Recommendation on Repeated Seasonal Influenza Vaccination
Download in PDF format
(1.37 MB, 41 pages)
Organization: Public Health Agency of Canada
Published: 2023-02-21
An Advisory Committee Statement (ACS)
National Advisory Committee on Immunization (NACI)
Preamble
The National Advisory Committee on Immunization (NACI) is an External Advisory Body that provides the Public Health Agency of Canada (PHAC) with independent, ongoing and timely medical, scientific, and public health advice in response to questions from PHAC relating to immunization.
In addition to burden of disease and vaccine characteristics, PHAC has expanded the mandate of NACI to include the systematic consideration of programmatic factors in developing evidence based recommendations to facilitate timely decision-making for publicly funded vaccine programs at provincial and territorial levels.
The additional factors to be systematically considered by NACI include: economics, ethics, equity, feasibility, and acceptability. Not all NACI Statements will require in-depth analyses of all programmatic factors. While systematic consideration of programmatic factors will be conducted using evidence-informed tools to identify distinct issues that could impact decision-making for recommendation development, only distinct issues identified as being specific to the vaccine or vaccine-preventable disease will be included.
This statement contains NACI's independent advice and recommendations, which are based upon the best current available scientific knowledge. This document is being disseminated for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph. Recommendations for use and other information set out herein may differ from that set out in the product monographs of the Canadian manufacturers of the vaccines. Manufacturer(s) have sought approval of the vaccines and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of PHAC's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Table of contents
- Summary of the information contained in this NACI statement
- I. Introduction
- II. Methods
- III. Results
- III.1 Study characteristics
- III.2 Evidence for vaccine efficacy and effectiveness of repeated vaccination compared to vaccination in current season only
- III.3 Evidence for vaccine effectiveness of repeated vaccination compared to vaccination in prior season only
- III.4 Evidence for vaccine effectiveness of repeated vaccination compared to no vaccination
- IV. Discussion
- V. Recommendations
- VI. Research priorities
- Additional tables
- List of abbreviations
- Acknowledgements
- Appendix A: Search strategies and results
- Appendix B: Flow diagram
- References
Summary of the information contained in this NACI statement
The following highlights key information for immunization providers. Please refer to the remainder of the statement for details.
1. What
Influenza is a respiratory illness caused primarily by influenza A and B viruses. The burden of influenza varies from year to year. Prior to the COVID-19 pandemic, influenza was responsible for an estimated 12,200 hospitalizations and 3,500 deaths annually in Canada. Influenza vaccination is repeated annually due to waning immunity and the tendency of influenza viruses to frequently mutate, requiring changes in the vaccine formulation.
Some studies from different influenza seasons have suggested that receiving the seasonal influenza vaccine in one or more previous seasons may reduce the effectiveness of the vaccine against strains circulating in the current season, while other studies have not.
2. Who
This Statement applies to all individuals 6 months of age and older who are not contraindicated to receive the influenza vaccine.
3. How
The seasonal influenza vaccine should be offered to all individuals 6 months of age and older on an annual basis, regardless of whether they received a seasonal influenza vaccine in prior seasons.
4. Why
Annual influenza vaccination reduces the morbidity and mortality associated with influenza infection. Overall, the evidence shows no difference in the effectiveness of repeated influenza vaccination compared to vaccination in the current season only. Of all the seasons investigated across many studies, only during two influenza seasons was repeated vaccination across seasons associated with a reduced effectiveness against influenza A(H3N2), compared to vaccination in the current season only. Further evaluation of the effects of repeated influenza vaccination on vaccine effectiveness (VE) is needed as there is currently no predictable association that could inform vaccine program decisions from year to year. Also, repeated vaccination including the current season is consistently more effective than no vaccination in the current season.
I. Introduction
Influenza is a respiratory illness caused primarily by influenza A and B viruses. Prior to the COVID-19 pandemic, influenza was estimated to cause approximately 12,200 hospitalizationsFootnote 1 and 3,500 deathsFootnote 2 annually in Canada. Although the epidemiology of influenza has changed during the course of the COVID-19 pandemic, seasonal influenza presents an ongoing disease burden in Canada during the fall and winter months, which varies from year to year. To reduce the morbidity and mortality associated with influenza, the National Advisory Committee on Immunization (NACI) recommends annual influenza vaccination for everyone 6 months of age and older who does not have contraindications to the vaccineFootnote 3. Influenza vaccination must be repeated annually due to waning of vaccine and infection-induced immunity against influenza over time and because influenza viruses frequently undergo antigenic drift. As a result, the World Health Organization (WHO) convenes twice a year to assess the currently circulating influenza strains and to recommend which strains should be used in the influenza vaccine for the upcoming Northern and Southern Hemisphere influenza seasonsFootnote 4.
However, there is a growing body of evidence that explores the potential negative effects of repeated seasonal influenza vaccination on current season VE. This issue was first studied in the 1970sFootnote 5, and since then several studies have indicated a potential negative impact of prior influenza vaccination on current season influenza VE5 Footnote 6Footnote 7 Footnote 8Footnote 9 Footnote 10. The most prominent theory explaining this phenomenon is the antigenic distance hypothesisFootnote 7Footnote 11. This hypothesis theorizes that influenza vaccination in the prior season may negatively interfere with the VE in the current season if the antigenic distance (difference) between the prior and current season's vaccine strain is small, but the antigenic distance between the prior season's vaccine strain and the current season's circulating strain is largeFootnote 7. Furthermore, additional observations and theories suggest that immune "imprinting" for influenza responses can be linked to birth cohort and influenced by early exposures that happened in previous seasons, notably the first influenza virus exposure of lifeFootnote 12Footnote 13. It is not yet well understood how repeated vaccination may impact influenza vaccine immune response. The current overview does not aim to address theories of how differences in VE due to repeated influenza vaccination may occur, but rather to determine the overall impact of this phenomenon and to provide an evidence base for population-level and individual-level vaccination decisions regarding annual influenza vaccination.
The primary objective of this overview of reviews is:
- To summarize the evidence from systematic reviews on the effects of repeated seasonal influenza vaccination on VE, vaccine efficacy and immunogenicity
II. Methods
II.1 Research question
What are the effects of repeated seasonal influenza vaccination on VE, efficacy, and immunogenicity?
P (population): Adults and children
I (intervention): Seasonal influenza vaccination in prior season(s) and current season
C (comparison): Seasonal influenza vaccination in prior season(s) only OR in current season only OR unvaccinated in any season included in the study
O (outcome): VE, vaccine efficacy, or immunogenicity in the current season
S (study design): Systematic review and meta-analysis
An a priori search strategy was developed in collaboration with a federal reference librarian of the Health Library of Health Canada and PHAC that included search terms for "influenza", "repeated vaccination", "systematic review", and "meta-analysis". The complete search strategy can be found in Appendix A. The search was limited to studies published in the English or French language and to a publication date of 2016 to June 2019. NACI was already aware of two systematic reviews that were published in 2017Footnote 14Footnote 15; therefore, the search was restricted to systematic reviews (SRs) and meta-analyses (MAs) published in 2016 or later to ensure that any additional recent and relevant SRs/MAs were captured. No limitation was placed on the types of primary study designs included in the SR/MA.
Inclusion criteria:
- The study is a SR/MA;
- The study assesses the effects of repeated influenza vaccination on VE, efficacy or immunogenicity.
Exclusion criteria:
- The study only presents primary research;
- The study is in language other than English or French;
- The study only includes non-human studies;
- The date of publication of the study is prior to 2016.
Abstracts and titles of records retrieved by the database search were loaded into DistillerSR (Evidence Partners, Ottawa, Canada) for screening. If the abstract and title met the inclusion criteria, or if it was not possible to determine eligibility based on the abstract and title alone, the full text was assessed for eligibility. Two reviewers independently screened titles, abstracts, and full texts for eligibility. Full texts that met all inclusion criteria were further assessed for the relevance of the SR/MA's PICO, as compared to the PICO formulated a priori by the NACI Influenza Working Group (outlined above) and for quality. SRs/MAs that were not considered sufficiently relevant for NACI's purposes or were not of sufficient quality were excluded from synthesis. This approach to the inclusion of systematic reviews into public health guidance was based on the methodology proposed within the Project on a Framework for Rating Evidence in Public Health (PRECEPT)Footnote 16 and was initially developed by the United States Agency for Healthcare Research and Quality (AHRQ)Footnote 17. The quality of the SRs/MAs were assessed using AMSTAR 2Footnote 18, which is a tool specifically designed to examine SR/MA quality. SRs/MAs for which reviewers had many serious concerns across AMSTAR 2 domains would be excluded.
Data from included SRs/MAs were extracted using a template with variables defined a priori. Extracted pooled effect estimates from SRs/MAs were assumed to represent pooled unadjusted estimates, unless otherwise specified. Quality assessment and data extraction were completed independently by two reviewers. Any disagreements during eligibility assessment, quality assessment, or data extraction were discussed until a consensus was reached. Results of subgroup analyses that included only one study were not extracted. Evidence was synthesized narratively, and estimates from all included SRs/MAs were discussed, regardless of primary study overlap.
III. Results
III.1 Study characteristics
Through a comprehensive literature search performed on October 27, 2017 and updated on June 3, 2019, five SRs/MAs were identified as eligible for inclusion in the evidence synthesis; two through MedlineFootnote 19Footnote 20, one through PROSPEROFootnote 21, and two that had previously been identified by expertsFootnote 14Footnote 15. All five of the identified SRs/MAs sufficiently aligned with this overview's PICO (Table 1). No new or ongoing SRs/MAs eligible for inclusion were identified through additional PROSPERO search updates conducted through March 2022. A complete PRISMA flow diagram can be found in Appendix B, and a full list of excluded studies and reason for exclusion is available upon request. None of the SRs/MAs included primary studies that assessed immunogenicity. Additional inclusion and exclusion criteria outlined for each SR/MA that were not specified by this overview's PICO are detailed in Table 2.
| PICO | Criteria | Ramsay et al. 2019Footnote 15 | Bartoszko et al. 2018Footnote 21 | Morimoto et al. 2018Footnote 20 | Belongia et al. 2017Footnote 14 | Caspard et al. 2016Footnote 19 |
|---|---|---|---|---|---|---|
| Population | All ages included | Yes | Yes | Yes | Yes | Partial (studies on adults 18 years of age and older excluded) |
| Intervention/ Comparison | Seasonal influenza vaccination in the prior influenza season and in the current season | Yes | Yes | Yes | Yes | Yes |
| Seasonal influenza vaccination in the prior influenza season only | Yes | Yes | Yes | Yes | Yes | |
| Seasonal influenza vaccination in the current influenza season only | Yes | Yes | Yes | Yes | Yes | |
| Unvaccinated with influenza vaccination in both the prior influenza season and in the current season | Yes | Yes | No | Yes | Yes | |
| Any seasonal influenza vaccine used for vaccination | Yes | Yes | Yes | Yes | No (only included studies on live attenuated influenza vaccine) | |
| Outcomes | Studies investigating vaccine effectiveness or efficacy | Yes | Yes | Yes | Yes | Yes |
| Studies investigating immunogenicity | No | No | No | No | No | |
|
||||||
Results from the AMSTAR 2 quality assessment are presented in Table 3. For this review, none of the domains within AMSTAR 2 were highlighted as "critical". The SRs/MAs conducted by Bartoszko et al., Morimoto et al., and Ramsay et al. were similar in quality and had minor differences across domains. Importantly, The SR/MA conducted by Belongia et al. was judged to be of lower quality primarily due to the lack of a documented risk of bias (RoB) appraisal of included studies. None of the SRs/MAs included a list of excluded studies or reported the funding sources for included primary studies. In addition, none of the SRs/MAs provided a full investigation of heterogeneity within the results; however, most studies discussed important, non-measured factors that would impact VE in the discussion (e.g., history of natural infection). Two of the reviews searched the grey literature (i.e., trial registries)Footnote 14Footnote 21, three assessed the quality of the included studiesFootnote 15Footnote 20Footnote 21, and two assessed the likelihood of publication biasFootnote 20Footnote 21. The SR/MA conducted by Caspard et al. had a large number of serious concerns across almost all AMSTAR 2 domains. Of particular note, no evidence for a priori design was provided, study selection and data extraction were not performed in duplicate, no quality assessment was specified, and heterogeneity was not assessed. In addition, a fixed effects model was used to estimate the efficacy of the influenza vaccines, which, given the expected differences in estimates across seasons, would not be appropriate; a random effects model would be preferred and was used in all other included SRs/MAs. Due to the limitations of the Caspard et al. SR/MA regarding these AMSTAR 2 domains, this SR/MA was excluded from evidence synthesis.
| PICO(T) | Criteria | Ramsay et al. 2019Footnote 15 | Bartoszko et al. 2018Footnote 21 | Morimoto et al. 2018Footnote 20 | Belongia et al. 2017Footnote 14 | Caspard et al. 2016Footnote 19 |
|---|---|---|---|---|---|---|
| Intervention/ Comparison |
Also included studies with vaccination in 2 or more prior influenza seasons | Included (Excluded from meta-analysis) |
Included | Included | Excluded | Unknown (Not excluded) |
| Vaccination with a monovalent pandemic influenza vaccine | Unknown (Not excluded) |
Not explicitly excluded | Excluded | Excluded | Unknown (Not excluded) |
|
| Outcomes | Influenza infection defined as medically-attended and laboratory confirmed by reverse transcriptase-polymerase chain reaction (RT-PCR) | Included | Included | Included | Included | Unknown (Not excluded) |
| Influenza infection defined as medically-attended and laboratory confirmed by any method | Unknown (Not included) |
Included | Included | Unknown (Not included) |
Unclear (Method of laboratory confirmation not stated) | |
| Study design | RCT | Unknown (Not included) |
Included | Included | Included | Included |
| Observational studies | Included | Included | Unknown (Not included) |
Partially included (includes only test-negative case-controls, case-controls, and cohort, others not included) | Excluded | |
| Cost-effectiveness studies, review articles | Unknown (Not included) |
Unknown (Not included) |
Unknown (Not included) |
Excluded | Excluded | |
| Conference abstract or proceeding | Excluded | Unknown (Not excluded) |
Unknown (Not included) |
Unknown (Not excluded) |
Unknown (Not excluded) |
|
| Article is an interim VE report that was superseded by an end-of-season report | Excluded | Unknown (Not excluded) |
Unknown (Not included) |
Unknown (Not excluded) |
Unknown (Not excluded) |
|
| Study did not apply standard symptom criteria for enrollment | Unknown (Not excluded) |
Unknown (Not excluded) |
Unknown (Not excluded) |
Excluded | Unknown (Not excluded) |
|
| Study used a convenience sample of clinical diagnostic tests as opposed to predefined screening criteria | Unknown (Not excluded) |
Unknown (Not excluded) |
Unknown (Not excluded) |
Excluded | Unknown (Not excluded) |
|
| Timing | Study reported current season VE for pre-2009 seasonal influenza | Unknown (Not excluded) |
Unknown (Not excluded) |
Unknown (Not excluded) |
Excluded | Unknown (Not included) |
|
||||||
The two studies that assessed the quality of included observational studies found that the RoB for included observational studies was low according to the Newcastle-Ottawa ScaleFootnote 15Footnote 21. The evidence for laboratory confirmed influenza (LCI) infection from randomized controlled trials (RCTs) included by Bartoszko et al. was determined by the authors to have a serious RoB, according to Cochrane's RoB tool for RCTs, due to improper allocation concealment, loss to follow-up and private or unclear fundingFootnote 21. Of the RCT studies included by Morimoto et al., the authors considered three to have a high RoB, two to have a low RoB, and three to have an unclear RoBFootnote 20. Belongia et al. did not perform a quality assessment of their included studies; however, the quality of all their included studies was examined in at least one other SR/MAFootnote 14 (see Figure 1).
All four SRs/MAs that were included contained a systematic review and a meta-analysis of the effects of repeated influenza vaccination on vaccine efficacy or effectiveness, and analyzed findings from a total of 24 unique primary studies. There was substantial overlap in the primary studies included in the SRs/MAs, with findings from 24 of 48 primary studies (50%) assessed in more than one SR/MA. Details on primary study overlap among the included SRs/MAs can be seen in Figure 1.
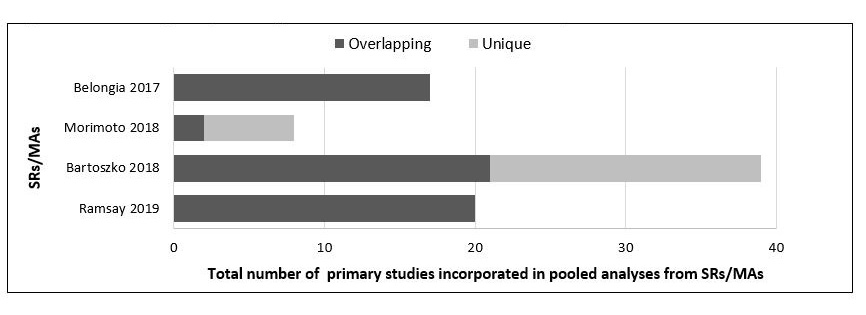
Figure 1: Text description
Figure 1 shows a horizontal stacked bar graph providing details on the degree of primary study overlap among the included systematic reviews and meta-analyses. The following information is depicted:
| Ramsay 2019 | Bartoszko 2018 | Morimoto 2018 | Belongia 2017 | |
|---|---|---|---|---|
| Overlapping | 20 | 21 | 2 | 17 |
| Unique | 0 | 18 | 6 | 0 |
| Total included in MA | 20 | 39 | 8 | 17 |
| AMSTAR 2 criteria | Ramsay et al. 2019Footnote 15 | Bartoszko et al. 2018Footnote 21 | Morimoto et al. 2018Footnote 20 | Belongia et al. 2017Footnote 14 | Caspard et al. 2016Footnote 19 |
|---|---|---|---|---|---|
| 1. Did the research questions and inclusion criteria for the review include the components of PICO? | Yes | Yes | Yes | Yes | Yes |
| 2. Did the review rerort contain an explicit statement that the review methods were established prior to its conduct and did the report justify any significant deviations from the protocol? | Yes | Yes | No | No | No |
| 3. Did the review authors explain their selection of the study designs for inclusion in the review? | No | No | Yes | No | No |
| 4. Did the review authors use a comprehensive literature search strategy? | Partial Yes | Partial Yes | No | No | No |
| 5. Did the review authors perform study selection in duplicate? | Yes | Yes | Yes | No | No |
| 6. Did the review authors perform data extraction in duplicate? | Yes | Yes | Yes | Yes | No |
| 7. Did the review authors provide a list of excluded studies and justify the exclusions? | No | No | No | No | No |
| 8. Did the review authors describe the included studies in adequate detail? | Yes | Yes | Yes | Yes | Yes |
| 9. Did the review authors use a satisfactory technique for assessing the RoB in individual studies that were included in the review? | Yes | Yes | Yes | No | No |
| 10.Did the review authors report on the funding sources for the studies included in the review? | No | No | No | No | No |
| 11. If meta-analysis was performed, did the review authors use appropriate methods for statistical combination of results? | Yes | Yes | Yes | Yes | No |
| 12. If meta-analysis was performed, did the review authors assess the potential impact of RoB in individual studies on the results of the meta-analysis or other evidence synthesis? | YesTable 3 Footnote a | No | Yes | No | No |
| 13. Did the review authors account for RoB in individual studies when interpreting/discussing the review results? | Yes | Yes | No | No | No |
| 14. Did the review authors provide a satisfactory explanation for, and discussion of, any heterogeneity observed in the review results? | No | No | No | No | No |
| 15. If they performed quantitative synthesis, did the review authors carry out an adequate investigation of publication bias (small study bias) and discuss its likely impact on the review results? | No | Yes | Yes | No | No |
| 16. Did the review authors report any potential sources of conflict of interest, including any funding they received for conducting the review? | Yes | Yes | Yes | Yes | Yes |
| Total (out of 16) | 10.5 | 10.5 | 10 | 5 | 3 |
|
|||||
Two of the SRs/MAs included primary studies with RCT and observational designsFootnote 14Footnote 21, one included only RCTsFootnote 20, and one included only observational studiesFootnote 15. A test-negative case-control design was the most common type of observational study design of the included primary studies. The SR/MA conducted by Bartoszko et al. had the least restrictive study selection criteria and included the largest number of studies. Two SRs/MAs included only primary studies that confirmed influenza infection by RT-PCRFootnote 14Footnote 15. Bartoszko et al. included studies which confirmed influenza infection by RT-PCR or viral culture as the primary outcome, and by any laboratory method as a secondary outcome. A sensitivity analysis performed by the authors indicated that the inclusion of studies that did not confirm influenza infection by RT-PCR or viral culture did not significantly alter the effect estimates; therefore, the authors chose to include these studies in their final meta-analysis. Morimoto et al. also included studies that defined LCI as confirmed by RT-PCR and serology and/or culture; however, no sensitivity analysis for method of laboratory confirmation was performed.
All four SRs/MAsFootnote 14Footnote 15Footnote 20Footnote 21 reported pooled effect estimates for vaccine efficacy or effectiveness of repeated influenza vaccination using a random effects model; however, each used a different method to combine primary study data. Belongia et al. calculated separately the pooled, unadjusted VE of vaccination in two consecutive seasons (i.e., the current and prior season), vaccination in the current season only, and vaccination in the prior season only, with no vaccination in both the current and prior seasons as a referent. Ramsay et al. pooled the differences in adjusted VE estimates for the different scenarios to control for within-study confounding. Bartoszko et al. calculated the unadjusted odds ratios (ORs) of medically-attended, LCI, comparing individuals with vaccination in two consecutive seasons to individuals with vaccination in the current season only. Morimoto et al. calculated the relative risk (RR) for medically-attended, LCI in individuals with vaccination in two consecutive seasons compared to individuals who were vaccinated in the current season only.
III.2 Evidence for vaccine efficacy and effectiveness of repeated vaccination compared to vaccination in current season only
In general, influenza vaccination in two consecutive seasons did not have a negative or positive effect on VE in comparison to vaccination in the current season only; however, there were two circumstances in which a potential negative effect was demonstrated. One SR/MA demonstrated a pooled negative effect of vaccination in two consecutive seasons for VE against influenza A(H3N2) in the 2010–2011 influenza seasonFootnote 21 and another SR/MA found a pooled negative effect for VE against influenza A(H3N2) in the 2014–2015 influenza seasonFootnote 15.
In addition, the odds of having medically-attended LCI were statistically significantly higher when the seasonal influenza vaccine was administered over multiple (three or more) consecutive seasonsFootnote 21, compared to the current season only; however, data on this exposure were limited (refer to section III.2.3 for further information).
III.2.1 Vaccine effectiveness by influenza type and subtype
Three SRs/MAs reported pooled VE stratified by influenza type and subtype comparing participants vaccinated in the prior and current season with participants vaccinated in the current season onlyFootnote 14Footnote 15Footnote 21.
Influenza A(H1N1): All three SRs/MAs assessed the effect of repeated vaccination on VE against influenza A(H1N1). Belongia et al. excluded studies which reported current season VE for pre-2009 seasonal influenza; therefore, the estimates represent the VE against influenza A(H1N1)pdm09 specifically, whereas Ramsay et al. and Bartoszko et al. pooled estimates for VE against influenza A(H1N1) during any season. The meta-analyses conducted by Belongia et al. and Ramsay et al. assessed the effect of receiving seasonal influenza vaccine in the current and prior seasons, whereas the pooled estimates reported by Bartoszko et al. included estimates from studies which assessed the effect of receiving seasonal influenza vaccine in the current seasons and seasonal or monovalent pandemic vaccine in the prior season. None of the SRs/MAs showed differences in VE for those vaccinated in two consecutive seasons and those vaccinated in the current season only for influenza A(H1N1).
Bartoszko et al.Footnote 21 found that the unadjusted odds of medically-attended, LCI A(H1N1) were statistically similar among participants vaccinated in two consecutive seasons and among participants vaccinated in the current season only. This result was consistent when the OR was calculated using estimates from RCTs [OR: 0.86, 95% confidence interval (CI): 0.38 to 1.96%, I2: 0%] and from observational studies [OR: 0.87, 95% CI: 0.67 to 1.12%, I2: 46%]. Ramsay et al.Footnote 15 found no statistically significant difference in adjusted VE against influenza A(H1N1) when influenza vaccination in two consecutive seasons was compared to vaccination in current season only (pooled VE difference: 3%, 95% CI: -8 to 13%, I2: 0%). Belongia et al.Footnote 14 did not directly compare VE between the two groups; however, they reported similar (i.e., widely overlapping 95% CI) pooled estimates of unadjusted VE against influenza A(H1N1)pdm09 for participants who received influenza vaccine in two consecutive seasons (pooled VE: 67%, 95% CI: 53 to 78%, I2: 69%) and for participants who received influenza vaccine in the current season only (pooled VE: 58%, 95% CI: 48 to 67%, I2: 0%).
Influenza A(H3N2): All three SRs/MAs assessed the effect of repeated seasonal vaccination on VE against influenza A(H3N2). However, results from the SRs/MAs were inconsistent.
Similar to the findings for influenza A(H1N1), Bartoszko et al. did not find a statistically significant difference in the pooled unadjusted odds of having medically-attended, LCI A(H3N2) between participants who received an influenza vaccine in the current and prior season compared with participants who received the vaccine in the current season only [OR (RCTs): 0.71, 95% CI: 0.37 to 1.34%, I2: 0%; OR (observational): 1.09, 95% CI: 0.86 to 1.38%, I2: 70%]. The pooled VE results reported by Belongia et al. showed that, while influenza vaccination in the current season only produced statistically significant VE against influenza A(H3N2) infection (pooled VE: 39%, 95% CI: 16 to 55%, I2: 73%), influenza vaccination in two consecutive seasons did not (pooled VE: 17%, 95% CI: -10 to 37%, I2: 86%). Ramsay et al. also did find a statistically significant difference in the pooled adjusted VE against influenza A(H3N2) when vaccination in two consecutive seasons was compared to vaccination in the current season only (pooled VE difference: -20%, 95% CI: -36 to -4%, I2: 35%). The authors noted that this appeared to be driven by estimates from the 2014–2015 influenza season, whose results are discussed further in Section III.2.2.
Influenza B: All three SRs/MAs assessed the effect of repeated seasonal vaccination on VE against influenza B. The SRs/MAs had concordant results, demonstrating no apparent difference between vaccination in two consecutive seasons and vaccination in the current season only for influenza B. There were no statistically significant differences in the season specific estimates for adjusted VE against influenza B between vaccination in two consecutive seasons and vaccination in the current season only in the analyses by Ramsay et al., except for the overall seasons pooled VE estimate where the upper limit of the CI was close to the null (pooled VE difference: -11%, 95% CI: -20 to -2%, I2: 0%). Similarly, Bartoszko et al. did not find a statistical difference in the pooled ORs of influenza B infection, comparing vaccination in two consecutive seasons with vaccination in the current season only, derived from either RCT or observational study designs [OR (RCTs): 0.85, 95% CI: 0.36 to 2.02%, I2: 15%; OR (observational): 1.13, 95% CI: 0.85 to 1.50%, I2: 52%]. Belongia et al. also reported similar VE against influenza B between vaccination in consecutive seasons [pooled VE: 55%, 95% CI: 38 to 67%, I2: not reported (NR)] and vaccination in the current season only (pooled VE: 61%, 95% CI: 43 to 74%, I2: NR). Belongia et al. was the only SR/MA that reported VE against the different influenza B lineages; the authors found similar pooled unadjusted VE between the two groups against influenza B/Yamagata [pooled VE (consecutive seasons): 57%, 95% CI: 47 to 65%, I2: NR; pooled VE (current season only): 62%, 95% CI: 46 to 73%, I2: NR] and against B/Victoria [pooled VE (consecutive seasons): 62%, 95% CI: 45 to 74%, I2: NR; pooled VE (current season only): 67%, 95% CI: 41 to 81%, I2: NR].
III.2.2 Vaccine effectiveness by influenza season where repeat effects were observed
Three of the four SRs/MAs examined VE stratified by influenza season. Belongia et al. assessed pooled VE against influenza A(H1N1)pdm09 in 2010–2011 and 2013–2014 and against influenza A(H3N2) in 2014–2015. Ramsay et al. assessed VE against influenza A(H1N1) and B in 2010–2011 to 2014–2015, and against influenza A(H3N2) in 2007–2008, 2011–2012, 2012–2013, and 2014–2015; however, not all analyses included data from more than one primary study. Bartoszko et al. assessed the effect of repeated vaccination on VE against influenza A(H3N2) during nine different influenza seasons (2008–2009 to 2016–2017), but only reported an effect estimate for the 2010–2011 season and narratively described the results for the other seasons. All estimates compared vaccination in two consecutive seasons to vaccination in the current season only.
None of the SRs/MAs found statistically significant differences in VE between vaccination in the current and prior season and vaccination in the current season only for influenza A(H1N1), A(H3N2), or B in any specific influenza season apart from the two listed belowFootnote 14Footnote 15Footnote 21 (data not shown, please refer to original studies for full details).
2010–2011: Bartoszko et al. completed a post-hoc subgroup meta-analysis of unadjusted estimates by season, and found that during the 2010–2011 influenza season, the odds of having medically-attended, LCI A(H3N2) were statistically significantly higher among those vaccinated with seasonal influenza vaccine over two consecutive seasons compared to those vaccinated in the current season only (OR: 1.98, 95% CI: 1.32 to 2.97%, I2: 0%) (I2 estimate received by request). Belongia et al. and Ramsay et al. did not have a VE estimate against influenza A(H3N2) for the 2010–2011 season.
2014–2015: Ramsay et al. found that repeated vaccination was statistically significantly less effective against influenza A(H3N2) in the 2014–2015 season than vaccination in the current season only (pooled adjusted VE difference: -54%, 95% CI: -88 to -20%, I2: 29%). Belongia et al. found that although the direction of the point estimates for vaccination in two consecutive seasons and for vaccination in the current season only differed, the CIs for the two estimates greatly overlapped, to the point that one estimate's CI completely encompassed the other's [pooled VE (consecutive): -9%, 95% CI: -26 to 6%, I2: NR; pooled VE (current only): 36%, 95% CI: -32% to 69%, I2: NR]. As well, both CIs crossed zero, indicating that neither demonstrated statistically significant VE against medically-attended influenza A(H3N2) during the 2014–2015 season. Bartoszko et al. noted in their SR/MA that they did not observe a statistically significant difference in pooled unadjusted VE during 2014–2015 among repeated vaccinees compared to current season only vaccinees (OR: 1.34, 95% CI: 0.97 to 1.83, I2: 70%) (effect estimate received by request); however, the trend appeared to follow that shown in the other SRs/MAs.
III.2.3 Vaccine effectiveness in individuals vaccinated over three or more consecutive seasons
Only the SR/MA by Bartoszko et al. assessed influenza VE over three or more consecutive seasons. The authors compared the current season VE of individuals vaccinated consecutively across three, four or more, and five or more influenza seasons compared with individuals vaccinated in the current season only, by pooling data from two RCTs (five estimates) and 3–4 observational studies (3–6 estimates). In observational studies, the pooled unadjusted odds of medically-attended, LCI among individuals vaccinated in three (OR: 1.97, 95% CI: 1.14 to 3.39%, I2: 60%), four or more (OR: 1.40, 95% CI: 1.03 to 1.88%, I2: 54%), and five or more (OR: 1.57, 95% CI: 1.23 to 2.02%, I2: 5%) consecutive seasons were higher relative to individuals vaccinated in the current season only. The pooled estimate from the two RCTs did not find a statistically significant difference in the unadjusted odds of having medically-attended, LCI among those with vaccination over three consecutive seasons compared with those with vaccination in the current season only (OR: 1.06, 95% CI: 0.65 to 1.75%, I2: 0%).
III.2.4 Vaccine efficacy and effectiveness by vaccine type
Two studies examined vaccine efficacy or effectiveness stratified by type of seasonal influenza vaccineFootnote 20Footnote 21. Bartoszko et al. pooled data from four RCTs (eight estimates) and 27 observational studies (40 estimates) separately to assess the unadjusted VE of repeated vaccination compared with vaccination in the current season only for inactivated influenza vaccines (IIV). The authors found that the odds of having medically-attended, LCI were not statistically significantly different among participants with repeated IIV vaccination over two consecutive seasons and participants vaccinated with IIV in the current season only [OR (RCTs): 0.87, 95% CI: 0.59 to 1.30%, I2: 28%; OR (observational): 1.14, 95% CI: 0.98 to 1.33%, I2: 63%].
The authors also conducted a subgroup meta-analysis of two RCTs (two estimates) on the comparative VE for live attenuated influenza vaccine (LAIV) and did not find a statistically significant difference in the odds of having medically-attended, LCI between the two vaccination scenarios (OR: 1.16, 95% CI: 0.58 to 2.32%, I2: 69%).
Morimoto et al. assessed vaccine efficacy by vaccine type against medically-attended influenza infection in children (six estimates). The authors found that the risk of having medically-attended, LCI was not statistically significantly different among children who received IIV during two consecutive seasons compared to the current season only (matched cases: RR: 1.16, 95% CI: 0.28 to 4.76%, I2: 0%; mismatched cases: RR: 1.08, 95% CI: 0.27 to 4.37%, I2: 0%). Please refer to Section III.2.8 for Morimoto et al.'s definition of matched and mismatched cases. The same was true for matched cases of children who received LAIV (RR: 0.61, 95% CI: 0.24-1.57%, I2: 46.3%); however, children who received LAIV in two consecutive seasons and had a mismatched case of influenza had significantly higher risk of medically-attended, LCI infection (RR: 2.03, 95% CI: 1.20-3.41%, I2: 0%).
III.2.5 Prior season vaccination with monovalent pandemic influenza vaccine
One SR/MA reported estimates involving prior vaccination with monovalent pandemic influenza vaccineFootnote 21. Bartoszko et al. pooled data from seven observational studies (number of estimates not reported) to examine the odds of having medically-attended, laboratory-confirmed seasonal influenza comparing participants who received monovalent pandemic influenza vaccine in the prior season and seasonal influenza vaccine in the current season relative to participants who received seasonal influenza vaccine in the current season alone. No difference in the pooled unadjusted odds was detected between either group (OR: 0.97, 95% CI: 0.59 to 1.60%, I2: NR). The authors did not report whether the pooled estimate included studies for which participants received an adjuvanted or unadjuvanted monovalent pandemic vaccine.
III.2.6 Vaccine efficacy and effectiveness by age group
Two SRs/MAs assessed vaccine efficacy or effectiveness by age groupFootnote 20Footnote 21. Overall, there appeared to be no significant difference in VE based on age group.
Two separate subgroup meta-analyses comparing VE by age group were completed by Bartoszko et al., which was the only SR/MA to report on VE stratified by age. One was a subgroup meta-analysis of 14 observational studies (20 estimates) that compared unadjusted VE of vaccination in consecutive seasons and vaccination in the current season only for children (17 years of age or younger), adults (18–64 years of age), and older adults (65 years of age and older) [OR (children): 0.93, 95% CI: 0.51 to 1.69%, I2: 78%; OR (adults): 0.95, 95% CI: 0.75 to 1.21%, I2: 34%; OR (older adults): 0.78, 95% CI: 0.61 to 1.01%, I2: 0%], while the other subgroup meta-analysis of four RCTs (eight estimates) compared unadjusted VE for the two vaccination scenarios in children and adults [OR (children): 1.07, 95% CI: 0.63 to 1.80, I2: 59%; OR (adults): 0.79, 95% CI: 0.50 to 1.24%, I2: 0%]. Results from these subgroup meta-analyses showed that the odds of medically-attended LCI were not statistically significantly different between the two vaccination scenarios for any of the age groups assessed by pooled estimates from RCTs or observational studies.
Morimoto et al. assessed vaccine efficacy against any medically-attended influenza in children (six studies, six estimates) and in adults 30–60 years of age (one study, three estimates). The authors found that the risk of having medically-attended, LCI was not statistically significantly different among children or adults who had received influenza vaccination over two consecutive seasons compared to those that had received the vaccine in the current season only (children: RR: 1.31, 95% CI: 0.79 to 2.16%, I2: 37.6%; adults: RR:1.12, 95% CI: 0.65 to 1.92%, I2: 19.1%).
III.2.7 Vaccine effectiveness by underlying comorbidity
A subgroup meta-analysis of 11 observational studies (12 estimates) conducted by Bartoszko et al. found that there was no statistically significant difference in the unadjusted odds of having medically-attended, LCI between vaccination in two consecutive seasons and vaccination in the current season only in subgroups with no reported comorbidities (OR: 1.06, 95% CI: 0.59 to 1.93%, I2: 81%) or in subgroups with one or more reported comorbidities (OR: 0.95, 95% CI: 0.69 to 1.54%, I2: 63%). There was substantial heterogeneity in both estimates. No other SRs/MAs assessed efficacy or effectiveness by underlying comorbidity.
III.2.8 Vaccine efficacy and effectiveness by vaccine match
Bartoszko et al. conducted a subgroup meta-analysis of five RCTs (nine estimates) and a subgroup meta-analysis of 27 observational studies (39 estimates) to assess the comparative effectiveness of repeated influenza vaccination in scenarios where the circulating influenza strains in the current influenza season were a match to vaccine strains, and scenarios where they were a mismatch to vaccine strains. The odds of having medically-attended, LCI did not differ significantly between individuals vaccinated in consecutive seasons and individuals vaccinated in the current season only for influenza seasons when the vaccine matched the circulating strains [OR (RCTs): 0.73, 95% CI: 0.42 to 1.26%, I2: 0%; OR (observational): 1.00, 95% CI: 0.80 to 1.26%, I2: 46%] or for when the vaccine was a mismatch for circulating strains [OR (RCTs): 0.96, 95% CI: 0.61 to 1.51%, I2: 50%; OR (observational): 1.26, 95% CI: 1.00 to 1.58%, I2: 73%]. Vaccine match and mismatch were determined based on what had been reported in the primary study, and if not reported, were based on SR/MA author judgement. However, the authors did not report how vaccine match and mismatch were defined; therefore, these results should be interpreted with caution.
Morimoto et al. assessed vaccine efficacy by vaccine match in children and in adults. The authors defined cases as matched or mismatched to the vaccine strain based on antigenic characterization by hemagglutinin inhibition assay. The vaccine was considered to match the circulating strain if it was the same subtype (influenza A) or lineage (influenza B) and antigenically similar to the vaccine strain. Meta-analysis results showed that the risk of having medically-attended, LCI was not statistically significantly different between matched cases in children (RR: 0.64, 95% CI: 0.33 to 1.22%, I2: 17.3%) or mismatched cases in adults (RR: 1.35, 95% CI: 0.77 to 2.38%, I2: 0%); however, as reported in Section III.2.4, children who had been vaccinated in two consecutive seasons were more at risk of influenza infection caused by an influenza virus not contained within the vaccine than those who had only been vaccinated in the current season (RR: 2.04, 95% CI: 1.29 to 3.22%, I2: 0%). No meta-analysis was conducted for matched cases in adults, as there was only one estimate availableFootnote 20.
III.3 Evidence for vaccine effectiveness of repeated vaccination compared to vaccination in prior season only
Two of the four SRs/MAs assessed VE of repeated vaccination compared to VE of vaccination in the prior season only. Ramsay et al. conducted three meta-analyses, stratified by influenza type, to examine the difference in adjusted VE between vaccination in the current and prior seasons and vaccination in the prior season only. For influenza A(H1N1), pooled data from 13 observational studies (16 estimates) showed statistically significantly higher adjusted VE among recipients vaccinated over the two most recent influenza seasons compared to vaccination in the prior season only (pooled VE difference: 25%, 95% CI: 14 to 35%, I2: 0%). Similar findings were also shown for influenza B, which were based on pooled data from 10 observational studies (13 estimates) (pooled VE difference: 18%, 95% CI: 3 to 33%, I2: 26%).
However, pooled data from 11 observational studies (14 estimates) found no statistically significant difference in adjusted VE against influenza A(H3N2) between the two vaccination scenarios (pooled VE difference: 7%, 95% CI: -7 to 21%, I2: 4%). VE estimates from the meta-analyses completed by Belongia et al. showed similar VE estimates for vaccination in consecutive seasons and for vaccination in the prior season only for influenza A(H1N1) [pooled VE (consecutive): 67%, 95% CI: 53 to 78%, I2: 69%; pooled VE (prior only): 46%, 95% CI: 29% to 59%, I2: 40%], influenza A(H3N2) [pooled VE (consecutive): 17%, 95% CI: -10 to 37%, I2: 86%; pooled VE (prior only): 9%, 95% CI: -10 to 25%, I2: 48%], and influenza B [pooled VE (consecutive): 55%, 95% CI: 38 to 67%, I2: NR; pooled VE (prior only): 25%, 95% CI: 4 to 42%, I2: NR].
III.4 Evidence for vaccine effectiveness of repeated vaccination compared to no vaccination
Two SRs/MAs reported VE of repeat vaccination compared to no vaccination. The SR/MA conducted by Belongia et al. reported the pooled VE of repeated influenza vaccination with reference to persons who were unvaccinated in both the current and prior season. Based on a meta-analysis of unadjusted estimates, vaccination in the current and prior season showed statistically significant VE against influenza A(H1N1) (pooled VE: 67%, 95% CI: 53 to 78%, I2: 69%) and influenza B (pooled VE: 55%, 95% CI: 38 to 67%, I2: NR). However, vaccination in the current and prior season did not produce statistically significant VE against influenza A(H3N2) (pooled VE: 17%, 95% CI: -10 to 37%, I2: 86%). A separate meta-analysis of three studies that assessed VE during specific influenza seasons found that repeated vaccination was not effective only during the 2014–2015 influenza season. Therefore, the authors concluded that the low VE during this season was driving the overall absence of statistically significant VE against influenza A(H3N2).
Bartoszko et al. concluded that, based on data pooled from five RCTs (nine estimates) and from 28 observational studies (40 estimates), vaccination in two consecutive seasons was statistically significantly effective against any influenza strain when no vaccination in either season was used as a reference [pooled VE (RCTs): 71%, 95% CI: 62 to 78%, I2: NR; pooled VE (observational): 41%, 95% CI: 30 to 51%, I2: NR].
IV. Discussion
For most estimates included in the SRs/MAs, there was no significant difference in vaccine efficacy or effectiveness between vaccination in two consecutive seasons and vaccination in the current season only. When stratified by season, the majority of estimates demonstrated that there was no significant difference in VE for vaccination in two consecutive seasons and vaccination in the current season only. However, there were some exceptions. Notably, two SRs/MAs demonstrated that repeated vaccination had a statistically significantly lower VE compared to vaccination in the current season only; one SR/MA found a lower VE against influenza A(H3N2) during 2010–2011Footnote 21, and the other SR/MA found a lower VE against influenza A(H3N2) during 2014–2015 and against influenza B, but only in the pooled overall estimateFootnote 15. During the 2014–2015 Northern Hemisphere influenza season, the influenza A(H3N2) component of the vaccine was unchanged from the 2013–2014 seasonFootnote 22 and was mismatched with the circulating strain, a situation in which repeated vaccination is predicted by the antigenic distance hypothesis to negatively interfere with VE7,11. However, the authors of this study noted that their estimate was largely driven by the 2014–2015 season. The 2010–2011 influenza season was the first post-2009 pandemic season and also contained a different influenza A(H3N2) vaccine component than the 2009 Northern Hemisphere vaccineFootnote 22. Therefore, it is important to consider that factors besides vaccine virus components may be affecting VE estimates.
Vaccination in the current season appeared to offer the best protection against influenza, regardless of previous season's vaccination status, since vaccination in the current season only and vaccination in two consecutive seasons was consistently more effective than vaccination in the prior season only and no vaccination in either season. The only instance when vaccination in two consecutive seasons was not significantly more effective than vaccination in the prior season only was in 2014–2015 against influenza A(H3N2). Firm conclusions on the difference between vaccination in consecutive seasons and vaccination in the prior season only could not be drawn from the indirect comparisons, as many of the 95% CIs for these VE estimates were slightly overlappingFootnote 23.
The one SR/MA that assessed the effect of vaccination over three or more consecutive influenza seasons showed that, based on meta-analyses of observational studies, the odds of medically-attended, LCI was greater among those vaccinated in three, four or more, and five or more consecutive influenza seasons compared to those vaccinated in the current season only. However, these estimates were based on a small number of studies and were not adjusted for confounding, which may be important as there could be important underlying differences between individuals who receive the influenza vaccine annually and individuals who do not regularly receive the vaccine (e.g., individuals at high-risk of influenza infection may be more likely to receive the vaccine annually and to seek medical attention for influenza-like illness). Therefore, the current evidence is insufficient to draw firm conclusions on the effect of vaccination in three or more consecutive seasons. A recent study by Kwong et al., which was not captured by any of the included SRs/MAs due the recency of publication, assessed the effect of repeated influenza vaccination on older adults over 10 previous seasons in CanadaFootnote 21. The authors of this study found a statistically significant trend towards decreasing VE for those vaccinated in the current season as the number of previous vaccinations increased. However, the opposite is true for those unvaccinated in the current season – as the number of previous vaccinations increased, protection in the current season also increased, which implies increasing residual protection from previous vaccinations. Regardless of the number of previous vaccinations however, vaccination in the current season provided some benefit, and was superior to remaining unvaccinated. This aligns with the findings presented in this overview for studies that assessed VE over a shorter period of time.
There was substantial heterogeneity for some of the pooled effect measures included in this overview, which indicates the presence of important underlying factors that may make meta-analysis of the data inappropriate. This was expected for estimates that pooled data across multiple influenza seasons, as VE is highly variable year to year. This was demonstrated by multiple SRs/MAs, as estimates from all SRs/MAs for specific influenza seasons tended to have little to no heterogeneity, suggesting that season-specific characteristics may account for most of the heterogeneity in other sub-analyses. However, despite seasonal differences explaining some of the heterogeneity present, further heterogeneity still exists. Some of this could be explained by differences in the local epidemiology, especially given that all SRs/MAs pooled estimates from multiple countries. Circulating influenza strains may differ by location, not just by hemisphere, and therefore estimates that pool data from many different countries could have substantial heterogeneity due to the varying contexts.
Influenza VE is also likely affected by many other factors, including vaccine strain match to circulating strainsFootnote 24, initial exposure to influenza virusFootnote 25, egg-adaptiveFootnote 25Footnote 26 mutations in the vaccine virusesFootnote 26Footnote 27, and possibly other currently unknown factors. In addition, these factors likely have complex interactions with each other, as suggested in a recent article by Skowronski et al.Footnote 28 The degree to which repeated vaccination and these other factors affect VE is not fully understood, and varies season to season, making it extremely difficult to predict far enough in advance of the next influenza season to make vaccine policy or administration practice changes. Therefore, a better understanding of the underlying immunological mechanisms and factors affecting the immune response to influenza vaccination are necessary to improve influenza vaccine development and programs.
Finally, addressing programmatic factors such as ethics, equity, feasibility and acceptabilityFootnote 29 as future evidence emerges on this topic will remain important. Guidance on influenza immunization upholds the core ethical dimensions for public health by aiming to prevent future disease, but it must be given in the challenging context of parameters that vary from season to season and are very difficult to predict (such as vaccine to circulating strain match or mismatch, and variable clinical disease severity). It is also important to consider that the effectiveness of a vaccine may have a significant impact on vaccine acceptabilityFootnote 30, which in turn may affect the uptake and impact of an immunization program. Therefore, despite negative interference occurring inconsistently in the literature summarized, the potential for reduced VE is of concern. As new vaccine products are added and as evidence emerges, including new studies examining the effect of pre-existing immunity on influenza vaccine responsesFootnote 31Footnote 32Footnote 33, NACI will continue to monitor the evidence for this phenomenon, and will issue new guidance as needed.
IV.1 Limitations
This overview was designed to assess the effects of repeated influenza vaccination on VE, efficacy, and immunogenicity for the purpose of providing guidance on annual influenza vaccination. All SRs/MAs that were included contained a systematic review and a meta-analysis of the effects of repeated influenza vaccination on vaccine efficacy or effectiveness but did not provide an evaluation of immunogenicity. Through this lens, additional evidence is necessary for the outcomes in this overview to determine the effect of repeated vaccination over time and across multiple influenza seasons. However, pooling data across seasons and from different geographic locations, as done by the SRs/MAs included in this overview, is insufficient to determine the potential causes of and mechanisms behind the effect of repeated vaccination on VE, and is expected to give estimates with high heterogeneity, since VE is affected by variables that often change season to season (e.g., circulating strain, vaccine match, etc.).
The SRs/MAs included for review all had similar research questions, as well as inclusion and exclusion criteria; as a result, there was significant overlap (46%) in the primary studies included for evidence synthesis in the SRs/MAs. Therefore, we caution that, while the results appear to draw data from many studies and populations, the SRs/MAs used much of the same data to produce the pooled estimates. Despite the different methods used by the SRs/MAs to pool data across studies (VE, difference in VE, RR, and OR), the results and conclusions of the SRs/MAs were generally consistent with each other, strengthening the reliability of the conclusions drawn from this evidence synthesis. The SRs/MAs were of good quality based on AMSTAR 2. The primary studies included in the SRs/MAs were also generally of good quality; the RoB was low for observational studies, which formed the majority of the evidence base. However, authors noted a high RoB for RCTs. Separate meta-analyses were completed for estimates from RCTs and from observational studies. The findings for most outcomes were similar; therefore, it does not appear that the high RoB for the included RCTs significantly affected the results of the meta-analyses.
The SRs/MAs by Bartoszko et al. and Morimoto et al. included studies that confirmed influenza infection using RT-PCR, which is the gold standard for influenza virus detection due to its higher sensitivity and specificityFootnote 34Footnote 35, but also studies using influenza infection confirmed by laboratory methods other than RT-PCR. Bartoszko et al. included these studies after determining that their inclusion did not significantly alter effect estimates, which alleviates some of the concerns with including studies that detected influenza virus by other laboratory methods for their SR/MA. Of note, studies using laboratory methods other than RT-PCR represented a small proportion of the total number of included studies (14%).
This overview included SRs/MAs that presented pooled effect estimates for direct comparisons (pooled difference in VE, RR, OR) and indirect comparisons, such as comparing separate pooled VE estimates for different vaccination scenarios which used unvaccinated in either season as a reference. Since the purpose of this overview was to determine the effects of repeated vaccination compared to either vaccination in the current season only, vaccination in the prior season only, or no vaccination, an effect estimate from a direct comparison is more appropriate for answering this overview's research question than an indirect comparison, as estimates with slightly overlapping CIs could still be significantly differentFootnote 23.
How some subgroups were assessed, and which subgroups were not assessed, presented particular limitations. Bartoszko et al. assessed influenza VE by vaccine match or mismatch, but did not specify how a match or mismatch had been defined, which presents difficulties for interpreting these findings. In addition, none of the SRs/MAs assessed the efficacy or effectiveness of adjuvanted, high dose, cell-based, or egg-based influenza vaccines, which are all different formulations of influenza vaccine authorized for use in Canada.
V. Recommendations
1. NACI continues to recommend that seasonal influenza vaccine should be offered annually to everyone 6 months of age and older who does not have contraindications to the vaccine, irrespective of previous seasons' influenza vaccination status. (Strong NACI Recommendation)
- NACI concludes that there is fair evidence to recommend annual influenza vaccination, irrespective of whether an individual received the seasonal influenza vaccine in previous seasons (Grade B Evidence).
Summary of evidence
- Repeated vaccination across seasons, including the current season, was consistently more effective than no vaccination in the current season.
- In general, the evidence shows no significant difference or predictable trend in vaccine efficacy or effectiveness between vaccinations in two consecutive seasons compared to vaccination in the current season only.
- Of all the seasons investigated across many studies, only two influenza seasons indicated that VE of vaccination over consecutive seasons was statistically significantly lower than vaccination in the current season only. These notable seasons were influenza A(H3N2) in 2010–2011Footnote 21, and influenza A(H3N2) in 2014–2015Footnote 15. These findings were not statistically significant in all SRs/MAs which assessed VE in these two seasons; however, a trend towards lower VE for repeated vaccination was consistent for the 2014–2015 season across all studiesFootnote 14Footnote 21.
- Evidence on the effects of repeated vaccination over three or more consecutive seasons was limited and is insufficient to draw firm conclusions at this point in time.
- Given the complex interplay between immune imprinting (such as previous exposures through vaccination and natural infection), circulating virus types, and individual characteristics, it is not currently feasible nor warranted to modify existing annual influenza vaccination programs to account for potential negative or positive interference effects related to repeated influenza vaccination across seasons.
VI. Research priorities
Research to address the following outstanding questions is encouraged:
New and emerging research priorities
Further evaluation of VE stratified by characteristics in addition to influenza strain type and subtype would allow for better identification of when the effects of repeated influenza vaccination should be considered and which specific populations may be affected.
- Further evaluation of the effects of long-term repeated influenza vaccination on VE over more than 2 consecutive seasons.
- Further evaluation of the effects of repeated influenza vaccination on VE stratified by age group and vaccine type.
- Investigation of the effects of repeated influenza vaccination on severe influenza-related outcomes, such as hospitalization and death.
- Evaluation of the effects of repeated influenza vaccination that accounts for previous influenza exposure through vaccination and/or natural infection.
- Further investigation of the immunological mechanisms underlying the effects of repeated influenza vaccination on VE, including the antigenic distance hypothesis and immunological imprinting.
Additional tables
| Strength of NACI recommendation Based on factors not isolated to strength of evidence (e.g. public health need) |
Grade of evidence Based on assessment of the body of evidence |
|---|---|
Strong
|
|
Discretionary
|
|
| Study details | Summary | |||||
|---|---|---|---|---|---|---|
| Study | Vaccine | Study design | Participants | Summary of key findings | Level of evidence | Quality |
Bartoszko JJ, McNamara IF, Aras OA, Hylton DA, Zhang YB, Malhotra D, Hyett SL, Morassut RE, Rudziak P, Loeb M. Does consecutive influenza vaccination reduce protection against influenza: A systematic review and meta-analysis. Vaccine. 2018 Jun 7;36(24):3434-44.Footnote 21 |
Seasonal influenza vaccine |
SR/MA Included: RCTs, quasi-RCTs, observational studies Influenza seasons: 23 seasons between 1983–1994 and mid 2016–2017 Funding: Canadian Institute for Health Research Foundation Grant |
Number of participants (RCTs): 11,987 Number of participants (observational): 28,627 Age range: all ages |
Primary findings: OR was assessed by determining the unadjusted odds of influenza infection, confirmed by any laboratory test, between vaccination in consecutive seasons and vaccination in the current season only. Any strain (RCT) Any strain (Obs) A(H1N1) (RCT) A(H1N1) (Obs) A(H3N2) (RCT) A(H3N2) (Obs) B (RCT) B (Obs) The VE of repeated vaccination was also assessed by pooling the unadjusted VE against influenza infection, confirmed by any RT-PCR. Unvaccinated in the current and prior seasons was the reference category: Current and prior season (RCT) Current and prior season (Obs) Current season only (RCT) Current season only (Obs) Subgroup meta-analyses: IIV (RCT) IIV (Obs) LAIV (RCT) Unadjusted odds of influenza infection, confirmed by any laboratory test, between vaccination with monovalent pandemic influenza vaccine in the prior season and seasonal influenza vaccine in the current season compared to vaccination in the current season only: Observational Protection against any influenza strain by age: Aged 17 years or younger (RCT) Aged 17 years or younger (Obs) Aged 18–64 years (RCT) Aged 18–64 years (Obs) Aged 65 years and older (Obs) Protection against any influenza strain by vaccine match: Match (RCT) Match (Obs) Mismatch (RCT) Mismatch (Obs) Protection against any influenza strain by presence of underlying comorbidity: No reported comorbidities (Obs) 1 or more comorbidities reported (Obs) Protection against any influenza strain when vaccinated in three consecutive seasons compared to current season only: RCT Obs Protection against any influenza strain when vaccinated in four or more consecutive seasons compared to current season only: Obs Protection against any influenza strain when vaccinated in five or more consecutive seasons compared to current season only: Obs Protection against influenza A(H3N2) in 2010–2011: Obs The odds of influenza A(H3N2) infection were not statistically significantly different between repeated vaccination and vaccination in the current season only for any other specific influenza season. |
SR/MA |
See Table 3. |
Morimoto N, Takeishi K. Change in the efficacy of influenza vaccination after repeated inoculation under antigenic mismatch: A systematic review and meta-analysis. |
Seasonal influenza vaccine |
SR/MA Included: RCT Influenza seasons (SR): 22 seasons between 1972-1973 and 2010-2011 Influenza seasons (MA): 9 seasons between 1973-1974 and 2009-2010 Funding: Study was not funded |
Number of observations (MA): 4541 Age range: all ages |
Primary findings: Vaccine efficacy was assessed by calculating the relative risk (RR) of medically-attended influenza infection for vaccination in two consecutive seasons compared to vaccination in the current season only. Medically-attended influenza was defined as acute respiratory illness (defined as the presence of fever, cough, headache, myalgia, sore throat or other respiratory symptoms) plus laboratory confirmation of influenza virus. Influenza infection was confirmed by RT-PCR or serology and/or culture. Vaccine efficacy against any medically-attended influenza: Children Adults Subgroup meta-analyses: Vaccine efficacy by vaccine match* against any medically-attended influenza: Matched (Children) Matched (Adults) Mismatched (Children) Mismatched (Adults) * Antigenically matched is defined as a vaccine that was deemed to match circulating strains with the same subtype and were antigenically similar. Vaccine efficacy by vaccine type and match* against any medically-attended influenza in children**: Matched (IIV) Matched (LAIV) Mismatched (IIV) Mismatched (LAIV) * Antigenically matched is defined as a vaccine that was deemed to match circulating strains with the same subtype and were antigenically similar. ** Number of estimates included in analysis was not reported for this measure. |
SR/MA |
See Table 3. |
Ramsay LC, Buchan SA, Stirling RG, Cowling BJ, Feng S, Kwong JC, Warshawsky BF. The impact of repeated vaccination on influenza vaccine effectiveness: A systematic review and meta-analysis. BMC Med, 2019;17(1):9. Retraction of: Ramsay LC, Buchan SA, Stirling RG. The Impact of Repeated Vaccination on Influenza Vaccine Effectiveness: A Systematic Review and Meta-Analysis. BMC Med. 2017;15(1):159Footnote 15 |
Seasonal influenza vaccine |
SR/MA Included: observational studies Influenza seasons: Funding: study was not funded |
Number of participants: NR Age range: all ages |
Primary findings: VE was assessed by determining the difference in adjusted VE against influenza infection, confirmed by any RT-PCR, between: VE for vaccination in two consecutive seasons minus VE for vaccination in the current season only: A(H1N1) A(H3N2) B VE for vaccination in two consecutive seasons minus VE for vaccination in the prior season only: A(H1N1) A(H3N2) B Subgroup findings: VE against A(H3N2) for vaccination in two consecutive seasons minus VE for vaccination in the current season only in 2014–2015 A(H3N2) Vaccination in two consecutive seasons was equally as effective as vaccination in current season only in all other season specific pooled analyses. |
SR/MA |
See Table 3. |
Belongia EA, Skowronski DM, McLean HQ, Chambers C, Sundaram ME, De Serres G. Repeated annual influenza vaccination and vaccine effectiveness: Review of evidence. Expert Rev Vaccines. 2017;16(7):723–36Footnote 14 |
Seasonal influenza vaccine |
SR/MA Included: test-negative case-control, case-control, cohort, RCTs Influenza seasons: Funding: study was not funded |
Number of participants: NR Age range: 2 years of age and older |
Primary findings: VE was assessed by pooling the unadjusted VE against influenza infection, confirmed by any RT-PCR. Unvaccinated in the current and prior seasons was the reference category for all following scenarios: VE for vaccination in two consecutive seasons: A(H1N1)pdm09 A(H3N2) B B/Yam B/Vic VE for vaccination in current season only: A(H1N1)pdm09 A(H3N2) B B/Yam B/Vic VE for vaccination in prior season only A(H1N1)pdm09 A(H3N2) B B/Yam B/Vic |
SR/MA |
See Table 3. |
Abbreviations – CI: confidence interval, IIV: inactivated influenza vaccine, LAIV: live attenuated influenza vaccine, MA: meta-analysis, NR: not reported, obs.: observational study, OR: odds ratio, PICO: population, intervention, comparator, and outcome, RCT: randomized controlled trial, RR: relative risk, RT-PCR: reverse-transcriptase polymerase chain reaction, SR/MA: systematic review and meta-analysis, VE: vaccine effectiveness |
||||||
List of abbreviations
- CI
- Confidence interval
- IIV
- Inactivated influenza vaccine
- LCI
- Laboratory confirmed influenza
- LAIV
- Live attenuated influenza vaccine
- MA
- Meta-analysis
- NACI
- National Advisory Committee on Immunization
- NR
- Not reported
- OR
- Odds ratio
- PHAC
- Public Health Agency of Canada
- PICO
- Population, intervention, comparator, and outcome
- RCT
- Randomized controlled trial
- RoB
- Risk of Bias
- RR
- Relative risk
- RT-PCR
- Reverse transcriptase-polymerase chain reaction
- SR
- Systematic review
- SR/MA
- Systematic review and meta-analysis
- VE
- Vaccine effectiveness
Acknowledgments
This NACI Statement was prepared by: K Young, MK Doll, J Przepiorkowski, L Zhao, R Harrison, I Gemmill, J Papenburg, and A Sinilaite on behalf of NACI.
NACI gratefully acknowledges the contribution of: P Doyon-Plourde, A Gil, L Glandon (Health Library, HC), A House, SJ Ismail, M Laplante, R Stirling, C Tremblay, M Tunis, M Xi, L Glandon (Health Library, HC), A House, M Laplante, R Stirling, and M Tunis.
NACI Influenza Working Group
Members: J Papenburg (Chair), P De Wals, D Fell, I Gemmill, R Harrison, J Langley, A McGeer, and D Moore.
Former working group members: N Dayneka, K Klein, D Kumar, M Lavoie, J McElhaney, S Smith, and B Warshawsky.
Liaison representatives: L Grohskopf (Centers for Disease Control and Prevention [CDC], United States).
Ex-officio representatives: C Bancej (Centre for Immunization and Respiratory Infectious Diseases [CIRID], PHAC), J Reiter (First Nations and Inuit Health Branch [FNIHB], Indigenous Services Canada [ISC]), and J Xiong (Biologics and Genetic Therapies Directorate [BGTD], Health Canada [HC]).
NACI
Members: S Deeks (Chair), R Harrison (Vice-Chair), M Andrew, J Bettinger, N Brousseau, H Decaluwe, P De Wals, E Dubé, V Dubey, K Hildebrand, K Klein, J Papenburg, A Pham-Huy, B Sander, S Smith, and S Wilson.
Former NACI members: M Lavoie, C Quach, C Rotstein, M Salvadori and N Sicard
Liaison representatives: L Bill / M Nowgesic (Canadian Indigenous Nurses Association), LM Bucci (Canadian Public Health Association), E Castillo (Society of Obstetricians and Gynaecologists of Canada), A Cohn (Centers for Disease Control and Prevention, United States), L Dupuis (Canadian Nurses Association), D Fell (Canadian Association for Immunization Research and Evaluation), S Funnell (Indigenous Physicians Association of Canada), J Hu (College of Family Physicians of Canada), M Lavoie (Council of Chief Medical Officers of Health), D Moore (Canadian Paediatric Society), M Naus (Canadian Immunization Committee), and A Ung (Canadian Pharmacists Association).
Former liaison representatives: J Brophy (Canadian Association for Immunization Research and Evaluation), A Cohn (CDC, United States), J Emili (College of Family Physicians of Canada), K Klein (Council of Chief Medical Officers of Health), and A Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada).
Ex-Officio representatives: V Beswick-Escanlar (National Defence and the Canadian Armed Forces), E Henry (Centre for Immunization and Respiratory Infectious Diseases (CIRID), PHAC), M Lacroix (Public Health Ethics Consultative Group, PHAC), C Lourenco (Biologic and Radiopharmaceutical Drugs Directorate, Health Canada), D MacDonald (COVID-19 Epidemiology and Surveillance, PHAC), S Ogunnaike-Cooke (CIRID, PHAC), K Robinson (Marketed Health Products Directorate, HC), G Poliquin (National Microbiology Laboratory, PHAC), and T Wong (First Nations and Inuit Health Branch, Indigenous Services Canada).
Former ex-officio representatives: K Barnes (National Defence and the Canadian Armed Forces), J Gallivan (Marketed Health Products Directorate, HC), J Pennock (CIRID, PHAC), and R Pless (BGTD, HC).
Appendix A: Search strategy and results
Outlined below are the search terms formatted for the respective databases; this list was developed in collaboration with a librarian at the federal Health Library. Please note the Medline table for a breakdown of search concepts.
OvidMEDLINE
Database(s): Ovid MEDLINE(R) Epub Ahead of Print, In-Process & Other Non-Indexed Citations, Ovid MEDLINE(R) Daily, Ovid MEDLINE and Versions(R)
| ID | Searches | Results |
|---|---|---|
| 1 | influenza vaccines/ or influenza, human/pc | 26844 |
| 2 | (influenza, human/ or exp influenzavirus a/ or exp influenzavirus b/) and (exp vaccines/ or exp vaccination/) | 18873 |
| 3 | ((influenza* or flu or H?N?) adj5 (vaccin* or immuni* or inoculat*)).tw,kf,kw. | 31281 |
| 4 | 1 or 2 or 3 | 40820 |
| 5 | (repeat* or annual* or yearly or consecutive* or ((each or every) adj3 (year* or season*))).tw,kf,kw. | 1162272 |
| 6 | 4 and 5 | 4070 |
| 7 | limit 6 to (meta analysis or "review" or systematic reviews) | 763 |
| 8 | (meta analysis or "review" or systematic reviews).pt. | 2592121 |
| 9 | meta-analysis/ or systematic review/ or meta-analysis as topic/ or "meta analysis (topic)"/ or "systematic review (topic)"/ | 114346 |
| 10 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*)) or (quantitative adj3 (review* or overview* or synthes*)) or (integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or meta analy* or metaanaly*).tw,kf,kw. | 231991 |
| 11 | 8 or 9 or 10 | 2660082 |
| 12 | 6 and 11 | 708 |
| 13 | 7 or 12 | 773 |
| 14 | limit 13 to yr="2016 -Current" | 87 |
| 15 | limit 14 to (English or French) | 86 |
86 results
EMBASE
Database: EMBASE 1974 to 2017 October 27
| ID | Searches | Results |
|---|---|---|
| 1 | influenza vaccine/ or influenza vaccination/ or exp influenza/pc or exp influenza virus/pc | 42496 |
| 2 | (exp influenza/ or exp influenza virus/) and (vaccine/ or virus vaccine/ or inactivated virus vaccine/ or vaccination/) | 12988 |
| 3 | ((influenza* or flu or H?N?) adj5 (vaccin* or immuni* or inoculat*)).tw,kw. | 35784 |
| 4 | 1 or 2 or 3 | 56476 |
| 5 | (repeat* or annual* or yearly or consecutive* or ((each or every) adj3 (year* or season*))).tw,kw. | 1472575 |
| 6 | 4 and 5 | 5508 |
| 7 | limit 6 to (meta analysis or "systematic review" or "review") | 927 |
| 8 | (meta analysis or "systematic review" or "review").pt. | 2348984 |
| 9 | meta analysis/ or review/ or systematic review/ or "meta analysis (topic)"/ or "systematic review (topic)"/ | 2472401 |
| 10 | ((systematic* adj3 (review* or overview*)) or (methodologic* adj3 (review* or overview*)) or (quantitative adj3 (review* or overview* or synthes*)) or (integrative adj3 (review* or overview*)) or (collaborative adj3 (review* or overview*)) or meta analy* or metaanaly*).tw,kw. | 268815 |
| 11 | 8 or 9 or 10 | 2663316 |
| 12 | 6 and 11 | 964 |
| 13 | 7 or 12 | 964 |
| 14 | limit 13 to yr="2016 -Current" | 114 |
| 15 | limit 14 to (English or French) | 110 |
110 results
Cochrane Library (Wiley interface)
| ID | Searches | Results |
|---|---|---|
| 1 | MeSH descriptor: [Influenza Vaccines] this term only | 1572 |
| 2 | MeSH descriptor: [Influenza, Human] this term only and with qualifier(s): [Prevention & control - PC] | 1198 |
| 3 | MeSH descriptor: [Influenza, Human] this term only | 1674 |
| 4 | MeSH descriptor: [Influenzavirus A] explode all trees | 895 |
| 5 | MeSH descriptor: [Influenzavirus B] explode all trees | 268 |
| 6 | MeSH descriptor: [Vaccines] explode all trees | 9061 |
| 7 | MeSH descriptor: [Vaccination] explode all trees | 2618 |
| 8 | (#3 or #4 or #5) and (#6 or #7) | 1298 |
| 9 | ((influenza* or flu or H?N?) and (vaccin* or immuni* or inoculat*)):ti,ab,kw (Word variations have been searched) | 3988 |
| 10 | #1 or #2 or #8 or #9 | 4145 |
| 11 | (repeat* or annual* or yearly or consecutive* or ((each or every) near/3 (year* or season*))):ti,ab,kw (Word variations have been searched) | 103447 |
| 12 | #10 and #11 Publication Year from 2016 to 2017 | 78 |
78 results
SCOPUS
( TITLE-ABS-KEY ( ( influenza* OR flu OR h?n?) W/5 ( vaccin* OR immuni* OR inoculat*))) AND ( TITLE-ABS-KEY ( repeat* OR annual* OR yearly OR consecutive* OR ( ( each OR every) W/3 ( year* OR season*)))) AND ( TITLE-ABS-KEY ( ( systematic* W/3 ( review* OR overview*)) OR ( methodologic* W/3 ( review* OR overview*)) OR ( quantitative W/3 ( review* OR overview* OR synthes*)) OR ( integrative W/3 ( review* OR overview*)) OR ( collaborative W/3 ( review* OR overview*)) OR meta AND analy* OR metaanaly*)) AND ( LIMIT-TO ( PUBYEAR, 2017) OR LIMIT-TO ( PUBYEAR, 2016))
18 results
ProQUEST Public Health
Database: Public Health Database, narrowed by: Entered date: 2016 - 2017; source type: Scholarly Journals
TI,AB,SU((influenza* or flu or H?N?) NEAR/5 (vaccin* or immuni* or inoculat*)) AND TI,AB,SU(repeat* or annual* or yearly or consecutive* or ((each or every) NEAR/3 (year* or season*))) AND ((systematic* NEAR/3 (review* or overview*)) or (methodologic* NEAR/3 (review* or overview*)) or (quantitative NEAR/3 (review* or overview* or synthes*)) or (integrative NEAR/3 (review* or overview*)) or (collaborative NEAR/3 (review* or overview*)) or meta analy* or metaanaly*)Limits applied
81 results
PROSPERO
(influenza* or flu) and (vaccin* or immuni* or inoculat*) and (repeat* or annual* or yearly or consecutive* or "each year" or "every year" or "each season" or "every season")
25 results
Appendix B: Flow diagram
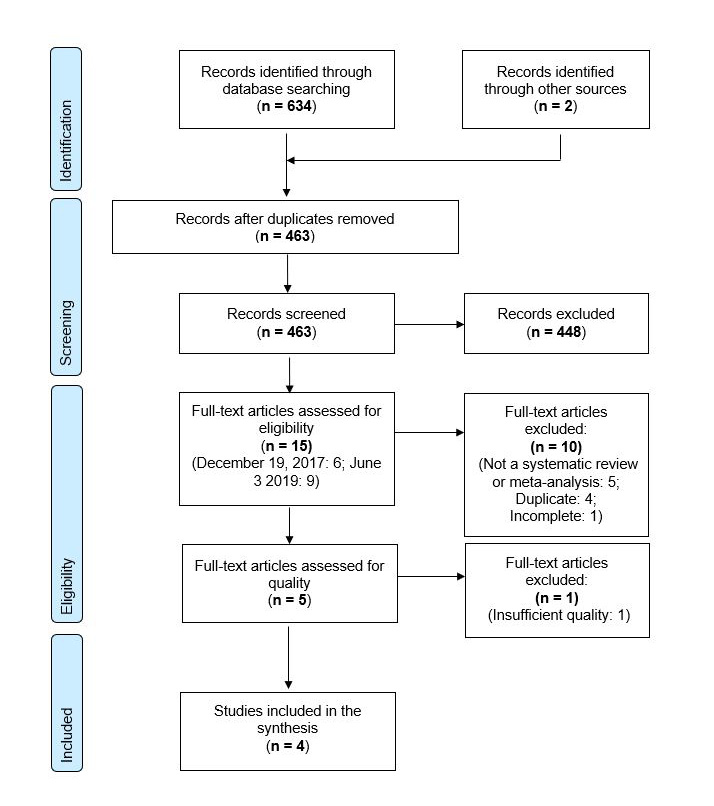
Text description
The PRISMA flow diagram describes the process by which articles were selected for the literature review. The process is broken down into four stages: Identification, Screening, Eligibility and Included.
Stage 1: Identification
- 634 records were identified through a database search performed on October 27, 2017 and updated on June 3, 2019.
- 2 records had been previously identified through additional sources.
- 463 records remained after duplicates were removed.
Stage 2: Screening
- 463 records were then screened.
- Of these 463 records, 448 records were excluded.
Stage 3: Eligibility
- 15 full-text articles were assessed for eligibility.
- Of these 15 full-text articles, 10 were excluded. The exclusion breakdown is as follows: 5 were not a systematic review or meta-analysis; 4 were duplicates; 1 was incomplete.
- 5 full-text articles were assessed for quality.
- Of these 5 full-text articles, 1 was excluded due to insufficient quality.
Stage 4: Included
- 4 articles were included in the final synthesis.
References
- Footnote 1
-
Schanzer DL, McGeer A, Morris K. Statistical estimates of respiratory admissions attributable to seasonal and pandemic influenza for Canada. Influenza Other Respir Viruses. 2013 Sep;7(5):799–808.
- Footnote 2
-
Schanzer DL, Sevenhuysen C, Winchester B, Mersereau T. Estimating Influenza Deaths in Canada, 1992–2009. Nishiura H, editor. PLoS ONE. 2013 Nov 27;8(11):e80481.
- Footnote 3
-
National Advisory Committee on Immunization. Canadian Immunization Guide Chapter on Influenza and Statement on Seasonal Influenza Vaccine for 2022–2023 [Internet]. Public Health Agency of Canada. 2022. Available from: https://www.canada.ca/en/public-health/services/publications/vaccines-immunization/canadian-immunization-guide-statement-seasonal-influenza-vaccine-2022-2023.html
- Footnote 4
-
World Health Organization. Influenza vaccine viruses and reagents [Internet]. Vol. 2017. [cited 2017 Nov 28]. Available from: http://www.who.int/influenza/vaccines/virus/en/
- Footnote 5
-
Hoskins TW, Davies JR, Smith AJ, Et. Al. Assessment of Inactivated Influenza-A Vaccine After Three Outbreaks of Influenza A at Christ's Hospital. The Lancet. 1979;313(8106):33–5.
- Footnote 6
-
Sullivan SG, Kelly H. Stratified Estimates of Influenza Vaccine Effectiveness by Prior Vaccination: Caution Required. Clin Infect Dis. 2013;57(3):474–6.
- Footnote 7
-
Skowronski DM, Chambers C, De Serres G, Et. Al. Serial Vaccination and the Antigenic Distance Hypothesis: Effects on Influenza Vaccine Effectiveness During A (H3N2) Epidemics in Canada, 2010–2011 to 2014–2015. J Infect Dis. 2017;215(7):1059–99.
- Footnote 8
-
McLean HQ, Thompson MG, Sundaram ME, Et. Al. Influenza Vaccine Effectiveness in the United States During 2012-2013: Variable Protection by Age and Virus Type. J Infect Dis. 2015;211(10):1529–40.
- Footnote 9
-
Puig-Barbera J, Burtseva E, Yu H, Et. Al. Influenza Epidemiology and Influenza Vaccine Effectiveness During the 2014-2015 Season: Annual Report from the Global Influenza Hospital Surveillance Network. BMC Public Health. 2016;16(Suppl 1):757.
- Footnote 10
-
Thompson MG, Naleway A, Fry AM, Et. Al. Effects of Repeated Annual Inactivated Influenza Vaccination among Healthcare Personnel on Serum Hemagglutinin Inhibition Antibody Response to A/Perth/16/2009 (H3N2)-like virus during 2010-11. Vaccine. 2016;34(7):981–8.
- Footnote 11
-
Smith DJ, Forrest S, Ackley DH, Et. Al. Variable Efficacy of Repeated Annual Influenza Vaccination. Proc Natl Acad Sci U S A. 1999;96(24):14001–6.
- Footnote 12
-
Flannery B, Smith C, Garten RJ, Et. Al. Influence of Birth Cohort on Effectiveness of 2015–2016 Influenza Vaccine Against Medically Attended Illness Due to 2009 Pandemic Influenza A(H1N1) Virus in the United States. J Infect Dis. 2018;218(2):189–96.
- Footnote 13
-
Gostic KM, Ambrose M, Worobey M, Et. Al. Potent Protection Against H5N1 and H7N9 Influenza via Childhood Hemagglutinin Imprinting. Science. 2016;354(6313):722–6.
- Footnote 14
-
Belongia EA, Skowronski DM, McLean HQ, Et. Al. Repeated Annual Influenza Vaccination and Vaccine Effectiveness: Review of Evidence. Expert Rev Vaccines. 2017;16(7):1–14.
- Footnote 15
-
Ramsay LC, Buchan SA, Stirling RG, Et. Al. The Impact of Repeated Vaccination on Influenza Vaccine Effectiveness: A Systematic Review and Meta-Analysis. BMC Med. 2019;17(1):9. Retraction of: Ramsay LC, Buchan SA, Stirling RG. The Impact of Repeated Vaccination on Influenza Vaccine Effectiveness: A Systematic Review and Meta-Analysis. BMC Med. 2017;15(1):159.
- Footnote 16
-
Harder T, Remschmidt C, Haller S, Et. Al. Use of Existing Systematic Reviews for Evidence Assessments in Infectious Disease Prevention: A Comparative Case Study. Syst Rev. 2016;5(1):171.
- Footnote 17
-
Robinson KA, Whitlock EP, Oneil ME, Et. Al. Integration of Existing Systematic Reviews into New Reviews: Identification of Guidance Needs. Syst Rev. 2014;3(1):60.
- Footnote 18
-
Shea BJ, Reeves BC, Wells G, Et. Al. AMSTAR 2: A Critical Appraisal Tool for Systematic Reviews that Include Randomised or Non-Randomised Studies of Healthcare Interventions, or Both. BMJ. 2017;358:j4008.
- Footnote 19
-
Caspard H., Heikkinen T., Belshe R.B., Et. Al. A Systematic Review of the Efficacy of Live Attenuated Influenza Vaccine upon Revaccination of Children. Hum Vaccines Immunother. 2016;12(7):1721–7.
- Footnote 20
-
Morimoto N, Takeishi K. Change in the Efficacy of Influenza Vaccination After Repeated Inoculation Under Antigenic Mismatch: A Systematic Review and Meta-Analysis. Vaccine. 2018;36(7):949–57.
- Footnote 21
-
Bartoszko JJ, McNamara IF, Aras OAZ, Et. Al. Does Consecutive Influenza Vaccination Reduce Protection Against Influenza: A Systematic Review and Meta-Analysis. Vaccine. 2018;36(24):3434–44.
- Footnote 22
-
World Health Organization. Recommended Composition of Influenza Virus Vaccines for Use in the 2021- 2022 Northern Hemisphere Influenza Season. Meet Rep [Internet]. 2021 [cited 2018 Mar 13]; Available from: https://cdn.who.int/media/docs/default-source/influenza/202102_recommendation.pdf?sfvrsn=8639f6be_3&download=true
- Footnote 23
-
Schenker N, Gentleman JF. On Judging the Significance of Differences by Examining the Overlap Between Confidence Intervals. Am Stat. 2001;55(3):182–6.
- Footnote 24
-
Belongia EA, Simpson MD, King JP, Et. Al. Variable Influenza Vaccine Effectiveness by Subtype: A Systematic Review and Meta-Analysis of Test-Negative Design Studies. Lancet Infect Dis. 2016;16(8):942–51.
- Footnote 25
-
Francis T. On the Doctrine of Original Antigenic Sin. Proc Am Philos Soc. 1960;104(6):572–8.
- Footnote 26
-
Zost S, Parkhouse K, Et. Al. Contemporary H3N2 Influenza Viruses have a Glycosylation Site that Alters Binding of Antibodies Elicited by Egg-Adapted Vaccine Strains. Proc Natl Acad Sci U A. 2017;114(47):12578–83.
- Footnote 27
-
Skowronski D, Janjua N, De Serres G. Low 2012-13 Influenza Vaccine Effectiveness Associated with Mutation in the Egg-Adapted H3N2 Vaccine Strain not Antigenic Drift in Circulating Viruses. PLoS One. 2014;9(3):e92153.
- Footnote 28
-
Skowronski D, Sabaiduc S, Leir S. Paradoxical Clade- and Age-Specific Vaccine Effectiveness during the 2018/19 Influenza A(H3N2) Epidemic in Canada: Potential Imprint-Regulated Effect of Vaccine (I-REV). Euro Surveill. 2019;24(46):1900585.
- Footnote 29
-
Ismail SJ, Hardy K, Tunis MC, Et. Al. A Framework for the Systematic Consideration of Ethics, Equity, Feasibility, and Acceptability in Vaccine Program Recommendations. Vaccine. 2020;38(36):5861–76.
- Footnote 30
-
Gates A, Gates M, Rahman S. A Systematic Review of Factors that Influence the Acceptability of Vaccines among Canadians. Vaccine. 2021;39(2):222–36.
- Footnote 31
-
Auladell M, Phuong H, Mai L. Influenza Virus Infection History Shapes Antibody Responses to Influenza Vaccination. Nat Med. 2022;28(2):363-372.
- Footnote 32
-
Moritzky S, Richards K, Glover M. The Negative Effect of Pre-Existing Immunity on Influenza Vaccine Responses Transcends the Impact of Vaccine Formulation Type and Vaccination History [published online ahead of print, 2022 Feb 24]. J Infect Dis. 2022;jiac068.
- Footnote 33
-
Snape N, Anderson G, Irving L. Vaccine Strain Affects Seroconversion after Influenza Vaccination in COPD Patients and Healthy Older People. NPJ Vaccines. 2022;7(1):8.
- Footnote 34
-
Centers for Disease Control and Prevention. Information on Rapid Molecular Assays, RT-PCR, and other Molecular Assays for Diagnosis of Influenza Virus Infection. Centers for Disease control and Prevention [Internet]. [cited 2018 Mar 13]. Available from: https://www.cdc.gov/flu/professionals/diagnosis/molecular-assays.htm
- Footnote 35
-
Merckx J, Wali R, Schiller I. Accuracy of Novel and Traditional Rapid Tests for Influenza Infection Compared with Reverse Transcriptase Polymerase Chain Reaction: A Systematic Review and Meta-Analysis. Ann Intern Med. 2017;167(6):394–409
