Update on HIV-1 Strain and Transmitted Drug Resistance in Canada: Findings from the Canadian HIV Strain and Drug Resistance Surveillance Program, 2012-2013

Download the alternative format
(PDF format, 1.24 MB, 48 pages)
Organization: Public Health Agency of Canada
Date published: 2017-12-01
Acknowledgements: We acknowledge the provincial HIV/AIDS coordinators, laboratories, healthcare providers, and reporting physicians for providing the data required to publish this report. Please refer to Appendix VI for a list of these contributors.
N.B. This document must be cited as the source for any information extracted and used from it.
Suggested citation: Public Health Agency of Canada. Update on HIV-1 Strain and Transmitted Drug Resistance in Canada: 2012-2013. Centre for Communicable Diseases and Infection Control, Public Health Agency of Canada, 2017.
Table of Contents
- List of Figures
- List of Tables
- List of Appendices
- Acronyms and Abbreviations
- Executive Summary
- Introduction
- Methods
- Results
- Limitations
- Conclusion
- References
- Appendices
List of Figures
- Figure 1: Overview of the Canadian HIV Strain and Drug Resistance Surveillance Program
- Figure 2: Overview of specimens in the Canadian HIV Strain and Drug Resistance Surveillance Program, 2012-2013
- Figure 3: Number and proportion of non-B subtypes by province, 2012-2013
- Figure 4: Number and proportion of non-B subtypes by age group, 2012-2013
- Figure 5: Number and proportion of specimens with drug resistance by province, 2012-13
- Figure 6: Number and proportion of specimens with drug resistance by age group, 2012-2013
- Figure 7: Distribution of resistant specimens by drug class, 2012-2013 (n=251)
- Figure 8: Proportion of drug class resistance by province, 2012-2013
- Figure 9: Resistance to commonly prescribed NNRTI drugs, 2012-2013
- Figure 10: Resistance to commonly prescribed NRTI drugs, 2012-2013
- Figure 11: Resistance to commonly prescribed PI drugs, 2012-2013
List of Tables
- Table 1: Sample characteristics of individuals newly diagnosed with HIV vs. those included in SDR Program, 2012-2013
- Table 2: Number and proportion of HIV-1 subtypes, 2012-2013
- Table 3: Number and proportion of B and non-B subtypes by sex and race/ethnicity, 2012-2013
- Table 4: Number and proportion of B and non-B subtypes by sex and exposure category, 2012-2013
- Table 5: Number and proportion of B and non-B subtypes by race/ethnicity and exposure category, 2012-2013
- Table 6: Number and proportion of specimens with drug resistance by sex and race/ethnicity, 2012-2013
- Table 7: Number and proportion of specimens with drug resistance by sex and exposure category, 2012-2013
- Table 8: Number and proportion of specimens with drug resistance by race/ethnicity and exposure category, 2012-2013
- Table 9: Number and proportion of specimens with drug resistance (single class only and MDR) by subtype, 2012-2013
- Table 10: Number and proportion of specimens with drug resistance (any class and MDR) by subtype 2012-2013
- Table 11: Comparison of the number and proportion of drug resistant specimens in 2008 vs. 2012-2013
- Table 12: Number and proportion of HIV-1 subtypes among those whom a first genotyping assay was carried out in Quebec, 2012-2013
- Table 13: Number and proportion of HIV-1 subtypes by year in Quebec, 2012-2013
- Table 14: Number and proportion of HIV-1 subtypes by sex in Quebec, 2012-2013
- Table 15: Number and proportion of HIV-1 subtypes by age group in Quebec, 2012-2013
- Table 16: Distribution of transmitted drug resistance among treatment-naïve individuals by age group in Quebec, 2012-2013
- Table 17: Distribution of transmitted drug resistance by recent versus established infection in Quebec, 2012-2013
List of Appendices
- Appendix I: Exposure category classification
- Appendix II: HIV strain and drug resistance in Quebec, 2012-2013
- Appendix III: List of HIV-endemic countries
- Appendix IV: Number and proportion of drug resistance mutations by drug class, 2012-2013
- Appendix V: Drug resistance grading system
- Appendix VI: Provincial SDR Program Partners
Acronyms and Abbreviations
- 3TC
- Lamivudine
- AB
- Alberta
- ABC
- Abacavir
- AIDS
- Acquired immune deficiency syndrome
- ART
- Antiretroviral therapy
- ATV/r
- Atazanavir/Ritonavir
- AZT
- Zidovudine
- BC
- British Columbia
- CCDIC
- Centre for Communicable Diseases and Infection Control
- CF
- Clotting factor
- CRF
- Circulating recombinant form
- D4T
- Stavudine
- DDI
- Didanosine
- DNA
- Deoxyribonucleic acid
- DR
- Drug resistance
- DRV/r
- Darunavir/Ritonavir
- EFV
- Efavirenz
- ETR
- Etravirine
- FPV/r
- Fosamprenavir/Ritonavir
- FTC
- Emtricitabine
- HIV
- Human immunodeficiency virus
- HR
- High risk
- IDU
- Injection drug use
- IDV/r
- Indinavir/Ritonavir
- LPV/r
- Lopinavir/Ritonavir
- MB
- Manitoba
- MDR
- Multi-drug resistance
- MSM
- Men who have sex with men
- MSM/IDU
- Men who have sex with men and inject drugs
- NFV
- Nelfinavir
- NHRL
- National HIV and Retrovirology Laboratories
- NIR
- No identifiable risk
- NNRTI
- Non-nucleoside reverse transcriptase inhibitor
- NRTI
- Nucleoside reverse transcriptase inhibitor
- NS
- Nova Scotia
- NVP
- Nevirapine
- ON
- Ontario
- PCR
- Polymerase chain reaction
- PEP
- Post-exposure prophylaxis
- PHAC
- Public Health Agency of Canada
- PI
- Protease inhibitor
- PrEP
- Pre-exposure Prophylaxis
- RNA
- Ribonucleic acid
- RPV
- Rilpivirine
- RT
- Reverse transcriptase
- SDR
- Strain and drug resistance
- SK
- Saskatchewan
- SQV/r
- Saquinavir/Ritonavir
- TDF
- Tenofovir disoproxil fumarate
- TPV/r
- Tipranavir
- URF
- Unique recombinant form
- WHO
- World Health Organization
Executive Summary
HIV is a significant public health challenge. A key component in monitoring the epidemic is surveillance on HIV subtypes and drug resistance. Subtype B historically constituted over 95% of new diagnoses in Canada; however, the proportion of non-B subtypes has gradually been increasing and now represents about 20% of new diagnoses. Surveillance on HIV subtypes informs vaccine development and provides the opportunity to examine potential variation in transmission risk and treatment success by subtype. Moreover, national HIV drug resistance surveillance is important since transmission of drug-resistant HIV impacts treatment and also prevention and control activities. In 1998, the Public Health Agency of Canada (PHAC) created the Canadian HIV Strain and Drug Resistance Surveillance Program (SDR Program) to monitor HIV-1 subtypes and pre-treatment antiretroviral resistance in Canada. Six provinces collaborate with PHAC on this initiative: British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, and Nova Scotia.
This report provides information about subtype and drug resistance mutations in people newly diagnosed with HIV-1 in 2012 and 2013 in Canada. Residual HIV diagnostic blood samples from these individuals, who were assumed to have never been on treatment, were sent from provincial laboratories to the National HIV and Retrovirology Laboratories (NHRL) for genotyping, which detects the HIV subtype and drug resistance to three classes of antiretrovirals. Laboratory results were then linked to epidemiological data collected as part of HIV case surveillance for further analysis. This report examines HIV subtypes and drug resistance by sex, age group, race/ethnicity and exposure category. Comparison to previous reports is limited due to an absence of data from 2009 to 2011. Additionally, results from the province of Quebec, where strain and drug resistance is monitored independently of the SDR Program, are included (see Appendix II ).
A total of 1,811 specimens were analyzed for this report, representing 56.1% of individuals diagnosed with HIV in the six SDR Program provinces in 2012 and 2013. Overall, 20.4% of specimens were non-B subtypes. Females had a higher proportion of non-B subtypes than males (46.0% vs. 14.0% respectively). Higher proportions of non-B subtypes were found in the 30-34, 35-39, and 40-44 age groups (25.3%, 24.0%, and 22.8% respectively) as compared to other age groups. Individuals of Black race/ethnicity had a greater proportion (62.1%) of non-B subtypes compared to those in other groups, and individuals in the heterosexual/endemic exposure category had a greater proportion (85.0%) of non-B subtypes as compared to other exposure categories. In comparison to 2008, there was a higher proportion of non-B subtype specimens in the SDR Program in 2012-2013 (11.6% vs. 20.4% respectively).
With respect to drug resistance, 13.9% of specimens were resistant to at least one drug class and 2.3% were resistant to multiple classes. Males had a higher proportion of drug resistance than females (14.6% vs. 11.0% respectively). Also, the under 25 age group had the greatest proportion of drug resistance (21.0%) of all age groups. People of Indigenous or White race/ethnicity had greater proportions of drug resistance (14.6% and 14.4% respectively) than other race/ethnicity groups. When examined by exposure category, the men who have sex with men (MSM) and injection drug use (IDU) exposure categories had the greatest proportions of drug resistance (16.0% and 18.3% respectively). Furthermore, prevalence of drug resistance was greater among subtype B specimens (15.5% vs. 7.6% in non-B specimens, respectively). Compared to 2008, there was a similar proportion of specimens with drug resistance in the SDR Program in 2012-2013 (13.4% vs. 13.9% respectively).
Going forward, the SDR Program is exploring new ways to improve the monitoring of strain and drug resistance, and will continue to be an important part of the national HIV surveillance efforts.
Introduction
Despite advances in our understanding of HIV and the gains made by antiretroviral therapy (ART), HIV remains a significant public health challenge. A key component in monitoring the epidemic is surveillance on HIV subtypes and drug resistance. HIV-1 takes the form of a number of different subtypes, and subtype B has historically constituted over 95% of all new diagnoses in CanadaFootnote 1. However, with changing patterns of migration, non-B subtypes, which are typically found in sub-Saharan Africa and AsiaFootnote 2, now represent a much larger proportion. Surveillance on HIV subtypes provides information on the specific subtypes that occur in Canada. This could help assess the usefulness of a potential vaccine since any vaccine will likely be subtype-specific. It also provides the opportunity to examine potential differences in transmission risk and treatment effectiveness by subtype as there is some limited evidence that the risk of HIV transmission differs by subtypeFootnote 3 (though further research is needed to understand this more completely).
ART has significantly altered the course of HIV and AIDS. The use of ART has meant that people with HIV are living longer with a life expectancy approaching that of people without HIVFootnote 4. However, as the availability and use of ART continues to grow, the need to monitor and control drug resistance to HIV medications has emerged as a global priority. The development and transmission of drug-resistant HIV limit treatment options and may contribute to increased rates of treatment failure, ultimately impacting HIV prevention and control. The prevalence of drug resistance in newly diagnosed HIV infections reflects the extent to which drug-resistant strains of HIV are being transmitted, and so can help assess the effectiveness of prevention and counselling programs. For these reasons, the World Health Organization (WHO) recommends the establishment of national HIV drug resistance surveillance programs and WHO has published a new five-year global action plan for 2017-2021 to support a co-ordinated international effort to prevent, monitor and respond to the emergence of HIV drug resistanceFootnote 5.
The Canadian HIV Strain and Drug Resistance Surveillance Program (SDR Program) was initiated by the Public Health Agency of Canada (PHAC) in 1998 in collaboration with six provinces (British Columbia, Alberta, Saskatchewan, Manitoba, Ontario, and Nova Scotia), to monitor HIV-1 subtypes and antiretroviral resistance in Canada. Through surveillance of diagnostic HIV samples, the scope of this program is to assess population-based transmitted drug resistance, that is, presumed infection with drug resistant HIV strains (as opposed to acquired drug resistance which may develop over the course of treatment). Furthermore, since HIV-2 is rare in Canada, the SDR Program only monitors HIV-1.
The first SDR Program report was published in 2002, with subsequent reports in 2004, 2005 and 2012, covering the period up to December 2008.Footnote 1 This report provides information on HIV subtype and drug resistance among treatment-naïve individuals newly diagnosed with HIV-1 in 2012 and 2013.
Note to readers
This report differs from previous reports in that limited time trends are reported. After the release of the HIV-1 Strain and Transmitted Drug Resistance in Canada Surveillance Report to December 31, 2008 the National HIV and Retrovirology Laboratories (NHRL) were relocated from Ottawa to the JC Wilt Infectious Diseases Research Centre in Winnipeg. During this time, limited genotyping was performed. Due to the break in continuity of data, this report provides limited comparison of results from 2012 and 2013 to previous years.
Methods
Specimen and Epidemiological data collection
Figure 1 provides an overview of the SDR Program. When an individual presents for HIV testing, or has a positive result on a rapid HIV test, a diagnostic blood specimen is sent to the provincial laboratory for HIV testing. Blood specimens that test positive for HIV are then stored by the provincial laboratory. Specimens are chosen for the SDR Program and sent to the NHRL based on eligibility criteria outlined by each participating province. Some provinces will review their data and exclude specimens if it is found that the individual was previously diagnosed with HIV or had been exposed to ART. Some provinces will also exclude specimens based on age, the residing out of province at the time of diagnosis, and an insufficient volume of specimen. In Ontario, due to relatively large number of HIV diagnoses compared to other provinces, a random sample of specimens was selected, representing 53.9% of total HIV diagnoses in Ontario from 2012 to 2013.
For each individual newly diagnosed with HIV, non-nominal epidemiological data is gathered and submitted to the provincial public health department. The data include sex, age group, race/ethnicity and likely method of exposure to HIV (i.e. exposure category; for more information on exposure category classification see Appendix I ). The provincial public health department then forwards this data to the Centre for Communicable Diseases and Infection Control (CCDIC) at PHAC, which coordinates the SDR Program. This process differs slightly in Ontario, where epidemiological data is gathered by the provincial laboratory and sent to CCDIC. PHAC shares provincial SDR analysis results with provincial partners.
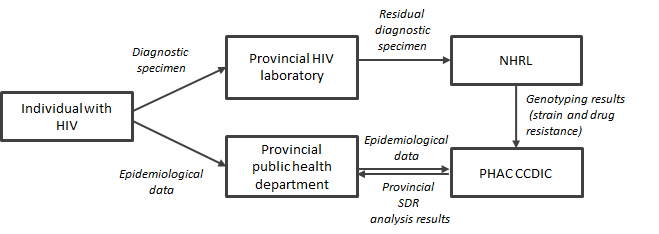
Figure 1 - Text Description
When an individual presents for HIV testing, or has a positive result on a rapid HIV test, a diagnostic blood specimen is sent to the provincial laboratory for HIV testing. Blood specimens that test positive for HIV are then stored by the provincial laboratory. Specimens are chosen for the SDR Program and sent to the NHRL based on eligibility criteria outlined by each participating province.
For each individual newly diagnosed with HIV, non-nominal epidemiological data is gathered and submitted to the provincial public health department. The data include sex, age group, race/ethnicity and likely method of exposure to HIV (i.e. exposure category). The provincial public health department then forwards this data to the Centre for Communicable Diseases and Infection Control (CCDIC) at PHAC, which coordinates the SDR Program. This process differs slightly in Ontario, where epidemiological data is gathered by the provincial laboratory and sent to CCDIC. PHAC shares provincial SDR analysis results with provincial partners.
Genotyping to determine HIV subtype and drug resistance is conducted at the NHRL. Specimens are classified as drug resistant if they have at least one mutation found on the World Health Organization’s consensus list of mutations for surveillance of transmitted HIV-1 drug resistance.
HIV Genotyping
Genotyping to determine HIV subtype and drug resistance is conducted at the NHRL. Aliquots of HIV diagnostic serum specimens are received on dry ice at the NHRL where they are coded and stored at -80°C. HIV RNA is extracted from the specimens using semi-automated robotic technology. The purified RNA is reverse transcribed and undergoes nested PCR with pol specific primers encompassing the entire protease gene and the first 235 amino acids of reverse transcriptase. The primers are designed to efficiently amplify all Group M HIV subtypes. Amplified nucleic acid is purified and the DNA sequence determined using dye terminator methodology on an ABI 3130XL genetic analyzer. The viral nucleic acid sequence is determined for both strands with sets of overlapping primers covering the entire protease and most of the reverse transcriptase genes. The Stanford HIV Drug Resistance Database Calibrated Population Resistance ToolFootnote 6 is used to interpret the genetic sequences for drug resistance mutations. Drug resistance is tested against three classes of antiretrovirals: non-nucleoside reverse transcriptase inhibitors (NNRTIs), nucleoside reverse transcriptase inhibitors (NRTIs), and protease inhibitors (PIs). Specimens are classified as drug resistant if they have at least one mutation found on the World Health Organization’s consensus list of mutations for surveillance of transmitted HIV-1 drug resistanceFootnote 7.
Analysis and Interpretation
CCDIC links specimen subtype and drug resistance data received from NHRL with corresponding epidemiological data provided by provincial public health departments.
For this report, the distribution of HIV subtypes and drug resistance was analyzed by sex, age group, race/ethnicity, and exposure category. No statistical techniques were applied to account for missing data. Additionally, this report includes an independent section of HIV strain and drug resistance findings from Quebec (see Appendix II ).
Results
Among 3,226 newly diagnosed cases of HIV from 2012 to 2013 in the six provinces participating in the SDR Program, genotyping results were received for 1,811 specimens (56.1%) (see Figure 2). Reasons for unsuccessful completion of genotyping included no leftover specimen or a quantity of leftover specimen insufficient for genotyping (n=1 151). Some specimens submitted to the NHRL for genotyping were unable to be sequenced (n=264).
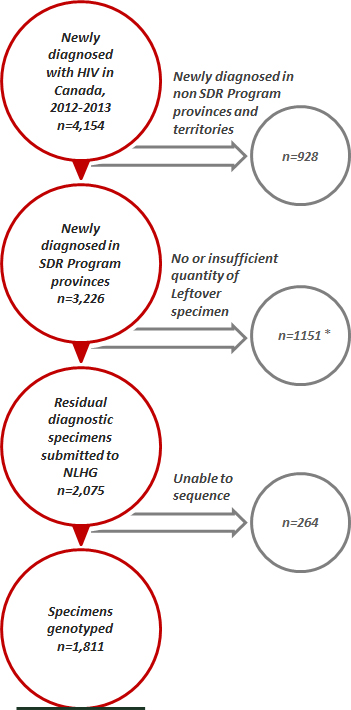
Figure 2 - Footnote * Ontario submitted a random sample of approximately 500 specimens per year; this number includes those samples not submitted
Figure 2 - Text Description
There are 4,154 newly diagnosed cases with HIV in Canada for 2012-2013. Of those, 928 cases were in non Strain and Drug Resistance (SDR) program provinces and territories. There are 3226 newly diagnosed cases in the SDR program provinces of which 1151 cases were not able to provide Genotyping data. Of the 2075 remaining cases, only 1811 specimens were able to provide genotyping data for this report.
Sample characteristics
Characteristics of individuals newly diagnosed with HIV are shown in Table 1, and are compared to those included in the SDR Program.
| Total HIV diagnoses in SDR Provinces Table 1 - Footnote * |
HIV diagnoses included in SDR program |
||
|---|---|---|---|
| Sample Characteristics | n | n | row % |
| Province | |||
| British Columbia | 506 | 309 | 61.1 |
| Alberta | 500 | 276 | 55.2 |
| Saskatchewan | 315 | 213 | 67.6 |
| Manitoba | 188 | 88 | 46.8 |
| Ontario | 1684 | 907 | 53.9 |
| Nova Scotia | 35 | 18 | 51.4 |
| Sex | |||
| Male | 2480 | 1443 | 58.2 |
| Female | 736 | 363 | 49.3 |
| Unknown | 12 | 5 | 41.7 |
| Age group | |||
| <20 | 82 | 33 | 40.2 |
| 20-29 | 749 | 434 | 57.9 |
| 30-39 | 965 | 533 | 55.2 |
| 40-49 | 870 | 493 | 56.7 |
| 50+ | 563 | 318 | 56.5 |
| unknown | 1 | 0 | 0 |
| Race/ethnicity | |||
| White | 1182 | 758 | 64.1 |
| Black | 502 | 219 | 43.6 |
| Indigenous | 471 | 301 | 63.9 |
| Asian or East/Southeast Asian | 155 | 96 | 61.9 |
| South/ West Asian or Arab | 118 | 72 | 61 |
| Latin American | 116 | 63 | 54.3 |
| Other | 41 | 27 | 65.9 |
| Unknown | 643 | 275 | 42.8 |
| Exposure category | |||
| MSMTable 1 - Footnote † | 1316 | 813 | 61.8 |
| MSM/IDUTable 1 - Footnote ‡ | 68 | 37 | 54.4 |
| IDUTable 1 - Footnote § | 398 | 235 | 59 |
| Heterosexual/EndemicTable 1 - Footnote ¦ | 358 | 147 | 41.1 |
| Heterosexual/Non-Endemic | 563 | 368 | 65.4 |
| Other | 88 | 26 | 29.5 |
| Unknown | 437 | 185 | 42.3 |
| Total | 3228 | 1811 | 56.1 |
Of the SDR Program sample, the majority (79.7%) were male. Individuals aged 20-49 formed the largest age group in the sample (80.6%). Races/ethnicities which made up >10% of the sample included White (41.9%), Indigenous (16.6%) and Black (12.1%) with a large subgroup where race/ethnicity data was unknown (15.1%). The men who have sex with men (MSM) exposure category represented 44.9% of the total sample.
HIV-1 Subtypes
Subtype Distribution
Of the 1,811 specimens genotyped, 1,441 (79.6%) were subtype B (see Table 2). Of non-B subtypes, there were 26 unique strains; most were subtype C (8.9% of the total sample and 43.5% of non-B subtypes). Other notable subtypes were: A (3.2%), CRF02_AG (2.6%), CRF01_AE (1.3%) and B recombinant (1.0%).
| HIV-1 subtype | n | % | % in non-B samples |
|---|---|---|---|
| B | 1441 | 79.6 | - |
| C | 161 | 8.9 | 43.5 |
| A | 58 | 3.2 | 15.7 |
| CRF02_AG | 47 | 2.6 | 12.7 |
| CRF01_AE | 24 | 1.3 | 6.5 |
| G | 10 | 0.6 | 2.7 |
| D | 4 | 0.2 | 1.1 |
| F | 3 | 0.2 | 0.8 |
| H | 3 | 0.2 | 0.8 |
| Other CRFsTable 2 - Footnote * | |||
| CRF07_BC | 7 | 0.4 | 1.9 |
| CRF06_CPX | 4 | 0.2 | 1.1 |
| CRF12_BF | 3 | 0.2 | 0.8 |
| CRF20_BG | 3 | 0.2 | 0.8 |
| CRF19_CPX | 2 | 0.1 | 0.5 |
| CRF24_BG | 2 | 0.1 | 0.5 |
| CRF18_CPX | 1 | 0.1 | 0.3 |
| CRF43_02G | 1 | 0.1 | 0.3 |
| URFsTable 2 - Footnote † | |||
| B recombinant | 19 | 1.0 | 5.1 |
| AD recombinant | 4 | 0.2 | 1.1 |
| BD recombinant | 4 | 0.2 | 1.1 |
| A recombinant | 2 | 0.1 | 0.5 |
| AC recombinant | 2 | 0.1 | 0.5 |
| AB recombinant | 1 | 0.1 | 0.3 |
| C recombinant | 1 | 0.1 | 0.3 |
| D recombinant | 2 | 0.1 | 0.5 |
| G recombinant | 1 | 0.1 | 0.3 |
| GF recombinant | 1 | 0.1 | 0.3 |
| Total | 1811 | - | - |
NON-B subtypes by province
Figure 3 shows the number and proportion of non-B subtypes by province. The prevalence of non-B subtypes was greatest in Manitoba (38.6%), ranged from 5.6% to 27.9% in other provinces, and was lower in British Columbia and Nova Scotia.
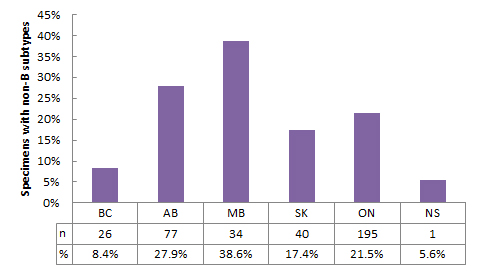
Figure 3 - Text Description
| BC | AB | MB | SK | ON | NS | |
|---|---|---|---|---|---|---|
| % | 8.4% | 27.9% | 38.6% | 17.4% | 21.5% | 5.6% |
| n | 26 | 77 | 34 | 40 | 195 | 1 |
NON-B subtypes by epidemiological characteristics
Sex and race/ethnicity
Table 3 shows the number and proportion of B and non-B subtypes by sex and race/ethnicity. Non-B subtypes comprised 46.0% of specimens from females, versus 14.0% of specimens from males. Females had a higher proportion of non-B subtypes than males across all race/ethnicity categories.
| Male | Female | TotalTable 3 - Footnote ‡ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Race/ethnicity | n | non-B | % | n | non-B | % | n | non-B | % |
| White | 677 | 40 | 5.9 | 80 | 18 | 22.5 | 757 | 58 | 7.7 |
| Black | 132 | 59 | 44.7 | 87 | 77 | 88.5 | 219 | 136 | 62.1 |
| Indigenous | 171 | 21 | 12.3 | 130 | 27 | 20.8 | 301 | 48 | 15.9 |
| AsianTable 3 - Footnote * | 136 | 33 | 24.3 | 7 | 6 | 85.7 | 143 | 39 | 27.3 |
| OtherTable 3 - Footnote † | 102 | 13 | 12.7 | 13 | 6 | 46.2 | 115 | 19 | 16.5 |
| Unknown | 225 | 36 | 16.0 | 46 | 33 | 71.7 | 271 | 69 | 25.5 |
| Total | 1443 | 202 | 14.0 | 363 | 167 | 46.0 | 1806 | 369 | 20.4 |
Overall, individuals of Black race/ethnicity had a greater proportion of non-B subtypes (62.1%), while individuals of White race/ethnicity had the lowest proportion of non-B subtypes (7.7%). Given that non-B subtypes is most prevalent in HIV-endemic countries, it is likely that the increased proportion of non-B subtypes is due to over half (57.5%) of individuals of Black race/ethnicity being classified in the heterosexual/endemic exposure category (see Table 5).
Age
Figure 4 displays the number and proportion of non-B subtypes by age group. The proportion of non-B subtypes ranged from 9.9% and 21.0% between age ranges.

Figure 4 - Text Description
| Age | % | n |
|---|---|---|
| <25 | 21.0% | 186 |
| 25-29 | 13.2% | 281 |
| 30-34 | 13.2% | 304 |
| 35-39 | 15.3% | 229 |
| 40-44 | 9.9% | 263 |
| 45-49 | 13.5% | 230 |
| 50-54 | 11.3% | 151 |
| 55+ | 15.6% | 167 |
Sex and exposure category
Table 4 displays the number and proportion of non-B subtypes by sex and exposure category. Individuals in the heterosexual/endemic exposure category had a greater proportion (85.0%) of non-B subtypes, followed by those in the heterosexual/non-endemic exposure category (25.5%). Females had a higher proportion of non-B subtypes than males across all exposure categories.
| Male | Female | TotalTable 4 - Footnote * | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure category | n | non-B | % | n | non-B | % | n | non-B | % |
| MSM | 813 | 68 | 8.4 | - | - | - | 813 | 68 | 8.4 |
| MSM/IDU | 37 | 4 | 10.8 | - | - | - | 37 | 4 | 10.8 |
| IDU | 149 | 11 | 7.4 | 86 | 7 | 8.1 | 235 | 18 | 7.7 |
| Heterosexual/Endemic | 65 | 49 | 75.4 | 82 | 76 | 92.7 | 147 | 125 | 85.0 |
| Heterosexual/Non-Endemic | 222 | 39 | 17.6 | 146 | 55 | 37.7 | 368 | 94 | 25.5 |
| Other | 13 | 5 | 38.5 | 13 | 6 | 46.2 | 26 | 11 | 42.3 |
| Unknown | 144 | 26 | 18.1 | 36 | 23 | 63.9 | 180 | 49 | 27.2 |
| Total | 1443 | 202 | 14.0 | 363 | 167 | 46.0 | 1806 | 369 | 20.4 |
Race/ethnicity and exposure category
Table 5 displays the number and proportion of B and non-B subtypes by race/ethnicity and exposure category. A greater proportion of non-B subtypes (for which the sample size was greater than two) were found in individuals of Black race/ethnicity who were in the heterosexual/endemic exposure category (85.7%), individuals of Black race/ethnicity who were in the heterosexual/non-endemic exposure category (45.8%) and individuals of Indigenous race/ethnicity who were in the heterosexual/non-endemic exposure category (23.8%).
| White | Black | Indigenous | Asian | Other | Unknown | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure category | n | non-B | % | n | non-B | % | n | non-B | % | n | non-B | % | n | non-B | % | n |
| MSM | 481 | 23 | 4.8 | 59 | 10 | 16.9 | 20 | 1 | 5.0 | 104 | 20 | 19.2 | 78 | 11 | 14.1 | 71 |
| MSM/IDU | 21 | 1 | 4.8 | 2 | 0 | 0.0 | 7 | 0 | 0.0 | 4 | 2 | 50.0 | 2 | 0 | 0.0 | 1 |
| IDU | 84 | 4 | 4.8 | 1 | 0 | 0.0 | 131 | 14 | 10.7 | 0 | 0 | 0.0 | 9 | 0 | 0.0 | 10 |
| Heterosexual /Endemic |
2 | 1 | 50.0 | 126 | 108 | 85.7 | 0 | 0 | 0.0 | 1 | 1 | 100 | 3 | 2 | 66.7 | 15 |
| Heterosexual /Non-Endemic |
149 | 27 | 18.1 | 24 | 11 | 45.8 | 126 | 30 | 23.8 | 24 | 12 | 50.0 | 20 | 6 | 30.0 | 25 |
| Other | 8 | 1 | 12.5 | 5 | 5 | 100 | 7 | 2 | 28.6 | 1 | 1 | 100 | 1 | 0 | 0.0 | 4 |
| Unknown | 13 | 1 | 7.7 | 2 | 2 | 100 | 10 | 1 | 10.0 | 9 | 3 | 33.3 | 2 | 0 | 0.0 | 149 |
| Total | 758 | 58 | 7.7 | 219 | 136 | 62.1 | 301 | 48 | 15.9 | 143 | 39 | 27.3 | 115 | 19 | 16.5 | 275 |
Discussion
Overall, there was a greater proportion of non-B subtype specimens in the SDR Program from 2012 to 2013 as compared to specimens in the SDR Program in 2008Footnote 1 (20.4% vs. 11.6% respectively). Subtypes C, A, CRF02_AG and CRF01_AE continued to represent the highest proportion of non-B subtypes.
Drug Resistance
Drug resistance by province
Mutations associated with resistance to at least one class of drugs were present in 251 (13.9%) of 1,811 specimens from 2012 to 2013. Figure 5 displays the number and proportion of specimens with drug resistance by province. Nova Scotia has been excluded due to its low number of specimens (n=2). Saskatchewan had a greater proportion of specimens with drug resistance (17.4%) followed by Manitoba and Ontario (15.9% and 14.7% respectively). Prevalence of drug resistance in other provinces ranged between 10.7% and 11.6%.
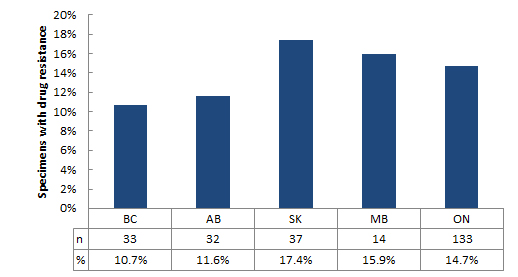
Figure 5 - Text Description
| BC | AB | SK | MB | ON | |
|---|---|---|---|---|---|
| % | 10.7% | 11.6% | 17.4% | 15.9% | 14.7% |
| n | 33 | 32 | 37 | 14 | 133 |
Drug resistance by epidemiological characteristics
Sex and race/ethnicity
Table 6 displays the number and proportion of specimens with drug resistance by sex and race/ethnicity. Overall proportions of drug resistance were highest among members of the Indigenous (14.6%) and White (14.4%) race/ethnicity categories. Individuals of Black race/ethnicity had the lowest proportion of drug resistance (11.9%). A higher proportion of drug resistant specimens were observed in males compared to females across all race/ethnicity categories except the “Other” category.
| Males | Females | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Race/ethnicity | n | DRTable 6 - Footnote * | % | n | DR | % | n | DR | % |
| White | 677 | 101 | 14.9 | 80 | 8 | 10.0 | 757 | 109 | 14.4 |
| Black | 132 | 18 | 13.6 | 87 | 8 | 9.2 | 219 | 26 | 11.9 |
| Indigenous | 171 | 29 | 17.0 | 130 | 15 | 11.5 | 301 | 44 | 14.6 |
| Asian | 136 | 19 | 14.0 | 7 | 0 | 0.0 | 143 | 19 | 13.3 |
| Other | 102 | 12 | 11.8 | 13 | 2 | 15.4 | 115 | 14 | 12.2 |
| Unknown | 225 | 32 | 14.2 | 46 | 7 | 15.2 | 271 | 39 | 14.4 |
| Total | 1443 | 211 | 14.6 | 363 | 40 | 11.0 | 1806 | 251 | 13.9 |
Age
The number and proportion of specimens with drug resistance by age group is shown in Figure 6. Specimens from the <25 and 20-24 age groups had greater proportions of drug resistance (21.0% and 20.9% respectively). The proportion of drug resistant samples among the other age categories ranged from 9.9% to 16.7%, and was lowest in the 40-44 age group.
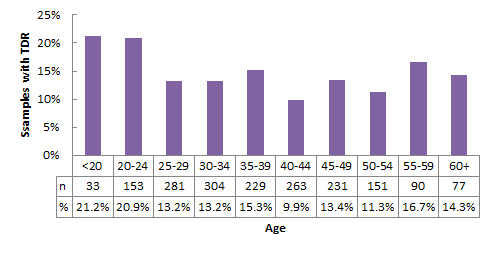
Figure 6 - Text Description
| Age | % | n |
|---|---|---|
| <20 | 21.2% | 33 |
| 20-24 | 20.9% | 153 |
| 25-29 | 13.2% | 281 |
| 30-34 | 13.2% | 304 |
| 35-39 | 15.3% | 229 |
| 40-44 | 9.9% | 263 |
| 45-49 | 13.4% | 231 |
| 50-54 | 11.3% | 151 |
| 55-59 | 16.7% | 90 |
| 60+ | 14.3% | 77 |
Sex and exposure category
Table 7 displays the number and proportion of specimens with drug resistance by sex and exposure category. Specimens from individuals in the IDU and MSM exposure categories had greater proportions of drug resistance at 18.3% and 16.0% respectively. Specimens from individuals in the heterosexual/non-endemic exposure category had the lowest proportion of drug resistance at 8.7%. Specimens from females had higher proportions of drug resistance in all exposure categories other than the IDU exposure category.
| Male | Female | TotalTable 7 - Footnote † | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure category | n | DRTable 7 - Footnote * | % | n | DR | % | n | DR | % |
| MSM | 813 | 130 | 16.0 | - | - | - | 813 | 130 | 16.0 |
| MSM/IDU | 37 | 5 | 13.5 | - | - | - | 37 | 5 | 13.5 |
| IDU | 149 | 31 | 20.8 | 86 | 12 | 14.0 | 235 | 43 | 18.3 |
| Heterosexual/Endemic | 65 | 6 | 9.2 | 82 | 9 | 11.0 | 147 | 15 | 10.2 |
| Heterosexual/Non-Endemic | 222 | 19 | 8.6 | 146 | 13 | 8.9 | 368 | 32 | 8.7 |
| Other | 13 | 1 | 7.7 | 13 | 2 | 15.4 | 26 | 3 | 11.5 |
| Unknown | 144 | 19 | 13.2 | 36 | 4 | 11.1 | 180 | 23 | 12.8 |
| Total | 1443 | 211 | 14.6 | 363 | 40 | 11.0 | 1806 | 251 | 13.9 |
Race/ethnicity and exposure category
Table 8 displays the number and proportion of specimens with drug resistance by race/ethnicity and exposure category. Drug resistance was greatest among individuals of Indigenous race/ethnicity who were in the MSM/IDU exposure category (28.6%), individuals of Indigenous race/ethnicity who were in the MSM exposure category (25%) and individuals of White race/ethnicity who were in the IDU exposure category (22.6%). However, due to small cell sizes, caution should be used in interpreting these data.
| White | Black | Indigenous | Asian | Other | Unknown | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Exposure category | n | DR | % | n | DR | % | n | DR | % | n | DR | % | n | DR | % | n |
| MSM | 481 | 79 | 16.4 | 59 | 10 | 16.9 | 20 | 5 | 25.0 | 104 | 18 | 17.3 | 78 | 9 | 11.5 | 71 |
| MSM/IDU | 21 | 2 | 9.5 | 2 | 0 | 0.0 | 7 | 2 | 28.6 | 4 | 0 | 0.0 | 2 | 1 | 50.0 | 1 |
| IDU | 84 | 19 | 22.6 | 1 | 0 | 0.0 | 131 | 22 | 16.8 | 0 | 0 | 0.0 | 9 | 1 | 11.1 | 10 |
| Heterosexual/Endemic | 2 | 0 | 0.0 | 126 | 12 | 9.5 | 0 | 0 | 0.0 | 1 | 0 | 0.0 | 3 | 0 | 0.0 | 15 |
| Heterosexual/Non-Endemic | 149 | 8 | 5.4 | 24 | 4 | 16.7 | 126 | 13 | 10.3 | 24 | 0 | 0.0 | 20 | 3 | 15.0 | 25 |
| Other | 8 | 0 | 0.0 | 5 | 0 | 0.0 | 7 | 2 | 28.6 | 1 | 0 | 0.0 | 1 | 0 | 0.0 | 4 |
| Unknown | 13 | 1 | 7.7 | 2 | 0 | 0.0 | 10 | 0 | 0.0 | 9 | 1 | 11.1 | 2 | 0 | 0.0 | 149 |
| Total | 758 | 109 | 14.4 | 219 | 26 | 11.9 | 301 | 44 | 14.6 | 143 | 19 | 13.3 | 115 | 14 | 12.2 | 275 |
Drug resistance by drug class
Figure 7 displays the distribution of drug resistance by drug class. Over three-quarters (83.7%) of specimens with drug resistance were resistant only to one class of medications. Of 251 specimens with any drug resistance, single-class resistance to NNRTI predominated (45.0%). Of specimens that were multi-drug resistant (MDR), that is, resistant to two or more drug classes, resistance to both NNRTI and NRTI was most common (12.0% of all specimens with drug resistance). A detailed list of the number and proportion of drug resistance mutations by drug class can be found in Appendix IV.
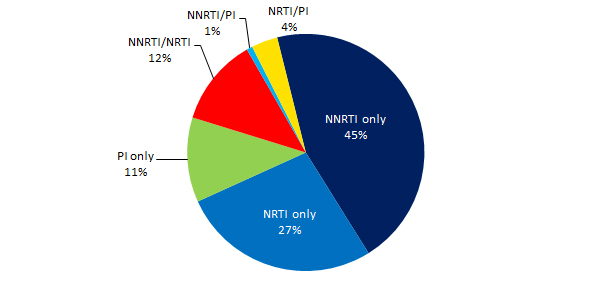
Figure 7 - Text Description
| Drug resistance | n | % |
|---|---|---|
| NNRTI only | 113 | 5.2 |
| NRTI only | 68 | 3.9 |
| PI only | 29 | 1.8 |
| NNRTI/NRTI | 30 | 1.8 |
| NNRTI/PI | 2 | 0.1 |
| NRTI/PI | 9 | 0.6 |
The distribution of drug class resistance by province is shown in Figure 8. Nova Scotia was excluded due to a low number of specimens (n=2). NNRTI resistance was predominant in Saskatchewan (81.1% of all drug resistant specimens), NRTI resistance was predominant in Manitoba (64.3% of all drug resistant specimens), PI resistance was greatest in BC (24.2% of all drug resistant specimens), and MDR was predominant in Ontario (25.6% of all drug resistant specimens).
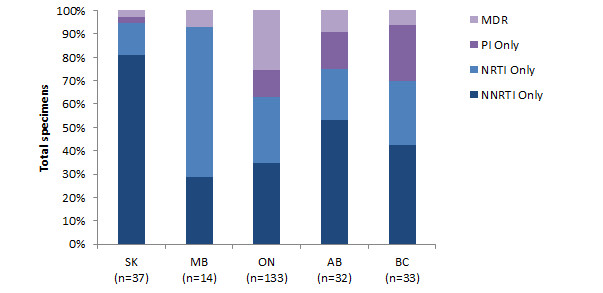
Figure 8 - Text Description
| NNRTI Only | NRTI Only | PI Only | MDR | |
|---|---|---|---|---|
| SK (n=37) |
14.1% | 2.3% | 0.5% | 0.5% |
| MB (n=14) |
4.5% | 10.2% | 0.0% | 1.1% |
| ON (n=133) |
5.1% | 4.2% | 1.7% | 3.7% |
| AB (n=32) |
6.2% | 2.5% | 1.8% | 1.1% |
| BC (n=33) |
4.5% | 2.9% | 2.6% | 0.6% |
Drug resistance by subtype
Table 9 and Table 10 display the number and proportion of specimens with drug resistance by subtype. Subtype B specimens had a similar proportion of MDR to non-B subtype specimens (2.3% and 2.2% respectively) but a higher proportion of overall drug resistance (15.5% and 7.6% respectively).
| Subtype B | Non-B subtypes | Total | ||||
|---|---|---|---|---|---|---|
| Drug resistance | n | % | n | % | n | % |
| None | 1218 | 84.5 | 342 | 92.4 | 1560 | 86.1 |
| Single class resistance | ||||||
| NNRTI only | 100 | 6.9 | 13 | 3.5 | 113 | 6.2 |
| NRTI only | 65 | 4.5 | 3 | 0.8 | 68 | 3.8 |
| PI only | 25 | 1.7 | 4 | 1.1 | 29 | 1.6 |
| Multi-class resistance | ||||||
| NNRTI/NRTI | 23 | 1.6 | 7 | 1.9 | 30 | 1.7 |
| NNRTI/PI | 2 | 0.1 | 0 | 0.0 | 2 | 0.1 |
| NRTI/PI | 8 | 0.6 | 1 | 0.3 | 9 | 0.5 |
| NNRTI/NRTI/PI | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total specimens | 1441 | 100% | 370 | 100% | 1811 | 100% |
| Subtype B | Non-B subtypes | Total | ||||
|---|---|---|---|---|---|---|
| Drug resistance | n | % | n | % | n | % |
| None | 1218 | 84.5 | 342 | 92.4 | 1560 | 86.1 |
| Total any drug resistance | 223 | 15.5 | 28 | 7.6 | 251 | 13.9 |
| Total any NNRTI | 125 | 8.7 | 20 | 5.4 | 145 | 8.0 |
| Total any NRTI | 96 | 6.7 | 11 | 3.0 | 107 | 5.9 |
| Total any PI | 35 | 2.4 | 5 | 1.4 | 40 | 2.2 |
| Total any MDR | 33 | 2.3 | 8 | 2.2 | 41 | 2.3 |
Drug resistance to commonly prescribed HIV drugs
The following section reports levels of drug resistance to commonly prescribed antiretrovirals. The analysis was completed using the Stanford HIV Drug Resistance Database Genotypic Resistance Interpretation Algorithm which assigns resistance mutations as either potential low, low, intermediate, or high level resistanceFootnote 8. More information about these interpretations can be found in Appendix V.
Non-Nucleoside Reverse Transcriptase Inhibitors
Figure 9 shows resistance to commonly prescribed NNRTI drugs. In total, 145 specimens (8.0% of all specimens and 57.8% of all drug-resistant specimens) were resistant to at least one NNRTI. All NNRTI resistant specimens had some form of resistance to Nevirapine (NVP; 100%) and Efavirenz (EFV; 100%), with specimens resistant to NVP almost exclusively (99.3%) displaying high-level resistance.
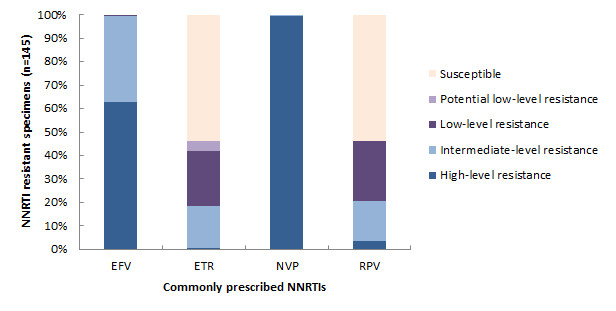
Figure 9 - Text Description
| ATV/r | DRV/r | FPV/r | IDV/r | LPV/r | NFV | SQV/r | TPV/r | |
|---|---|---|---|---|---|---|---|---|
| High-level resistance | 8 | 0 | 5 | 8 | 8 | 20 | 8 | 0 |
| Intermediate-level resistance | 2 | 0 | 7 | 11 | 2 | 2 | 11 | 10 |
| Low-level resistance | 11 | 2 | 9 | 2 | 11 | 12 | 5 | 0 |
| Potential low-level resistance | 15 | 0 | 12 | 12 | 11 | 1 | 0 | 5 |
| Susceptible | 3 | 37 | 6 | 6 | 7 | 4 | 15 | 24 |
Nucleoside Reverse Transcriptase Inhibitors (NRTI)
The levels of resistance to commonly prescribed NRTI drugs is shown in Figure 10. In total, 107 specimens (5.9% of all specimens and 42.6% of all drug-resistant specimens) were resistant to at least one NRTI. The majority of specimens that showed resistance to an NRTI were resistant to Zidovudine (AZT; 89.7%), Abacavir (ABC; 90.7%), Stavudine (D4T; 92,5%), and/or Didanosine (DDI; 98.1%). In comparison, less than one quarter of specimens were resistant to Lamivudine (3TC) and/or Emtricitabine (FTC) (18.7% for both).
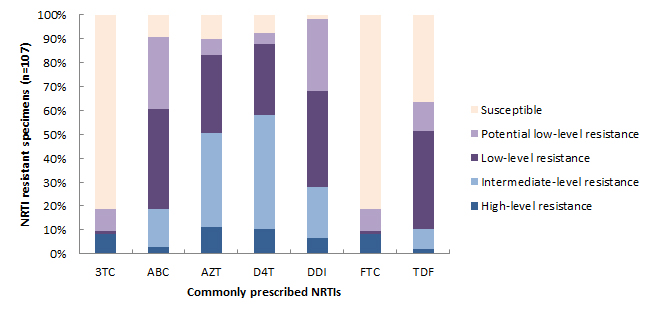
Figure 10 - Text Description
| 3TC | ABC | AZT | D4T | DDI | FTC | TDF | |
|---|---|---|---|---|---|---|---|
| High-level resistance | 9 | 3 | 12 | 11 | 7 | 9 | 2 |
| Intermediate-level resistance | 0 | 17 | 42 | 51 | 23 | 0 | 9 |
| Low-level resistance | 1 | 45 | 35 | 32 | 43 | 1 | 44 |
| Potential low-level resistance | 10 | 32 | 7 | 5 | 32 | 10 | 13 |
| Susceptible | 87 | 10 | 11 | 8 | 2 | 87 | 39 |
Protease Inhibitors
Figure 11 shows the levels of resistance to commonly prescribed PI drugs. In total, 40 specimens (2.2% of all specimens and 15.9% of all drug-resistant specimens) had resistance to at least one PI; 89.7% were resistant to Nelfinavir (NFV). In comparison, only 5.1% and 38.5% of specimens were resistant to Darunavir/Ritonavir (DRV/r) and Tipranavir (TPV/r) respectively.
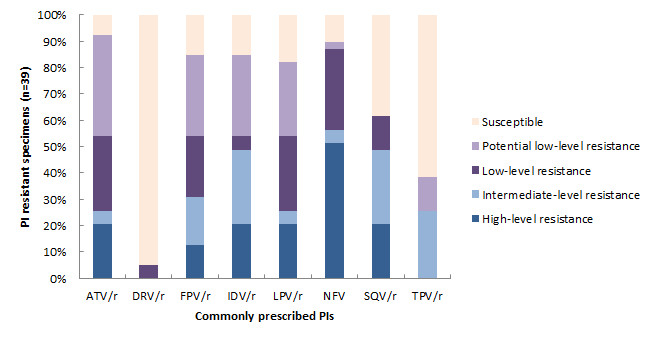
Figure 11 - Text Description
| EFV | ETR | NVP | RPV | |
|---|---|---|---|---|
| High-level resistance | 91 | 1 | 144 | 5 |
| Intermediate-level resistance | 53 | 26 | 1 | 25 |
| Low-level resistance | 1 | 34 | 0 | 37 |
| Potential low-level resistance | 0 | 6 | 0 | 0 |
| Susceptible | 0 | 78 | 0 | 78 |
Discussion
Overall, the proportion of specimens with drug resistance in the SDR Program from 2012 to 2013 was relatively similar to the proportion of specimens with drug resistance in the SDR Program in 2008 (13.9% vs. 13.4% respectively). Table 11 displays a comparison of the number and proportion of drug resistant specimens in 2008 vs. 2012-2013.
| 2008 | 2012-2013 | |||
|---|---|---|---|---|
| Drug resistance | n | % | n | % |
| NNRTI only | 29 | 49.2 | 113 | 45.0 |
| NRTI only | 19 | 32.2 | 68 | 27.0 |
| PI only | 6 | 10.2 | 29 | 11.0 |
| MDR | 5 | 8.5 | 41 | 17.0 |
| Total any DR | 59 | 100.0 | 251 | 100.0 |
Limitations
The data presented in this report are subject to a few limitations. First, the SDR Program aims to monitor transmitted drug resistance in treatment-naïve individuals. As surveillance is based on residual diagnostic specimens, it is assumed that these individuals have never been on treatment for HIV. However, it is possible that in some cases individuals were previously exposed to ART, such as through pre-exposure prophylaxis (PrEP) or post-exposure prophylaxis (PEP). Secondly, in some instances, sample sizes were too small to allow for meaningful interpretation and caution should be used when comparing between groups. Finally, as previously discussed, this report presents limited time trend comparisons due to a gap in data between 2008 and 2012-2013.
Conclusion
The SDR Program is an integral part of the national effort to combat the HIV epidemic in Canada. From 2012 to 2013 the program collected and genotyped 1811 specimens from individuals newly diagnosed with HIV in six provinces, linking the results to epidemiological data. Of those specimens that were successfully genotyped, 13.9% exhibited some form of drug resistance, which was similar to the proportion of specimens in 2008 with drug resistance (13.4%).
Drug resistance surveillance allows for enhanced monitoring of HIV infection in Canada, contributing knowledge on circulating strains of HIV and transmitted drug resistance particularly within populations at increased risk for HIV in order to focus prevention and control efforts. Going forward, the SDR Program is exploring new ways to continue surveillance on HIV strain and drug resistance that would realize resource efficiencies. This information will continue to guide clinicians, public health professionals, HIV program managers and policymakers in developing treatment options, public health recommendations, and targeted programs for intervention.
References
Appendices
Appendix I : Exposure category classification
HIV cases are assigned by provinces and territories to a single exposure category based on data on risk factors received as part of routine case surveillance. These exposure categories are defined below.
If a case reports more than one risk factor, exposure category hierarchies developed by each province is used to classify the case according to the exposure category listed first (or highest) in the hierarchy. For example, people who inject drugs may also be at risk of HIV infection through heterosexual sexual activity. However, injection drug use (IDU) is accepted as the higher risk activity with greater likelihood of transmission of HIV. The exception to this is men who have sex with men (MSM) and who have also injected drugs, as those activities are generally accepted to have fairly equivalent, and it is not known which factor led to HIV infection. Such cases are classified in the combined exposure category MSM/IDU.
For most analyses in this report, the Perinatal, Receipt of blood, and Receipt of CF (clotting factor) exposure categories have been combined with the “Other” exposure category. Furthermore, the Heterosexual/Non-Endemic-HR and Heterosexual/Non-Endemic-NIR exposure categories have been combined into one Heterosexual/non-endemic exposure category.
Exposure Categories
- Perinatal:
- Transmission of HIV from a woman infected with HIV to her infant, either in utero, during childbirth or through breastfeeding
- MSM/IDU:
- Men who have sex with men and use injection drugs
- MSM:
- Men who have sex with men (this category includes men who report either homosexual or bisexual sexual contact)
- IDU:
- Injection drug use
- Receipt of CF:
- Recipient of pooled concentrates of clotting factor VIII or IX for treatment of hemophilia/coagulation disorder
- Receipt of blood:
- Recipient of transfusion prior to 1985 of whole blood or blood components such as packed red cells, plasma, platelets or cryoprecipitate
- Heterosexual/Endemic:
- Born in a country where HIV is endemic (see Appendix III)
- Heterosexual/Non-Endemic-HR:
- Heterosexual contact with someone who is either HIV-infected or who is at increased risk of HIV infection (e.g. a person who injects drugs, a bisexual male or a person from an HIV-endemic country)
- Heterosexual/Non-Endemic-NIR:
- Heterosexual contact is the only risk factor reported and nothing is known about the HIV-related factors associated with the partner
- Other:
- Mode of HIV transmission is known but cannot be classified into any of the major exposure categories listed above (e.g. a recipient of semen from an HIV-positive donor)
- Unknown:
- The exposure category is not reported or unknown.
Adapted from: Public Health Agency of Canada. HIV and AIDS in Canada: Surveillance Report to December 31. 2014. Ottawa: Minister of Public Works and Government Services Canada; 2015.
Appendix II : HIV strain and drug resistance in Quebec, 2012-2013
Introduction
Genotyping is carried out as part of clinical follow-up of HIV-infected patients to determine resistance to antiretrovirals. In the event of therapeutic failure, genotyping serves as a valuable tool to guide clinicians in determining the optimal treatment strategy specific to the patient’s HIV-1 resistance profile. In the province of Quebec, antiretroviral therapy is universally available for all HIV-infected persons.
HIV genotyping carried out as part of routine clinical follow-up was initiated in October 2001 via a network of three laboratories located at the Hôpital Notre-Dame (HND) at the Centre hospitalier de l'Université de Montréal (CHUM), the McGill AIDS Centre at the Jewish General Hospital (JGH) and the Laboratoire de santé publique du Québec (LSPQ). A clinical advisory committee is in place which determines indications for HIV genotyping and reviews the need to add new analytical tests to this program. Since 2001, clinical indications for HIV genotyping include therapeutic failure, perinatal transmission, pregnant women who test positive for HIV, and primary HIV infection. The latter is defined as a newly diagnosed HIV infection where seroconversion likely occurred within the six months prior to collection of the diagnostic specimen. In 2004, genotyping was initiated for patients with established HIV infection for the purpose of assessing antiretroviral resistance in treatment-naïve individuals who have been seropositive for at least six months.
Methodology
The three laboratories in the network use standardized gene amplification equipment and sequencing methods. In 2012 and 2013, the Quebec program issued drug resistance reports from virtual phenotyping (VirtualPhenotype or vircoTYPE, Virco) using analytic methods developed by Virco (Janssen diagnostics). Despite the decentralized nature of testing, genotyping data as well as interpretation of antiretroviral resistance results generated by the three laboratories are compiled in a unique database. Non-nominal sociodemographic data, clinical information pertaining to the prescribed genotyping test, and measures of HIV viral load are added to the genotyping results. Viral load may be assessed using the same sample submitted for genotyping, or may have been carried out on a previous sample up to two months prior to the genotyping test. Provision of clinical justification and viral load data is not a mandatory requirement for genotyping to be carried out.
Overall, the provincial program database serves as a management and internal quality control tool. Analytical data are captured directly from results provided by sequencing assays, while resistance interpretations and HIV-1 subtypes are obtained from secondary reports. Sociodemographic data are requested by each of the laboratories on a retroactive basis and are integrated into the database according to a predetermined schedule. Each centre uses a unique identifier to track records. As these data are non-nominal, it is not possible to identify patients who are tested more than once. Procedures pertaining to the integrated management of laboratory analyses, such as periodic archiving, also limit the ability to monitor individual patient results over time.
For the above reasons and in an attempt to exclude duplicates, data presented in this report are based on selective extracts from the database. Data have been refined based on comparisons of unique identifiers, date of birth, resistance profiles and nucleotide sequences. For example, where nucleotide sequences from patients with the same date of birth had less than a 2.0% difference, only the earlier sequence was included for analysis. Comparison of resistance profiles enabled validation of the cases selected. The same methodology is routinely used to detect and control cross-contamination in laboratories. Although not perfect, this method enables a certain degree of precision in identifying the first genotyping test carried out for a patient by the provincial program. Results presented by year are based on the date of specimen collection.
Results
Distribution of HIV-1 Subtypes
The data presented in this section are based on drug resistance reports from the first HIV genotyping test carried out by the Quebec program (since 2001) for each patient registered in the pgDB in 2012 and 2013. All patients for whom a HIV genotyping was performed were included.
Table 12 shows that a variety of HIV subtypes circulated in the province of Quebec during the period studied, but the B subtypes still predominated (80.9%). Since 2001, there was a slow but steady increase in the proportion of non-B subtypes among persons treated with antiretrovirals and monitored through genotype testing. However, among non-B subtypes, there was no increase in any particular subtype in relation to other subtypes over time ( Table 13 ). The proportion of sequenced isolates accounted for by subtype C specimens raised up to 6.6% in 2005, but decreased in subsequent years and stayed stable at around 5% in 2012-13. These proportions did not change from 2012 to 2013 ( Table 13 ).
HIV-1 subtype |
Number | Proportion (%) | % in non-B samples |
|---|---|---|---|
| B | 1077 | 81.0 | - |
| C | 62 | 4.7 | 24.5 |
| A/AE | 71 | 5.3 | 28.1 |
| AG | 61 | 4.6 | 23.7 |
| D | 8 | 0.6 | 3.2 |
| F | 12 | 0.9 | 4.7 |
| G | 13 | 1.0 | 5.1 |
| H | 0 | 0.0 | 0.0 |
| J | 1 | 0.1 | 0.4 |
| K | 4 | 0.3 | 1.6 |
| Other CRFs | 22 | 1.7 | 8.7 |
| Indeterminate (non-B) | 0 | 0.0 | 0.0 |
| Total | 1331 |
| Year | HIV-1 subtype | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B n (%) |
C | A/AE | AG | D | F | G | H | J | K | Other CRFs | Indet. | Total | |
| 2012 | 541(81.5) | 36 | 30 | 32 | 0 | 4 | 7 | 0 | 1 | 3 | 10 | 0 | 664 |
| 2013 | 536 (80.4) | 26 | 41 | 29 | 8 | 8 | 6 | 0 | 0 | 1 | 12 | 0 | 667 |
| Total | 1077 (80.9) | 62 | 71 | 61 | 8 | 12 | 13 | 0 | 1 | 4 | 22 | 0 | 1331 |
Almost 77% of genotyping tests were carried out among men ( Table 14 ). This proportion is comparable to the distribution of newly diagnosed HIV cases by sex in Quebec during the period studied. The proportion of B subtypes was higher among men (86.4%) compared to women (62.6%). Thus, the prevalence of non-B subtypes among women was 2.78 times than the rate observed among men.
Sex |
HIV-1 subtype | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B n (%) |
C | A/AE | AG | D | F | G | H | J | K | Other CRFs | Indet. | Total | |
| Men | 884(86.4) | 35 | 49 | 27 | 4 | 4 | 3 | 0 | 0 | 4 | 13 | 0 | 1023 |
| Women | 186 (62.2) | 26 | 22 | 33 | 4 | 8 | 10 | 0 | 1 | 0 | 9 | 0 | 299 |
| Unknown | 7 (77.8) | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 9 |
| Total | 1077 (80.9) | 62 | 71 | 61 | 8 | 12 | 13 | 0 | 1 | 4 | 22 | 0 | 1331 |
The distribution of subtypes by age group is presented in Table 15. There was a difference in the proportion of non-B subtypes among younger persons compared to older persons. The proportion of non-B subtypes decreased with age, from 58.8% in children under 20 to 11.6% among persons aged 50 years or older, however, caution should be used with interpretation given the small number of individuals in the <20 age group. In general, the prevalence of non-B subtypes was higher in age groups of persons under 40 years as compared to persons in age groups over 40 years.
| Age group | HIV-1 subtype | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B n (%) |
C | A/AE | AG | D | F | G | H | J | K | Other CRFs | Indet. | Total | |
| <15 | 4 (28.6) | 4 | 2 | 4 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 15 |
| 15-19 | 10 (52.6) | 2 | 6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 19 |
| 20-29 | 170 (81.3) | 12 | 9 | 11 | 2 | 3 | 1 | 0 | 0 | 0 | 1 | 0 | 209 |
| 30-39 | 241 (74.1) | 22 | 18 | 26 | 2 | 3 | 3 | 0 | 1 | 0 | 9 | 0 | 325 |
| 40-49 | 340 (82.9) | 11 | 24 | 14 | 2 | 3 | 5 | 0 | 0 | 3 | 8 | 0 | 410 |
| 50-59 | 230 (88.1) | 8 | 8 | 4 | 2 | 2 | 3 | 0 | 0 | 1 | 3 | 0 | 261 |
| 60+ | 82 (89.1) | 3 | 4 | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 92 |
| Total | 1077 (80.9) | 62 | 71 | 61 | 8 | 12 | 13 | 0 | 1 | 4 | 22 | 0 | 1331 |
Primary HIV-1 drug resistance
Transmission of antiretroviral resistant HIV is concerning from a clinical point of view because it is associated with reduced treatment options for patients. Transmission of drug resistant strains also complicates the selection of appropriate medication for post-exposure prophylaxis. The distribution of transmitted drug resistance is presented in Table 16 . Results in this section are based on samples from patients who have never been on antiretroviral treatment. Only patients for whom HIV genotyping was indicated as a result of a new HIV diagnosis (recent or established) or perinatal transmission were included in order to exclude patients who were not treatment-naïve. Resistance to antiretrovirals was assessed by the presence of primary mutations as per the World Health Organization’s List of mutations for surveillance of transmitted drug resistant HIV: 2009 updateFootnote 7. These mutations are considered to be induced specifically by antiretroviral treatment. Mutations associated with HIV-1 subtype polymorphisms were excluded from this list.
| Age group | WT | NRTI | NNRTI | PI | MDR | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | |
| <15 | 6 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 6 |
| 15-19 | 8 | 100.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 8 |
| 20-29 | 146 | 92.4 | 0 | 0.0 | 10 | 6.3 | 0 | 0.0 | 2 | 1.3 | 158 |
| 30-39 | 207 | 93.2 | 3 | 1.4 | 9 | 4.1 | 2 | 0.9 | 1 | 0.5 | 222 |
| 40-49 | 186 | 92.5 | 4 | 2.0 | 10 | 5.0 | 1 | 0.5 | 0 | 0.0 | 201 |
| 50-59 | 114 | 91.2 | 5 | 4.0 | 4 | 3.2 | 1 | 0.8 | 1 | 0.8 | 125 |
| 60+ | 46 | 93.9 | 1 | 2.0 | 2 | 4.1 | 0 | 0.0 | 0 | 0.0 | 49 |
| Total | 713 | 92.7 | 13 | 1.7 | 35 | 4.6 | 4 | 0.5 | 4 | 0.5 | 769 |
During the study period, the majority (92.7%) of the genotyped specimens were wild-type. Mutations conferring resistance to NNRTIs were most common among drug resistant specimens. MDR (viruses with mutations conferring resistance to three classes of drugs), was identified in only four cases. The presence of mutations conferring antiretroviral resistance in persons who have never received treatment suggests that transmission of drug resistant HIV is occurring. Overall, transmitted drug resistance rates were similar in age groups 20 years and above. There was no drug resistance seen among infants and young adults (i.e. those <20 years old).
Table 17 shows the distribution of transmitted drug resistance in pre-treatment specimens of persons with recent compared to established HIV infection. The proportion with resistant strains was similar in both groups. A higher proportion of mutations associated with NNRTI resistance were found among recent infections, whereas a higher proportion of mutations associated with NRTI resistance were found among established infections.
Time of infection |
WT | NRTI | NNRTI | PI | MDR | Total | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | |
| Recent infection | 219 | 93.6 | 3 | 1.3 | 11 | 4.7 | 1 | 0.4 | 0 | 0.0 | 234 |
| Established infection | 494 | 92.3 | 10 | 1.9 | 24 | 4.5 | 3 | 0.6 | 4 | 0.7 | 535 |
| Total | 713 | 92.7 | 13 | 1.7 | 35 | 4.6 | 4 | 0.5 | 4 | 0.5 | 769 |
Discussion
To place the Quebec data presented in this report in proper context, it is important to reiterate that in Quebec, samples submitted for HIV genotyping are not usually the same specimens as those collected for laboratory confirmation of HIV infection. In addition, HIV-positive persons may be asymptomatic and only become aware of their serostatus several years later. From the time of infection to the initiation of antiretroviral treatment, some mutations associated with drug resistance, especially those that reduce viral fitness may disappear from circulating subtypes in the absence of selective pressure exercised by drugs. For example, the M184V mutation, which confers resistance to lamivudine, quickly disappears after treatment is stopped. On the other hand, mutations in positions 103, 181 and 190 that are induced by NNRTIs may persist for several months or years, even in the absence of selective pressure.
In summary, aggregate data presented by the Quebec provincial program on HIV drug resistance testing show an evolving distribution of HIV subtypes. Subtype B was most prevalent among adults over 50 years of age; however, the relative proportion decreased in younger age groups. This coincides with evolution of the HIV epidemic over the past decade, which has been impacted by changes in patterns of HIV transmission and international travel. Transmitted drug resistance did not appear to increase during the period studied. New treatment options that facilitate compliance, as well as the introduction of new classes of drugs will likely contribute to a reduction in therapeutic failure, which in turn may decrease the transmission of antiretroviral resistant viruses. Nonetheless, continued epidemiological monitoring is essential as it allows for improvement in treatment options for postexposure prophylaxis.
Authors: Hugues Charest, Linda Lemieux and Michel Desrochers (Laboratoire de santé publique du Québec/Institut national de santé publique du Québec).
Collaborators: Isabelle Hardy and Michel Roger (l'Hôpital Notre-Dame du Centre hospitalier universitaire de l'Université de Montréal) and Bluma Brenner and Mark Wainberg (McGill AIDS Center, Jewish General Hospital in Montréal).
Appendix III : List of HIV-endemic countries
HIV-endemic countries
- Angola
- Anguilla
- Antigua and Barbuda
- Bahamas
- Barbados
- Benin
- Bermuda
- Botswana
- British Virgin Islands
- Burkina Faso
- Burma (Myanmar)
- Burundi
- Cambodia
- Cameroon
- Cape Verde
- Cayman Islands
- Central African Republic
- Chad
- Congo (Democratic Rep)
- Dahomey (Benin)
- Djibouti
- Dominica
- Dominican Republic
- Equatorial Guinea
- Eritrea
- Ethiopia
- French Guiana
- Gabon
- Gambia
- Ghana
- Grenada
- Guadeloupe
- Guinea
- Guinea-Bissau
- Guyana
- Haiti
- Honduras
- Ivory Coast
- Jamaica
- Kenya
- Lesotho
- Liberia
- Malawi
- Mali
- Martinique
- Montserrat
- Mozambique
- Namibia
- Netherlands Antilles
- Niger
- Nigeria
- Republic of the Congo
- Rwanda
- Saint Lucia
- Senegal
- Sierra Leone
- Somalia
- South Africa
- St. Kitts and Nevis
- St. Vincent and the Grenadines
- Sudan
- Suriname
- Swaziland
- Tanzania
- Thailand
- Togo
- Trinidad and Tobago
- Turks and Caicos Islands
- Uganda
- United States Virgin Islands
- Zaire (Congo DR)
- Zambia
- Zimbabwe
Public Health Agency of Canada. People from Countries where HIV is Endemic - Black people of African and Caribbean descent living in Canada: Public Health Agency of Canada; 2010.
| Drug Class | Mutation(s) | nAppendix IV - Footnote * | % | |
|---|---|---|---|---|
| NRTI (Total=107) | M41L | 56 | 52.3 | |
| T215E | 29 | 27.1 | ||
| T215S | 17 | 15.9 | ||
| L210W | 14 | 13.1 | ||
| T215C | 12 | 11.2 | ||
| M184V | 9 | 8.4 | ||
| D67N | 8 | 7.5 | ||
| K219Q | 7 | 6.5 | ||
| V75M | 6 | 5.6 | ||
| T69D | 5 | 4.7 | ||
| L74I | 5 | 4.7 | ||
| T215D | 5 | 4.7 | ||
| D67G | 2 | 1.9 | ||
| K70R | 2 | 1.9 | ||
| K65R | 1 | 0.9 | ||
| K70E | 1 | 0.9 | ||
| F116Y | 1 | 0.9 | ||
| Q151M | 1 | 0.9 | ||
| K219R | 1 | 0.9 | ||
| NNRTI (Total=145) | K103N | 82 | 56.6 | |
| G190A | 33 | 22.8 | ||
| Y181C | 22 | 15.2 | ||
| K101E | 3 | 2.1 | ||
| K101P | 2 | 1.4 | ||
| K103S | 2 | 1.4 | ||
| V106A | 2 | 1.4 | ||
| Y181V | 2 | 1.4 | ||
| Y188L | 2 | 1.4 | ||
| G190E | 2 | 1.4 | ||
| P225H | 2 | 1.4 | ||
| V106M | 1 | 0.7 | ||
| PI (Total=40) | M46I | 12 | 30 | |
| L90M | 9 | 22.5 | ||
| G48V | 8 | 20.0 | ||
| I54T | 8 | 20.0 | ||
| V82A | 8 | 20.0 | ||
| M46L | 4 | 10.0 | ||
| F53L | 2 | 5.0 | ||
| I54L | 2 | 5.0 | ||
| I54V | 2 | 5.0 | ||
| V82M | 2 | 5.0 | ||
| I85V | 2 | 5.0 | ||
| D30N | 1 | 2.5 | ||
| F53Y | 1 | 2.5 | ||
| G73S | 1 | 2.5 | ||
| N88D | 1 | 2.5 | ||
Appendix V : Drug resistance grading system
The following grading system is from the Stanford HIV Drug Resistance Database Genotypic Resistance Interpretation Algorithm release notes.Footnote d
- Susceptible:
- No evidence of reduced antiretroviral susceptibility compared with a wild-type virus.
- Potential low-level resistance:
- The sequence may contain mutations indicating previous antiretroviral exposure or may contain mutations that are associated with drug resistance only when they occur with additional mutations.
- Low-level resistance:
- The virus encoded by the submitted sequence may have reduced in vitro antiretroviral susceptibility or that patients harboring viruses with the submitted mutations may have a suboptimal virological response to treatment with the antiretroviral.
- Intermediate resistance:
- high likelihood that a drug's activity will be reduced but that the drug will likely retain significant remaining antiviral activity.
- High-level resistance:
- The predicted level of resistance is similar to those observed in viruses with greater levels of in vitro drug resistance or that clinical data exist demonstrating that patients infected with viruses having such mutations usually have little or no virological response to treatment with the antiretroviral.
Appendix VI : Provincial SDR Program Partners
British Columbia
- Mel Krajden, BC Centre for Disease Control
- Jason Wong, BC Centre for Disease Control
- Elsie Wong, Field Surveillance Officer
Ontario
- Vanessa Allen, Public Health Ontario
- Alex Marchand-Austin, Public Health Ontario
- Doug Sider, Public Health Ontario
- Ashleigh Sullivan, Field Surveillance Officer
Alberta
- Carmen Charlton, Alberta Provincial Laboratory for Public Health
- Sumana Fathima, Alberta Health
- Mariam Osman, Alberta Health
- Sabrina Plitt, Field Surveillance Officer
Saskatchewan
- Helen Bangura, Saskatchewan Ministry of Health
- Patty Beck, Saskatchewan Ministry of Health
- Greg Horsman, Saskatchewan Disease Control Laboratory
- Suresh Khatkar, Field Surveillance Officer
- Paul Levett, Saskatchewan Disease Control Laboratory
- John Manalo, Saskatchewan Ministry of Health
- Valerie Mann, Saskatchewan Ministry of Health
Manitoba
- Jared Bullard, Cadham Provincial Lab
- Paul Van Caeseele, Cadham Provincial Lab
- Kamran Kadkhoda, Cadham Provincial Lab
- Carla Loeppky, Manitoba Health
- Tracey Russnak-Redden, Field Surveillance Officer
Nova Scotia
- Todd Hatchette, Queen Elizabeth II Health Sciences Lab
- Elaine Holmes, Nova Scotia Department of Health and Wellness
- Marie LaFreniere, Field Surveillance Officer