Canadian Antimicrobial Resistance Surveillance System: 2025 key findings
Download in PDF format
(1.9 MB, 26 pages)
Organization: Public Health Agency of Canada
On this page
- Understanding antimicrobial resistance and use
- Trends in antimicrobial resistance and antimicrobial use
- Integration of One Health data and health equity
- Policy implications and research advances
- Conclusion
- Acknowledgements
- References
Understanding antimicrobial resistance and use
Antimicrobial resistance (AMR) remains one of the world’s most pressing public health threats. It occurs when microorganisms – including bacteria, fungi, viruses, and parasites – evolve to withstand medicines that were once effective. As AMR spreads, infections become harder – and sometimes impossible – to treat. This not only affects how we manage common infections but also puts patients at risk during essential medical procedures like surgery, chemotherapy, dialysis, and organ transplants, in the event they acquire an infection.
Globally, AMR was associated with nearly 4.7 million deaths in 2021, of which 1.14 million were estimated to be directly attributable to resistant infections Footnote 1, and this burden is projected to increase, reaching 1.91 million AMR-attributable deaths annually by 2050Footnote 1. The Council of Canadian Academies projects that if resistance to first-line antimicrobials among human infections rises from 26% in 2018 to 40% by 2050, 13,700 people in Canada could die each year due to AMR, with an estimated $388 billion loss to gross domestic product and $120 billion in healthcare costsFootnote 2. AMR’s economic and social impacts extend across sectors. By 2050, AMR could cost Canadian agriculture $11 billion annually, exceeding the economic shock of COVID-19Footnote 3.
Canada’s response to AMR is grounded in collaboration across sectors and jurisdictions to protect the health of humans, animals, and the environment. In 2023, the federal, provincial, and territorial (FPT) Ministers of Health and Agriculture released the Pan-Canadian Action Plan on Antimicrobial Resistance (PCAP)Footnote 4Footnote 5Footnote 6 – a 5-year action plan (2023-2027) that established FPT commitments to address AMR across sectors. Ten priority actions guide Canada’s multi-sectoral and multi-jurisdictional response across five pillars: research and innovation; surveillance; antimicrobial stewardship; infection prevention and control (IPC); and leadership.
The role of surveillance
Robust surveillance underpins Canada’s ability to detect, understand, and respond to emerging public health threats. The Canadian Antimicrobial Resistance Surveillance System (CARSS) serves as the national focal point for AMR and antimicrobial use (AMU) surveillance data. It consolidates and highlights evidence and trends from Public Health Agency of Canada (PHAC) surveillance and antimicrobial stewardship programs and partners across the human, animal, food, and environmental sectors, collectively monitoring AMR and AMU across Canada. CARSS provides relevant, timely, accurate and comprehensive information to stakeholders to support research, public health policy, and actions. Together, CARSS and its contributing programs provide reliable Canadian data to international networks, including the World Health Organization (WHO) Global Antimicrobial Resistance and Use Surveillance System (GLASS)Footnote 7, the WHO Gonococcal Antimicrobial Surveillance Program (GASP)Footnote 8, the Food and Agriculture Organisation of the United Nations (FAO) InFARMFootnote 9, and the World Organisation for Animal Health (WOAH) ANIMUSEFootnote 10. These collaborations support global monitoring and response efforts and align with PCAP and Office of the Auditor General (OAG) recommendationsFootnote 11.
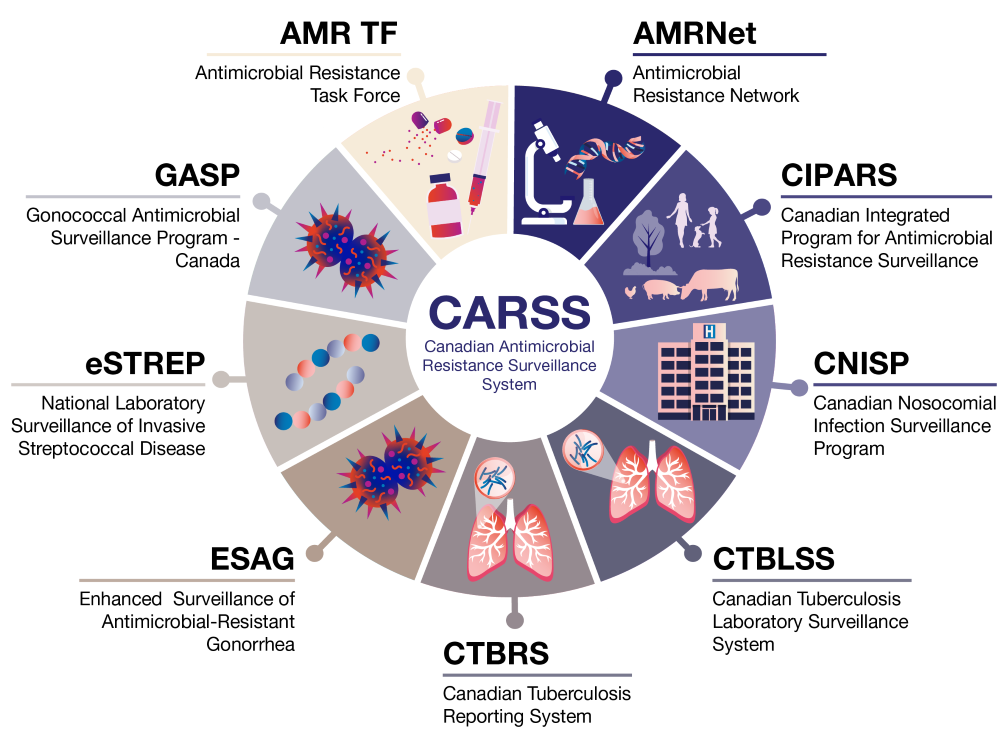
Figure 1: Text description
Image illustrating the structure of the Canadian Antimicrobial Resistance Surveillance System (CARSS), showing a central CARSS circle surrounded by nine connected surveillance programs: Antimicrobial Resistance Task Force (AMR TF), Antimicrobial Resistance Network (AMRNet), Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), Canadian Nosocomial Infection Surveillance Program (CNISP), Canadian Tuberculosis Laboratory Surveillance System (CTBLSS), Canadian Tuberculosis Reporting System (CTBRS), Enhanced Surveillance of Antimicrobial-Resistant Gonorrhea (ESAG), National Laboratory Surveillance of Invasive Streptococcal Disease (eSTREP), and Gonococcal Antimicrobial Surveillance Program - Canada (GASP), each represented by a labeled wedge with a small illustrative icon.
Surveillance also plays a critical role in identifying populations that are disproportionately affected by AMR, including First Nations, Inuit, and Métis communities, gay, bisexual and other men who have sex with men (GBMSM), unhoused populations, and individuals with chronic medical conditions. This supports the application of a health equity lens in AMR policy, ensuring that surveillance data inform inclusive and equitable public health actions.
Canada’s priority AMR pathogens
In 2025, PHAC published an update to national AMR threat prioritization, which was first conducted in 2015Footnote 12. This updated prioritization considered factors like disease trends, morbidity, and health equity using national data (2017–2022). Drug-resistant gram-negative bacteria and drug-resistant sexually transmitted infections (STIs) are emerging as top threats. Canada is the first country to formally include health equity as a criterion in this type of prioritization activityFootnote 13Footnote 14.
| Priority tier (2025) | Pathogen | Status/Change from 2015 priority list |
|---|---|---|
| High (Tier 1) |
Carbapenem-resistant Enterobacterales | No change in priority tier compared to 2015 |
| Drug-resistant Neisseria gonorrhoeae | Moved up in priority tier compared to 2015 (from Tier 2) | |
| Carbapenem-resistant Pseudomonas aeruginosa | Moved up in priority tier compared to 2015 (from Tier 3) | |
| Carbapenem-resistant Acinetobacter spp. | Moved up in priority tier compared to 2015 (from Tier 2) | |
| Candida auris Footnote * | New addition to 2025 priority list | |
| Extended-spectrum β-lactamase producing Enterobacterales | No change in priority tier compared to 2015 | |
| Medium-High (Tier 2) |
Drug-resistant Shigella spp. | Moved up in priority tier compared to 2015 (from Tier 4) |
| Mycoplasma genitalium | New addition to 2025 priority list | |
| Drug-resistant Streptococcus pneumoniae | Moved up in priority tier compared to 2015 (from Tier 3) | |
| Methicillin-resistant Staphylococcus aureus | Moved down in priority tier compared to 2015 (from Tier 1) | |
| Vancomycin-resistant Enterococcus spp. | No change in priority tier compared to 2015 | |
| Drug-resistant non-typhoidal Salmonella spp. | Moved up in priority tier compared to 2015 (from Tier 3) | |
| Medium-Low (Tier 3) |
Clindamycin-resistant invasive group A Streptococcus | Moved down in priority tier compared to 2015 (from Tier 2) |
| Drug-resistant influenza A | Moved up in priority tier compared to 2015 (from Tier 4) | |
| Drug-resistant human immunodeficiency virus | Moved up in priority tier compared to 2015 (from Tier 4) | |
| Drug-resistant group B Streptococcus | No change in priority tier compared to 2015 | |
| Clostridioides difficile | Moved down in priority tier compared to 2015 (from Tier 1) | |
| Multi-drug resistant Mycobacterium tuberculosis | Moved down in priority tier compared to 2015 (from Tier 2) | |
| Drug-resistant Aspergillus spp. | No change in priority tier compared to 2015 | |
| Drug-resistant typhoidal Salmonella spp. | Moved up in priority tier compared to 2015 (from Tier 4) | |
| Low (Tier 4) |
Drug-resistant Haemophilus influenzae | No change in priority tier compared to 2015 |
| Drug-resistant Helicobacter pylori | Moved down in priority tier compared to 2015 (from Tier 3) | |
| Drug-resistant Candida spp., excluding Candida auris | Moved down in priority tier compared to 2015 (from Tier 3) | |
| Drug-resistant Campylobacter spp. | Moved down in priority tier compared to 2015 (from Tier 2) | |
| Drug-resistant Bacteroides spp. | Moved down in priority tier compared to 2015 (from Tier 3) | |
| Ureaplasma spp. | New addition to 2025 priority list | |
| Drug-resistant Treponema pallidum | No change in priority tier compared to 2015 | |
| Drug-resistant Chlamydia trachomatis | No change in priority tier compared to 2015 | |
| Drug-resistant pulmonary non-tuberculosis Mycobacteria | No change in priority tier compared to 2015 | |
Trends in antimicrobial resistance and antimicrobial use
The 2025 CARSS report highlights important progress towards improving national AMR surveillance and antimicrobial stewardship across One Health sectors. This report also confirms that national AMR threats continue to increase, placing added pressure on healthcare systems and public health programs. These findings have significant implications for Canadian health policy, health equity, and domestic and international commitments related to AMR and AMU actions.
AMR is not evenly distributed across pathogens or populations. The CARSS report helps to identify AMR organisms that pose the greatest threat to Canada, highlights populations at greatest risk, and informs public health interventions to protect the health of all people who live in Canada.
| Pathogen | Status/TrendFootnote * |
|---|---|
| Tier 1: High-priority group | |
| Carbapenemase-producing Enterobacterales (CPE) infections | Trending up |
| Drug-resistant Neisseria gonorrhoeae infections | Trending up |
| Carbapenemase-producing Acinetobacter spp. (CPA) infections | Low/stable |
| Candida auris (C. auris) Footnote ** | New Low/trending up |
| Extended-spectrum β-lactamase (ESBL)-producing Enterobacterales infections | Trending up |
| Tier 2: Medium-high priority group | |
| Drug-resistant Shigella spp. infections | New emergingFootnote *** |
| Mycoplasma genitalium infections | New emergingFootnote *** |
| Drug-resistant Streptococcus pneumoniae (Invasive Pneumococcal Disease (IPD)) infections | Trending up |
| Methicillin-resistant Staphylococcus aureus (MRSA) (bloodstream infections) | Stable |
| Vancomycin-resistant Enterococcus spp. (VRE) (bloodstream infections) | Trending up |
| Drug-resistant non-typhoidal Salmonella infections | Trending up |
| Tier 3: Medium-low priority group | |
| Clindamycin and/or macrolide-resistant invasive group A Streptococcus (iGAS) infections | Trending up |
| Clostridioides difficile infections (CDI)Footnote **** | Stable |
| Multi-drug resistant Mycobacterium tuberculosis (TB) infections | Low/stable |
| Drug-resistant typhoidal Salmonella infections | High/stable |
| Tier 4: Low priority group | |
| Drug-resistant Campylobacter infections | High/stable |
Key messages: Antimicrobial resistance
- Carbapenemase-producing Enterobacterales (CPE) remain a high‑priority and escalating threat in Canada. Though previously attributed to travel and healthcare exposure abroad, more recent genomic and phenotypic surveillance shows increasing evidence of transmission likely occurring within Canadian healthcare facilities. Hence, increased efforts focused on screening, the adoption of rapid laboratory algorithms for carbapenemase detection, enhanced inter-facility communication with standardized case definitions are important. In addition, increased research and access to therapeutic agents, both antimicrobials and alternatives (e.g. phage therapy) should be prioritized.
- Candida auris is an emerging multidrug-resistant fungal pathogen that is posing both significant IPC and laboratory detection challenges. It can also be resistant to multiple classes of antifungal medications, making treatment quite difficult. Though case counts are high internationally (e.g. USA), thus far, numbers in Canada remain low. However, increased vigilance is important with regards to this emerging pathogen. Early detection, strict IPC measures including environmental cleaning and cohorting, enhanced laboratory capacity for species identification and antifungal susceptibility testing, coordinated national surveillance, and reporting and outbreak response are all essential to limit the spread.
- Vancomycin-resistant Enterococcus (VRE) bloodstream infections (BSIs) have been steadily on the rise, and the majority of cases are healthcare-associated. In some jurisdictions, the discontinuation of routine VRE screening has coincided with rising rates of VRE-BSIs, which suggests a potential gap in IPC practices and underscores the importance of continued surveillance and targeted mitigation strategies.
- Although healthcare-associated (HA) methicillin-resistant Staphylococcus aureus (MRSA) bloodstream infections (BSI) and Clostridioides difficile infections (CDI) show stable incidence over the last five years, both remain among the top antimicrobial-resistant organisms in hospitals and continue to place significant burden on IPC resources. Stable incidence should not be interpreted as low risk; it likely reflects ongoing, effective IPC practices within healthcare settings. In addition, increased research and access to therapeutic agents, both antimicrobials and alternatives (e.g. phage therapy, potential vaccine development, improved access to fecal transplant in the case of CDI) should be prioritized.
- Considering methicillin-resistant Staphylococcus aureus (MRSA) infections that occur outside the healthcare setting, certain vulnerable populations are at higher risk. This can include children, athletes, incarcerated individuals, GBMSM, people who inject drugs (PWID), elderly individuals with comorbidities, First Nations, Inuit and Métis communities, and unhoused individuals. IPC and antimicrobial stewardship play key roles, and surveillance efforts should increase focus on monitoring community transmission.
- Increased cases of drug-resistant Neisseria gonorrhoeae are being detected, including those resistant to first-line therapies. Of note, the first extensively drug-resistant case was identified, which prompted updates to national treatment guidelines. Furthermore, the first documented treatment failure in Canada was reported, which initiated an International Health Regulation notice. Treatment guidelines now emphasize updated empiric therapy, test-of-cure, and enhanced surveillance. Expanding enhanced surveillance (including molecular resistance markers), targeted antimicrobial stewardship in sexual health programs, ensuring comprehensive partner notification, and maintaining accessible culture facilities could help contain spread and preserve remaining treatment options.
- Multidrug-resistant (MDR) Shigella outbreaks and rising cases of Mycoplasma genitalium, including dual macrolide- and quinolone-resistant strains, are occurring among GBMSM, unhoused populations, PWID, and are often linked with sexual networks. These populations may experience delayed diagnosis, interrupted treatment, and challenges with partner management, which can increase transmission and adverse health outcomes. Surveillance and interventions should integrate a health equity lens, including low-barrier clinics, outreach testing, culturally appropriate partner services, expedited partner therapy where permitted, and point-of-care testing for timely treatment and follow-up.
- Increased number of invasive pneumococcal disease (IPD) cases identified as multi-drug resistant (MDR), of which the majority are vaccine-preventable serotypes, particularly in adults. Young children (age <5) and older adults (age >65) are the most impacted, highlighting the ongoing need for strengthened vaccination programs and targeted communication to high-risk groups.
- Invasive group A Streptococcus (iGAS) infections continue to rise in Canada. Increased rates have been particularly noted among populations experiencing homelessness, PWID, and First Nation, Inuit, and Métis communities. While there is currently no documented resistance to first-line therapy (penicillin), resistance to macrolides and clindamycin is increasing—an important consideration given their use as alternative or adjunctive treatments. These trends underscore the importance of promoting accurate assessment and possible testing for true penicillin allergy, as unnecessary avoidance of penicillin may contribute to greater reliance on these second-line therapies.
- Multi-drug resistant (MDR) Tuberculosis (TB) rates remain low and stable. However, the majority of resistant cases are identified among individuals born outside of Canada. Continued efforts to enhance awareness, improve access to information, and support early detection are needed among both healthcare providers and affected communities. In addition, ongoing challenges related to drug shortages and access, particularly for MDR-TB treatment, highlight the need for continued focus and coordinated efforts in this area.
- Typhoidal Salmonella, generally associated with travel and often causing invasive disease, continues to exhibit extremely high resistance to ciprofloxacin, a recommended treatment option. Alternative antimicrobials should be considered.
Key messages: Antimicrobial use (consumption)
This section provides an integrated view of how antimicrobials are used across human, animal, and food production sectors in Canada. These findings underscore the need to sustain and expand antimicrobial stewardship initiatives, optimize prescribing practices, and strengthen One Health data integration to guide coordinated, evidence-based actions across sectors.
| Sector | 2020-2024 AMU trend |
|---|---|
| Hospital | Trending up |
| Community | Trending up |
| Veterinary antimicrobial salesFootnote * | Plateaued / Stable |
| Sentinel terrestrial farms: Broiler chicken, turkey, grower-finisher pigs, and beef feedlotFootnote * | Trending down |
| Sentinel terrestrial farms – dairy cattleFootnote ** | Trending up |
| Aquaculture operationsFootnote 15Footnote ** | Trending down |
| International context: Human AMU | Canada ranks 23rd lowest out of 65 comparable countriesFootnote 7 and lower than the Organisation for Economic Co-operation and Development (OECD)Footnote 16 average in 2024 |
| International context: Veterinary antimicrobial sales | Canada ranked 4th highest among 31 European network countries in 2022Footnote 17 |
- Antimicrobial use in humans has returned to pre-COVID levels after notable declines in 2019-2020, reflecting the resumption of routine healthcare activity and prescribing patterns in both hospital and community sectors.
- Antimicrobial use in the community sector: Canada is exceeding antimicrobial prescribing guidelines recommended by the WHO AWaRe program, reporting that more than 70% of prescriptions fall within the AccessFootnote 18Footnote 19 category. This suggests that the majority of AMU in the community sector consists of first- or second-line treatment options for common infections, generally with a narrower spectrum of activity. However, this alone does not necessarily indicate judicious use, and efforts are still required to identify and minimize suboptimal prescribing practices.
- Antimicrobial use in the hospital sector: Over 1 in 4 prescriptions remain inappropriate or suboptimal, underscoring ongoing opportunities for antimicrobial stewardship initiatives.
- The quantity of medically important antimicrobials sold for use in animals has plateaued between 2019 and 2023.
- The quantity of antimicrobials consumed by broiler chickens, turkeys, grower-finisher pigs, and beef feedlot decreased, but increased on dairy farms, which may be partly due to improved reporting.
- The quantity of antimicrobials consumed by aquaculture declined between 2019 and 2022.
Integration of One Health data and health equity
CARSS continues to highlight that AMR is a One Health issue, with many high priority pathogens (e.g., CPE, ESBLs, MDR S. pneumoniae) crossing human, animal, and environmental sectors.
Integrated surveillance along the food chain showcases emerging concerns for several types of AMR pathogens. For example:
- ESBL-producing non-typhoidal Salmonella trends have increased across humans, animals, and food sources;
- Ciprofloxacin resistance in Campylobacter has increased in animal and food isolates; in humans, the rate of resistance has remained relatively stable, though the overall proportion of resistance was high;
- Nalidixic acid resistance in Salmonella Enteritidis from poultry continues to increase - notable, given this serovar was once fully susceptible to all tested antimicrobials.
Expanded surveillance now monitors AMR in select bacteria from water, farm environments, feed ingredients, and mixed feeds intended for animals. Resistance to ciprofloxacin has been detected in isolates from surface water and in isolates derived from environments with sick animals. Resistant non-typhoidal Salmonella serovars capable of causing human illness have been detected in feed ingredients and mixed feeds. These findings underscore the interconnectedness of AMR transmission across sectors and highlight the importance of maintaining integrated surveillance to detect emerging risks along the food chain.
The expansion of environmental surveillance marks a major milestone for Canada. The Environmental Surveillance Strategic Framework (ESSF) provides the first federal roadmap for monitoring resistance in water, soil, and wildlife, while wastewater pilot projects enable community-level signal detection. Integrated with human and animal surveillance, these initiatives support a comprehensive view of the development and transmission of AMR, and contribute to a sustainable One Health approach.
Prioritizing health equity is critical. AMR does not affect all communities equally. Targeted surveillance and interventions for high-risk populations, including First Nation, Inuit, and Métis communities, GBMSM, unhoused populations, and those with limited healthcare access, help reduce disproportionate burden and prevent onward transmission.
Policy implications and research advances
Canada has strengthened its policy tools and research base:
- 2025 AMR Pathogen Prioritization considered health equity as a criterion for the first time, ensuring AMR pathogens disproportionately affecting marginalized groups are included in policy planningFootnote 13Footnote 14.
- Genomics Research and Development Initiative (GRDI) on AMR–One Health (2022–2027), a multi-departmental initiative, continued to build on efforts that started in 2013. To date, GRDI has produced 88 peer-reviewed publications, 140 public communication activities, and developed novel genomic approaches to advance the understanding of AMR transmission between One Health sectors – all of which contribute to AMR policy and effective One Health actionFootnote 20Footnote 21.
- Antimicrobial stewardship and regulatory efforts in the agriculture sector successfully reduced the use of Veterinary Category I drugs to less than 2%, and aligned national prescribing with OECD best practices.
Policy recommendations
Findings from the 2025 CARSS report reinforce the need for sustained and coordinated action.
Reinforce antimicrobial stewardship
- Strengthen hospital antimicrobial stewardship programs, increase education and awareness among the general public, antimicrobial prescribers and producers, utilize audit/feedback tools, and increase access to evidence-based guidelines.
Maintain IPC and vaccination efforts
- Strengthen IPC measures across healthcare and community settings, continue to promote recommended immunizations, and implement directed strategies to prevent transmission of priority antimicrobial-resistant organisms.
Advance One Health integration
- Fully integrate environmental data and maintain collaboration between human, animal, and food surveillance programs to understand AMR transmission pathways between One Health sectors.
Address health equity
- Target surveillance and interventions toward high-risk populations experiencing higher AMR burden.
Sustain investment in innovation and research
- Support genomic surveillance, rapid diagnostics, as well as novel therapies and vaccines to help reduce AMR burden and enhance Canada’s global leadership in AMR response.
Conclusion
Canada has made measurable improvements to national AMR and AMU surveillance. However, AMR is dynamic and the Canadian AMR landscape continues to evolve. Of concern include the following:
- Escalating rates of gram-negative organisms, including carbapenemase‑producing organisms
- Increasing drug-resistant STIs, including gonorrhea
- The emergence of MDR C. auris, Mycoplasma genitalium, and drug-resistant Shigella infections
- Rising rates of MDR S. pneumoniae
While some antimicrobial resistant organisms (such as healthcare-associated MRSA and C. difficile) remain stable, they continue to impose a heavy burden on healthcare systems.
In parallel, AMU continues to increase in both human and some animal sectors. Given that AMU is a primary driver of AMR, ongoing and integrated surveillance of both AMR and AMU in Canada is essential. Core public health principles remain critical to reducing the overall burden of AMR and preserving the effectiveness of existing antimicrobials. This includes:
- Strong antimicrobial stewardship,
- Effective infection prevention and control practices, and
- Sustained immunization efforts.
Continued investment, collaboration, and innovation across sectors will be required to safeguard treatment options, protect vulnerable populations, and sustain Canada’s leadership in the global response to AMR.
Acknowledgements
The 2025 CARSS report reflects the collaborative effort of national AMR and AMU surveillance and antimicrobial stewardship communities, including the following PHAC programs and their partners:
- Antimicrobial Resistance Task Force (AMR TF), Stewardship Initiatives Division and Surveillance Integration and Transformation Division
- Antimicrobial Resistance Network (AMRNet)
- Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS)
- Canadian Nosocomial Infection Surveillance Program (CNISP)
- Canadian Tuberculosis Laboratory Surveillance System (CTBLSS) and Canadian Tuberculosis Reporting System (CTBRS)
- Enhanced Surveillance of Antimicrobial-Resistant Gonorrhea (ESAG) and Gonococcal Antimicrobial Surveillance Program of Canada (GASP-Canada)
- National Laboratory Surveillance of Invasive Streptococcal Disease (eSTREP)
- National Microbiology Laboratory (NML)
References
- Footnote 1
-
Naghavi M, Vollset SE, Ikuta KS, et al. Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050. The Lancet 2024;404:1199–226 doi:10.1016/S0140-6736(24)01867-1. Retrieved from https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(24)01867-1/fulltext
- Footnote 2
-
Council of Canadian Academies. When Antibiotics Fail 2019. Retrieved from https://cca-reports.ca/wp-content/uploads/2023/05/Updated-AMR-report_EN.pdf
- Footnote 3
-
World Organisation for Animal Health. The State of the World's Animal Health 2025 2025:1–120 doi:rg/10.20506/woah.3586. Retrieved from https://www.woah.org/app/uploads/2025/05/the-state-of-the-worlds-animal-health-2025.pdf
- Footnote 4
-
Public Health Agency of Canada. Pan-Canadian Action Plan on Antimicrobial Resistance. Public Health Agency of Canada 2023:1–39. Retrieved from /content/canadasite/en/public-health/services/publications/drugs-health-products/pan-canadian-action-plan-antimicrobial-resistance.html
- Footnote 5
-
Public Health Agency of Canada. Building Momentum: Activities Underway to Address Antimicrobial Resistance in Canada - Compendium to the Pan-Canadian Action Plan on Antimicrobial Resistance. Public Health Agency of Canada 2023:1–26. Retrieved from /content/canadasite/content/dam/phac-aspc/documents/services/publications/drugs-health-products/pan-canadian-action-plan-antimicrobial-resistance/building-momentum-activities-underway-address-antimicrobial-resistance-canada.pdf
- Footnote 6
-
Public Health Agency of Canada. Pan-Canadian Action Plan on Antimicrobial Resistance: Year 1 Progress Report (June 2023 to May 2024). Public Health Agency of Canada 2023:1–28. Retrieved from /content/canadasite/content/dam/phac-aspc/documents/services/publications/drugs-health-products/pan-canadian-action-plan-antimicrobial-resistance-year-1-progress-report-2023-2024/pan-canadian-action-plan-antimicrobial-resistance-year-1-progress-report-2023-2024.pdf
- Footnote 7
-
World Health Organization (WHO). GLASS dashboard. Retrieved from https://worldhealthorg.shinyapps.io/glass-dashboard/_w_d0eefc1c7910477e9588360b22a487be/#!/home
- Footnote 8
-
World Health Organization (WHO). The Gonococcal Antimicrobial Surveillance Programme (GASP). Retrieved from https://www.who.int/initiatives/gonococcal-antimicrobial-surveillance-programme
- Footnote 9
-
Food and Agriculture Organisation of the United Nations (FAO).InFARM: The international FAO antimicrobial resistance monitoring system. Retrieved from https://infarm.fao.org/
- Footnote 10
-
World Organisation for Animal Health (WOAH).ANIMUSE. Retrieved from https://amu.woah.org/amu-system-portal/home
- Footnote 11
-
Office of the Auditor General of Canada. 2023 Reports 5 to 9 of the Auditor General of Canada to the Parliament of Canada—Gaps remain in Canadian surveillance data and access to antimicrobial drugs. Retrieved from https://www.oag-bvg.gc.ca/internet/English/mr_20231019_e_44353.html
- Footnote 12
-
Garner MJ, Carson C, Lingohr EJ, et al. An Assessment of Antimicrobial Resistant Disease Threats in Canada. PLOS ONE 2015;10:e0125155 doi:10.1371/journal.pone.0125155. Retrieved from https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0125155
- Footnote 13
-
Public Health Agency of Canada (PHAC). (2025). Canada's priority antimicrobial-resistant pathogens. Retrieved from /content/canadasite/en/public-health/services/antimicrobial-resistance/health-professionals/priority-pathogens.html
- Footnote 14
-
Abdesselam K, Ngendabanka R, Muchaal PK, et al. Canada’s 2025 AMR priority pathogens: Evidence-based ranking and public health implications. PLOS ONE 2025;20:e0330128 doi:10.1371/journal.pone.0330128. Retrieved from https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0330128
- Footnote 15
-
Fisheries and Oceans Canada. National Aquaculture Public Reporting Data. Retrieved from https://open.canada.ca/data/en/dataset/288b6dc4-16dc-43cc-80a4-2a45b1f93383
- Footnote 16
-
Organisation for Economic Co-operation and Development (OECD). Prescribing in primary care. Retrieved from https://data-explorer.oecd.org/vis?tm=primary%20care&pg=0&snb=44&vw=ov&df[ds]=dsDisseminateFinalDMZ&df[id]=DSD_HCQO%40DF_PC&df[ag]=OECD.ELS.HD&df[vs]=1.1&dq=.A...._T.OBS&pd=2015%2C&to[TIME_PERIOD]=false
- Footnote 17
-
European Medicines Agency. Sales of veterinary antimicrobial agents in 31 European countries in 2022: Trends from 2010 to 2022 2023 doi:10.2809/766171. Retrieved from https://www.ema.europa.eu/en/documents/report/sales-veterinary-antimicrobial-agents-31-european-countries-2022-trends-2010-2022-thirteenth-esvac-report_en.pdf
- Footnote 18
-
World Health Organization (WHO). WHO Antibiotics Portal. Retrieved from https://aware.essentialmeds.org/groups
- Footnote 19
-
World Health Organization (WHO). The WHO AWaRe (Access, Watch, Reserve) antibiotic book. Geneva 2022.
- Footnote 20
-
National Research Council (NRC). (2021). Antimicrobial resistance (the AMR project) - Genomics R&D Initiative (GRDI). Retrieved from https://grdi.canada.ca/en/projects/antimicrobial-resistance-amr-project
- Footnote 21
-
National Research Council (NRC). (2025). Antimicrobial Resistance – One Health (AMR-OH project) - Genomics R&D Initiative (GRDI). Retrieved from https://grdi.canada.ca/en/projects/antimicrobial-resistance-one-health-amr-oh-project
