Centre for Biosecurity Annual Report 2016-2017

Download the entire report
(PDF format, 1.12 Mb, 21 pages)
Organization: Public Health Agency of Canada
Type: Report
Published: November 2017
Cat.: HP42-1E-PDF
ISSN: 2561-3642
Pub.: 170299
Related topics
A Year of Transition
Promoting safe science through regulatory change
Centre for Biosecurity Annual Report 2016-2017
On December 1, 2015, the Human Pathogens and Toxins Act and Human Pathogens and Toxins Regulations entered into full force, marking a new phase in the regulation of biosafety and biosecurity in Canada.
This report tells the story of how the Centre took action to ensure a successful transition to the new regulatory regime by managing change, promoting compliance among regulated parties, and influencing global biosafety and biosecurity regulatory efforts leading up to and throughout the ensuing year.
Questions or comments? Please contact the Centre for Biosecurity.
Introduction
Research and diagnostic work with pathogens and toxins is paramount to public health, science and innovation. But it also poses risks to public health and safety that need to be addressed and managed at a national level, supported by modern legislative authorities. When the Human Pathogens and Toxins Act (HPTA) and the new Human Pathogens and Toxins Regulations (HPTR) came into full force on December 1, 2015, it marked the culmination of a near 10-year effort within the Public Health Agency of Canada (PHAC) to reshape how biosafety and biosecurity are regulated across the country.
The new regulatory regime brought significant changes to our roles and responsibilities at the Centre for Biosecurity. Before December 2015, oversight was largely limited to issuing import permits under the Human Pathogens Importation Regulations to individual principal investigators. Today, under the HPTA and HPTR, that oversight role has expanded and the Centre is now mandated to deliver a national program that encompasses mandatory licensing covering a range of controlled activities, security clearances to work with or have access to a subset of high-risk pathogens and toxins, incident and exposure reporting, pathogen risk assessments, standards and guidelines development, and more.
Taking on this new scope of work required a culture shift within the Centre for Biosecurity. Engaging with regulated parties at the organizational level instead of individual researchers - on a broader set of compliance requirements -has required an evolution in its policies and procedures. Overseeing the new regulatory framework has demanded greater cross-functional collaboration within the Centre as well.
Things also changed quite significantly for those who work with pathogens and toxins every day. Under the new regulatory regime, a licence is now required for any facility conducting controlled activities with human pathogens and toxins, whether imported or domestically acquired. Depending on the nature of their work, some facilities also need to demonstrate that appropriate institutional controls are in place. To be considered compliant with the HPTR, all affected parties had to submit their licence applications and supporting documents to the Centre for Biosecurity by February 29, 2016.
The new regulatory regime supports a stronger culture of biosafety and biosecurity in laboratories across the country, reducing the risk of an accidental or deliberate release in the communities in which they are located. There is now a clearer picture of who is working with pathogens and toxins, with the confidence that organizations with laboratory facilities are following appropriate standards for biosafety and biosecurity. Within those organizations, biological safety officers now serve as the Centre's primary points of contact, promoting greater institutional accountability and good practices throughout their operations.
In order to ensure a smooth transition to the new regime, nearly everyone in the Centre stepped outside their day-to-day role at some point to ensure applicants were fully supported through the licensing process. Going forward, as focus shifts toward an ongoing program of compliance monitoring and enforcement, the Centre will continue to provide regulated parties with the guidance they need to understand why compliance is important and how to achieve it. By monitoring and evaluating the impact of the new regulatory framework, the Centre can develop better guidelines and tools as well as more efficient stakeholder outreach programs, all with the aim of helping organizations across the country improve their own biosafety and biosecurity practices. The Centre will also continue to engage with its domestic and international colleagues to facilitate coordinated pathogen oversight in Canada and influence the uptake of best practices internationally.
In reading this inaugural report, it should be noted that data from the fourth quarter of 2015-16 has also been included.
Our Mission
To establish and maintain a strong and comprehensive safety and security regime which prevents, detects and responds to the risks posed by the use of human pathogens and toxins.
The Centre for Biosecurity protects Canadians from health and safety risks associated with the use of pathogens and toxins by administering and enforcing the Human Pathogens and Toxins Act and Human Pathogens and Toxins Regulations, as well as certain provisions of the Health of Animals Regulations related to the importation of terrestrial animal pathogens.
The Centre also promotes coordinated pathogen oversight and capacity building between pathogen regulators and security partners domestically and internationally, and helps Canada meet its obligations under the International Health Regulations and the Biological Toxins and Weapons Convention.
The Centre for Biosecurity is Canada’s national authority for the biosafety and biosecurity of human and terrestrial animal pathogens and select biological toxins.
What is Biosafety?
The containment principles, technologies and practices used to prevent unintentional exposure to or accidental release of pathogens and toxins.
What is Biosecurity?
The institutional and personal security measures used to prevent loss, theft, misuse, diversion or intentional release of pathogens, toxins, and other related assets.
What is a pathogen?
Pathogens are microorganisms that can cause disease. They range from common bacteria like Salmonella to viruses of significant public health concern like Ebola.
What is a toxin?
A biological toxin is a poisonous substance produced or derived from a microorganism that can cause adverse health effects in humans or animals, such as botulinum neurotoxin and anthrax toxin.
| RG1 | RG2 | RG3 | RG4 |
|---|---|---|---|
| Individual risk: Low |
Individual risk: |
Individual risk: High |
Individual risk: High |
| Community risk: Low |
Community risk: Low |
Community risk: Low |
Community risk: High |
| E.g. Brewer’s yeast | E.g. Salmonella | E.g. Rabies | E.g. Ebola |
|
The HPTA applies to all persons/facilities in Canada conducting controlled activities with RG 2, 3 or 4 human pathogens and toxins. |
|||
Who Works with Human Pathogens and Toxins - And Why?
Human pathogens and toxins are used by organizations in a wide range of sectors for many different purposes: teaching and research at universities, disease diagnosis at hospitals and public health facilities, vaccine development in the pharmaceutical industry, quality control in the food industry, and more.
The licences issued to date by the Centre are distributed as follows:
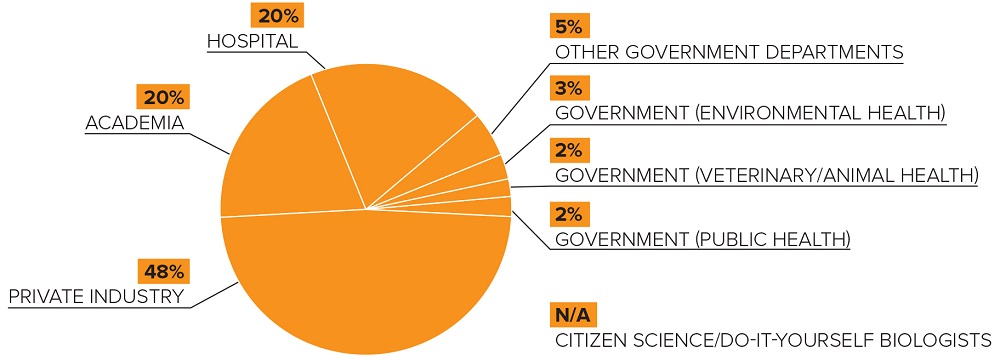
Figure 1 - Text Description
Pie chart showing the sector-by-sector distribution of licenses issued by the Centre:
- Government (public health): two percent
- Government (veterinary and animal health): two percent
- Government (environmental health): three percent
- Other government departments: five percent
- Hospitals: twenty percent
- Academia: twenty percent
- Private industry: forty-eight percent
- Citizen science and do-it-yourself biologists: not applicable
What Our Program Covers
The Centre delivers a national program that includes:
- Licensing
- Security clearances for individuals accessing high-risk pathogens
- Incident/exposure reporting
- Pathogen risk assessments
- Standard and guidance development and implementation
- Stakeholder outreach and engagement
- Compliance monitoring, verification and enforcement (i.e., inspections, audits, document reviews)
Our Scope to Date
Pathogen and Toxin Licences
Unless exempted or excluded, a licence is required to conduct the following controlled activities with human pathogens and biological toxins in Canada:
- possessing, handling, using
- producing
- storing
- permitting access to
- transferring
- importing or exporting
- releasing or abandoning
- disposing
1,550
total licences issued (includes active, amended or cancelled licences)
951
active licences (as of March 31, 2017)
93%
of active licences are for RG2 pathogens
264
PAOs reviewed
All licence applicants who intend to conduct scientific research must submit a Plan for Administrative Oversight (PAO) for Pathogens and Toxins in a Research Setting, which sets out how biosafety and biosecurity risks will be administratively managed at the institutional level.
366
security clearances issued
Security clearances are required for all individuals who work with or have access to a subset of RG3 and RG4 pathogens and toxins known as security-sensitive biological agents (SSBAs).
18
biosecurity plans reviewed
All facilities require a biosecurity plan. Facilities that work with SSBAs are required to submit their existing plan with their licence application, outlining the security measures in place to prevent the theft or diversion of biological agents and facility assets.
Licences Issued per Month
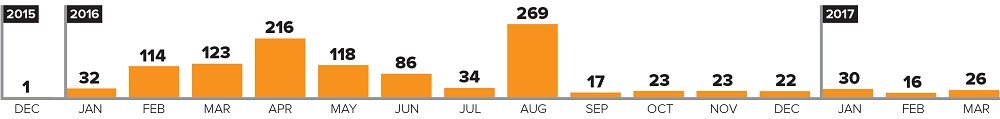
Figure 2 - Text Description
Bar graph showing number of licenses issued per month from December 2015 to March 2017.
In 2015:
- December: one
In 2016:
- January: thirty-two
- February: one hundred and fourteen
- March: one hundred and twenty-three
- April: two hundred and sixteen
- May: one hundred and eighteen
- June: eighty-six
- July: thirty-four
- August: two hundred and sixty-nine
- September: seventeen
- October: twenty-three
- November: twenty-three
- December: twenty-two
In 2017:
- January: thirty
- February: sixteen
- March: twenty-six
Compliance Monitoring, Verification and Enforcement
The Centre uses a risk-based approach to monitor and verify compliance with the HPTA/HPTR and to address any non-compliances.
2
RG4 + SSBA Licensees Inspected
15
RG3 + SSBA Licensees Inspected
2
SSBA Toxin Licensees Inspected
13
RG2 Licensees Inspected
13
Designated inspectors
Biological Safety Officers
A biological safety officer is the individual designated by a licence holder for overseeing an organization's biosafety and biosecurity practices.
856
biological safety officers across Canada
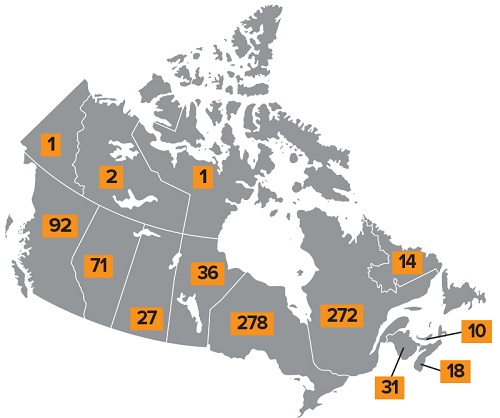
Figure 1 - Text Description
Map showing the distribution of biological safety officers across Canada:
- Yukon: one
- Northwest Territories: two
- Nunavut: one
- British Columbia: ninety-two
- Alberta: seventy-one
- Saskatchewan: twenty-seven
- Manitoba: thirty-six
- Ontario: two hundred and seventy-eight
- Quebec: two hundred and seventy-two
- New Brunswick: thirty-one
- Nova Scotia: eighteen
- Prince Edward Island: ten
- Newfoundland and Labrador: fourteen
Incident Awareness
Licence holders are required to report four types of laboratory incidents to the Centre, categorized as follows:
- Exposure Related Laboratory Incidents:
- exposure to a pathogen or toxin that has or may have caused a disease
- exposure to a pathogen or toxin that has or may have caused a disease
- Non-Exposure Related Laboratory Incidents:
- inadvertent release of a pathogen or toxin
- inadvertent possession or production of a pathogen or toxin
- missing, lost or stolen pathogen or toxin
Top three root causes for a notification about an exposure or laboratory-acquired infection (LAI):
- Standards, policies and procedures
- Communications
- Management oversight
Required Notifications
Laboratory Incident Notifications
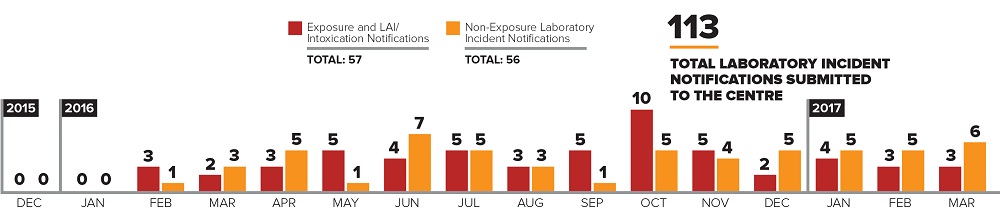
Figure 1 - Text Description
Bar graph showing the number of laboratory incident notifications (LINs) submitted to the Centre between December 2015 and March 2017.
- Total LINs: one hundred and thirteen
- Total exposure and laboratory-acquired infection/intoxication notifications: fifty-seven
- Total non-exposure LINs: fifty-six
Exposure and laboratory-acquired infection/Intoxication notifications by month:
- In 2015:
- December: zero
- In 2016:
- January: zero
- February: three
- March: two
- April: three
- May: five
- June: four
- July: five
- August: three
- September: five
- October: ten
- November: five
- December: two
- In 2017:
- January: four
- February: three
- March: three
- Non-exposure LINs by month:
- In 2015:
- December: zero
- In 2016:
- January: zero
- February: one
- March: three
- April: five
- May: one
- June: seven
- July: five
- August: three
- September: one
- October: five
- November: four
- December: five
- In 2017:
- January: five
- February: five
- March: six
Managing the Transition
The Centre for Biosecurity knew from the start that a smooth transition to the new regulatory regime would depend on giving organizations across the country the knowledge and tools to navigate the application process and address any challenges they encountered.
Through an "all hands on deck" effort that brought in personnel from all corners of the organization, the Centre worked closely with its many partners and stakeholders to streamline the licence and security clearance application processes, and to support regulated parties in strengthening their internal controls so they can meet the highest standards of biosafety and biosecurity at their facilities.
| December 1, 2015 | February 29, 2016 | January 2017 |
|---|---|---|
| Canada's new Human Pathogens and Toxins Regulations, along with the remaining sections of the 2009 Human Pathogens and Toxins Act, came into full force. | Hundreds of organizations had 90 days to file a licence application to be in compliance with the new regulatory regime. | With foresight, flexibility and determination, the Centre for Biosecurity facilitated the transition every step of the way - and by January 2017 had processed all 893 licence applications received during the transition period. |
One Portal for All
To make the licence application process as efficient as possible and reduce regulatory and administrative burden, on December 1, 2015, the Centre replaced its former paper-based permit application system with a significantly more efficient online portal.
The new Biosecurity Portal allows regulated parties to securely exchange information with the Centre, streamlining licence submission, tracking and amendments. It also provides one centralized location for creating, storing and submitting incident and exposure reports, as well as ready access to the Centre's full list of biological agents.
Ease of use was a top priority when developing the Biosecurity Portal, which features clear on-screen instructions and offers pop-up prompts to give applicants extra help when needed.
+200 Calls a Month
In the push to get applications submitted through the Biosecurity Portal, the Centre saw monthly call volumes spike from the usual average of 40 to more than 200. Fielding those calls was crucial: the Centre was determined that users’ unfamiliarity with the portal would not be a limiting factor in the licence-application process.
To support the portal’s launch, the Centre hosted online WebEx tutorials to walk applicants through the submission process and had staff from all parts of the organization field calls from users needing step-by-step support.
Centre staff also benefit from an enhanced customer relationship management (CRM) solution that provides a comprehensive compliance history of every licensed institution: improving the internal administration of licensing, compliance monitoring and verification, and reporting and stakeholder engagement activities, while also expediting the review of licence amendments and renewal applications.
Since going live, the Biosecurity Portal has been updated twice in response to stakeholder input. Additional enhancements are planned for early 2017–2018.
Getting Everyone in the System
To be considered compliant with the new regime, organizations had to submit a licence application by the end of February 2016. With the goal of getting each and every prospective applicant into the system before the deadline, the Centre coordinated a comprehensive push for enrollment, which included everything from communiques to emails and direct phone calls to remind regulated parties of their requirements. A total of 893 applications were received through the Biosecurity Portal by deadline, triggering a new phase of work: verifying data integrity. Throughout spring 2016, staff worked with applicants to improve the detail and accuracy of their submitted information, creating high-quality “tombstone” data entries that would provide the foundation for all ongoing activities from inspections to licence renewals.
The Centre began issuing licences as soon as the first applications were received in December 2015. Once again, concerted effort led to a successful outcome: all applications received by the February deadline were processed by January 2017.
Strengthening Licence Holders’ Internal Accountability
Under Canada’s previous import-based regime, the Centre for Biosecurity engaged one-on-one with individual principal investigators on a transactional basis to issue import permits for human pathogens and toxins. Today, with the HPTA/HPTR in place, the Centre issues licences that authorize an organization to conduct controlled activities with human pathogens and toxins in Canada, with mandatory conditions related to biosafety and biosecurity that must be met by every person conducting activities under that licence. These licences also authorize the importation of terrestrial animal pathogens regulated under the Health of Animals Regulations.
Within an organization, the licence holder has overall accountability for ensuring compliance with the HPTA/HPTR, and must designate a biological safety officer to oversee the biosafety and biosecurity practices at its facilities. The Centre modified its policies and communication approach to reflect this new single-point-of-contact structure and to empower biological safety officers to carry out their functions effectively.
Some facilities are required to submit additional documents to support their licence application. For example, facilities that conduct scientific research must submit a plan for administrative oversight (PAO), setting out how biosafety and biosecurity risks will be administratively managed in research settings. Similarly, facilities working with a subset of high-risk pathogens and toxins known as security-sensitive biological agents (SSBAs) must submit their existing biosecurity plan with their application, which outlines the security measures in place to prevent the theft or diversion of biological agents and facility assets.
Based on queries received and challenges observed, the Centre actively issued guidance throughout the year to assist regulated parties in developing their plans.
Going forward into 2017–2018, the Centre will continue to develop and refine its standard operating procedures for managing the new regime and respond to feedback from regulated parties — with the aim of ensuring licence holders have the greatest possible clarity about how to manage risks within their operations for the safety and security of Canadians.
The Advantage of Administrative Efficiency
A risk-based approach to licensing aims to minimize administrative burden to the extent possible, especially for facilities working with lower-risk pathogens. For example:
- Because a licence combines many controlled activities into one authorization, importing laboratories are no longer required to obtain individual import permits.
- The maximum term of a licence varies from one to five years depending on the risk group of the human pathogens involved. Most laboratories working with RG2 pathogens are eligible for a five-year licence.
- Renewing a licence is fast and easy through the Biosecurity Portal, which carries over previously provided information so there is no need to fill out the entire application every time.
Promoting Compliance
Evidence shows that compliance is higher when regulated parties understand why it is important and how to achieve it. Recognizing that fact, the Centre for Biosecurity engaged in a comprehensive campaign of outreach, communications and training last year, providing clear, consistent and easy-to-access information to organizations across the country ahead of the February 2016 deadline for submitting licence applications.
Central to that effort were an updated version of the Canadian Biosafety Standard (CBS) that clarified the new requirements for safe handling and storing of pathogens and toxins, along with a revised Canadian Biosafety Handbook providing essential guidance on how to meet the requirements described in the CBS. The Centre also made several upgrades to the CBS app for mobile devices to help organizations more easily identify the requirements that apply to their own facilities, including the ability to browse requirements by containment level and work type.
Complementing these were the Canadian Biosafety Guidelines, a series of guidance documents providing details on concepts such as how to develop a comprehensive biosecurity plan, as well as biosafety directives, advisories and notifications for particular pathogens to communicate changes in containment requirements or operational practices. Periodic newsletters provided additional information on specific biosafety and biosecurity topics over the course of the year, and also reported on key exposure incidents with important lessons learned to be applied by the broader laboratory community
By actively working with its regulated parties to promote compliance and facilitate implementation of the new regulatory requirements, the Centre is reducing the need for more resource-intensive enforcement actions down the road.
To help Canadian laboratories comply with their regulatory requirements, the Centre for Biosecurity has taken a proactive approach that emphasizes engagement, education and easy access to guidance and resources.
2,325
downloads of the CBS Biosafety App
3,733
subscribers to the Centre’s newsletters and email blasts
3,760
downloads of the Pathogen Safety Data Sheet App
A One-Stop Source of Biosafety Know-How
The Centre offers free online training and resources on laboratory biosafety and biosecurity through its e-Learning Portal. It features more than 20 courses and videos on a wide range of topics, including general introductions to biosafety and biosecurity, an overview of microbiology, pathogen and toxin risk assessments, laboratory-acquired infections, biomedical waste management, and more. The courses were updated and expanded in February 2017 to reflect the latest best practices.
10,974
Courses completed in 2016–17.
Managing Compliance in Relation to Risk
The Centre takes a risk-based approach to compliance monitoring, verification and enforcement, seeking to bring regulated parties into compliance using the most appropriate level of intervention.
The frequency of on-site inspections and targeted document reviews is based on a number of factors, including licence term, the complexity of the work carried out at a given facility, the associated safety risks and the facility’s compliance history. The Centre’s responses to non-compliance depend on a number of factors, and can range from issuing notices or changing a licence’s terms and conditions to suspending/cancelling a licence or pursuing prosecution for an offense.
Following the push to review and issue licence applications during the first half of the year, inspections under the Human Pathogens and Toxins Regulations began in earnest in October 2016. The Centre’s team of 13 designated inspectors carried out a total of 32 compliance monitoring and verification inspections and an additional 4 RG3 pre-licensing inspections.
Monitoring compliance right to the border
Participating in Canada Border Services Agency’s Single Window Initiative gives the Centre access to data that can be used to monitor and verify the regulatory compliance of facilities importing pathogens into Canada.
Accountability at the Institutional Level
As each licensed organization now has a designated biological safety officer responsible for the oversight of biosafety and biosecurity, the Centre can more effectively promote and monitor compliance at the institutional level. Rather than liaising with individual researchers or investigators, the Centre works closely with the biological safety officers so that regulatory requirements are understood, risks are identified and proactively addressed, and any necessary corrective actions are implemented. Biological safety officers also make sure lessons learned are applied consistently throughout their organizations via training and policy development.
Mandatory reporting of laboratory incidents gives the Centre a clearer picture of what is happening in licensed Canadian facilities — enabling data collection at the national level that can be used to update and strengthen biosafety and biosecurity standards and guidelines. By rapidly detecting incidents and recognizing trends, the Centre can also inform biological safety officers and licence holders in a timely, consistent way about how to respond, investigate and discover root causes, allowing them to improve or reinforce their biosafety practices and prevent future occurrences. To date, the Centre has processed notifications related to 113 laboratory incidents including laboratory-acquired infections and exposures, inadvertent releases, and lost or stolen pathogens.
Reaching Out to Citizen Scientists
Significant advancements in life science research are transforming the way biological experiments are conducted. “Do it yourself” (DIY) biology is a growing global movement of professional scientists, engineers, students, amateurs and hobbyists who pursue biology outside traditional academic and industrial laboratory settings. The Centre has been engaging with members of the Canadian DIY biology community since 2014 to promote biosafety and biosecurity awareness.
In March 2016, the Centre convened the first DIY Biology Canadian Summit, bringing together the DIY biology community, academia and other federal departments to examine how the DIY biology movement affects Canada’s science and innovation landscape, discuss the potential risks to public health and safety, and explore ways to support these activities while ensuring a culture of safety.
The Centre is also involved in the annual International Genetically Engineered Machine (iGEM) Competition as a judge and a member of the safety committee, reaching out to high school and undergraduate students involved in synthetic biology to instill good biosafety and risk-management practices into their projects - and to link iGEM participants with biosafety professionals in their home countries.
Strengthening Global Biosafety and Biosecurity
Successfully re-designated as a World Health Organization collaborating Centre for Biosafety and Biosecurity
Because an infectious disease threat anywhere can quickly become an infectious disease threat everywhere.
The Centre works globally to build international capacity in biosafety and biosecurity.
Through active leadership and global knowledge sharing on the safe use of pathogens and toxins, the Centre for Biosecurity is helping make Canada - and the world - a safer place.
Stronger, more standardized international approaches to biosafety and biosecurity mean better protection for Canadians at home and abroad. Even as the Centre carried out its transition agenda last year, it continued to share knowledge and pursue regulatory alignment with partners around the world.
Key to those efforts was its re-designation in 2016 as a World Health Organization (WHO) Collaborating Centre for Biosafety and Biosecurity. The WHO extends the Centre’s international reach, allowing it to influence safe and secure practices for human pathogens and toxins in laboratories around the world. In return, the WHO and its members benefit from Canadian expertise and experience to improve inspection processes, strengthen data collection and analysis, build policy tools, establish networks of trusted experts and regulators, and improve incident reporting worldwide.
By creating opportunities to promote safe, secure and practical biosafety and biosecurity solutions abroad - and for our country to learn from the experiences of others - this work with the WHO provides the foundation for most of the Centre’s international activities.
A Government of Canada team player
In carrying out its international work, the Centre often collaborates with Government of Canada agencies to make the best use of the country’s resources. Global Affairs Canada, for example, provides funding for a portion of the Centre’s international programming through its Global Partnership Program, which supports a wide range of activities designed to strengthen biological security and enhance the capacity of partner countries to prevent, detect and respond to biological threats.
Preventing the Spread of Infectious Diseases
As polio gets pushed to the brink of eradication, reducing the number of poliovirus containment facilities around the world minimizes the risk of the disease re-emerging. Last year, the Centre continued to coordinate Canada's implementation of the latest poliovirus biocontainment requirements under the WHO's Polio Eradication and Endgame Strategic Plan - playing a key role in global and regional programs to verify that safe handling and storage practices are followed wherever the virus is kept and contributing to stronger global lab auditing.
8
Canadian labs working with poliovirus, down from 23 in 2009 — part of the WHO’s commitment to decrease the global number of facilities holding the virus.
While some international efforts focus on promoting the safe handling, storage, use and disposal of human pathogens and toxins, others - like the Biological Toxins and Weapons Convention (BTWC) - aim to prohibit the development, production and stockpiling of biological weapons. In Canada, the HPTA/HPTR is the primary legal instrument for implementing the requirements of the BTWC. The Centre routinely provides technical expertise to Global Affairs Canada in support of its leadership on this file, and last year it supported Canada's delegation on biosafety and biosecurity at the 2016 BTWC Review Conference.
Bringing Regulations into Alignment
One of the ways the Centre contributes to stronger global biosafety and biosecurity is by helping national and regional health organizations around the world develop or strengthen their oversight frameworks. As one of six co-leads on the Global Health Security Agenda (GHSA) Action Package for Biosafety and Biosecurity, for example, the Centre provides policy development and planning support for tools that countries can use to build, implement and maintain their biosafety and biosecurity oversight frameworks. In 2016, the Centre transferred the role of secretariat of this group from Canada to Denmark.
As part of its ongoing work with the GHSA, last year the Centre produced a toolkit to help developing countries create their own national biosafety and biosecurity oversight frameworks in consideration of their local realities. Up to five countries are expected to pilot the toolkit in 2017, with the Centre supporting a facilitated launch.
Partners in Biosafety and Biosecurity
Consisting of biosafety and biosecurity regulatory officials from around the world, the International Experts Group of Biosafety and Biosecurity Regulators (IEGBBR) encourages coordination among national regulators when developing biosafety and biosecurity regulations, with the end goal being international regulatory convergence. In September 2016, with funding from Global Affairs Canada’s Global Partnership Program, the Centre helped establish a formal secretariat for the IEGBBR and will provide the secretariat function until December 2017. Also, as a member of the European Enforcement Project on Genetically Modified Organisms, the Centre has the opportunity to share lessons learned and inform the development of regulations in the European Union with Canadian best practices (and vice versa).
The Centre’s bilateral work with countries like the United States also helps advance Canada’s biosafety and biosecurity interests. Its ongoing implementation of the 2015–2018 action plan for the Canada–U.S. Beyond the Border initiative, for example, aims to improve cooperation on pathogen control and enhance information-sharing about biological threats between the two countries. Last year, the Centre delivered a number of presentations and participated in meetings with the U.S. Centers for Disease Control, sharing best practices and further discussing ways to increase regulatory alignment.
Pathogen Safety Data Sheets (PSDSs) are technical documents produced by the Agency which give organizations across Canada and around the world instant access to information on the hazards of various pathogens and how to work with them safely in a laboratory setting.
180
PSDS Available On-Line
Most downloaded data sheets:
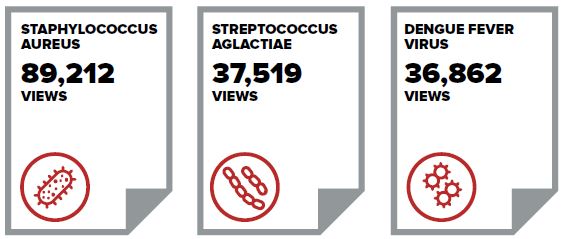
Figure 5 - Text Description
Image showing the three most downloaded Pathogen Safety Data Sheets
- Staphylococcus Aureus: Eighty-nine thousand two hundred and twelve views
- Streptococcus Aglactiae: Thirty-seven thousand five hundred and nineteen views
- Dengue Fever Virus: Thirty-six thousand eight hundred and sixty-two views
1.8 Million+
Page Views
PSDS website access by country:
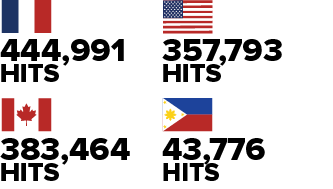
Figure 6 - Text Description
Image showing the top four countries accessing the Pathogen Safety Data Sheet website:
- France: four hundred forty-four thousand nine hundred and ninety-one hits
- Canada: three hundred eighty-three thousand four hundred and sixty-four hits
- United-States of America: three hundred fifty-seven thousand seven hundred and ninety-three hits
- Philippines: Forty-three thousand seven hundred and seventy-six hits