Tuberculosis: Drug resistance in Canada 2015

Download the alternative format
(PDF format, 1.55 MB, 58 pages)
Organization: Public Health Agency of Canada
Date published: 2017-04-03
Acknowledgements
The Surveillance and Epidemiology Division, Centre for Communicable Diseases and Infection Control at the Public Health Agency of Canada would like to acknowledge the members of the Canadian Tuberculosis Laboratory Technical Network and their teams as well as colleagues at the National Microbiology Laboratory for their contribution to and participation in the Canadian Tuberculosis Laboratory Surveillance System.
Table of Contents
- Acknowledgements
- List of Figures
- List of Tables
- List of Appendices
- Acronyms and Abbreviations
- Introduction
- Background
- Methods
- Results
- Discussion
- Conclusion
- Appendices
- References
List of Figures
- Figure 1: Number of Mycobacterium tuberculosis complex isolates tested by province or territory of origin, 2015
- Figure 2: Percentage of isolates tested with any resistance to isoniazid, pyrazinamide, rifampin and ethambutol, 2015
- Figure 3: Percentage of isolates tested with any resistance to isoniazid, pyrazinamide, rifampin and ethambutol, 2005 to 2015
- Figure 4: Tuberculosis drug resistance patterns as a percentage of isolates tested, 2015
- Figure 5: Tuberculosis drug resistance patterns as a percentage of isolates tested, 2005 to 2015
List of Tables
- Table 1: Critical concentrations for routine testing of anti-tuberculosis drugs
- Table 2: Total number of Mycobacterium tuberculosis complex isolates by reporting and originating province/territory, 2015
- Table 3: Total number of Mycobacterium tuberculosis complex isolates and number and percentage identified with any resistance, as multidrug and as extensively drug resistant in Canada, 2005 to 2015
- Table 4: Overall pattern of reported tuberculosis drug resistance in Canada, 2005 to 2015
- Table 5: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Alberta, 2005 to 2015
- Table 6: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for British Columbia, 2005 to 2015
- Table 7: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Manitoba, 2005 to 2015
- Table 8: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for New Brunswick, 2005 to 2015
- Table 9: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Newfoundland and Labrador, 2005 to 2015
- Table 10: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Northwest Territories, 2005 to 2015
- Table 11: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Nova Scotia, 2005 to 2015
- Table 12: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Nunavut, 2005 to 2015
- Table 13: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Ontario, 2005 to 2015
- Table 14: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Prince Edward Island, 2005 to 2015
- Table 15: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Quebec, 2005 to 2015
- Table 16: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Saskatchewan, 2005 to 2015
- Table 17: Results for routine drug susceptibility testing of Mycobacterium tuberculosis complex isolates to anti-tuberculosis drugs for Yukon, 2005 to 2015
- Table 18: Multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis isolates by province/territory of origin, 2015
- Table 19: Provincial/territorial breakdown by any resistance, multidrug-resistant tuberculosis and extensively drug-resistant tuberculosis in Canada, 2005 to 2015
- Table 20: Tuberculosis drug resistance by sex and age group in Canada, 2015
List of Appendices
- Appendix I: Participating Laboratories of the Canadian Tuberculosis Laboratory Technical Network (CTLTN)
- Appendix II: Provincial/territorial laboratory drug susceptibility testing capacity
- Appendix III: M. tuberculosis Complex Antimicrobial Susceptibility Reporting Form
- Appendix IV: Data Tables
Acronyms and Abbreviations
- AK
- Amikacin
- Alta.
- Alberta
- B.C.
- British Columbia
- BCG
- Bacillus Calmette-Guérin
- CCDIC
- Centre for Communicable Diseases and Infection Control
- CI
- Confidence interval
- CLSI
- Clinical and Laboratory Standards Institute
- CM
- Capreomycin
- CTBLSS
- Canadian Tuberculosis Laboratory Surveillance System
- CTLTN
- Canadian Tuberculosis Laboratory Technical Network
- EMB
- Ethambutol
- ETH
- Ethionamide
- INH
- Isoniazid
- KM
- Kanamycin
- LIN
- Linezolid
- M. africanum
- Mycobacterium africanum
- M. bovis
- Mycobacterium bovis
- M. canetti
- Mycobacterium canetti
- M. caprae
- Mycobacterium caprae
- M. microti
- Mycobacterium microti
- M. pinnipedii
- Mycobacterium pinnipedii
- M. tuberculosis
- Mycobacterium tuberculosis
- Man.
- Manitoba
- MDR-TB
- Multidrug-resistant tuberculosis
- MOX
- Moxifloxacin
- MTBC
- Mycobacterium tuberculosis complex
- N.B.
- New Brunswick
- N.L.
- Newfoundland and Labrador
- NRCM
- National Reference Centre for Mycobacteriology
- N.S.
- Nova Scotia
- Nvt.
- Nunavut
- N.W.T.
- Northwest Territories
- OFL
- Ofloxacin
- Ont.
- Ontario
- PAS
- Para-aminosalicylic acid
- P.E.I.
- Prince Edward Island
- PHAC
- Public Health Agency of Canada
- ProvLab
- Provincial Laboratory of Public Health (Alberta)
- PZA
- Pyrazinamide
- Que.
- Quebec
- RBT
- Rifabutin
- RMP
- Rifampin
- Sask.
- Saskatchewan
- SM
- Streptomycin
- TB
- Tuberculosis
- XDR-TB
- Extensively drug-resistant tuberculosis
- Y.T.
- Yukon
Introduction
Drug-resistant strains of tuberculosis (TB) pose a threat to Canadian TB prevention and control efforts. Although drug-resistant TB is not a major problem in Canada, it has the potential to become one because Canadians frequently travel abroad and many individuals immigrate to Canada from countries with high TB rates and associated drug resistance.
The Canadian Tuberculosis Laboratory Surveillance System (CTBLSS) was created in 1998 as part of Canada’s response to a growing worldwide concern about TB drug resistance. It was established by the Health Canada Division of Tuberculosis Prevention and Control in collaboration with the Canadian Tuberculosis Laboratory Technical Network (CTLTN) and participating laboratories. The CTBLSS was designed to monitor emerging trends and patterns in TB drug resistance in Canada and is currently managed by the Centre for Communicable Diseases and Infection Control (CCDIC) within the Public Health Agency of Canada (PHAC).
This report is part of an annual surveillance report series that describes data collected through the CTBLSS. Specifically, this report provides details on TB drug resistance in Canada for the period 2005 to 2015, with a focus on 2015.
The data presented in this report are intended to inform public health action as well as policy and program development and assessment.
Background
Patterns of drug resistance
TB drug resistance is identified through susceptibility testing of clinical specimens collected from individuals with culture-positive TB.Footnote 1 People with TB are said to have drug-resistant TB if the strain of Mycobacterium tuberculosis causing their disease is resistant to one or more of the four first-line drugs: isoniazid, rifampin, pyrazinamide or ethambutol. The following resistance patterns are described in this report:
- Monoresistance – defined as resistance to one first-line anti-tuberculosis drug only (isoniazid, rifampin, ethambutol or pyrazinamide).
- Polyresistance (other patterns) – defined as resistance to more than one first-line anti- tuberculosis drug, not including the isoniazid and rifampin combination.
- Multidrug-resistant tuberculosis (MDR-TB) – defined as resistance to isoniazid and rifampin with or without resistance to other anti-tuberculosis drugs.
- Extensively drug-resistant TB (XDR-TB) – defined as resistance to isoniazid and rifampin and any fluoroquinolone and at least one of the three injectable second-line drugs (amikacin, capreomycin or kanamycin).Footnote 2
TB drug resistance testing standards in Canada
The mission of the CTLTN is to promote excellence, standardization and quality assurance in mycobacteriology services. The CTLTN is a pan-Canadian network of technical and scientific heads of provincial and territorial TB laboratories (see Appendix I: Participating Laboratories of the Canadian Tuberculosis Laboratory Technical Network (CTLTN)).
The goals of the CTLTN are to:
- standardize laboratory methodologies;
- improve biosafety operational practices and physical requirements;
- implement biosafety guidelines;
- participate in national surveillance and proficiency programs; and
- exchange services and information about new technologies.
Laboratory testing methods in Canada, including drug selection and the critical concentrations used for routine drug susceptibility testing, follow recommended laboratory standards.Endnote i,Footnote 3,Footnote 4 Participating CTLTN laboratories perform routine susceptibility testing of Mycobacterium tuberculosis complex (MTBC) isolates against first-line anti-tuberculosis drugs using fluorometric proportion method BACTEC® MGIT 960. Table 1 provides a list of recommended first-line and second-line anti-tuberculosis drugs and the recommended critical concentrations to be used for testing.Footnote 3,Footnote 4
Second-line drug susceptibility testing varies across jurisdictions. Typically, however, isolates are tested for resistance to amikacin, kanamycin, capreomycin, ethionamide, linezolid, ofloxacin, moxifloxacin, para-aminosalicylic acid and rifabutin.
Methods
Overview of the Canadian Tuberculosis Laboratory Surveillance System
The CTBLSS is an isolate-based surveillance system designed to collect data on TB drug resistance from across Canada. Each year, drug susceptibility test results for isolates tested in the previous calendar year are voluntarily submitted to PHAC by provincial TB laboratories for inclusion in the CTBLSS. Not all provinces and territories, however, have the capacity to perform drug susceptibility testing. Those without the capacity prepare the isolates and forward them to other provincial laboratories for testing. In some instances, the laboratory that tests the sample sends the results to PHAC on behalf of the originating province or territory. For further details on provincial/territorial laboratory drug susceptibility testing capacity, please refer to Appendix II.
Data are submitted to PHAC either through the manual completion of a standard reporting form (Appendix III: M. tuberculosis Complex Antimicrobial Susceptibility Reporting Form) or electronically. Standardized data recoding procedures are applied to all data to create a national dataset. The following information is submitted to PHAC:
- the date the isolate or specimen was received at the laboratory;
- the isolate or specimen identification number provided by the laboratory;
- the province where the isolate was tested;
- the province/territory from which the isolate originated;
- the sex of the individual from whom the isolate was collected;
- the date of birth or age at time of testing of the individual from whom the isolate was collected;
- the name(s) of the drug(s) tested;
- the concentration at which the drug(s) was (were) tested; and
- drug susceptibility result (sensitive/resistant/not done).
Data are submitted for confirmed cases of MTBC demonstrated on culture, including M. tuberculosis, M. africanum, M. canetti, M. caprae, M. microti, M. pinnipedii or M. bovis. Results may be submitted at the species level or for MTBC only without species identification. Some laboratories also submit results for the M. bovis Bacillus Calmette-Guérin (BCG) strain, a complication of TB vaccination often found in immunocompromised patients. These results are excluded from this report because this strain is not infectious.
All participating laboratories test for resistance to the first-line antibiotics isoniazid, ethambutol and rifampin. Although the Canadian Tuberculosis Standards (7th edition) recommends that laboratories perform drug susceptibility testing to pyrazinamideFootnote 1, British Columbia does not routinely test for resistance to this drug. If resistance to any first-line drug is detected, British Columbia will subsequently test the isolate for resistance to pyrazinamide.
Results of second-line drug susceptibility testing are submitted for isolates showing resistance to isoniazid and rifampin. To rule out XDR-TB, laboratories are asked to report results for at least one of the fluoroquinolones (ofloxacin, moxifloxacin or levofloxacin) and at least one of the injectable agents (amikacin, kanamycin and capreomycin).
Tabulation and presentation of results
This report provides an overview of TB drug resistance in Canada for the period 2005 to 2015. Data are presented by province/territory and by age group and sex where feasible. Data from 2015 (the most recent reporting year for which data are available) are highlighted as are important trends over time.
The data presented in this report were extracted from the CTBLSS database on February 28, 2016 and have been validated by the reporting laboratories. Results from cultures that grow in a given year are included in the statistics for that calendar year; otherwise the results are reflected in the subsequent year’s report. For example, if a specimen was received by the laboratory on December 20, 2015 and the culture did not grow M. tuberculosis until January 2016, these results would be reflected in the 2016 report.
Samples submitted to the laboratory for drug susceptibility testing may be collected at the time of the individual’s diagnosis or at any time during treatment. Depending on the treatment duration, an individual may be tested multiple times over several years until cured or until the prescribed treatment is completed. If two specimens are confirmed to be from the same individual in a given calendar year, only the most recent susceptibility result is retained. Therefore, the number of isolates described in this report is not equal to the number of culture-positive cases reported through the case-based surveillance system over the same time period where each individual with culture-positive TB is only reported once in the year of diagnosis.
No statistical procedures were used for comparative analyses in this report, nor were any statistical techniques applied to account for missing data. Data in tables with small cell sizes (n £ 5) were not suppressed, since disclosure is not deemed to pose any risk of identifying individuals. These procedures are consistent with PHAC’s Directive for the collection, use and dissemination of information relating to public health.Footnote 5
Results
In 2015, anti-tuberculosis drug susceptibility test results for 1,352 isolates were reported to PHAC. Of these, 13 (1.0%) isolates were identified as M. bovis BCG and were excluded from further analyses. Of the remaining 1,339 isolates analyzed, 788 (58.8%) were reported as MTBC where the species was known (772 were M. tuberculosis, ten were M. africanum and six were M. bovis) and 551 (41.2%) were MTBC of an unknown species (data not shown). Figure 1 shows the number of MTBC isolates tested by province or territory of origin. Table 2 provides a breakdown of the number of isolates by reporting and originating province or territory.
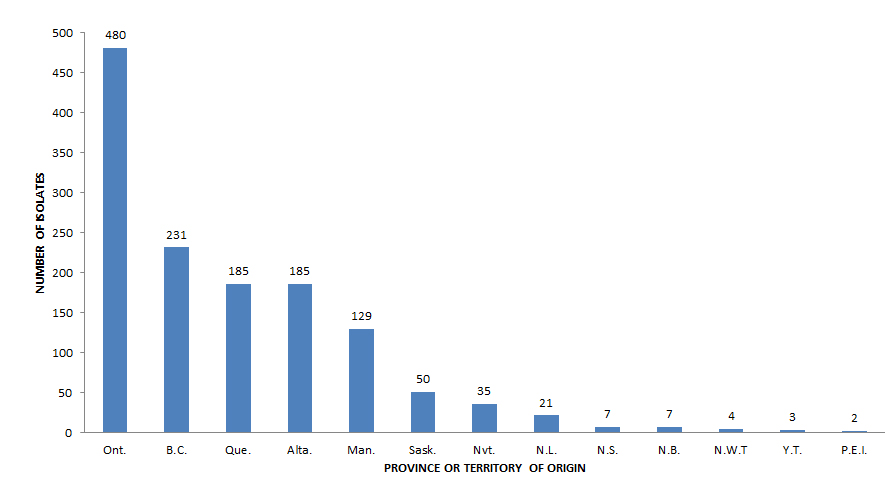
Figure 1 - Text Description
| Province or territory of origin | Number of reported isolates |
|---|---|
| Ont. | 480 |
| B.C. | 231 |
| Que. | 185 |
| Alta. | 185 |
| Man. | 129 |
| Sask. | 50 |
| Nvt. | 35 |
| N.L. | 21 |
| N.S. | 7 |
| N.B. | 7 |
| N.W.T | 4 |
| Y.T. | 3 |
| P.E.I. | 2 |
For the period 2005 to 2015, drug susceptibility test results were reported for 14,776 isolates (Table 3). Of the results received during this period, 1,402 (9.5%) were resistant to one or more of the first-line drugs, 178 isolates (1.2%) were identified as multidrug-resistant and 7 (< 0.1%) were identified as extensively drug-resistant (Table 3).
Any first-line drug resistance
In 2015, rifampin and ethambutol sensitivity results were available for all 1,339 isolates tested, isoniazid sensitivity results were available for 1,336 of the 1,339Endnote ii isolates tested and 1,111 (83.0%) isolates were tested for resistance to pyrazinamide (Table 4). As a percentage of the isolates tested, 110 (8.2%) were resistant to isoniazid, 38 (3.4%) were resistant to pyrazinamide 24 (1.8%) were resistant to rifampin, and 10 (0.7%) were resistant to ethambutol (Figure 2 ).
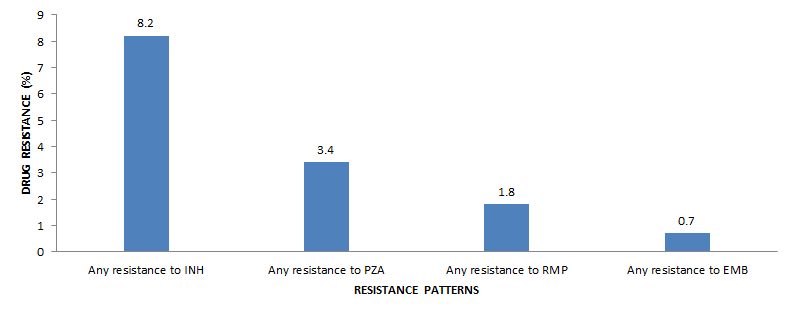
Figure 2 - Text Description
| Resistance pattern | Drug resistance (%) |
|---|---|
| Any resistance to INH | 8.2 |
| Any resistance to PZA | 3.4 |
| Any resistance to RMP | 1.8 |
| Any resistance to EMB | 0.7 |
Figure 3 shows the changes over time in the percentage of isolates resistant to each of the first-line drugs for the period 2005 to 2015. During this period of time, 8.0% (range: 6.7% to 9.2%) of all isolates tested were resistant to isoniazid (Table 4) whereas resistance shown to ethambutol, rifampin or pyrazinamide has remained below 4% (Figure 3).
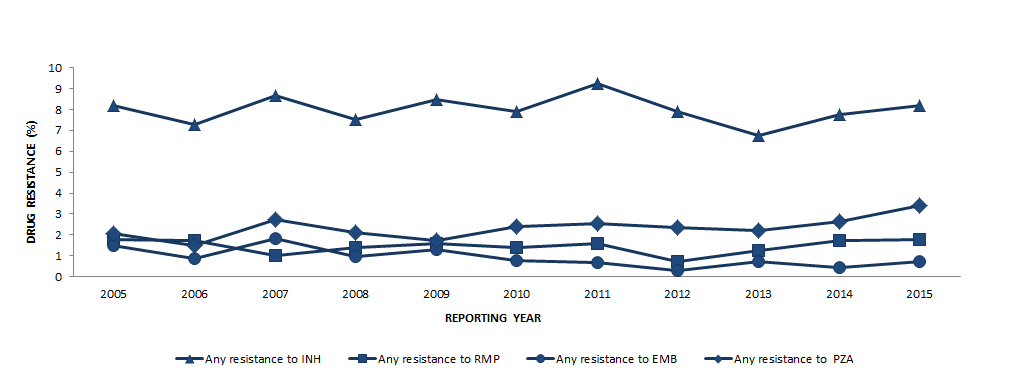
Figure 3 - Text Description
| Any resistance to INH | Any resistance to RMP | Any resistance to EMB | Any resistance to PZA | |
|---|---|---|---|---|
| 2005 | 8.2 | 1.8 | 1.5 | 2.1 |
| 2006 | 7.3 | 1.7 | 0.9 | 1.5 |
| 2007 | 8.7 | 1.0 | 1.8 | 2.7 |
| 2008 | 7.5 | 1.4 | 1.0 | 2.1 |
| 2009 | 8.5 | 1.6 | 1.3 | 1.7 |
| 2010 | 7.9 | 1.4 | 0.8 | 2.4 |
| 2011 | 9.2 | 1.6 | 0.7 | 2.6 |
| 2012 | 7.9 | 0.7 | 0.3 | 2.4 |
| 2013 | 6.7 | 1.2 | 0.7 | 2.2 |
| 2014 | 7.8 | 1.7 | 0.4 | 2.6 |
| 2015 | 8.2 | 1.8 | 0.7 | 3.4 |
Monoresistance
In 2015, of the 139 TB isolates (10.4% of all isolates tested) reported to be resistant to at least one of the four first-line drugs (Table 4), the majority (n = 114; 82.0%) were monoresistant. Of these, 85 (74.6%) were isoniazid monoresistant, 27 (23.7%) were pyrazinamide monoresistant and 2 (1.8%) were rifampin monoresistant. No isolates were ethambutol monoresistant (Table 5 to Table 17; data not tabulated across tables).
For the period 2005 to 2015, 7.9% of all isolates tested were monoresistant, ranging from a high of 9.1% in 2012 to a low of 6.7% in 2013 (Table 4). During this time, 30 isolates (0.2% of all isolates tested) were identified as rifampin-monoresistant, which is an uncommon resistance patternFootnote 1. Of these, 16 (53.3%) originated from British Columbia; six (20.0%) from Ontario, three (10.0%) from Quebec, two (6.7%) from Alberta, and one (3.3%) each from Saskatchewan, Northwest Territories and Nunavut. On average, one to three rifampin-monoresistant isolates were reported each year from 2005 to 2015 (Table 5 to Table 17; data not tabulated across tables).
Polyresistant, multidrug-resistant and extensively drug-resistant TB
In 2015, after excluding isolates resistant to both isoniazid and rifampin, three isolates (0.2%) were resistant to two or more of the first-line drugs and were therefore classified as polyresistant (Table 4). Two of these were resistant to isoniazid and ethambutol and one was resistant to isoniazid and pyrazinamide.
For the period 2005 to 2015, 54 (0.4%) isolates were identified as polyresistant (Table 4). Of these, 26 (48.1%) were resistant to isoniazid and ethambutol, 21 (38.9%) were resistant to isoniazid and pyrazinamide, and one was resistant to ethambutol and pyrazinamide. The remaining six isolates were resistant to isoniazid, ethambutol, and pyrazinamide (Table 5 to Table 17; data not tabulated across tables)
With respect to first-line drug resistance, 22 (1.6%) isolates were isoniazid and rifampin resistant (identifying them as at least MDR-TB) in 2015. Of these, 11 (50.0%) were resistant to only isoniazid and rifampin. In addition to being isoniazid and rifampin resistant, three were also resistant to pyrazinamide, and one to ethambutol. Seven isolates were resistant to all four of the first-line drugs (Table 18).
To rule out XDR-TB, all 22 isolates resistant to both isoniazid and rifampin were subsequently tested for resistance to select second-line drugs. Of these, three isolates were resistant to at least one of the injectable agents (amikacin, capreomycin or kanamycin) but susceptible to the fluoroquinolones, and one isolate was resistant to a fluoroquinolone but susceptible to all of the injectable agents (Table 5 to Table 17; data not tabulated across tables). Because none of the 22 isoniazid- and rifampin-resistant isolates were resistant to both an injectable agent and a flouroquinolone, all 22 were classified as MDR-TB and none were classified as XDR-TB (Table 18). Figure 4 presents patterns of TB drug resistance as a percentage of all isolates tested in 2015.
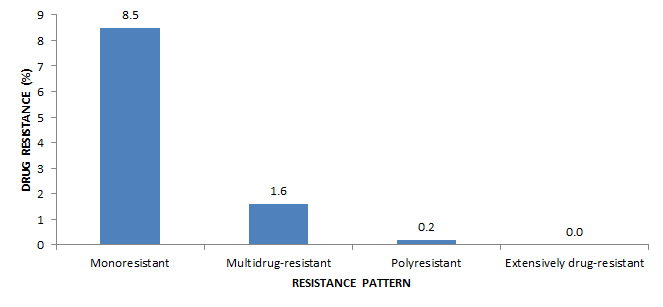
Figure 4 - Text Description
| Resistance pattern | Drug Resistance (%) |
|---|---|
| Monoresistant | 8.5 |
| Multidrug-resistant | 1.6 |
| Polyresistant | 0.2 |
| Extensively drug-resistant | 0.0 |
For the period 2005 to 2015, 178 isolates were classified as MDR-TB, representing 1.2% of isolates tested over this time (Table 4) and seven isolates were classified as XDR-TB, representing less than 0.1% of the isolates tested. An average of 16 MDR-TB isolates were reported each year, ranging from a low of eight in 2012 (0.6% of all isolates tested in 2012) to a high of 22 in 2005 and in 2015 (1.6% of all isolates tested in each respective year).
Figure 5 shows the overall pattern of reported TB drug resistance as a percentage of isolates tested for the period 2005 to 2015. While there have been small fluctuations in the percentage of isolates showing various resistance patterns, there has been no notable change during this time.
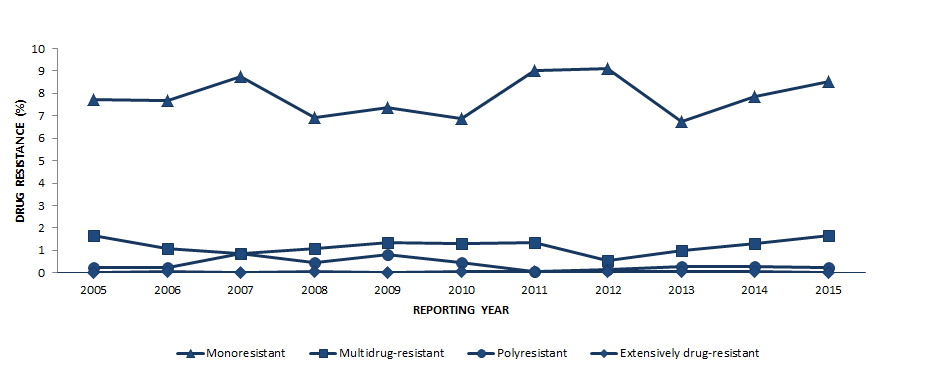
Figure 5 - Text Description
| Resistance pattern | 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoresistant | 7.7 | 7.7 | 8.8 | 6.9 | 7.4 | 6.9 | 9.0 | 9.1 | 6.7 | 7.8 | 8.5 |
| Multidrug-resistant | 1.6 | 1.1 | 0.9 | 1.1 | 1.4 | 1.3 | 1.4 | 0.6 | 1.0 | 1.3 | 1.6 |
| Polyresistant | 0.2 | 0.2 | 0.9 | 0.4 | 0.8 | 0.5 | 0.1 | 0.1 | 0.3 | 0.3 | 0.2 |
| Extensively drug-resistant | 0.0 | 0.1 | 0.0 | 0.1 | 0.0 | 0.1 | 0.1 | 0.1 | 0.1 | 0.1 | 0.0 |
Geographical distribution
In 2015, the majority (90.4%) of isolates originated from five provinces: Ontario, British Columbia, Quebec, Alberta and Manitoba. Saskatchewan accounted for fewer than 4% of reported isolates while the northern territories (Northwest Territories, Nunavut and Yukon) and the Atlantic provinces (New Brunswick, Newfoundland and Labrador, Nova Scotia and Prince Edward Island) together accounted for 5.9% of reported isolates Table 2 .
In 2015, all isolates from the Northwest Territories, Yukon, Newfoundland and Labrador, Nova Scotia, and Prince Edward Island were susceptible to all first-line drugs tested. Of the 22 MDR-TB isolates, 14 originated from Ontario, two from each Alberta, British Columbia, and Quebec and one from each Manitoba and New Brunswick (Table 19).
For the period 2005 to 2015, all 178 MDR-TB isolates originated from seven provinces: Alberta, British Columbia, Manitoba, New Brunswick, Ontario, Quebec and Saskatchewan. Of the seven isolates identified as XDR-TB, five originated from Ontario, one from Manitoba and one from Quebec (Table 19).
Table 5 through Table 17 present complete resistance profiles for all the isolates tested for the period 2005 to 2015, by province and territory.
Demographic information
In 2015, age and/or date of birth was reported for all 1,339 individuals from whom isolates were collected (Table 20). Of the 139 drug-resistant isolates, none were from individuals under the age of 15 years. The majority of isolates with any resistance were collected from individuals between 15 and 34 years of age. Of the 22 MDR-TB isolates, 36.4% (n=8) were collected from individuals between 25 and 34 years of age.
In 2015, sex was known for 1,331 (99.4%) of the 1,339 individuals from whom isolates were collected (Table 20). Males accounted for 55.1% of all reported isolates. Females accounted for 54.0% of isolates showing any resistance, and males accounted for 54.5% of MDR-TB isolates.
Discussion
In many parts of the world, drug resistance is a major challenge to preventing and controlling TB. Eastern Europe and Central Asia continue to have the world’s highest proportion of MDR-TB cases.Footnote 6
Tuberculosis strains that are resistant to both isoniazid and rifampin pose a considerable challenge to treatment and prevention efforts because effective anti-tuberculosis drugs are limited. Data published by the World Health Organization show that, globally, in 2014 about 3.3% (95% CI: 2.2%–4.4%) of new TB cases and 20% (95% CI: 14%–27%) of previously treated TB cases were MDR-TB.Footnote 6 Although the data captured through the CTBLSS do not distinguish between isolates from new versus previously treated cases of TB, the fact that only 1.6% of isolates tested in 2015 were MDR-TB is a considerably lower finding than global estimates. In addition, the fact that only seven XDR-TB cases were identified over the period 2005 to 2015 indicates that XDR-TB in Canada is rare.
Strengths and limitations
The CTBLSS is the result of a successful collaboration between federal, provincial and territorial governments and the CTLTN. The primary objective of the CTBLSS is to monitor emerging trends and patterns in resistance to anti-tuberculosis drugs in Canada. This report presents detailed data on the extent of first- and second-line TB drug resistance in Canada, disaggregated by province/territory and, where feasible, by sex and/or age group. As the primary source of national data on TB drug resistance in Canada, the data within this report provide timely information for public health action, as well as policy and program development and assessment.
Prior to analysis and report preparation, all data were reviewed for errors, inconsistencies and completeness. Submitting laboratories were provided with a summary report of their data for review. Following validation by the reporting laboratories, the data were integrated into the CTBLSS database. Nevertheless, like most surveillance data, the data in this report are subject to possible coding, reporting and processing errors.
Previously published data may be updated based on late reporting or revisions from participating laboratories. Any revisions to previously reported data are reflected in subsequent reports. Therefore, the data presented in this report are considered the most up-to-date and replace those previously published in this report series.
Although efforts are made to ensure that multiple records for any one individual in a given year are removed, given the minimal identifying information available for each isolate (age and sex), it is possible that multiple records from one individual are included in the database. This bias is likely minimal given the validation process with the data providers.
Demographic and clinical data collected through the CTBLSS are limited. No data are collected on the ethnic origin, diagnostic/clinical status or the treatment outcome of the individual from whom the sample was collected. Additional demographic and clinical information would facilitate a more in-depth epidemiological assessment of drug resistance patterns in Canada. What’s more, differentiation between primary and acquired drug resistance and differing resistance patterns among new cases in comparison to re-treatment cases are not possible based on data collected through this surveillance system. However, the Tuberculosis in Canada report, which provides an overview of the overall number of reported active TB cases and corresponding incidence rates in Canada by select demographic and clinical characteristics, presents case-based (vs. isolate-based) data on primary and acquired drug resistance in Canada that are not presented here. Together, these two reports provide a more comprehensive overview of TB case and drug resistance surveillance data from a national perspective.
Typically, only MDR-TB isolates or other extensive resistance patterns will undergo select second-line drug sensitivity testing. Although the Clinical and Laboratory Standards Institute (CLSI) recommends that isoniazid-monoresistant isolates as well as other polyresistant, non-MDR isolates be tested for second-line drug resistance, this is not universally reported in Canada. Other isolates that are not MDR-TB may be resistant to fluoroquinolones because of the widespread use of these antibiotics for other respiratory infections. To some extent, this limits our understanding of the emergence of second-line drug resistance within Canada.
Conclusion
TB drug resistance is an important global public health concern, but it is not a significant problem in Canada. In 2015, 10.4% of all isolates tested were resistant to at least one of the four first-line drugs; the majority were resistant to only one drug (82.0%), and 1.6% were identified as MDR-TB and none as XDR-TB. TB drug resistance levels have been stable over the past 10 years and have remained below the global average since national surveillance began. However, with growing worldwide concern about resistance and the emergence of XDR-TB, the CTBLSS remains vital to the monitoring of TB drug resistance in Canada.
Appendices
Appendix I: Participating Laboratories of the Canadian Tuberculosis Laboratory Technical Network (CTLTN)
Alberta
Provincial Laboratory of Public Health
Edmonton/Calgary
Cary Shandro
Technologist Mycobacteriology
Dr. Greg Tyrrell
Clinical Microbiologist
Dr. Graham Tipples
Medical/Scientific Director
British Columbia
British Columbia Centre for Disease Control
Public Health Microbiology and Reference Laboratory
Vancouver
Dr. Mabel Rodrigues
Mycobacteriology/TB Laboratory, Team Lead
Dr. Mel Krajden
Medical Microbiologist/ Director, Laboratory Services
Manitoba
Diagnostics Services Manitoba
Health Sciences Centre
Winnipeg
Assunta Rendina
Charge Technologist, Mycobacteriology
Doug Swidinsky
Senior Technologist
Dr. Heather Adam
Clinical Microbiologist
New Brunswick
Department of Laboratory Medicine
Saint John Regional Hospital
Saint John
Hope MacKenzie
MLT3-Supervisor CL3 Lab
Dr. Duncan Webster
Medical Microbiologist/ Infectious Disease
Dr. Tarek Rahmeh
Laboratory Director
Newfoundland and Labrador
Newfoundland and Labrador Public Health Laboratory
St. John's
Sherry Baird
Tech II
Dr. George Zahariadis.
Director & Divisional Chief of the Public Health Laboratory and Microbiology Services
Northwest Territories
Stanton Territorial Hospital
Yellowknife
Sherrill Webber
Tech II, Microbiology
Carolyn Russell
Laboratory Supervisor
Cheryl Case
Manager
Therapeutic & Diagnostic Services
Nova Scotia
Department of Pathology & Laboratory Medicine
Queen Elizabeth II Health Sciences Centre
Halifax
Darlene McPhee (MLTC/ temporary supervisor)
Division of Medical Microbiology
Dr. David Haldane,
Director, Provincial Public Health Laboratory Network and Special Pathogens
Dr. Todd Hatchette
Director, Pathology and Laboratory Medicine
Nunavut
Qikiqtani General Hospital
Iqaluit
Sonia Marchand
Laboratory Health
Ontario
Public Health Ontario Laboratories
Public Health Ontario
Toronto
Kevin May
Operational Lead, Mycobacteriology
Dr. Frances Jamieson
Medical Microbiologist -TB and Mycobacteriology
Kirby Cronin
Laboratory Liaison Technical Officer (PHAC)
Alex Marchand-Austin
Manager, Laboratory Surveillance and Data Management
Quebec
Laboratoire de santé publique du Québec
Institut national de santé publique du Québec
Sainte-Anne-de-Bellevue
Hafid Soualhine
Head, Mycobacteriology & Aerobic Actinomycetes
Dr. Jean Longtin
Director
Saskatchewan
Saskatchewan Disease Control Laboratory
Regina
Rita Thomas
Technologist, TB/Bacteriology
Dr. David Farrell
Director of Bacteriology/ Associate Clinical Director
Dr. Paul Levett
Clinical Director
Dr. David Alexander
Microbiologist
Dr. Greg Horsman
Medical Director
Federal
National Microbiology Laboratory
Public Health Agency of Canada
Winnipeg
Joyce Wolfe
Senior Expert Program Delivery
Kym Antonation
Chief, Bioforensics Assay Development and Diagnostics
National Microbiology Laboratory
Appendix II: Provincial/territorial laboratory drug susceptibility testing capacity
- The British Columbia Public Health Microbiology and Reference Laboratory at the British Columbia Centre for Disease Control tests and reports first-line susceptibility results for British Columbia and Yukon.
- The Provincial Laboratory of Public Health (ProvLab) in Alberta tests and reports results for Alberta and Northwest Territories.
- Public Health Ontario Laboratories test and report results for Ontario and Nunavut.
- The National Reference Centre for Mycobacteriology (NRCM)Endnote iii located in Manitoba does first-line susceptibility testing for Newfoundland and Labrador, Manitoba, New Brunswick, Nova Scotia and Prince Edward Island. In this case, the NRCM returns test results to the originating province and the originating province submits their results to PHAC.
- All the remaining provinces conduct their own first-line testing and do not routinely report results for any other jurisdiction.
- Four laboratories in Canada conduct second-line drug susceptibility testing: the ProvLab in Alberta, the Public Health Ontario Laboratories, the Laboratoire de santé publique du Québec and the NRCM.
- The NRCM tests the susceptibility of isolates to second-line drugs for all provinces and territories that do not conduct such testing at their laboratories. Upon request, the NRCM also tests isolates submitted by any provincial laboratory to confirm resistance patterns. Results from testing done by NRCM are returned to the provincial laboratory that submitted the isolates and the provincial laboratory then reports the results to PHAC.
Appendix III: M. tuberculosis Complex Antimicrobial Susceptibility Reporting Form
Please note
The PDF fillable form in this section is available here:

Download the alternative format
(PDF format, 76 KB, 1 page)
Organization: Public Health Agency of Canada
Date published: 2015-02-04
Appendix IV: Data Tables
| ANTI-TUBERCULOSIS DRUGS | CRITICAL CONCENTRATIONSTable 1 - Footnote * (mg/L) BACTEC® 960 | COMMENTS | |
|---|---|---|---|
| FIRST-LINE | Isoniazid (INH) | 0.1 | When resistance to INH is 0.1 mg/L, tests are repeated with INH 0.4 mg/L to determine the level of resistance. Nevertheless, the isolate is reported as resistant using the 0.1 mg/L cut-off level. |
| Rifampin (RMP) | 1.0 | - | |
| Ethambutol (EMB) | 5.0 | - | |
| Pyrazinamide (PZA) | 100.0 | Routine testing is not performed for isolates from British Columbia. | |
| SECOND-LINE | Amikacin (AK) | 1.0 | - |
| Capreomycin (CM) | 2.5 | - | |
| Ethionamide (ETH) | 5.0 | - | |
| Kanamycin (KM) | 2.5 | - | |
| Linezolid (LIN) | 1.0 | - | |
| Moxifloxacin (MOX) | 0.3 | - | |
| Ofloxacin (OFL) | 2.0 | - | |
| Para-amino salicylic acid (PAS) | 4.0 | - | |
| Rifabutin (RBT) | 0.5 | - | |
| Streptomycin (SM) | 1.0 | - | |
|
|||
| REPORTING PROVINCE/TERRITORY | CANADA | ORIGINATING PROVINCE/ TERRITORY | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N.L. | P.E.I. | N.S. | N.B. | Que. | Ont. | Man. | Sask. | Alta. | B.C. | Y.T. | N.W.T | Nvt. | ||
| N.L. | 21 | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N.S. | 9 | 0 | 2 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| N.B. | 7 | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Que. | 183 | 0 | 0 | 0 | 0 | 183 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ont. | 517 | 0 | 0 | 0 | 0 | 2 | 480 | 0 | 0 | 0 | 0 | 0 | 0 | 35 |
| Man. | 129 | 0 | 0 | 0 | 0 | 0 | 0 | 129 | 0 | 0 | 0 | 0 | 0 | 0 |
| Sask. | 50 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 50 | 0 | 0 | 0 | 0 | 0 |
| Alta. | 190 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 185 | 1 | 0 | 4 | 0 |
| B.C. | 233 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 230 | 3 | 0 | 0 |
| TOTAL | 1,339 | 21 | 2 | 7 | 7 | 185 | 480 | 129 | 50 | 185 | 231 | 3 | 4 | 35 |
| Abbreviations: Alta.= Alberta; B.C.= British Columbia; Man.= Manitoba; N.B.= New Brunswick; N.L.= Newfoundland and Labrador; N.S.= Nova Scotia; Nvt.= Nunavut; N.W.T.= Northwest Territories; Ont.= Ontario; P.E.I.= Prince Edward Island; Que.= Quebec; Sask.= Saskatchewan; Y.T.= Yukon. | ||||||||||||||
| TOTAL NUMBER OF REPORTED MTBC ISOLATES | RESISTANT TO ONE OR MORE FIRST-LINE DRUGS | MULTIDRUG-RESISTANT TBTable 3 - Footnote * | EXTENSIVELY DRUG-RESISTANT TBTable 3 - Footnote † | ||||
|---|---|---|---|---|---|---|---|
| NUMBER | PERCENT (%) | NUMBER | PERCENT (%) | NUMBER | PERCENT (%) | ||
| 2005 | 1,335 | 128 | 9.6 | 22 | 1.6 | 0 | 0.0 |
| 2006 | 1,389 | 126 | 9.1 | 15 | 1.1 | 1 | 0.1 |
| 2007 | 1,267 | 133 | 10.5 | 11 | 0.9 | 0 | 0.0 |
| 2008 | 1,356 | 116 | 8.6 | 15 | 1.1 | 1 | 0.1 |
| 2009 | 1,331 | 127 | 9.5 | 18 | 1.4 | 0 | 0.0 |
| 2010 | 1,279 | 112 | 8.8 | 17 | 1.3 | 1 | 0.1 |
| 2011 | 1,319 | 139 | 10.5 | 18 | 1.4 | 1 | 0.1 |
| 2012 | 1,404 | 139 | 9.9 | 8 | 0.6 | 1 | 0.1 |
| 2013 | 1,381 | 112 | 8.1 | 14 | 1.0 | 1 | 0.1 |
| 2014 | 1,376 | 131 | 9.5 | 18 | 1.3 | 1 | 0.1 |
| 2015 | 1,339 | 139 | 10.4 | 22 | 1.6 | 0 | 0.0 |
| TOTAL | 14,776 | 1,402 | 9.5 | 178 | 1.2 | 7 | <0.1 |
Abbreviations: MTBC= Mycobacterium tuberculosis complex
|
|||||||
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | TOTAL | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Total number of isolates tested | 1,335 | 100.0 | 1,389 | 100.0 | 1,267 | 100.0 | 1,356 | 100.0 | 1,331 | 100.0 | 1,279 | 100.0 | 1,319 | 100.0 | 1,404 | 100.0 | 1,381 | 100.0 | 1,376 | 100.0 | 1,339 | 100.0 | 14,776 | 100.0 |
| Isolates tested that were susceptible | 1,207 | 90.4 | 1,263 | 90.9 | 1,134 | 89.5 | 1,240 | 91.4 | 1,204 | 90.5 | 1,167 | 91.2 | 1,180 | 89.5 | 1,265 | 90.1 | 1,269 | 91.9 | 1,245 | 90.5 | 1,200 | 89.6 | 13,374 | 90.5 |
| Isolates showing any resistance to first-line drugs | ||||||||||||||||||||||||
| Any resistance to isoniazid | 109 | 8.2 | 101 | 7.3 | 110 | 8.7 | 102 | 7.5 | 113 | 8.5 | 101 | 7.9 | 122 | 9.2 | 111 | 7.9 | 93 | 6.7 | 107 | 7.8 | 110 | 8.2 | 1,179 | 8.0 |
| Any resistance to rifampin | 24 | 1.8 | 24 | 1.7 | 13 | 1.0 | 19 | 1.4 | 21 | 1.6 | 18 | 1.4 | 21 | 1.6 | 10 | 0.7 | 17 | 1.2 | 24 | 1.7 | 24 | 1.8 | 215 | 1.5 |
| Any resistance to ethambutol | 20 | 1.5 | 12 | 0.9 | 23 | 1.8 | 13 | 1.0 | 17 | 1.3 | 10 | 0.8 | 9 | 0.7 | 4 | 0.3 | 10 | 0.7 | 6 | 0.4 | 10 | 0.7 | 134 | 0.9 |
| Any resistance to pyrazinamideTable 4 - Footnote * | 22 | 2.1 | 16 | 1.5 | 27 | 2.7 | 22 | 2.1 | 18 | 1.7 | 25 | 2.4 | 28 | 2.3 | 33 | 2.8 | 26 | 2.2 | 30 | 2.6 | 38 | 3.4 | 285 | 2.4 |
| Total number isolates resistant to one or more first-line TB drugs | 128 | 9.6 | 126 | 9.1 | 133 | 10.5 | 116 | 8.6 | 127 | 9.5 | 112 | 8.8 | 139 | 10.5 | 139 | 9.9 | 112 | 8.1 | 131 | 9.5 | 139 | 10.4 | 1,402 | 9.5 |
| Monoresistant | 103 | 7.7 | 107 | 7.7 | 111 | 8.8 | 94 | 6.9 | 98 | 7.4 | 88 | 6.9 | 119 | 9.0 | 128 | 9.1 | 93 | 6.7 | 108 | 7.8 | 114 | 8.5 | 1,163 | 7.9 |
| Multidrug resistantTable 4 - Footnote † | 22 | 1.6 | 15 | 1.1 | 11 | 0.9 | 15 | 1.1 | 18 | 1.4 | 17 | 1.3 | 18 | 1.4 | 8 | 0.6 | 14 | 1.0 | 18 | 1.3 | 22 | 1.6 | 178 | 1.2 |
| Polyresistant | 3 | 0.2 | 3 | 0.2 | 11 | 0.9 | 6 | 0.4 | 11 | 0.8 | 6 | 0.5 | 1 | 0.1 | 2 | 0.1 | 4 | 0.3 | 4 | 0.3 | 3 | 0.2 | 54 | 0.4 |
| Extensively drug resistantTable 4 - Footnote ‡ | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 | 1 | 0.1 | 0 | 0.0 | 1 | 0.1 | 1 | 0.1 | 1 | 0.1 | 1 | 0.1 | 1 | 0.1 | 0 | 0.0 | 7 | <0.1 |
|
||||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 5 - Footnote * | 129 | 100.0 | 104 | 100.0 | 98 | 100.0 | 134 | 100.0 | 159 | 100.0 | 107 | 100.0 | 156 | 100.0 | 163 | 100.0 | 154 | 100.0 | 181 | 100.0 | 185 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 115 | 89.1 | 95 | 91.3 | 92 | 93.9 | 123 | 91.8 | 145 | 91.2 | 96 | 89.7 | 133 | 85.3 | 148 | 90.8 | 140 | 90.9 | 165 | 91.2 | 164 | 88.6 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 10 | 7.8 | 7 | 6.7 | 5 | 5.1 | 8 | 6.0 | 8 | 5.0 | 6 | 5.6 | 14 | 9.0 | 10 | 6.1 | 9 | 5.8 | 11 | 6.1 | 15 | 8.1 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 1 | 1.0 | 1 | 1.0 | 0 | 0.0 | 3 | 1.9 | 0 | 0.0 | 2 | 1.3 | 3 | 1.8 | 4 | 2.6 | 0 | 0.0 | 3 | 1.6 |
| Subtotal - Monoresistant TB | 10 | 7.8 | 8 | 7.7 | 6 | 6.1 | 8 | 6.0 | 12 | 7.5 | 6 | 5.6 | 16 | 10.3 | 13 | 8.0 | 13 | 8.4 | 12 | 6.6 | 18 | 9.7 |
| Polyresistant | ||||||||||||||||||||||
| INH & EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & EMB & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 |
| INH & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 1 | 0.9 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| Subtotal - Polyresistant | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 2 | 1.3 | 2 | 1.9 | 0 | 0.0 | 1 | 0.6 | 1 | 0.6 | 0 | 0.0 | 1 | 0.5 |
| Multidrug-resistant TBTable 5 - Footnote † | ||||||||||||||||||||||
| INH & RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & OFL & MOX | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| INH & RMP & EMB & PZA & SM & OFL & MOX & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.7 | 0 | 0.0 | 1 | 0.9 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & ETH | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| INH & RMP & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 1.3 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 |
| INH & RMP & PZA & SM & ETH | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & SM & OFL & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM | 1 | 0.8 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 |
| INH & RMP & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Multidrug-resistant TB | 4 | 3.1 | 1 | 1.0 | 0 | 0.0 | 2 | 1.5 | 0 | 0.0 | 3 | 2.8 | 7 | 4.5 | 1 | 0.6 | 0 | 0.0 | 4 | 2.2 | 2 | 1.1 |
| Extensively drug-resistant TBTable 5 - Footnote ‡ | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number isolates resistant to one or more first-line TB drugs | 14 | 10.9 | 9 | 8.7 | 6 | 6.1 | 11 | 8.2 | 14 | 8.8 | 11 | 10.3 | 23 | 14.7 | 15 | 9.2 | 14 | 9.1 | 16 | 8.8 | 21 | 11.4 |
Abbreviations: AK=amikacin;EMB=ethambutol; ETH=ethionamide; INH=isoniazid; KM=kanamycin; MOX=moxifloxacin; OFL=ofloxacin; PAS=para-aminosalicylic acid; PZA=pyrazinamide; RBT=rifabutin; RMP=rifampin; SM=streptomycin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 6 - Footnote * Table 6 - Footnote † | 204 | 100.0 | 275 | 100.0 | 231 | 100.0 | 254 | 100.0 | 239 | 100.0 | 204 | 100.0 | 194 | 100.0 | 254 | 100.0 | 223 | 100.0 | 270 | 100.0 | 231 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 182 | 89.2 | 257 | 93.5 | 210 | 90.9 | 230 | 90.6 | 215 | 90.0 | 185 | 90.7 | 170 | 87.6 | 231 | 90.9 | 204 | 91.5 | 235 | 87.0 | 199 | 86.1 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 11 | 5.4 | 7 | 2.5 | 13 | 5.6 | 18 | 7.1 | 22 | 9.2 | 16 | 7.8 | 21 | 10.8 | 21 | 8.3 | 19 | 8.5 | 25 | 9.3 | 27 | 11.7 |
| RMP | 2 | 1.0 | 6 | 2.2 | 0 | 0.0 | 3 | 1.2 | 1 | 0.4 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 2 | 0.7 | 1 | 0.4 |
| EMB | 4 | 2.0 | 3 | 1.1 | 4 | 1.7 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| Subtotal - Monoresistant TB | 17 | 8.3 | 16 | 5.8 | 17 | 7.4 | 21 | 8.3 | 23 | 9.6 | 18 | 8.8 | 22 | 11.3 | 21 | 8.3 | 19 | 8.5 | 27 | 10.0 | 29 | 12.6 |
| Polyresistant | ||||||||||||||||||||||
| INH & EMB | 1 | 0.5 | 0 | 0.0 | 2 | 0.9 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| INH & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 2 | 0.7 | 0 | 0.0 |
| Subtotal - Polyresistant TB | 1 | 0.5 | 0 | 0.0 | 2 | 0.9 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 2 | 0.7 | 1 | 0.4 |
| Multidrug-resistant TBTable 6 - Footnote ‡ | ||||||||||||||||||||||
| INH & RMP | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & KM & CM & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & ETH & RBT & PAS | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & KM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & OFL & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & RBT | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & AK & KM & CM & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 |
| INH & RMP & EMB & SM & ETH & RBT | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 |
| INH & RMP & EMB & SM & ETH & RBT & PAS | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 1 | 0.4 |
| INH & RMP & PZA & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 |
| INH & RMP & PZA & SM & RBT | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & SM & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.7 | 0 | 0.0 |
| INH & RMP & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 |
| Subtotal - Multidrug-resistant TB | 4 | 2.0 | 2 | 0.7 | 2 | 0.9 | 3 | 1.2 | 0 | 0.0 | 1 | 0.5 | 1 | 0.5 | 2 | 0.8 | 0 | 0.0 | 6 | 2.2 | 2 | 0.9 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 22 | 10.8 | 18 | 6.5 | 21 | 9.1 | 24 | 9.4 | 24 | 10.0 | 19 | 9.3 | 24 | 12.4 | 23 | 9.1 | 19 | 8.5 | 35 | 13.0 | 32 | 13.9 |
Abbreviations: CM=capreomycin; EMB=ethambutol; ETH=ethionamide; INH=isoniazid; KM=kanamycin; MOX=moxifloxacin; OFL=ofloxacin; PAS=para-aminosalicylic acid; PZA=pyrazinamide; RBT=rifabutin; RMP=rifampin; SM=streptomycin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 7 - Footnote * | 94 | 100.0 | 119 | 100.0 | 84 | 100.0 | 116 | 100.0 | 106 | 100.0 | 113 | 100.0 | 97 | 100.0 | 123 | 100.0 | 148 | 100.0 | 125 | 100.0 | 129 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 92 | 97.9 | 113 | 95.0 | 75 | 89.3 | 111 | 95.7 | 99 | 93.4 | 99 | 87.6 | 90 | 92.8 | 113 | 91.9 | 144 | 97.3 | 120 | 96.0 | 125 | 96.9 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 2 | 2.1 | 6 | 5.0 | 7 | 8.3 | 4 | 3.4 | 4 | 3.8 | 10 | 8.8 | 5 | 5.2 | 10 | 8.1 | 4 | 2.7 | 5 | 4.0 | 3 | 2.3 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 0 | 0.0 | 1 | 1.2 | 0 | 0.0 | 1 | 0.9 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Monoresistant TB | 2 | 2.1 | 6 | 5.0 | 8 | 9.5 | 4 | 3.4 | 5 | 4.7 | 11 | 9.7 | 5 | 5.2 | 10 | 8.1 | 4 | 2.7 | 5 | 4.0 | 3 | 2.3 |
| Polyresistant | ||||||||||||||||||||||
| INH & EMB | 0 | 0.0 | 0 | 0.0 | 1 | 1.2 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 1 | 1.2 | 0 | 0.0 | 2 | 1.9 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TBTable 7 - Footnote † | ||||||||||||||||||||||
| INH & RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & AK & KM & CM & ETH & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 |
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 1 | 0.9 | 2 | 2.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.8 |
| Extensively drug-resistant TBTable 7 - Footnote ‡ | ||||||||||||||||||||||
| INH & RMP & EMB & PZA & KM & OFL & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 2 | 2.1 | 6 | 5.0 | 9 | 10.7 | 5 | 4.3 | 7 | 6.6 | 14 | 12.4 | 7 | 7.2 | 10 | 8.1 | 4 | 2.7 | 5 | 4.0 | 4 | 3.1 |
Abbreviations: AK=amikacin; CM=capreomycin; EMB=ethambutol; ETH=ethionamide; INH=isoniazid; KM=kanamycin; MOX=moxifloxacin; OFL=ofloxacin; PAS=para-aminosalicylic acid; PZA=pyrazinamide; RBT=rifabutin; RMP=rifampin; SM=streptomycin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 8 - Footnote * | 5 | 100.0 | 3 | 100.0 | 5 | 100.0 | 3 | 100.0 | 10 | 100.0 | 9 | 100.0 | 5 | 100.0 | 4 | 100.0 | 3 | 100.0 | 6 | 100.0 | 7 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 4 | 80.0 | 3 | 100.0 | 5 | 100.0 | 3 | 100.0 | 10 | 100.0 | 7 | 77.8 | 5 | 100.0 | 3 | 75.0 | 2 | 66.7 | 6 | 100.0 | 5 | 71.4 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 33.3 | 0 | 0.0 | 0 | 0.0 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| Subtotal - Monoresistant TB | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 1 | 33.3 | 0 | 0.0 | 1 | 14.3 |
| Polyresistant | ||||||||||||||||||||||
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TBTable 8 - Footnote † | ||||||||||||||||||||||
| INH & RMP & PZA & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 14.3 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 25.0 | 1 | 33.3 | 0 | 0.0 | 2 | 28.6 |
Abbreviations: EMB=ethambutol; ETH=ethionamide; INH=isoniazid; PZA=pyrazinamide; RBT=rifabutin; RMP=rifampin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZA | 6 | 100.0 | 11 | 100.0 | 5 | 100.0 | 5 | 100.0 | 10 | 100.0 | 9 | 100.0 | 5 | 100.0 | 5 | 100.0 | 11 | 100.0 | 6 | 100.0 | 21 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 5 | 83.3 | 11 | 100.0 | 5 | 100.0 | 5 | 100.0 | 10 | 100.0 | 9 | 100.0 | 5 | 100.0 | 5 | 100.0 | 11 | 100.0 | 6 | 100.0 | 21 | 100.0 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 1 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Monoresistant TB | 1 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Polyresistant | ||||||||||||||||||||||
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TB | ||||||||||||||||||||||
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 1 | 16.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Abbreviations: EMB=ethambutol; INH=isoniazid; PZA=pyrazinamide; RMP=rifampin. | ||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZA | 6 | 100.0 | 4 | 100.0 | 14 | 100.0 | 13 | 100.0 | 10 | 100.0 | 5 | 100.0 | 8 | 100.0 | 6 | 100.0 | 3 | 100.0 | 2 | 100.0 | 4 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 6 | 100.0 | 3 | 75.0 | 14 | 100.0 | 13 | 100.0 | 9 | 90.0 | 4 | 80.0 | 8 | 100.0 | 6 | 100.0 | 3 | 100.0 | 2 | 100.0 | 4 | 100.0 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Monoresistant TB | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Polyresistant | ||||||||||||||||||||||
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TB | ||||||||||||||||||||||
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 0 | 0.0 | 1 | 25.0 | 0 | 0.0 | 0 | 0.0 | 1 | 10.0 | 1 | 20.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Abbreviations: EMB=ethambutol; INH=isoniazid; PZA=pyrazinamide; RMP=rifampin. | ||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 11 - Footnote * | 7 | 100.0 | 8 | 100.0 | 5 | 100.0 | 3 | 100.0 | 7 | 100.0 | 8 | 100.0 | 7 | 100.0 | 9 | 100.0 | 9 | 100.0 | 8 | 100.0 | 7 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 6 | 85.7 | 8 | 100.0 | 5 | 100.0 | 3 | 100.0 | 7 | 100.0 | 5 | 62.5 | 7 | 100.0 | 9 | 100.0 | 8 | 88.9 | 5 | 62.5 | 7 | 100.0 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 1 | 11.1 | 3 | 37.5 | 0 | 0.0 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Monoresistant TB | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 25.0 | 0 | 0.0 | 0 | 0.0 | 1 | 11.1 | 3 | 37.5 | 0 | 0.0 |
| Polyresistant | ||||||||||||||||||||||
| INH & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 12.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TB | ||||||||||||||||||||||
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 1 | 14.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 37.5 | 0 | 0.0 | 0 | 0.0 | 1 | 11.1 | 3 | 37.5 | 0 | 0.0 |
Abbreviations: EMB=ethambutol; INH=isoniazid; PZA=pyrazinamide; RMP=rifampin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZA | 28 | 100.0 | 37 | 100.0 | 25 | 100.0 | 51 | 100.0 | 50 | 100.0 | 71 | 100.0 | 64 | 100.0 | 65 | 100.0 | 42 | 100.0 | 66 | 100.0 | 35 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 28 | 100.0 | 37 | 100.0 | 24 | 96.0 | 51 | 100.0 | 49 | 98.0 | 70 | 98.6 | 62 | 96.9 | 65 | 100.0 | 42 | 100.0 | 66 | 100.0 | 33 | 94.3 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 0 | 0.0 | 0 | 0.0 | 1 | 4.0 | 0 | 0.0 | 1 | 2.0 | 1 | 1.4 | 1 | 1.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.7 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Monoresistant TB | 0 | 0.0 | 0 | 0.0 | 1 | 4.0 | 0 | 0.0 | 1 | 2.0 | 1 | 1.4 | 2 | 3.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.7 |
| Polyresistant | ||||||||||||||||||||||
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TB | ||||||||||||||||||||||
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 0 | 0.0 | 0 | 0.0 | 1 | 4.0 | 0 | 0.0 | 1 | 2.0 | 1 | 1.4 | 2 | 3.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 5.7 |
| Abbreviations: EMB=ethambutol; INH=isoniazid; PZA=pyrazinamide; RMP=rifampin. | ||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 13 - Footnote * | 553 | 100.0 | 567 | 100.0 | 538 | 100.0 | 479 | 100.0 | 488 | 100.0 | 496 | 100.0 | 507 | 100.0 | 493 | 100.0 | 511 | 100.0 | 457 | 100.0 | 480 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 487 | 88.1 | 504 | 88.9 | 466 | 86.6 | 427 | 89.1 | 428 | 87.7 | 456 | 91.9 | 454 | 89.6 | 429 | 87.0 | 458 | 89.6 | 407 | 89.1 | 421 | 87.7 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 44 | 8.0 | 39 | 6.9 | 50 | 9.3 | 33 | 6.9 | 39 | 8.0 | 27 | 5.4 | 39 | 7.7 | 45 | 9.1 | 27 | 5.3 | 30 | 6.6 | 30 | 6.3 |
| RMP | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 2 | 0.4 | 1 | 0.2 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 7 | 1.3 | 9 | 1.6 | 9 | 1.7 | 6 | 1.3 | 4 | 0.8 | 2 | 0.4 | 6 | 1.2 | 10 | 2.0 | 8 | 1.6 | 11 | 2.4 | 15 | 3.1 |
| Subtotal - Monoresistant TB | 51 | 9.2 | 49 | 8.6 | 61 | 11.3 | 40 | 8.4 | 44 | 9.0 | 29 | 5.8 | 45 | 8.9 | 57 | 11.6 | 37 | 7.2 | 42 | 9.2 | 45 | 9.4 |
| Polyresistant | ||||||||||||||||||||||
| EMB & PZA | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & EMB | 2 | 0.4 | 3 | 0.7 | 1 | 0.2 | 2 | 0.4 | 3 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| INH & EMB & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & PZA | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 2 | 0.4 | 1 | 0.2 | 0 | 0.0 |
| Subtotal - Polyresistant TB | 2 | 0.4 | 3 | 0.5 | 4 | 0.7 | 4 | 0.8 | 5 | 1.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 3 | 0.6 | 1 | 0.2 | 0 | 0.0 |
| Multidrug-resistant TBTable 13 - Footnote † | ||||||||||||||||||||||
| INH & RMP | 0 | 0.0 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & AK & CM & RBT | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & CM & RBT | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & AK & CM & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & CM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & RBT | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 |
| INH & RMP & EMB & PZA & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & AK & KM & CM & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| INH & RMP & EMB & PZA & SM & ETH & RBT | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & OFL & MOX & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & OFL & RBT | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & RBT | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 3 | 0.6 |
| INH & RMP & EMB & RBT | 0 | 0.0 | 2 | 0.4 | 1 | 0.2 | 1 | 0.2 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & AK & CM | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & ETH & RBT | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & KM & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & OFL & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & OFL & MOX & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & OFL & RBT | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & RBT | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 |
| INH & RMP & ETH & RBT | 0 | 0.0 | 1 | 0.4 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 |
| INH & RMP & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & OFL & ETH & RBT & PAS | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & ETH & RBT | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & SM & AK & KM & CM & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| INH & RMP & PZA & SM & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| INH & RMP & PZA & SM & OFL & MOX & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 |
| INH & RMP & RBT | 3 | 0.5 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM & CM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 |
| INH & RMP & SM & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 |
| INH & RMP & SM & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 |
| INH & RMP & SM & KM & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM & OFL & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM & RBT | 2 | 0.4 | 0 | 0.0 | 0 | 0.0 | 3 | 0.6 | 1 | 0.2 | 1 | 0.2 | 1 | 0.2 | 1 | 0.2 | 2 | 0.4 | 1 | 0.2 | 5 | 1.0 |
| Subtotal - Multidrug-resistant TB | 13 | 2.4 | 10 | 1.8 | 7 | 1.3 | 7 | 1.5 | 11 | 2.3 | 10 | 2.0 | 7 | 1.4 | 5 | 1.0 | 13 | 2.5 | 6 | 1.3 | 14 | 2.9 |
| Extensively drug-resistant TBTable 13 - Footnote ‡ | ||||||||||||||||||||||
| INH & RMP & AK & CM & OFL & ETH & RBT | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & CM & OFL & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & KM & OFL & MOX & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & KM & OFL & MOX & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & KM & OFL & MOX & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 |
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 | 0 | 0.0 | 1 | 0.2 | 1 | 0.2 | 0 | 0.0 | 1 | 0.2 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 66 | 11.9 | 63 | 11.1 | 72 | 13.4 | 52 | 10.9 | 60 | 12.3 | 40 | 8.1 | 53 | 10.5 | 64 | 13.0 | 53 | 10.4 | 50 | 10.9 | 59 | 12.3 |
Abbreviations: AK=amikacin; CM=capreomycin; EMB=ethambutol; ETH=ethionamide; INH=isoniazid; KM=kanamycin; MOX=moxifloxacin; OFL=ofloxacin; PAS=para-aminosalicylic acid; PZA=pyrazinamide; RBT=rifabutin; RMP=rifampin; SM=streptomycin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZA | 1 | 100.0 | 0 | - | 0 | - | 0 | - | 1 | 100.0 | 1 | 100.0 | 3 | 100.0 | 0 | - | 0 | - | 2 | 100.0 | 2 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 1 | 100.0 | 0 | - | 0 | - | 0 | - | 1 | 100.0 | 1 | 100.0 | 2 | 66.7 | 0 | - | 0 | - | 2 | 100.0 | 2 | 100.0 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| RMP | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 1 | 33.3 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Monoresistant TB | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 1 | 33.3 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| Polyresistant | ||||||||||||||||||||||
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TB | ||||||||||||||||||||||
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 0 | 0.0 | 0 | - | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 | 1 | 33.3 | 0 | - | 0 | - | 0 | 0.0 | 0 | 0.0 |
| Abbreviations: EMB=ethambutol; INH=isoniazid; PZA=pyrazinamide; RMP=rifampin. | ||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 15 - Footnote * | 226 | 100.0 | 201 | 100.0 | 200 | 100.0 | 210 | 100.0 | 171 | 100.0 | 197 | 100.0 | 205 | 100.0 | 209 | 100.0 | 205 | 100.0 | 186 | 100.0 | 185 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 207 | 91.6 | 173 | 86.1 | 177 | 88.5 | 188 | 89.5 | 156 | 91.2 | 179 | 90.9 | 180 | 87.8 | 187 | 89.5 | 187 | 91.2 | 167 | 89.8 | 167 | 90.3 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 14 | 6.2 | 21 | 10.4 | 12 | 6.0 | 15 | 7.1 | 7 | 4.1 | 11 | 5.6 | 18 | 8.8 | 13 | 6.2 | 12 | 5.9 | 10 | 5.4 | 8 | 4.3 |
| RMP | 0 | 0.0 | 1 | 0.5 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 4 | 1.8 | 4 | 2.0 | 4 | 2.0 | 4 | 1.9 | 2 | 1.2 | 5 | 2.5 | 6 | 2.9 | 9 | 4.3 | 4 | 2.0 | 7 | 3.8 | 6 | 3.2 |
| Subtotal - Monoresistant TB | 18 | 8.0 | 26 | 12.9 | 17 | 8.5 | 19 | 9.0 | 9 | 5.3 | 16 | 8.1 | 24 | 11.7 | 22 | 10.5 | 16 | 7.8 | 17 | 9.1 | 15 | 8.1 |
| Polyresistant | ||||||||||||||||||||||
| INH & EMB | 0 | 0.0 | 0 | 0.0 | 3 | 1.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| INH & PZA | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 1 | 0.5 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 4 | 2.0 | 1 | 0.5 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| Multidrug-resistant TBTable 15 - Footnote † | ||||||||||||||||||||||
| INH & RMP & EMB & ETH & RBT | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| INH & RMP & EMB & PZA & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & ETH | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & KM & ETH & RBT & PAS | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & PZA & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & RBT | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & EMB & SM & RBT | 0 | 0.0 | 1 | 0.5 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 |
| INH & RMP & PZA & ETH & RBT | 1 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 |
| INH & RMP & PZA & SM & AK & KM & CM | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & PZA & SM & KM & CM & ETH | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.6 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 2 | 1.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 |
| Subtotal - Multidrug-resistant TB | 1 | 0.4 | 2 | 1.0 | 2 | 1.0 | 2 | 1.0 | 6 | 3.5 | 1 | 0.5 | 1 | 0.5 | 0 | 0.0 | 1 | 0.5 | 2 | 1.1 | 2 | 1.1 |
| Extensively drug-resistant TBTable 15 - Footnote ‡ | ||||||||||||||||||||||
| INH & RMP & EMB & PZA & SM & AK & KM & CM & OFL & MOX & ETH & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 0.5 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 19 | 8.4 | 28 | 13.9 | 23 | 11.5 | 22 | 10.5 | 15 | 8.8 | 18 | 9.1 | 25 | 12.2 | 22 | 10.5 | 18 | 8.8 | 19 | 10.2 | 18 | 9.7 |
Abbreviations: AK= amikacin; CM= capreomycin; ETH= ethionamide; KM= kanamycin; MOX=moxifloxacin; OFL= ofloxacin; PAS= para-aminosalicylic acid; RBT= rifabutin; RMP= rifampin; SM= streptomycin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZA | 74 | 100.0 | 58 | 100.0 | 60 | 100.0 | 81 | 100.0 | 77 | 100.0 | 54 | 100.0 | 66 | 100.0 | 72 | 100.0 | 71 | 100.0 | 63 | 100.0 | 50 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 72 | 97.3 | 57 | 98.3 | 59 | 98.3 | 79 | 97.5 | 72 | 93.5 | 51 | 94.4 | 62 | 93.9 | 68 | 94.4 | 69 | 97.2 | 60 | 95.2 | 49 | 98.0 |
| Monoresistant TB | ||||||||||||||||||||||
| INH | 2 | 2.7 | 1 | 1.7 | 1 | 1.7 | 2 | 2.5 | 3 | 3.9 | 2 | 3.7 | 4 | 6.1 | 1 | 1.4 | 1 | 1.4 | 0 | 0.0 | 0 | 0.0 |
| RMP | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.6 | 0 | 0.0 |
| EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 3 | 4.2 | 1 | 1.4 | 1 | 1.6 | 1 | 2.0 |
| Subtotal - Monoresistant TB | 2 | 2.7 | 1 | 1.7 | 1 | 1.7 | 2 | 2.5 | 3 | 3.9 | 2 | 3.7 | 4 | 6.1 | 4 | 5.6 | 2 | 2.8 | 2 | 3.2 | 1 | 2.0 |
| Polyresistant | ||||||||||||||||||||||
| INH & EMB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & PZA | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.6 | 0 | 0.0 |
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.6 | 0 | 0.0 |
| Multidrug-resistant TBTable 16 - Footnote * | ||||||||||||||||||||||
| INH & RMP & RBT | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| INH & RMP & SM | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 1 | 1.3 | 1 | 1.9 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 2 | 2.7 | 1 | 1.7 | 1 | 1.7 | 2 | 2.5 | 5 | 6.5 | 3 | 5.6 | 4 | 6.1 | 4 | 5.6 | 2 | 2.8 | 3 | 4.8 | 1 | 2.0 |
Abbreviations: EMB=ethambutol; INH=isoniazid; PZA=pyrazinamide; RBT = rifabutin; RMP=rifampin; SM=streptomycin.
|
||||||||||||||||||||||
| REPORTING YEAR | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2005 | 2006 | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | ||||||||||||
| n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | n | % | |
| Isolates tested for resistance to INH, RMP, EMB & PZATable 17 - Footnote * | 2 | 100.0 | 2 | 100.0 | 2 | 100.0 | 7 | 100.0 | 3 | 100.0 | 5 | 100.0 | 2 | 100.0 | 1 | 100.0 | 1 | 100.0 | 4 | 100.0 | 3 | 100.0 |
| Isolates susceptible to all first-line TB drugs | 2 | 100.0 | 2 | 100.0 | 2 | 100.0 | 7 | 100.0 | 3 | 100.0 | 5 | 100.0 | 2 | 100.0 | 1 | 100.0 | 1 | 100.0 | 4 | 100.0 | 3 | 100.0 |
| Monoresistant TB | ||||||||||||||||||||||
| Subtotal - Monoresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Polyresistant | ||||||||||||||||||||||
| Subtotal - Polyresistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Multidrug-resistant TB | ||||||||||||||||||||||
| Subtotal - Multidrug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Extensively drug-resistant TB | ||||||||||||||||||||||
| Subtotal - Extensively drug-resistant TB | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Total number of isolates resistant to one or more first line drugs | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
Abbreviations: EMB=ethambutol; INH=isoniazid; PZA=pyrazinamide; RMP=rifampin.
|
||||||||||||||||||||||
| CANADA | ORIGINATING PROVINCE/TERRITORY | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N.L. | P.E.I. | N.S. | N.B. | Que. | Ont. | Man. | Sask. | Alta. | B.C. | Y.T. | N.W.T | Nvt. | ||
| INH and RMP | 11 | 0 | 0 | 0 | 0 | 1 | 7 | 1 | 0 | 1 | 1 | 0 | 0 | 0 |
| INH and RMP and PZA | 3 | 0 | 0 | 0 | 1 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| INH and RMP and EMB | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| INH and RMP and EMB and PZA | 7 | 0 | 0 | 0 | 0 | 1 | 5 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Total number of Multidrug-resistant TBTable 18 - Footnote * | 22 | 0 | 0 | 0 | 1 | 2 | 14 | 1 | 0 | 2 | 2 | 0 | 0 | 0 |
| Extensively drug-resistant TBTable 18 - Footnote † | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
Abbreviations: Alta.= Alberta; B.C.= British Columbia; Man.= Manitoba; N.B.= New Brunswick; N.L.= Newfoundland and Labrador; N.S.= Nova Scotia; Nvt.= Nunavut; N.W.T.= Northwest Territories; Ont.= Ontario; P.E.I.= Prince Edward Island; Que.= Quebec; Sask.= Saskatchewan; Y.T.= Yukon.
|
||||||||||||||
| ORIGINATING PROVINCE/TERRITORY | TOTAL NUMBER OF REPORTED MTBC ISOLATES | RESISTANT TO ONE OR MORE FIRST-LINE DRUGS | MULTIDRUG-RESISTANT TBTable 19 - Footnote * | EXTENSIVELY DRUG-RESISTANT TBTable 19 - Footnote † | |||
|---|---|---|---|---|---|---|---|
| NUMBER | PERCENT (%) | NUMBER | PERCENT (%) | NUMBER | PERCENT (%) | ||
| Ont. | 5,569 | 632 | 11.3 | 103 | 1.8 | 5 | 0.1 |
| B.C. | 2,579 | 261 | 10.1 | 23 | 0.9 | 0 | 0.0 |
| Que. | 2,195 | 227 | 10.3 | 20 | 0.9 | 1 | 0.0 |
| Alta. | 1,570 | 154 | 9.8 | 24 | 1.5 | 0 | 0.0 |
| Man. | 1,254 | 73 | 5.8 | 5 | 0.4 | 1 | 0.1 |
| Sask. | 726 | 28 | 3.9 | 2 | 0.3 | 0 | 0.0 |
| Nvt. | 534 | 7 | 1.3 | 0 | 0.0 | 0 | 0.0 |
| N.L. | 94 | 1 | 1.1 | 0 | 0.0 | 0 | 0.0 |
| N.S. | 78 | 8 | 10.3 | 0 | 0.0 | 0 | 0.0 |
| N.W.T. | 75 | 3 | 4.0 | 0 | 0.0 | 0 | 0.0 |
| N.B. | 60 | 7 | 11.7 | 1 | 1.7 | 0 | 0.0 |
| Y.T. | 32 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| P.E.I. | 10 | 1 | 10.0 | 0 | 0.0 | 0 | 0.0 |
| CANADA | 14,776 | 1,402 | 9.5 | 178 | 1.2 | 7 | 0.0 |
Abbreviations: Alta.= Alberta; B.C.= British Columbia; Man.= Manitoba; N.B.= New Brunswick; N.L.= Newfoundland and Labrador; N.S.= Nova Scotia; Nvt.= Nunavut; N.W.T.= Northwest Territories; Ont.= Ontario; P.E.I.= Prince Edward Island; Que.= Quebec; Sask.= Saskatchewan; Y.T.= Yukon.
|
|||||||
| AGE GROUP AND SEX | ISOLATES REPORTED | RESISTANT TO ONE OR MORE FIRST-LINE DRUGS | MULTIDRUG RESISTANTTable 20 - Footnote * | EXTENSIVELY DRUG RESISTANTTable 20 - Footnote † | |||||
|---|---|---|---|---|---|---|---|---|---|
| NUMBER | PERCENT (%) | NUMBER | PERCENT (%) | NUMBER | PERCENT (%) | NUMBER | PERCENT (%) | ||
| < 1 | Male | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Female | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 3 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 1 to 4 | Male | 3 | 0.2 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Female | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Unknown | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 6 | 0.4 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 5 to 14 | Male | 10 | 0.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Female | 10 | 0.7 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 20 | 1.5 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| 15 to 24 | Male | 86 | 6.4 | 9 | 6.5 | 2 | 9.1 | 0 | 0.0 |
| Female | 82 | 6.1 | 12 | 8.6 | 1 | 4.5 | 0 | 0.0 | |
| Unknown | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 170 | 12.7 | 21 | 15.1 | 3 | 13.6 | 0 | 0.0 | |
| 25 to 34 | Male | 119 | 8.9 | 9 | 6.5 | 3 | 13.6 | 0 | 0.0 |
| Female | 122 | 9.1 | 17 | 12.2 | 5 | 22.7 | 0 | 0.0 | |
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 241 | 18.0 | 26 | 18.7 | 8 | 36.4 | 0 | 0.0 | |
| 35 to 44 | Male | 95 | 7.1 | 5 | 3.6 | 1 | 4.5 | 0 | 0.0 |
| Female | 99 | 7.4 | 12 | 8.6 | 1 | 4.5 | 0 | 0.0 | |
| Unknown | 2 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 196 | 14.6 | 17 | 12.2 | 2 | 9.1 | 0 | 0.0 | |
| 45 to 54 | Male | 107 | 8.0 | 13 | 9.4 | 4 | 18.2 | 0 | 0.0 |
| Female | 69 | 5.2 | 7 | 5.0 | 2 | 9.1 | 0 | 0.0 | |
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 176 | 13.1 | 20 | 14.4 | 6 | 27.3 | 0 | 0.0 | |
| 55 to 64 | Male | 94 | 7.0 | 10 | 7.2 | 0 | 0.0 | 0 | 0.0 |
| Female | 62 | 4.6 | 4 | 2.9 | 1 | 4.5 | 0 | 0.0 | |
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 156 | 11.7 | 14 | 10.1 | 1 | 4.5 | 0 | 0.0 | |
| 65 to 74 | Male | 82 | 6.1 | 8 | 5.8 | 1 | 4.5 | 0 | 0.0 |
| Female | 64 | 4.8 | 10 | 7.2 | 0 | 0.0 | 0 | 0.0 | |
| Unknown | 2 | 0.1 | 2 | 1.4 | 0 | 0.0 | 0 | 0.0 | |
| Total | 148 | 11.1 | 20 | 14.4 | 1 | 4.5 | 0 | 0.0 | |
| 75+ | Male | 135 | 10.1 | 9 | 6.5 | 1 | 4.5 | 0 | 0.0 |
| Female | 87 | 6.5 | 12 | 8.6 | 0 | 0.0 | 0 | 0.0 | |
| Unknown | 1 | 0.1 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 223 | 16.7 | 21 | 15.1 | 1 | 4.5 | 0 | 0.0 | |
| Unknown | Male | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 |
| Female | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Unknown | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | 0 | 0.0 | |
| Total | Male | 733 | 54.7 | 63 | 45.3 | 12 | 54.5 | 0 | 0.0 |
| Female | 598 | 44.7 | 74 | 53.2 | 10 | 45.5 | 0 | 0.0 | |
| Unknown | 8 | 0.6 | 2 | 1.4 | 0 | 0.0 | 0 | 0.0 | |
| TOTAL | 1,339 | 100.0 | 139 | 100.0 | 22 | 100.0 | 0 | 0.0 | |
|
|||||||||