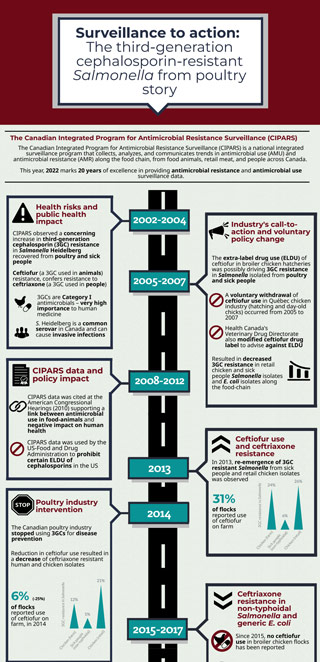
Surveillance to action: the third-generation cephalosporin-resistant Salmonella from poultry story
Download the alternative format
(PDF format, 324 Kb, 1 page)
- Organization: Public Health Agency of Canada
- Date published: 2022-12-12
- Pub.: 220511
- Cat.: HP40-325/3-2022E-PDF
- ISBN: 978-0-660-45984-4
The Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS)
The Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) is a national integrated surveillance program that collects, analyzes, and communicates trends in antimicrobial use (AMU) and antimicrobial resistance (AMR) along the food chain, from food animals, retail meat, and people across Canada.
This year, 2022 marks 20 years of excellence in providing antimicrobial resistance and antimicrobial use surveillance data.
2002 to 2004
Health risks and public health impact
- CIPARS observed a concerning increase in third-generation cephalosporin (3GC) resistance in Salmonella Heidelberg recovered from poultry and sick people
- Ceftiofur (a 3GC used in animals) resistance, confers resistance to ceftriaxone (a 3GC used in people)
- 3GCs are Category I antimicrobials – very high importance to human medicine
- S. Heidelberg is a common serovar in Canada and can cause invasive infections
2005 to 2007
Industry's call-to-action and voluntary policy change
- The extra-label drug use (ELDU) of ceftiofur in broiler chicken hatcheries was possibly driving 3GC resistance in Salmonella isolated from poultry and sick people
- A voluntary withdrawal of ceftiofur use in Québec chicken industry (hatching and day-old chicks) occurred from 2005 to 2007
- Health Canada's Veterinary Drug Directorate also modified ceftiofur drug label to advise against ELDU
- Resulted in decreased 3GC resistance in retail chicken and sick people Salmonella isolates and E. coli isolates along the food-chain
2008 to 2012
CIPARS data and policy impact
- CIPARS data was cited at the American Congressional Hearings (2010) supporting a link between antimicrobial use in food-animals and negative impact on human health
- CIPARS data was used by the US-Food and Drug Administration to prohibit certain ELDU of cephalosporins in the US
2013
Ceftiofur use and ceftriaxone resistance
- In 2013, re-emergence of 3GC resistant Salmonella from sick people and retail chicken isolates was observed
- 31% of flocks reported use of ceftiofur on farm

Figure 1 - Text Description
| Source | 3GC resistance in Salmonella in 2013 |
|---|---|
| Chicken (farm) | 24% |
| Sick people (non-typhoidal) | 6% |
| Chicken (retail) | 26% |
2014
Poultry industry intervention
- The Canadian poultry industry stopped using 3GCs for disease prevention
- Reduction in ceftiofur use resulted in a decrease of ceftriaxone resistant human and chicken isolates
- 6% (-25%) of flocks reported use of ceftiofur on farm, in 2014
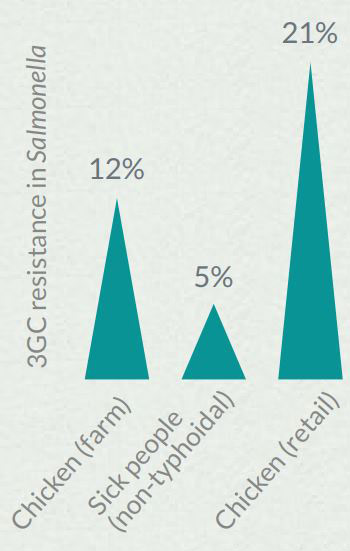
Figure 2 - Text Description
| Source | 3GC resistance in Salmonella in 2014 |
|---|---|
| Chicken (farm) | 12% |
| Sick people (non-typhoidal) | 5% |
| Chicken (retail) | 21% |
2015 to 2017
Ceftriaxone resistance in non-typhoidal Salmonella and generic E. coli
- Since 2015, no ceftiofur use in broiler chicken flocks has been reported
- Ceftriaxone resistance decreased in E. coli and Salmonella isolates from chickens and chicken meat
- A continued decrease in ceftriaxone resistant chicken and chicken meat and human isolates were linked to the ceftiofur ban
2018
A shifting story: 3GC resistance and serovars
- In 2018, the continued decrease in ceftriaxone resistance in Salmonella serovars in sick people was observed
- But… the majority of these resistant isolates were serovars Infantis, followed by Heidelberg
- In chickens and chicken meat, most ceftriaxone-resistant Salmonella isolates were S. Kentucky, followed by S. Heidelberg and S. Infantis
- There were some increases in ceftriaxone resistance in 2018 compared to 2017, most notable in Salmonella isolated from on farm chickens
2019 and ongoing
Continued surveillance
- Overall, there has been a desired decrease in 3GC resistance trend; low level ceftriaxone resistance remains
- However, while not as frequent compared to other serovars occurring in people, S. Kentucky
is likely acting as a reservoir for these resistant genes - Use of other antimicrobials may also maintain resistance due to co-selection of genes
- The poultry industry has committed to removing the preventive use of other types of antimicrobials that are important to human medicine
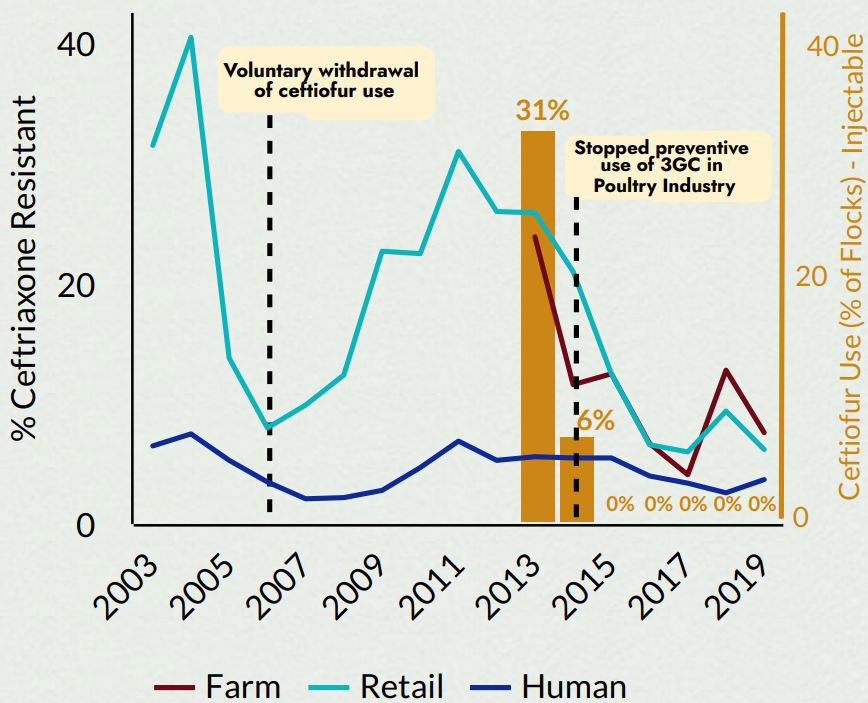
Figure 3 - Text Description
| Period | Farm (%) | Retail (%) | Human (%) | Ceftiofur Use (% of flocks) - injectable |
|---|---|---|---|---|
| 2003 | n/a | 31.5 | 6.5 | n/a |
| 2004 | n/a | 40.5 | 7.5 | n/a |
| 2005 | n/a | 13.8 | 5.3 | n/a |
| 2006 | n/a | 8 | 3.5 | n/a |
| 2007 | n/a | 9.9 | 2.1 | n/a |
| 2008 | n/a | 12.4 | 2.2 | n/a |
| 2009 | n/a | 22.7 | 2.8 | n/a |
| 2010 | n/a | 22.5 | 4.7 | n/a |
| 2011 | n/a | 31 | 6.9 | n/a |
| 2012 | n/a | 26 | 5.3 | n/a |
| 2013 | 23.9 | 25.9 | 5.6 | 31 |
| 2014 | 11.6 | 21 | 5.5 | 6 |
| 2015 | 12.5 | 12.5 | 5.5 | 0 |
| 2016 | 6.7 | 6.6 | 4 | 0 |
| 2017 | 4.1 | 6 | 3.4 | 0 |
| 2018 | 12.8 | 9.4 | 2.6 | 0 |
| 2019 | 7.6 | 6.2 | 3.7 | 0 |
2005 to 2007: Voluntary withdrawal of ceftiofur use 2015: Stopped preventive use of 3GC in Poultry Industry | ||||
Key points
- CIPARS surveillance data identified a concerning trend, which led to government action (label warnings), a voluntary industry intervention and actions in another country
- The reduction in ceftiofur use and associated decrease in ceftriaxone resistance compared to pre-2014 data in chickens and humans is a good example of a successful intervention to limit antimicrobial resistance
- Low level ceftriaxone resistance remains despite reduction in antimicrobial use, possibly caused by some bacteria and serovars maintaining resistance genes
- A change in antimicrobial use, even with those of lesser importance to human medicine, may result in a shift in resistance trends