Management of the 2022 Mpox (Monkeypox) Outbreak in Canada

Download the alternative format
(PDF format, 1.53 MB, 17 pages)
- Organization: Public Health Agency of Canada
- Date published: August 2024
Table of Contents
- Context
- Objective
- 1.0 Overview of outbreak in Canada
- 2.0 Public health interventions
- 2.1 Coordinated communications and response
- 2.2 Surveillance, reporting and laboratory capacity
- 2.3 Medical countermeasures
- 2.4 Travel health advice and border measures
- 2.5 Public health measures guidance
- 2.6 Scientific evidence generation and monitoring
- 2.7 One Health
- 2.8 International collaboration
- 3.0 Conclusion
Context
Mpox (monkeypox) is a viral infectious disease caused by an Orthopoxvirus, which is closely related to the variola virus that causes smallpox. There are 2 clades of the monkeypox virus (MPXV): Clade I (former Congo Basin or Central African) and Clade II (former West African). The subtype Clade IIb has been implicated in the 2022 outbreak.
Mpox is usually a self-limited infection. It presents as a rash accompanied by general symptoms such as: fever, chills, headache, exhaustion, swollen lymph nodes, and pain in the muscles, joints, or back. Most people recover on their own after a few weeks. However, in rare circumstances, people can get severely sick or even die from complications.
Since May 2022, there have been cases of mpox in many countries where the disease is not commonly found, including Canada. Most confirmed mpox cases initially reported travel to countries in Europe (including the Canary Islands) and North America. This represents the first instance of concurrent transmission of mpox in non-endemic and endemic countries, in widely disparate geographical areas. Another important new feature of the 2022 global mpox outbreak is that the main route of transmission is not zoonotic (meaning that it spreads from animals to humans), but instead close person-to-person contact, especially sexual contact.
On July 23, 2022, as the number of newly detected cases surpassed 16,000 in 75 countries and territories, the World Health Organization (WHO) Director-General declared the global mpox outbreak a public health emergency of international concern (PHEIC).
Objective
The goal of this report is to summarize the Public Health Agency of Canada's (PHAC) management of the mpox outbreak in Canada up to December 31, 2022. As health is a shared responsibility in Canada, PHAC and other federal government departments, worked with provinces and territories (PTs) and Indigenous partners to monitor and respond to the outbreak of mpox in Canada.
PHAC's response was informed by the Federal, Provincial and Territorial (FPT) Public Health Response Plan for the Management of the Monkeypox Outbreak which took a whole-of-Canada approach to controlling the disease while still reflecting the respective responsibilities of each level of government. Driven by the goal of containing the outbreak, the plan and its actions support the following FPT objectives in response to the mpox outbreak:
- reduce the health impact of mpox on the people of Canada
- rapidly stop the chains of transmission of mpox in Canada
- minimize the risk of mpox becoming established in people and animals in Canada
- ensure Canada's public health response and clinical management of mpox are based on the best available and up-to-date scientific evidence and expert input.
Note about terminology
On November 28, 2022, the World Health Organization recommended the new preferred term "mpox" over the previous terminology "monkeypox" to refer to the disease caused by the monkeypox virus. This change in terminology was adapted to address growing reports of racism and stigma in the wake of the 2022-23 global mpox outbreak. While the name of the disease changed to mpox, the virus responsible for the disease continues to be referred to as "monkeypox virus" or MPXV.
1.0 Overview of outbreak in Canada
1.1 Initial detection
An initial cluster of cases was first detected in London, UK, on May 16, 2022, with no link to travel to mpox-endemic countries. Subsequently, cases were reported from an increasing number of regions, predominantly from Europe and the Americas, but also from Asia, Oceania and non-endemic African countries.
On May 17, 2022, the United States (US) Centers for Disease Control and Prevention (CDC) notified PHAC of a suspected case of mpox in the US, with travel history to Canada (Montreal, Quebec). This US resident tested positive for mpox on May 18, 2022. Concurrently, Montreal public health was investigating a cluster of cases of genital ulcerations and notified PHAC, prompting evaluation for mpox. On May 19, 2022, the first 2 confirmed cases of mpox in Canada were reported to PHAC. The Agency actively responded to this outbreak in the initial days by engaging with affected provinces and their Chief Medical Officers of Health, informing the WHO of suspect cases, creating awareness and communicating information about this disease and escalating its response position to increased vigilance and readiness.
While these first cases were reported to PHAC on May 19, 2022, information later shared by PTs collected during public health investigations describe the earliest case with an epidemiological date of April 28, 2022 (the earliest of symptom onset, lab report, or public health report date), as shown in Figure 1.
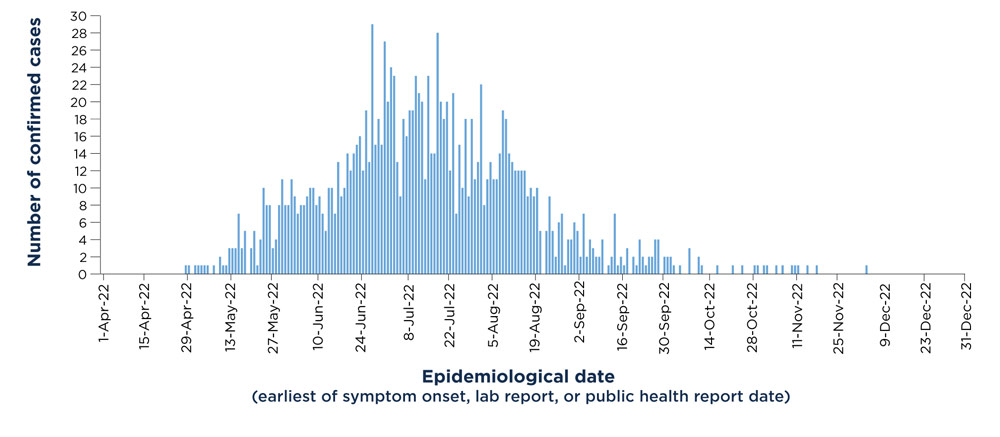
Figure 1 - Text equivalent
| Epidemiological date (earliest of symptom onset, lab report, or public health report date) | Number of Confirmed Cases |
|---|---|
| 1-Apr-22 | 0 |
| 2-Apr-22 | 0 |
| 3-Apr-22 | 0 |
| 4-Apr-22 | 0 |
| 5-Apr-22 | 0 |
| 6-Apr-22 | 0 |
| 7-Apr-22 | 0 |
| 8-Apr-22 | 0 |
| 9-Apr-22 | 0 |
| 10-Apr-22 | 0 |
| 11-Apr-22 | 0 |
| 12-Apr-22 | 0 |
| 13-Apr-22 | 0 |
| 14-Apr-22 | 0 |
| 15-Apr-22 | 0 |
| 16-Apr-22 | 0 |
| 17-Apr-22 | 0 |
| 18-Apr-22 | 0 |
| 19-Apr-22 | 0 |
| 20-Apr-22 | 0 |
| 21-Apr-22 | 0 |
| 22-Apr-22 | 0 |
| 23-Apr-22 | 0 |
| 24-Apr-22 | 0 |
| 25-Apr-22 | 0 |
| 26-Apr-22 | 0 |
| 27-Apr-22 | 0 |
| 28-Apr-22 | 1 |
| 29-Apr-22 | 1 |
| 30-Apr-22 | 0 |
| 1-May-22 | 1 |
| 2-May-22 | 1 |
| 3-May-22 | 1 |
| 4-May-22 | 1 |
| 5-May-22 | 1 |
| 6-May-22 | 0 |
| 7-May-22 | 1 |
| 8-May-22 | 0 |
| 9-May-22 | 2 |
| 10-May-22 | 1 |
| 11-May-22 | 1 |
| 12-May-22 | 3 |
| 13-May-22 | 3 |
| 14-May-22 | 3 |
| 15-May-22 | 7 |
| 16-May-22 | 3 |
| 17-May-22 | 5 |
| 18-May-22 | 0 |
| 19-May-22 | 3 |
| 20-May-22 | 5 |
| 21-May-22 | 1 |
| 22-May-22 | 4 |
| 23-May-22 | 10 |
| 24-May-22 | 8 |
| 25-May-22 | 8 |
| 26-May-22 | 3 |
| 27-May-22 | 4 |
| 28-May-22 | 8 |
| 29-May-22 | 11 |
| 30-May-22 | 8 |
| 31-May-22 | 8 |
| 1-Jun-22 | 11 |
| 2-Jun-22 | 9 |
| 3-Jun-22 | 7 |
| 4-Jun-22 | 8 |
| 5-Jun-22 | 8 |
| 6-Jun-22 | 9 |
| 7-Jun-22 | 10 |
| 8-Jun-22 | 10 |
| 9-Jun-22 | 8 |
| 10-Jun-22 | 9 |
| 11-Jun-22 | 7 |
| 12-Jun-22 | 5 |
| 13-Jun-22 | 10 |
| 14-Jun-22 | 10 |
| 15-Jun-22 | 7 |
| 16-Jun-22 | 13 |
| 17-Jun-22 | 9 |
| 18-Jun-22 | 10 |
| 19-Jun-22 | 14 |
| 20-Jun-22 | 12 |
| 21-Jun-22 | 14 |
| 22-Jun-22 | 15 |
| 23-Jun-22 | 16 |
| 24-Jun-22 | 12 |
| 25-Jun-22 | 19 |
| 26-Jun-22 | 13 |
| 27-Jun-22 | 29 |
| 28-Jun-22 | 15 |
| 29-Jun-22 | 18 |
| 30-Jun-22 | 15 |
| 1-Jul-22 | 27 |
| 2-Jul-22 | 20 |
| 3-Jul-22 | 24 |
| 4-Jul-22 | 23 |
| 5-Jul-22 | 13 |
| 6-Jul-22 | 9 |
| 7-Jul-22 | 18 |
| 8-Jul-22 | 16 |
| 9-Jul-22 | 19 |
| 10-Jul-22 | 19 |
| 11-Jul-22 | 23 |
| 12-Jul-22 | 21 |
| 13-Jul-22 | 20 |
| 14-Jul-22 | 11 |
| 15-Jul-22 | 23 |
| 16-Jul-22 | 14 |
| 17-Jul-22 | 14 |
| 18-Jul-22 | 28 |
| 19-Jul-22 | 20 |
| 20-Jul-22 | 18 |
| 21-Jul-22 | 20 |
| 22-Jul-22 | 12 |
| 23-Jul-22 | 21 |
| 24-Jul-22 | 7 |
| 25-Jul-22 | 15 |
| 26-Jul-22 | 10 |
| 27-Jul-22 | 18 |
| 28-Jul-22 | 9 |
| 29-Jul-22 | 18 |
| 30-Jul-22 | 11 |
| 31-Jul-22 | 13 |
| 1-Aug-22 | 22 |
| 2-Aug-22 | 8 |
| 3-Aug-22 | 11 |
| 4-Aug-22 | 13 |
| 5-Aug-22 | 11 |
| 6-Aug-22 | 11 |
| 7-Aug-22 | 14 |
| 8-Aug-22 | 19 |
| 9-Aug-22 | 18 |
| 10-Aug-22 | 14 |
| 11-Aug-22 | 13 |
| 12-Aug-22 | 12 |
| 13-Aug-22 | 12 |
| 14-Aug-22 | 12 |
| 15-Aug-22 | 12 |
| 16-Aug-22 | 9 |
| 17-Aug-22 | 10 |
| 18-Aug-22 | 9 |
| 19-Aug-22 | 10 |
| 20-Aug-22 | 5 |
| 21-Aug-22 | 0 |
| 22-Aug-22 | 5 |
| 23-Aug-22 | 9 |
| 24-Aug-22 | 5 |
| 25-Aug-22 | 2 |
| 26-Aug-22 | 6 |
| 27-Aug-22 | 7 |
| 28-Aug-22 | 1 |
| 29-Aug-22 | 4 |
| 30-Aug-22 | 4 |
| 31-Aug-22 | 6 |
| 1-Sep-22 | 5 |
| 2-Sep-22 | 2 |
| 3-Sep-22 | 7 |
| 4-Sep-22 | 2 |
| 5-Sep-22 | 4 |
| 6-Sep-22 | 3 |
| 7-Sep-22 | 2 |
| 8-Sep-22 | 2 |
| 9-Sep-22 | 4 |
| 10-Sep-22 | 0 |
| 11-Sep-22 | 1 |
| 12-Sep-22 | 2 |
| 13-Sep-22 | 7 |
| 14-Sep-22 | 1 |
| 15-Sep-22 | 2 |
| 16-Sep-22 | 1 |
| 17-Sep-22 | 3 |
| 18-Sep-22 | 0 |
| 19-Sep-22 | 2 |
| 20-Sep-22 | 1 |
| 21-Sep-22 | 4 |
| 22-Sep-22 | 2 |
| 23-Sep-22 | 1 |
| 24-Sep-22 | 2 |
| 25-Sep-22 | 2 |
| 26-Sep-22 | 4 |
| 27-Sep-22 | 4 |
| 28-Sep-22 | 0 |
| 29-Sep-22 | 2 |
| 30-Sep-22 | 2 |
| 1-Oct-22 | 2 |
| 2-Oct-22 | 1 |
| 3-Oct-22 | 0 |
| 4-Oct-22 | 1 |
| 5-Oct-22 | 0 |
| 6-Oct-22 | 0 |
| 7-Oct-22 | 3 |
| 8-Oct-22 | 0 |
| 9-Oct-22 | 0 |
| 10-Oct-22 | 2 |
| 11-Oct-22 | 1 |
| 12-Oct-22 | 0 |
| 13-Oct-22 | 0 |
| 14-Oct-22 | 0 |
| 15-Oct-22 | 0 |
| 16-Oct-22 | 1 |
| 17-Oct-22 | 0 |
| 18-Oct-22 | 0 |
| 19-Oct-22 | 0 |
| 20-Oct-22 | 0 |
| 21-Oct-22 | 1 |
| 22-Oct-22 | 0 |
| 23-Oct-22 | 0 |
| 24-Oct-22 | 1 |
| 25-Oct-22 | 0 |
| 26-Oct-22 | 0 |
| 27-Oct-22 | 0 |
| 28-Oct-22 | 1 |
| 29-Oct-22 | 1 |
| 30-Oct-22 | 0 |
| 31-Oct-22 | 1 |
| 1-Nov-22 | 1 |
| 2-Nov-22 | 0 |
| 3-Nov-22 | 0 |
| 4-Nov-22 | 1 |
| 5-Nov-22 | 0 |
| 6-Nov-22 | 1 |
| 7-Nov-22 | 0 |
| 8-Nov-22 | 0 |
| 9-Nov-22 | 1 |
| 10-Nov-22 | 1 |
| 11-Nov-22 | 1 |
| 12-Nov-22 | 0 |
| 13-Nov-22 | 0 |
| 14-Nov-22 | 1 |
| 15-Nov-22 | 0 |
| 16-Nov-22 | 0 |
| 17-Nov-22 | 1 |
| 18-Nov-22 | 0 |
| 19-Nov-22 | 0 |
| 20-Nov-22 | 0 |
| 21-Nov-22 | 0 |
| 22-Nov-22 | 0 |
| 23-Nov-22 | 0 |
| 24-Nov-22 | 0 |
| 25-Nov-22 | 0 |
| 26-Nov-22 | 0 |
| 27-Nov-22 | 0 |
| 28-Nov-22 | 0 |
| 29-Nov-22 | 0 |
| 30-Nov-22 | 0 |
| 1-Dec-22 | 0 |
| 2-Dec-22 | 0 |
| 3-Dec-22 | 1 |
| 4-Dec-22 | 0 |
| 5-Dec-22 | 0 |
| 6-Dec-22 | 0 |
| 7-Dec-22 | 0 |
| 8-Dec-22 | 0 |
| 9-Dec-22 | 0 |
| 10-Dec-22 | 0 |
| 11-Dec-22 | 0 |
| 12-Dec-22 | 0 |
| 13-Dec-22 | 0 |
| 14-Dec-22 | 0 |
| 15-Dec-22 | 0 |
| 16-Dec-22 | 0 |
| 17-Dec-22 | 0 |
| 18-Dec-22 | 0 |
| 19-Dec-22 | 0 |
| 20-Dec-22 | 0 |
| 21-Dec-22 | 0 |
| 22-Dec-22 | 0 |
| 23-Dec-22 | 0 |
| 24-Dec-22 | 0 |
| 25-Dec-22 | 0 |
| 26-Dec-22 | 0 |
| 27-Dec-22 | 0 |
| 28-Dec-22 | 0 |
| 29-Dec-22 | 0 |
| 30-Dec-22 | 0 |
| 31-Dec-22 | 0 |
1.2 Outbreak progression and spread
National data trends
Cases of mpox were reported by 9 provinces and 1 territory and were largely centered in metropolitan areas of Ontario, Quebec, and British Columbia. The outbreak in Canada peaked in late June/early July 2022, when 25 to 30 new cases were being reported per day. Since the peak, the number of cases declined rapidly. From April 28 to December 31, 2022, the total number of confirmed cases of mpox in Canada was 1,460, based on publicly reported data from PT websites.
Detailed case information was shared with PHAC by PTs for 1,396 cases. Of these cases, almost all were among individuals 18 years of age or older who identify as male (98%), and only 27 non-male cases were reported (13 cis-women; 14 gender-diverse). The median age of cases was 36 years. Most cases (96% of cases with information available) were reported among gay, bisexual and other men who have sex with men (GBMSM). Forty-four hospitalizations were reported across Canada, and 3 cases were admitted to ICU. No deaths associated with mpox were reported in Canada.
International data trends
From January 1, 2022 to December 28, 2022, 110 Member States across all 6 WHO regions reported over 83,751 laboratory confirmed cases, including 75 deaths (for current statistics please refer to the WHO). Similar to what has been observed in Canada, most cases have occurred among males (96.6%), typically in their 30s (median age: 34), identifying as men who have sex with men (84.4%). A small number of cases have been reported in women (3.4%) and children under 18 (1.0%) (WHO).
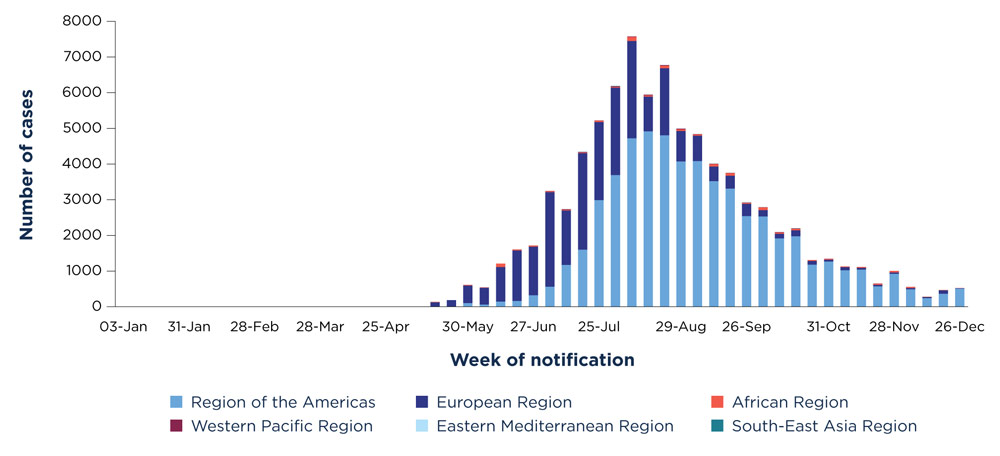
Figure 2 - Text equivalent
| Week of notification | African Region | Eastern Mediterranean Region | European Region | Region of the Americas | South-East Asia Region | Western Pacific Region |
|---|---|---|---|---|---|---|
| 03-Jan | 1 | 0 | 0 | 0 | 0 | 0 |
| 10-Jan | 2 | 0 | 0 | 0 | 0 | 0 |
| 17-Jan | 1 | 0 | 0 | 0 | 0 | 0 |
| 24-Jan | 0 | 0 | 0 | 0 | 0 | 0 |
| 31-Jan | 0 | 0 | 0 | 0 | 0 | 0 |
| 07-Feb | 0 | 0 | 0 | 0 | 0 | 0 |
| 14-Feb | 3 | 0 | 0 | 0 | 0 | 0 |
| 21-Feb | 1 | 0 | 0 | 0 | 0 | 0 |
| 28-Feb | 4 | 0 | 0 | 0 | 0 | 0 |
| 07-Mar | 5 | 0 | 0 | 0 | 0 | 0 |
| 14-Mar | 2 | 0 | 0 | 0 | 0 | 0 |
| 21-Mar | 0 | 0 | 0 | 0 | 0 | 0 |
| 28-Mar | 1 | 0 | 0 | 0 | 0 | 0 |
| 04-Apr | 2 | 0 | 0 | 0 | 0 | 0 |
| 11-Apr | 1 | 0 | 0 | 0 | 0 | 0 |
| 18-Apr | 2 | 0 | 0 | 0 | 0 | 0 |
| 25-Apr | 2 | 0 | 0 | 0 | 0 | 0 |
| 02-May | 0 | 0 | 1 | 0 | 0 | 0 |
| 09-May | 4 | 0 | 2 | 0 | 0 | 0 |
| 16-May | 12 | 0 | 121 | 0 | 0 | 2 |
| 23-May | 0 | 4 | 183 | 0 | 0 | 0 |
| 30-May | 18 | 5 | 484 | 105 | 0 | 3 |
| 06-Jun | 15 | 5 | 465 | 61 | 0 | 2 |
| 13-Jun | 99 | 0 | 964 | 146 | 0 | 1 |
| 20-Jun | 26 | 1 | 1410 | 163 | 0 | 4 |
| 27-Jun | 30 | 0 | 1360 | 321 | 0 | 3 |
| 04-Jul | 22 | 0 | 2648 | 563 | 0 | 12 |
| 11-Jul | 22 | 1 | 1522 | 1173 | 1 | 18 |
| 18-Jul | 16 | 9 | 2705 | 1600 | 2 | 13 |
| 25-Jul | 40 | 2 | 2182 | 2992 | 3 | 7 |
| 01-Aug | 20 | 4 | 2448 | 3691 | 7 | 20 |
| 08-Aug | 113 | 2 | 2723 | 4725 | 0 | 14 |
| 15-Aug | 33 | 2 | 970 | 4919 | 2 | 22 |
| 22-Aug | 69 | 1 | 1879 | 4806 | 1 | 18 |
| 29-Aug | 44 | 3 | 854 | 4073 | 2 | 20 |
| 05-Sep | 39 | 8 | 707 | 4085 | 0 | 6 |
| 12-Sep | 70 | 4 | 407 | 3521 | 3 | 8 |
| 19-Sep | 73 | 2 | 359 | 3313 | 0 | 8 |
| 26-Sep | 31 | 11 | 341 | 2543 | 2 | 2 |
| 03-Oct | 76 | 3 | 175 | 2532 | 0 | 7 |
| 10-Oct | 35 | 4 | 126 | 1917 | 0 | 12 |
| 17-Oct | 46 | 1 | 161 | 1977 | 6 | 7 |
| 24-Oct | 28 | 0 | 100 | 1180 | 1 | 1 |
| 31-Oct | 21 | 0 | 51 | 1266 | 1 | 6 |
| 07-Nov | 22 | 0 | 83 | 1025 | 0 | 0 |
| 14-Nov | 25 | 0 | 46 | 1041 | 1 | 3 |
| 21-Nov | 35 | 6 | 39 | 571 | 0 | 4 |
| 28-Nov | 43 | 0 | 35 | 923 | 0 | 3 |
| 05-Dec | 33 | 1 | 30 | 490 | 0 | 2 |
| 12-Dec | 12 | 1 | 25 | 241 | 3 | 0 |
| 19-Dec | 10 | 0 | 93 | 366 | 0 | 1 |
| 26-Dec | 8 | 0 | 6 | 510 | 0 | 0 |
2.0 Public health interventions
Guided by the temporary recommendations of the WHO in relation to the multicountry outbreak of mpox and the Federal, Provincial, and Territorial Public Health Response Plan for the Management of the Monkeypox Outbreak developed in August 2022, the following key response activities were undertaken to address the outbreak.
2.1 Coordinated communications and response
Several collective measures were undertaken to coordinate the Health Portfolio response to the outbreak, raise awareness, and empower communities to adopt public health measures, to protect groups at highest risk of mpox infection.
2.1.1 Emergency management governance
PHAC's emergency management and response functions are guided by the Health Portfolio Emergency Response Plan (HPERP) and the Federal/Provincial/Territorial Public Health Response Plan for Biological Events (PHRPBE).
On May 24, 2022, the Health Portfolio Operations Centre activated to a level 2 (Increased Vigilance and Readiness) and established an Incident Management Structure to coordinate the activities described in Section 2.
In July 2022, following the WHO announcement declaring the global mpox outbreak a PHEIC, under the framework of the FPT PHRPBE, it was determined that a coordinated FPT response was required, and a Special Advisory Committee (SAC) on mpox was established.
The mandate of the SAC on mpox was to provide advice, including technical and operational recommendations, to the Conference of FPT Deputy Ministers of Health pertaining to the coordination, planning and response across the health sector. Members of the SAC acted as liaisons to the health care sector within their respective jurisdictions and provided jurisdictional input to ensure that the full continuum of the health sector was considered in the response. On December 15, 2022, as mpox cases continued to trend downwards nationally and triggers for de-activation were met, a unanimous decision was made to de-activate the SAC on mpox, while still recognizing the importance of continued monitoring and reporting.
2.1.2 Communications
Coordinated communications was an integral part of the mpox response. As part of this work, PHAC:
- Established a bilingual central webpage to provide regular epidemiological updates on the mpox outbreak, public health information, a living evidence profile and guidance for health care professionals at Canada.ca/Monkeypox.
- Raised public awareness about mpox symptoms, transmission, public health measures and vaccination using a range of communications platforms, such as: Chief Public Health Officer and Deputy Chief Public Health Officer-led press conferences, websites, social media, social networking applications, paid advertising campaigns and community engagement with key populations.
- Developed tailored communications that were responsive to the needs of key affected populations / communities and health professionals, with consideration given to language barriers and the need for culturally appropriate materials.
- Coordinated a series of webinars with the National Collaborating Centres for Infectious Disease (NCCID) targeted to clinicians.
- Targeted public health professionals through two Infectious Disease (ID) news briefs in the Canada Communicable Disease Report (CCDR)
2.1.3 Stakeholder engagement
To raise awareness among populations most affected by mpox, PHAC worked and continues to liaise with various stakeholders through its strong sexually transmitted and blood borne infections (STBBI) networks. PHAC also collaborated with the federal two-spirit, lesbian, gay, bisexual, transgender, queer, intersex, and people who identify as part of a sexual and gender diverse communities, who use additional terminologies (2SLGBTQI+) Secretariat. Community-based organizations have experience in addressing stigma and discrimination, and PHAC actively adopted their advice and recommendations to ensure that information about mpox was communicated in a timely and appropriate way. In collaboration with these organizations, PHAC:
- Provided targeted funding to community-based organizations working with GBMSM in Canada for prevention, education, awareness, and anti-stigma activities.
- Used social media and provided education materials for special events, including Pride celebrations.
- Created and distributed a stakeholder toolkit that contained content and resources that organizations could tailor to fit their needs and be effective locally.
- Launched a student ambassador program at colleges and universities in Canada to further educate on transmission, symptoms, and prevention of mpox.
2.1.4 Field epidemiologist mobilization
PHAC provided epidemiological support to one of the affected local health jurisdictions in response to the outbreak.
2.2 Surveillance, reporting and laboratory capacity
The FPT response included increased surveillance, reporting and data sharing to better understand the affected populations, track the evolution of the outbreak, identify specific risks, and inform timely public health action.
2.2.1 National case definitions and surveillance system
PHAC established national case definitions and an mpox surveillance system, in collaboration with FPT partners. PT health authorities were engaged through the Public Health Network (PHN) and other FPT tables to share information and develop a coordinated approach to support effective and timely outbreak management in Canada.
2.2.2 Reporting
PHAC used data submitted by PT partners to create a national weekly epidemiological summary report distributed to internal stakeholders to monitor the national and international situation on mpox. The report included information on incidence, transmission, case severity, clinical features, publicly available data from the WHO and summaries of recent literature and scientific findings. Public-facing epidemiological updates were posted weekly on Canada.ca, comprising a summary of case counts and high-level demographics. As per the requirements of the International Health Regulations (IHR) (2005), PHAC, along with 109 other WHO member states, responded to requests for verification, shared information and inter-jurisdictional notifications to facilitate international contact tracing. In addition, PHAC submitted de-identified case data to the WHO on a weekly basis to support monitoring of the global mpox outbreak.
2.2.3 Laboratory capacity
As part of their mandate in emergency preparedness and to provide specialized services to identify diseases other labs may not be able to detect, PHAC's National Microbiology Laboratory (NML) had an existing laboratory test for mpox. This test was developed by NML's Special Pathogens Program and is a PCR (Polymerase Chain Reaction) test that targets multiple parts of the mpox viral genome. Following the detection of the first cases in Canada, the test was rapidly further validated using the clinical specimens to confirm accuracy in the context of the outbreak strain.
Following confirmation of performance, NML turned its attention to enabling provincial laboratories to do the testing themselves. This was important to increase timeliness of results, to facilitate contact tracing and the use of therapeutics. These technology transfer activities were coordinated through the Canadian Public Health Laboratory Network. In addition, NML performed whole genome sequencing on positive samples to study the evolution of mpox and help link cases and look at patterns of introduction and transmission through molecular epidemiology. The Agency also shared proficiency panels (DNA sequencing) to enhance mpox diagnostic testing internationally.
PHAC's Centre for Biosecurity actively engaged Canadian laboratories and published the Pathogen Safety Data Sheet - Monkeypox virus, a technical document describing the hazardous properties of the virus for laboratory workers. The Centre for Biosecurity also published the Biosafety Advisory on the monkeypox virus which assists laboratories to implement proper biosafety protocols to safely handle MPXV.
2.2.4 Wastewater surveillance
In May 2022, the NML committed to exploring the monitoring of MPXV in wastewater as a public health surveillance tool to support the ongoing outbreak response. In late June 2022, a wastewater diagnostic test was validated and used to undertake weekly wastewater testing in 9 provinces and 1 territory. Wastewater sampling, an additional surveillance tool, was offered as an early warning system to monitor levels of MPXV circulating in communities across Canada, especially in cases that were undetected.
2.3 Medical countermeasures
To manage the mpox outbreak in Canada, several medical countermeasures were utilized. This included the procurement and distribution of therapeutics to treat affected individuals and vaccines to provide pre-exposure and post-exposure prophylaxis for those at highest risk of mpox.
2.3.1. Vaccination
Imvamune® (Modified Vaccinia Ankara, Bavarian Nordic) was approved by Health Canada in 2020, for immunization against mpox in adults 18 years of age and older at high risk for exposure. Interim guidance on the use of Imvamune® in the context of ongoing mpox outbreaks was initially issued by the National Advisory Committee on Immunization (NACI) on June 10, 2022, with an update on September 23, 2022.
From May to December 31, 2022, PHAC's National Emergency Strategic Stockpile (NESS) deployed over 145,000 doses of Imvamune® to PTs. On September 19, 2022, PHAC procured additional Imvamune® vaccine for 2023-2024 to protect Canadians against mpox going forward.
Vaccine safety surveillance that detects, assesses, understands and communicates adverse events following immunization (AEFIs) was conducted through systems already in place, namely the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS) and the Canada Vigilance Program.
2.3.2. Therapeutics
Tecovirimat (TPOXX®) (an antiviral developed specifically to treat orthopoxviruses, particularly smallpox) was approved by Health Canada for the treatment of smallpox disease in adults and some pediatric patients in November 2021. Canada's Drug Agency (CDA, formerly the Canadian Agency for Drugs and Technologies in Health) published a Health Technology Review of Tecovirimat for the Treatment of Human Monkeypox outlining implementation advice on July 12, 2022, and subsequently updated its implementation advice on September 28, 2022.
Since May 2022, the NESS deployed over 165 treatment courses of Tecovirimat (TPOXX®) to PTs as assessed via Requests for Assistance (RFA).
2.4 Travel health advice and border measures
PHAC aims to protect the health of Canadians travelling internationally and to reduce the risk of importing infectious diseases to Canada.
2.4.1 Travel health advice
The PHAC travel health notices (THNs) outline potential health risks to Canadian travellers and recommend ways to help reduce them. A THN, mpox (Monkeypox): Advice for Travellers, was posted on June 7th, 2022, for individuals planning travel to non-endemic countries reporting cases of mpox. PHAC continues to monitor mpox internationally and update travel health advice as appropriate.
2.4.2 Border measures
No border measures or enhanced screening measures were implemented in response to the mpox outbreak. PHAC provided guidance to frontline border officials on the assessment and management of symptomatic travellers and/or cases and close contacts.
2.5 Public health measures guidance
Through FPT collaboration, guidance documents were developed to equip public health programs and professionals with the tools necessary to minimize the risk of onward transmission and support the management of cases. The central webpage provided regular updates on the mpox outbreak and facilitated access to published guidance for the population and for health professionals.
2.6 Scientific evidence generation and monitoring
The collective FPT public health response to mpox was evidence-informed and grounded in science. PHAC demonstrated scientific leadership, contributed to community engagement, and enhanced the evidence base for the mpox response during this outbreak. PHAC implemented the following approaches to support foundational knowledge and scientific evidence to guide decision-making.
2.6.1 Monitoring science, emerging evidence, and research plan
PHAC activated an evidence support system, commissioning and mobilizing three rapid evidence profiles and convening internal scientific and medical experts for emerging science knowledge sharing. They coordinated an external expert panel of clinical, laboratory and public health experts, which met on multiple occasions to identify knowledge gaps, define science priorities, provide expert input to PHAC guidance, and coordinate research efforts. These academic and clinical researchers were convened from several jurisdictions to promote collaboration on key priorities, including vaccine effectiveness studies.
Additionally, PHAC developed a research plan to address needs within the Canadian context, to inform and align with the WHO Research and Development Blueprint. PHAC also provided input on priorities for the Canadian Institutes of Health Research (CIHR) and International Development Research Centre (IDRC) funding of mpox research co-led by Canadian and African researchers.
2.6.2 Therapeutics evaluation
Early research studies suggested that Tecovirimat (TPOXX®) would be effective, and it was recommended as the primary treatment to be administered in severe cases of mpox. During 2022, Tecovirimat (TPOXX®) was being used off-label to treat mpox, and there was a need for further evidence of its effectiveness for this use. The NML was able to isolate the virus from one of the first mpox cases identified in Canada and used it to evaluate tecovirimat (TPOXX®) effectiveness in animal models. This investigation found that Tecovirimat (TPOXX®) was able to significantly reduce the amount of virus in study animals to the point where they did not display any obvious clinical signs of disease.
2.6.3 Animal studies
Animal studies were performed at the PHAC's NML on samples submitted from provinces with confirmed cases of mpox. Using a mouse model, it was demonstrated that the MPXV circulating in Canada in 2022 was less lethal than historic samples suggesting there could be less death expected in humans. In endemic countries rodents are thought to be the main reservoirs for MPXV. To investigate a potential host species in North America, the NML experimentally infected deer mice with the present strain of MPXV. This resulted in a self-limiting infection, suggesting these mice would not be a viable animal reservoir for mpox in North America.
2.6.4 Modelling
PHAC modellers developed two mathematical compartment-type models to explore and forecast the mpox epidemic in Canada and assess the impact of different interventions including behaviour change and vaccination. An importation model was adapted to assess likely numbers of imported cases. Modelling updates were presented regularly to support decision making within the Agency. Additionally, modelling results were also presented at the WHO mpox data and modelling meetings to support the global effort. These peer sharing meetings helped to validate and strengthen these models as well as share the Canadian approach with the world.
2.6.5 Risk assessment
PHAC's Centre for Integrated Risk Assessment (CIRA) leads coordinated risk assessment activities to anticipate, detect and assess public health risks to Canadians. CIRA, in collaboration with other PHAC programs, produced an initial threat assessment on mpox in Canada on May 17, 2022. This group evaluated the risks on an ongoing basis and published two rapid risk assessments (RRA). These assessments provided the Agency with a series of recommended actions over the course of the outbreak to best respond to this event.
Additionally, PHAC's Centre for Biosecurity, completed a comprehensive pathogen risk assessment to evaluate the risks of MPXV in a laboratory setting. Monkeypox virus is classified as a Risk Group 3 human and animal pathogen, as well as a Security Sensitive Biological Agent. These classifications dictate the containment level at which MPXV must be handled in a laboratory, including the physical containment, operational practice, performance, and verification testing requirements, as well as enhanced biosecurity measures.
2.7 One Health
The Agency also applied a One Health approach to the outbreak, recognizing that the health of people is connected to animals and our shared environments. PHAC led an FPT working group with experts from human, animal, and environmental health with the goal of preventing spread from infected people into Canadian animal populations. Key outcomes included:
- implementing passive surveillance of suspect animal infections of mpox within Canada
- developing animal testing capacity, led by the Canadian Food Inspection Agency (CFIA)
- developing and issuing guidance for public health authorities and the public on preventing transmission of mpox between humans and animals
- raising awareness of the specialty pet industry, highlighting high-risk animal species (rodents) and the potential for mpox transmission from humans to animals
- collaborating on pathway exposure analysis, led by the CFIA, on how animals could be exposed to MPXV via waste products from infected humans in Canada
- event-based surveillance of reports of animal mpox infections outside of Canada.
2.8 International collaboration
PHAC led on international engagement and coordination with various bilateral and multilateral international stakeholders, both to share intelligence and technical information and to facilitate collaboration. Notably, Canada engaged with the United Kingdom, Africa Centres for Disease Control and Prevention and Chile about early mpox research, enhancing diagnostic capacities, and to share national mpox vaccination strategies respectively.
3.0 Conclusion
Building on experience from previous emergency management events, the Agency responded promptly to the mpox outbreak in a wide-ranging and comprehensive manner, the timelines of which are illustrated in Figure 3. Canada was seen as a global leader in its response to this outbreak.
Canada was one of the first nations to position and implement vaccine regimes within PTs based on NACI guidance. Over 119,800 doses of the vaccine were estimated to have been administered during the outbreak. In addition, it took an international lead in wastewater monitoring. The Agency regularly engaged with international partners to support the global response.
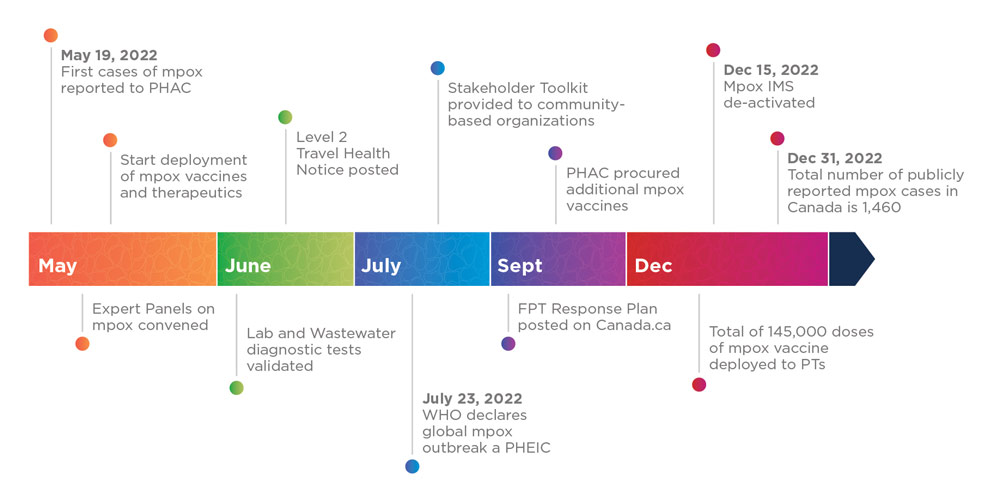
Figure 3 - Text equivalent
On May 19, 2022, the first cases of mpox were reported to PHAC. Throughout May, PHAC convened Expert Panels on mpox and started the deployment of mpox vaccines and therapeutics to PTs. In June 2022, PHAC validated lab and wastewater diagnostic tests, and posted a Level 2 Travel Health Notice for mpox. On July 23, 2022, the WHO declared the global mpox outbreak a Public Health Emergency of International Concern (PHEIC). Also in July, PHAC shared a Stakeholder Toolkit with community-based organizations. In September 2022, PHAC posted its FPT Response Plan on Canada.ca, and also procured additional mpox vaccines. By December 2022, a total of 145,000 doses of the mpox vaccine had been deployed to PTs. On December 15, 2022, PHAC de-activated the mpox IMS and by December 31, 2022, a total 1,460 mpox cases had been publicly reported in Canada since the beginning of the outbreak in May 2022.
The Agency collaborated early and often with the PTs through the development of guidance documents, deployment of vaccines for all PTs, acting as the national reference laboratory and providing confirmatory testing and training, including wastewater surveillance and various research studies; however, the critical element of Canada's strategy included community engagement.
Stakeholder engagement was a foundational pillar of Canada's response; PHAC engaged early and often with stakeholders, including community-based organizations that were supporting populations that were impacted by the outbreak. These actions continued to inform PHAC's approaches, from risk communication to research priorities to improve PHAC's response and help address stigma and discrimination within the GBMSM community most affected by this outbreak.
Rapid dissemination and communication of information supported several behavioral practices and protective measures, including using masks, hand washing and sexual health measures such as limiting sexual contact with multiple partners.
This unprecedented outbreak, arising from the novel introduction of a known pathogen, has illustrated the need to remain abreast of the scientific expertise and knowledge networks that enable effective, timely and evidence-informed public health response across Canada. However, there is a continued need to maintain baseline monitoring to prevent another resurgence of this disease. Following an internal evaluation of the response, PHAC will continue to work with all levels of government and community stakeholders to improve the Agency's knowledge, preparedness, and response initiatives.
Finally, in the past few years, global health has faced significant challenges due to PHEICs such as COVID-19, Ebola and mpox. However, applications of a variety of tools, including raising awareness through risk communication, building diagnostic and scientific capacity, and providing access to vaccines and treatments have helped generate a rigorous response to these emerging diseases. While recognizing global health inequities are an ongoing challenge, PHAC employs a One Health approach to recognize, understand, and manage the interface between humans, animals, and environments. This is critical in achieving proactive positive public health outcomes for new emerging zoonotic diseases worldwide.