. However, once available, one dose may not be fully protectiveFootnote 5 and two doses may be required. See The Canadian Pandemic Influenza Plan for the Health Sector - Annex D.
- Individuals who recover from infection caused by the pandemic influenza strain should be immune to further infection from that specific strain.
- The novel pandemic influenza strain and first human cases of influenza caused by the pandemic viral strain will likely be identified outside of Canada.
- Surveillance measures are in place to detect influenza-like illness (ILI) and severe respiratory illness (SRI) across Canada.
2. Infection Prevention and Control Assumptions used in Annex F
A well functioning IPC program working in concert with a well functioning OH program, is the basis for an effective IPC response during an influenza pandemicFootnote 26-29. Well functioning IPC programs should prevent, limit or control the acquisition of healthcare-associated infections (HAIs) for everyone (i.e., patients, HCWs, visitors, contractors, etc.) in the healthcare setting.
Recommendations in this Annex are based on the assumption that an effective and fully supported IPC program is functioning within each healthcare settingFootnote 2, Footnote 3, Footnote 30 Footnote 31. An effective IPC program should consist of the following:
- Adequate numbers of trained Infection Control Professionals for the population size and case-mix of the healthcare organization who are able to carry out the pandemic influenza planning and implementation activities recommended in this documentFootnote 32-36.
- A HAI surveillance program that is capable of tracking trends in key HAIs, including respiratory infections Footnote 33.
- Infection prevention and control measures such as "Routine Practices"Footnote 2, Footnote 3 to ensure that all patients are cared for in a manner that prevents or minimizes the transmission of infection from an individual and/or environment to another person.
- A HCW 's decision to wear PPE as part of Routine Practices should be based on his/her assessment of the risk of exposure to blood, body fluids, non-intact skin and excretions or secretions, including respiratory secretions.
- Infection prevention and control measures such as "Additional Precautions"Footnote 2, Footnote 3 to provide guidance for the care of patients with infections insufficiently contained by Routine Practices. These patients should be cared for with additional measures to prevent the transmission of specific infectious agents or infectious syndromes spread via contact, droplet or airborne mechanisms.
- Contact Precautions (see Section V.4.4.1.), Droplet Precautions (see Section V.4.4.2.) and Airborne Precautions (see Section V.4.4.3.), are based on the three modes of exposure and transmission of infectious diseases.
- All HCWs decisions about whether the patient requires Additional Precautions should be based on an assessment of the presence of a specific infectious agent or syndrome (diagnosed or suspected).
- Pandemic Influenza Precautions is a synthesis of Additional Precautions critical to the prevention and control of pandemic influenza virus in healthcare settings.
- Elements of Routine Practices and Additional Precautions (RPAP) include policies and procedures for:
- Hand hygieneFootnote 10, Footnote 11 for HCWs.
- Respiratory hygiene for patients, HCWsFootnote 3.
- Infected source control, for example:
- Patient spatial separation policies and practices;
- Processes and procedures to identify and limit/modify clinical procedures with increased risk of infectious agent exposure;
- A screening program for early identification of patients, and HCWs with acute respiratory infections;
- Means to apply Additional PrecautionsFootnote 2,Footnote 3 when patients or residents with a specific infectious agent are identified;
- Processes to ensure appropriate immunization of patients (for HCWs see Section V.3.);
- Processes to identify and manage outbreaks of infectious agents, including outbreaks caused by respiratory viruses.
- Patient assessment, placement, and movement within the facility.
- Aseptic technique.
- Reprocessing medical equipment.
- Cleaning the patient environment.
- Handling of medical waste.
- Handling of patient care linens.
- Visitor access policies and practices.
3. Occupational Health Assumptions
A well functioning OH program working in concert with a well functioning IPC program, is the basis for an effectiveFootnote 26, Footnote 28, Footnote 29, Footnote 37. Well functioning OH programs should identify workplace hazards and provide appropriate processes and training to ensure employees can perform their duties in an environment that minimizes exposure to environmental hazards (e.g., Respiratory protection). The OH program should also provide required immunization to employees.
The OH recommendations in this document are based on the assumption that the healthcare setting has a functioning OH program that is working in concert with a functioning IPC program. This assumption is the basis for an effective response to protect HCWs from acquiring the pandemic influenza virus while at work during an influenza pandemic.
Agencies that provide contract workers (e.g., HCWs) to a healthcare organization should ensure they are trained to meet the Occupational Health and Occupational Health and Safety requirements of the receiving organization, including fit testing for the N95 respirators used in the organization. Depending on the jurisdiction, either or both the contracting agency or the providing agency may hold the responsibility to provide the training.
An effective OH infectious disease program should consist of:
- A hazard assessment process to evaluate the workplace to identify, assess and analyze risks related to work activities that may result in exposure to the identified biological hazards, including infectious agents.
- The application of systematic controls and personal protective equipment (i.e., engineering and administrative controls, and the use of PPE) to enable employees to perform their duties in an environment that minimizes their risk of exposure to hazards, including infectious agents.
- The cumulative impact of utilizing all three levels of control will provide more protection than the application of any one control level alone. The degree of protection offered by effective engineering and administrative controls are greater and more systematic than those provided with the use of personal protective equipment (PPE) alone.
- Provision of the necessary resources (e.g., adequate numbers of gloves, gowns) to HCWs to perform their work activities safely.
- Measures to ensure appropriate immunization and immunization documentation of HCWs.
- Measures to ensure that policies, procedures and programs are consistent with current recommendations, achieve their stated objectives, and are in compliance with current workplace occupational health and safety legislation and regulations (e.g., Occupational Health and Safety, Workplace Safety, Labour codes).
- A Respiratory Protection Program (RPP) focused on the respiratory protection needs of all HCWs. The program should provide health screening, fit testing, and instruction in the care, use and limitations of respirators for all HCWs who may wear a respirator or other respiratory protective device during the provision of health care (see Section V.6.2.4.).
- Respiratory protection requires the use of a respirator to prevent inhalation of chemical or biological hazards.
- The processes of fit testing and the frequency of fit testing should be in compliance with relevant (federal, provincial, territorial) regulations. In the absence of regulations from the jurisdictional region, the frequency of fit testing should be in compliance with the Canadian Standards Association standardsFootnote 15.
- Each time HCWs put on a respirator, they should perform a seal check (previously referred to as a "fit-check") to enable proper functioning of the respiratorFootnote 15.
- Facial hair may interfere with the seal of the respirator and as a result the respirator may not form a tight facial seal. Healthcare organizations should develop policies related to facial hair and the use of respirators. These policies should be in compliance with relevant occupational health and safety legislations and regulations.
- Fit testing results are NOT transferable between respirator manufacturers or models. Note: Powered air purifying respirators are NOT recommended for influenza care. Other options are available for healthcare workers with facial hair and should be made available if required. (See Section V.6.2.4.e.).
- Healthcare organizations that perform AGMPs (see Section V.4.4.4.), and/or care for patients infected with airborne infectious agents (e.g., tuberculosis) should have an active RPP.
- Healthcare organizations that require personnel to wear respirators should have written policies and procedures for their RPP.
Note: The use of N95 respirators in the prevention of most respiratory virus infections, including influenza, remains controversialFootnote 38, Footnote 39.
NOTE: During an influenza pandemic, HCWs, like others in the larger community, are at risk of exposure to the pandemic influenza viral strain as they go about their daily activities in the community (e.g., grocery shopping, attending school meetings, caring for ill family members, playing group sports).
4. Principles of Influenza Exposure and Transmission in Healthcare
4.1. Basic Principles of Infectious Disease Epidemiology
Epidemiology is the study of the distribution and determinants of health-related states or events in specified populations and the application of this study to the control of health problems Footnote 40. The main purpose of infectious disease epidemiology is to assist in the prevention of infection through an understanding of its distribution (i.e., of person, place and time), factors that affect its natural history, and factors that influence the acquisition of disease.
Acquisition of infection is the result of a set of complex interrelationships between the infectious agent/infected source, the susceptible host and the environment. The "epidemiological triangle" Footnote 41 can be used to describe and understand the relationship between these three key elements. Figure 1 provides a visual representation of the interaction of the three elements as it relates to infectious disease, and influenza in particular. In this document, the relationship between the elements found in the epidemiological triangle will provide the basis for describing a) the process of exposure to and transmission of the influenza virus and b) interventions to minimize (prevent and control) transmission of the influenza virus among patients, HCWs, visitors, contractors, etc. while present (e.g., working, receiving care, visiting, volunteering) in healthcare settings.
Figure 1. Epidemiological Triangle – acquisition and transmission of infection
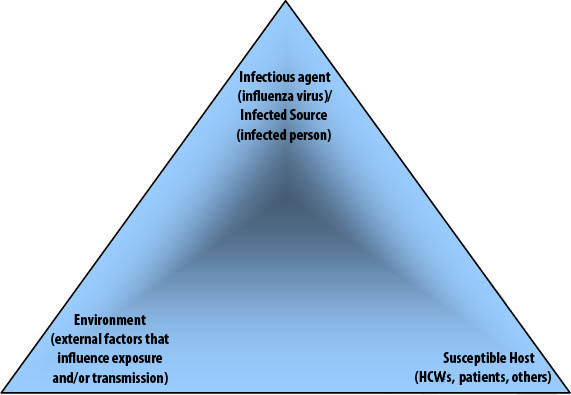
4.1.1. Infectious Agent/Infected Source
The infectious agent is a microorganism that causes, or has the potential to cause an infection. Characteristics of the infectious agent (e.g., pathogenicity, virulence, and infectious dose), symptoms and behaviours of the infected source may influence the possibility of exposure to, and transmission of, an infectious agent to a susceptible host. In the healthcare setting "infected source" describes a person with an infection caused by an infectious agent. The infected source may be a patient, HCW, visitor, etc.
4.1.2. Susceptible Host
A "susceptible host" is an individual not possessing sufficient immunity against a particular infectious agent to prevent contracting an infection when exposed to an infectious agent. A susceptible host must be exposed to an infectious agent/infected source in a manner that will enable the acquisition of an infection. The integrity of a susceptible host's internal defences, both innate (e.g., normal flora, intact skin, neutrophils, macrophages) and acquired (antibodies, cell-mediated responses), may impact the host's ability to prevent disease after exposure to the infectious agent. Host defences may be altered by age, co-morbidities, immunization status, genetic factors, medications, and invasive medical procedures that predispose the susceptible host to infection. In the healthcare setting, the susceptible host may be a patient, HCW, visitor, etc.
4.1.3. Environment
The "environment" includes all factors, external to either the susceptible host or the infected source, that may assist or impede the exposure to, or transmission of, the infectious agent from the infected source to the susceptible host. The environment may be conducive to the survival and transmission of the infectious agent, potentially increasing the size of the dose to which the host is exposed.
The environment may play a larger role than previously appreciated in the transmission of certain pathogens Footnote 42, reinforcing the importance of minimizing environmental contamination by patient excretions and secretions, avoiding unnecessary hand contact with environmental surfaces and ensuring that adequate resources (e.g., housekeeping personnel) are available for cleaning patient care equipment and horizontal surfaces in the patient's environment.
The environmental risk can be minimized by the use of the Hierarchy of Controls (i.e., engineering and administrative controls, as well as the availability and use of PPE). The concept of the Hierarchy of Controls will be utilized throughout this Annex and is described in detail in Section V.6. In healthcare settings, the impact of these control measures frequently overlap (e.g., the effectiveness of hand hygiene may be influenced by the placement of alcohol-based hand rubs [ABHRs] dispensers and dedicated hand washing sinks [engineering controls], policies and procedures for performing hand hygiene [administrative controls] and the availability and use of PPE [e.g., gloves]).
4.2. Epidemiological Triangle Applied to Pandemic Influenza
While the concepts of infected source and susceptible host are discussed below as occurring separately in individuals, it is critical to remember that each person (patient, HCW, etc.) has the potential to be either (e.g., a HCW may be a susceptible host who acquires influenza in the community and then becomes an infected source at work in the healthcare setting).
As per the assumptions in Section V.1., V.2., and V.3., the pandemic influenza virus will be a human influenza virus to which a large number of the population will be susceptible. The pandemic influenza virus is expected to be clinically and epidemiologically similar to other known influenza virus strains.
4.2.1. Pandemic Influenza: Infectious Agent/Infected Source
For a discussion regarding the potential characteristics of the pandemic influenza virus refer to the section entitled Public Health Assumptions (see Section V.1.). It should be noted that the actual virulence, pathogenicity, shedding, incubation period and period of communicability of a specific strain cannot be determined until the pandemic has been declared and sufficient epidemiologic information has been obtained. Further characterization of the influenza pandemic strain will continue to be developed as the pandemic progresses and new information becomes available.
Infected sources include all individuals present in the healthcare setting who are infected with the pandemic influenza virus and are within the period of communicability (see Section V.1.a.), including patients, HCWs, visitors, etc. The identification of infected sources may be difficult as individuals infected with the pandemic influenza virus may be able to transmit influenza up to 24 hours before symptom onset (see Section V.1.a.). The similarity of influenza symptoms to other respiratory illnesses may also make it difficult to definitively diagnose influenza.
In general, factors that increase the source's ability to transmit infection include the frequency of coughing and sneezing, the concentration of infectious agents in the respiratory secretions, and the stage of illness Footnote 43. Infected sources, who are unable to comply with respiratory hygiene (e.g., children and individuals with cognitive impairment) are more likely to expose and potentially transmit their infection to othersFootnote 44, Footnote 45.
4.2.2. Pandemic Influenza: Susceptible Host
Susceptible hosts include all non-immune individuals in the healthcare setting including HCWs, patients and visitors who may be exposed to an infected source or environmental contamination. Susceptible hosts that become infected with the pandemic influenza virus may in turn become infected sources. Note that the pandemic influenza viral strain is likely to be a new strain with most individuals likely to be susceptible at the beginning of the pandemic.
The risk of pandemic influenza infection and subsequent disease will be dependent on the likelihood of exposure to and susceptibility of a specific hostFootnote 5.
- In the inter-pandemic period, immunity to seasonal influenza is provided from high annual influenza immunization and/or past influenza infections in the population. During an influenza pandemic caused by a novel influenza strain, the lack of immunity against the pandemic influenza viral strain may result in a greater number of susceptible hosts (who may subsequently become infected sources) and thus potentially greater spread of disease.
- Individuals with underlying medical conditions may be at higher risk of serious complications if they become infected with the pandemic influenza virus.
The National Advisory Committee on Immunization has described medical conditions that place individuals at higher risk of complications should they acquire influenza Footnote 17. (See Canadian Immunization Guide Seventh Edition - 2006).- Individuals with the following medical conditions are at higher risk of complications should they acquire influenza:
- Cardiac or pulmonary disorders, including bronchopulmonary dysplasia, cystic fibrosis and asthma
- Diabetes mellitus and other metabolic disorders
- Cancer, immunodeficiency, immunosuppression due to underlying disease and or therapy
- Renal disease
- Anemia and hemoglobinopathy
- Conditions that compromise the management of respiratory secretions and are associated with an increased risk of aspiration
- Pregnant women
- Infants and young children
- Individuals greater than 65 years of age
- Individuals with the following medical conditions are at higher risk of complications should they acquire influenza:
- During the pandemic period, healthcare organizations with a large number of vulnerable patients will have a susceptible host population at high risk of serious complications.
4.2.3. Pandemic Influenza: Environment
The environment includes the physical area in which interactions and/or activities bring an infectious agent/infected source together with a susceptible host. During a pandemic wave, the increase in the number of infected sources (i.e., surge) will greatly increase the risk of exposure of a susceptible host. In the healthcare setting this means bringing a person with influenza or object contaminated with the influenza virus and a susceptible host together in a way that may allow the influenza virus to pass from the source to the host. For example, an infected individual's hands may be "the environmental factor"that brings infected source and susceptible host together.
In most healthcare organizations, the areas with the greatest risk will be where patient care is delivered.
4.3. Pandemic Influenza: Modes of Exposure and Transmission
The following information is based on the Public Health Assumptions discussed in Section V.1., specifically, that the incubation period and communicability of the pandemic virus may be similar to other known human influenza viruses. However, the exact nature of the pandemic influenza virus and pandemic influenza infection (e.g., period of communicability, severity of illness) may be unclear until an actual influenza pandemic occurs. The three modes of potential respiratory pathogen exposure/transmission (i.e., contact, droplet, and airborne) (see Figure 2) will be discussed separately; however, these modes of transmission do overlap. The recommendations in this document will interpret and apply evidence for contact, droplet and airborne as a continuum.
4.3.1. Pandemic Influenza: Exposure
Exposure to an influenza virus occurs when a susceptible host comes into contact with an infected source or contaminated environment (e.g., inanimate/animate objects or via virus particles in the air). Figure 2 illustrates the continuum of infectious agent exposure that may be relevant to a susceptible host when touching an infected source or a contaminated environment (e.g., less than two metres away from an infected source, face-to-face) and when a susceptible host inhales an infectious agent (as an aerosol or droplet)Footnote 46-48.
Pandemic influenza virus will be a human influenza virus to which there is global susceptibility. The pandemic influenza virus will likely behave clinically and epidemiologically similar to other known seasonal influenza virus strains.
Figure 2: Exposure to Particles Develped by the ANNEX F Working Group, 2008; * See Glossary
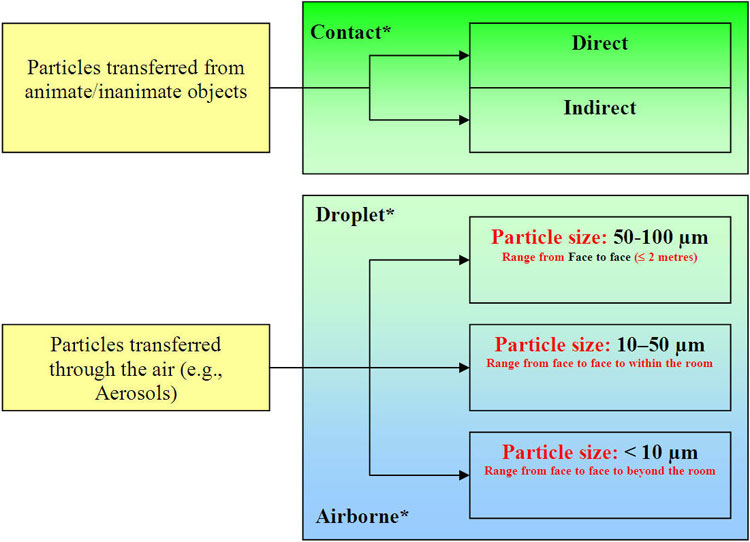
Text equivalent
Exposure to Particles Develped by the ANNEX F Working Group, 2008
Figure 2 illustrates the continuum of infectious agent exposure that may impact a susceptible host when touching an infected source or a contaminated environment.
Particles transferred from animate/inanimate objects are considered direct or indirect contact exposure.
Particles transferred through the air (e.g., Aerosols) are primarily considered:
- Droplet exposure if the particle size is:
- 50-100 µm – face to face range (≤ 2 metres)
- 10-50 µm – range from face to face to within the room (overlaps with Airborne – see 2.a. below)
- Airborne exposure if the particle size is:
- 10-50 µm – range from face to within the room (overlaps with Droplets see 1.b. above)
- < 10 µm – range from face to face to beyond the room
The overlapping region with particles from 10-50 µm may constitute either droplet or airborne exposure on the circumstances of the source, host and environment.
Figure 3 Deposition regions of the respiratory tract for the various particle sizesFootnote 49 . Used with Permission.
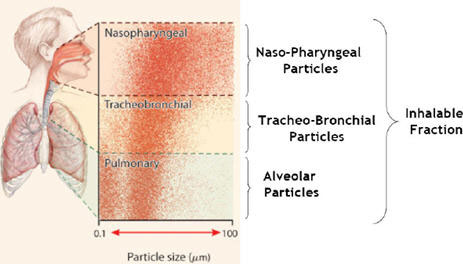
Text equivalent
Deposition regions of the respiratory tract for the various particle sizes
Figure 3 provides a visual representation of the penetration of inhalable particles into a person’s airway. Particles with a diameter of 0.1 to 100 µm fall primarily in the naso-pharyngeal and tracheal-bronchial areas of the upper respiratory tract. Particles with a diameter of 0.1 µm to 10 µm penetrate as far as the alveolar ducts.
Recent literature has demonstrated that aerosols contain both droplets and airborne sized particles that can be found in the air at close proximity to a coughing/sneezing source (less than two metres)Footnote 46-54 . In addition, a portion of larger particles (droplets) may desiccate (become smaller) while in the air and become, in effect, droplet nuclei. Polymerase Chain Reaction (PCR) has identified influenza ribonucleic acid (RNA) in small aerosols produced by persons with influenza; however, the relationship between the presence of these small segments of influenza RNA to the actual infectivity of small aerosol particles has not been demonstratedFootnote 39 Footnote 49.
Particles with a diameter of 0.1µm to 10µm may penetrate as far as the alveolar ducts (i.e., beyond the upper respiratory system) but may also be deposited at any point in the respiratory tract (Figure 3). Aerosols with a larger diameter (10µm -100µm) can be deposited on influenza receptors, which are predominantly found in the upper airway (e.g., nasopharynx).
4.3.2. Pandemic Influenza: Transmission
Receptors for human influenza virus are predominantly located on the nasopharyngeal mucosa. Transmission of infection occurs when influenza viruses penetrate a susceptible host's defences and are deposited on viral receptors in the upper respiratory tract Footnote 49. Transmission of the human influenza virus depends on the exposure of a susceptible host to a sufficient concentration (infectious dose) and attachment to a receptor of viable human strain viral particles Footnote 50. Published clinical observationsFootnote 50, Footnote 51 suggest that influenza transmission usually occurs when the susceptible host and infectious source are within close proximity (less than two metres)Footnote 46-54.
Human-to-human transmission of the influenza virus appears to be similar to the transmission of other human influenza viruses (e.g., seasonal influenza) occurring primarily either directly or indirectly through close unprotected contact with large respiratory droplets. The contribution of close range exposure to smaller droplet nuclei to transmission of influenza is unknown, but may be more prominent under special conditions (e.g., aerosol-generating procedures). Therefore, IPC precautions for patients with suspected, probable or confirmed pandemic influenza virus infection, as well as those with other respiratory pathogens that cause ILI symptoms, should focus on controlling the spread of respiratory dropletsFootnote 46.
Exposure to the influenza virus does not necessarily result in its transmission and subsequent infection. A susceptible host may come in contact with (i.e., be exposed to) an infectious agent/infected source and NOT acquire influenza infection (i.e., transmission does not occur). The probability of infection (transmission) is dependent on a number of factors, including host mucosal immunity, infectious dose, viability and virulence of the infectious agent, and the effective implementation of Routine Practices and Additional Precautions within an organization's healthcare service environment.
4.4. Modes of Exposure to Pandemic influenza
4.4.1. Pandemic Influenza: Contact Exposure and transmission
Pandemic influenza contact exposure may occur, when infectious agents are transferred through direct physical contact between an infected source and a susceptible host or through the transfer of the infectious agent to a susceptible host via an intermediate objectFootnote 2, Footnote 3.
Figure 4: Direct contact where there is skin to skin contact between two persons
Infectious agents, including influenza and other respiratory viruses that are expelled in large droplets, remain viable in droplets that settle on objects in the immediate environment of the patient. In one study, both influenza A and B viruses were shown to survive on hard, non-porous surfaces for 24 to 48 hours, on cloth, paper and tissue for eight to twelve hours and on hands for five minutes Footnote 52. Hands can be contaminated with influenza virus by contact with an infected source or by contact with contaminated inanimate surfaces or objects in the immediate environment of a source with influenza infection. Contact exposure includes direct and indirect contact:
- Direct contact exposure may occur when the transfer of the pandemic influenza virus results from direct physical contact between an infected source and a susceptible host (e.g., hands of infected source to the mucus membranes of a susceptible host).
- Indirect contact exposure involves the passive transfer of pandemic influenza virus to a susceptible host via an intermediate object, such as contaminated hands that are not cleaned between episodes of patient care, contaminated instruments that are not cleaned between patients/uses or other inanimate objects/environmental surfaces in the patient's immediate environment.

Figure 5: Indirect contact where there is contact with an inanimate object which may serve as the vehicle for transmission of pathogens
Pandemic influenza contact transmission may occur when contact exposure leads to an infectious dose of viable pandemic influenza particles from an infected/contaminated source being inoculated onto mucus membranes, (i.e., eyes, nose and mouth) and overcomes other host defences.
4.4.2. Pandemic Influenza: Droplet Exposure and Transmission
Pandemic influenza droplet exposure may occur when droplets containing an infectious agent are propelled a short distance (less than two metres)Footnote 46-54 through the air and deposited on a person's mucous membranesFootnote 2, Footnote 3. Infectious droplets are generated naturally from an infected source primarily during coughing and sneezingFootnote 50-54, or through AGMPs (see Section V.4.4.4.). AGMPs may also result in the generation of smaller infectious droplets, which can travel further than those generated spontaneously from patientsFootnote 54-58.
Droplets of various sizes (see Figure 2) containing influenza virus may contaminate the immediate environment when they settle on surfaces, and contribute to contact transmission Footnote 58.
Pandemic influenza droplet transmission may occur when droplets that contain an infectious dose of viable influenza particles are propelled a short distance (less than two metres)Footnote 46-54 through the air and come into contact with influenza virus receptors in the susceptible host's upper airway, and overcome other host defences.

Figure 6: Droplet transmission, where large respiratory particles travel up to 2 meters
4.4.3. Pandemic Influenza: Airborne Exposure and Transmission
Pandemic influenza airborne exposure may occur if small particles (i.e., aerosols containing droplet nuclei) with viable influenza virus are generated and propelled over short or long distances and then inhaled by a susceptible host. Aerosols containing viable influenza virus may be generated naturally from an influenza infected source during coughing and sneezing. However, the contribution of close range exposure to small aerosols carrying viable influenza viruses is not well documented but is theorized to be more common during AGMPs. Airborne exposure may result almost immediately after generation i.e., the direct projection of an aerosol containing viable amounts of influenza virus through the air, and directly captured by a host's respiratory system. Exposure may also occur for longer periods as droplet nuclei can remain suspended in the air for a period of timeFootnote 39, Footnote 49, Footnote 53-73 before settling out of the air during which time a host may inhale the suspended aerosol.

Figure 7: Airborne transmission whereby small particles travel long distances
Pandemic influenza airborne transmission may occur when viable viral particles contained in aerosolized secretions from an infected source are propelled a short distance (less than two metres)Footnote 46-54 through the air, are inhaled, come into contact with influenza virus receptors in the susceptible host's upper airway and cause disease. Polymerase Chain Reaction (PCR) has identified influenza RNA in small aerosols produced by persons with influenza; however, the viability (infectivity) of these small segments of influenza RNA has not been demonstrated, thus, the clinical importance of influenza airborne transmission remains controversialFootnote 50,Footnote 51,Footnote 58.
Figure 3 depicts the various regions of the respiratory tract along with the size classification of particles and their corresponding region of deposition Footnote 49.
4.4.4. Pandemic Influenza: Aerosol-Generating Medical Procedures
AGMPs (see Section V.6.2.4. and Section VII.1.5.4.) are medical procedures that can generate aerosols as a result of manipulation of a person's airway. There are several types of AGMPs associated with a documented increased risk of tuberculosis (TB) or SARS transmission. It should be acknowledged that there is an evidence base and consensus of opinion regarding the spread of infection via droplets and aerosols by these procedures for TB or SARS. Further research may provide additional evidence regarding the hazards that exist from AGMPs, and performed when other organisms are present Footnote 9. The risk of infection transmission via droplet nuclei and aerosols may increase during AGMPs because of the potential to generate a high volume of respiratory aerosols that may be propelled over a longer distance than that involved in natural dispersion patternFootnote 46Footnote 60. These procedures include:
- Intubation and related procedures (e.g., manual ventilation, open endotracheal suctioningFootnote 48, Footnote 55, Footnote 61,Footnote 62.
- Cardiopulmonary resuscitation Footnote 62.
- BronchoscopyFootnote 63, Footnote 64.
- Sputum induction Footnote 65.
- Nebulized TherapyFootnote 66, Footnote 67.
- Surgery and autopsyFootnote 68, Footnote 69.
- Bi-level Positive Airway Pressure (i.e., BiPAP) Footnote 70.
There is debate as to whether other medical procedures that result in the generation of aerosols or cough induction lead to the transmission of infection. To date, however, there is no published literature that documents the transmission of respiratory infections, including TB, SARS and influenza, as a result of these procedures. Examples of such procedures include:
Chest physiotherapy
High-frequency oscillatory ventilation
Tracheostomy care
Nasopharyngeal swabs, Nasopharyngeal aspirates
NOTE: AGMPs performed on patients with no symptoms of influenza should be carried out using RPAP recommendations.
4.4.5. Pandemic Influenza: Continuum of droplet and airborne exposures
The probability of airborne exposure to an infectious aerosol is influenced by several factors in addition to the proximity of the infected source and susceptible host. These include the particle sizes containing the infectious agent, the viability of the infectious agent, and the animate and inanimate environment of a room (e.g., the concentration of the viral particles in the aerosol, the concentration of aerosol in the room, the relative humidity, the direction of air flow and the number of air changes per hour in the room).
Particles of a variety of sizes are expelled from the human airway during coughing, sneezing, talking, and medical procedures. The initial size of these particles and the distance they will be propelled is dependent on the force generated by the individual or the procedure. Large particles (greater than 10 µm diameter) will fall quickly (in a few seconds) to the groundFootnote 46, Footnote 49, Footnote 54. However, smaller particles may remain suspended for a significantly longer time: tens of seconds for a droplet of 10 µm diameter and minutes or hours longer for smaller droplet nuclei. The particles that remain aloft for minutes or hours (less than 10 µm diameter) can be carried by air currents over a measurable distance, including beyond the room, and are considered to represent an airborne exposure. To date there is no human evidence to suggest that viable infectious influenza virus particles are carried measurable distances beyond the two metreFootnote 46-54 bed space.
The likelihood of airborne exposure to the pandemic influenza virus will depend, principally on the size of the particles in aerosols (see Figure 2) generated by the infected source. Airborne exposure is highly dependent on environmental factors, (i.e., humidity and air changes per hour in the room). Influenza virus particles have been detected in the airspace (less than two metres)Footnote 46-54 around a person with diagnosed influenza using polymerase chain reaction; however, viability (and thus infectivity) of these viral particles is unknownFootnote 71,Footnote 72.
Note: The previous one metre recommendation for spatial separation was extrapolated from studies regarding the transmission of meningococcal disease Footnote 73 from coughing and sneezing. However, studies of individuals who expelled respiratory droplets through coughs and/or sneezes demonstrate that the distance these respiratory droplets travel approaches two metresFootnote 46-54.
5. Literature Reviews that Examine the Modes of Transmission of Influenza
There have been three recent systematic reviews on the transmission and control of seasonal influenza: Brankston et al. (2007) Footnote 50, Council of Canadian Academies (2007) Footnote 58 and Jefferson et al. (2009) Footnote 51. The methodology for selecting the original articles is clearly outlined in these reviews. Their findings are summarized here.
5.1. Transmission of Influenza A in Human Beings by Brankston et al. Footnote 50
Objective: To review the evidence about the routes of seasonal influenza transmission.
Conclusions:
- Natural influenza transmission in human beings occurs over short distances, rather than long distances.
- Natural influenza transmission occurs primarily via the droplet and contact routes.
- The airborne route (i.e., long range) is neither the predominant mode of transmission nor a frequent enough occurrence to be of significant concern when considering control measures for most clinical settings.
5.2. Expert Panel on Influenza and Personal Protective Respiratory Equipment by the Council of Canadian Academies
(Council of Canadian Academies ) Footnote 58
Objective: In 2007, the Public Health Agency of Canada commissioned the Council of Canadian Academies to examine how and where seasonal influenza is transmitted as well as to assess the respective contribution that respirators and masks each make in the prevention of seasonal influenza and/or pandemic influenza transmission Footnote 58.
Conclusions:
- Modes of influenza transmission
- Ballistic, nasopharyngeal-, tracheobronchial- and alveolar-sized particles (see Aerosols in glossary) are all emitted from the human respiratory tract.
- Note: In the CCA review, ballistic particles refers to particles with a mean aerodynamic diameter greater than 100 µm. They are predominantly affected by gravity and have a low probability of being inhaled, settling out of the air in seconds.
- Evidence about the relative contribution of the different modes of transmission to the spread of influenza is sparse and inconclusive.
- Influenza is transmitted primarily at short range.
- Influenza can be transmitted through inhalation of tracheobronchial- and alveolar-sized particles at short range.
- Deposition of nasopharyngeal-sized particles in the upper respiratory tract can cause influenza.
- Contact transmission can occur. The current weight of evidence suggests that transmission of influenza by inhalation of droplets (i.e., direct contact) is more probable than by indirect contact.
- The Academy stated that the evidence was lacking to determine whether long-range transmission of influenza (i.e., more than two metres) occurs however, the Academy did not rule this out.
- Protective measures against influenza transmission
- The primary elements of protection against influenza transmission are engineering and administrative controls. When exposure to an infected person is required or unavoidable, personal protective equipment including respiratory protection is required.
- N95 respirators protect against the inhalation of nasopharyngeal-, tracheobronchial- and alveolar-sized particles.
- Masks worn by an infected source may play a role in the prevention of influenza transmission by reducing the amount of infectious material that is expelled into the environment.
- Both masks and respirators offer a physical barrier, preventing contact with contaminated hands and ballistic trajectory particles.
- The efficiency of the filters in masks to block penetration of alveolar- and tracheobronchial-sized particles is highly variable when combined with the inability of masks to form a facial seal. These factors suggest masks offer no significant protection against the inhalation of alveolar- and tracheobronchial-sized particles.
- The efficiency of the filters of masks to block penetration of nasopharyngeal-sized particles is unknown. The lack of a sealed fit on a mask may allow for the inhalation of an unknown quantity of nasopharyngeal-sized particles.
5.3. Interventions for the Interruption and Reduction of Respiratory Viruses by Jefferson et al. Footnote 51
Objectives: This Cochrane collaborative reviewed the evidence related to the effectiveness of physical interventions to interrupt or reduce the spread of respiratory viruses (excluding vaccines and antiviral drugs).
Conclusions:
- Interventions such as hand washing, wearing a mask and isolating potentially infected patients were effective in preventing the spread of respiratory virus infections.
- Most effort should be concentrated on reducing transmission from young children in the community.
- The following interventions be implemented, preferably in a combined fashion, to diminish transmission of viral respiratory disease.
- Encourage frequent hand washing with or without adjunct antiseptics.
- Use barrier measures such as gloves, gowns, and masks with filtration apparatuses.
- Maintain a high level of diagnostic suspicion for respiratory viruses and institute timely isolation of likely cases.
5.4. Synthesis of Assumptions Used in this Annex to Describe the Risks of Pandemic Influenza Virus Transmission in Healthcare
Due to conflicting descriptions in the literature and assertions regarding modes of influenza transmission and effective preventative measures, the Expert Working Group developing this Annex developed a synthesis of assumptions, conclusions and principles regarding the transmission of influenza in order to provide the foundation for the prevention and control recommendations outlined in this Annex.
5.4.1. Assumptions About the Impact of Pandemic Influenza on Healthcare Organizations
- The number of individuals seeking medical assessments for ILI may rise substantially during an influenza pandemic due to an increased volume of patients with ILI.
- The number of individuals requiring treatment may also rise depending on the virulence of the pandemic influenza viral strain.
- As the pandemic spreads, there may be increased demands on healthcare resources and supplies due to increased numbers of patients with ILI seeking care. Medical suppliers may have difficulty meeting the surge in demand.
- Additional HCWs may be required to provide for the surge in ill patients noted above.
- HCWs may develop influenza from exposures in the larger community outside the healthcare setting at the same rate as the general population and may be unavailable for work. As a result, personnel shortages in programs offered by the healthcare organization may occur.
- Patients with risk factors placing them at high risk of complications should they develop influenza, should be identified and efforts made to reduce their risk of exposure to the pandemic influenza virus.
- Severity indicators for unanticipated risk groups may be identified as the pandemic progresses.
5.4.2. Assumptions about Pandemic Influenza Transmission
- The pandemic influenza virus will be a human influenza virus to which there will be global susceptibility. The pandemic influenza virus should behave in a manner clinically and epidemiologically similar to other known influenza virus strains.
- Pandemic influenza will be an acute respiratory infection spread primarily in the community, and will spread rapidly in the community, once introduced.
- The majority of pandemic influenza transmissions will occur at short range.
- Several modes of pandemic influenza exposure and transmission (contact, droplet, airborne) may contribute simultaneously to the transmission of influenza.
- The relative contribution of each mode of influenza transmission is undefined.
- Droplets generated by coughing and sneezing may lead to the generation of viable infectious aerosols.
- Aerosols generated by AGMPs on patients with pandemic influenza may have the potential to transmit potentially viable influenza to susceptible hosts.
- Survival of aerosolized pandemic influenza virus will depend upon environmental conditions, e.g., temperature, relative humidity, number of room air changes, etc.
- Whether an individual acquires pandemic influenza will depend on the inhaled influenza virus dose, the viability of the inhaled virus (particles), the host's immune response, innate barrier defences in the respiratory tract and the presence and contact with virus-specific receptors in a susceptible host's respiratory tract.
5.4.3. Assumptions About the Potential Spread of Pandemic Influenza in Healthcare Settings
- Without consistent use of effective controls, pandemic influenza will spread in the healthcare setting because of the concentration of infected sources (patients, HCWs) and susceptible hosts (patients, HCWs, etc.).
- The need to establish processes to separate patients with ILI symptoms from those without influenza will impact all patient care areas, including specialty areas, physician's clinics and home care services.
- Organizational implementation of systematic controls, (i.e., engineering and administrative controls and the use of PPE) PPE availability, accessibility and appropriate use should decrease the likelihood of a susceptible host being exposed to an infected source within the healthcare setting.
- RPAP protocols and practices, including the use of Pandemic Influenza Precautions, should be effective in minimizing the transmission of influenza viruses to any susceptible host in any healthcare setting.
- HCWs with ILI may be a source of infection for other HCWs Footnote 74.
6. Hierarchy of Controls in the Inter-pandemic and Pandemic Periods
6.1. Background on the Hierarchy of Controls
Collaboration between IPC professionals, Occupational Health professionals and healthcare facility building personnel (e.g., engineers) has led to better understanding and application of a tiered framework of measures/interventions that allows healthcare organizations to comprehensively evaluate the risk of exposure to infectious and other hazards in the workplace and the effectiveness of the healthcare organization's mitigation responses. The framework is known as the Hierarchy of Controls and involves an understanding of engineering, administrative and personal protective equipment (PPE) controlsFootnote 6-8. Understanding the Hierarchy of Controls should enable healthcare organizations to determine how the environment (e.g., infrastructure, equipment, processes and practices) increases or decreases a susceptible host's (i.e., patient, HCW, visitor, etc.) likelihood of exposure to infectious agents/infected sources within the healthcare setting. As such, all three levels of control are important and healthcare organizations should not focus on the implementation of one to the exclusion of the others as this may result in poorly protected patients, HCWs, etc.
6.1.1. Engineering ControlsFootnote 6, Footnote 7
In the Hierarchy of Controls, the engineering control aims to reduce the risk of exposure to infectious agents/infected sources by applying infrastructural methods of minimization, isolation or removal of the hazard.
Engineering controls are infrastructure controls that control the hazard (i.e., infectious agents, infected sources, and environment) at the source and therefore, do not depend on individual HCW's knowledge and compliance. These controls are usually established and managed within the building structure and as such, eliminate HCW's choice about their application and reduce the opportunity for error.
Engineering controls are those elements of the healthcare organization's physical plant/infrastructure that function to prevent exposure to and/or transmission of the influenza virus to the susceptible host, at the source or along the path of the hazard. Engineering controls include facility designFootnote 75-77, room design, ventilation systems, room air flow, human traffic patterns, positioning of alcohol-based hand rub (ABHR) dispensers and dedicated hand washing sinks, physical barriers to separate patients in multi-bed wards and patients in waiting areas, etc. For example, adherence to spatial separation requirements (e.g., planning for a high proportion of single patient rooms or alternatively, two metre separation between beds) when designing new healthcare facilities, planning renovations to existing facilities or re-organizing patient care areas should enhance a healthcare organization's ability to prevent the transmission of all infectious agents including seasonal and pandemic influenza virusFootnote 75-77. An organization's inability to ensure appropriate, functioning engineering controls may result in unnecessary exposure to a pandemic influenza virus for all individuals in a healthcare setting.
6.1.2. Administrative ControlsFootnote 6, Footnote 7
Administrative controls include the policies, procedures, and patient care practices intended to prevent exposure to and/or transmission of an infectious agent to a susceptible host during the provision of health care. To be effective in preventing of transmission of influenza and/or detecting cases of influenza, administrative controls must be implemented at the first encounter with an infected source and be continued until the infected source leaves the healthcare setting, or is no longer infectious. Ineffective or inconsistent application of administrative controls may lead to unnecessary exposure to a pandemic influenza virus for people present in a healthcare setting (i.e., patients, HCWs, etc.).
Inherent in the development of administrative controls to prevent the transmission of influenza and other infections is the commitment by the healthcare organization to provide the necessary resources to implement the controls. Examples of administrative controls to prevent the transmission of infectious agents within a healthcare setting are policies and procedures for management of outbreaks caused by the influenza virus or other respiratory viruses; passive and active screening processes to identify and appropriately isolate patients with a transmissible infection; availability and accessibility of hand and respiratory hygiene supplies, processes to enable appropriate immunization; etc.
The IPC and OH administrative controls that are central to pandemic influenza preparedness in healthcare settings include:
- Source control policies
- Point of Care Risk Assessment to protect HCWs and other patients (see Section V.7.3.).
- Measures to limit the introduction of the influenza virus into identified areas of the healthcare setting.
- Measures to separate infected sources and susceptible hosts, (e.g., fitness-for-work policies, policies for spatial/social distancing).
- Fitness-for-work policies, including policies that support absences due to illness (see Section V.6.2.2.).
- Policies to evaluate and address the risk of HCWs at high risk of severe complications from influenza (see Section V.6.2.3.).
- Respiratory protection policies and programs including policies to address individuals that cannot establish a tight facial seal when using respirators (e.g., facial deformities, men with beards for religious reasons) (see Section V.6.2.4.).
- Pandemic influenza specific education and skills training for HCWs (see Section VI.4.).
- Effective implementation of Routine Practices and Additional Precautions protocols including Pandemic Influenza Precautions (see Section V.6.2.6.).
6.1.3. Personal Protective EquipmentFootnote 6, Footnote 7
PPE refers to the availability and appropriate use of gowns, gloves, masks, respirators, face shields or other eye protection, which a susceptible host may wear to provide a physical barrier between themselves and an infectious agent/infected source. The following discussion of the availability and training in the care, use and limitations of PPE is applicable to all healthcare settings including prehospital care, acute care, long-term care, ambulatory care (including physicians' offices), community clinic care and professional home care.
The effective and appropriate use of PPE is dependant on the user's compliance and competence in using the PPE. The level of protection provided by PPE controls may be more easily compromised and as a result, ineffective protection from an infectious agent/infected sourceFootnote 6-8, Footnote 15, Footnote 78.
PPE effectiveness is highly dependent on appropriate use therefore HCWs should be fully knowledgeable of the care, use and limitations of specific PPE available for their use. The healthcare organization has a critical role in ensuring the availability of appropriate PPE for use by patients, HCWs, etc., who may have close contact with an infectious agent/infected source.
Healthcare organizations should ensure that HCWs have access to the PPE appropriate to the patient care being provided and have been provided with training on the choice, care, use and limitations of the appropriate PPE for specific situations.
HCWs should select PPE compatible with the hazard likely to be encountered during the patient care interaction (based on the Point of Care Risk Assessment (see Section V.7.3.). The selected PPE should maximize protection, dexterity and comfort. Inappropriate application and removal of PPE may result in exposure to the influenza virus through self-contamination. Please refer to Appendix B for a guide to safe putting on and removing of PPE.
Specific recommendations for when and where to put on PPE during the pandemic period will be found in Section V.6.2.6. of this document. During an influenza pandemic, the selection and use of PPE may need to be re-evaluated as part of the ORA, as knowledge of the pandemic virus evolves and if PPE stockpiles are depleted.
6.2. Application of the Hierarchy of Controls in the Inter-pandemic and Pandemic Periods
A major component of any healthcare organization's preparation and planning for pandemic influenza should be to ensure that the Hierarchy of Controls utilized in the healthcare setting is effective in preventing infectious disease transmission through the provision of health care during the inter-pandemic period. It is also important that each organization evaluate their ability to prevent and contain the transmission of the influenza virus within various healthcare settings during the pandemic. This review and evaluation is referred to as an Organizational Risk Assessment (ORA).
6.2.1. Organizational Risk Assessment
During the inter-pandemic period, an organization should be prepared to conduct an ORA (see Appendix C) that includes:
- An inventory of:
- the numbers and types of patients cared for and
- the numbers and types of healthcare services provided and
- An evaluation of:
- The availability and effectiveness of the organization's physical plant/infrastructure (i.e., engineering controls) and use of PPE supplies (i.e., PPE controls) and
- Policies and procedures to manage infectious agents/infected sources (patients, HCWs, etc.) (i.e., administrative controls and PPE controls) and
- HCWs' knowledge of and skills of and adherence to the array of engineering, administrative and PPE controls in their organization to prevent the transmission of HAIs and
- The effectiveness of the organization's communication plan related to influenza.
The ORA, completed in the inter-pandemic period should form the basis of an organization's IPC and OH plan (IPC/OH plan) for an influenza pandemic. Re-evaluation of the ORA may be necessary, especially after major organizational changes, (e.g., building renovations/construction, depletion of human resources, etc.).
Throughout the pandemic period, a healthcare organization should be prepared to:
- Re-evaluate the effectiveness and the application of its Hierarchy of Controls;
- Review and, if necessary, re-assess the ORA (see Section VII. and Appendix C) done in the inter-pandemic period, as each wave of pandemic influenza enters the region where the organization is located;
- Promptly address personnel and supplies shortages requiring remedial action
The ORA should be reassessed based:
- on new information on the emerging epidemiology and the characteristics of the pandemic influenza virus strain;
- the availability of an effective vaccine;
- the impact of the pandemic on HCWs, patients and the community;
- depletion of stockpiled equipment; and
- education and training of HCWs etc.
6.2.2. Administrative Controls: Fitness-for-work
Fitness-for-work policies should include measures to identify HCWs with respiratory infections and determine their ability to work along with the need for modified work assignments as required. Fitness-for-work policies should prevent an infected source from exposing patients and HCWs (i.e., susceptible hosts) to the influenza virus (i.e., infectious agent) in the healthcare setting. The fitness-for-work concept is applicable to all sectors of health care including prehospital care, acute care, long-term care, ambulatory care (including physicians' offices), community clinic care and professional home care (see Section VII. for specific recommendations for each sector of care).
For HCWs, actual fitness-for-work during a pandemic wave will be influenced by the person's susceptibility to the pandemic influenza viral strain; immunization status related to the pandemic influenza viral strain; and willingness/ability/need to use antiviral medicationFootnote 1,Footnote 19.
- A HCW may be considered fit-for-work if he/she:
- Is asymptomatic (as per self assessment for ILI, see Appendix A)
- In a severe or prolonged pandemic wave or when multiple waves occur close together, extreme staffing shortages may compromise the safety of patients, HCWs, visitors, etc. Organizations may need to consider some HCWs "fit-for-work with restrictions". HCWs may have symptoms of influenza but may be deemed "fit-for-work with restrictions" if ALL of the following apply. The HCW:
- Has mild influenza symptoms; and
- Feels well enough to work; and
- Will be assigned only to patients with influenza; and
- Will pay meticulous attention to respiratory and hand hygiene; and
- Will wear a mask at all times when in common areas.
6.2.3. Administrative Controls: Work Evaluation
The healthcare organization is responsible for ensuring that measures are in place to evaluate and educate self identified HCWs at increased risk of severe complications from influenza (see Section V.4.2.2.b.). These individuals should be offered an assessment by an occupational health clinician and be provided with counselling and education. This should include information pertaining to the severe outcomes of influenza and reinforcement of protective measures such as PCRA, appropriate use of PPE, access to vaccine as soon as available, use of early antiviral treatment, respiratory and hand hygiene, etc.
These work evaluation measures are applicable to all sectors of healthcare including prehospital, acute care, long-term care, ambulatory care (including physicians' offices), community clinic care and professional home care.
6.2.4. Administrative Controls: An Active Respiratory Protection Program
The RPP (see Section V.4.4.4.) should be designed to ensure that all HCWs who need to wear a respirator for their work have been appropriately:
- fit tested, and
- trained in the care, use and limitations of available PPE, and
- have access to recommended respirator models and sizes.
The RPP measures are fully applicable to all sectors of health care including prehospital, acute care, long-term care, ambulatory care (including physicians' offices), community clinic care and professional home care.
Respiratory protection requires the use of a respirator classified as N95 or higher filtration to prevent inhalation of aerosols containing infectious particlesFootnote 6, Footnote 15.
Healthcare organizations should select and stock an adequate supply and variety of respirator makes and models to accommodate the anticipated demand and the physical diversity of their workforce (e.g., a smaller face frame will require a smaller fitting respirator). When respirators are being selected by the organization, those with inherently good fit characteristics are preferred. An exact description of "inherently good fit characteristics" has not been fully defined. A respirator has inherently good fit characteristics if during the preliminary steps of fit-testing, a large proportion of HCWs in the organization can establish an effective facial seal with the model.
The following should also be considered:
- Respirators are available in a number of different shapes and sizes. These may need to be obtained from a number of different manufacturers to fit the range of facial structures within the organization's workforce.
- In most Canadian jurisdictions, HCWs who need to wear a tight fitting respirator require formal fit testing Footnote 15. Most jurisdictions require that fit-testing be repeated on a set schedule (e.g., at least every 2 years as per the CSA standard or as defined by jurisdictional regulations), or more frequently if facial conditions change (e.g., weight gain/loss, dental work, etc.).
- Each time HCWs put on a respirator, they should perform a seal check (previously referred to as a "fit-check") to enable proper functioning of the respirator Footnote 15.
- HCWs should be knowledgeable of the applications, advantages and limitations, and proper use of the specific respirator model(s) that they have been fitted for.
- Healthcare organizations should develop policies for healthcare workers that are unable to form a tight facial seal when wearing a respirator (e.g., facial scarring, men with beards for religious reasons, etc.) Footnote 15.
- Reusable (e.g., elastomeric) respirators should be available for these individuals. The advantages and limitations associated with the use of reusable respirators must be carefully considered. As well, the provision and use of reusable respirators requires education, training, and a successful fit test.
- Because influenza can be droplet spread, contamination of the surface of the respirator may occur. Between uses, all respirator components must be properly reprocessed or replaced according to the CSA standard and the manufacturer's instructions. HCWs should also be aware that communication between HCWs and patients may be difficult or compromised when wearing PAPRs.
- A powered air purifying respirator (PAPR) with a loose-fitting hood may be considered for those who cannot wear either a disposable or reusable respirator (e.g., men with full beards for religious reasons). The PAPR must have high efficiency particulate filters. Provision and use of a PAPR requires extensive education and training. Extreme care must be taken when removing the PAPR to prevent self contamination from the PAPR surfacesFootnote 46, Footnote 50, Footnote 52, Footnote 54, Footnote 58, Footnote 61, Footnote 71.
*Note: PAPRs are NOT recommended for the care of patients with infections transmitted by the droplet route, including influenza,
In the inter-pandemic period, healthcare organizations should continually maintain their RPP and provide education and training for HCWs about the potential need for and appropriate use of respirators during an influenza pandemic. The RPP should continue to operate during the pandemic period to orient and train new personnel, and to ensure all HCWs are utilizing the respiratory protective equipment appropriately. This is especially significant if an organization needs to change the brand and/or model of respirator available for use (e.g., accessing emergency stockpiles). Note: that fit testing results are specific to make, model and individual fit tested and are NOT transferable between respirator manufacturers or models. Because of anticipated scarcity of all supplies, organizations should continue to evaluate the availability of respiratory protection supplies and the ability of the RPP to adapt and train HCWs in the use of new PPE, including respirators acquired from regional, provincial/territorial or national stockpiles.
Healthcare organizations should be aware of the brand(s) of respirators stockpiled by regional and national organizations and be prepared to repeat a fit test for the new make, model and size of respirator (as per jurisdictional fit-testing requirements) as these stockpiles are accessed. Strategies should also be developed to manage respirator supply shortages, should this occur.
6.2.5. Administrative Controls: Routine Practices and Additional Precautions
Routine Practices and Additional Precautions are administrative controls that, when used in conjunction with other control measures and procedures, are effective in preventing the transmission of infectious agents (including influenza) in every healthcare settingFootnote 2, Footnote 3. RPAP are fully applicable to all sectors of health care including prehospital, acute care, long-term care, ambulatory care (including physicians'offices), community clinic care and professional home care.
Pandemic Influenza Precautions is a concept utilized in this Annex to identify all of the components of RPAP that are particularly relevant to the prevention and control of exposure to and transmission of influenza during a pandemic.
During the inter-pandemic period, preventing the transmission of seasonal influenza in a healthcare setting is best achieved through:
- application of Routine Practices, plus Contact and Droplet Precautions, and
- provision of annual influenza immunization (e.g., patients, HCWs)Footnote 17, Footnote 18.
During the pandemic period, application of RPAPFootnote 2, Footnote 1 measures should minimize or prevent the transmission of all infections, including pandemic influenza, in all healthcare settings. During the early waves of the influenza pandemic, an effective vaccine against the pandemic influenza virus may not be available Footnote 5 making the application of RPAP (including hand hygiene, respiratory hygiene and appropriate use of PPE) critical to the prevention and control of pandemic influenza.
6.2.6. Administrative Controls - Pandemic Influenza Precautions
Pandemic Influenza Precautions is a concept utilized in this Annex to identify all of the key components of the RPAP protocolsFootnote 2, Footnote 3 that are particularly relevant to the prevention and control of exposure to and transmission of influenza during a pandemic. Appropriate application of Pandemic Influenza Precautions should interrupt transmission of the influenza virus by droplet, contact and airborne routes. The following key components are described in greater detail below:
- Hand Hygiene (see Section V.6.2.6.1.);
- Appropriate Use of PPE (see Section V.6.2.6.2.)
- Masks and Respirators (see Section V.6.2.6.3.);
- Eye Protection and Face Shields (see Section V.6.2.6.4.);
- Gloves (see Section V.6.2.6.5.);
- Gowns (see Section V.6.2.6.6.);
- Environmental Hygiene Programs (Housekeeping, Laundry and Waste) (see Section V.6.2.6.7.).
6.2.6.1. Hand Hygiene
Hand Hygiene should be performed frequentlyFootnote 10, Footnote 11. Performing hand hygiene with ABHR, if hands are not visibly soiled, will inactivate the virusFootnote 11, Footnote 79. Soap and water should be used if hands are visibly soiled. Dispensers for ABHR should be easily accessible by patients, HCWs, visitors, contractors, etc. Products for performing hand hygiene should be located at all points of care (e.g., bedside) and at entrances to the healthcare settings.
6.2.6.2. Appropriate Use of Personal Protective Equipment
HCW's decision to wear PPE, should be informed by an organization's policies and procedures and be based on an understanding of the patient's infectious disease status and the mode of transmission of the infectious agent. PPE should be worn regardless of vaccination status or recovery from laboratory confirmed influenza as other respiratory viruses may be circulating. A Point of Care Risk Assessment (PCRA) (see Section V.7.3. and Appendix D) should be performed prior to every patient interaction to determine what level of respiratory and other personal protection is required to provide care to a specific patient with specific symptoms.
6.2.6.3. Masks and Respirators
The PCRA will enable the HCWs to determine when to wear a mask or respirator. The decision to wear a mask (e.g., for droplet protection) or a respirator (e.g., for respiratory protection) when providing patient care during the pandemic period should be based on an assessment of the risk of exposure to the pandemic influenza viral strain from the patient, the procedure and the environment as follows:
HCWs should wear a mask and face or eye protection when the HCW will be working within two metres of an influenza patient (or someone with ILI symptoms)Footnote 15, Footnote 80-82.
HCWs should wear a respirator and face or eye protection when the HCW will be working within two metres of an influenza patient (or someone with ILI symptoms)Footnote 15, Footnote 80-82 and
- The patient is coughing forcefully (see Glossary) and
- The patient is unable or unwilling to comply with respiratory hygiene (e.g., coughing or sneezing into sleeve, using tissues or wearing a mask).
A respirator is recommended for all HCWs present in a room where an AGMP (see Section V.4.4.4.) is being performed on a patient with symptoms compatible with the pandemic influenza strain.
- When either a mask or respirator is wornFootnote 2, Footnote 3, Footnote 83, Footnote 84, it should:
- Be put on and worn appropriately to prevent self-contamination;
- Be removed carefully by the straps or ties;
- Cover the nose, mouth and chin;
- Be discarded immediately after use into an appropriate, preferably hands-free waste receptacle (i.e., disposed of when removed from the face);
- Not be touched on its external surface with the hands in order to avoid self-contamination;
- Not dangle around the neck;
- Be changed if it becomes wet or soiled (from the wearer's respiration or through an external splash).
- Be changed if breathing becomes difficult.
- Hand hygiene should be performed immediately before and after removing a mask or respirator.
- In designated influenza patient care areas, admission isolation or cohort care areas, the mask or respirator may be worn for sequential care of influenza patients. Gloves and gowns must be changed between patients.
6.2.6.4. PPE - Eye Protection and Face ShieldsFootnote 2, Footnote 3
- Eye protection or face shields should be worn whenever a mask or respirator is worn as per Routine Practices.
- Eye protection or face shields should be removed immediately after use and discarded promptly into an appropriate, preferably hands-free receptacle. If eye protection is reusable, place in appropriate area for cleaning and re-processing.
- Hand hygiene should be performed immediately before and after removal of eye protection or face shields.
- Prescription glasses are NOT adequate for eye protection; additional eye protection should be worn over glasses.
- HCWs should avoid touching their faces with their hands to prevent self-contamination.
- In a designated influenza assessment, admission, isolation or cohort area, eye protection or face shields may be worn for sequential care of influenza patients. Gloves and gowns must be changed between patients.
6.2.6.5. PPE - GlovesFootnote 2, Footnote 3
- Gloves should be worn when coming within two metres of a patient with symptoms of ILI.
- Gloves should be removed and discarded immediately upon leaving the patient's room or bed space.
- Hand hygiene should be performed immediately after removal of gloves.
- When caring for a number of influenza patients, gloves MUST be changed between patients, including within designated influenza assessment, admission isolation or cohort care areas.
6.2.6.6. PPE - GownsFootnote 2, Footnote 3
- Gowns are not recommended for the routine care of patients with influenza or symptoms of ILI, unless contact with clothing or skin of the patient or contact with the patient's immediate (i.e. within two metres) environment is anticipated.
- Long-sleeved gowns are recommended if skin or clothing may be contaminated during patient care.
- If a gown is worn, it should be removed immediately after the indication for their use and placed into an appropriate, preferably hands-free receptacle.
- Hand hygiene should be performed immediately after removal of gowns.
- Gowns must be changed between ALL patients. When caring for a number of influenza patients, gowns MUST be changed between patients, including within designated influenza assessment, admission isolation or cohort care areas.
6.2.6.7. Environmental Hygiene Programs (Housekeeping, Laundry and Waste)Footnote 11, Footnote 85-87
- Clutter and entertainment items such as magazines, books and toys in waiting areas should be removed to prevent cross contamination and allow for ease of cleaning.
- Hospital-grade disinfectants can be used for environmental cleaning as these disinfectants readily inactivate the influenza virus.
- Meticulous daily cleaning of environmental surfaces.
- Surfaces frequently touched by the hands of HCWs and patients, such as, medical devices and knobs for adjustment or opening, should be cleaned and disinfected with disinfectant wipes or hospital grade disinfectant at least twice daily and when visibly contaminated.
- Non-critical medical devices and medical equipment (e.g., oximetres, intravenous infusion pumps, armrests, examining tables, stretchers, etc.) should be cleaned and disinfected before use by a patient and in between patients.
- Linen contaminated with secretions from patients with ILI symptoms does NOT require special handling.
- Waste from patients with ILI symptoms does NOT require special handling.
- Dishes (e.g., disposable dishes) used by patients with ILI symptoms do NOT require special handling.
6.3. Use of Personal Protective Equipment to Ensure Availability of Supplies During an Influenza Pandemic
A recent study suggests that due to major increases in usage of all gowns, gloves, masks, and face protectors, PPE may be difficult to obtain during later phases of the pandemic Footnote 88. Even with jurisdictional stockpiling of PPE during the inter-pandemic period, pandemic planners anticipate that PPE supplies in specific healthcare settings or regions may be depleted Footnote 5, especially during the later stages of an influenza pandemic wave.
The World Health Organization Footnote 9 has proposed a series of actions to encourage the appropriate use of PPE during an influenza pandemic. These actions are described below from a Canadian perspective:
- The provision of PPE supplies throughout a pandemic should be a jurisdictional and institutional priority, and availability of supply should be addressed during pandemic planningFootnote 89-92.
- Stockpiling PPE, such as masks, respirators, face shields, etc. for use during an influenza pandemic should be considered during the inter-pandemic period.
- The federal and most provincial and territorial governments are developing stockpiles of PPE, including respirators, for use when an organization's PPE supply has been depleted during a pandemic wave.
- HCWs may experience anxiety as a result of a pandemic. Such fear may increase the propensity for overuse or misuse of PPE and the misuse/theft/hoarding of PPE supplies.
- Disposable PPE should not be reusedFootnote 93-95.
- Reuse of disposable PPE may not provide the same protective efficacy and safety as new PPE.
- Reuse of disposable PPE or inappropriate PPE disposal may result in influenza virus exposure through direct contact with the used piece of respiratory equipment followed by self inoculation from contaminated hands.
- When resources are limited and disposable PPE is not available, reusable items, properly reprocessed as per the item manufacturer's instructions, may be considered.
- Healthcare organizations should educate HCWs to appropriately select and use PPE by performing a PCRA prior to every patient encounter (see Section V.6.2.6.2.).
- Unnecessary use of PPE may be avoided by maximizing the provision of clinical care during each entry to a patient's room or bed space Footnote 96.
- If the supply of respirators becomes severely limited (e.g., during a prolonged pandemic wave) organizations should consider prioritizing use of respirators to care of patients with known airborne infections (e.g., tuberculosis patients) and to higher risk AGMPs (see Section V.4.4.4., items a. to g.).
7. Risk Assessment – A method to prevent/minimize Pandemic Influenza exposure and/or transmission in healthcare settings
7.1. Background
For the purpose of this Annex, the evaluation of risk is the analysis of the probability and impact of pandemic influenza virus exposure and/or transmission to and from individuals (patients, HCWs, visitors, contractors, etc.) and/or environments in a healthcare setting.
A risk assessment is a systematic process for identifying, assessing and integrating quantitative information and qualitative judgements about possible and probable adverse conditions and/or events and the impact of these conditions/events on individuals, facilities and the larger communityFootnote 6, Footnote 8, Footnote 98, Footnote 99. Risk assessment, then, is a systematic process to identify, evaluate and mitigate the risk of exposure to and/or transmission of the pandemic influenza virus in the healthcare setting beginning in the inter-pandemic period and continuing throughout the pandemic period. Risk assessments for pandemic influenza should take into consideration the likelihood of transmission of the pandemic influenza virus to any and all individuals within a healthcare setting, and the impact of transmission on individuals, facilities and the larger community.
Within the healthcare organization, performance of risk assessments should NOT be static exercises, nor should they be the sole responsibility of either the organization or the HCW. Knowledge, infrastructure, and patient populations are constantly changing. In preparation for and managing the need to care for patients with ILI symptoms, both healthcare organizations and HCWs have risk assessment responsibilities to ensure the safety of all patients, HCWs, visitors, contractors, etc.
In this document, "Organizational Risk Assessments" (ORAs) refer to risk assessments undertaken by the healthcare organization to evaluate the state of readiness of its IPC and OH programs to respond to the arrival and continuation of an influenza pandemic. Point of Care Risk Assessments (PCRAs) will refer to risk assessments undertaken by HCWs as they go about their daily work activities (healthcare organizations should provide PCRA training to all HCWs that will need to perform PCRAs) (see Section V.7.3.).
7.2. Performance of Organizational Risk Assessments
Organizational Risk Assessments are central to any healthcare organization's preparation and planning for the protection of all individuals (i.e., patients, HCWs, visitors, contractors, etc.) from healthcare-associated infections when they enter a healthcare setting. ORAs are the evaluation of both the hazards and the application of the Hierarchy of Controls. Inter-pandemic ORAs should lead to the planning and preparation needed to ensure that the risk of transmitting the pandemic influenza strain within the healthcare setting is as low as possible and that inadvertent exposure to respiratory viruses (and other infectious agents) is minimized.
The ORA should be conducted during the inter-pandemic period and re-evaluated as new information, directions and regulations become available as well as when major re-organization/restructuring and building/renovation take place. During the pandemic period, the ORA may need to be re-evaluated each time there is a pandemic and/or with evolving knowledge of the actual pandemic strain.
The ORA should characterize the organizations' patient population (size and case-mix), level and intensity of health care provided, and resources available, including skilled workers (e.g., clinical, non-clinical, volunteers, etc.) and various PPE. The ORA will need to evaluate the effectiveness of existing control measures and the breadth of the Hierarchy of Controls in light of what is presently known about the influenza virus, influenza outbreaks, and assumptions about the influenza pandemic.
The recommendation for performing an inter-pandemic ORA applies to all sectors of health care including prehospital, acute care, long-term care, ambulatory care (including physicians’ offices), community clinic care and professional home care.
To conduct a robust ORA (see Appendix C) related to pandemic influenza in the inter-pandemic period, an organization should:
- Determine situations/conditions where pandemic influenza hazards might exist.
- Evaluate the potential for exposure to and/or transmission of the pandemic influenza virus in that situation/condition.
- Determine the consequences of exposure to the pandemic influenza virus (i.e., predicted virulence, complications, etc.).
- Determine the consequences of transmission of the pandemic influenza virus on individuals (i.e., patients, HCWs, visitors, contractors, etc.), organizations and the community.
- Assess control measures (i.e., engineering, administrative, and PPE) to mitigate the hazard in the specific healthcare setting and identify where gaps exist that may require remediation.
- Assess the availability of human resources and supplies as the pandemic progresses.
- Assess communication strategies to ensure accurate, concise, two-way sharing of information (internal and external) is practiced.
7.2.1. Performance of Organizational Risk Assessments in the Inter-Pandemic Period
An ORA, completed in the inter-pandemic period, should serve as a primary planning tool for the healthcare organization's pandemic influenza IPC/OH plan. The gaps identified in the inter-pandemic period should be addressed (e.g., partitions installed, development of administrative policies and procedures to increase safety when building infrastructure limits engineering controls) in the inter-pandemic period for the organization to be prepared for an influenza pandemic. When remediation of engineering controls is not feasible, healthcare organizations may need to expand and enhance administrative controls to reduce the hazard not addressed by engineering controls (e.g., patient flow, natural ventilation). An increase in the number and type of PPE required by HCWs, visitors, contractors, etc. may also be required.
Organizations should provide education and skills training for HCWs regarding the organization's ORA and its impact on their practice. Organizations should share results of the ORA with staff health and safety representatives so that HCWs can routinely perform effective and efficient PCRA (see Section V.7.3.1.) for influenza risk before every interaction with a potentially infectious agent/infected source during the inter-pandemic and pandemic periods.
Organizational Risk Assessments should be occurring at all jurisdictional levels. Ongoing, systematic evaluation of the ORA is recommended to ensure that:
- Policies, procedures and programs are consistent with changing jurisdictional recommendations and
- Policies, procedures and programs are adapted to changing knowledge of the pandemic virus (and its impact) and
- Policies, procedures and programs achieve their stated objectives and
- Policies, procedures and programs are in compliance with current legislation and regulations.
7.2.2. Performance of Organizational Risk Assessments in the Pandemic Period
Healthcare organizations should be prepared to re-evaluate the inter-pandemic ORA and communicate changes to HCWs throughout the pandemic period. In addition, the healthcare organization should continually evaluate the evolving risk posed by a wave of pandemic influenza moving through a specific patient population. The availability of an effective vaccine, the impact of the pandemic on HCWs, patients and the community, depletion of stockpiled equipment and the effectiveness and availability of antivirals may impact the organization and may lead to organizational changes to engineering and administrative controls and PPE utilized by HCWs. Communication with HCWs in a timely and consistent manner should enable appropriate PCRAs to be undertaken.
7.3. Performance of Point of Care Risk Assessments
The healthcare organization should train all HCWs in the use and application of Point of Care Risk Assessments (PCRAs) and should be an activity performed by HCWs before every patient interaction, in any/every healthcare setting (see Appendix D) to:
- Evaluate the likelihood of exposure to an infectious agent/infected source,
- For a specific interaction (e.g., task anticipated, equipment to be used, task organization, period of time).
- With a specific patient (e.g., immunocompromised, infants/children, not capable of self care/hand hygiene, poor-compliance with respiratory hygiene, copious respiratory secretions, frequent cough/sneeze, early stage of influenza illness, etc.).
- In a specific environment (e.g., single rooms, shared rooms/washrooms, hallway, assessment/triage areas, therapy departments, diagnostic departments, spatial separation, housekeeping, shared patient-care equipment, etc.).
- Choose the appropriate safe work practices (administrative and PPE controls) to minimize the risk of everyone’s (patients, HCWs, visitors, contractors, etc.) exposure to an infectious agent or infected source (see Section V.6.2.).
HCWs are already familiar with performing PCRAs, although it is unlikely that they name the process a PCRA. For example, HCWs routinely perform a PCRA when they evaluate a patient and situation to:
- Determine the possibility of blood or body fluid exposure.
- Choose appropriate PPE to care for a patient with an infectious disease.
- Modify when, where and how to safely perform a procedure.
HCWs already engage in PCRAs many times a day for their health and safety and the health and safety of patients and others in the healthcare setting. The PCRA in this document introduces the application of risk assessment for care of patients with ILI symptoms.
7.3.1. Point of Care Risk Assessments for Routine Practices During the Inter-Pandemic Period
Prior to any patient interaction, all HCWs have a professional responsibility to assess the infectious risk posed to themselves and other patients, visitors, HCWs by a patient, situation, or procedure. This risk assessment is based on professional judgement (i.e., knowledge, skills, reasoning and education) about the clinical situation as well as up to date information on how the specific healthcare setting has implemented engineering and administrative controls and use and availability of PPE.
PCRA training and education should be provided by the healthcare organization to ensure that HCWs have the knowledge, skills and resources to routinely perform PCRAs for pandemic influenza risk before every interaction with a potentially infectious agent/infected source during the pandemic period.
A PCRA is an activity whereby HCWs (in any healthcare organization):
- Evaluates the likelihood of exposure to infectious agents
- for a specific interaction
- with a specific patient
- in a specific environment (e.g., single room, hallway)
- under available conditions (e.g., air exchanges in a large waiting area compared to air changes in an airborne infection isolation room)
- Chooses the appropriate actions/PPE needed to minimize the risk of exposure for the specific patient, other patients in the environment, the HCW, visitors, contractors, etc.
7.3.2. Point of Care Risk Assessments During the Pandemic Period
During an influenza pandemic all HCWs should assess the influenza risk posed by a specific patient, situation or procedure to themselves, to their patients, to visitors and to other personnel interacting with patients. This pandemic period PCRA is based on professional judgement and informed decision making and should be consistent with an understanding of the epidemiology of the pandemic virus and the engineering and administrative controls and PPE available within a specific setting.
As new information about the risk posed by the pandemic influenza virus becomes available, organizations should integrate this into the ORA and aid HCWs to integrate this new information into their PCRA(s).
To facilitate the integration, healthcare organizations should provide HCWs with the knowledge, skills and resources to routinely perform PCRAs for pandemic influenza risk before every interaction with a potentially infected source during the pandemic period. In order to perform an effective PCRA, HCWs should have adequate knowledge of the following (see Section V. for in depth discussion):
- Epidemiology of the illness caused by the pandemic influenza virus (e.g., screening criteria provided by public health organizations).
- Pandemic influenza virus characteristics, virulence, reservoirs, infectivity, mode of transmission, incubation period, period of communicability.
- Transmission factors, e.g., type of exposure, inoculum, host factors, control methods.
The PCRA should provide answers to key questions including:
- What symptoms is the patient exhibiting?
- Is the patient able and willing to practice respiratory hygiene?
- What contact is the HCW or other person going to have with the patient?
- What task(s)/procedure(s) is the HCW going to perform?
- What is the location, proximity to others?
- If the patient is to be moved, are there any considerations for the transport (e.g., spatial separation, respiratory hygiene, hand hygiene, communication with receiving site)?
Using the epidemiologic triangle discussed in Section V.4., Table 1 provides an overview of some of the factors that should be taken into consideration for a PCRA during the pandemic period. Note that in practice these factors are inter-related and should not be considered in isolation.
| Higher Transmission Risk | Lower Transmission Risk | |
|---|---|---|
| Infectious Agent/Infected Source |
|
|
| Environment |
|
|
| Susceptible Host Patient |
|
|
| Susceptible Host - HCW |
|
|
For an infected source, the PCRA should evaluate the changing nature of an infected source's symptoms and environment to determine the appropriate PPE for HCWs, and visitors. The PCRA should also determine if there is a need to move the patient to another area, the need for patient masking, and any other practice changes to provide safe care, even when a patient's condition is changing.
For the environment, the PCRA should evaluate the patient's environment and determine the environment needed for the planned interaction or procedure to proceed safely. To assess the environment's risk appropriately, HCWs should understand the organization's ORA as it relates to patient care.
For a susceptible host (other patients, HCWs, visitors, contractors, etc.) a PCRA should evaluate whether:
- The patient has recently developed ILI symptoms (i.e., the susceptible host patient has become an infectious source); and
- Whether the risk posed by an infected source has increased or decreased since the last PCRA.
The PCRA should lead to a determination of:
- The appropriate PPE for the susceptible host to safely care for or visit the infected source;
- If there is a need to move the infected source or the roommates to another area, and
- Any other practice changes (e.g., spatial distancing, choice of PPE, use of mask on infected source, etc.) recommended to address the change in risk for influenza acquisition.
7.3.3. Integration of Information From the PCRA with the Knowledge About the Organization's Pandemic Influenza ORA
The PCRA should be completed based on the most recent information from the ORA. For example:
- A decision by the organization to cohort symptomatic patients in one area of the hospital may impact the HCW's evaluation of where to house a highly vulnerable patient, or
- A decision by the local health authority to open an influenza assessment centre may impact a physician's decision about where to see symptomatic patients.
The evolving nature of the influenza pandemic, together with unique patient characteristics, precludes using a "one time" assessment to determine the risk posed by any given interaction with an infected source. Therefore, a PCRA before each patient interaction is essential.
By answering the following questions, HCWs can integrate the information obtained from the PCRA with the knowledge of the organization's pandemic influenza ORA.
- What does the ORA (see Appendix C) tell the HCW about the engineering and administrative controls available for caring for this patient, undergoing this task or procedure, in this environment (room)?
- What does the PCRA (see Appendix D) tell the HCW about recommended changes in routine patient care practices for this patient, undergoing this procedure, in this environment (room)? Is a room change necessary?
- Based on the answers to these first two questions, what is the appropriate PPE (see Section V.6.2.6.2.) for this HCW, and this patient undergoing this procedure, in this environment (room)?
VI. Planning for an Influenza Pandemic – Using the Organizational Risk Assessment to develop the Pandemic Influenza Infection Prevention and Control and Occupational Health Plan
During an influenza pandemic, how well a healthcare organization is able to prevent the transmission of the pandemic virus in the healthcare setting will largely be determined by the strength of its inter-pandemic planning activities and routine HAI prevention and control strategies. Effective IPCFootnote 2, Footnote 3 (see Section V.2.) and OH Footnote 1 (see Section V.3.) programs that include seasonal influenza immunization campaigns and seasonal influenza HAI prevention strategies should have a significant impact on the effectiveness of all healthcare-associated pandemic influenza prevention strategies Footnote 100. An Organizational Risk Assessment (ORA) (see Section V.7. and Appendix C) that evaluates the organization's core functions and the effectiveness of existing IPC and OH programs and readiness to respond to an influenza pandemic should provide the basis for all IPC and OH pandemic planning.
The pandemic influenza planning recommendations in this section have corresponding action recommendations in Section VII.
This Section provides recommendations for all healthcare organizations for performing and using the ORA for pandemic influenza planning activities and subsequent actions that need to take place in the inter-pandemic period to prepare for an influenza pandemic.
- Section VI.1. provides recommendations for using the ORA to evaluate the thoroughness and effectiveness of existing IPC and OH programs;
- Section VI.2. provides specific IPC and OH planning recommendations to prepare healthcare organization to safely care for patients and protect other patients, HCWs, visitors, contractors, etc. during an influenza pandemic;
- Section VI.3. provides recommendations to enable the organization to plan for the impact that a substantial number of HCWs with ILI symptoms during an influenza pandemic may pose; and
- Section VI.4. provides recommendations on education and skills training to ensure HCWs are prepared to care for patients during an influenza pandemic.
1. Performing an Inter-Pandemic ORA to Evaluate Existing IPC/OH Programs
Healthcare organizations should perform an ORA to identify the comprehensiveness of its existing Hierarchy of Controls, with a focus on existing IPC and OH programs (and elements of other programs that impact the control of HAIs). Consideration as to how these controls relate to what is currently known about disease transmission should also be reviewed, (e.g., medical instrument reprocessing, air handling systems, etc.). In preparation for an influenza pandemic, the ORA should be broad (e.g., public health, other organizations) and include all elements that potentially impact the spread of respiratory viruses including a pandemic influenza virus (see Appendix C for an ORA tool that can be used in all healthcare settings).
1.1. Evaluation of the Existing OH Program for the ORA
The OH (see Section V.3.) component of the ORA should include evaluation of Occupational Hygiene and Occupational Health and Safety activities related to the prevention and control of transmissible infections in HCWs, including:
- Pre-placement screening, assessment, and immunization of HCWs;
- The workplace infections hazard assessment;
- The Respiratory Protection Program; and
- Measures to identify and track HCWs with acute infections that pose a risk to other HCWs and/or patients.
1.1.1. Pre-Placement Screening, Assessment, and Immunization of HCWs Footnote 1
The evaluation should include:
- Measures to ensure that all HCWs, and contractors are offered appropriate immunizations and are fully immunizedFootnote 17, Footnote 101, Footnote 102 for the following:
- Hepatitis B immunization (three doses)Footnote 7, Footnote 103.
- A tetanus booster (preferably one dose containing acellular pertussis, if not previously received) Footnote 17.
- HCWs without proof of measles, mumps, rubella (MMR) and varicella immunity should be given the appropriate vaccine unless contraindicated Footnote 17.
- Pneumococcal vaccine should be offered to HCWs who are considered at increased risk of Streptococcus pneumoniae Footnote 17.
- Evaluation of tuberculosis status.
- Optimizing uptake of annual (seasonal) influenza vaccine by HCWs.
- Implement tracking to determine seasonal influenza immunization uptake by HCWsFootnote 18, Footnote 101, Footnote 102.
1.1.2. Workplace Infectious Hazard Assessment
The Workplace Infectious Hazard Assessment Process should enable an organization to:
- Evaluate the entire healthcare workplace to identify potential infectious hazards related to work activities;
- Assess and analyze the infectious risk associated with exposure to the identified work related hazard.
1.1.3. Respiratory Protection ProgramFootnote 15, Footnote 16, Footnote 59
The RPP (see Section V.6.2.4.) provides health screening, fit testing/re-testing and training on the appropriate use of respirators (e.g., performance of the seal check every time a respirator is worn) to all HCWs who may wear a respirator.
- All healthcare organizations (including prehospital, acute, non-acute, long-term, ambulatory, home, clinic and community care) where AGMPs are performed and patients with airborne respiratory pathogens (e.g., tuberculosis, measles) are cared for, should have a RPP in place. Note: jurisdictional legislation and regulations may apply. For example, in LTC organizations, the ORA (see Appendix C) should facilitate the identification of HCWs at greatest risk of workplace exposure to pandemic influenza. These may include HCWs performing AGMPs and HCWs working with cognitively impaired residents or residents with aggressive behaviour or residents who are unable or unwilling to comply with respiratory hygiene practices during an influenza outbreak.
- Powered Air Purifying Respirators (PAPRs) are NOT recommendedFootnote 46, Footnote 50, Footnote 52, Footnote 54, Footnote 61, Footnote 71 when providing care to patients with influenza (see Section V.6.2.4.e. for further details regarding the use of respirators).
- If HCWs cannot be fit tested for a respirator (e.g., men with beards for religious reasons) they should be restricted from performing AGMPs on patients with pandemic influenza. Consideration may be given to restricting these individuals from providing direct patient care to patients with ILI symptoms for whom a PCRA has indicated that respiratory protective equipment should be worn.
1.1.4. Identification of HCWs With Acute Infections
Healthcare organizations should be able to identify HCWs with acute infections through activities that include:
- Policies for employees' self-reporting of acute infectious respiratory illness (e.g., ILI symptoms) to OH departments or other responsible bodies, as per applicable jurisdictional laws and labour regulations (see Appendix A for the Influenza Self Assessment Tool).
- A system for reporting occupational illness/injury internally and externally as required by governing federal/ provincial/ regional legislation or regulation.
- A systematic process for recording and tracking HCWs absenteeism due to infection (specifically clusters of respiratory infections).
- A systematic process for linking clusters of respiratory infections in HCWs with clusters of respiratory infections in patients.
- Procedures to manage ill (i.e., ILI symptoms) personnel so they do not expose others in the workplace (e.g., patients, other HCWs, visitors, contractors, etc.)Footnote 104, Footnote 105. (See Section VI.3.)
- A systematic process to assess and identify whether there are clusters of ill HCWs.
1.2. Organizational Evaluation of the Existing IPC Program for the ORA
The ORA should include evaluation of the organization's IPC (see Section V.2.) program to assess the following:
- The number of trained Infection Control Professionals for the size and complexity of the organization's patient populationFootnote 35, Footnote 106.
- Effectiveness of the HAI surveillance program to identify and track trends and outbreaks of respiratory virus infection within the organizationFootnote 31, Footnote 106.
- Systems to enable the acquisition and maintenance of adequate quantities of equipment/products/materials that may be needed to prevent exposure to and transmission of infections in the organization.
- Effectiveness of the application of RPAPFootnote 2, Footnote 3 policies and procedures to prevent or minimize the transmission of an infectious agent from an infected source or contaminated environment to a susceptible host including:
- Establishment of a PPE and hand hygiene compliance audits.
- HCWs training in the selection, putting on and taking off PPE safely along with the care, use and limitations of PPE (see Section V.6.3.).
- Use of glass or acrylic partitions as barriers to protect triage and reception personnel.
- A system for rapidly identifying patients with symptoms of acute respiratory infections and/or accommodation for their assessment in a separate space away from patients without symptoms.
- Policies/processes for the early recognition (e.g., a screening program), containment, investigation, and reporting of any individuals (patients, HCWs, visitors, contractors, etc.) in the healthcare setting, with ILI symptoms (see Appendix A).
- Processes to minimize the generation of and exposure to infectious aerosols created during AGMPs (see Section VII.1.5.4. for suggested practices) Footnote 107.
- Promotion of and adherence to effective hand hygiene practices (see Section V.6.2.6.1.) by all individuals in the healthcare organization including patients, HCWs, visitors, contractors, etc.Footnote 10, Footnote 11.
- The use of ABHRs is the preferred method of hand hygiene in all healthcare settings. Hand washing with soap and water is recommended when hands are visibly soiledFootnote 10, Footnote 11.
- Placement of ABHR dispensers at point of care, and in triage, assessment, waiting and patient-care areas for easy access and use by patients, HCWs, visitors, contractors, etc.
- Consideration of individual bottles of ABHR available for use by HCWs in settings where wall/bedside-mounted containers are not feasible.
- Infected source control programs to minimize face-to-face contact between infected sources, (i.e., patients, HCWs, visitors, contractors, etc.) with ILI symptoms, and susceptible hosts (i.e., other patients, HCWs, visitors, contractors, etc.) including:
- Preparation of educational materials on the prevention of respiratory virus transmission for use by patients, HCWs, visitors, contractors, etc. including but not limited to information on respiratory hygiene and hand hygiene.
- Development of signage (multilingual as required) to encourage adherence to respiratory hygiene and hand hygiene by all individuals within the healthcare setting.
- Placement of dispensers for masks and tissues at point of care, in triage, assessment, waiting and patient-care areas for easy access and use by patients, HCWs, visitors, contractors, etc.
- Placement of closed, hands-free waste containers in triage, assessment, waiting and patient-care areas Footnote 108.
- Placement of point-of-use sharp containers in triage, assessment, and patient care areas Footnote 103.
- Processes to ensure that pneumococcal immunization is offered to high-risk patients to minimize influenza morbidity and mortality related to secondary bacterial pneumonia Footnote 17.
- Processes to ensure that seasonal influenza immunization is offered to all residents of long-term care facilities (and high risk individuals accessing healthcare in the community)Footnote 17, Footnote 18.
1.3. Deficiencies in OH and IPC Programs Identified by the ORA.
Existing OH and IPC programs that function effectively should form the basis of a healthcare organization's preparation and planning for an influenza pandemic. Organizations may need to commit human and financial resources to address deficiencies identified by the inter-pandemic ORA such as ensuring:
- An appropriate number of conveniently located, dedicated sinks for hand washing, hand soap, hand lotion and single-use paper towelsFootnote 10, Footnote 11.
- Adherence to appropriate spatial separation (i.e., two metre separation or use of partitions), to decrease the risk of infectious agent/infected source exposure for susceptible hosts (e.g., patients and visitors) in clinical and waiting areasFootnote 46-54, Footnote 75.
- Maintenance of peak operation of the HVAC systems in accordance with available guidelines and regulationsFootnote 75, Footnote 77, Footnote 108.
- Maintenance of housekeeping, laundry and waste management standards in accordance with available guidelines and regulations Footnote 11.
- Maintenance of standards for cleaning, disinfecting and sterilizing patient-care equipment in accordance with available guidelines, standards and regulationsFootnote 2, Footnote 3, Footnote 11, Footnote 104-114.
2. Establishing a Pandemic Influenza IPC/OH Plan for the Management of Pandemic Influenza in All Healthcare Organizations
2.1. Developing the Pandemic Influenza IPC/OH Plan
Based on the inter-pandemic ORA, healthcare organizations should develop a pandemic influenza IPC/OH plan which incorporates IPC and Occupational Hygiene principles and is integrated with the existing IPC and OH programs.
All organizations responsible for existing and temporary prehospital, acute, non-acute, long-term, ambulatory (including physicians' offices), home care, healthcare clinics and other community healthcare settings should have a pandemic influenza IPC/OH plan.
The organization should establish a multi-disciplinary team to lead the development and implementation of the pandemic influenza IPC/OH plan.
- The pandemic influenza IPC/OH planning team should liaise closely with the organization's pandemic influenza planning team.
- The pandemic influenza IPC/OH plan should be integrated with existing IPC and OH programs.
The planning team should ensure that the pandemic influenza IPC/OH plan:
- Is reviewed annually, potentially during the seasonal influenza campaign, and updated according to emerging knowledge, regulations and legislation.
- Includes measures to identify and manage respiratory illness affecting HCWs.
- Includes plans for evaluating and counselling HCWs at risk of severe complications should they acquire influenza Footnote 17.
- Includes recommendations for how patient accommodation will be handled to reduce transmission.
- Includes policies to deal with patient visitation during the pandemic waves.
- Provides a clear screening method to identify patients and visitors with ILI symptoms upon entry into the healthcare setting.
- Consider developing policies to limit visitation (see Section VII.1.7.).
- Consider developing multi-lingual signage for entrances to healthcare settings, that should provide patients, HCWs, visitors, contractors, etc., with:
- Directions to influenza assessment and admission areas;
- Instructions regarding respiratory hygiene;
- Instructions regarding hand hygiene.
- Includes specific pandemic influenza education and skills training for HCWs, including how to do Point of Care Risk Assessments (see Section VI.4.).
- Includes recommendations for the delivery of a pandemic influenza vaccine (when it becomes available) according to the Canadian Pandemic Influenza Plan for the Health Sector (CPIP) Footnote 5 (see Annex E of the CPIP The Canadian Pandemic Influenza Plan for the Health Sector - Annex E) and provincial/territorial/regional or local pandemic influenza vaccine distribution initiatives.
- Includes recommendations for the use and distribution of antiviral medications according to the CPIP Annex on antiviralsFootnote 5, Footnote 19 (see Annex D of the CPIP The Canadian Pandemic Influenza Plan for the Health Sector - Annex D) and provincial/territorial/regional or local pandemic influenza antiviral medication distribution initiatives.
2.2. Planning for the Accommodation and Cohorting of Patients/Residents/Clients
Single rooms are preferred for patients/residents admitted with influenza to acute care facilities, LTC facilities or other healthcare settingsFootnote 2, Footnote 3. However, during an influenza pandemic wave, healthcare organizations may not have sufficient numbers of single rooms to accommodate all inpatients with ILI symptoms. The use of temporary healthcare settings and the use of influenza cohorts may provide options when caring for large numbers of patients with ILI symptoms.
- The IPC/OH plan should provide for appropriate spatial separation between patients/residents/clients with ILI symptoms or infected with the pandemic influenza virus (infected sources) and patients/residents without influenza (susceptible hosts)Footnote 46-54, Footnote 115-117 by predetermining:
- The need for physical barriers (e.g., glass/acrylic partitions in entrances to assessment centres) to separate infectious agents/infected sources from susceptible hosts (other patients, HCWs, visitors, contractors, etc.).
- The location of influenza care/isolation areas and non-influenza care areas (i.e., these areas should be identified in acute care, long-term care, and community infirmary settings).
- The location of influenza care areas within specialty units (e.g., Trauma Intensive Care Units, Coronary Care Units, Maternity Units, Neonatal units).
- The location of separate assessment areas for patients/residents with ILI symptoms and those without influenza symptoms.
- The location of separate admission holding areas for patients and LTC residents with ILI symptoms and those without influenza symptoms.
- A process for alternative methods of ambulatory care and home care delivery to decrease the numbers of vulnerable people potentially exposed to influenza through time spent waiting for service in the healthcare setting.
- Provision should be made to establish inpatient units to cohort patients/residents with ILI symptoms separately from non-influenza patients/residents during a pandemic wave. Criteria should also be developed for closing inpatient units at the end of the wave. Opening and closing patient/resident units should be based on the needs and requirements that emerge during the pandemic.
- An active screening process should be established to separate patients/residents with ILI symptoms from non-influenza patients/residents as soon as influenza symptoms are identified.
- LTC facilities should identify areas to hold newly admitted residents for one incubation period.
- LTC facilities should identify a separate area to isolate residents that develop ILI symptoms.
- Cohorting of non-influenza patients/residents who are at high risk of severe complications if they were to be infected with influenza should be considered Footnote 17.
- Any patient/resident admitted from the community to a non-influenza cohort should be assumed to have been exposed to influenza in the community (i.e., maintain a high index of suspicion during the incubation period).
- Patients/residents should be closely monitored (i.e., every four to six hours) for influenza symptoms for the duration of the incubation period.
- Non-influenza patients/residents without high risk of severe influenza complications should be accommodated as per the organization's routine patient or residential accommodation system (e.g., medical, surgical).
- Assume that all acute care patients and newly admitted LTC residents admitted to a non-influenza cohort have been exposed to influenza in the community.
- Processes should be established to monitor for symptoms of influenza upon admission and every four to six hours for the duration of the incubation period.
- Processes should be developed to separate patients/residents with ILI symptoms from non-influenza patients as soon as symptoms are noted.
- Plans should be developed to cohort confirmed influenza patients/residents and patients/residents with ILI symptoms including those who also require specialty care or assessment for other conditions (e.g., Trauma Intensive Care Units, Coronary Care Units, Maternity Units, Neonatal units).
- A plan to establish and maintain spatial separation of two metres between influenza and non-influenza patients should be developed.
- Wherever possible plan to use physical barriers to minimize exposure to other patients, HCWs, visitors, contractors, etc., from patients/residents with ILI symptoms.
- Patients/residents who are immune to the pandemic influenza strain (i.e., those who were immunized at least two weeks previously or who have recovered from the pandemic strain of influenza) may be accommodated in the area most appropriate to their care needs.
- The influenza vaccine may not be fully efficacious in providing immunity. Immunized patients/residents should continue to be assessed for signs of influenza.
2.2.1. Use of Patient Rooms During the Pandemic Period
Single rooms are preferred for patients with ILI symptoms (seasonal and pandemic influenza).
Note: Ambulatory care settings including physicians' offices and other outpatient settings. Processes should be established for waiting areas to enable the spatial separation of infected sources and susceptible hosts (e.g., two metres, partitions, immediate placement of patients with ILI symptoms into an examination room).
2.2.2. Planning Airborne Infection Isolation Rooms During a Pandemic Period
Patients/residents with influenza caused by the pandemic strain do not require airborne infection isolation.
- AGMPs (see Section V.4.4.4. and Section VII.1.5.4.) on patients with influenza caused by the pandemic strain should be performed in a designated airborne infection isolation room or other rooms with enhanced air exchanges and air exhausted to the outside, if feasibleFootnote 2, Footnote 3, Footnote 35.
- As the number of airborne infection isolation rooms is limited in most healthcare settings, these rooms should be prioritized for patients with known or suspected airborne infections (e.g., tuberculosis, measles, varicella and disseminated zoster) or those undergoing sputum induction or bronchoscopyFootnote 60-70 over patients with ILI symptoms.
- Moving influenza patients/residents to an airborne infection isolation room for treatment should not be considered if it compromises the delivery of care.
2.2.3. Temporary Healthcare Settings
- To limit confusion, whenever possible, the temporary setting's pandemic influenza IPC/OH plan should be integrated with the parent organization's pandemic influenza IPC/OH plan.
- The temporary setting's pandemic influenza IPC/OH plan should be based on published IPC recommendationsFootnote 2, Footnote 3, Footnote 10, Footnote 11.
- The temporary setting's pandemic influenza IPC/OH plan should be integrated with federal/provincial/territorial/regional pandemic influenza contingency plans.
- Pandemic influenza planning should ensure that all HCWs working in temporary healthcare settings (e.g., housekeeping and laundry workers and workers handling waste) are offered appropriate immunizations.
- Reprocessing of reusable medical instruments should not be undertaken in temporary settings.
2.3. Planning for Transfer/Transport of Patients with ILI symptoms Within and Between Healthcare Settings
Prior determination of an organization's patient transportation/transfer policies during a pandemic period should enable the application of consistent care policies. Planners should consider the organization's patient population, the possibility of HCWs and other staff shortages, and the impact of transportation/transfer policies and capacities on the medical care and recovery of patients.
In preparation for an influenza pandemic, the organization should:
- Plan to limit the movement of patients with ILI symptoms to moves that are medically necessary.
- For patients that must be moved between departments, units or organizations, formal communication processes should be established to ensure that the transporting agency, and the receiving department, unit or facility is made aware of the patient's ILI symptoms, diagnosis and laboratory results (i.e., direct communication with the staff of the receiving department, unit or facility).
- Ensure transfer/transport personnel perform a PCRA (see Appendix D) and put on appropriate PPE (see Section V.6.2.6.2.) for the transport.
- When transfer/transport is necessary plans should be in place to teach patients with ILI symptoms (if able) the following functions:
- Perform hand hygiene prior to transfer/transport.
- Wear a mask (NOT a respirator) for the duration of transfer/transport (if tolerated).
- Practice respiratory hygiene during transport.
2.4. Planning for Visitors: Responsibilities and Restrictions
Prior determination of an organization's visitor policies during the pandemic period should enable the application of consistent restrictions. Planners should consider their patient population, the possibility of HCWs and other personnel shortages, and the impact of restrictions on care and recovery of patients.
- In preparation for an influenza pandemic, the organization should plan that asymptomatic visitors may visit asymptomatic patients/residents in accordance with the organization's visitation policies.
- Organizations should identify processes for visitors who wish to visit a patient with ILI symptoms. Visitors should:
- Consider NOT visiting if they are at high risk of complications should they contract influenza (e.g., immunosuppressed, pregnant).
- Perform hand hygiene on entry to and exit from the patient's room.
- Consider wearing the same PPE that HCWs are using/wearing if they will be within two metres of the patient they are visiting.
- Restrict the visit to one patient only to prevent inadvertent influenza transmission to multiple patients.
- The organization should identify processes to enable the identification of visitors with ILI symptoms upon entry into a healthcare setting.
- Symptomatic visitors should be prevented from visiting except under exceptional circumstances (see Section VI.2.4.).
- Consideration should be given to identifying prominent areas at the entrances of the healthcare setting for all visitors to perform an influenza self-assessment under the direction of organizational personnel to monitor for any ILI symptoms (see Appendix A).
- Organizations should identify areas where visitors can perform hand hygiene upon entering a healthcare setting and on entrance to and exit from a patient's room.
- Organizations should identify methods to ensure that all visitors receive respiratory hygiene instructions prior to or immediately upon entry into the healthcare setting.
- Organizations should plan for further visitor restrictions if an outbreak or active transmission of pandemic influenza is occurring in the facility. During a facility influenza outbreak, consideration should be given to:
- Restricting visitors who have not yet had the pandemic strain of influenza.
- Restricting visitors who have not been immunized against the pandemic strain in the prior two weeks.
- The organization should plan for special exemptions for a visitor with ILI symptoms (e.g., if the visitor is a close relative of a terminally ill patient or a parent of a sick/admitted child).
- The organization should ensure that symptomatic visitors do not have opportunity to expose other patients, HCWs, visitors, contractors, etc., to influenza while in the healthcare setting.
- The organization should plan to provide resources (i.e., equipment and direction) to enable the ill visitor to receive instruction on proper mask wearing and removal, hand hygiene and respiratory hygiene including:
- Putting on a mask upon entering and wearing the mask for the duration of the time in the healthcare setting.
- Performing hand hygiene upon entering the healthcare setting and prior to entering and leaving a patient's room.
- Observing respiratory hygiene throughout the time in the healthcare setting.
- Ill visitors should restrict their visit to a single patient (terminally ill adult or sick child). Under no condition should they visit anyone other then the designated patient/resident.
- Children with ILI symptoms, who are relatives of a terminally ill patient, may visit if the parents or guardians provide strict supervision of the child.
- Parents or guardians should ensure that the sick child/visitor wear a mask and practice strict hand and respiratory hygiene.
- Under no condition should visiting children with ILI symptoms visit any other area/room/patient in the facility (e.g., cafeterias, common areas, play areas, etc.).
- Patients who have received ill visitors should be monitored for one incubation period after the last visit by an ill visitor.
3. Planning for the Identification and Management of HCWs with ILI Symptoms
During the pandemic period, it is likely that most cases of influenza will be caused by the pandemic strain. When HCWs are in the community, (e.g., at home, at school, shopping) they will have the same risk of acquiring influenza as the general population.
Note: The pandemic influenza planning recommendations in this section have corresponding action recommendations in Section VII.1.9.
- Processes should be established for HCWs to perform a daily self assessment for influenza (see Appendix A) to determine their influenza status and thus their ability to work with susceptible hosts (patients, other HCWs, visitors, etc.).
- Processes should be established to accommodate potential staffing shortages (e.g., a number of HCWs, and/or the HCW's family members may acquire influenza while in the community).
- In situations of severe personnel shortages, consider establishing criteria for allowing HCWs that have mild influenza to come to work, if they feel well enough. These HCWs should only work with patients with ILI symptoms. See Section V.6.2.2.
- Criteria should be established to evaluate and counsel self identified HCWs at high risk of influenza complications. See Section V.6.2.2.
- Policies and procedures should be established to identify, track and manage organizational clusters of ill HCWs including processes, case definitions, surveillance methodology, and outbreak response directives. Report the cluster to public health authorities, as required.
- For planning purposes, HCWs who have recovered from laboratory confirmed influenza acquired during the pandemic may be considered immune to the pandemic influenza strain.
- For planning purposes, two weeks after vaccination, HCWs who have been immunized against the pandemic influenza strain may be considered immune for purposes of work placement. For more recommendations regarding pandemic influenza vaccine, please refer to Annex D of the CPIP Footnote 5 The Canadian Pandemic Influenza Plan for the Health Sector - Annex D and the National Advisory Committee on Immunization Canadian Immunization Guide Seventh Edition - 2006Footnote 17, Footnote 18.
- Vaccinated individuals should continue to do daily self-assessment for influenza. Please refer to the Influenza Assessment Tool in Appendix A.
- Vaccinated individuals should continue to use appropriate PPE to protect against new strains of influenza and other respiratory agents.
- For more information and recommendations for antiviral use during an influenza pandemic, please refer to Annex E of the CPIP The Canadian Pandemic Influenza Plan for the Health Sector - Annex E Footnote 5.
4. Planning and Providing Pandemic Influenza Education and Skills Training for HCWs in All Healthcare Organizations
During the inter-pandemic period, all healthcare organizations, including agencies that supply contract personnel to healthcare settings, should provide appropriate education and skills training for all HCWs regarding prevention and control of pandemic influenza in health care.
- Education and skills training related to seasonal influenza and pandemic influenza should be provided to HCWs on all shifts, in all departments.
- Building on an organization's seasonal influenza campaign may be an appropriate time to provide pandemic influenza education and skills training.
- The education and skills training on pandemic influenza should be appropriate to the audience.
- A variety of education methods may be employed, e.g., postings in elevators and at entrances, brochures, newsletters, websites, mini-education sessions during shift change, use of role plays or other scenarios, etc.
- Pandemic influenza education and skills training, given in the inter-pandemic period, should include:
- A detailed review of the organization's pandemic influenza IPC/OH plan, including how it can be accessed.
- An explanation of how the organization plans to communicate their evolving plans to HCWs.
- An explanation of the organizations' ORA (see Appendix C) and how personnel should apply the relevant changes in the ORA to their practice.
- A discussion of processes for HCWs, at high risk of complications due to influenza, to self-identify and seek guidance from the OH clinician (e.g., early antiviral treatment, immunization).
- An explanation of why and how to perform the daily Influenza Self Assessment to identify ILI symptoms and/or determine whether symptoms are compatible with influenza (see Appendix A).
- An explanation of how full application of RPAP measures plus Pandemic Influenza Precautions should minimize or prevent the transmission of all infections, including pandemic influenza, in all healthcare settings. This should include an explanation of the importance of:
- The use of Pandemic Influenza Precautions (see Section V.6.2.6.) for all patients with ILI symptoms.
- The appropriate use of PPE (see Appendix D) for caring for patients with ILI symptoms.
- Strict adherence to hand hygiene practices as a key strategy in preventing transmission of the pandemic influenza virus in healthcare settingsFootnote 10, Footnote 11.
- The impact of respiratory hygiene in minimizing influenza transmission.
- Procedures for accessing immunization for the pandemic strain when vaccine becomes available, as per the Annex D of the CPIP The Canadian Pandemic Influenza Plan for the Health Sector - Annex D.
- Procedures for access and use of various antiviral medications for the purpose of treatment and outbreak response during a pandemic, as per Annex E of the CPIP The Canadian Pandemic Influenza Plan for the Health Sector - Annex E.
- A review of plans for temporary pandemic influenza assessment centres and temporary influenza hospitals (see Section VI.2.2.3.).
- A discussion of appropriate use of PPE based on PCRAs; including any PPE prioritization strategies.
- Training to perform efficient and accurate PCRAs (see Appendix D) before every patient contact during the pandemic period.
In addition, all HCWs should have the following education and skills training in order to perform effective PCRAs and proficiently and safely care for patients while protecting themselves. Consideration should be given to including the following elements in training materials:
- Information on novel influenza strains that may lead to an influenza pandemic.
- The expected clinical presentation of the pandemic strain including the clinical presentation in very young children and the frail elderly with influenza and other upper respiratory tract infections.
- A discussion of the risk posed by the pandemic influenza strain and possible complications for high-risk groups (see Section V.4.2.2.b.).
- A review of the organization's pandemic influenza ORA findings and any corrective action taken.
- Provision of skills training on performing PCRAs during an influenza pandemic (see Appendix D).
- Provision of skills training on how to integrate and apply knowledge acquired from specific PCRAs with knowledge of the organization's ORA (see Appendix C) (e.g., available engineering controls and administrative policies and procedures to prevent and control pandemic influenza transmission in the healthcare setting).
Pandemic influenza education and skills training should be intensified when an influenza pandemic is imminent.
VII. Pandemic Period: Recommendations to Prevent the Spread of Pandemic Influenza in Existing Healthcare Settings
The recommendations that follow have been adapted to a variety of healthcare settings and should apply to all settings where health care is provided (e.g., prehospital, acute, non-acute, long-term, ambulatory, home, clinic and community care settings).
Specific recommendations for the prevention and control of pandemic influenza in acute care settings can be found in Section VII.1.; recommendations for long-term care can be found in Section VII.2.; for ambulatory care in Section VII.3.; for community care settings in Section VII.4.; home care in Section VII.5.
The primary goal of the recommendations in Section VII is to reduce the opportunity for exposure to and transmission of the pandemic strain of the influenza virus to patients, HCWs, visitors, etc., by minimizing the time infected sources and susceptible hosts co-mingle in areas within the healthcare setting.
1. Pandemic Period Recommendations for Acute Care Settings
1.1. Implementation of the Pandemic Influenza IPC/OH Plan for the Acute Care Setting
Based on the planning done for the pandemic influenza IPC/OH plan (see Section VI.) the following recommendations should be implemented once an influenza pandemic is declared in the local area.
Note: The trigger for some influenza pandemic activities may be determined by local governments/jurisdictional authorities irrespective of pandemic influenza declarations.
1.2. Acquisition of Up To Date Information on this Pandemic Influenza Viral Strain.
The organization needs to ensure that they and their HCWs are accessing the most up to date information on the epidemiology, clinical presentation and IPC/OH recommendations available throughout the pandemic wave. For example, the Public Health Agency of Canada’s websiteFootnote 118 and websites from the provincial/territorial and local public health jurisdictions should be reviewed regularly.
1.3. Implementation of Pandemic Influenza Precautions in Acute Care Settings
Pandemic Influenza Precautions (see Section V.6.2.6.) in acute care settings includes:
- Signage should be placed at specific locations (e.g., entrances, etc.) indicating the location of masks and ABHR.
- Frequent hand hygiene (see Section V.6.2.6.1.) and respiratory hygiene by patients, HCWs, visitors, contractors, etc.
- Place ABHRs at points of care and at entrances to and exits from healthcare settings and patient-care units.
- Performance of a PCRA (see Appendix D), by HCWs, prior to every patient encounter.
- Appropriate use of Personal Protective Equipment.
- Masks and Respirators (see Section V.6.2.6.3.).
- Facial/eye protection or face shield (see Section V.6.2.6.4.).
- Gloves (see Section V.6.2.6.5.).
- Gowns (see Section V.6.2.6.6.).
- Appropriate housekeeping, laundry and waste management activities (see Section V.6.2.6.7.).
- Implementation of engineering and administrative controls (as per the IPC/OH pandemic influenza plan) to enable rapid and sustainable separation of infected sources from susceptible hosts using:
- Separate assessment and care areas
- Temporary partitions
- Two metre distancing
- Passive screening indicated by signage or active screening as determined by the organization’s IPC/OH pandemic plan for the specific healthcare setting to:
- Provide directions to the pandemic influenza assessment and admission areas,
- Provide training in the performance of respiratory hygiene and hand hygiene,
- Provide guidance in the performance of Influenza Self Assessments (see Appendix A).
1.4. Triage and Assessment
1.4.1. Upon Entry Into the Healthcare Setting, Patients Should Be Separated (Triaged) Into Those Requiring Assessment For
- Influenza
- Other conditions as well as ILI symptoms (e.g., patient with arrhythmias, trauma)
- Other conditions with NO ILI symptoms
1.4.2. Influenza Assessment
The influenza assessment process should be organized to minimize crowding and provide for appropriate spatial separation (two metres) between infected sources and susceptible hosts in assessment cubicles, waiting areas and treatment areas.
- Whenever possible, single rooms should be used for patients with ILI symptoms.
- When single rooms are not possible, ensure that spatial separation recommendations are applied (i.e., two metre distancing between influenza and non-influenza patients or use of temporary physical barriers).
- Respiratory Hygiene should be used, including the use of masks by patients, if tolerated.
- Consider cohorting patients with similar symptoms in the same area (a two metre separation is not needed when patients have similar symptoms/diagnosis).
- Assessment staff should be evaluating not only the patient's symptoms, but also the symptoms of the person accompanying the patient.
- If the person accompanying the patient has ILI symptoms and the patient has no symptoms (i.e., presenting with "non-influenza" complaints):
- Consider the patient exposed to influenza;
- The patient should be monitored every four to six hours for ILI symptoms (see Appendix A);
- Request that another person without ILI symptoms accompany the patient. However, if this is not possible, the accompanying person with ILI symptoms may stay with this patient;
- The accompanying person with ILI symptoms should be informed that if they leave the patient's bedside, they should leave the patient area and immediately leave the facility.
- The accompanying person with ILI symptoms should be asked to wear a mask and instructed in respiratory and hand hygiene.
- The accompanying person with ILI symptoms should be informed that they may NOT go to the cafeteria, visit other patients, or wait in any public area.
- If the person accompanying the patient has ILI symptoms and the patient has no symptoms (i.e., presenting with "non-influenza" complaints):
1.5. Admission Process
- The admission process should be organized to minimize crowding and provide for appropriate spatial separation (two metres) between infectious agents, infected sources, and susceptible hosts.
- Where ever feasible, physical barriers (i.e., glass/acrylic partitions) should be used to minimize exposure of assessment, reception and admission personnel to patients with ILI symptoms.
- When physical barriers are not possible, spatial separation recommendations should be applied (i.e., two metres between non-infected personnel and patients with ILI symptoms).
- Separate influenza and non-influenza cohorts should be maintained for the duration of the pandemic (i.e., locally).
1.5.1. Open Separate Influenza and Non-Influenza Inpatient Care Areas
All inpatient care areas should be organized to have separate influenza care areas and non-influenza care areas. The opening and use of these areas will be dependent on the emerging epidemiology, severity of the pandemic and the impact on the organization.
There will be specialty care areas where separate units or rooms are not feasible because of care requirements (e.g., intensive care). For these areas, consider using temporary partitions (e.g., glass or acrylic) between patients with ILI symptoms and patients without ILI symptoms.
A two metre distance between patients with ILI symptoms and patients without influenza symptoms should be maintained.
Any patient admitted to a non-influenza cohort should be assumed to have been potentially exposed to influenza in the community (i.e., maintain a high index of suspicion for ILI symptoms for the incubation period).
Note: A two metre distance is not needed between patients infected with the same infectious agent (e.g., in influenza cohorts).
1.5.2. Use of Single Rooms
Whenever possible, single patient rooms should be utilized for inpatients with ILI symptoms.
1.5.3. Use of Airborne Infection Isolation Rooms
Note: Airborne infection isolation rooms are not required for the routine care of influenza patients.
Room prioritization processes should be implemented for available airborne infection isolation rooms as per the organization's pandemic influenza IPC/OH plan.
- Airborne infection isolation rooms should be prioritized for patients with known or suspected airborne infectionsFootnote 2, Footnote 3 including:
- Tuberculosis,
- Measles,
- Chickenpox, and
- Disseminated zoster.
- Airborne infection isolation rooms may be utilized for AGMPs on patients with ILI symptoms when possible.
1.5.4. Urgent AGMPS
- Urgent AGMPs (e.g., intubation associated with cardiac arrest) should not be delayed by transferring patients to single rooms or airborne infection isolation rooms (see Section V.4.4.4., Section V.6.2.4. and Section VII.1.5.4.).
- Ensure availability and use of appropriate PPE and spatial distancing for everyone in the room.
- When performing an AGMP in a patient's room all non-essential individuals should leave the room.
- All personnel in the room where an AGMP is being performed should wear appropriately fitted respirators.
- In multi-bed rooms draw curtains around beds of other patients. Personnel involved in performing the AGMP should wear a respirator; patients in the multi-bed room are not required to wear respirators.
- Maintain a two metre spatial separation between the patient requiring the AGMP and patients without ILI symptoms.
- Enhance monitoring of roommates to every four to six hours for one incubation period following the AGMP.
- Note: When responding to a code (cardiac arrest) on a patient with ILI symptoms, the delay in life saving treatment in order to transfer the arresting patient to a single room or airborne infection isolation room is inappropriate.
- Source control strategies can reduce the level of aerosol generation and, therefore, the risk posed by performing an AGMP on a patient with symptoms compatible with pandemic influenza. (See Section V.4.4.4.).
- Medical practices that may reduce aerosol generation include: Using appropriate patient sedation.
- Limiting AGMPs to those that are medically necessary.
- Performing AGMPs with skilled/experienced personnel.
- Anticipating and controlling processes for AGMPS wherever possible.
- Limiting the number of HCWs in the room when AGMPs are performed.
- Using metre dose inhalers (MDIs) to avoid nebulization.
- Organizing workflow to maintain optimum room ventilation (e.g., keep ventilation areas free of materials to ensure safe levels of air filtration and direction of air flow).
- Using closed suction systems wherever possible.
1.5.5. Placement of Patients WITHOUT ILI symptoms
As soon as non-influenza patients are identified (i.e., those requiring acute-care assessment for conditions other than influenza), they should be directed to specific non-influenza assessment or waiting areas.
Non-influenza inpatient areas should be physically separate from the influenza areas to reduce influenza exposure.
- Inpatients without symptoms of influenza should be cared for using RPAP recommendations as indicated by their illnesses.
- Consider monitoring for symptoms of influenza (see Appendix A) on admission and every four to six hours for one incubation period (see Assumptions, Section V.1.a.).
- Patients who develop ILI symptoms should be separated from non-influenza patients as soon as symptoms are noted.
- Patients, who are at high risk of serious complications should they acquire influenza, should be quickly identified and separated (e.g., at least two metres, use of partitions) from individuals with ILI symptoms (see Section V.4.2.2.b.).
- Patients at high risk of severe complications should be closely monitored for influenza symptoms every four hours for one incubation period (see Section V.4.2.2.b.) after admission then every shift for the duration of admission (see Appendix A).
- Plans should be made for early antiviral treatment of patients with ILI symptoms.
1.5.6. Placement of Patients WITH ILI symptoms
- Patients who primarily require assessment and care for ILI symptoms should be directed to influenza cohort areas.
- Wherever possible, place patients with ILI symptoms in single rooms.
- If single rooms are unavailable, a two metre separation between patients should be maintained, and curtains should be pulled.
1.5.7. Placement of Patients WITH ILI symptoms and Another Medical Condition
- Patients who have ILI symptoms and also require acute-care assessment for other medical conditions, should quickly be directed to areas that have the resources to assess/care for the “other medical condition” while preventing the spread of influenza to asymptomatic patients, HCWs, visitors, contractors, etc.
- Cohort areas for patients with ILI symptoms who also require care within specialty units (e.g., Trauma Intensive Care Units, Coronary Care Units, Maternity Units, Neonatal units) should be established utilizing single rooms or physical partitions in multi-bed rooms.
- When single rooms or physical barriers are not possible, spatial separation (two metres between influenza and non-influenza patients) should be maintained.
- Patients with ILI symptoms who require treatment for other medical conditions may be cohorted together in multi-bed rooms.
1.5.8. Placement of Patients Immune to Influenza
- Patients who are immune to influenza include those who:
- Were immunized against the pandemic influenza strain at least two weeks prior; or
- Have recovered from laboratory confirmed pandemic influenza
- Patients who have recovered from laboratory confirmed influenza may be accommodated in the area most appropriate to their care needs.
- As immunization may not be fully protective, consider assessing immunized inpatients for signs of influenza every four to six hours for one incubation period (see Section V.1.a.).
1.6. Transfer/Transport of Patients with ILI symptoms Within (i.e., Intra-Facility) and Between (i.e., Inter-Facility) Healthcare Settings
- The movement of patients with ILI symptoms should be limited to those transfers/transports that are medically necessary.
- When transfer/transport (intra-facility or inter-facility) is necessary, HCWs should perform a PCRA (see Appendix D) to determine the range of Pandemic Influenza Precautions (see Section V.6.2.6.) that are recommended for care before, during and after the transport of the patient.
- Formal communication processes should be established to ensure that the transporting agency and the receiving department, unit, or facility, is made aware of the patient’s ILI symptoms, diagnosis and lab results (e.g., direct communication with personnel of the receiving department, unit or facility) so that the transferring personnel and the receiving area can rapidly initiate Pandemic Influenza Precautions during transport and upon arrival.
- When transport (intra-facility or inter-facility) is necessary, patients with ILI symptoms should be taught to:
- Perform hand hygiene.
- Wear a mask (NOT a respirator) for the duration of transport (if tolerated).
- Practice respiratory hygiene during transport.
1.7. Visitor Responsibilities and Restrictions
Visitors with symptoms of influenza should NOT visit except in very exceptional circumstances (see Exemptions below in Section VII.1.7.3.)
1.7.1. Visitors and Influenza Assessment
- Before entering the healthcare setting, visitors should perform an influenza self assessment to identify ILI symptoms and should not enter the area if ILI symptoms are present (see Section VI.2.4. and Appendix A).
- Consideration may be given to posting the influenza assessment guide on the organization’s web site so that visitors can self-assess prior to arriving at the healthcare setting.
- Asymptomatic visitors may visit without infection prevention restrictions.
1.7.2. Visitors With No Symptoms of Influenza Visiting a Patient With ILI symptoms
The visitor should:
- Consider NOT visiting if they (the visitor) are at high risk of complications should they contract influenza (e.g., cardio-pulmonary disease, immuno-suppressed, pregnant, etc.).
- Be restricted to visiting one patient per hospital visit to prevent inadvertent influenza transmission to other patients or the visitor.
1.7.3. Exceptional Circumstances for Visitors With ILI symptoms
- Visitors who have ILI symptoms should NOT visit unless they are:
- Close relatives of terminally ill patients.
- Parents of sick children.
- Able to wear a mask and comply with respiratory and hand hygiene.
- Children with ILI symptoms, who are relatives of terminally ill patients, may visit under extremely strict supervision by parents or guardians. Parents or guardians should ensure that the sick child wears a mask and practices strict hand and respiratory hygiene.
- Under no condition should visiting children with ILI symptoms visit any other area/room/patient in the facility, or visit an open unit with vulnerable patients (e.g., Neonatal Intensive Care Unit).
- The organization should ensure that symptomatic visitors do not have opportunity to expose other patients, HCWs, visitors, contractors, etc., to influenza while in the facility.
- Ill visitors should restrict their visit to a single patient (terminally ill adult or sick child).
- Ill visitors should not visit any other patient, or utilize any other area of the facility (e.g., public areas, waiting areas, lounges, etc.).
- The organization should provide resources (i.e., equipment and direction) to enable the ill visitor to do the following:
- Put on a mask upon entering the healthcare setting.
- Remove the mask and place in appropriate receptacle upon leaving the healthcare setting.
- Perform hand hygiene upon entering the healthcare setting and prior to entering and leaving a patient’s room and after removal of the mask when leaving the setting.
- Practice respiratory hygiene.
- Ill visitors should report to the nursing station prior to entering the patient’s room:
- To receive instruction on proper mask wearing and removal; and hand and respiratory hygiene.
- For assessment of infection prevention and control practices.
- Monitor patients for ILI symptoms for one incubation period after the last visit of an ill visitor.
1.8. Pandemic Period: Work Assignments during the Pandemic Period
(See Planning Recommendations in Section VI.3.)
- During the pandemic period, HCWs should perform and interpret a daily influenza self assessment to determine their influenza status and thus their ability to work.
- HCWs should know how to perform and interpret the daily influenza self assessment (see Appendix A) to determine their personal influenza status and thus their fitness-for-work.
- HCWs who develop ILI symptoms while on duty should report the occurrence and should be relieved of their duties.
- HCWs considered fit-for-work (see Section V.6.2.2.a.)
- Is asymptomatic (as per self assessment for ILI, see Appendix A).
- HCWs who are considered unfit-for-work should, at a minimum, not report to work for at least one period of communicability after the onset of symptoms.
- HCWs considered fit-for-work with restrictions (see Section V.6.2.2.b.).
- In a severe or prolonged pandemic where personnel shortages compromise patient safety, organizations may allow a person with mild influenza symptoms to return to work.
- Consideration should be given to assigning these personnel to influenza cohort areas.
- HCWs who self identify that they are at high risk of complications related to influenza, including pregnant HCWs (see Section V.6.2.3.) should be offered an assessment by an occupational health clinician (or if unavailable their personal clinician in conjunction with infection prevention and control or public health personnel). These HCWs should be provided with counselling and education including information pertaining to the severe outcomes of influenza and reinforcement of protective measures such as PCRA, appropriate use of PPE, access to treatment and use of antiviral medication. (See Section V.6.2.3.).
- (See Annex E of the CPIPFootnote 5 for recommendations on the use of antiviral medication The Canadian Pandemic Influenza Plan for the Health Sector - Annex E).
1.9. Pandemic Period: Pandemic Influenza Education and Skills Training for Healthcare Workers in Acute Care Settings
Education and skills training should be reinforced when an influenza pandemic is imminent. (See Planning Recommendations in Section VI.4.)
1.10. Influenza Outbreak Detection and Management for Acute Care Settings
Outbreaks may occur in non-influenza cohort areas. The influenza virus may be introduced by patients who were incubating influenza at the time of admission to the healthcare settings and HCWs or visitors with ILI symptoms. (See Section V.1.a.).
1.10.1. Detection of a New Influenza Case Among Cohorts of Non-Influenza Patients
- Any patient admitted to a non-influenza cohort/patient area may have been exposed to influenza in the community and may be incubating influenza when admitted to a healthcare setting.
- All new non-influenza admissions should be closely monitored for symptoms compatible with pandemic influenza every four to six hours for one incubation period (see Section V.1.a.) and then once a shift for the duration of admission.
1.10.2. Separate Patients Who Develop ILI symptoms From Non-Influenza Patients
- Geographic boundaries of exposed area(s) should be defined (consider the physical design of the unit/area). Consider that all patients and HCWs in that geographic area exposed to influenza.
- Patients who have developed ILI symptoms should be transferred to the influenza care unit and cohorted if medically and/or logistically feasible.
- If transfer is not possible, symptomatic patients should be cohorted as much as feasible within the unit.
- Consider identifying specific HCWs to work with the symptomatic cohort to decrease potential exposure to other susceptible patients.
1.10.3. Outbreak Declaration
- An outbreak may be declared when two or more patients in separate rooms (in a non influenza cohort or care area) develop ILI symptoms within one incubation period. This definition may vary from one jurisdiction to another.
- Infection prevention and control, occupational health and local public health departments should be notified when an outbreak is identified.
- When an outbreak is declared:
- Convene an outbreak management team.
- Activate policies on communication strategies for outbreak management as per organizational policy and procedure.
- Close the unit or area to admission/discharge/transfer (perform a risk benefit analysis before closing the unit/area).
- Consider antiviral prophylaxis for all new admissions to the unit where the outbreak is occurring if closure of the unit is not feasible (see CPIPFootnote 5 Annex E The Canadian Pandemic Influenza Plan for the Health Sector - Annex E).
- Antiviral prophylaxis should be strongly considered for all individuals (i.e., patients and HCWs) within the exposed unit/area, unless medically contraindicated (see CPIPFootnote 5 Annex E The Canadian Pandemic Influenza Plan for the Health Sector - Annex E).
- Intensify surveillance of new cases by monitoring all patients on the unit every four to six hours for the duration of the outbreak.
- Meticulous daily cleaning of environmental surfaces should be performed (see Section V.6.2.6.7.). All non-critical patient-care items should be cleaned between patient uses. Surfaces frequently touched by the hands of HCWs, or patients, such as the surfaces of medical equipment and knobs for adjustment or opening, door knobs, hand rails, etc., should be cleaned at least twice daily and when known to be contaminated.
1.10.4. Isolation of New Influenza Patients
- Newly identified patients with ILI symptoms should be isolated within their room while ensuring a two metre separation from any roommates who do not have ILI symptoms.
- Close the privacy curtains between the symptomatic patient and other patients, if they are not in a private room.
- Treat new influenza patients with antiviral medication, unless medically contraindicated.
- Instruct patients to practice frequent hand hygiene, utilize respiratory hygiene and remain within their own bed space.
1.10.5. Contact Tracing of Roommates
- For an outbreak, the definition of roommates includes present roommates and any patient that had shared the room within a previous one incubation period (see Section V.1.a.). For open units (e.g., the intensive care unit), consider "roommates" to be the susceptible patients on either side of the newly infected patient or any patient within two metres of the newly infected patient.
- Monitoring roommates for signs and symptoms of influenza should be increased to every four to six hours for one incubation period (see Section V.1.a.).
- Roommates should be started on antiviral prophylaxis, unless medically contraindicated (see CPIPFootnote 5 Annex E The Canadian Pandemic Influenza Plan for the Health Sector - Annex E).
- Roommates should be encouraged to practice frequent hand hygiene.
- Asymptomatic roommates should not be required to wear masks to prevent influenza transmission.
1.10.6. Limit Transfers of Exposed Patients
(See Section VI.2.3.)
- Consider limiting the transfer of all exposed patients who have NOT developed ILI symptoms. (See Section VII.1.10.8.).
- If an exposed patient must be transferred for medically necessary reasons:
- The transferring personnel/agent and receiving units/organizations should be informed of the outbreak in advance of the transfer.
- Notify the infection control professional in the receiving healthcare setting to ensure Pandemic Influenza Precautions are utilized (see Section V.6.2.6.).
- The transferred patient should be monitored every four to six hours for ILI symptoms, for one incubation period (see Section V.1.a.).
1.10.7. Outbreak Visitor Restrictions
- Consider posting multi-lingual signage at entrances to the unit/area to notify visitors of the outbreak.
- Consider limiting visitors (exceptions may be made for visitors to terminally ill patients).
- Discourage visitation from visitors who self identify as being at high risk of severe complications should they acquire influenza.
1.10.8. Declaring the End of an Influenza Outbreak
- The outbreak may be declared over after one period of communicability followed by one incubation period after the last case has been identified.
- If the last case was a personnel member who was sent home, the outbreak may be declared over when one incubation period (see Section V.1.a.) has passed after the personnel member was removed from the work environment, or one period of communicability plus one incubation period from the last patient case, whichever is longer.
2. Pandemic Period Recommendations for Infection Prevention and Control Activities in Long-Term Care LTC) Settings
The goal of the pandemic influenz IPC OH plan in LTC facilities is to keep the facility (or major areas of the facility) completely free of influenza.
Seasonal influenza is a major cause of illness and death for residents of LTC facilities. Based on experiences with seasonal influenza, it is likely that the residents of LTC facilities will be vulnerable to severe complications should they acquire influenza caused by the pandemic strain. Since, LTC facilities are relatively closed communities, visitors HCWs, and residents who have been on trips/visits into the community, or residents newly admitted from the community, are frequently the point of entry of the influenza virus into LTC facilities.
During a pandemic wave, it is likely that acute care facilities may only have the capacity to admit LTC residents who require a higher level of care than can be provided within the LTC facility. As a result, LTC facilities should be prepared to care for residents with influenza on–site.
2.1. Implementation of the Long-Term Care Organization's Pandemic Influenza IPC/OH Plan
Based on the planning done for the pandemic influenz IPC OH plan (see Section VI.) the following recommendations should be implemented once an influenza pandemic is declared in the local area.
Note: The trigger for some influenza pandemic activities may be determined by local governments/jurisdictional authorities irrespective of pandemic influenza declarations.
- During the pandemic wave, LTC organizations should remain open to admission of new or returning residents (see Section VII.2.6. below for further information).
- All LTC HCWs should begin practicing Pandemic Influenza Precautions (see Section V.6.2.6.) when a pandemic wave is imminent in the community where the LTC facility is located.
- Up to date information on the pandemic influenza viral strain should be obtained:
- Throughout the pandemic wave, the organization needs to ensure that they and thei HCWs are accessing the most up to date information on the epidemiology, clinical presentation an IPC OH recommendations available. For example, the Public Health Agency of Canada's websiteFootnote 118 and websites from the provincial/territorial and local public health jurisdictions should be reviewed regularly.
2.2. Influenza Monitoring for all LTC Residents
- All LTC residents should be monitored for signs of influenza at least once per shift (see Appendix A for an Influenza Self Assessment Tool).
- All residents HCWs, visitors, contractors, etc., should be practicing effective hand and respiratory hygiene as pe RPAP.
2.3. Acceleration of Pandemic Influenza Education and Skills Training for HCWs in LTC Organizations
In addition to the education and skills training undertaken in the inter-pandemic period (see Section VI.4.):
- All LTC HCWs should be informed of the goal to keep the LTC facility (or major areas of the facility) completely free of influenza.
- HCWs should be utilizing Routine Practices with all residents.
- HCWs should know how to apply Pandemic Influenza Precautions for residents with ILI symptoms (see Section V.6.2.6.).
- The organization should provid HCWs with information about the ORA for the specific LTC facility including how the LTC facility will manage:
- Room assignments, including the influenza admission area, the influenza isolation area and the resident care area.
- Visitor screening and visitor restrictions.
- Ability/need to perfor AGMPs in the facility.
- Staff that become ill with ILI symptoms.
2.4. Implement Pandemic Influenza Precautions
HCWs should utilize Pandemic Influenza Precautions (see Section V.6.2.6.). In LTC facilities Pandemic Influenza Precautions include:
- Performance of PCRA, b HCWs, prior to every resident encounter (see Section V.7.3.2.).
- Frequent hand hygiene and respiratory hygiene performed b HCWs require the following (see Section V.6.2.6.1.):
- ABHRs placed at entrances and exits of LTC facilities.
- ABHR dispensers placed at all residential points of care (i.e., at the bedside).
- Materials for respiratory hygiene placed at facility/setting entrances and at entrances to all care areas.
- Appropriate use of Personal Protective Equipment.
- Masks or Respirators (see Section V.6.2.6.3.).
- Eye protection and face shield (see Section V.6.2.6.4.).
- Gloves (see Section V.6.2.6.5.).
- Gowns (see Section V.6.2.6.6.).
- Appropriate housekeeping, laundry and waste management activities (see Section V.6.2.6.7.).
- Implement engineering and administrative controls (as per the LTC organization’ IPC OH pandemic influenza plan) to enable rapid and sustainable separation of infected sources from susceptible hosts using:
- Admission area and Influenza Isolation areas.
- Temporary partitions.
- Two metre distancing.
- Signage with instructions for passive screening of ILI or active screening/training to:
- Provide training in the performance of respiratory hygiene and hand hygiene.
- Provide guidance in the performance of Influenza Self Assessments (see Appendix A).
- One HCW per shift should be assigned the authority to relieve employees of their duties if they display ILI symptoms.
2.5. Implementation of General Source Control Plans
- Consider implementing active screening for ILI symptoms at all LTC facility’s entrances. Consideration may also be given to limiting the number of entrances in order to reduce the number of personnel required to carry out active screening.
2.6. Activities Outside the Long-Term Care Facility
Residents who return from medical appointments or procedures performed outside the LTC facility, other community activities (e.g., funerals) or home visits should be considered exposed to influenza and monitored every four to six hours for one incubation period (see Section V.1.a.). Based on the emerging epidemiology of the pandemic, consider implementing the following:
- Organized community social activities (e.g., taking residents to shopping malls) should be cancelled for the duration of the pandemic wave.
- Family home visits, especially to homes where family member has ILI symptoms should be discouraged.
- All outside appointments should be postponed, unless medically necessary.
2.7. Admission Area for New Admissions
During the pandemic wave, all new residents, coming from the community (e.g., from home or another residential setting) or acute care hospital should be considered exposed to influenza. Based on the emerging epidemiology, consider the following:
- Open an Admission Area for ALL new residents
- Newly admitted residents WITHOUT ILI symptoms should stay in the Influenza Admission Area for one incubation period (see Section V.1.a.) prior to transfer to the Resident Care Area.
- Residents in the Admission Area should be assessed every four to six hours for one incubation period (see Section V.1.a.) for ILI symptoms (see Appendix A).
- The Admission Area should be organized to minimize crowding.
- Wherever possible, single rooms should be utilized for residents in the Admission Area.
- HCWs in the Admission Area should be performin PCRAs (see Appendix D) prior to every "care-giving" contact with every resident.
- All HCWs in the Admission Area should be well prepared to perform influenza assessments, to recognize ILI symptoms, and to implement Pandemic Influenza Precautions as soon as a symptomatic resident is identified.
- Implement Pandemic Influenza Precautions if any resident in the Admission Area is identified with ILI symptoms and consider antiviral prophylaxis of roommates (see Section VII.2.8.).
- Appoint at least on HCW on every shift with the authority to implement Pandemic Influenza Precautions so they can be initiated without delay.
- Treat symptomatic residents with antiviral medications as appropriate.
- Identify and transfer any resident who develops ILI symptoms to the Influenza Isolation Area.
- If transfer is delayed HCWs should have the authority to confine the resident to his or her room, initiate Pandemic Influenza Precautions and ensure adequate separation from non-influenza residents (e.g., at least two metres, temporary partitions, move to a single room).
- If the facility is unable to establish a separate Admission Area, new residents who do not have ILI symptoms can be managed safely within the resident care area if the following criteria are strictly applied:
- Place resident in a single room if possible
- If a single room is not available, place resident in a multi-bed room where a two metre separation can be constantly maintained between residents.
- Monitor resident and all roommates for signs of influenza every four to six hours for one incubation period (see Section V.1.a.).
- Ensure resident is able and willing to comply with respiratory hygiene and hand hygiene.
- Instruct resident not to leave their bed space for one incubation period, except for medically necessary procedures.
- Utilize Pandemic Influenza Precautions for one incubation period for all resident care (see Section V.6.2.6.).
- Admission to the Influenza Admission Area is NOT necessary for the following resident:
- New admission from the community with proof of recovery from laboratory confirmed influenza during the pandemic influenza period.
- Based on the emerging epidemiology and vaccine effectiveness, was immunized against the influenza pandemic strain more than two weeks prior to this admission.
2.8. Influenza Isolation Area
- Influenza Isolation Areas may be opened (i.e., if the physical environment permits) to accommodate residents with ILI symptoms (i.e., new admissions, residents returning from the community, residents acquiring influenza while in the LTC facility).
- HCWs should utilize Pandemic Influenza Precautions (see Section V.6.2.6.) for care of every resident in the Influenza Isolation Area and every resident isolated in any other area in the LTC facility for ILI symptoms (in single or multiple bed rooms).
- Symptomatic residents should be treated with antiviral medications as indicated (se CPIPFootnote 5 Annex E The Canadian Pandemic Influenza Plan for the Health Sector - Annex E).
- HCWs in the Influenza Isolation Area should have access to adequate supplies for the care of a resident acutely ill with influenza (e.g., PPE including masks and respirators; clinical supplies for treatment and symptomatic relief of persons ill with influenza).
Expect that residents admitted to the Influenza Isolation Area will remain in the Influenza Isolation Area for a minimum of one period of communicability and until symptoms are improving before returning to the Resident Care area.
2.9. Resident Care Areas
As far as possible, Resident Care Areas should be kept free of influenza (i.e., residents HCWs that have ILI symptoms should be restricted from entering/working in the Resident Care Area).
- All residents should be monitored at least once a shift for ILI symptoms for the duration of the pandemic wave.
- HCWs should utilize Pandemic Influenza Precautions (see Section V.6.2.6.) to care for residents with ILI symptoms who are housed in the Resident Care Area.
- HCWs should perform PCRA (see Appendix D) prior to initiating care of any resident.
- At least on HCW, in every Resident Care Area on every shift, should have the authority to:
- Implement Pandemic Influenza Precautions without delay.
- Identify and transfer any resident who develops ILI symptoms to the Influenza Isolation Area (or appropriately isolate in room).
- Restrict resident to their room or bed space including for meals and all social activities.
- Establish and/or maintain adequate separation (i.e., at least two metres) between symptomatic and non-symptomatic residents.
- Begin treatment with antiviral medications as indicated.
- Relieve staff of their duties if they develop influenza symptoms.
- Residents with ILI symptoms, who reside in a multi-bed room, should be isolated in their room HCWs should:
- Manage the ill resident according to symptoms as well as the resident's level of cognitive function.
- Maintain a separation of two metres between the bed space of the infected resident and any susceptible roommates.
- Privacy curtains should be drawn between bed spaces at all times.
- Consider all residents who share a room with the ill resident, exposed to influenza.
- Influenza monitoring should be increased to every four to six hours in the exposed residents for one period of communicability plus one incubation period (see Section V.1.a.).
- Contact tracing should be initiated for ILI symptoms in any resident that shares or shared a room with an ill resident during one incubation period (see Section V.1.a.) prior to the onset of symptoms.
- Roommates should be started on antiviral prophylaxis, if indicated.
- Roommates or face-to-face contacts of the ill resident should NOT be transferred to any other room for one period of communicability plus one incubation period (after the last exposure to the ill resident).
- Roommates receiving prophylaxis should be allowed in common areas of the facility.
2.10. Visitor Restrictions and Exemptions
LTC facilities should implement the section on th IPC OH plan for visitors (see Section VI.2.4.). LTC facilities should also consider implementing visitor recommendations found in Section VII.1.7.
2.10.1. Visitors and Influenza Assessment
- Before entering the LTC facility, visitors should perform an influenza self-assessment for any ILI symptoms (see Appendix A).
- Consider instituting active visitor screening for ILI symptoms (i.e., assign personnel members to the door, to ensure every visitor is screened).
- Consideration may be given to posting the Influenza Assessment Tool (see Appendix A) on the organization’s web site so that visitors can self-assess prior to arriving at the LTC facility.
- If there is an outbreak or transmission of influenza within the LTC facility, visitors who have not yet had the pandemic strain of influenza or those who have not been immunized against the pandemic strain in the previous two weeks should not visit except in very exceptional circumstances.
- Asymptomatic visitors may visit without infection prevention restrictions.
2.10.2. Visitors With No Symptoms of Influenza Visiting a Resident With ILI symptoms
The visitor should:
- Consider NOT visiting until the period of communicability has passed.
- Be restricted to visiting one resident per visit to prevent inadvertent influenza transmission to other residents via the visitor.
2.10.3. Exceptional Circumstances for Visitors With ILI symptoms
Any visitor with ILI symptoms should be restricted from entering the LTC facility for a minimum of one communicability period from date of onset of their symptoms. However, exceptional circumstances may exist where an ill visitor will be allowed to visit a terminally ill resident.
- Visitors who have ILI symptoms should NOT visit unless they are:
- Close relatives of a terminally ill resident.
- Children with ILI symptoms, who are relatives of terminally ill patients, may visit under extremely strict supervision by parents or guardians. Parents or guardians should ensure that the sick child wears a mask and practices strict hand and respiratory hygiene.
- Under no condition should visiting children with ILI symptoms visit any other area/room/resident in the facility.
- The organization should ensure that symptomatic visitors do not have opportunity to expose other residents, to influenza while in the facility.
- Ill visitors should restrict their visit to a single terminally ill resident.
- Ill visitors should not visit any other resident or utilize any other area of the facility (e.g., public areas, waiting areas, lounges, etc.).
- The organization should provide resources (equipment and direction) to enable the ill visitor to do the following:
- Put on a mask appropriately upon entering the facility.
- Remove the mask safely upon leaving the facility.
- Perform effective hand hygiene upon entering the facility, prior to entering and leaving a patient's room and after removal of the mask when leaving the facility.
- Practice respiratory hygiene.
- Monitor residents for ILI symptoms for one incubation period after the last visit of an ill visitor.
- If active screening is not occurring at the entrances to the LTC facility, signage should direct ill visitors to report to the nursing station prior to entering a resident’s room.
- To receive instruction on proper mask wearing and removal; and hand and respiratory hygiene.
- For assessment of infection prevention and control practices.
2.11. LTC HCWs : Fitness-for-Work
- Initiate th IPC OH Pandemic Influenza Plan as per Section VI.3.
- During the pandemic wave HCWs should perform and interpret a daily influenza self assessment to determine their influenza status and thus their ability to work. Please refer to the Influenza Self Assessment Tool in Appendix A.
- HCWs who develop ILI symptoms while on duty should report the occurrence and be relieved of their duties.
- HCWs with ILI symptoms should remain off work for one period of communicability (see Section V.1.a.).
- HCWs should be referred for early antiviral treatment as required.
2.12. Pandemic Influenza Outbreak Detection and Response in LTC Facilities
- An outbreak may be declared when two or more residents in separate rooms (in the Residential Care Area or the Influenza Admission Area) develop ILI symptoms.
- When a resident develops ILI symptoms from an unknown/unidentified exposure while residing in the Resident Care Area (e.g., no trips into the larger community, no known contact with personnel or visitors with ILI symptoms), unit monitoring should be increased (i.e., beyond the infected sources' room) for additional cases of influenza illness.
- In this circumstance, all residents on the unit should be assessed for ILI symptoms every four to six hours for one incubation period.
- Infection prevention and control should be notified of all cases.
- Public Health authorities should be notified as per jurisdictional requirements.
- Outbreak recommendations found in Section VII.1.10. should be applied
- Establish an outbreak management team and activate communication strategies for outbreaks including:
- A method to track ILI symptoms i HCWs working in outbreak areas;
- A method to monitor residents developing ILI symptoms; and
- A method to perform contact tracing of residents who have moved to other units (i.e., within one incubation period).
- Residents who develop ILI symptoms should be separated from non-influenza residents if medically and logistically possible.
- Residents residing in the Resident Care Area who develop ILI symptoms should be transferred to the Influenza Isolation Area if medically/logistically appropriate and feasible.
- If possible, residents that require complex medical care should be transferred to an acute care hospital.
- Pandemic Influenza Precautions should be utilized for care of ALL symptomatic residents within the outbreak area.
- All newly symptomatic residents should be treated with antiviral medications as indicated (se CPIPFootnote 5 Annex E The Canadian Pandemic Influenza Plan for the Health Sector - Annex E).
- Antiviral prophylaxis should be provided to all residents within the outbreak unit/area, as per th CPIPFootnote 5 Annex E The Canadian Pandemic Influenza Plan for the Health Sector - Annex E.
- Consideration should be given to closing the unit or area to admission, discharge or transfer.
- All transfers of exposed residents (i.e., those residing within the defined outbreak area who have NOT developed ILI symptoms) should be limited for the duration of the outbreak, (i.e. one incubation period plus one period of communicability after the last patient develops symptoms).
- If an exposed resident must be transferred for medically necessary reasons, transport personnel and receiving units/organizations should be informed of the LTC facility’s outbreak and the need for Pandemic Influenza Precautions for this resident prior to the transfer. The resident should be monitored for one incubation period.
- Outbreak Visitor restrictions should be applied as per Section VII.1.10.
- The end of an influenza outbreak should be declared as per Section VII.1.10.
3. Ambulatory Care Clinics and Settings
3.1. Implementation of the Pandemic Influenza pandemic IPC/OH Plan
Based on the planning done for the pandemic influenza IPC/OH plan (see Section VI.) the following recommendations should be implemented once an influenza pandemic is declared in the local area.
Note: The trigger for some influenza pandemic activities may be determined by local governments/jurisdictional authorities irrespective of pandemic influenza declarations.
The IPC/OH recommendations for Ambulatory Care that follows should apply to stand- alone outpatient clinics, doctors’ offices, school-based health clinics and other settings where health services are provided to susceptible hosts (e.g., physiotherapy practices, outpatient laboratories, adult day programs, foot care clinics, wellness clinics). If services or programs are not essential, organizations should consider cancelling the service/program until after the pandemic wave has passed.
The goal of the pandemic influenza IPC/OH plan for ambulatory care clinics should be to reduce or limit the time (e.g., waiting, receiving care) an infected source (i.e., patient with ILI symptoms) is in contact with a susceptible host (e.g., HCWs, patients and family without influenza) and to protect HCWs, other patients, visitors, etc. while the care, program or service is provided.
- All HCWs in the Ambulatory Clinic/Group Setting should be informed and practicing Pandemic Influenza Precautions with all clients who have ILI symptoms as per Section V.6.2.6.
- HCWs should perform a PCRA if providing care or within two metres of any patient/client as per Section V.7.3.2. and Appendix D.
- ABHR, masks, respirators, gloves, gowns, and eye or face protection should be available and utilized as per Section V.6.2.6.
- HCWs in Ambulatory Care Clinics wearing respirators for respiratory protection (e.g. performing aerosol-generating procedures) require fit-testing as per jurisdictional (federal/provincial/territorial/local) directives as per Section V.6.2.6.3.
- HCWs: Fitness-for-Work
- Initiate the ambulatory care clinic’s IPC/OH Pandemic Influenza Plan as per Section VI.3.
- During the pandemic wave, HCWs should perform and interpret the daily influenza self assessment to determine their influenza status and ability to work. Refer to the Influenza Self Assessment Tool in Appendix A.
- HCWs who develop ILI symptoms while on duty should report these and be relieved of their duties.
- Alternative care plans that have been developed by pandemic influenza provincial/territorial/regional authorities should be initiated.
- If the service or program is essential:
- Patient appointments should be evaluated and, where possible, prioritize visits to those patients for whom acute care hospitalization may be prevented (i.e., where possible prevent patients with other medical conditions from deteriorating and requiring acute hospitalization).
- Consideration should be given to implementing a telephone triage plan to screen patients/clients for ILI symptoms prior to arriving at the clinic, if appropriate.
- If patients/clients with ILI symptoms are identified:
- Cancel/postpone appointments if medically appropriate, based on an assessment by a health professional, until the period of communicability (see Section V.1.a.) has passed.
- Re-direct patients/clients who need medical assessment for ILI symptoms to their primary care provider or local influenza assessment clinics/centres where available and appropriate.
- Consider implementing a process for prescription renewal (as developed in the clinic’s pandemic influenza IPC/OH plan) that does not require a patient/client to visit the clinic/office (e.g., telephone renewals).
- Consider scheduling appointments for patients with ILI symptoms at the same time, if appropriate and feasible.
- If the service or program is essential:
- Other general source control activities:
- Post multi-lingual signage at all entrances to the clinic for HCWs, etc.
- Instructions and equipment (e.g., mask, tissues, ABHR station) for hand hygiene and respiratory hygiene should be available for anyone entering the ambulatory care area.
- Signs with clear directions to influenza assessment clinic/centres (potentially at another location) should be posted for any patient/client who requires assessment of ILI symptoms.
- Any patients/clients with ILI symptoms that attend the clinic should put on a mask and perform hand hygiene as soon as they enter the clinic area.
- Separation of two metres between the infected source (i.e., patient/client) and susceptible hosts (i.e., other clients, HCWs, etc.) should be established and maintained (e.g., move patients/clients with ILI symptoms into examination rooms on arrival).
- Pandemic Influenza Precautions should be utilized when within two metres of an infected client (see Section V.6.2.6.).
- When the ambulatory clinic is situated within a larger healthcare facility, patients with ILI symptoms should not leave the ambulatory care area during their visit except for medically essential procedures or to leave the facility.
- Hands-free garbage receptacles should be provided in adequate numbers, in convenient locations.
- Magazines and toys should be removed from waiting rooms to reduce opportunity for exposure to contaminated articles (i.e., decrease potential for contact exposure) and to facilitate cleaning.
- Post multi-lingual signage at all entrances to the clinic for HCWs, etc.
4. Community Settings with Infirmaries
The goal of the pandemic influenza IPC/OH plan for Community Settings with Infirmaries should be to reduce the likelihood of susceptible hosts (patients, HCWs, etc.) acquiring pandemic influenza while in the infirmary AND of pandemic influenza outbreaks starting in the infirmary and then spreading throughout the organization.
4.1. Implementation of the Pandemic Influenza IPC/OH Plan
Based on the planning done for the pandemic influenza IPC/OH plan (see Section VI.) the following recommendations should be implemented once an influenza pandemic is declared in the local area.
Note: The trigger for some influenza pandemic activities may be determined by local governments/jurisdictional authorities irrespective of pandemic influenza declarations.
This section is directed to existing infirmaries within community-based organizations (e.g., infirmaries in correctional facilities, boarding schools, etc.) as outlined below;
- Initiate the community setting’s IPC/OH Pandemic Influenza Plan as per Section VI.3.
- All HCWs in the infirmary should be informed and practicing Pandemic Influenza Precautions with all clients who have ILI symptoms as per Section V.6.2.6.
- All HCWs in community settings with infirmaries wearing respirators for personal protection (e.g. performing aerosol-generating procedures) require fit-testing as per jurisdictional (federal/provincial/territorial/local) directives. See Section V.6.2.6.3.
- HCWs should perform a PCRA before entering the bed space (within two metres of) of any patient (see Appendix D).
- ABHR, masks, respirators, gloves, gowns, and eye or face protection should be utilized as per Section V.6.2.6.
- HCWs: Fitness-for-Work
- During the pandemic wave, HCWs should perform and interpret the daily influenza self assessment to determine their influenza status and thus their ability to work. Please refer to the Influenza Self Assessment Tool in Appendix A.
- HCWs who develop ILI symptoms while on duty should report the occurrence and be relieved of their duties.
- All patients who attend the infirmary should be screened for ILI symptoms (see Appendix A).
- Consider establishing an Influenza Isolation Area if the physical environment permits, for patients with ILI symptoms who require admission to the infirmary (e.g., single room).
- Consider transferring these patients into the general infirmary area after one period of communicability or when symptoms resolve.
- If transfer to another healthcare setting is required, recommendations in Section VII.1.6. should be followed.
- Evaluate patient appointments, and where possible, prioritize infirmary visits to those patients for whom acute care hospitalization (for influenza or other medical conditions) may be prevented (i.e. where possible prevent patients with other medical condition from deteriorating and requiring hospitalization in an acute care facility).
- All patients admitted to the infirmary should be monitored every four to six hours for ILI symptoms for one incubation period and then once a shift for the duration of their stay.
- The infirmary should be monitored for an Influenza Outbreak as outlined in Section VII.1.10.
5. Home Care Settings Where Care or Service is Provided by Regulated and Unregulated HCWs
5.1. Implementation of the Pandemic Influenza IPC/OH Plan
Based on the planning done for the pandemic influenza IPC/OH plan (see Section VI.) the following recommendations should be implemented once an influenza pandemic is declared in the local area.
Note: The trigger for some influenza pandemic activities may be determined by local governments/jurisdictional authorities irrespective of pandemic influenza declarations.
- Initiate the home care organization’s IPC/OH Pandemic Influenza Plan as per Section VI.3.
- All HCWs providing home care and service should be informed and be practicing Pandemic Influenza Precautions with all clients who have ILI symptoms. (See Section V.6.2.5.).
- All HCWs providing home care and service should receive training for conducting PCRAs, respiratory and hand hygiene, selecting and wearing PPE, and the use of Pandemic Influenza Precautions. (See Section VI .4.).
- HCWs should have access to ABHR, and sufficient and appropriate PPE for the care of clients with ILI symptoms.
- Volunteers should also have access to PPE for unexpected contact with clients with ILI symptoms (e.g., when delivering meals).
- All HCWs working for home care organizations wearing respirators for respiratory protection (e.g. performing aerosol-generating procedures) require fit-testing as per jurisdictional (federal/provincial/territorial/local) directives, (See Section V.6.2.3.).
- HCWs should perform a PCRA before entering the home of any patient (see Appendix D).
- ABHR, masks, respirators, gloves, gowns, and eye or face protection should be provided to HCWs as per Section V.6.2.6.
- HCWs: Fitness-for-Work (see Section V.6.2.2.)
- During the pandemic wave, HCWs should perform and interpret a daily influenza self assessment to determine their influenza status and thus their ability to work. Please refer to the Influenza Self Assessment Tool in Appendix A.
- HCWs who develop ILI symptoms while on duty should report the occurrence and be relieved of their duties.
- Care and services for patients for whom hospitalization (i.e., for influenza or other medical conditions) may be prevented should be the priority (e.g., where possible prevent patients with other medical condition from deteriorating and requiring acute care hospitalization).
- Telephone screening of patients and families should be undertaken prior to arriving at the home. If telephone screening is not possible, upon arrival at the home, the patient and family should be screened for ILI symptoms (see Appendix A) while maintaining a two metre distance from patient and family. If patients/clients/family members with ILI symptoms are identified:
- Cancel/postpone home visit appointments if medically appropriate until the period of communicability (see Section V.1.a.) has passed.
- Direct clients who need medical assessment for ILI symptoms to their primary care providers or local Influenza Assessment Centre, if available.
- Implement respiratory hygiene as required (i.e., have the client/patient wear a mask or cover their nose and mouth when coughing or sneezing).
- Maintain a separation of two metres when not wearing PPE between a person with ILI symptoms and susceptible HCWs.
- Utilize Pandemic Influenza Precautions when within two metres of a client/patient with ILI symptoms (see Section V.6.2.6.).
- Family members with ILI should ask to remain in their own rooms if possible.
- Clients without ILI symptoms should be given information on the prevention of influenza to reduce the possibilities of influenza exposure. Clients should:
- Clean their hands frequently with soap and water or wash with ABHRs when hands are not visibly soiled.
- Ensure household surfaces (e.g., counter tops, frequently touched surfaces) are cleaned as needed with regular household cleaning products.
- Restrict visitors that have ILI symptoms (i.e., for up to one period of communicability after onset of symptoms) from entering the client’s room.
- If the patient is terminally ill, the patient and family may decide whether or not to allow visitors with ILI symptoms.
- Ensure all visitors practice hand and respiratory hygiene.
- Avoid crowds and waiting areas where people with ILI symptoms are gathered.
- Request that visitors and family members entering the home and when within two metres of the client perform an influenza self assessment for ILI. Use Influenza Self Assessment Tool (for household members). See Appendix A.
- Maintain a two metre separation between the client (i.e., susceptible host) and individuals with ILI symptoms (i.e., infected sources), whenever possible.
- Open windows in the home when feasible, to provide natural ventilationFootnote 9.
Appendix A - Influenza Self Assessment Tool
This tool is to be used for assessment of symptoms of influenza as follows:
- For HCWs: For self-assessment prior to arriving in the workplace
- For patients: Early identification of patients with emerging ILI symptoms.
- For visitors and families: To assess ILI symptoms.
This tool is not intended to be used as a clinical management tool (see CPIP Annex G The Canadian Pandemic Influenza Plan for the Health Sector - Annex G) for surveillance or for influenza cohort assignment.
Do you have the following symptoms?
- New onset or worsening of existing cough;AND/OR
- Fever (> 38°C) Note: Fever may be absent in the elderly or newbornsPLUS
- Abrupt onset of any of the following:
- Headache
- Sore throat
- Joint pain
- Muscle pain
- Severe fatigue
The presence of a. and/or b., plus one of c. requires further assessment.
- Patients should be managed as per the organizational Pandemic Influenza IPC/OH plan.
- HCWs should be assessed by an Occupational Health Clinician or delegate for assessment and diagnosis of influenza.
- Visitors should not enter the healthcare setting until they are assessed by their family physician or at an Influenza Assessment Centre.
Appendix B – Recommended Steps for Putting on and Taking off PPE
Images used with permission from the Ontario Ministry of Health and Long Term CareFootnote 119
Images developed by Kevin Rostant. Some images adapted from Northwestern Ontario Infection Control Network — NWOICN
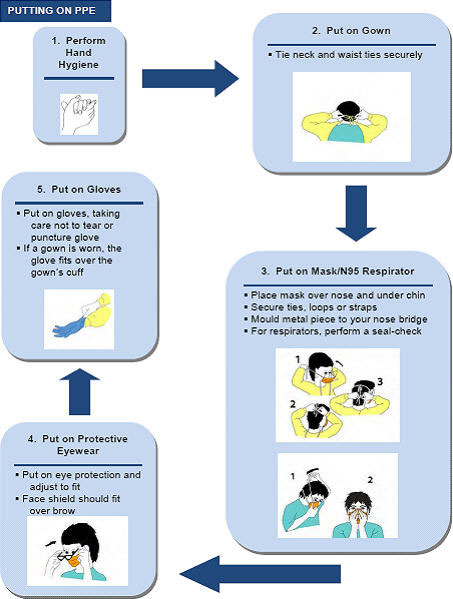
Putting on PPE Text Equivalent
Putting on PPE
- Wash hands/perform hand hygiene
- Put on gown
- Tie neck and waist ties securely.
- Put on mask or N95 respirator
- Place mask over nose and under chin.
- Secure ties, loops or straps.
- Mould metal piece to your nose bridge.
- For respirators, perform a seal-check.
- Put on protective eyewear
- Adjust eyewear to fit.
- Face shield should fit over brow.
- Put on Gloves
- Put on gloves, taking care not to tear or puncture the glove.
- If a gown is worn, the glove fits over the gown’s cuff.
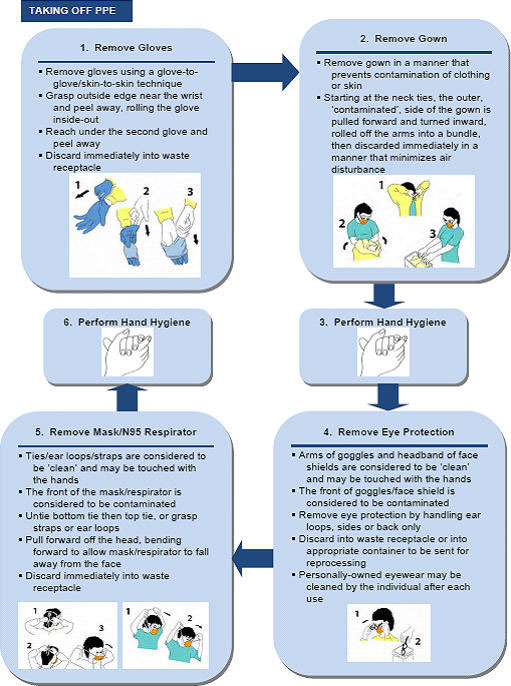
Taking off PPE Text Equivalent
Taking off PPE
- Remove gloves
- Use glove to glove, skin to skin technique.
- Grasp outside edge near the wrist and peel away, rolling the glove inside out.
- Reach under the second glove and peel away.
- Discard immediately into a waste receptacle.
- Remove gown
- Remove gown in a manner that prevents skin or clothes contamination.
- Starting at the neck ties, the outer contaminated side of the gown is pulled forward and turned inward, rolled off the arms into a bundle, then discarded immediately in a manner that minimizes air disturbance.Â
- Wash hands/perform hand hygiene
- Remove eye protection
- Arms of goggles and headbands of face shields are considered to be clean and may be touched with the hands.
- The front of goggles/face shield is considered to be contaminated.
- Remove eye protection by handling ear loops, sides or back only.
- Discard into waste receptacle or into appropriate container to be sent for reprocessing.
- Personally-owned eyewear may be cleaned by the individual after each use.
- Remove mask or N95 respirator
- Ties/ear loops/straps are considered to be clean and may be touched with the hands.Â
- The front of the mask/respirator is considered to be contaminated.Â
- Untie bottom tie then top tie or grasp the straps or ear loops.
- Pull forward off the head bending forward to allow mask/respirator to fall away from the face.
- Discard immediately into waste receptacle.Â
- Wash hands/perform hand hygiene
Additional optional opportunities for hand hygiene include:
- *between steps 1 and 2
- *between steps 4 and 5, and
- before leaving the care area
Appendix C - Check List - Organizational Risk Assessment for Pandemic Influenza
Purpose of the Check List - Organizational Risk Assessment for Pandemic Influenza:
The Organizational Risk Assessment check list is a tool for evaluating the availability and effectiveness of a healthcare organization's physical plant/infrastructure and adherence to engineering, administrative and personal protective equipment (PPE) controls to prevent the transmission of healthcare-associated infection related to respiratory viruses, including influenza. Performing the Organizational Risk Assessment during the inter-pandemic period, along with periodic reassessment, should enable healthcare settings to prepare, plan, and manage an influenza pandemic, so that the risk of healthcare workers (HCWs), patients, visitors, acquiring influenza in the healthcare organization, is minimized.
The check list is organized into four distinct parts:
- Evaluating the thoroughness and effectiveness of the organization's existing infection prevention and control (IPC) and occupational health (OH) programs;
- Planning for the management of pandemic influenza in existing and temporary healthcare settings;
- Planning for the identification and management of HCWs with symptoms compatible with the pandemic influenza virus;
- Planning for the education and skills training for HCWs on pandemic influenza.
| # | Item | Activity | Completed | In Progress | Not Started | Not Applicable | Comments |
|---|---|---|---|---|---|---|---|
| Evaluation of Existing Occupational Health Program (see Section VI.1.1.) | |||||||
| 1 | Immunization program | Develop and implement protocols for immunization of all HCWs including pre-placement screening and assessment. This includes assessment of tuberculosis status, offering appropriate immunizations to all HCWs without proof of immunity, including, housekeeping, laundry workers, and workers handling waste. Immunizations includes: hepatitis B; annual influenza (see item 2 below); tetanus booster; measles; mumps; acellular pertussis; pneumococcal; rubella (MMR) and varicella. (See Section VI.1.1.1.). | |||||
| 2 | Annual influenza prevention program | Develop and implement annual influenza prevention protocols to include HCWs education, annual immunization and strategies to monitor and improve immunization rates. (See Section VI.1.1.1.). | |||||
| 3 | Workplace hazard assessment | Develop and implement a process to evaluate the healthcare workplace to identify potential infectious hazards (and all other health and safety hazards) related to work activities with regular, ongoing inspections and evaluations, and implementation of correction measures. (See Section VI.1.1.2.) | |||||
| 4 | Respiratory protection program | Develop and implement a respiratory protection program for use with respirators (N95 or higher filtration), in healthcare organizations where personnel are required to wear respiratory protective equipment. This includes health screening, fit testing/re-testing, and training to all HCWs who may need to wear a respirator for the performance of AGMPs. (See Section V.6.2.4.). | |||||
| 5 | Surveillance program - HCWs with symptoms of acute respiratory infections | Establish a process for early identification, containment, investigation and reporting of HCWs with symptoms of acute infections. This includes: policies for HCWs and the self assessment and reporting of acute infection illness to OH; a process for recording HCWs absenteeism due to infection; procedures to manage ill HCWs so they do not expose others in the workplace, and a process to assess whether clusters of ill personnel are associated with outbreaks/clusters of HAIs, including influenza, in patient populations. (See Section VI.3.). | |||||
| Evaluation of Existing Infection Prevention and Control Program (see Section VI.1.2.) | |||||||
| 6 | IPC professionals | Evaluate and implement appropriate measures to ensure an adequate number of trained Infection Control Practitioners for the size and complexity of the patient population. (See Section VI.1.2.). | |||||
| 7 | Surveillance program for Healthcare-associated infections (HAIs) | Develop and implement an HAI surveillance program to track trends and identify and manage outbreaks of respiratory virus infections. This includes a systematic process to assess whether clusters of ill HCWs with respiratory illness can be associated with outbreaks/clusters of HAIs, including influenza, in patient populations. (See Section VI.1.2.). | |||||
| 8 | Influenza screening program | Develop and implement methods of early recognition, containment, investigation and reporting of individuals (patients, visitors, etc.) in the healthcare setting with ILI symptoms. (See Section VI.1.2.). | |||||
| 9 | Supplies and PPE equipment | Identify measures to obtain and maintain adequate quantities of equipment/products/materials needed for IPC program to prevent exposure to and transmission of respiratory viruses. This includes determining requirements, timely purchases and storage. (See Section V.6.3.). | |||||
| 10 | Routine practices and additional precautions (RPAP) program | Develop and implement a RPAP program, with protocols, education and training, to prevent or minimize the transmission of an infectious respiratory agent from an infected source or contaminated environment to a susceptible host. (See Section V.2.). | |||||
| a) Hand Hygiene | Develop and implement hand hygiene protocols and an ongoing education and training program for HCWs, patients, visitors, etc. in all healthcare organizations. This includes establishing compliance audits and implementation of measures for improving hand hygiene compliance. (See Section V.6.2.6.1.). | ||||||
| b) Personal protective equipment (PPE) | Implement appropriate use of gowns, gloves, eye or facial protection, masks and respirators and establish HCWs PPE compliance audits, with measures for improving PPE compliance. (See Section VI.3.). | ||||||
| c) Infected source controls | Develop and implement a program, including protocols, education and training, to minimize face-to-face contact between infected sources, with ILI symptoms , and susceptible hosts. This includes respiratory hygiene, signage, dispensers for masks and tissues, supplies, use and maintenance of personal protective equipment (PPE), alcohol-based hand rub (ABHR), handling of waste and sharps, and patient immunization. (See Section VI.1.2.). | ||||||
| i) Respiratory hygiene | Develop and implement a respiratory hygiene protocol and an education and training program on source control measures for respiratory hygiene. This includes processes to minimize the generation of and exposure to infectious aerosols created during aerosol-generating medical procedures. (See Section VII.1.5.4.). | ||||||
| ii) Signage | Develop and place signage (in multiple languages) throughout the organization to educate and encourage hand hygiene and respiratory hygiene by all individuals within the healthcare organization. (See Section VII.1.4.). | ||||||
| iii) Dispensers for masks and tissues | Place dispensers for masks and tissues at entrances, point of care, in triage, assessment, waiting and patient-care areas, for easy use by patients, HCWs, visitors, etc. (see Section VI.1.2.). Develop instructional materials for the public for putting on and taking off PPE. | ||||||
| iv) Alcohol-based hand rub (ABHR) | Implement point of care ABHR in all patient-care areas including, triage, assessment, waiting and all other patient-care areas, with the installation of ABHR dispensers. Provide individual bottles of ABHR for use by HCWs where wall/bedside mounted containers are not appropriate. (See Section V.6.2.6.1.). | ||||||
| v) Waste containers | Place closed, hands-free waste containers for use in triage, assessment, waiting and patient-care areas. (See Section V.6.2.6.7.). | ||||||
| vi) Sharps containers | Provide adequate numbers and maintenance of point-of-use sharps containers for use in triage, assessment, and patient-care areas. (See Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care). | ||||||
| viii) Patient immunization | Develop processes for offering pneumococcal immunization to high-risk patients in Acute care and long-term care facilities. (See Section VI.1.2.). | ||||||
| d) Engineering and administrative controls | Ensure adequate human and financial resources to support the structural and system changes recommended for engineering and administrative controls, and the availability and use of PPE. This includes sinks for hand washing, spatial separation, heating, ventilation and air conditioning (HVAC) systems, housekeeping, laundry and waste management, cleaning, disinfection and sterilization. (See Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care). | ||||||
| i) Hand-wash sinks | Ensure appropriate number of, and conveniently located, dedicated sinks for hand washing, hand soap, hand lotion and single-use paper towels. (See Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care). | ||||||
| ii) Spatial separation and spacing | Implement a process to identify patients with acute respiratory infections and implement measure to ensure adherence to appropriate spatial separation requirements to decrease infectious disease exposure for patients and visitors in clinical and waiting areas. (See Section VI.2.2.). This includes,
|
||||||
| iii) HVAC systems | Develop a process/plan for the maintenance and operation of the HVAC system in accordance with available guidelines and regulations. (See Section VI.1.2.). | ||||||
| iv) Housekeeping, laundry, waste management | Develop and implement standards for housekeeping, laundry and waste management, in accordance with available guidelines and regulations. (See Section V.6.2.6.7.). | ||||||
| v) Cleaning, disinfection, sterilization | Develop and implement, along with regular monitoring, standards for cleaning, disinfection and sterilization, in accordance with available guidelines, standards and regulations. (See Section V.2.). | ||||||
| Item | Activity | Completed | In Progress | Not Started | Not Applicable | Comments | |
|---|---|---|---|---|---|---|---|
| 1 | IPC/OH Pandemic planning team | Establish a multi-disciplinary team to lead the development and operationalization of the pandemic influenza IPC/OH plan, with defined roles and responsibilities for the preparedness, response, and recovery planning (see Section VI.2.1.). This includes ensuring the integration of the pandemic influenza:
|
|||||
| 2 | Activities of the planning team | Ensure the requirements of the pandemic influenza IPC/OH plan includes how to review the influenza pandemic plan, the cohorting and transferring/transporting of patients, surveillance, visitation, education and training, immunization, use of antivirals, equipment/supplies, and waste management. (See Section VI.2.1.). | |||||
| a) How often to review the plan | Develop a process to review the influenza pandemic plan annually and update according to emerging knowledge, legislation, and regulations. (See Section VI.2.1.). | ||||||
| b) Patient accommodation | Develop strategies and structures to accommodate patients with influenza as per Section VI.2.2., to include; Use of single rooms for patients with any type of influenza is preferred (i.e., seasonal or pandemic strain), Cohort patients with ILI symptoms, when single rooms are not available, AGMPs, during the Inter-pandemic period - patients with seasonal influenza do not require isolation rooms for AGMPs, Pandemic period - patients with influenza, caused by the pandemic strain, may utilize airborne infection isolation rooms, however, ensure these rooms are prioritized for patients with known or suspected airborne infections, e.g., TB, measles, varicella, disseminated zoster. | ||||||
| c) Transferring/Transporting patients | Develop a strategy to identify how patients with ILI symptoms should be transferred/transported intra- and inter-facility, as per Section VI.2.3. | ||||||
| d) Surveillance and management of respiratory illness | Develop measures to identify and manage respiratory illness affecting HCWs including, plans to address self identified HCWs at high risk of complications related to influenza, including pregnant HCWs. These individuals should be offered counselling and assessment from an occupational health clinician (or if unavailable, their personal clinician in conjunction with infection prevention and control or public health personnel). These individuals should be provided with counselling and education, including information pertaining to the severe outcomes of influenza, reinforcement of protective measures such as PCRA, appropriate use of PPE, access to treatment and antiviral medication. See Section VI.3. and Part 3 below. (See Section V.3.). Develop measures to identify and manage respiratory illnesses related to pandemic influenza affecting HCWs. This includes,
|
||||||
| e) Visitation | Develop guidelines and policies to deal with patient visitation during the pandemic waves, as per Section VI.2.3. | ||||||
| f) Education and training | Develop and implement specific pandemic influenza education and skills training for HCWs, including how to do a Point of Care Risk Assessment. (See Check List Part 4, below). (See Section VI.4.). | ||||||
| g) Immunization | Develop protocols on the delivery of pandemic influenza vaccine (when it becomes available) according to the Canadian Pandemic Influenza Plan (see CPIP - Annex D The Canadian Pandemic Influenza Plan for the Health Sector - Annex D) and provincial/territorial/regional or local pandemic influenza vaccine distribution initiatives. | ||||||
| h) Antivirals | Develop protocols on the use and distribution of antiviral medications according to the Canadian Pandemic Influenza Plan (see CPIP-Annex E The Canadian Pandemic Influenza Plan for the Health Sector - Annex E) and provincial/territorial/regional or local pandemic influenza antiviral medication distribution initiatives. | ||||||
| i) Equipment and supplies | Develop strategies/measures for obtaining and maintaining adequate supplies (including stockpiling) of equipment/products/materials needed to prevent exposure to and transmission of pandemic influenza in the healthcare organization. This includes supplies of ABHR, soaps, single-use towels, masks, respirators, eye protection/face shields, gloves, gowns, sharps containers, etc. (See Section V.6.3.). | ||||||
| j) Waste management | Identify measures to handle the anticipated increase in waste generated due to the increased use of single use PPE. (See Section V.6.2.6.7.). | ||||||
| k) Signage | Prepare and place signage (in multiple languages) to educate and inform HCWs, patients and visitors with direction to assessment areas, and instructions regarding respiratory and hand hygiene. (See Section VI.2.1.). | ||||||
| l) Spatial separation | Develop strategies for ensuring appropriate spatial separation between patients with ILI symptoms, those infected with the pandemic influenza virus, and patients without influenza in clinical and waiting areas, in all existing and temporary healthcare settings. (See Section VI.2.2.). This includes predetermining,
|
||||||
| m) Temporary healthcare settings | Develop a plan for each existing and temporary healthcare setting. The parent organization that is responsible for a temporary setting is also responsible for the planning for that setting. (See Section VI.2.2.3.). Ensure the temporary settings' pandemic influenza IPC/OH plan as outlined below:
|
| Item | Activity | Completed | In Progress | Not Started | Not Applicable | Comments | |
|---|---|---|---|---|---|---|---|
| 1 | Self-assessments | Develop and implement an education program for HCWs on influenza self assessment (as per the Influenza Self Assessment Tool in Appendix A) to determine their influenza status and ability to work. Develop a process to monitor and evaluate the effectiveness and compliance of ongoing HCWs self assessments. | |||||
| 2 | Surveillance | Establish policies and procedures to identify, track, and manage organizational clusters of ill HCWs to include processes, case definitions, surveillance methodology, and outbreak response directives. | |||||
| 3 | Immunity to influenza | Develop and implement a process to identify HCWs who have immunity to influenza. Immunity to influenza may include,
|
|||||
| 4 | Staffing shortage | Develop processes to accommodate significant HCWs staffing shortages due to the acquisition of influenza while in the community. This includes criteria for,
|
|||||
| 5 | Antivirals | Develop protocols on the routine use of antivirals in accordance to Annex E, CPIP The Canadian Pandemic Influenza Plan for the Health Sector - Annex E. This includes:
|
| Item | Activity | Completed | In Progress | Not Started | Not Applicable | Comments | |
|---|---|---|---|---|---|---|---|
| 1 | Education and skills training program | Develop and implement an education and skills training program for infection prevention and control related to seasonal and pandemic influenza for all HCWs. This includes the timing, appropriateness, and delivery method. | |||||
| a) Timing | Education and training should be delivered on an annual basis to all HCWs on all shifts, in all departments, and be intensified when an influenza pandemic is imminent. | ||||||
| b) Appropriate to audience | Education and skills training on pandemic influenza should be appropriate to the audience. | ||||||
| c) Delivery method | A variety of education methods, (e.g., postings in elevators and at entrances, brochures, newsletters, websites, mini education sessions during shift change, use of role play, or other scenarios, etc.) should be used. | ||||||
| 2 | Education and skills training content | Develop and implement a comprehensive education and skills training program to include the following content,
|
Appendix D - Point of Care Risk Assessment Tool for Pandemic Influenza
Prior to any patient interaction, all HCWs have a responsibility to always assess the infectious risk posed to themselves and to other patients, visitors, and HCWs by a patient, situation or procedure. This risk assessment is based on professional judgement about the clinical situation and up-to-date information on how the specific healthcare organization has designed and implemented engineering and administrative controls, along with the availability and use of PPE.
It is expected that the healthcare organization has taken the following measures:
- The Organizational Risk Assessment has been done (as per Section VI. and Appendix C), and engineering and administrative controls are in place for the healthcare setting.
- Infection Prevention and Control and Occupational Health programs are in place for the healthcare setting.
The Point of Care Risk Assessment (PCRA) is an activity performed by HCWs before every patient interaction, in any existing or temporary healthcare setting to:
-
Evaluate the likelihood of exposure to the pandemic influenza virus;
- For a specific interaction (e.g., performing, assisting with, aerosol-generating medical procedures, other clinical procedure/interaction, non-clinical interaction (i.e., admitting, teaching patient/family), transferring/transporting patients, direct face-to-face interaction with patients, etc.).
- With a specific patient (e.g., infants/young children, patients not capable of self care/ hand hygiene, have poor-compliance with respiratory hygiene, copious respiratory secretions, frequent cough/sneeze, early stage of influenza illness, etc.).
- In a specific environment (e.g., single rooms, shared rooms/washrooms, hallway, influenza assessment areas, emergency departments, public areas, therapeutic departments, diagnostic imaging departments, housekeeping, etc.).
- Under available conditions (e.g., air exchanges in a large waiting area or in an airborne infection isolation room, patient waiting areas).
AND
-
Choose the appropriate actions/PPE needed to minimize the risk of patients, HCWs and visitors from exposure to the infectious agent/infected source.
The PCRA is not a new concept, but one that is currently performed regularly by HCWs many times a day for their safety and the safety of patients and others in the healthcare setting. For example, when HCWs evaluate a patient and situation to determine the possibility of blood or body fluid exposure or choose appropriate PPE to care for a patient with an infectious disease, these actions are both included in a PCRA.
It is the responsibility of the healthcare organization to provide PCRA training and education to ensure that all HCWs have the knowledge, skills and resources recommended to routinely perform PCRAs for pandemic influenza risk before every interaction with a potentially infectious agent/ infected source during the pandemic period.
The classification and assessment of risk are based on current knowledge, Public Health assumptions, and the assumptions regarding the transmission of influenza (as per Annex F, Section V). When other factors (e.g., provincial/territorial labour regulations, clinical presentations suggestive of other infectious agents, etc.) not stated in this PCRA tool warrant a higher level of personal protective equipment, the application of higher level of protection should be undertaken. The examples of situations and patient presentations contained in this Appendix are not meant to be exhaustive, nor comprehensive.
The PCRA tool consists of tables 1 to 4. A step-by-step description on how to use them follows:
- Step 1: In Table 1, choose one of the physical setting and the level of patient interaction (in the highlighted column) using the description and example columns in the table.
- Step 2: In Table 2, choose one of the patient clinical status and source control capability (in the highlighted column) using the description and patient presentation column in the table.
- Step 3: Using the matrix on Table 3, match the physical setting and level of patient interaction from Table 1 (Step 1) with the patient clinical status and source control capability identified from Table 2 (Step 2), to determine the appropriate level of precautions.
- Step 4: From Table 4, determine what specific measures and personal protective equipment are indicated for the level of precautions identified in Table 3 (Step 3).
The PCRA tool is to complement, and be used in conjunction with, the content and recommendations stated in the Annex F of the Canadian Pandemic Influenza Plan for the Health Sector, and does not replace existing Infection Prevention and Control and Occupational Health programs of healthcare settings.
| Physical Setting and Level of Patient Interaction | Description | Example |
|---|---|---|
| No Patient Interaction, Non-Clinical | Area with no patient access (restricted areas). | Non-clinical setting (medical record department, administrative office, central pharmacy, information technology office, central storage area, mail room, central maintenance areas, business office, etc.). |
| No Direct Patient Interaction and No Indirect Contact | No face-to-face interaction and no indirect contact with patients. | Hallways, cafeteria, public areas, clinical areas with no patient access (charting room, office, storage room, staff lounge, medication room, etc.), totally enclosed reception/triage areas. |
| Indirect Contact | No direct patient interactions; indirect contact only. Contact with patient environment or contaminated inanimate objects. | Discharge patient room cleaning, equipment cleaning. |
| Direct Patient Interaction | Direct, face to face interaction with patient (within 2m from patient). | Providing patient care, home care visit, assisting with Activity of Daily Living (ADL), diagnostic imaging, phlebotomy services, physiotherapy, occupational therapy, recreational therapy, intra-hospital transport/portering, non-enclosed triage/registration area, cleaning patient bed space while occupied, routine ambulance or inter-facility transport. |
| Direct Patient Interaction with Potential for Aerosol Generation | Performing and/or assisting with Aerosol-Generating Medical Procedures (AGMPs) | Open endotracheal suctioning, bronchoscopy, endotracheal intubation, tracheostomy procedures, nebulized therapy, cardio-pulmonary resuscitation. |
| Patient Clinical Status and Source Control Capability | Description | Patient Presentation |
|---|---|---|
| Recovered from Influenza | Patient recovered from influenza | Influenza infected patient, beyond the known period of communicability |
| Influenza | 1) Patient with ILI symptoms with cough | Cough of any intensity and Adherence with respiratory hygiene Adherence to hand hygiene |
| 2) Patient with ILI symptoms with weak or no cough | Weak or no cough and Not adherent with respiratory hygiene Not adherent to hand hygiene | |
| Influenza and Forceful Cough and Not Compliant | Patient with ILI symptoms | Forceful cough and Not adherent with respiratory hygiene Not adherent to hand hygiene |
| Influenza and AGMP | Patient with ILI symptoms | And an Aerosol-Generating Medical Procedure (AGMP) is being performed |
Note: If more than one risk level identified (e.g., multiple concurrent patient interactions), select the higher risk level.
| Physical Setting and Level of Patient Interaction | |||||
|---|---|---|---|---|---|
| Patient Clinical Status and Source Control Capability | No Patient Interaction Non clinical | No Direct or Indirect Patient Interaction | Indirect Contact | Direct Patient Interaction | Direct Patient Interaction with AGMP |
| Recovered from Influenza | I | I | II | II | II |
| Influenza Compliant | I | I | II | III | IV |
| Influenza and Forceful Cough and Not Compliant | I | I | II | IV | IV |
| Influenza and AGMP | I | I | II | IV | IV |
Note: It is anticipated that the majority of patients with pandemic influenza will be cared for using level II and III and a minority would be cared for using level IV precautions.
| Hand hygiene | Respiratory hygiene | Respirator | Mask | Eye Protection | Gown | Gloves | |
|---|---|---|---|---|---|---|---|
| Level I | Yes | Yes | No Patient Contact – Not Recommended | ||||
| Level II | Yes | Yes | No, Except as per Additional PrecautionsTable 4 - Footnote * | As Per Routine Practices and Contact Precautions | |||
| Level III | Yes | Yes | No, Except as per Additional PrecautionsTable 4 - Footnote * | Yes | Yes | As Per Routine Practices and Contact Precautions | |
| Level IV | Yes | Yes | Yes | No | Yes | As Per Routine Practices and Contact Precautions | |
|
|||||||