Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings
Part A: Introduction to routine practices and additional precautions
I. Introduction
Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care represent the IPC practices to be used in all healthcare settings in Canada and the expected processes and practices of care. The objective of this guideline is to identify and promote IPC practices and precautions for preventing the transmission of infection in all healthcare settings. This guideline is designed for use by infection control professionals (ICPs). It is recommended that individuals who lack IPC expertise seek the expertise of ICPs in their organization or region for assistance.
This revision promotes the consistent application of routine practices and additional precautions across the continuum of care, and outlines modifications in the application of additional precautions outside of acute care. This guideline should be used to develop specific recommendations for local use, taking into consideration local conditions, such as the type of facilities available, risk of acquisition of infection, type of healthcare setting, type of care, and level of education and awareness of the healthcare workers (HCWs) providing the care. Included in this document are the principles necessary to prevent transmission of microorganisms from patient to patient, patient to HCW and HCW to patient across the continuum of care. This document does not provide a comprehensive approach to outbreak recognition, reporting and management, but does provide recommendations intended to prevent some of the most common outbreak situations (e.g., respiratory hygiene to prevent respiratory virus outbreaks and environmental cleaning and hand hygiene to prevent outbreaks of Clostridium difficile and norovirus). For the purposes of this document, the term "patient" will be used to include those receiving health care who are traditionally/routinely referred to as patients, clients or residents. Principles of transmission, as well as routine practices and additional precautions, are outlined for acute care, LTC, ambulatory care, prehospital care and home care settings. For the purpose of this document, acute care includes ambulatory care settings such as hospital emergency departments, and free-standing or facility-associated ambulatory (day) surgery or other invasive day procedures (e.g., endoscopy units, hemodialysis, ambulatory wound clinics).
A. Principles upon which this document is based
This document recognizes certain principles:
- Consistent application of routine practices is expected for the care of all patients, at all times, across the continuum of care.
- Adherence to routine practices can reduce the transmission of microorganisms in healthcare settings.
- Individual components of routine practices are determined by a point-of-care risk assessment (PCRA) (i.e., one that includes an assessment of the task/care to be performed, the patient's clinical presentation, physical state of the environment and the healthcare setting).
- Microorganisms may be transmitted from symptomatic and asymptomatic individuals, emphasizing the importance of adhering to routine practices at all times for all patients in all healthcare settings.
- In addition to routine practices, precautions should be used for patients with suspected or known infections or colonization with microorganisms for which routine practices are insufficient to prevent transmission.
- Additional precautions should be used empirically, based on the patient's condition or clinical presentation. These may need to be modified or discontinued based on the specific microorganism identified.
- The primary goal of IPC programs is to reduce the risk of acquiring a healthcare- associated infection (HAI) to a minimum level; zero risk may not be attainable in every circumstance, but should nevertheless be strived for. The consequences of cross-transmission of microorganisms should be balanced against the consequences (adverse effects and cost) of precautions taken.
- Application of additional precautions may vary between acute care, LTC, ambulatory care, prehospital care and home care settings. Local epidemiology should be considered in the application of additional precautions.
Major changes with this revision include:
- Expecting HCWs to use ABHR at the point-of-care as the preferred method of hand hygiene in all healthcare settings unless exceptions apply (i.e., when hands are visibly soiled with organic material, if exposure to norovirus and potential spore-forming pathogens such as Clostridium difficile is strongly suspected or proven, including outbreaks involving these organisms).
- Preferring single inpatient rooms rather than multipatient rooms with designated private toilets and patient sinks and accessible designated HCW handwashing sinks.
- Implementing respiratory hygiene, a strategy involving a combination of measures designed to minimize the transmission of respiratory pathogens, across the continuum of care.
- Changing the recommendation for spatial separation between a patient with a suspected or confirmed droplet transmissible respiratory infection who is coughing (infected source) and another patient without that infection (susceptible host) from one metre to two metres. When using a risk assessment, one metre may be sufficient for young children and others whose cough is not forceful enough to propel the droplets as far as two metres.
- Implementing strategies to reduce aerosol generation when performing aerosol generating medical procedure (AGMPs) on patients with signs and symptoms of suspected or confirmed tuberculosis (TB), SARS or respiratory infection with an emerging respiratory pathogen. (refer to Part A, Section II, C, 2c for discussion on AGMPs, and Part B, Section IV, subsection iii, 1b for strategies to reduce aerosol generation.) Routine practices and contact and/or droplet precautions, as indicated, are necessary for AGMPs on other patients.
- Changing to a recommendation that adult patients with known or suspected viral respiratory infections be placed on contact and droplet precautions (which is the current practice in pediatrics).
- Reaffirming the recommendation that HCWs follow aseptic technique for invasive procedures and in the handling and delivery of parenteral medications and intravenous systems.
- An expectation that healthcare organizations should perform an Organizational Risk Assessment (ORA) — that is, evaluating the healthcare environment to identify the risk of exposure to microorganisms and implementing appropriate control measures (i.e., healthcare facility design, and cleaning, disinfection and sterilization of patient care equipment).
- Emphasizing the expectation that HCWs perform a PCRA prior to each patient interaction, taking into consideration the patient, patient environment and nature of the interaction.
B. Routine practices
Routine practices are the IPC practices for use in the routine care of all patients at all times in all healthcare settings and are determined by the circumstances of the patient, the environment and the task to be performed.
Performing an Organizational Risk Assessment (ORA) (Refer Part A, Section III, B) and addressing deficiencies provides the framework to ensure that appropriate components in the hierarchy of controls related to routine practices are in place in order to minimize the risk of exposure to and transmission of microorganisms within healthcare settings.
A PCRA is performed by HCWs to determine the appropriate IPC measures for safe patient care (i.e., to protect the patient from transmission of microorganisms) and to protect the HCW from exposure to microorganisms (e.g., from sprays of blood, body fluids, respiratory tract or other secretions or excretions and contaminated needles and other sharps). (Refer Part A, Section III, C).
Routine practices include:
- Point-of-care risk assessment
- Hand hygiene program (including point-of-care ABHR)
- Source control (triage, early diagnosis and treatment, respiratory hygiene, spatial separation)
- Patient placement, accommodation, and flow
- Aseptic technique
- Use of PPE
- Sharps safety and prevention of bloodborne pathogen transmission
- Management of the patient care environment
- Cleaning of the patient care environment
- Cleaning and disinfection of non-critical patient care equipment
- Handling of waste and linen
- Education of patients, families and visitors
- Visitor management
C. Additional precautions
Additional precautions are applied when the transmission characteristics of, or impact of, infection with a specific microorganism (e.g., microorganisms with a low infectious dose such as Shigella spp., or microorganisms spread by the droplet route such as respiratory syncytial virus [RSV], or epidemiologically significant microorganisms such as antibiotic-resistant organisms [AROs]) or syndromes are not fully prevented by routine practices. These precautions should also be used when medical procedures increase the risk of transmission of a specific infectious agent (e.g., AGMPs) or when the clinical situation prevents consistent application of routine practices (e.g., care of the young child, incontinent adult or cognitively impaired individual). How additional precautions are applied is specific to the care setting (acute care, ambulatory care, prehospital care, LTC and home care).
Additional precautions are conventionally divided into:
- contact precautions, for microorganisms of very low infective dose or situations where heavy contamination of the patient's environment is anticipated.
- droplet precautions, for microorganisms primarily transmitted by the large droplet route.
- airborne precautions, for microorganisms transmitted through the air over extended time and distance by small particles.
Some infections may need a combination of additional precautions (contact, droplet, airborne), since some microorganisms can be transferred by more than one route. The application of routine practices continues even with the application of additional precautions.
Performing an ORA (refer Part A, Section III, B) and addressing deficiencies provides the framework to ensure that appropriate components in the hierarchy of controls (refer to Part A, Section III, A) related to additional precautions are in place to minimize the risk of exposure to and transmission of infectious agents within healthcare settings.
D. Evolution of isolation precautions
Isolation precautions have evolved from the concept of "fever hospitals" for the care of patients with specific communicable pathogens of major public health concern, such as smallpox, diphtheria and TBFootnote 1. As these diseases became less prevalent, care was transferred to special isolation wards in general hospitals and eventually to single rooms on regular patient care wards. Over time, isolation precautions were extended to all patients with infections considered to be transmissible. Infectious diseases were classified into categories, according to the presumed major mechanism of transmission, and specific precautions were recommended for each transmission categoryFootnote 2. A preprinted card listed the precautions to be taken for each selected category. Category-based precautions were simple to learn and implement. However, dissatisfaction with category-based precautions developed. Mechanisms of disease transmission did not always fit into the assigned categories, resulting in excessive or inadequate use of barrier techniques. Healthcare workers needed to have more flexibility in applying isolation precautionsFootnote 3, Footnote 4.
As a result, an alternative disease-specific system was developed, whereby isolation precautions were fine-tuned according to the needs of the individual patient and microorganism. Hospitals could choose between category or disease-specific systemsFootnote 3. Specific barrier techniques (e.g., single room, air control, gloves, gowns and masks) were assigned according to the patient's diagnosis or symptoms or the microorganism isolated, as well as to patient behaviours or characteristics (e.g., age, mental status, mobility, continence). Isolation precautions were written or selected from check boxes on an isolation card. Disease-specific precautions eliminated unnecessary measures, permitting more efficient use of facilities and materials. Compliance was expected to be higher, since these recommendations were more epidemiologically sound. There was an increased emphasis on decision making on the part of the HCW. However, there were a number of drawbacks. This system required more knowledge, initiative and responsibility on the part of HCWs. Selecting the appropriate techniques for individual patients was time consuming. There was a risk of error when HCWs were not adequately informed, when the diagnosis was incorrect or when personnel were rushedFootnote 5, Footnote 6.
The most dramatic modification in isolation precautions occurred after the realization that the human immunodeficiency virus (HIV) could be transmitted from patients with unrecognized infection to HCWsFootnote 7. Initiation of bloodborne pathogen precautions, based on symptoms or diagnosis, was no longer adequate. The response to this problem was the extension of the use of blood and body fluid precautions to all patients. These precautions became known as universal blood and body fluid precautions. Universal precautions included use of barrier precautions, such as gloves for contact with blood and certain other body fluids; gown, masks and eye protection in situations with potential for contamination of skin or clothing or for splashes with these fluids; measures to prevent injuries from contaminated needles and other sharp items; and protocols for blood spill clean-up and laboratory safety.
Universal precautions were developed with the primary purpose of protecting the HCW from exposure to bloodborne pathogens, and were based on the principle that it was not possible to know which patients harboured bloodborne pathogens. Universal precautions were used in conjunction with category - or disease-specific isolation systems for patients with specific symptoms or infectionsFootnote 8, Footnote 9.
There was also concern that diagnosis-driven precautions were inadequate, in that they did not address potential transmission from body substances of asymptomatic colonized patients. To address this concern, a new isolation system, called body substance isolation, was created, in which barrier precautions were tailored to the activity performed rather than the diagnosis. This system extended barrier precautions to all direct contact with blood, body fluids, secretions and moist body substances, and with non-intact skinFootnote 10, Footnote 11, Footnote 12.
Gloves were used for all such contacts. Gowns, masks and eye protection were recommended for procedures in which soiling or splashing was anticipated. The principles of body substance isolation were that all persons harbour potentially pathogenic agents in moist body sites and substances and that all persons are at risk of acquiring microorganisms from inoculation of mucous membranes and non-intact skin. The goal was to prevent transmission by preventing contamination of the HCW's hands. There was confusion over whether or not handwashing was indicated after removal of gloves. Body substance precautions were not intended for control of droplet and airborne transmitted microorganismsFootnote 13, Footnote 14.
The US Centers for Disease Control and Prevention (CDC) revised its isolation guidelines in 1996 by selecting what were considered to be the best recommendations from each of the previous systemsFootnote 13. These guidelines applied only to acute care inpatient facilities. A two-tiered system was developed, with standard precautions for all patients and three categories of transmission-based precautions for specific infections that warranted additional measures. Standard precautions addressed the concern of transmission by contact with asymptomatic patients and with contaminated sources in the environment of the infected or colonized patient. Gloves were recommended for all contacts, as indicated in body substance isolation and, in addition, for contact with contaminated items. The three categories of additional precautions were based on known or presumed routes of transmission (e.g., airborne, droplet and contact) and patient characteristics. Contact precautions were more extensive than previously specified, in that barrier techniques were recommended for all persons entering the patient's roomFootnote 13. The 2007 Guideline for Isolation Precautions: Preventing Transmission of Infectious Agents in Healthcare SettingsFootnote 15 provides recommendations that can be applied in all healthcare settings and introduced a number of new elements into standard precautions.
E. History of Canada's isolation guidelines
Infection control precautions and isolation guidelines were originally published by the Steering Committee on Infection Control Guidelines Development, as convened by the Bureau of Communicable Disease Epidemiology of the Laboratory Centre for Disease Control, Health Canada, in 1985. These guidelines, revised in 1990Footnote 16, were written from a disease-specific perspective, listing specific precautions for diseases and microorganisms. The 1990 revision added symptoms as a basis for determining isolation precautions. Separate documents were issued in 1987, 1988 and 1989 outlining universal precautionsFootnote 17, Footnote 18, Footnote 19, which were incorporated into the 1990 revision. Infection control guidelines for LTC were published in 1986 and revised in 1994Footnote 20. These did not address specific issues related to isolation in LTC facilities, but referred to the 1990 Health Canada guidelines for isolation and precaution techniquesFootnote 16. In 1996, recommendations to prevent transmission of TB were publishedFootnote 21. Revised guidelines for preventing transmission of bloodborne pathogensFootnote 22,and vancomycin-resistant enterococci (VRE)Footnote 23 were published in 1997.
Revised guidelines for isolation and precautions, Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care, were published in 1999Footnote 24. The term "routine practices" was chosen to emphasize that this is the level of care that should be provided for all patients at all times, in all healthcare settings. When routine practices are insufficient, "additional precautions" should be used. The 1999 Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Health Care provided recommendations that were specific to acute care, LTC, ambulatory care and home care settings. Recommendations for acute care settings did not differ in principle from the standard precautions and transmission-based precautions published by the CDC in 1996, although more details were included in the Canadian document.
F. Changing populations and healthcare delivery systems
Over the past decade, healthcare systems have continued to be restructured. The patient population in acute care hospitals has continued to shift toward a group at higher risk for HAIs. New technologies and aggressive treatments, many of which compromise host defences, have permitted patients with previously fatal diseases to survive. Organ and hematopoietic stem cell transplants, HIV, and an aging population have also added to the number of high-risk patients. The shift has resulted in: increased acuity of illness in acute care facilities; increased level of acuity in LTC (providing complex care such as intravenous therapy, hemodialysis or ventilation therapy); performance of invasive procedures and complex treatments in day treatment or outpatient settings exposing this population to the risk of HAIs; and transfer of care for many similar conditions or treatments to the home or outpatient settings. In addition, an aging population has increased the demand for healthcare services at the same time as the nation is experiencing a shortage of HCWs.
There is the potential for HAI across the continuum of care, from prehospital care to acute care hospitals, rehabilitation centers, LTC facilities, nursing homes, adult residential care, ambulatory care centres and home care. Transfers of patients between facilities and between different levels of care within facilitiesFootnote 25, and transfers back to Canada, from a foreign country, of patients who had trauma (such as returning soldiersFootnote 26 or people who have been hospitalized in a foreign country) are frequent and increase the risk for transmission of antimicrobial-resistant microorganisms.
G. Burden of healthcare-associated infections
Healthcare-associated infections (e.g., surgical site infections, central venous catheter-associated bloodstream infections) result in a substantial burden of disease in Canadians, and are an important public health problemFootnote 27, Footnote 28, Footnote 29. They are also a burden on Canada's healthcare system and a barrier to timely access to care for all Canadians.
There has been no comprehensive survey of the occurrence of HAIs in Canada; however, it is generally estimated that 5%-10% of hospitalized Canadians will develop a HAIFootnote 30. A survey of sentinel Canadian hospitals in February 2002 by Gravel et al. found that 10.5% of adult inpatients and 9.1% of paediatric inpatients had a HAI on the surveyFootnote 28, Footnote 29.In a repeat survey in 2009, involving a similar hospital group, Gravel et al. found that 12.3% of adult patients and 7.2% of paediatric patients had a HAI on the day of the survey. Between the two surveys, the number of patients on isolation precautions had nearly doubled (from 7.7% to 14.8%), largely due to the impact of C. difficile infection and AROs (personal communication, Canadian Nosocomial Infection Surveillance Program 2010). Extrapolating from US data, Zoutman et al. estimated that each year, approximately 220,000 HAIs occur in Canada, as do more than 8,000 deaths attributable to HAIsFootnote 27. Healthcare-associated infections vary in type, frequency and severity. For example, healthcare-associated urinary tract infections are among the most common of all HAIs, but result in less serious patient impactFootnote 31. In contrast, the less common ventilator-associated pneumonia has a case mortality rate exceeding 10%Footnote 32.
Healthcare-associated infections are also costly to treat. In the US, it is estimated that the attributable cost of treating HAIs range from US$1,257 for urinary tract infections to US$9,986 for ventilator-associated pneumoniaFootnote 30. In a study to determine the incremental cost attributable to methicillin-resistant Staphylococcus aureus (MRSA) in a Canadian hospital, patient-specific hospitalization costs for a cohort of patients with hospital-acquired MRSA and a matched comparison group of uninfected patients were investigated. The median total hospitalization cost per nosocomial MRSA patient (colonized and infected) was $14,841, whereas the corresponding cost for those in the uninfected comparison group was $5,844, which suggests an incremental cost of $8,997 per nosocomial MRSA patient. The incremental cost to prevent a case of nosocomial MRSA was $19.77. The authors suggested the cost-effectiveness ratio can be improved by decreasing hospital length of stayFootnote 33.
Patients with HAIs occupy scarce hospital beds (e.g., healthcare-acquired surgical site infections prolong hospital stay by a mean of 25.7 days)Footnote 30, and investigation and treatment of these infections consumes other scarce healthcare resources. Healthcare-associated infections are therefore a significant barrier to access to care for other health conditions.
All healthcare interventions have potential risks, including risk of infection, and potential benefits. Currently, not all HAIs are preventable. However, HAIs are not inevitable; it has been known for many yearsFootnote 34 that organized approaches to HAI prevention are highly effective in reducing their frequency. The gap between those that can be prevented and those that are currently being prevented exists because of a lack of awareness and implementation of prevention strategies by frontline HCWs and inadequate prioritization of HAI prevention strategies by healthcare managers and administrators.
Application of Routine Practices and Additional Precautions for Preventing the Transmission of Infection in Healthcare Settings is an important component of a comprehensive approach to HAI prevention. By adopting the recommendations in this document across the continuum of care, the burden of HAIs on Canadians and Canada's healthcare system can be reduced.
H. Balancing risk and benefit in preventing cross-transmission
Ideally, care should be provided in a manner that maximizes the probability that all transmission of potential microorganisms from all patients — asymptomatic colonized as well as symptomatic — in all healthcare settings will be prevented. In reality, this is currently not achievable. Transmission of microorganisms in the healthcare setting cannot always be prevented, and attempts to do so would entail additional costs and restrictive measures that would interfere with the quality of life for the patient or avoidance of potentially beneficial medical procedures or interventions. Thus, IPC practices should be tailored to the level of care that is being provided and the inherent risk to the individual and the population if infection occurs. Precautions that may be justified in terms of risk-benefit in an intensive care unit (ICU) or acute care ward may not be of equal benefit or indicated for a patient in LTC.
Unnecessary use of additional precautions is to be avoided. It is clear that isolation practices can be stigmatizing and psychologically damaging, and run some risk of having adverse effects on the quality of health care delivered (e.g., medical errors)Footnote 35, Footnote 36, Footnote 37, Footnote 38, Footnote 39. Furthermore, unnecessary isolation practices are expensive and consume scarce healthcare resources that could be used to benefit other patients. Consequently, only IPC isolation practices that are clearly indicated in the setting where the care is provided should be implemented, and they should be discontinued as soon as appropriate.
In most instances, the precautions to apply are clear-cut, based on the evidence available. In other situations, certain measures may need to be modified for different types of healthcare settings, based on assessment of risks and benefits. The benefit of reducing risk of transmission needs to be balanced against the cost (in quality of life, adequacy of medical care and monetary outlay) of the precautions taken to achieve this reduction in risk.
II. Principles of transmission of microorganisms
A. Chain of infection
Epidemiologic analysis helps us prevent disease by explaining the distribution of illness (in terms of person, place and time) and identifying modifiable factors that affect its occurrence and outcomes. It provides the rationale for control measures to minimize transmission of microorganisms, and ultimately to reduce the incidence of HAIs in patients and occupational infections in HCWs.
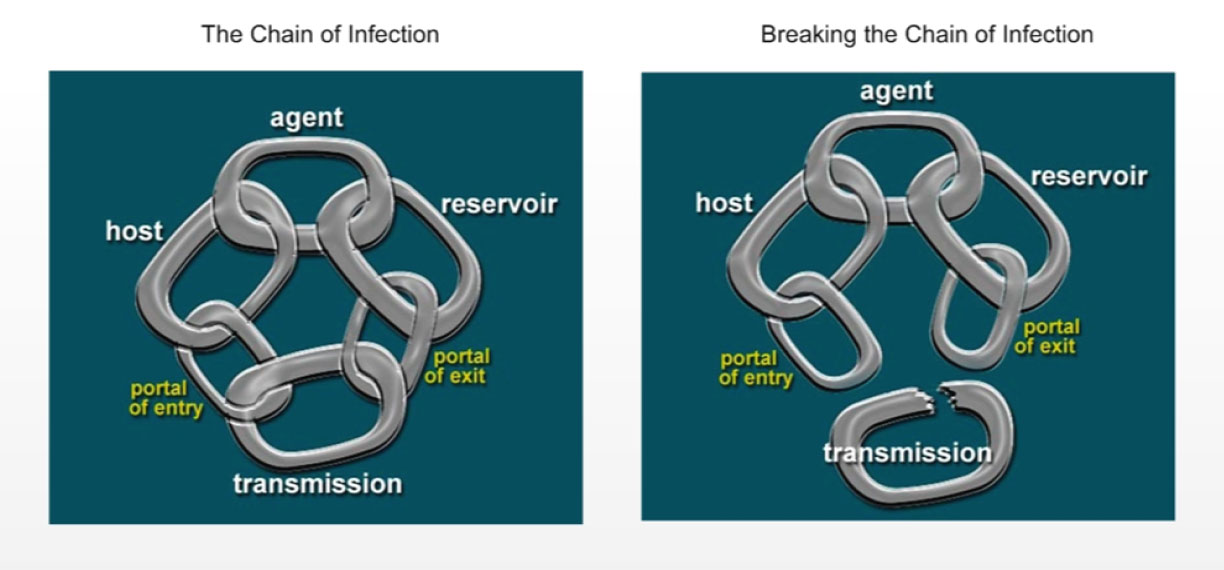
Transmission of microorganisms may result in transient carriage or long-term colonization, asymptomatic infection or clinical disease. The presence of microorganisms in or on a host, with growth and multiplication but without tissue invasion or cellular injury, is referred to as colonization. Infection is the condition in which microorganisms are able to multiply within the body and cause a response from the host's immune defences. Infection may or may not lead to clinical disease (symptomatic infection). The establishment of infection involves a set of complex interrelationships between the source of the infectious agent (microorganism), the susceptible host and the environment, and requires the transmission of microorganisms from the source to a susceptible host. One framework for understanding this complex relationship is the chain of infection, which can have six links (as shown in Figure 1a): the infectious agent, reservoir, portal of exit, mode of transmission, portal of entry and susceptible host. Breaking any one of the links in the chain of infection will prevent infection from occurring (Figure 1b).
Figure 1a and 1b: Chain of infection
Permission for use of graphics provided by Dr. Donna Moralejo,
Associate Professor, Memorial University School of Nursing, St. John's Newfoundland
Text description
Figure 1a shows the chain of infection, which is one framework for understanding the complex relationship, which can have six links: the infection agent, reservoir, portal of exit, mode of transmission, portal of entry and susceptible host.
Figure 1b shows that breaking any of the links in the chain of infection as shown in Figure 1a will prevent infection from occurring.
A brief explanation of each link follows:
1. Infectious agents (microorganisms)
These include bacteria, viruses, fungi and parasites. They can be either endogenous flora (i.e., patient's own microorganisms) or exogenous flora (i.e., microorganisms external to the patient, for example from other individuals, plants or inanimate objects). Regardless of whether they are from other parts of the body or from another person or object, microorganisms are considered to be transient flora if they are temporarily carried by the patient (refer to Part A, Section II, B). Antimicrobials, disinfectants and hand hygiene with ABHRs kill microorganisms, breaking this link in the chain of infection, where applicable. The characteristics of a particular microorganism affect the ease of its transmission. Microorganisms that can survive environmental conditions and remain viable on inanimate objects, such as patient care equipment, are more likely to be transmittedFootnote 40, Footnote 41, Footnote 42, Footnote 43, as are those with a low infective dose (e.g., Shigella)Footnote 44.
2. Reservoirs in health care
Humans, animals and the environment are reservoirs of infectious agents (microorganisms) relevant to health care. Hand hygiene following contact with individuals or their environment, preoperative skin preparation and cleaning the environment all reduce the number of microorganisms present in a reservoir, breaking this link in the chain of infection (refer to Part A, Section II, B).
3. Portals of exit
A portal of exit is the route by which an infectious agent (microorganism) leaves the reservoir, although not all reservoirs have an obvious portal of exit (e.g., the environment). Infectious agents are contained in blood, body fluids, excretions, secretions and skin of human reservoirs, depending on the agent, and leave the reservoir through the respiratory, gastrointestinal or integumentary (skin/mucous membranes) system. Reduction of excretions or secretions or covering portals of exit (e.g., dressings on wounds, masks), break this link in the chain of infection.
4. Routes of transmission
Routes of transmission of infectious agents (microorganisms) are conventionally categorized into five routes: contact, droplet, airborne, common vehicle and vectorborne. It should be recognized that the transmission of the many varieties of microorganisms and infections they may cause cannot always be precisely circumscribed within a limited number of carefully contained transmission modes. Nevertheless, these transmission categories have proven very useful in describing the spread of microorganisms in populations. The routes of transmission vary with the microorganisms involved, and some microorganisms can be transferred by more than one route (refer to Part A, Section II, C). The appropriate use of barriers and adherence to hand hygiene break this link in the chain of infection.
5. Portals of entry
A portal of entry is the route by which an infectious agent enters the host. Examples include mucous membranes of the respiratory tract, the gastrointestinal tract, the urinary tract, breaks in the skin (e.g., wounds) and devices such as intravenous lines. This link in the chain of infection can be broken by protecting portals of entry by covering wounds, wearing PPE, reducing breaches in the mechanical barriers of the skin and mucous membranes, using sterilized equipment when required, or by performing hand hygiene so that hands do not transfer microorganisms to a portal of entry.
6. Susceptible host
An individual must be susceptible to the infectious agent (microorganism) for an infection to occur. Humans do not become infected with most animal viruses because they do not have the appropriate cell receptors, and individuals with circulating antibodies to vaccine-preventable diseases do not get the infection because the immune response prevents the infectious agent from multiplying (refer to Part A, Section II, D). This link in the chain of infection can be broken by ensuring host defences are maximized (e.g., through immunization, optimal nutrition, reduction of smoking and control of diabetes).
B. Sources or reservoirs of infectious agents (microorganisms)
The sources or reservoirs of infectious agents transmitted in health care may be human or environmental. Portals of exit vary by reservoir and infectious agent.
1. Human sources
Source individuals may have active disease, be in the asymptomatic and/or incubation period of an infection or may be transiently or indefinitely colonized with microorganisms, particularly on the skin and mucous membranes. Human reservoirs include patientsFootnote 45, Footnote 46, Footnote 47, Footnote 48, Footnote 49, Footnote 50, Footnote 51, Footnote 52, HCWsFootnote 53, Footnote 54, Footnote 55, Footnote 56, Footnote 57, Footnote 58, Footnote 59, Footnote 60, Footnote 61, Footnote 62, Footnote 63, household members and other visitorsFootnote 64, Footnote 65, Footnote 66, Footnote 67, Footnote 68.
Transmission of microorganisms in health care is increased by the presence of patients who visibly soil the environment or cannot maintain appropriate hygiene, including respiratory hygiene; patients who are cognitively impaired; patients with uncontained secretions or excretions; patients with wound drainage that cannot be contained by a dressing; patients with fecal incontinence if stools cannot be contained in incontinence products or infant diapers; and those with viral respiratory or gastrointestinal infectionsFootnote 48, Footnote 69, Footnote 70, especially infants.
2. Animal sources
This is not a common or usual mode of transmission of HAI in most care settings, although the advent of pet therapy in acute care and the presence of companion animals in home and LTC provides some opportunity for zoonotic infectionFootnote 71, Footnote 72. Recently researchers have demonstrated transfer of MRSA and C. difficile to canine visitors, emphasizing the importance of hand hygiene before and after contact with animals in healthcare settingsFootnote 73, Footnote 74.
3. Environmental sources
Environmental factors may either assist or impede the transmission of microorganisms. The environment may play a larger role in the survival and growth of certain microorganisms than previously appreciated, reinforcing the importance of minimizing environmental contamination by patient secretions and excretions, avoiding unnecessary hand contact with environmental surfaces and ensuring high standards for environmental cleaning are maintained.
Respiratory virusesFootnote 75, Footnote 76, Footnote 77, rotavirus, norovirusFootnote 78, Footnote 79, Footnote 80, Footnote 81 and C. difficile sporesFootnote 82, Footnote 83 persist for prolonged periods in the environment and may be a source of transmission. The role of the environment is increasingly recognized as an important source of patient-to-patient transmission of AROsFootnote 84, Footnote 85.
The mobile environment (i.e., equipment and items that are shared between patients), if not cleaned between uses, may increase the chance of exposure to the microbial flora of other patients, and also be a source of transmission. Examples of items implicated in the transmission of infection or known to be an environmental source of contamination are listed in List 1.
List 1: Examples of environmental sources of contamination
- 1a. Patient care items implicated in the transmission of infection
- Contaminated blood pressure cuffs in the transmission of C. difficileFootnote 86, Klebsiella spp.Footnote 87
- Contaminated thermometers in the transmission of VRE and C. difficileFootnote 42, Footnote 88, Footnote 89, Footnote 90
- Ultrasonic nebulizers in the transmission of MRSAFootnote 91
- Reusable fingerstick blood sampling devices in the transmission of hepatitis BFootnote 92
- Environmental surfaces near infant bedside, such as countertops, crib sides, pacifiers, toys in the transmission of RSVFootnote 77
- Toys in the transmission of multiresistant Pseudomonas aeruginosaFootnote 93
- 1b. Patient care items contaminated but not clearly implicated in the transmission of infection
- Call bells contaminated with VREFootnote 94
- Bedside tables, bedrails and furniture contaminated with VREFootnote 94, Footnote 95, Footnote 96 and MRSAFootnote 70
- Tourniquets, monitoring devices, otoscopes, stethoscopesFootnote 97, Footnote 98, Footnote 99, Footnote 100, Footnote 101, Footnote 102, Footnote 103, Footnote 104
- ComputersFootnote 105, Footnote 106, Footnote 107, Footnote 108, computer keyboards, faucetsFootnote 109
- ToysFootnote 110, Footnote 111, Footnote 112
- Furnishings, mattresses, curtains, linenFootnote 113, Footnote 114, Footnote 115, Footnote 116, Footnote 117, Footnote 118
- Apparel, necktiesFootnote 119, Footnote 120, medical chartsFootnote 121
C. Exposure to and routes of transmission of infectious agents
1. Exposure to infectious agents (microorganisms)
Exposure occurs when a susceptible host comes into contact with an infected source or contaminated environment (e.g., inanimate/animate object or particles in the air). Not all exposures lead to transmission and resultant infection. The probability of transmission and infection is further dependent on a number of factors, including host susceptibility, presence of host receptors for the microorganism, microorganism inoculum size, viability and virulence, and the effectiveness of the hierarchy of controls (refer to Part A, Section III, A) utilized by an organization and the individual barriers worn by a HCW.
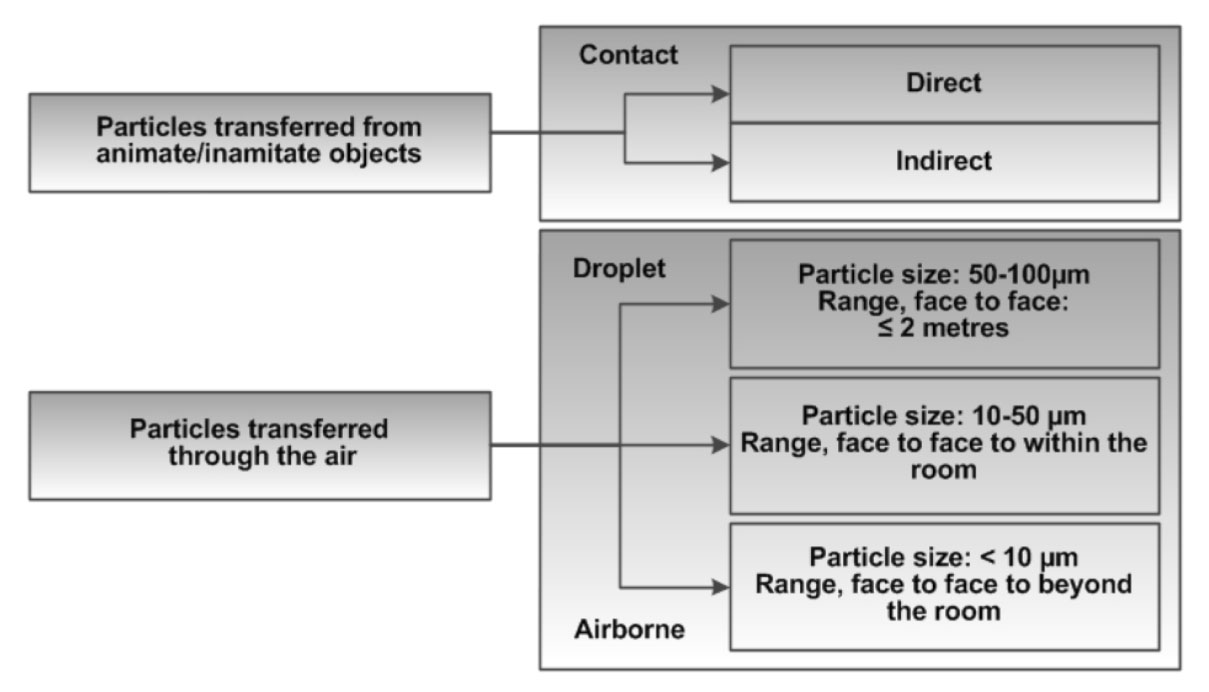
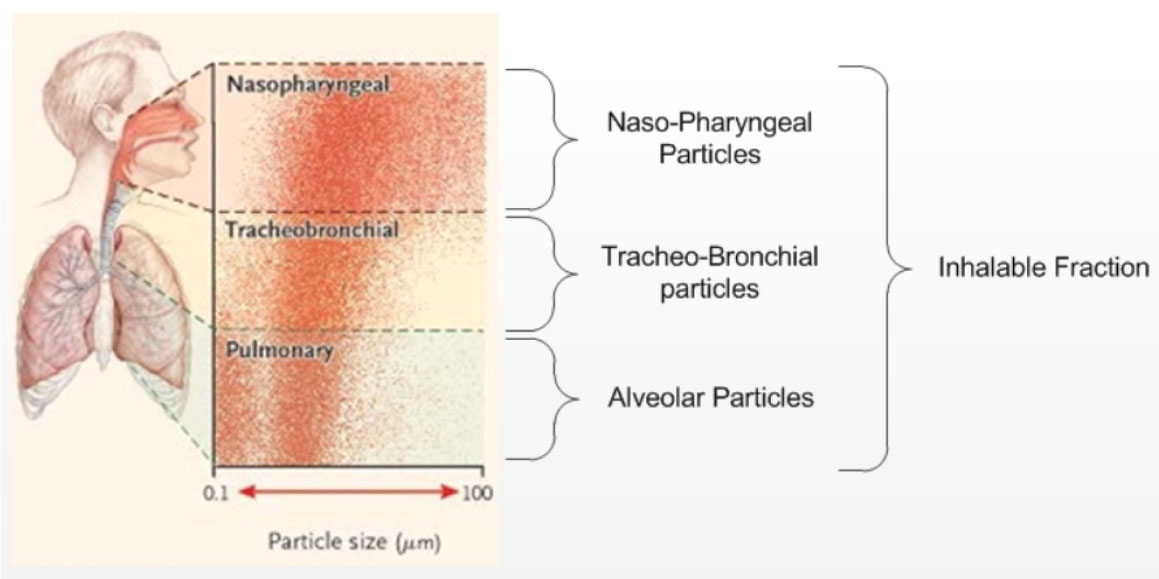
Figure 2 illustrates the continuum of infectious agent exposure specific to the contact, droplet or airborne routes that may be relevant to a susceptible host when having contact with an infected source or a contaminated environment (physical or passive, face-to-face contact or close contact (within two metres of an infected coughing source) and when a susceptible host inhales a microorganism (as an aerosol or droplet). Research has demonstratedFootnote 122, Footnote 123, Footnote 124 that both droplet and airborne-sized particles can be found in the air at close proximity (up to two metres) to a coughing/sneezing source. In addition, a portion of larger particles (droplets) may desiccate (and so become smaller) while in the air and become, in effect, droplet nuclei. Particles with a diameter of 1 µm to 10 µm may penetrate as far as the alveolar ducts (i.e., beyond the vocal cords), but may also be deposited at any point in the respiratory tract, as shown in Figure 3.
Figure 2. Exposure to Particles
Developed by the Canadian Pandemic Influenza Plan - ANNEX F Working Group, 2008
Text description
Figure 2 illustrates the continuum of infectious agent exposure specific to the contact, droplet or airborne routes that may be relevant to a susceptible host when having contact with an infected source or a contaminated environment (physical or passive, face-to-face contact or close contact (within two metres of an infected coughing source) and when a susceptible host inhales a microorganism (as an aerosol or droplet). Research has demonstrated that both droplet and airborne-sized particles can be found in the air at close proximity (up to two metres) to a coughing/sneezing source. In addition, a portion of larger particles (droplets) may desiccate (and so become smaller) while in the air and become, in effect, droplet nuclei.
Figure 3: Deposition regions of the respiratory tract for the various particle sizesFootnote 125
Text description
Figure 3 shows that particles with a diameter of 1 µm to 10 µm may penetrate as far as the alveolar ducts (i.e., beyond the vocal cords), but may also be deposited at any point in the respiratory tract.
a. Continuum of droplet and airborne exposure
The probability of airborne exposure to an infectious aerosol is influenced by several factors, in addition to the proximity of the infected source to the host. These include the particle sizes containing the infectious agent, the viability of the infectious agent and the animate and inanimate environment of a room (e.g., the concentration of the viral particles in droplet nuclei, the concentration of aerosol in the room, the relative humidity, the direction of air flow and the number of air changes per hour [ACH] in the room).
Particles of a variety of sizes are expelled from the human airway during coughing, sneezing, talking and medical procedures. The size of these particles and the distance they will be propelled is dependent on the force generated by the individual or the procedure. Large particles (greater than 10 µm) will fall quickly (in a few seconds) to the groundFootnote 125. However, smaller particles may remain suspended for a significantly longer time: tens of seconds for a droplet 10 µm in diameter and minutes or hours for small droplet nuclei. The particles that remain aloft for minutes or hours (less than 10 µm in diameter) can be carried by air currents over a measurable distance, including beyond the room, and are considered to represent an airborne exposure.
2. Routes of transmission
In IPC terminology, routes of transmission of microorganisms have conventionally been classified as contact, droplet, airborne, common vehicle and vectorborne. The routes of transmission vary with the microorganisms involved. For most microorganisms, transmission may primarily be by one route, such as direct or indirect contact (e.g., rotavirus or C. difficile), by droplet route (e.g., pertussis) or by airborne route (e.g., Mycobacterium tuberculosis). Some infectious agents, however, may be transmitted by more than one route (e.g., RSV can be transmitted by both the droplet and contact routes).
a. Contact exposure and transmission
Contact exposure occurs when microorganisms are transferred through physical contact between an infected source and a host, or through the passive transfer of the microorganisms to a host via an intermediate objectFootnote 24. Hands can be contaminated by contact with an infected source or by contact with contaminated inanimate surfaces or objects in the immediate environment of an infected sourceFootnote 77, Footnote 126, Footnote 127, Footnote 128.
Contact exposure includes both direct contact and indirect contact:

Figure 4: Direct contact where there is skin to skin contact between two persons
- Direct contact exposure occurs when the transfer of microorganisms results from direct physical contact between an infected or colonized source and a host (body surface to body surface without barriers), such as shaking hands, as shown in Figure 4.
- Indirect contact exposure involves the passive transfer of microorganisms to a host via an intermediate object, such as contaminated hands that are not cleaned between episodes of patient careFootnote 129, Footnote 130, contaminated patient care equipment (e.g., commodes, wheelchairs, base of electronic thermometers, blood pressure cuffs, monitoring equipment)Footnote 90, Footnote 92, Footnote 131, Footnote 132, surfaces such as bedrailsFootnote 77 that are not appropriately cleaned and disinfected between patients, or devices that have manufacturing defects that impede appropriate reprocessing. Other inanimate objects in the patient's environment that may be involved include computersFootnote 105, Footnote 106, Footnote 107, Footnote 108, Footnote 109, toysFootnote 93, Footnote 110 and electronic recreational devices that are not cleaned or disinfected between patients, as shown in Figure 5.

Figure 5: Indirect contact where there is contact with an inanimate object which may serve as the vehicle for transmission of pathogens
Contact transmission occurs when contact exposure leads to an infectious dose of viable microorganisms from an infected/contaminated source, resulting in colonization and/or infection of a susceptible host.
Microorganisms transmitted by the contact route include many of the epidemiologically significant microorganisms in healthcare settings, such as C. difficile, AROs (e.g., MRSA, VRE), and the viruses that cause gastroenteritis (refer to Appendix VI). Other infectious agents, especially respiratory viruses (e.g., RSV, influenza, parainfluenza and rhinovirus) that are expelled in large droplets, remain viable in droplets that settle on objects in the immediate environment of the patient and survive long enough on surfaces to be picked up on the hands of patients or HCWsFootnote 75, Footnote 76, Footnote 124, Footnote 133.
Refer to List 3 and Table 5 for the list of microorganisms transmitted by the contact route. Prevention and control of infectious agents transmitted by the contact route involve adhering to routine practices and contact precautions.
b. Droplet exposure and transmission

Figure 6: Droplet transmission, where large respiratory particles travel up to 2 meters
Droplet exposure may occur when droplets that contain microorganisms are propelled a short distance (i.e., within 2 metres)Footnote 122, Footnote 123, Footnote 124 through the air and are deposited on the mucous membranes of a host. Droplets may also contaminate the immediate environment when they settle on surfaces and may contribute to contact transmission, as shown in Figure 6.
Droplets are generated naturally from an infected source, primarily during coughing, sneezing or talkingFootnote 134, or artificially through AGMPs. Aerosol-generating medical procedures may also result in the generation of smaller infectious droplets that can travel farther than those generated spontaneously from patients (refer to Part A, Section II, C, 2c, for further discussion on AGMPs). The coughs and sneezes of some individuals (e.g., young children or frail elderly) may not be forceful enough to propel droplets as far as two metresFootnote 135.
Droplets of various sizes (refer to Figure 2) may contaminate the immediate environment when they settle on surfaces. Some microorganisms may remain viable for extended periods of time and contribute to contact transmission (e.g., many respiratory viruses)Footnote 136. Large aerosol particles (i.e., greater than 10 µm in diameter) will fall to the surface in a few seconds, and droplet exposure can only occur if the source and host are in close proximity (within two metres). Some microorganisms expelled in large droplets are very fragile and do not survive outside the human host or on surfaces (e.g., Bordetella pertussis, meningococcus).
Droplet transmission occurs when the droplets that contain an infectious dose of viable particles are propelled a short distance (i.e., less than two metres) through the air and are deposited on the mucous membranes of the eyes, nose or mouth of a susceptible host, and overcome other host defences.
Microorganisms transmitted by the droplet route include viruses that cause respiratory tract infections (e.g., RSV, influenza, parainfluenza, rhinovirus, adenovirus), rubella, mumps and Bordetella pertussis.
Refer to List 4 and Table 5, for the list of infectious agents transmitted by the droplet route. Prevention and control of infections transmitted by the droplet route involve immunization for those that are vaccine preventable and adhering to routine practices and droplet precautions.
c. Airborne exposure and transmission
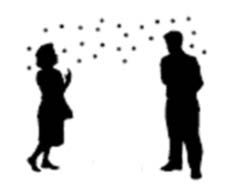
Figure 7: Airborne transmission whereby small particles travel long distances
Airborne exposure may occur if small particles (i.e., aerosols containing droplet nuclei) with viable microorganisms are generated, propelled over short or long distances, and inhaled. Aerosols containing viable microorganisms are generated naturally from an infected source during coughing, sneezing and talking, or artificially through AGMPs. Airborne exposure may result immediately after generation (i.e., the direct projection of an aerosol containing viable amounts of microorganisms through the air, and directly captured by a susceptible host's respiratory system) or after a longer period of time. Droplet nuclei can remain suspended in the air for a period of time before settling out of the air, during which time a susceptible host may inhale the suspended aerosol, as shown in Figure 7.
Airborne transmission may occur when viable microorganisms contained in aerosolized secretions from an infected source are propelled a short (i.e., within two metres) or long (i.e., greater than two metres) distance through the airFootnote 122, Footnote 123, Footnote 124 are inhaled, come into contact with receptors in a susceptible host's airway, overcome host defences and cause disease. For transmission of infection to occur, the microorganisms contained in the particles must be capable of remaining viable in the air for a prolonged period of time, and the susceptible host must be exposed to a sufficient concentration (infectious dose) of these viable microorganisms. Infection can result only if the appropriate receptors for the infectious agents are present at the site of exposure. Figure 3 depicts the various regions along the respiratory tract with the size classification of particles and their corresponding region of depositionFootnote 125.
Varicella zoster virus (chickenpox)Footnote 137, Mycobacterium tuberculosisFootnote 138, Footnote 139, Footnote 140, rubeola virus (measles)Footnote 141, Footnote 142 and smallpox and monkeypoxFootnote 143, Footnote 144 are infectious agents that are transmitted by the airborne route. Measles transmission has been reported up to 90 minutes after the index case has left the roomFootnote 141, Footnote 145.
Refer to List 5 and Table 5, for the list of microorganisms transmitted by the airborne route. Prevention and control of infections transmitted by the airborne route involves vaccination against vaccine preventable viruses, and adhering to routine practices and airborne precautions, as outlined in Part B, Section IV, subsection iii. Specifics related to airborne precautions are that only immune HCWs work with patients infected with chickenpox or measles and that airflow is controlled. Control of airflow ensures that ventilation systems provide adequate rates of air exchange and appropriate pressure differentials to maintain direction of flowFootnote 146, Footnote 147 for an airborne infection isolation room (AIIR).
Appendix VIII provides information regarding the length of time it takes for the removal of airborne particles from a room with no ongoing aerosol-generating source. There is time needed before the room is safe for a new patient or staff to enter without a respirator.
Aerosol-generating medical procedures
Aerosol-generating medical procedures are medical procedures that can generate aerosols as a result of artificial manipulation of a person's airway. Several types of AGMPS have been associated with an increased risk of TB or SARS transmissionFootnote 148. It should be acknowledged that while there is some evidence and consensus of opinion regarding the spread of infections by these procedures, further research is needed to provide additional evidence regarding the hazards that exist from these procedures. The risk of infection transmission may increase during AGMPs because of the potential to generate a high volume of respiratory aerosols that may be propelled over a longer distance than that involved in natural dispersion patternsFootnote 122, Footnote 149. These procedures include:
- intubation and related procedures (e.g., manual ventilation, open endotracheal suctioning)Footnote 150, Footnote 151, Footnote 152
- cardiopulmonary resuscitationFootnote 152
- bronchoscopyFootnote 153
- sputum inductionFootnote 154
- nebulized therapyFootnote 155, Footnote 156
- non-invasive positive pressure ventilation (continuous or bilevel positive airway pressure)Footnote 157
There is debate about whether other types of AGMPs may result in the generation of aerosols that can lead to transmission. However, there is no published literature that documents the transmission of respiratory infections, including TB, SARS and influenza, by the following meansFootnote 136, Footnote 158, Footnote 159, Footnote 160:
- high-frequency oscillatory ventilation
- tracheostomy care
- chest physiotherapy
- nasopharyngeal swabs, nasopharyngeal aspirates
Patients should be carefully assessed for signs or symptoms of suspected or confirmed TB, SARS or respiratory infection with an emerging pathogen for which transmission routes are not yet fully knownFootnote 150, Footnote 151, Footnote 152, Footnote 153, Footnote 154, Footnote 155, Footnote 156 prior to performing AGMPs, and strategies to reduce aerosol generation should be implemented (refer to Part B, Section IV, subsection iii, 1b). Strategies to reduce aerosol generation should also be implemented when AGMPs are necessary on patients with viral hemorrhagic feversFootnote 161. For novel influenza viruses or the emergence of new pathogens, refer to the PHAC website for specific guidance documents (http://www.phac-aspc.gc.ca/nois-sinp/guide/pubs-eng.php) Routine practices and contact and/or droplet precautions, as indicated, should be maintained for AGMPs on patients with no signs or symptoms of suspected or confirmed TB, SARS or emerging respiratory infections. Other procedures that may generate aerosols that have been shown to transmit TB include procedures that may aerosolize viable tubercle bacilli (e.g., irrigation) of nonrespiratory lesionsFootnote 162, Footnote 163, Footnote 164 and use of oscillating saws during autopsy on patients with TBFootnote 165, Footnote 166. Airborne precautions are recommended when performing these procedures on patients with suspected or confirmed TB.
d. Common vehicle transmission
Figure 8: An example of common vehicle transmission is a contaminated multi-dose vial
Common vehicle transmission refers to a single contaminated source, such as food, multi-dose vialsFootnote 167, Footnote 168, Footnote 169, Footnote 170, Footnote 171, Footnote 172, Footnote 173, intravenous fluidsFootnote 174 or equipment, which serves to transmit infection to multiple hosts. Control is by maintenance of appropriate standards in the preparation of food and medications and in decontamination of equipment as shown in Figure 8.
e. Vectorborne transmission

Figure 9: Disease transmitted by insects is an example of vectorborne transmissions
Vectorborne transmission refers to transmission by insect vectors and is prevented by appropriate hospital construction and maintenance, closed or screened windows and proper housekeepingFootnote 175. Such transmission has rarely, if ever, been reported in Canadian healthcare settings. Refer to figure Figure 9.
D. Host Factors
Microorganisms have to gain access to a susceptible host, by a receptive portal of entry, for transmission to occur. The risk of transmission is influenced by the susceptibility of the host. The host's defences, if normal, may be able to eliminate a few microorganisms but be overwhelmed by many, while an immunocompromised host may not be able to eliminate even a few. Host defences, both non-specific (e.g., normal flora, intact skin, neutrophils, macrophages) and specific (antibodies, cell-mediated responses), may be altered by extremes of age, underlying disease (e.g., diabetesFootnote 176, Footnote 177, HIVFootnote 178, malignancy/transplantationFootnote 179), genetic factors or medications. Additional factors that may facilitate acquisition of microorganisms are invasive/surgical procedures, radiation therapy, breaks in the skin and breaching of normal barriers such as occurs with the presence of invasive medical devices (e.g., endotracheal tubes, indwelling urethral catheters and intravascular devices)Footnote 180, Footnote 181, Footnote 182, and provision of wound care.
E. Outcomes of Transmission of Infectious Agents (Microorganisms)
Whether or not transmission results in colonization, asymptomatic infection or clinical disease (symptomatic infection) depends on the pathogenicity and virulence of the infectious agent (microorganism), the inoculum size and the integrity of host defences (refer to Part A, Section II, D). Pathogenicity refers to the ability of the microorganism to cause disease (i.e., harm the host). Some microorganisms are inherently pathogenic and cause disease in any susceptible host (e.g., varicella), whereas others are opportunists causing infection only under special circumstances (e.g., coagulase-negative staphylococci in people who have prosthetic devices). Virulence refers to the intensity of pathogenicity and is related to the ability to cause morbidity and mortality (e.g., Ebola has high virulence; rhinovirus has low virulence). Several factors contribute to the virulence of a microorganism: toxin production, invasiveness, presence of capsule, adherence mechanisms and ability to survive in host cells. Inoculum size refers to the number of microorganisms transmitted to the host. Some microorganisms are highly pathogenic and need only a low inoculum to cause disease (e.g., Shigella).
1. Colonization
The presence of microorganisms in or on a host with growth and multiplication but without tissue invasion or cellular injury is referred to as colonization. With most microorganisms, colonization is far more frequent than clinical disease. Colonization of the nasopharynx with aerobic Gram-negative rods occurs with increased severity of illness, malnutrition, major surgery, alcoholism and diabetesFootnote 183. Colonization with Staphylococcus aureus is common in normal healthy persons. Some patient populations are heavily colonized with S. aureus (e.g., hemodialysis patients, injection drug users, and patients with diabetes mellitus or skin disorders)Footnote 184.
Disturbance of the normal flora by antimicrobials enhances overgrowth of endogenous aerobic Gram-negative rods and enterococci, and increases risk of colonization with exogenous microorganisms, including antimicrobial-resistant bacteria and yeastFootnote 40, Footnote 183. The presence of normal or endogenous bowel flora is a defence mechanism against colonization of the gastrointestinal tract by exogenous microorganisms. The endogenous flora (e.g., bacteria residing in the respiratory or gastrointestinal tract) can also be a cause of HAIsFootnote 185, Footnote 186, Footnote 187, Footnote 188, Footnote 189, Footnote 190, Footnote 191, Footnote 192, Footnote 193. Once acquired, prolonged carriage of antimicrobial-resistant organisms (AROs) may be the norm in some patient populations. Colonization with resistant strains of Pseudomonas aeruginosa or Burkholderia cepacia is common in persons with cystic fibrosis. Persistent VRE colonization has been demonstrated in dialysisFootnote 194 and otherFootnote 195, Footnote 196 patient populations.
2. Subclinical/Asymptomatic Infection
Infection may or may not be associated with clinical disease (illness). Infection may cause cellular and tissue changes that may be detectable in the absence of overt signs and symptoms. This is a subclinical or asymptomatic infection.
3. Clinical Disease/Symptomatic Infection
When sufficient cellular and tissue changes occur to produce overt signs and symptoms, the individual has clinical disease, which may range from mild to severe, depending on the microorganism and the health status of the host.
III. Control measures to reduce healthcare worker exposure to and transmission of microorganisms
A. Hierarchy of controls to reduce exposure to and transmission of infectious agents
Collaboration between IPC/OH professionals and healthcare building engineers has led to better understanding and application of a tiered framework of measures/interventions that enables healthcare organizations to comprehensively evaluate the risk of HCW (including volunteers) exposure to microorganisms and other hazards in the workplace and the effectiveness of the healthcare organization's mitigation responses.
The ideal approach to containment of a hazard is to implement a hierarchy of controls. The first level of control is engineering interventions. If this level of control is not possible or adequate, then administrative interventions are used. Last in the hierarchy of controls is PPE. Personal protective equipment is not the first control measure to use, as its use is dependent on the variable of worker adherence. An understanding of the engineering, administrative (including patient care practices) and PPE controls enables healthcare organizations to determine how the healthcare environment in each setting (e.g., infrastructure, equipment, processes and practices) increases or decreases a susceptible host's (e.g., patient, HCW, visitor) likelihood of exposure to a microorganism/reservoir within the healthcare setting.
1. Engineering controls
The engineering control tier reduces the risk of exposure to an infectious agent/infected source hazard by applying methods of isolation or ventilation. Engineering controls do not depend on an individual's compliance with exposure prevention strategies. These controls are usually established and controlled within the building structure, thereby eliminating an individual's choice about their application, and reducing the opportunity for individual error. As such, they provide more effective protection.
2. Administrative controls
The administrative control tier provides an infrastructure of policies, procedures and patient care practices intended to prevent exposure to and/or transmission of microorganisms to a susceptible host during the provision of health care. To be effective in preventing the transmission of microorganisms and/or detecting cases of infection, administrative controls are best implemented at the point of first encounter with an infected source and continued until the infected source leaves the healthcare setting or is no longer infectious. Inherent in the development of administrative controls to prevent transmission of infection is the commitment, by the healthcare organization, to provide the necessary resources to implement the controls.
3. Personal protective equipment
Although the use of PPE controls are the most visible in the hierarchy of controls, PPE controls are the weakest tier in the hierarchy of controls, and should not be relied on as a stand-alone primary prevention program. The PPE tier refers to the availability and appropriate use of barriers that a susceptible host may wear to provide a physical barrier between him/her and an infectious agent/infected source. These barriers include gloves, gowns, masks, facial protection, eye protection (including face shields, or masks with visor attachments) and respirators. The healthcare organization plays a critical role in ensuring the availability of appropriate PPE for use by patients, HCWs, visitors, contractors, etc., to prevent exposure to an infectious agent/infected source.
A singular focus on availability and use of various PPE to the exclusion of other tiers in the hierarchy of controls will result in suboptimal protection of all people in the healthcare setting, including patients, HCWs and other staff. The effective and appropriate use of PPE is the control that is most reliant on the user's adherence and competence and, therefore, the control most easily compromised (resulting in ineffective protection from an infectious agent/infected source). The use of PPE is the final step in the hierarchy of controls to minimize exposure and subsequent transmission (refer to Appendix X).
List 2: Examples of control measures according to hierarchy of controls
- 2a. Tier 1: Examples of engineering controls
- Source control:
- single rooms, with private toilets, patient sink, designated staff handwashing sinks
- AIIRs
- signage to direct patients to separate entrances (during community outbreaks) for patients symptomatic with respiratory infections
- physical barriers (e.g., partitions in triage areas to prevent exposure to patients symptomatic with respiratory infections)
- appropriate spatial separation (in patient rooms, waiting areas and in the home)
- appropriate ventilation and, in the home, natural ventilation when appropriate
- Installation of:
- point-of-care ABHR
- point-of-use sharps containers
- appropriately functioning, accessible dispensers for hand hygiene products (ABHR, soap, lotion, paper towels) and respiratory hygiene/cough etiquette products
- designated handwashing sinks for HCW use
- Appropriate number of commodes
- Appropriate supply of and accessibility of PPE
- Appropriate number of accessible no-touch waste receptacles for disposal of paper towels, tissues, masks, gloves, etc.
- 2b. Tier 2: Examples of administrative controls
- Appropriate resources for diagnosis and treatment of infection or colonization, and for immunization of patients and staff
- Organizational support for effective IPC and OH services and for management of outbreaks
- Appropriate OH and safety policies, including preplacement assessment, work restrictions, respiratory protection program, sharps safety and prevention of exposure to bloodborne pathogens and immunization programs
- Education of HCWs
- Policies, procedures and resources to support the application of:
- point-of-care risk assessment
- point-of-care ABHR as the standard of care in all healthcare settings
- routine practices as the standard of care for all patients in all healthcare settings
- source control (instructions for patients)
- Patient placement, accommodation and flow
- 2c. Tier 3: Examples of personal protective equipment to prevent exposure of patients, healthcare workers and other staff
- Following PCRA, PPE for the appropriate application of routine practices and additional precautions may include:
- gloves
- gowns
- masks (surgical or procedure masks used by HCW and/or infectious source)
- facial protection (masks and eye protection, or face shields, or masks with visor attachment)
- respirators (refer to Appendix V, glossary)
B. Role of the organization to reduce exposure to and transmission of infectious agents
1. Organizational risk assessment
A major responsibility of any healthcare organization is the evaluation (i.e., ORA) of the components in the hierarchy of controls to minimize the risk of exposure to and transmission of microorganisms within healthcare settings. This ORA is central to any healthcare organization's preparation and planning to protect all individuals (e.g., patient, HCW, visitor, contractor) from HAIs in all healthcare settings. Organizations have a responsibility to provide information and train HCWs regarding the organization's ORA and its impact on their practice. For example, the availability of functioning AIIRs may affect when and where AGMPs are performed and may influence the PCRA performed by HCWs.
An ORA should be conducted on an annual basis and re-evaluated when major reorganization/restructuring and building/renovation take place. The need for an ORA applies to all levels of healthcare settings, including prehospital care, acute care, LTC, ambulatory care and home care settings. Ongoing systematic evaluation of the ORA is important to ensure that policies, procedures and programs:
- are consistent across the organization
- achieve their stated objectives
- are in compliance with current applicable regulations
The ORA will characterize the organization's patient population, level and intensity of health care provided and resources available, including the variously skilled workers. It will need to evaluate the effectiveness of present control measures and the breadth of the hierarchy of controls to prevent HAIs.
To conduct the ORA an organization will need to:
- determine situations/conditions where infectious microorganisms (hazards) might exist
- evaluate the potential for exposure to and/or transmission of the microorganism
- determine the consequences of exposure to the microorganism
- determine the severity of illness caused by the microorganism
- determine the consequences of transmission of the microorganism on individuals, organizations and the community
- assess available control measures in place (e.g., engineering, administrative and PPE) to mitigate exposure to or transmission of the microorganism in the specific healthcare setting
2. Organizational control measures
Once the ORA is completed, control measures can be implemented to address any areas of concern. Such control measures, described below, can be at one or more of the three levels of the hierarchy of controls. Appropriate ventilation and hospital design (e.g., AIIRs, single patient rooms) are engineering controls, whereas education of HCWs, routine practices and additional precautions and OH (e.g., respiratory protection programs) are administrative controls.
Engineering controls—Healthcare facility design, renovation and construction
Facility design is an example of engineering controlFootnote 197, Footnote 198, Footnote 199, Footnote 200, Footnote 201, Footnote 202, Footnote 203. Room design, ventilation systems, room air flow and human traffic patterns, positioning of ABHR dispensers and designated handwashing sinks, and physical barriers to separate patients in multi-bed wards and patients in waiting areas are all examples of engineering controls. Adherence to spatial separation recommendations (i.e., preferably a high proportion of single patient rooms or, alternatively, a two-metre separation between patient spaces) when designing new healthcare facilities, planning renovations to existing facilities or reorganizing patient care areas will enhance a healthcare organization's ability to prevent transmission of infections.
Healthcare facility design related to IPC also includes appropriate number, location, and type of AIIRs; location(s) of special ventilation and filtration, such as emergency department triage and waiting areas; air handling and ventilation needs in surgical services and laboratories; local exhaust systems for hazardous agents and other special areas; water systems to limit Legionella species and waterborne opportunistic pathogens; and consideration of preferred surface characteristics (of the ideal product) such asFootnote 201, Footnote 202:
- ease of maintenance/repair and cleanability
- does not support microbial growth
- nonporous, smooth
- durable
- sustainable
- ease of installation, demolition and replacement
- seamless
- resilient, impact resistant
Infection prevention and control professionals should be included from the beginning of projects (i.e., when designing new healthcare facilities, planning renovations to existing facilities or reorganizing patient care areas) until the project endsFootnote 197, Footnote 198, Footnote 202, Footnote 203, Footnote 204, Footnote 205, Footnote 206.
Engineering controls—Heating, ventilation and air conditioning in healthcare facilities
To ensure optimal performance of ventilation systems for removal of particulates and elimination of excess moisture, organizations have a responsibility to design, construct, install and maintain ventilation systems in accordance with engineering and manufacturers' recommendations. Recommendations for heating, ventilation and air conditioning systems particular to healthcare facilities have been publishedFootnote 146, Footnote 147, Footnote 207. Additional information specific to Mycobacterium tuberculosis can be found in the latest edition of The Canadian Tuberculosis Standards.
Healthcare settings that provide care for, or potentially care for, patients with suspected or confirmed airborne transmissible infections should have an adequate number of AIIRs (also called negative pressure rooms)Footnote 138, Footnote 208, Footnote 209, Footnote 210, Footnote 211. The ORA should determine the appropriate number of AIIRs required. Airborne infection isolation rooms are recommended for placement in the following areas in healthcare facilities, including but not limited to: emergency departments, critical care settings, inpatient units, bronchoscopy and autopsy suitesFootnote 138, Footnote 209, Footnote 210, Footnote 211, Footnote 212.
This guideline does not recommend specific ACH requirements but provides healthcare organizations with the recommendations that currently exist (refer to Table 1. Ventilation Recommendations for Selected Areas in Healthcare Facilities, (below). Further research in this area is needed including collaboration between experts in engineering and biomedical sciences to provide additional insights and clear evidence for ventilation requirements.
| Area | CSA, 2010 | ASHRAE, 2008 | CDC, 2005 |
|---|---|---|---|
| Autopsy suite | 20 ACH | 12 ACH | 12 ACH |
| Bronchoscopy, sputum induction rooms | 20 ACH | 12 ACH | 12 ACH |
| Airborne infection isolation rooms (AIIR) | 12 ACH | 12 ACH | |
New construction (existing) |
12 ACH (at least 6 ACH) |
- CSA Z317.2-10 Special Requirements for Heating, Ventilation, and Air Conditioning (HVAC) Systems in Health Care FacilitiesFootnote 146.
- ASHRAE American Society of Heating, Refrigerating and Air-conditioning Engineers, Ventilation of Health Care FacilitiesFootnote 147.
- CDC Centers for Disease Control, Guidelines for Preventing the Transmission of Mycobacterium Tuberculosis in Health-care SettingsFootnote 207.
Specific recommendations for heating, ventilation and air conditioning in operating room settings are beyond the scope of this document and are available from the Facility Guidelines InstituteFootnote 201 and Canadian Standards AssociationFootnote 146.
Engineering controls—Source control
Source control measures are used to contain microorganisms from dissemination from an infectious source. Instructions on how to comply with source control should be provided to patients and other persons with symptoms at the point of initial encounter in any healthcare setting (e.g., triage in emergency departments, acute assessment settings, reception and waiting areas in emergency departments, outpatient clinics and physician's offices) and in strategic places (e.g., elevators, cafeteria) within ambulatory and inpatient settings. Policies and procedures (administrative controls) should be implemented to develop a program for source control. Source control measures may include but are not limitedFootnote 124, Footnote 148:
- signage at entrances to healthcare settings for early recognition of symptoms (e.g., syndromic screening)
- separate entrances/waiting areas for symptomatic patients
- spatial separation
- physical barriers for acute assessment
- early identification, diagnosis and treatment of infection (e.g. TB, norovirus)
- respiratory hygiene
- hand hygiene
- patient placement (e.g., patient care areas, single rooms/AIIRs)
- strategies to reduce aerosols during AGMPs (refer to Part B, Section IV, subsection iii, 1b).
Engineering controls—Source control—Spatial separation
Appropriate spatial separation and spacing recommendations to decrease exposure to microorganisms for patients and visitors in clinical and waiting areas should be implemented. A two-metre spatial distance between a coughing/sneezing infected source (e.g., symptomatic individual with an acute respiratory illness) and an unprotected susceptible host (e.g., patients, HCWs, visitors, contractors) should be considered to prevent the transmission of droplet-borne infectious particlesFootnote 122, Footnote 123, Footnote 124.
Spatial separation recommendations should be included when designing new healthcare facilities or planning renovations to existing facilities (refer to Part A, Section III, B, 2).
Engineering controls—Source control—Respiratory hygiene
Respiratory hygiene refers to a combination of measures designed to minimize the transmission of respiratory pathogensFootnote 45, Footnote 48, Footnote 148, Footnote 213, Footnote 214, Footnote 215. These source control measures are targeted to all individuals with symptoms of respiratory infection, starting at the initial encounter in a healthcare setting, and are maintained throughout every encounter in the healthcare setting (e.g., triage in emergency departments, reception in ambulatory clinics or healthcare provider offices, and in strategic places such as elevators and cafeterias). Respiratory hygiene involves educating and encouraging all individuals (patients, HCWs and visitors) who have the physical and cognitive abilities to do so to practice respiratory hygiene. Specific measures may include instructional signs, education programs and provision of materials for respiratory hygiene (e.g., tissues, no-touch plastic-lined waste receptacles, ABHR).
Further information is available in the PHAC Infection Control Guideline for the Prevention of Healthcare-Associated PneumoniaFootnote 216.
Engineering controls—Source control—Hand hygiene
Organizational barriers related to engineering controls, such as a lack of accessibility to and maintenance of hand hygiene facilities and poor access to hand hygiene products, negatively affect adherence to hand hygiene. Organizations have the responsibility to ensure that such barriers are addressed. Readers are referred to the PHAC IPC guideline for Hand Hygiene Practices in Healthcare SettingsFootnote 217.
Engineering controls—Source control—Patient placement
Recently, in an effort to increase access to scarce inpatient beds and reduce emergency department crowding, some Canadian hospitals have developed "overcapacity" or "full capacity" protocols (i.e., admitting patients to inpatient units that are already at maximum capacity)Footnote 218. The Canadian Nurses Association (CNA) Position Statement, "Overcapacity Protocols and Capacity in Canada's Health System" noted such protocols may affect the safety of patients and nurses including increasing the number and severity of adverse events and have concerns regarding control of infectious diseaseFootnote 218. The CNA advises that hospitals take every necessary step to avoid use of overcapacity protocols and that an overcapacity protocol not be considered the norm in the delivery of hospital services. Hospitals that have short-term use of overcapacity or full capacity protocols should develop and implement policies and practices that minimize the risk of spread of infection through overcrowding and understaffing. Patients who present to hospital with acute transmissible infections (including but not limited to vomiting, diarrhea, fever, cough, coryza, shortness of breath) are not candidates for overcapacity placement.
Engineering controls—Source control—Strategies to reduce aerosols during aerosol-generating medical procedures
Refer to Part A, Section II, C, 2c for discussion on AGMPs and Part B, Section IV, subsection iii, 1b for strategies to reduce the risk of aerosol generation.
3. Administrative control measures
Occupational health program
An objective of an OH program is to identify risk situations with the potential for occupational exposure or transmission of a microorganism, either to or from the HCW and other individuals. Components of an OH program that support the use of routine practices and additional precautions to prevent exposure or transmission of microorganisms can be found in the PHAC IPC guideline Prevention and Control of Occupational Infections in Health CareFootnote 219 and include:
- preplacement assessment (at time of employment)
- ensuring immunity to vaccine preventable infectious diseases
- tuberculosis screening (preplacement and screening, as per organizational policies)
- annual influenza immunization
- policies for management of HCWs with infections
- management of latex and other glove component allergies
- prevention of exposure to bloodborne pathogens, including a sharps safety program (refer below)
- management of HCWs who cannot wear PPE (e.g., respirators)
Important components of an OH program that support the use of routine practices and additional precautions not in the PHAC IPC guideline Prevention and Control of Occupational Infections in Health CareFootnote 219 include:
- management of HCWs unable to comply with hand hygiene recommendations (for details refer to PHAC IPC guideline Hand Hygiene Practices in Healthcare SettingsFootnote 217
- respiratory protection program (refer below)
Occupational health program — Sharps safety and prevention of exposure to bloodborne pathogens
The prevention of sharps injury and HCW exposure to bloodborne pathogens is a component of routine practices.
Users of sharps require education and training about how to safely handle sharp devices to prevent injuries to themselves and others who may encounter the device during or after procedures. Safety programs should include a formal incident investigation for every sharp injury occurring in the work settingFootnote 220. Components of a sharps injury prevention program have been publishedFootnote 221, Footnote 222. The CDC workbook for designing, implementing and evaluating a sharps injury prevention program is available on the CDC website (http://www.cdc.gov/sharpssafety/resources.html).
Use of safety engineered devices, such as protected needle devices, needle-free systems with self-sealing ports and syringes with safety features, have been reported to reduce needlestick injuriesFootnote 220, and their use has been identified as a priority in risk-reduction strategiesFootnote 223. In some jurisdictions, these safety devices are required by regulation (refer to local regulations). The choice of specific needleless devices for a healthcare organization should be considered carefullyFootnote 224, Footnote 225, Footnote 226, Footnote 227, as some models have demonstrated a risk for patientsFootnote 228, Footnote 229, Footnote 230, Footnote 231, Footnote 232.
Occupational health program—Respiratory protection program
Respiratory protection specifies the use of a respirator to prevent inhalation of aerosols containing infectious particles. Respirators should be used for the care of patients with suspected or confirmed airborne respiratory pathogens (e.g., TB, measles), and in some situations when AGMPs are performed (refer to Part B, Section IV, subsection iii, 7). Healthcare organizations that use respirators should have a respiratory protection program in placeFootnote 233. The respiratory protection program should provide health screening, fit-testing/retesting and training to all HCWs who may wear a respirator. The organization should be committed to developing, implementing, maintaining and evaluating the respiratory protection program.
Healthcare organizations are responsible for choosing specific respirator brands, models and sizes to be used by their workforce, while taking into consideration the diversity of their workforce and patient population. Organizations should ensure their workforce has access to recommended respirator models and sizes, as required by local Labour Code and Occupational Health regulations.
Healthcare Organizations should consider the following:
- When respirators are being selected by the organization, those with inherently good fit characteristics are preferred.
- Respirators from more than one manufacturer may be needed to fit the range of ethnic groups/facial structures represented within the organization's workforce.
- Fit testing is used to evaluate how well a given respirator fits a given person by assessing leakage around the face seal. Published literature regarding fit-testing respirators in the healthcare setting is inconclusiveFootnote 234, Footnote 235, Footnote 236 however, most Canadian jurisdictions require fit testing for HCWs to determine their ability to obtain a satisfactory seal when using respiratorsFootnote 233. As a result, HCWs are referred to jurisdictional regulations regarding fit testing. In the absence of such regulation, consult your provincial/territorial public health authorities. Most jurisdictions specify that fit-testing be repeated on a set schedule (e.g., at least every 2 years)Footnote 233, or as defined by jurisdictional regulations, or more frequently if facial conditions change (e.g., weight gain/loss, dental work).
- If an organization chooses to change the brand and/or model of respirators available for use, it should be aware that fit-testing results are not transferable between respirator brands and/or models.
- Healthcare organizations should develop policies for HCWs who are unable to form a tight facial seal when wearing a respirator (e.g., facial deformities, men with beards).
Healthcare workers should consider the following:
- Healthcare workers should only use respirators to which they were fit-tested.
- Healthcare workers should be knowledgeable of the applications, advantages and limitations, and proper use of the specific respirator model(s) that they have been fitted for (refer to Appendix X).
- Each time HCWs put on a respirator, they are to perform a seal check (sometimes referred to as fit check) to enable proper functioning of the respiratorFootnote 233.
Education of healthcare workers
Education and training on IPC policies and procedures should be provided to all HCWs during their training in health professions, during employment orientation, as a result of special circumstances (e.g., outbreaks, new equipment/information) and on a regular basis. Healthcare organizations have the responsibility to provide the training, and HCWs have the responsibility to take advantage of educational opportunities. Planning and evaluating educational programs for an adult learner is complex, and appropriate resources should be consulted (e.g., Community and Hospital Infection Control Association-Canada, IPC core competencies for HCWsFootnote 237, planning programs for adult learners)Footnote 238. It is important that topics, methods and materials for education and training are appropriate to the level of the HCW understanding and responsibility. Content for routine practices and additional precautions education and training sessions should include, but are not limited to, the following principles:
- point-of-care risk assessment
- transmission of microorganisms (chain of infection)
- prevention of exposure to microorganisms (including source control)
- importance of immunization
- knowledge of immune status to vaccine preventable diseases (e.g., varicella)
- indications for hand hygiene (ABHR at point-of-care as preferred method unless exceptions apply (i.e., when hands are visibly soiled with organic material, if exposure to norovirus and potential spore-forming pathogens such as Clostridium difficile is strongly suspected or proven, including outbreaks involving these organisms)
- indications for and appropriate application of aseptic technique
- safe use and disposal of sharps
- cleaning and disinfection of non-critical patient care equipment between patients
- patient/visitor education
- indications for and appropriate use of PPE
- implementation of additional precautions
- modification of practices during outbreaks
- how to use Table 4 to implement additional precautions empirically
- how to use Table 5 to modify or discontinue additional precautions
Reprocessing of patient care equipment—Reprocessing reusable equipment
The appropriate reprocessing (i.e., cleaning, disinfection and sterilization) of reusable medical devices (e.g., equipment, instruments) is important in preventing the transmission of microorganisms, and an obligatory component of health care that must be performed according to published guidelinesFootnote 239, Footnote 240 and standardsFootnote 241, Footnote 242, Footnote 243, Footnote 244, Footnote 245.
Spaulding developed a system to classify the cleaning, disinfection and sterilization specifications for equipment used in patient careFootnote 246. This system divides medical devices, equipment and surgical materials into three categories (non-critical, semi-critical and critical), based on the potential risk of infection involved in their useFootnote 247. Healthcare workers need to be able to identify semi-critical and critical items for reprocessing by high level disinfection or sterilization. Healthcare workers also need to be able to identify non-critical equipment and ensure it has been cleaned before use (refer to item below).
Reprocessing of reusable medical devices can occur within a hospital or regional health facility, or it can be contracted to a third-party reprocessor. When third-party reprocessors are contracted, provincial/territorial regulations should apply. Reusable devices need to be reprocessed by trained personnel under the supervision of specially trained individuals. To the largest extent possible, reprocessing should be in a centralized location and audited on a regular basis. Where this is not achievable, single-use disposable devices are preferred.
Identification and reprocessing of prion-contaminated equipment (agents responsible for transmissible spongiform encephalopathies such as Creutzfeldt-Jakob disease) require more rigorous and highly specific processes. Readers should refer to specific PHAC guidanceFootnote 248, Footnote 249, Footnote 250 for further information.
Reprocessing of patient care equipment—Reprocessing and reuse of single-use medical devices
Devices designed and sold for single use are not intended for reprocessing and reuse. Nevertheless, a 2006 survey to investigate the practices of reprocessing and reusing single-use devices (SUDs) in Canadian acute-care hospitals found that 28% of hospitals reprocess SUDs, either by in-house or third-party reprocessingFootnote 251. These results were similar to a Canadian survey in 2000Footnote 252. Concerns related to reprocessing SUDs include the increased risk of patient adverse events, legal liability, ethical concerns and the cost-effectiveness of reprocessingFootnote 251. Reprocessing SUDs involves a process to ensure an original SUD previously used on one patient is safe for use on another patient, and includes cleaning, functional testing, repacking, relabeling, testing for pyrogenic substances and disinfection or sterilizationFootnote 253. Healthcare organizations contracting third-party reprocessors for this purpose must adhere to provincial/territorial legislation. At the time of this writing, there is no process to regulate third-party reprocessors of SUDs in Canada. For this reason, facilities that choose to reprocess SUDs must contract to Food and Drug Administration-regulated facilities in the US.
Reprocessing of patient care equipment—Cleaning and disinfection of non-critical patient care equipment
Contamination of patient care equipment, items in the patient environment and the patient's environment has been documented and implicated in transmission of infection. (Refer to List 1, Examples of environmental sources of contamination). Used or potentially contaminated items that have had contact with a patient's intact skin should always be cleaned and disinfected before use with another patient. Refer below for cleaning of the patient environment.
Environmental cleaning
Measures to minimize exposure to environmental contamination includeFootnote 239:
- dedicating non-critical medical equipment to a single patientFootnote 254
- assigning responsibility and accountability for routine cleaning and disinfection of patient care equipmentFootnote 255, Footnote 256, Footnote 257, Footnote 258
- ensuring environmental cleaning is done according to a set procedure and frequency, documented and supervised by adequately trained personnel and by dedicated personnel
- ensuring surfaces are constructed of materials that can be easily cleaned at the point-of- useFootnote 201, Footnote 202
- increasing the frequency of cleaning and disinfecting frequently touched surfacesFootnote 70, Footnote 82, Footnote 95, Footnote 254, Footnote 259, Footnote 260
- monitoring adherence to recommended environmental cleaning practicesFootnote 261, Footnote 262, Footnote 263
- ensuring rooms are terminally cleaned following patient discharge and after discontinuing precautionsFootnote 263 (refer to Appendix VII)
- determining what product to use for routine environmental cleaning
In situations of continued transmission of certain microorganisms (e.g., norovirus, rotavirus, C. difficile) use of specific disinfectant products may need to be consideredFootnote 78, Footnote 239, Footnote 264, Footnote 265. In outbreak situations or when there is continued transmission, rooms of C. difficile infection patients should be decontaminated and cleaned with chlorine-containing cleaning agents (at least 1,000 ppm) or other sporicidal agentsFootnote 43, Footnote 266, Footnote 267, Footnote 268, Footnote 269, Footnote 270, Footnote 271.
Additional information is available in the CDC/Healthcare Infection Control Practices Advisory Committee Guideline for Disinfection and Sterilization in Healthcare FacilitiesFootnote 239 and CDC's Guidelines for Environmental Infection Control in Health-Care FacilitiesFootnote 72.
Waste
Most waste generated in healthcare settings is no more hazardous than household wasteFootnote 272, Footnote 273, Footnote 274. Special handling of sharps and some biomedical waste (e.g., sponges, dressings or surgical drapes soaked with blood or secretions) may be required by local regulationsFootnote 275. Waste receptacles should be conveniently located and preferably no-touch. Local regulations may apply.
Additional information is available in the Canadian Standards Association Handling of Waste Materials in Health Care Facilities and Veterinary Health Care FacilitiesFootnote 275.
Linen
Although linen in healthcare facilities may become contaminated with pathogens, risk of transmission of disease is negligibleFootnote 117, Footnote 276, Footnote 277. Care should be taken in the handling of soiled linen to prevent dispersal of microorganismsFootnote 278, Footnote 279. Special handling of linen from patients on additional precautions is not requiredFootnote 276, Footnote 280
If laundry chutes are used, they should be properly designed, maintained and used in a manner to minimize dispersion of aerosols from contaminated laundryFootnote 281, Footnote 282.
Clean linen should be transported and stored in a manner to prevent inadvertent handling or contamination by dust, which may contain fungal spores harmful to immunocompromised patientsFootnote 72.
Additional information is available in the CDC's Guidelines for Environmental Infection Control in Health-Care FacilitiesFootnote 72.
Management of deceased bodies
There are no special recommendations when handling deceased bodies, preparing bodies for autopsy or transferring bodies to mortuary services. Routine practices properly and consistently applied and the additional precautions as indicated prior to death (contact or airborne) is sufficient. Droplet precautions are an exception and are not necessary postmortem. Some provinces and territories may have specified communicable disease regulations.
Management of pets/animals
The use of pet therapy in health care may have benefits to patients. Policies and procedures for animal health screening and IPC for animal-assisted interventions in healthcare facilities are an organizational responsibility. Recommendations for IPC practices related to animal health screening and interventions in healthcare facilities have been publishedFootnote 71, Footnote 72.
C. Role of the healthcare worker
1. Point-of-care risk assessment
Prior to every patient interaction, HCWs have a responsibility to assess the infectious risk posed to themselves and other patients, visitors and HCWs by a patient, situation procedure. The PCRA is an evaluation of the variables (risk factors) related to the interaction between the HCW, the patient and the patient's environment to assess and analyze their potential for exposure to infectious agents and identify risks for transmissionFootnote 283. This PCRA is based on judgement about the clinical situation (including the patient's clinical condition, physical, emotional and mental state) and up-to-date information on how the specific healthcare organization has designed and implemented engineering and administrative controls, availability and use of PPE. Control measures are based on the evaluation of the variables (risk factors) identified.
Healthcare workers should routinely perform PCRAs many times a day to apply control measures for their safety and the safety of patients and others in the healthcare environment.
For example, a PCRA is performed when a HCW evaluates a patient and situation to:
- determine the priority for single rooms or for roommate selection if rooms are to be shared by patients
- determine the possibility of exposure to blood, body fluids, secretions and excretions and non-intact skin and select appropriate control measures (e.g., PPE) to prevent exposure
- apply strategies to reduce aerosol generation during AGMPs (refer to Part B, Section IV, subsection iii, 1b)
- determine the need for additional precautions when routine practices are not sufficient to prevent exposure
Risk factors affecting control measures
Control measures to prevent exposure or transmission may differ according to specific microorganism, patient condition, situation or procedure and care setting. For example, measures to reduce the transmission of respiratory infections will differ from those to reduce the transmission of gastrointestinal infections. Certain patients (e.g., young children, incontinent adults and cognitively impaired individuals) or specific procedures on certain patients may increase risk of transmission, thereby requiring different control measures. Healthcare workers are at higher risk of exposure to respiratory viruses when providing care to patients who have copious respiratory secretions or frequent cough and are unable to perform self-care, including respiratory hygiene and hand hygiene. Procedures such as AGMPs have been shown to increase the transmission of TBFootnote 153 and SARSFootnote 150, Footnote 152, Footnote 284 and, therefore, specific control measures (refer to Part B, Section IV, subsection iii, 1b) should be used.
Some infections may be more readily transmitted in paediatric settings than in adult settings. Infection is a frequent cause of healthcare utilization by young children, who often harbour microorganisms, especially respiratory and gastrointestinal viruses that they may shed, even if asymptomaticFootnote 181, Footnote 285. Young children are also susceptible to many infections, as they may have not yet developed immunity to many microorganisms. The close proximity of large numbers of infectious persons and susceptible hosts favours transmission, as do behavioural characteristics of young children, such as incontinence, inadequate hygiene, frequent mouthing of hands and toys or other objects, drooling and direct contact between children during play. In addition, frequent hands-on contact from HCWs and parents may occur during basic care. Shared toys, playrooms and visiting siblings may also contribute to the transmission riskFootnote 181, Footnote 285.
There is variation of risk within different settings (e.g., prehospital, acute, LTC, ambulatory and home care). Therefore, control measures may often need to be modified, depending on the healthcare setting, as it would be inappropriate to impose the same level of precautions in each setting. The usual care model of LTC is to provide a home-like setting with participation in activities of daily living. There should be a balanced approach offering a safe environment without undue restrictive measures that could be detrimental to the individual's overall well-being or quality of lifeFootnote 286. There may be potential for increased risk of transmission with prehospital care, as it is an uncontrolled environmentFootnote 287.
The risk of cross-transmission may increase when patients share rooms instead of being accommodated in single patient care roomsFootnote 48, Footnote 201, Footnote 202, Footnote 288, Footnote 289, Footnote 290, Footnote 291, Footnote 292, Footnote 293, Footnote 294, Footnote 295, Footnote 296, Footnote 297, Footnote 298, Footnote 299, Footnote 300, Footnote 301, Footnote 302.
Knowledge and skills for point-of-care risk assessment
Healthcare workers should have sufficient knowledge, skills and resources to perform a PCRA before every interaction with a patient to apply appropriate control measures. In order to perform a PCRA, each HCW should have an understanding of the following principles, taking into consideration the level of care they are providing, their level of education and their specific job/responsibilities:
- the links in the chain of infection
- variables that influence transmission of microorganisms that may include type of exposure, size of inoculum, host susceptibility and control methods that reduce risk
- characteristics of the microorganisms that may include reservoirs, infectivity, mode of transmission, incubation period, period of communicability and virulence
- patient care practices and activities that contribute to exposure to microorganisms
- exposure risks specific to the healthcare setting
- environmental circumstances
- the level of risk and the appropriate control measures to be put in place to reduce the risk of transmission of microorganisms
- how to consult with IPC with concerns or questions
- control measures that may differ with different microorganisms and in different healthcare settings
Application of point-of-care risk assessments
When performing a PCRA, each HCW may consider asking questions to determine the risk of exposure and potential for transmission of microorganisms during patient interactions. Examples of such questions are:
- What contact is the HCW going to have with the patient?
- What task(s) or procedures(s) is the HCW going to perform? Is there a risk of splashes/sprays?
- If the patient has diarrhea, is he/she continent? If incontinent, can stool be contained in an adult incontinence product?
- Is the patient able and willing to perform hand hygiene?
- Is the patient in a shared room?
Tables 2 and 3 provide an overview of some of the risk factors identified in the questions above to consider when applying a PCRA, using C. difficile infection and seasonal influenza as examples. The tables outline how the risk of exposure and potential transmission changes, depending on variables in the infected source, environment and susceptible host. Risk factors to be considered as part of your PCRA, as outlined in Tables 2 and 3, include the following.
- An infected source: The PCRA should evaluate the changing nature of the infected source's symptoms and environment to determine the appropriate PPE for the HCW, other staff members and visitors. The PCRA should also determine if there is a need to move the patient to a single room with a private bathroom, and any other practice changes needed to address a change in a patient's condition.
- A susceptible host: The PCRA should evaluate whether the susceptible host has developed an infection such as C. difficile infection (e.g., cross-infection from a roommate/HCW) or whether the risk posed by an infected source has increased or decreased (e.g., diarrhea has increased or stools are now formed). The PCRA should lead to a determination of appropriate PPE that should be used to care for the patient in various situations. Examples include changing diaper products, taking a blood pressure or delivering meal trays with no patient or environmental contact, determining whether there is a need to move the patient or the roommates to another area, determining whether there is a need for enhanced housekeeping, and any other care practices required as a result of the change in risk for C. difficile acquisition.
| Source | Higher transmission risk | Lower transmission risk |
|---|---|---|
| Infectious agent/infected source | Frequent diarrhea | Formed stools |
| Incontinence | Continence | |
| Poor hygiene | Good hygiene | |
| Not capable of self-care due to physical condition, age or cognitive impairment | Capable of self-care | |
| Environment | High patient/nurse ratio | Low patient/nurse ratio |
| Shared bathroom, shared sink | Single room, private in-room toilet, designated patient handwashing sink | |
| Shared commode without cleaning between patients | Dedicated commode | |
| No hand hygiene at point-of-care | Hand hygiene at point-of-care | |
| No designated staff handwashing sink or sink is used for other purposes or sink is dirty | Accessible, designated, clean staff handwashing sink | |
| Inadequate housekeeping | Appropriate housekeeping | |
| Susceptible host (patient) | Receiving direct patient care | Capable of self-care |
| Poor personal hygiene | Good personal hygiene |
| Source | Higher transmission risk | Lower transmission risk |
|---|---|---|
| Infectious agent/infected source | Copious respiratory secretions | Minimal respiratory secretions |
| Frequent cough or sneeze | Infrequent cough or sneeze | |
| Poor compliance with respiratory hygiene | Compliance with respiratory hygiene practices | |
| Early stage of illness | Convalescent stage of illness | |
| Not capable of self-care | Capable of self-care | |
| Infants and children (potential prolonged viral shedding and environmental contamination) | Adults | |
| Immunocompromised (potential prolonged viral shedding) | Immunocompetent | |
| Inadequate patient placement or cohorting | Adequate patient placement, cohorting | |
| Environment | High patient/nurse ratio | Low patient/nurse ratio |
| Prolonged/frequent contact to infected source | Limited contact with infected source | |
| Shared room, washroom | Single room and washroom | |
| Inadequate housekeeping | Appropriate housekeeping | |
| Shared patient care equipment without cleaning between episodes of patient care | Dedicated equipment or cleaning and disinfection of equipment between uses | |
| Inadequate spatial separation between infected source and susceptible host (less than two metres) | Adequate spatial separation between infected source and susceptible host (at least two metres) | |
| Non-compliance with cleaning and disinfections standards | Compliance with cleaning and disinfection standards | |
| Susceptible host (patient) |
Not capable of self-care | Capable of self-care |
| Underlying disease | No underlying disease | |
| Susceptible | Immunized or recovered from disease | |
| Immunocompromised | Immunocompetent | |
| Susceptible host (HCWs or other staff) |
Inadequate application of engineering, administrative and PPE controls | Performs PCRA and chooses PPE appropriate to risk |
| Inadequate hand hygiene | Compliance with appropriate hand hygiene | |
| Infected source actively coughing and sneezing unable to contain secretions | Compliance with respiratory hygiene | |
| Not immunized against the circulating strain of influenza virus | Immunized against the circulating influenza virus more than two weeks prior to exposure | |
| Immunocompromised | Immunocompetent |
Applying control measures following point-of-care risk assessment
Additional precautions are to be applied as per the organizational policies and procedures. The PCRA of the circumstances of the patient, the environment and the task to be performed determine the control measures that should be used. Control measures are at the level of HCW patient care practices and PPE in the hierarchy of controls, and may include:
- hand hygiene, ensuring point-of-care ABHR is available and used (expected as the standard of care for all HCWs in all healthcare settings)
- patient placement and accommodation, prioritizing patients with uncontained wound drainage or uncontained diarrhea into a single room or placing a patient with suspected or confirmed airborne infection into an AIIR with the door closed
- treatment of active infection
- roommate selection for shared rooms or for transport in shared ambulances (and other types of transportation, such as air ambulances, taxis), considering the immune status of patients who will potentially be exposed to certain infections (e.g., measles, mumps, rubella, varicella)
- patient flow, restricting the movement of symptomatic patients within the specific patient care area/facility or outside the facility, as appropriate, for the suspected or confirmed microbial etiology
- work assignment, considering the immune status of HCWs who will potentially be exposed to certain infections (e.g., measles, mumps, rubella, and varicella)
- personal protective equipment selection, applying PPE appropriate to the suspected or confirmed infection or colonization
- cleaning and disinfecting non-critical patient care equipment and the patient environment
- handling of linen and waste
- restricting visitor access where appropriate
- reassessing the need for continuing or discontinuing additional precautions
2. Healthcare worker control measures to reduce exposure to and transmission of infectious agents
Routine practices
Routine practices are a comprehensive set of IPC measures that have been developed for use in the routine care of all patients at all times in all healthcare settings. Routine practices aim to minimize or prevent HAIs in all individuals in the healthcare setting, including patients, HCWs, visitors and contractors. Routine practices address infectious agent/infected source control, susceptible host protection and environmental hygiene utilizing aspects from all components of the hierarchy of controls.
All HCWs (e.g. physicians, nurses, allied HCWs, students, volunteers and others) are responsible for complying with routine practices and for tactfully calling infractions to the attention of offenders. No one is exempt from complying with routine practices.
Patients and visitors have a responsibility to comply with routine practices where indicated. Teaching patients and visitors basic principles (e.g., hand hygiene, use of PPE) is the responsibility of all HCWs.
Routine practices—Hand hygiene
The efficacy of hand disinfection in reducing nosocomial infection, as recognized by Semmelweis in 1847, has been repeatedly reaffirmedFootnote 303, Footnote 304. Use of ABHR has been shown to reduce HAI ratesFootnote 217, Footnote 305. Hand hygiene with point-of-care ABHR is the standard of care expected in all healthcare settings and of all HCWs.
A consistent trend demonstrating a reduction in infection rates related to improved hand hygiene has been reportedFootnote 305, Footnote 306, Footnote 307, Footnote 308, Footnote 309. However, sustaining improved hand hygiene rates and the reduction of HAIs is difficult, as a return to prestudy rates often occurs once the study is completed and interventions to promote hand hygiene are discontinuedFootnote 310, Footnote 311. Refer to the PHAC IPC guideline Hand Hygiene Practices in Healthcare SettingsFootnote 217 for further information.
Routine practices—Patient placement and accommodation
Accommodation of inpatients in single rooms facilitates IPC activities. Single rooms with a private toilet, designated patient handwashing sink and designated staff handwashing sink may reduce opportunities for cross-transmission between patients, particularly when the patient has poor hygiene, contaminates the environment, or cannot comply with IPC measures because of physical, behavioural and/or cognitive impairment(s)Footnote 201, Footnote 202, Footnote 289, Footnote 290, Footnote 291, Footnote 292, Footnote 293, Footnote 294, Footnote 295, Footnote 296, Footnote 297, Footnote 297, Footnote 299, Footnote 300, Footnote 301, Footnote 302. The HCW, in consultation with bed/accommodation coordinators and/or ICP professionals, as necessary, should select the most appropriate accommodation based on the PCRA and for prioritizing use of single rooms and AIIRs, if these are scarce.
Routine practices—Patient flow
Patient flow refers to patient transfer/transport within and outside of the facility and patient activity. There is a potential for exposure to and transmission of microorganisms as a result of patient activity and transport, due to inadvertent contact with other patients, patient care items and environmental surfaces. Patients should not be transported between patient care units, departments or facilities unless medically necessary. Frequent patient transfers should be avoided, as this increases the number of interactions with staff and other patients, providing opportunities for transmission to occurFootnote 25.
Routine practices—Aseptic technique for injections and intravascular and other invasive procedures
Aseptic technique is the purposeful prevention of transfer of microorganisms from the patient's body surface to a normally sterile body site or from one person to another by keeping the microbe count to an irreducible minimum. Aseptic techniques, sometimes referred to as sterile techniques, are measures designed to render the patient's skin, supplies and surfaces maximally free from microorganisms. Such practices are used when performing procedures that expose the patient's normally sterile sites (e.g., intravascular system, spinal canal, subdural space, urinary tract) in such a manner as to keep them free from microorganisms. Components of aseptic technique prior to a procedure may involve the following: preparing the patient's skin with an antiseptic; hand hygiene, preferably with ABHR or, if not accessible, an antimicrobial soap; sterile gloves, gowns, masks, equipment, and drapes; and maintaining a sterile field.
Infections may result from failure to use proper skin antisepsis prior to injection of medications, vaccines or venipunctureFootnote 312, Footnote 313. Chlorhexidine in alcohol inactivates microorganisms on the skin more effectively than most other antiseptics, and is the preferred antiseptic for skin preparation prior to insertion of central venous catheters and pulmonary artery cathetersFootnote 314, Footnote 315, Footnote 316, Footnote 317. Evidence suggests maximal aseptic barriers (including a head cap, mask, long-sleeved sterile surgical gown, sterile gloves and large (full bed) sterile drape during insertion) reduce infection rates associated with insertion of central venous cathetersFootnote 228, Footnote 318, Footnote 319, Footnote 320, Footnote 321. As reported studies differ in their patient populations, research designs and healthcare settings, additional investigation is warranted.
Meningitis has been reported after myelography and other spinal procedures and is usually caused by respiratory flora of the person performing the procedureFootnote 322, Footnote 323, Footnote 324, Footnote 325, Footnote 326, Footnote 327, Footnote 328, Footnote 329. The failure of the operator to wear a face mask during the procedureFootnote 325, Footnote 327, Footnote 329, Footnote 330, or to wear a mask properlyFootnote 328, has been implicated. Aseptic technique for sterile procedures, such as placing a catheter or injecting material into the spinal canal or subdural space (e.g., during myelograms, lumbar puncture, intrathecal chemotherapy, and spinal or epidural anesthesia), includes hand hygiene with ABHR, preparation of the site with an antiseptic, the use of a maskFootnote 331, use of sterile gloves and maintaining a sterile field.
Drapes are used to prevent transferring microorganisms from the environment to the patient during the procedure being performed. Masks are worn to prevent microorganisms carried in the HCWs nose and mouth from contaminating the sterile field.
Appropriate aseptic technique for the insertion of urinary catheters includes sterile equipment (e.g., gloves, drapes, sponges and catheters), a sterile or antiseptic solution for cleaning the meatus and a single-use packet of sterile lubricant jelly for insertionFootnote 31.
Aseptic technique for the withdrawal of medication or other sterile substances from any vial or other containers includes hand hygiene, the use of alcohol to prepare the rubber stopper or injection port (waiting for alcohol to dry), single-use sterile needles and syringes and following manufacturer's instructions. Transmission of hepatitis B and hepatitis C virus and other agents, has been related to the reuse of needles and/or syringes used to withdraw agents from multiuse vials, inappropriate use of glucose monitoring equipment, and reusing a single needle and syringe to administer medications to multiple patientsFootnote 92, Footnote 167, Footnote 168, Footnote 169, Footnote 170, Footnote 171, Footnote 172, Footnote 173, Footnote 174, Footnote 332.
Recommendations for injection safety are as followsFootnote 333:
- Do not administer medications from the same syringe to more than one patient, even if the needle is changed.
- Consider a syringe or needle to be contaminated after it has been used to enter or connect to a patient's intravenous infusion bag or administration set.
- Do not enter a vial or bag/bottle with a used syringe or needle.
- Do not use medications packaged as single-use vials for more than one patient.
- Assign medications packaged as multi-use vials to a single patient whenever possible.
- Follow proper IPC practices during the preparation and administration of injectable medications.
Routine practices—Personal protective equipment
Personal protective equipment consists of barriers worn by HCWs to protect the patient from transmission of microorganisms and to protect the HCW from exposure to bloodborne and other microorganisms (e.g., sprays of blood, body fluids, respiratory tract or other secretions or excretions). Healthcare organizations are responsible for ensuring that HCWs have access to the PPE appropriate to the work and patient care being provided and have received training on its use (as described in the role of the organization; refer to Part A, Section III, B).
Healthcare workers should be fully knowledgeable of the application and limitations of the specific PPE available for their use and be able to determine what is needed by assessing the risk of exposure to blood, body fluids, secretions and excretions, mucous membranes and non-intact skinFootnote 22, Footnote 219 during patient care interactions. The PCRA identifies hazards and enables the HCW to select PPE compatible with the hazard likely to be encountered during the patient care interaction. The selected PPE should maximize protection, with considerations for dexterity and comfort.
Performing a PCRA to determine whether PPE is necessary is also important to avoid over-reliance on PPE, misuse or waste. Over-reliance on PPE may result in a false sense of security. Misapplication or incorrect removal of PPE can result in inadvertent exposure of the HCWFootnote 334 or the patient to infectious agents or contamination of the patient's environmentFootnote 335. Wasting PPE can be avoided by maximizing the provision of clinical care during each entry into the patient's room.
The effectiveness of PPE is highly dependent on appropriate and proper use. Appropriate and proper use of PPE includes:
- point-of-care risk assessment to determine need for PPE
- using the correct technique for putting on and taking off PPE (refer to Appendix X)
- using the correct technique when wearing PPE (e.g., not to self-contaminate)
- discarding PPE into designated receptacles immediately after use, followed by hand hygiene
Routine practices—Gloves (refer also to Appendix IX)
The use of gloves is not a substitute for hand hygiene, but is an additional measure of protection. For routine practices, glove use is dependent on a PCRA of the patient, the environment and the interactionFootnote 336. Gloves are used to reduce the transmission of microorganisms from one patient to another or from one body site to another, and to reduce the risk of exposure of HCWs to blood, body fluids, secretions and excretions, mucous membranes, draining wounds and non-intact skin and for handling items or touching surfaces visibly or potentially soiledFootnote 22, Footnote 219, Footnote 337, Footnote 338. Gloves do not completely eliminate hand contaminationFootnote 337, as hands can become contaminated during the wearing of gloves through glove defects or during glove removalFootnote 339, Footnote 340, Footnote 341. Therefore, hand hygiene is necessary after the removal of gloves. Use of gloves may provide a false sense of security, leading to decreased hand hygieneFootnote 336, Footnote 342, Footnote 343, Footnote 344, Footnote 345.
It is important to assess and select the most appropriate glove to be worn for the circumstances. Glove selection should include assessment of its durability during use, the rigor and duration of the procedures being performed, the potential for exposure to infectious microorganisms or other hazardous substances and, ultimately, the safety of the user (e.g., latex allergies)Footnote 346 Factors such as comfort and fit are also important considerations.
Nonsterile disposable medical gloves for routine patient care are made from nitrile, latex or vinylFootnote 347. Powdered latex gloves have been associated with latex allergyFootnote 348. Latex-free alternatives must be used by persons with type I hypersensitivity to natural rubber and for care of patients with this type of latex allergyFootnote 346.
The barrier quality of medical examination gloves is influenced by glove material, production quality and stress during useFootnote 346, Footnote 347. Higher failure rates have been observed with vinyl gloves than with latex or nitrile gloves when tested under simulated and actual clinical conditionsFootnote 340, Footnote 341, Footnote 346, Footnote 347.
The integrity of latex gloves may be affected by the use of petroleum-based lotions or creamsFootnote 349, Footnote 350. Some ABHRs may interact with powder remaining on HCWs hands following the removal of powdered gloves and produce gritty particles on the handsFootnote 339, Footnote 341. Gloving hands that have not yet dried following the use of an ABHR may result in significant increase in glove perforationsFootnote 351.
Single-use gloves must never be washed with soap, chlorhexidine gluconate or alcohol for reuse, as washing affects their integrity and has not been shown to be effective in removing inoculated microorganismsFootnote 339, Footnote 352, Footnote 353.
The use of gloves to prevent the transmission of bloodborne pathogens is discussed in the PHAC IPC guideline Prevention and Control of Occupational Infections in Health CareFootnote 219.
Routine practices—Long-sleeved gowns and other apparel
Long-sleeved gowns are worn for routine practices, as indicated by the risk assessment, to protect uncovered skin and clothing during procedures and patient care activities likely to produce soiling or generate splashes or sprays of blood, body fluids, secretions or excretionsFootnote 22, Footnote 219. Gowns should be cuffed and cover the front and back of the HCW from the neck to mid-thigh. Gowns include isolation gowns (reusable/disposable, fluid repellent or sterile). The type of gown selected is based on the:
- anticipated degree of contact with infectious material
- potential for blood and body fluid penetration of the gown (fluid repellence when heavy liquid contamination is anticipated, such as in the operating theatre and during dialysis)
- requirement for sterility (e.g., operating theatre, central line insertion)
There is no evidence that the routine use of gowns for all patient care is beneficial in the prevention of HAIs, even in high-risk units (e.g., neonatal intensive care unit, ICU, haematopoietic stem cell transplant unit, burn unit)Footnote 354, Footnote 355, Footnote 356, Footnote 357. Universal gown use has had no effect on HAI rates in neonatalFootnote 358, Footnote 359 or paediatric ICUsFootnote 360, or on rates of neonatal colonization on postpartum wardsFootnote 361, Footnote 362.
In the laboratory setting, wearing of laboratory coats is considered PPE. Outside of the laboratory, apparel such as uniforms, laboratory coats and scrub suits may be worn by HCWs for purposes of comfort, convenience or identity, but do not have a role in the prevention of infection (i.e., they are not considered PPE). For aesthetic purposes and professional etiquette, HCW apparel and uniforms should be clean. The safety of home laundering HCWs uniforms has been investigated and no increase in infection rates has been detectedFootnote 363.
Routine practices—Facial protection
Transmission of hepatitis C has been reported by blood splash into the conjunctivaFootnote 364, Footnote 365 and HIV has been transmitted by splashes of blood onto the faceFootnote 366. A study to investigate the risk of contamination of radiologists' eyes during invasive vascular procedures determined 6.7% of procedures resulted in splashesFootnote 367. Facial protection includes masks and eye protection, or face shields or masks with visor attachment. Eye protection may include masks with built-in eye protection, safety glasses or face shields. The need for facial protection during routine patient care is determined by the PCRA of the patient interaction and the task to be performed. Interactions involving activities likely to generate coughing, splashes or sprays of blood, body fluids, secretions or excretions, and procedures that potentially expose the mucous membranes of the eyes, nose or mouth warrant facial protectionFootnote 22, Footnote 219.
Masks include surgical or procedure masks; no specific mask has been shown to be superior to another for achieving facial protection. Masks have several uses: as a barrier to protect from sprays or splashesFootnote 22, Footnote 219; as a barrier for infectious sourcesFootnote 368, Footnote 369; as a barrier when performing aseptic/sterile proceduresFootnote 331; and as a barrier to protect susceptible hosts when within two metres of patients on droplet precautionsFootnote 135, Footnote 213, Footnote 368, Footnote 369, Footnote 370, Footnote 371, Footnote 372, Footnote 373, Footnote 374, Footnote 375, Footnote 376.
Routine practices—Management of visitors
Visitation policies should be developed and implemented to balance the risk of transmission of infectious diseases and the promotion of patient and family-centered careFootnote 377. Visitors have been documented to transmit various infections, including TBFootnote 66, Footnote 378, pertussisFootnote 64, and respiratory viruses, in healthcare settingsFootnote 46, Footnote 67, Footnote 379, Footnote 380, Footnote 381, Footnote 382, Footnote 383. Exclusion of those with signs and symptoms of transmissible infections should reduce this risk. For essential visits, the visitor with an infection should be instructed on measures to take to reduce the risk of transmission (e.g., wear a mask for a respiratory tract infection, perform appropriate hand hygiene, remain in the patient's room, avoid public areas, avoid contact with other patients or with patient care equipment).
Additional precautions
Additional precautions are applied when the natural transmission characteristics of specific microorganisms (e.g., epidemiologically significant microorganisms including C. difficile, antibiotic-resistant microorganisms, viral gastroenteritis and emerging respiratory infections; refer to Appendix VI) or syndromes are not fully managed by routine practices. Additional precautions may be required when medical procedures artificially increase the risk of transmission of a specific microorganism or because of the clinical situation (e.g., care of a young child, incontinent adult or cognitively impaired individual). Additional precautions are specific to the care setting (e.g., acute care, ambulatory care, prehospital care, LTC, and home care). Additional precautions are conventionally divided into:
- contact precautions, for microorganisms of very low infective dose and/or situations where heavy contamination of the patient's environment is anticipated (refer to List 3)
- droplet precautions, for microorganisms transmitted by the large droplet route (refer to List 4)
- airborne precautions, for microorganisms transmitted over extended time and distance by small particles (refer to List 5)
Additional precautions—Implementing and discontinuing additional precautions
Additional precautions should be implemented as soon as disease or risk factors are suspected or identified. A confirmed diagnosis is not necessary for additional precautions to be applied.
The organization is responsible for:
- designating the personnel responsible on a day-to-day basis for implementing additional precautions
- specifying the notification processes once precautions have been initiated
- identifying the person responsible for modifying or discontinuing precautions
- identifying the person who has ultimate authority to make decisions regarding precautions, outbreak management and bed allocation
The HCW is responsible for:
- ensuring that appropriate additional precautions are taken for specific patients
- ensuring patients are not subjected to unnecessary additional precautions
- ensuring that precautions are reviewed daily, adjusted if indicated by new information and discontinued when no longer indicated
To minimize the transmission of microorganisms, patients should be assessed for evidence of infection or potential infections on admission (if an inpatient setting) or at the initial point of patient encounter and regularly throughout their stay, as per the PCRA. The results of the assessment should be communicated to other personnel providing care and be documented in the patient record. In situations where a patient has or is suspected of having a disease requiring additional precautions above and beyond routine practices, these precautions should be implemented as soon as indicated by triggering mechanisms such as diagnosis, symptoms of infection, laboratory information and assessment of risk factors. It is not necessary to wait for a specific diagnosis or microbiologic confirmation before initiating additional precautions when PCRA clearly indicates a clinical syndrome or risk factors related to a potentially transmissible infection.
All HCWs are responsible for complying with additional precautions (in addition to routine practices) and for tactfully calling infractions to the attention of offenders. Patients and visitors also have a responsibility to comply where indicated. Teaching the basic principles of routine practices and additional precautions is the responsibility of all HCWs.
Additional precautions—Accommodation
When availability of single rooms is limited, priorities for placement of patients in single rooms are determined by the PCRA. Priority for single rooms goes to patients:
- requiring additional precautionsFootnote 292, Footnote 299, Footnote 300, Footnote 301, Footnote 302
- identified as high risk for transmission of microorganismsFootnote 69 (e.g., stool incontinenceFootnote 290, Footnote 291, Footnote 384, uncontained secretions)Footnote 48, Footnote 385
- identified as being at higher risk of acquisition and adverse outcomes resulting from transmission of microorganisms (e.g., immunosuppressionFootnote 379, Footnote 386, Footnote 387, open wounds, indwelling catheters, anticipated prolonged length of stay)Footnote 388
- requiring dependence on HCWs for activities of daily livingFootnote 288
Factors to be considered with shared rooms (when single rooms are not available) include:
- selecting appropriate roommates
- avoiding placing patients at high risk of complications, if they become infected, in rooms with patients with transmissible infections, diarrhea or open wounds
- delineating the boundary of the potentially contaminated patient area within the shared room
- preventing transmission risks from sharing of sinks and toilets
- assessing activities of the roommates and their visitors
Assignment of patients known to be infected with the same microorganisms to the same room (cohorting), or assignment of infected and non-infected patients in separate wards or areas has been successful in controlling transmission of some microorganismsFootnote 285, Footnote 389, Footnote 390, Footnote 391, Footnote 392, Footnote 393, Footnote 394. The benefit of using cohorting for managing ARO outbreaks, including MRSAFootnote 47, Footnote 390 and VREFootnote 395, Footnote 396, Footnote 397, Gram-negative-resistant organismsFootnote 53, Footnote 398 and outbreaks due to other infectious agentsFootnote 399, Footnote 400, Footnote 401, is difficult to determine, as multiple other control measure were implemented during these published outbreaks.
Additional precautions—Accommodation—Airborne infection isolation rooms
Airborne infection isolation rooms (AIIRs) with negative pressure ventilation (i.e., with air flow from the outside corridor into a room through the doorway and exiting directly to the exterior of the building or filtered before recirculation) are designed for patients suspected or confirmed to have an infection transmitted by the airborne route including:
- measles
- respiratory (including pleuropulmonary or laryngeal) TB
- smallpox, monkeypox
- varicella (chickenpox)
- disseminated zoster
An AIIR should also be used for performing AGMPs on patients with TB, SARS, viral hemorrhagic fever and respiratory infection with an emerging pathogen for which transmission routes are not yet fully known (refer to Appendix VI, item 4).
In settings where AIIRs are limited, patients with proven or suspected infectious respiratory TB have priority. For measles, varicella and disseminated zoster, risk of transmission may be assessed in relation to the presence of non-immune patients or HCWs. Non-immune HCWs should not work with patients with measles, varicella or disseminated zoster. Non-immune patients should not share rooms with patients with measles, varicella or zoster.
When AIIRs are not available, the patient should be temporarily housed in a single room with the door closed, away from high-risk patients. Patients should be transferred as soon as medically feasible to a facility with AIIRs.
- Prehospital care: Patients should wear a mask and be transported separately. When transporting multiple patients, the risk of transmission should be considered as noted above and control measures applied (e.g., personnel in the ambulance should be limited to those medically necessary; if possible, a window in ambulance should be open; if possible, the window between the driver and the patient area of the ambulance should be closed).
- Ambulatory care: Patients should defer their appointment if possible or enter through a separate entrance. Upon arrival, patients should be asked to wear a mask, perform hand hygiene and be moved to an examining room with the door closed as soon as possible.
- Home care settings: Family members who have not been exposed or are not immune should avoid sharing airspace with the patient. Natural ventilation (e.g., open windows) will help disperse the microorganisms from the room.
Additional precautions—Patient flow
When additional precautions are necessary, patients should leave their rooms for medically necessary purposes only. Communication between the transporting area and the receiving area is important to ensure consistency of precautions and to decrease unnecessary waiting time in public areas. Source control measures (e.g., requesting that the patient perform hand hygiene before leaving their room, cover skin lesions, wear a mask) should be applied.
Additional precautions—Personal protective equipment—Gloves
Gloves are used for all care of patients on contact precautions. When worn appropriately, evidence has confirmed the effectiveness of gloves in preventing contamination of HCWs' hands, thereby reducing the potential transfer of microorganisms from colonized or infected patients to HCWs and from patient to patient via HCWs' handsFootnote 130, Footnote 337, Footnote 342, Footnote 402, Footnote 403. A prospective controlled trial of vinyl gloves to prevent the transmission of C. difficile demonstrated a significant decrease in the incidence of C. difficile-associated disease during a 6-month intervention periodFootnote 338. In one study, an outbreak of MRSA was controlled with the use of gloves for all contact with patients and their immediate environmentFootnote 404.
Gloves become contaminated during use and, if used inappropriately, can result in transmission of microorganismsFootnote 98, Footnote 343, Footnote 345, Footnote 405, Footnote 406. Transmission of C. difficileFootnote 86, MRSA and Acinetobacter spp.Footnote 407 has been associated with failure to change gloves between patients. Failing to change gloves between care activities and procedures with the same patient after contact with materials that may contain high concentrations of microorganisms (e.g., after handling an indwelling urinary catheterFootnote 408, or suctioning an endotracheal tube)Footnote 407 may result in contamination of clean body sites or the patient's environmentFootnote 86.
Additional precautions—Personal protective equipment—Long-sleeved gowns
The benefits of using gowns as a control measure to prevent transmission is difficult to determine, as the use of gowns and multiple interventions (e.g., gloves, increased emphasis on hand hygiene, isolation/cohorting) are often implemented concurrently and the individual benefits of these measures could not be identifiedFootnote 403, Footnote 409.
Gowns are used for contact precautions if direct contact of clothing with the patient or with contaminated environmental surfaces is anticipated. Although gowns may become contaminated with potential pathogens after caring for an infected or colonized patient (e.g., MRSAFootnote 70, VREFootnote 98 and C. difficileFootnote 119) there is no evidence that gowns have been involved in the transmission of these pathogens to others.
Additional precautions—Personal protective equipment—Facial protection
Facial protection includes masks and eye protection, or face shields or masks with visor attachment. Facial protection should be worn when within two metres of a coughing/sneezing patient with a suspected or confirmed transmissible respiratory infectionFootnote 216, Footnote 219.
The eye is an important portal of entry for some pathogens. Pathogens may be introduced into the eye directly via respiratory droplets generated during coughing or suctioning, or by self-inoculation if the eyes are touched with contaminated fingersFootnote 48. Wearing eye protection during all care of children with RSV has been shown to reduce the acquisition of this infection by HCWsFootnote 410, Footnote 411, probably by preventing hand-to-eye contact.
Additional precautions—Personal protective equipment—Respiratory protection
Respiratory protection from airborne infection includes the use of a respirator (refer to Appendix V, glossary) to prevent inhalation of airborne microorganismsFootnote 233. Respiratory protection may be necessary as a component of airborne precautions or a recommendation for performing AGMPs on certain patients. The use of a respirator or the need for airborne precautions is determined by a PCRA. Factors to be considered are the specific infectious agent, known or suspected infection status of the patient involved, the patient care activity to be performed, the immune status of the HCW and the patient's ability to perform respiratory hygiene (refer to Part A, Section III, B, 3).
Additional precautions—Management of visitors
Visitors should not have conditions that put them at risk for serious diseases if they acquire the patient's infection (e.g., a visitor with chronic lung disease could acquire a respiratory virus or a non-immune visitor could acquire varicella), and should comply with necessary precautions to prevent indirect transmission to other patients (e.g., hand hygiene, no sharing of personal items).
Generally, visitors should have access to the same PPE as staff when providing direct patient care. Evidence to support the use of PPE by visitors is lacking. The following should be considered when requesting that visitors wear PPE:
- Personal protective equipment may not be necessary if they have likely been exposed to the infection preadmission.
- Personal protective equipment may be appropriate for visitors who visit multiple patients in the facility. If used by visitors, the PPE should be changed before visiting a different patient.
Additional precautions—Epidemiologically significant organisms requiring additional precautions include the following diseases/conditions (refer also to Appendix VI)
- C. difficile
- certain AROs
- viral gastroenteritis
- emerging respiratory infections