Transfusion Transmitted Injuries Surveillance System (TTISS), 2018-2019
Download the alternative format
(PDF format, 241 Kb, 1 page)
- Organization:Public Health Agency of Canada
- Date published: 2023-02-16
TTISS project
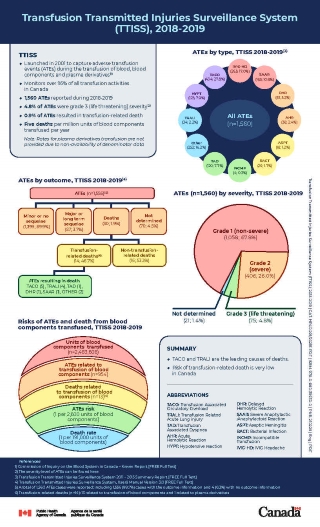
The Transfusion Transmitted Injuries Surveillance System (TTISS) captures data on adverse transfusion events (ATEs) during blood transfusions in all Canadian provinces and territories. The objective of the TTISS is to identify and estimate the risk of ATEs following transfusion. An ATE is an undesirable and unintended occurrence during or after the administration of blood, blood components (such as red blood cells, platelets, plasma, cryoprecipitates), or blood products (plasma derivatives such as immunoglobulin, albumin, coagulation factors).
The project was launched in 2001.Footnote 1 The TTISS now monitors over 95% of all transfusion activities across Canada.
Surveillance data summary
See below for a summary of data on ATEs captured by TTISS.
- 1,560 ATEs were reported during 2018 to 2019
- 4.8% of ATEs were considered life-threateningFootnote 2
- 0.9% of ATEs resulted in transfusion-related death
- TACO and TRALI are the leading causes of deaths
- The rate of death is five per million units of blood components transfused
Note: Rates for plasma derivative transfusions are not provided because the total number of units of plasma derivatives transfused is not collected in the provinces or territories.
ATEs by type
Please find below a breakdown of the counts and proportions of reported cases according to ATE types.
- Transfusion-associated circulatory overload (TACO): 434; 27.8%
- Immunoglobulin-induced headache (IVIG-HD): 265; 17.0%
- Other: 252; 16.2%
- Severe anaphylactic/anaphylactoid reaction (SAAR): 165; 10.6%
- Hypotensive reaction (HYPT): 123; 7.9%
- Transfusion associated dyspnea (TAD): 120; 7.7%
- Delayed haemolytic reaction (DHR): 81; 5.2%
- Acute haemolytic reaction (AHR): 38; 2.4%
- Transfusion-related acute lung injury (TRALI): 34; 2.2%
- Bacterial infection (BACT): 26; 1.7%
- Aseptic meningitis (ASPT): 18; 1.2%
- Incompatible transfusion (INCMP): 4; 0.3%
ATEs by outcome
Each reported adverse reaction is categorized by its resulting outcome. The outcome categories assesses whether the patient sustained any physiological or physical consequence (damage or impairment of a body function) following the development of the reaction.
| Outcome | Number of harms (n) | Percentage (%) |
|---|---|---|
| Minor or no sequelae (that is, no complications or conditions that arose from the ATE) | 1,399 | 89.9% |
| Major or long-term sequelae | 57 | 3.7% |
| Transfusion-related deathsTable 1 Footnote 3 | 14 | 0.9% |
| Non-transfusion-related deathsTable 1 Footnote 4 | 16 | 1.0% |
| Undetermined | 70 | 4.5% |
| ATEs | 1,556Table 1 Footnote 5 | 100% |
ATEs resulting in transfusion-related deaths
- TACO – 5
- TRALI – 4
- OTHER - 2
- TAD – 1
- DHR – 1
- SAAR – 1
- Total: 14
ATEs by severity
A non-severe ATE does not incur permanent damage or impairment of a bodily function. A patient with a severe ATE, on the other hand, requires hospitalization or a prolongation of hospitalization that is directly attributable to the reaction. A severe ATE can also cause persistent or significant incapacity or require medical intervention to preclude such damage.
| Severity | Number of occurrences (n) | Percentage (%) |
|---|---|---|
| Non-severe | 1,058 | 67.8% |
| Severe | 406 | 26.0% |
| Life-threatening | 75 | 4.8% |
| Undetermined | 21 | 1.4% |
Risks of ATEs from blood components transfused
- 2,493,606 units of blood components were transfused
- 954 ATEs were related to transfusion of blood components
- 13 deaths were related to transfusion of blood components
- The risk of an ATE is 1 per 2,600 units of blood components
- The death rate is 1 per 191,000 units of blood components