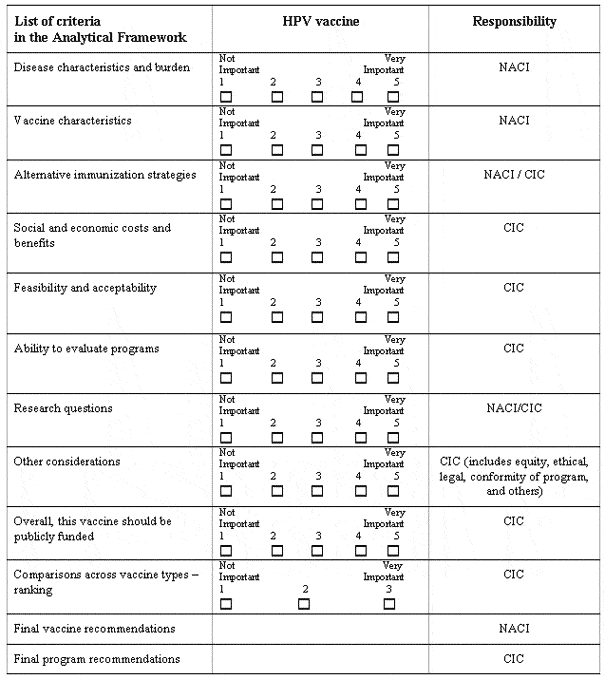
| Disease Characteristics and Burden
- Nature and characteristics of the infective agents
- Clinical manifestations and complications
- Epidemiology of the disease
- Specific populations affected and risk factors
- Current disease treatment and preventability
- Social impact of the disease
- Economic impact of the disease
Vaccine Characteristics
- Nature and characteristics of immunizing agent
- Characteristics of commercial products
- Storage, handling, product format
- Vaccine manufacturers, production capacity and supply
- Administration schedule, number of doses, combination with other vaccines
- Nature and characteristics of the immune response
- Immunogenicity in different population groups
- Short- and long-term direct and indirect protection
- Impact on reduction of burden of disease
- Safety: rates and severity of adverse effects, contra-indications, precautions
- Potential interaction with other vaccines
- Potential impacts on antibiotic resistance
Alternative Immunization Strategies and Programs
- Existing recommendations/guidelines for use of the vaccine
- Objectives of disease control/elimination/eradication at international, national, and/or provincial/territorial levels
- Alternative immunization strategies for meeting objectives
- Specific objectives in terms of reduction of incidence, complications, sequelae and mortality
- Specific objectives re coverage of specific groups
- Delivery strategy/system
Social and Economic Costs and Benefits
- Total and opportunity costs of program for families and the health system
- Evidence regarding short- and long-term effectiveness
- Evidence regarding social and economic benefits
- Other benefits
- Economic evaluation: net present costs and cost-benefit ratios
Feasibility and Acceptability of Alternative Programs
- Public perception of disease risk, severity, fear, need for control
- Demand for/acceptability of immunization for target groups
- Priority for approved program compared with other programs
- Expected date of licensure or current use of vaccine
|
- Integration of new program with existing programs and schedules
- Impacts on existing immunizations services and the health care sector
- Accessibility of target population/expected levels of uptake
- Availability of vaccine supply
- Availability of funding for vaccine purchase
- Availability of human, technical and financial resources
- Availability of appropriate documentation/consent forms
- Availability of system for recording/registering vaccine administration
- Availability of resources for marketing and communication
- Existence of operational planning and implementation committee
Ability to Evaluate Questions
- Desirability of evaluation to families, professionals
- Availability of information systems to measure coverage, utilization, quality
- Availability of information systems for monitoring reduction of disease incidence, complications, mortality
- Availability of system for monitoring adverse events associated with vaccine administration
- Availability of systems for linking health outcomes databases, immunization registries and population registries
Research Questions
- Ongoing and planned research projects in the fields of vaccine development, immunogenicity, efficacy and safety
- Identification of areas in previous sections in which research is needed to assist planning evaluation and decision-making
Other Considerations
- Equity of new program, including universality, accessibility and gratuity of services for the most vulnerable population groups
- Ethical considerations, including informed consent and protection of confidentiality of medical information
- Conformity of new program with planned or existing programs in other jurisdictions and countries
- Possible political benefits and risks associated with implementation of the new program
|