FluWatch report: March 22 to 28, 2020 (week 13)
Download the alternative format
(PDF format, 1.3 MB, 14 pages)
Organization: Public Health Agency of Canada
Date published: 2020-04-03
Related Topics
Overall Summary
- The percentage of tests positive for influenza fell below 5% this week. This suggests that Canada is nearing the end of the 2019-2020 influenza season at the national level.
- All indicators of influenza activity decreased compared to the previous week.
- Laboratory detections and syndromic indicators may be influenced by the COVID-19 pandemic. These data should be interpreted with caution.
- The 2019-2020 Seasonal Influenza Immunization Coverage Survey showed that coverage was low among adults aged 18-64 years (34%) and highest among seniors aged 65 years and older (70%).
- The highest cumulative hospitalization rates are among children under 5 years of age and adults 65 years of age and older. Hospitalizations among adults are predominantly due to influenza A, while those among children are due to a mix of influenza A and B.
On this page
- Influenza/ILI Activity (geographic spread)
- Laboratory Confirmed Influenza Detections
- Syndromic/Influenza-like Illness Surveillance
- FluWatchers
- Influenza Outbreak Surveillance
- Severe Outcomes Influenza Surveillance
- Influenza Strain Characterizations
- Antiviral Resistance
- Vaccine Monitoring
- Provincial and International Influenza Reports
Influenza/Influenza-like Illness (ILI) Activity (geographic spread)
During week 13, influenza activity was reported in 33 regions across Canada and 4 regions reported no influenza activity. Among the regions reporting influenza activity, 65% reported sporadic activity and 24 % reported localized activity (Figure 1).
Figure 1 - Map of influenza/ILI activity by province and territory, Canada, week 2020-13
Number of Regions Reporting in week 13: 37 out of 53
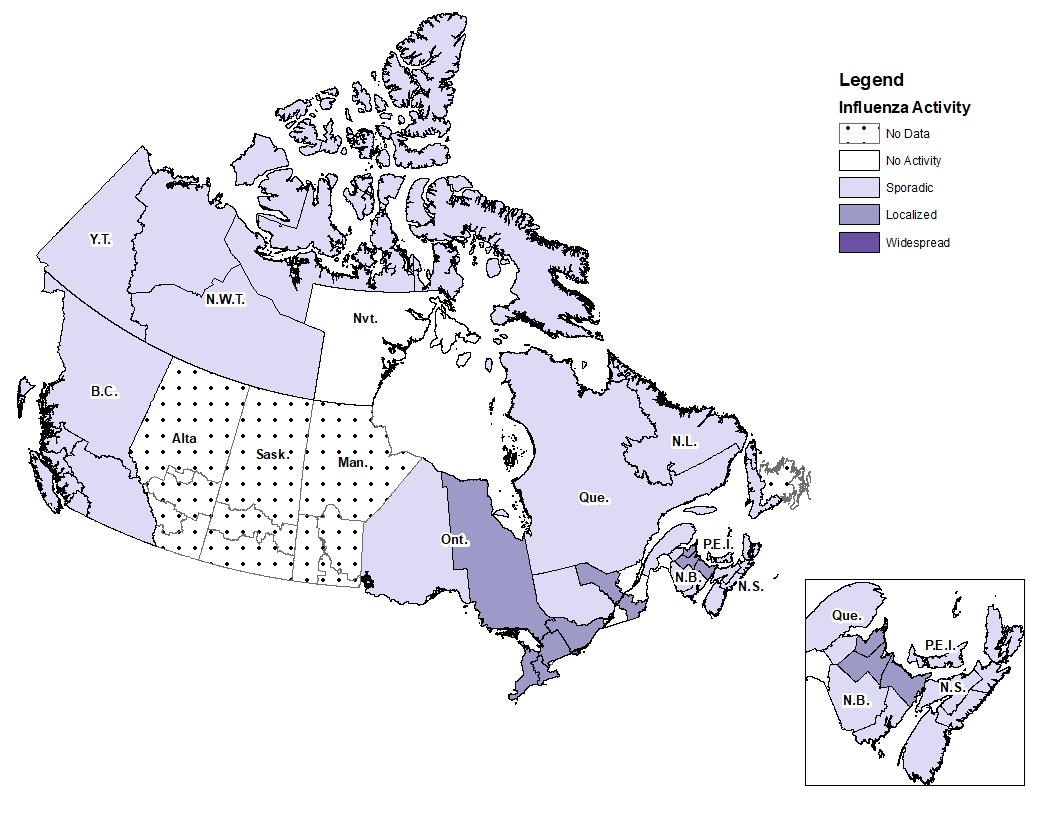
Figure 1 - Text equivalent
| Province | Influenza Surveillance Region | Activity Level |
|---|---|---|
| N.L. | Eastern | No Data |
| N.L. | Labrador-Grenfell | Sporadic |
| N.L. | Central | No Data |
| N.L. | Western | Sporadic |
| P.E.I. | Prince Edward Island | Sporadic |
| N.S. | Zone 1 - Western | Sporadic |
| N.S. | Zone 2 - Northern | Sporadic |
| N.S. | Zone 3 - Eastern | Sporadic |
| N.S. | Zone 4 - Central | Sporadic |
| N.B. | Region 1 | Localized |
| N.B. | Region 2 | Sporadic |
| N.B. | Region 3 | Sporadic |
| N.B. | Region 4 | No Activity |
| N.B. | Region 5 | Sporadic |
| N.B. | Region 6 | Localized |
| N.B. | Region 7 | Localized |
| Que. | Nord-est | Sporadic |
| Que. | Québec et Chaudieres-Appalaches | No Activity |
| Que. | Centre-du-Québec | Localized |
| Que. | Montréal et Laval | Sporadic |
| Que. | Ouest-du-Québec | Sporadic |
| Que. | Montérégie | No Activity |
| Ont. | Central East | Localized |
| Ont. | Central West | Localized |
| Ont. | Eastern | Localized |
| Ont. | North East | Localized |
| Ont. | North West | Sporadic |
| Ont. | South West | Localized |
| Ont. | Toronto | No Data |
| Man. | Northern Regional | No Data |
| Man. | Prairie Mountain | No Data |
| Man. | Interlake-Eastern | No Data |
| Man. | Winnipeg | No Data |
| Man. | Southern Health | No Data |
| Sask. | North | No Data |
| Sask. | Central | No Data |
| Sask. | South | No Data |
| Alta. | North Zone | No Data |
| Alta. | Edmonton | No Data |
| Alta. | Central Zone | No Data |
| Alta. | Calgary | No Data |
| Alta. | South Zone | No Data |
| B.C. | Interior | Sporadic |
| B.C. | Fraser | Sporadic |
| B.C. | Vancouver Coastal | Sporadic |
| B.C. | Vancouver Island | Sporadic |
| B.C. | Northern | Sporadic |
| Y.T. | Yukon | Sporadic |
| N.W.T. | North | Sporadic |
| N.W.T. | South | Sporadic |
| Nvt. | Qikiqtaaluk | Sporadic |
| Nvt. | Kivalliq | No Activity |
| Nvt. | Kitimeot | Sporadic |
Laboratory-Confirmed Influenza Detections
In week 13, the percentage of laboratory tests positive for influenza continued to decrease sharply to 2.5%. The percentage of positive tests crossed the seasonal threshold of 5%, suggesting that the end of the influenza season at the national level may have occurred last week.
Testing for influenza and other respiratory viruses may be influenced by the current COVID-19 pandemic. Changes in laboratory testing practices in the coming weeks may affect the comparability of data to previous weeks or previous seasons.
The following results were reported from sentinel laboratories across Canada (Figures 2 and 3):- 50% of detections were influenza A.
- Among subtyped influenza A detections, influenza A(H1N1) accounted for 66% of detections.
To date this season (weeks 35 to 13), all influenza types and subtypes have circulated. Among the 55,033 laboratory detections of influenza reported:
- 59% (32,547) were influenza A.
- Among subtyped influenza A detections (7,288), A(H1N1) is the predominant subtype this season (68%).
Detailed information on age and type/subtype has been received for 42,355 laboratory-confirmed influenza cases (Table 1). To date this season (weeks 35 to 13):
- Cases of influenza A(H1N1) (3,729) were primarily in adults; 26% 20-44 years, 26% 45-64 years and 28% 65 years of age and older.
- Among cases of influenza A(H3N2) (2,048), the largest proportion of cases was in adults 65 years of age and older (46%).
- Cases of influenza B (18,086) were primarily in younger age groups; 22% under 5 years of age, 33% 5-19 years and 31% between 20 and 44 years of age.
For more detailed weekly and cumulative influenza data, see the text descriptions for Figures 2 and 3 or the Respiratory Virus Detections in Canada Report.
Figure 2 - Number of positive influenza tests and percentage of tests positive, by type, subtype and report week, Canada, weeks 2019-35 to 2020-13
Number of Laboratories Reporting in Week 13: 33 out of 36
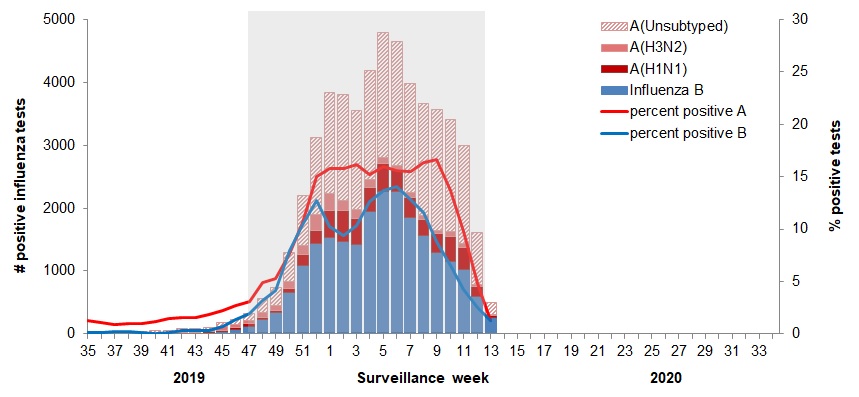
The shaded area indicates weeks where the positivity rate was at least 5% and a minimum of 15 positive tests were observed, signalling the period of seasonal influenza activity.
Figure 2 - Text equivalent
| Surveillance Week | A(Unsubtyped) | A(H3N2) | A(H1N1) | Influenza B | Percent Positive A | Percent Positive B |
|---|---|---|---|---|---|---|
| 35 | 10 | 16 | 0 | 2 | 1.3 | 0.1 |
| 36 | 11 | 13 | 2 | 2 | 1.1 | 0.1 |
| 37 | 5 | 17 | 2 | 5 | 0.9 | 0.2 |
| 38 | 11 | 15 | 3 | 6 | 1.0 | 0.2 |
| 39 | 11 | 21 | 2 | 3 | 1.0 | 0.1 |
| 40 | 34 | 9 | 1 | 2 | 1.2 | 0.1 |
| 41 | 34 | 18 | 0 | 5 | 1.4 | 0.1 |
| 42 | 54 | 12 | 1 | 14 | 1.6 | 0.3 |
| 43 | 44 | 13 | 7 | 17 | 1.6 | 0.3 |
| 44 | 43 | 23 | 16 | 17 | 1.8 | 0.3 |
| 45 | 57 | 57 | 20 | 39 | 2.2 | 0.7 |
| 46 | 82 | 43 | 23 | 77 | 2.7 | 1.4 |
| 47 | 118 | 49 | 33 | 124 | 3.1 | 1.9 |
| 48 | 225 | 67 | 42 | 223 | 4.9 | 3.2 |
| 49 | 281 | 79 | 41 | 336 | 5.3 | 4.1 |
| 50 | 463 | 100 | 73 | 654 | 7.7 | 8.0 |
| 51 | 794 | 149 | 169 | 1094 | 10.6 | 10.4 |
| 52 | 1223 | 267 | 197 | 1439 | 15.0 | 12.7 |
| 1 | 1620 | 261 | 431 | 1533 | 15.8 | 10.3 |
| 2 | 1690 | 165 | 493 | 1463 | 15.8 | 9.4 |
| 3 | 1575 | 139 | 417 | 1418 | 16.2 | 10.3 |
| 4 | 1727 | 133 | 370 | 1952 | 15.2 | 12.7 |
| 5 | 1983 | 97 | 440 | 2269 | 16.0 | 13.7 |
| 6 | 1970 | 75 | 344 | 2265 | 15.6 | 14.1 |
| 7 | 1741 | 76 | 321 | 1851 | 15.5 | 12.8 |
| 8 | 1768 | 74 | 255 | 1559 | 16.3 | 11.6 |
| 9 | 1928 | 58 | 303 | 1287 | 16.7 | 8.9 |
| 10 | 1773 | 81 | 399 | 1152 | 13.6 | 6.5 |
| 11 | 1552 | 70 | 354 | 1016 | 9.8 | 4.3 |
| 12 | 830 | 38 | 152 | 594 | 4.8 | 2.5 |
| 13 | 179 | 17 | 33 | 265 | 1.3 | 1.3 |
Figure 3 - Distribution of positive influenza specimens by type/subtype and province/territory, Canada, weeks 2019-35 to 2020-13
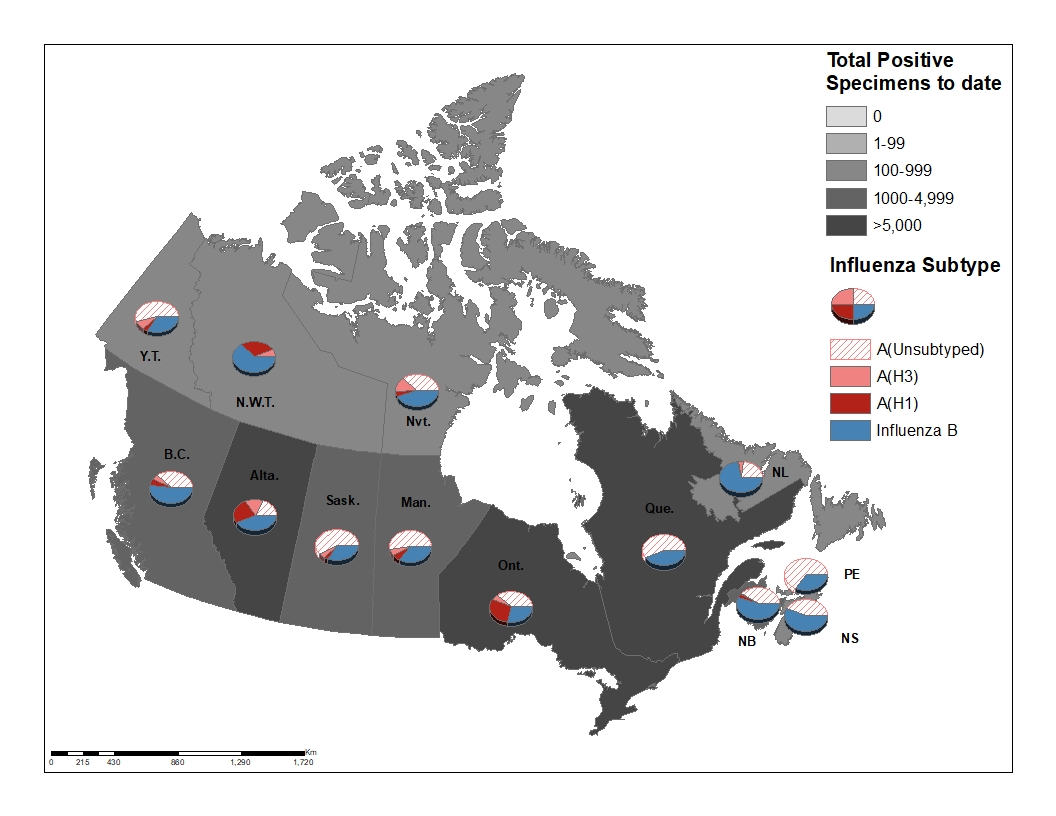
Figure 3 - Text equivalent
| ProvincesTable Figure 3 - Footnote 1 | Cumulative (August 25, 2019 to March 28, 2020) | |||||
|---|---|---|---|---|---|---|
| A Total | A(H1N1) | A(H3N2) | A(UnS)Table Figure 3 - Footnote 3 | B Total | A & B Total | |
| B.C. | 4072 | 276 | 243 | 1846 | 2552 | 6624 |
| Alta. | 4352 | 1721 | 1285 | 1346 | 3403 | 7755 |
| Sask. | 1370 | 59 | 102 | 1209 | 700 | 2070 |
| Man. | 1637 | 174 | 134 | 1329 | 883 | 2520 |
| Ont. | 6468 | 2563 | 459 | 3446 | 2554 | 9022 |
| Que. | 12827 | 0 | 0 | 12827 | 10025 | 22852 |
| N.B. | 1045 | 60 | 29 | 956 | 1292 | 2337 |
| N.S. | 195 | 3 | 2 | 190 | 238 | 433 |
| P.E.I. | 144 | 0 | 0 | 144 | 78 | 222 |
| N.L. | 200 | 8 | 33 | 159 | 491 | 691 |
| Y.T. | 70 | 4 | 8 | 55 | 35 | 105 |
| N.W.T | 107 | 87 | 19 | 1 | 186 | 293 |
| Nvt. | 60 | 5 | 14 | 41 | 49 | 109 |
| Canada | 32547 | 4960 | 2328 | 23549 | 22486 | 55033 |
| PercentageTable Figure 3 - Footnote 2 | 59% | 15% | 7% | 72% | 41% | 100% |
|
||||||
| Age groups (years) | Cumulative (August 25, 2019 to March 28, 2020) | ||||||
|---|---|---|---|---|---|---|---|
| Influenza A | B | Influenza A and B | |||||
| A Total | A(H1N1) | A(H3N2) | A (Un subtyped) Table 1 Footnote 1 | Total | # | % | |
| 0-4 | 3797 | 441 | 213 | 3143 | 4037 | 7834 | 18% |
| 5-19 | 2741 | 297 | 261 | 2183 | 5983 | 8724 | 21% |
| 20-44 | 5216 | 963 | 330 | 3923 | 5539 | 10755 | 25% |
| 45-64 | 5012 | 978 | 303 | 3731 | 1143 | 6155 | 15% |
| 65+ | 7503 | 1050 | 941 | 5512 | 1384 | 8887 | 21% |
| Total | 24269 | 3729 | 2048 | 18492 | 18086 | 42355 | 100% |
|
|||||||
Syndromic / Influenza-like Illness Surveillance
Healthcare Professionals Sentinel Syndromic Surveillance
In week 13, 1.1% of visits to healthcare professionals were due to influenza-like illness (ILI) which is a slight decrease from the previous week and around average for this time of year (Figure 4). This trend should be interpreted with caution as there continues to be a decreasing number of sentinels reporting. Given the evolving Canadian situation with COVID-19, we will continue to monitor this indicator closely.
Figure 4 – Percentage of visits for ILI reported by sentinels by report week, Canada, weeks 2019-35 to 2020-13
Number of Sentinels Reporting in week 13: 60
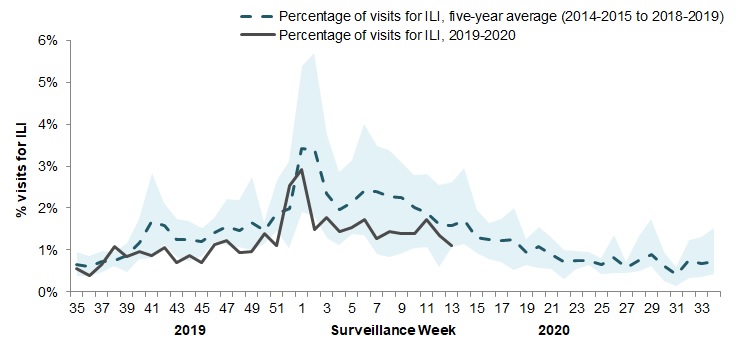
The shaded area represents the maximum and minimum percentage of visits for ILI reported by week from seasons 2014-2015 to 2018-2019
Figure 4 - Text equivalent
| Surveillance Week | 2019-2020 | Average | Min | Max |
|---|---|---|---|---|
| 35 | 0.6% | 0.6% | 0.4% | 0.9% |
| 36 | 0.4% | 0.6% | 0.4% | 0.9% |
| 37 | 0.7% | 0.7% | 0.5% | 1.0% |
| 38 | 1.1% | 0.7% | 0.6% | 1.0% |
| 39 | 0.8% | 0.9% | 0.5% | 1.2% |
| 40 | 1.0% | 1.2% | 0.8% | 1.7% |
| 41 | 0.9% | 1.7% | 0.8% | 2.8% |
| 42 | 1.1% | 1.6% | 1.2% | 2.1% |
| 43 | 0.7% | 1.2% | 0.8% | 1.7% |
| 44 | 0.9% | 1.2% | 0.7% | 1.7% |
| 45 | 0.7% | 1.2% | 0.9% | 1.5% |
| 46 | 1.1% | 1.4% | 1.2% | 1.8% |
| 47 | 1.2% | 1.6% | 1.1% | 2.2% |
| 48 | 0.9% | 1.5% | 1.1% | 2.2% |
| 49 | 1.0% | 1.7% | 1.0% | 2.8% |
| 50 | 1.4% | 1.5% | 1.1% | 1.7% |
| 51 | 1.1% | 1.9% | 1.4% | 2.7% |
| 52 | 2.5% | 2.0% | 1.0% | 3.1% |
| 1 | 2.9% | 3.4% | 1.9% | 5.4% |
| 2 | 1.5% | 3.4% | 1.8% | 5.7% |
| 3 | 1.8% | 2.3% | 1.3% | 3.7% |
| 4 | 1.4% | 2.0% | 1.1% | 2.9% |
| 5 | 1.5% | 2.1% | 1.4% | 3.1% |
| 6 | 1.7% | 2.4% | 1.4% | 4.0% |
| 7 | 1.3% | 2.4% | 0.9% | 3.5% |
| 8 | 1.4% | 2.3% | 0.8% | 3.4% |
| 9 | 1.4% | 2.3% | 0.9% | 3.1% |
| 10 | 1.4% | 2.0% | 1.0% | 2.8% |
| 11 | 1.7% | 1.9% | 1.1% | 2.8% |
| 12 | 1.4% | 1.6% | 0.6% | 2.6% |
| 13 | 1.1% | 1.6% | 1.1% | 2.6% |
FluWatchers
In week 13, 3,124 participants reported to FluWatchers, of which 0.9% (27) reported symptoms of cough and fever (Figure 5).The percentage of participants reporting cough and fever continue to decrease and are at the lowest levels ever observed. This may be due to social distancing measures implemented in recent weeks. Given the evolving Canadian situation with COVID-19, we will continue to monitor this indicator closely.
Among the 27 participants who reported cough and fever:
- 38% consulted a healthcare professional, a larger proportion than in recent weeks;
- 52% reported days missed from work or school, a similar proportion to the previous week, resulting in a combined total of 56 missed days of work or school.
If you are interested in becoming a FluWatcher, sign up today.
Figure 5 - Percentage of FluWatchers participants reporting cough and fever, Canada, weeks 2019-40 to 2020-13
Number of participants reporting in week 13: 3,124
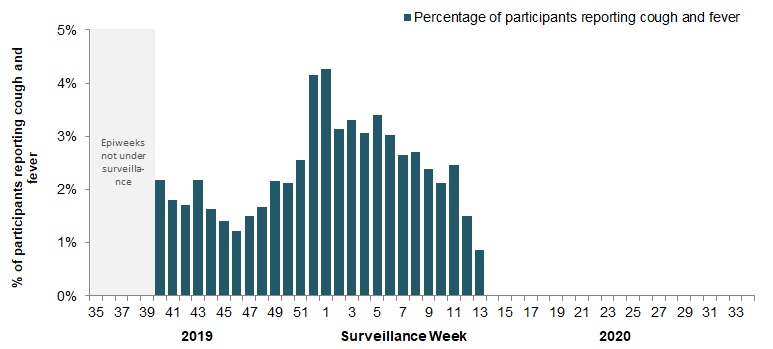
Figure 5 - Text equivalent
| Surveillance Week | % cough and fever |
|---|---|
| 40 | 2.2% |
| 41 | 1.8% |
| 42 | 1.7% |
| 43 | 2.2% |
| 44 | 1.6% |
| 45 | 1.4% |
| 46 | 1.2% |
| 47 | 1.5% |
| 48 | 1.7% |
| 49 | 2.2% |
| 50 | 2.1% |
| 51 | 2.6% |
| 52 | 4.1% |
| 1 | 4.3% |
| 2 | 3.1% |
| 3 | 3.3% |
| 4 | 3.1% |
| 5 | 3.4% |
| 6 | 3.0% |
| 7 | 2.7% |
| 8 | 2.7% |
| 9 | 2.4% |
| 10 | 2.1% |
| 11 | 2.5% |
| 12 | 1.5% |
| 13 | 0.9% |
Online Figure - Geographic distribution of FluWatchers participants reporting cough and fever, Canada, week 2020-13
Click on the map to access the link
Influenza Outbreak Surveillance
In week 13, a total of 5 outbreaks were reported: 3 in long term care facilities and 2 in acute care facilities (Figure 6).
To date this season, a total of 903 laboratory-confirmed influenza outbreaks have been reported; 65% (583) in long-term care facilities, 25% (221) in facilities categorized as ‘other’, 9% (84) in acute care facilities, and 2% (15) in schools/daycares. Of the 855 outbreaks where influenza type was reported, 88% (750) were due to influenza A. Among the 307 outbreaks for which the influenza A subtype was reported, 53% were associated with A(H1N1) and 47% with A(H3N2). To date this season, 175 ILI outbreaks have also been reported; 98% (171) in schools/daycares and 2% (4) in facilities categorized as ‘other’.
Figure 6 - Number of new outbreaks of laboratory-confirmed influenza by report week, Canada, weeks 2019-35 to 2020-13
Number of provinces and territories reporting in week 13: 10 out of 13
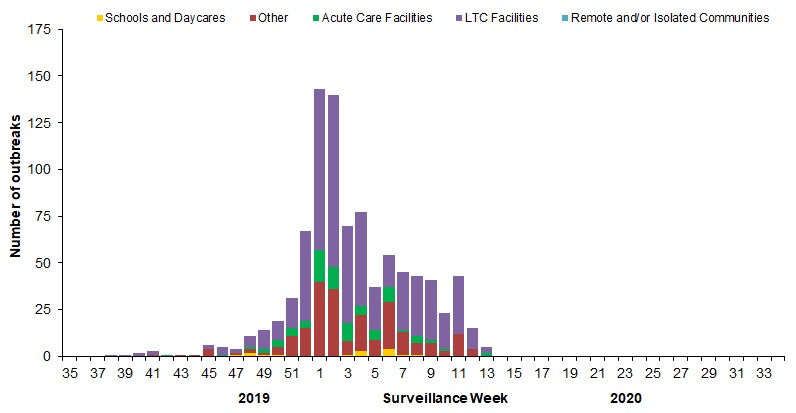
Figure 6 - Text equivalent
| Surveillance Week | Acute Care Facilities | Long Term Care Facilities | Other | Schools and Daycares | Remote and/or Isolated Communities |
|---|---|---|---|---|---|
| 35 | 0 | 0 | 0 | 0 | 0 |
| 36 | 0 | 0 | 0 | 0 | 0 |
| 37 | 0 | 0 | 0 | 0 | 0 |
| 38 | 0 | 1 | 0 | 0 | 0 |
| 39 | 0 | 1 | 0 | 0 | 0 |
| 40 | 0 | 2 | 0 | 0 | 0 |
| 41 | 0 | 2 | 1 | 0 | 0 |
| 42 | 1 | 0 | 0 | 0 | 0 |
| 43 | 0 | 0 | 1 | 0 | 0 |
| 44 | 0 | 0 | 1 | 0 | 0 |
| 45 | 0 | 2 | 4 | 0 | 0 |
| 46 | 1 | 4 | 0 | 0 | 0 |
| 47 | 0 | 2 | 1 | 1 | 0 |
| 48 | 1 | 6 | 2 | 2 | 0 |
| 49 | 2 | 10 | 1 | 1 | 0 |
| 50 | 4 | 10 | 4 | 1 | 0 |
| 51 | 4 | 16 | 11 | 0 | 0 |
| 52 | 4 | 48 | 15 | 0 | 0 |
| 1 | 17 | 86 | 40 | 0 | 0 |
| 2 | 12 | 92 | 36 | 0 | 0 |
| 3 | 10 | 52 | 7 | 1 | 0 |
| 4 | 5 | 50 | 19 | 3 | 0 |
| 5 | 5 | 23 | 9 | 0 | 0 |
| 6 | 8 | 17 | 25 | 4 | 0 |
| 7 | 1 | 31 | 12 | 1 | 0 |
| 8 | 4 | 32 | 6 | 1 | 0 |
| 9 | 2 | 32 | 7 | 0 | 0 |
| 10 | 1 | 19 | 3 | 0 | 0 |
| 11 | 0 | 31 | 12 | 0 | 0 |
| 12 | 0 | 11 | 4 | 0 | 0 |
| 13 | 2 | 3 | 0 | 0 | 0 |
Severe Outcomes Influenza Surveillance
Provincial/Territorial Influenza Hospitalizations and Deaths
To date this season, 2,333 influenza-associated hospitalizations were reported by participating provinces and territories Footnote 1.
- 69% of the cases were associated with influenza A.
- Of the 1,036 cases for which subtype was reported, 52% were associated with influenza A(H3N2).
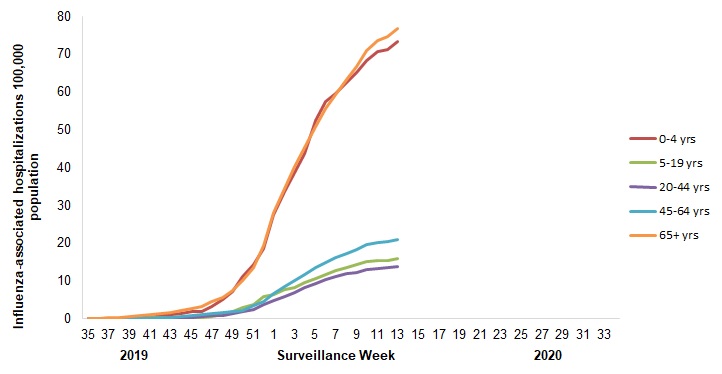
- The highest cumulative hospitalization rates up to week 13 were among adults 65 years of age and older (77/100,000 population) and children under 5 years of age (73/100,000 population).
285 ICU admissions and 102 deaths have been reported.
- 69% of the ICU admissions and 72% of the deaths were associated with influenza A.
Figure 7 - Cumulative rates of influenza-associated hospitalization by age group and epidemiological week, Canada, participating provinces and territories Footnote 1 weeks 2019-35 to 2020-13
Number of provinces and territories reporting in week 13: 4 out of 9
- Footnote ‡
-
Influenza-associated hospitalizations are reported by Alberta, Manitoba, New Brunswick, Newfoundland and Labrador, Northwest Territories, Nova Scotia, Prince Edward Island and Yukon. Only hospitalizations that require intensive medical care are reported by Saskatchewan.
Figure 7 - Text equivalent
| Surveillance Week | 0-4 yrs | 5-19 yrs | 20-44 yrs | 45-64 yrs | 65+ yrs |
|---|---|---|---|---|---|
| 35 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| 36 | 0.0 | 0.0 | 0.0 | 0.0 | 0.2 |
| 37 | 0.0 | 0.0 | 0.0 | 0.1 | 0.2 |
| 38 | 0.0 | 0.0 | 0.0 | 0.1 | 0.4 |
| 39 | 0.2 | 0.0 | 0.0 | 0.1 | 0.6 |
| 40 | 0.2 | 0.0 | 0.0 | 0.1 | 0.8 |
| 41 | 0.4 | 0.2 | 0.1 | 0.2 | 1.1 |
| 42 | 0.8 | 0.2 | 0.1 | 0.2 | 1.3 |
| 43 | 1.0 | 0.2 | 0.2 | 0.3 | 1.6 |
| 44 | 1.5 | 0.2 | 0.2 | 0.5 | 2.1 |
| 45 | 1.9 | 0.4 | 0.4 | 0.8 | 2.8 |
| 46 | 1.9 | 0.4 | 0.6 | 1.1 | 3.3 |
| 47 | 3.1 | 0.6 | 0.8 | 1.5 | 4.6 |
| 48 | 5.0 | 1.1 | 1.0 | 1.6 | 5.7 |
| 49 | 7.1 | 1.9 | 1.3 | 2.0 | 7.5 |
| 50 | 11.1 | 3.0 | 1.8 | 2.3 | 10.2 |
| 51 | 14.3 | 3.7 | 2.5 | 3.5 | 13.6 |
| 52 | 18.7 | 5.8 | 3.7 | 4.5 | 19.3 |
| 1 | 27.7 | 6.5 | 4.8 | 6.6 | 28.1 |
| 2 | 33.8 | 7.6 | 6.0 | 8.4 | 34.5 |
| 3 | 38.8 | 8.4 | 7.0 | 10.0 | 40.4 |
| 4 | 43.7 | 9.6 | 8.2 | 11.8 | 45.4 |
| 5 | 52.5 | 10.7 | 9.4 | 13.5 | 50.9 |
| 6 | 57.5 | 11.8 | 10.5 | 15.0 | 55.7 |
| 7 | 59.6 | 12.8 | 11.2 | 16.3 | 59.5 |
| 8 | 62.6 | 13.7 | 11.9 | 17.3 | 63.4 |
| 9 | 65.3 | 14.4 | 12.3 | 18.4 | 66.8 |
| 10 | 68.2 | 15.2 | 12.9 | 19.5 | 71.0 |
| 11 | 70.7 | 15.4 | 13.3 | 20.2 | 73.7 |
| 12 | 71.2 | 15.6 | 13.5 | 20.5 | 74.8 |
| 13 | 73.3 | 16.0 | 13.8 | 21.0 | 76.7 |
Pediatric Influenza Hospitalizations and Deaths
In week 13, four pediatric (≤16 years of age) laboratory-confirmed influenza-associated hospitalizations were reported by the Immunization Monitoring Program Active (IMPACT) network (Figure 8). Since the beginning of March, the weekly number of reported cases have dropped below the 5-year weekly average.
To date this season (weeks 35 to 13):
- 1,207 pediatric hospitalizations have been reported by the IMPACT network, of which 52% (622) were associated with influenza A and 49% (585) with influenza B.
- The largest proportion of hospitalizations (66%) were among children under 5 years of age (Figure 9).
- 203 ICU admissions were reported, of which 58% were associated with influenza A, and 61% were among children under 5 years of age.
- Five pediatric deaths have been reported.
Figure 8 - Number of pediatric (≤16 years of age) hospitalizations reported by the IMPACT network, by week, Canada, weeks 2019-35 to 2020-13
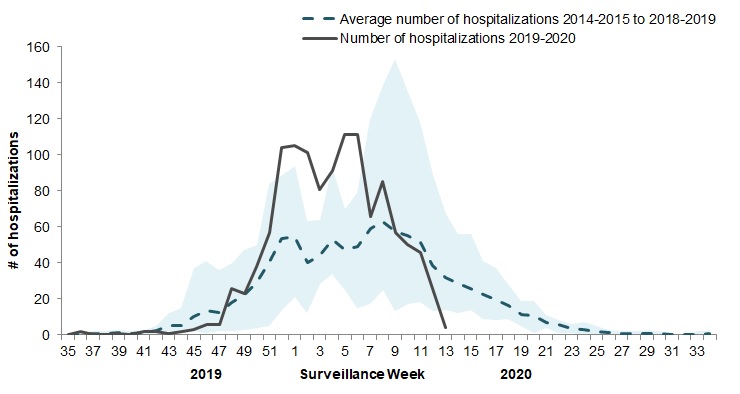
The shaded area represents the maximum and minimum number of cases reported by week from seasons 2014-15 to 2018-19
Figure 8 - Text equivalent
| Surveillance week | 2019-2020 | Average | Min | Max |
|---|---|---|---|---|
| 35 | 0 | 0 | 0 | 1 |
| 36 | 2 | 0 | 0 | 1 |
| 37 | 0 | 1 | 0 | 2 |
| 38 | 0 | 1 | 0 | 2 |
| 39 | 1 | 1 | 0 | 3 |
| 40 | 0 | 1 | 0 | 2 |
| 41 | 2 | 1 | 0 | 3 |
| 42 | 2 | 2 | 0 | 5 |
| 43 | 1 | 5 | 2 | 12 |
| 44 | 2 | 5 | 1 | 15 |
| 45 | 3 | 10 | 2 | 37 |
| 46 | 6 | 13 | 1 | 41 |
| 47 | 6 | 13 | 2 | 36 |
| 48 | 26 | 18 | 2 | 40 |
| 49 | 23 | 22 | 3 | 47 |
| 50 | 38 | 29 | 4 | 50 |
| 51 | 57 | 41 | 5 | 84 |
| 52 | 104 | 54 | 14 | 89 |
| 1 | 105 | 55 | 21 | 94 |
| 2 | 101 | 40 | 12 | 63 |
| 3 | 81 | 44 | 28 | 64 |
| 4 | 91 | 53 | 34 | 93 |
| 5 | 111 | 47 | 25 | 70 |
| 6 | 111 | 49 | 15 | 79 |
| 7 | 66 | 59 | 17 | 120 |
| 8 | 85 | 63 | 25 | 139 |
| 9 | 57 | 58 | 13 | 153 |
| 10 | 50 | 55 | 17 | 135 |
| 11 | 46 | 51 | 18 | 118 |
| 12 | 26 | 39 | 13 | 89 |
| 13 | 4 | 32 | 14 | 67 |
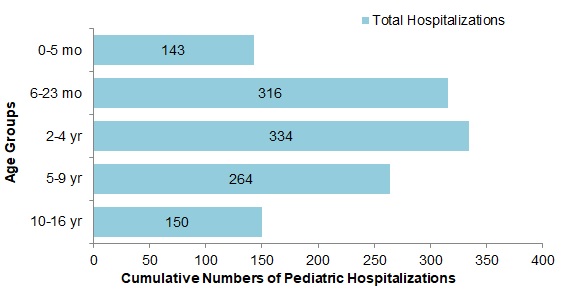
Figure 9 - Text Description
| Age Group | Total |
|---|---|
| 0-5 mo | 143 |
| 6-23 mo | 316 |
| 2-4 yr | 334 |
| 5-9 yr | 264 |
| 10-16 yr | 150 |
Adult Influenza Hospitalizations and Deaths
Surveillance of laboratory-confirmed influenza-associated adult (≥16 years of age) hospitalizations by the Canadian Immunization Research Network (CIRN) Serious Outcomes Surveillance (SOS) network began on November 1st for the 2019-20 season.
To date this season, 787 hospitalizations, 87 intensive care unit admissions, and 42 deaths have been reported (Figure 10).
- The majority of hospitalizations have been due to influenza A (79%), and among those subtyped (166) 92% were influenza A(H1N1).
- Among the 623 cases with influenza A, the largest proportion of hospitalizations were in adults 65 years of age and older (66%). Among the 153 cases with influenza B, 52% were in adults 65 years of age and older, and 28% of cases were between 16 and 34 years of age (Figure 11).
- 90% of hospitalized cases reported at least one type of comorbid condition.
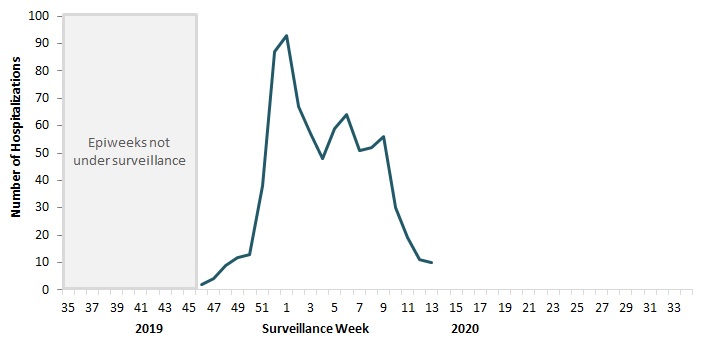
Figure 10 - Text Description
| Surveillance Week | Number of Hospitalizations |
|---|---|
| 35 | No data |
| 36 | No data |
| 37 | No data |
| 38 | No data |
| 39 | No data |
| 40 | No data |
| 41 | No data |
| 42 | No data |
| 43 | No data |
| 44 | No data |
| 45 | No data |
| 46 | 2 |
| 47 | 4 |
| 48 | 9 |
| 49 | 12 |
| 50 | 13 |
| 51 | 38 |
| 52 | 87 |
| 1 | 93 |
| 2 | 67 |
| 3 | 57 |
| 4 | 48 |
| 5 | 59 |
| 6 | 64 |
| 7 | 51 |
| 8 | 52 |
| 9 | 56 |
| 10 | 30 |
| 11 | 19 |
| 12 | 11 |
| 13 | 10 |
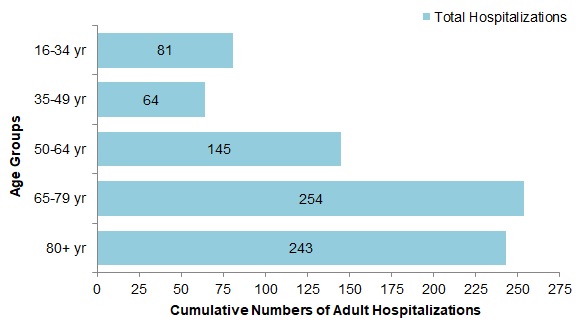
Figure 11 - Text Description
| Age Group | Total hospitalizations |
|---|---|
| 16-34 yr | 81 |
| 35-49 yr | 64 |
| 50-64 yr | 145 |
| 65-79 yr | 254 |
| 80+ yr | 243 |
Influenza Strain Characterizations
From September 1, 2019 to March 19, 2020, the National Microbiology Laboratory (NML) has characterized 1,224 influenza viruses (474 A(H1N1), 177 A(H3N2) and 573 influenza B) that were received from Canadian laboratories.
Influenza A(H3N2)
Over recent years, circulating strains of A(H3N2) have evolved, and are increasingly difficult to characterize by hemagglutination inhibition (HI) assay. Genetic characterization is established by sequencing the hemagglutinin (HA) gene of the influenza viruses to compare their genetic properties.
Antigenic Characterization:
Among the 55 influenza A(H3N2) viruses antigenically characterized to date, the majority (80%) showed reduced titer by HI assay to A Kansas/14/2017 using antiserum raised against egg-propagated A Kansas/14/2017. Eleven viruses were characterized as A Kansas/14/2017-like (Figure 12a).
Genetic Characterization:
Nearly all (98%) of the 160 A(H3N2) viruses genetically characterized this season belonged to genetic group 3C.2a1b based on sequence analysis of the HA gene. Three viruses belonged to the genetic group 3C.3a (Figure 13).
Group 3C.2a1b viruses analysed represent:
- 92% (35 out of 38) viruses that were also antigenically characterized.
- 100% (122 out of 122) viruses which did not grow to sufficient hemagglutination titer for antigenic characterization by HI assay.
A/Kansas/14/2017 belongs to genetic group 3C.3a and is the influenza A(H3N2) component of the 2019-20 Northern Hemisphere influenza vaccine.
Influenza A(H1N1)
Among the 474 A(H1N1) viruses characterized to date, 53% were antigenically similar to A/Brisbane/02/2018 by HI testing using antiserum raised against egg-propagated A/Brisbane/02/2018 (Figure 12 b).
A/Brisbane/02/2018 is the influenza A(H1N1) component of the 2019-20 Northern Hemisphere influenza vaccine.
Influenza B
Antigenic Characterization:
Among the 182 influenza B viruses antigenically characterized this season, the vast majority (180) belonged to the B/Victoria lineage. Two viruses were antigenically characterized as similar to B/Phuket/3073/2013 (B/Yamagata lineage).
The majority (89%, 161) of B/Victoria lineage viruses showed reduced titer by HI assay to B/Colorado/06/2017 using antiserum raised against cell culture-propagated B/Colorado/06/2017 (Figure 12c).
Sequence analysis of 150 B/Victoria lineage viruses with reduced titre to B/Colorado/06/2017 showed that 100% had a three amino acid deletion (162-164) in the HA gene and belong to the genetic subclade V1A.3 (3Del). Sequencing is pending for the remaining viruses.
Genetic Characterization:
Genetic characterization was also performed on 391 B/Victoria lineage viruses. All of these viruses had a three amino acid deletion (162-164) in the HA gene and belong to the genetic subclade V1A.3 (3Del).
To date, 100% (541) of influenza B/Victoria viruses genetically characterized belong to the genetic subclade V1A.3 (3Del) (Fig 13b). Viruses in this genetic subclade are antigenically distinct from the vaccine strain B/Colorado/06/2017, which belongs to genetic subclade V1A.1 (2Del).
The recommended influenza B components for the 2019-20 Northern Hemisphere influenza vaccine are B/Colorado/06/2017 (Victoria lineage) and B/Phuket/3073/2013 (Yamagata lineage). B/Phuket/3073/2013 is included in the quadrivalent influenza vaccine. The vaccine strain B/Colorado/06/2017 belongs to genetic subclade V1A.1.
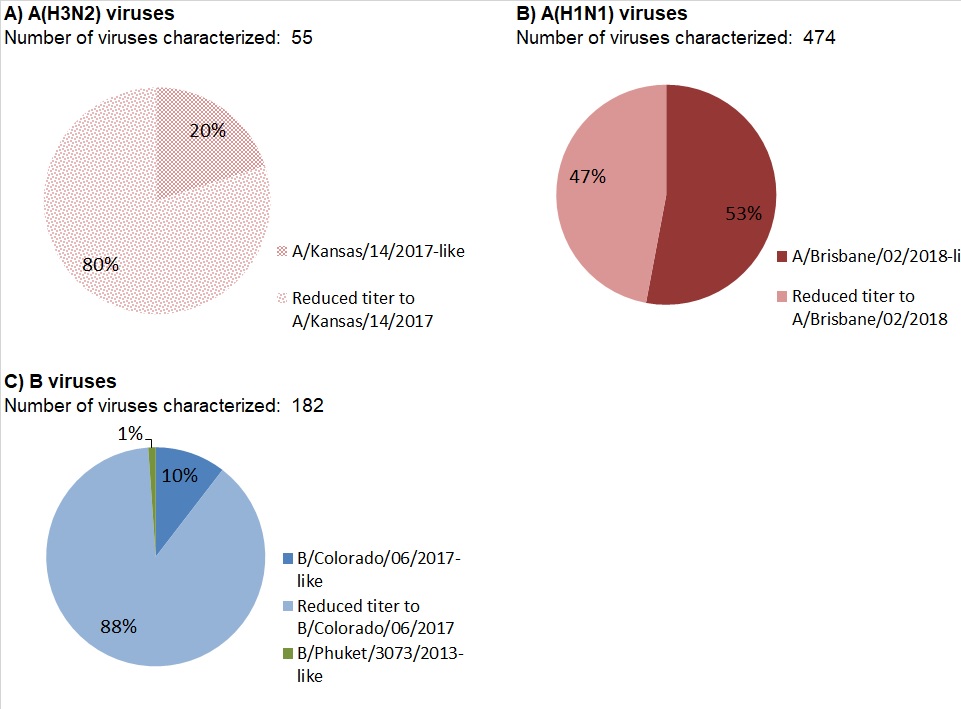
Figure 12 - Text Description
| Number of viruses characterized: 55 | ||
| Antigenic phenotype of A(H3N2) virus | Number of viruses | Percentage |
|---|---|---|
| A/Kansas/14/2017-like | 11 | 20% |
| Reduced titer to A/Kansas/14/2017 | 44 | 80% |
| Number of viruses characterized: 474 | ||
| Antigenic phenotype of A(H1N1) virus | Number of viruses | Percentage |
|---|---|---|
| A/Brisbane/02/2018-like | 251 | 53% |
| Reduced titer to A/Brisbane/02/2018 | 223 | 47% |
| Number of viruses characterized: 182 | ||
| Antigenic phenotype of influenza B virus | Number of viruses | Percentage |
|---|---|---|
| B/Colorado/06/2017-like | 19 | 10% |
| Reduced titer to B/Colorado/06/2017 | 161 | 88% |
| B/Phuket/3073/2013-like | 2 | 1% |
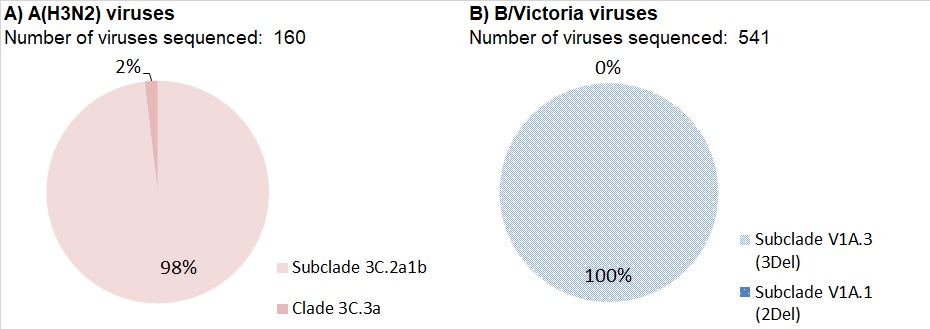
Figure 13 - Text Description
| Number of viruses sequenced: 160 | ||
| Genetic Clade of A(H3N2) virus | Number of viruses | Percentage |
|---|---|---|
| Subclade 3C.2a1b | 157 | 98% |
| Clade 3C.3a | 3 | 2% |
| Number of viruses sequenced: 541 | ||
| Genetic Clade of influenza B/Victoria virus | Number of viruses | Percentage |
|---|---|---|
| Subclade V1A.3 (3Del) | 541 | 100% |
| Subclade V1A.1 (2Del) | 0 | 0% |
Antiviral Resistance
The National Microbiology Laboratory (NML) also tests influenza viruses received from Canadian laboratories for antiviral resistance. From September 1, 2019 to March 19, 2020, the following results were reported:
Oseltamivir:
669 influenza viruses (155 A(H3N2), 251 A(H1N1) and 263 B) were tested for resistance to oseltamivir:
- All influenza A(H3N2) and B viruses were sensitive to oseltamivir.
- Among the A(H1N1) viruses tested, 250 (99.6%) were sensitive to oseltamivir and one virus was resistant to oseltamivir with the H275Y mutation in the neuraminidase gene.
Zanamivir:
669 influenza viruses (155 A(H3N2), 251 A(H1N1) and 263 B) were tested for resistance to zanamivir:
- All influenza viruses tested were sensitive to zanamivir.
Amantadine:
High levels of resistance to amantadine persist among influenza A(H1N1) and influenza A(H3N2) viruses. All viruses tested this season were resistant.
Vaccine Monitoring
Vaccine monitoring refers to activities related to the monitoring of influenza vaccine coverage and effectiveness.
Vaccine Coverage
The Seasonal Influenza Immunization Coverage Survey is an annual telephone survey conducted between January and February that collects information from Canadians on whether they received the annual seasonal influenza vaccine that season. Vaccine coverage is measured as the percentage of people who received the influenza vaccine in a specific influenza season.
In the 2019-20 influenza season, coverage was similar to the 2018-19 season, at:
- 34% among adults aged 18 to 64 years.
- 30% among adults aged 18-64 without chronic diseases.
- 44% among adults aged 18 to 64 years with chronic diseases.
- 70% among seniors (aged 65 years and older).
| Age group (years) | All | Male | Female | |||
|---|---|---|---|---|---|---|
| N | Vaccine Coverage % (95% CI) |
N | Vaccine Coverage % (95% CI) |
N | Vaccine Coverage % (95% CI) |
|
| All adults (≥18) | 3023 | 41.8 (39.7-43.9) | 1320 | 37.2 (34.1-40.2) | 1691 | 46.1 (43.2-49.0) |
| 18-64 | 2234 | 34.1 (31.8-36.5) | 1005 | 29.8 (26.5-33.1) | 1218 | 38.4 (35.1-41.7) |
| with chronic diseases | 668 | 43.6 (39.0-48.1) | 268 | 38.3 (31.5-45.2) | 397 | 47.9 (41.7-54.0) |
| without chronic diseases | 1558 | 30.0 (27.3-32.7) | 732 | 26.7 (22.9-30.5) | 818 | 33.5 (29.6-37.3) |
| ≥65 | 789 | 70.3 (66.7-73.8) | 315 | 67.2 (61.5-72.9) | 473 | 72.7 (68.3-77.1) |
CI: 95% confidence interval
|
||||||
Vaccine Effectiveness
The Canadian Sentinel Practitioner Surveillance Network (SPSN) provides estimates of the effectiveness of the seasonal influenza vaccine in preventing medically-attended illness due to laboratory-confirmed influenza among Canadians.
Based on data collected between November 1, 2019 and February 1, 2020, vaccine effectiveness (VE) was estimated to be 58% for any influenza, 44% for influenza A(H1N1), 62% for influenza A(H3N2), and 69% for influenza B. Substantial protection was observed among children 1 to 19 years of age against both influenza A and B. A good level of protection was also observed among working age adults (20-64 yrs) across all influenza types (Table 2). VE among adults 65 years and older, although imprecise due to small numbers, was lower at 18% (95% CI -59 to 58). The SPSN interim estimates are published and available online.
More information on the network and past VE findings can be viewed on the SPSN website.
Updated influenza vaccine effectiveness estimates will be published at the end of the 2019/2020 influenza season. At that time, sufficient data will likely be available to estimate VE by age-group, including adults 65 years and older with greater precision, as well as for influenza A subtypes.
| All ages | 1-19 years | 20-64 years | ||
|---|---|---|---|---|
| VE(%) (CI)Table 3 - Footnote * | N | VE(%) (CI)Table 3 - Footnote * | VE(%) (CI)Table 3 - Footnote * | |
| All Influenza | 58 (47, 66) | 2808 | 74 (59, 84) | 55 (41, 66) |
| Influenza A | 49 (34, 60) | 2128 | 70 (44, 84) | 45 (25, 59) |
| Influenza A(H1N1) | 44 (26, 58) | 1948 | - | - |
| Influenza A(H3N2) | 62 (37, 77) | 1561 | - | - |
| Influenza B | 69 (57, 77) | 2080 | 77 (59, 87) | 68 (51, 79) |
|
||||