Bar code standards for vaccine products in Canada (update 2014-2015)
Table of Contents
- The Benefits of Bar Coding
- Developing the Standards
- Global Standards
- Harmonizing Vaccine Bar Code Standards
- The Bar Code Standards for Levels of Packaging
- Vaccine Identification Database System (VIDS)
- Bar Code Standard Implementation Status
- Appendix A: Packaging Summary
- Appendix B: Attributes included in VIDS
The Bar Code Standards for Vaccine Products in Canada have been developed in order to provide clarification and consistency of the implementation of bar coding standards for vaccine products in Canada. Vaccine products in Canada use the Global Trade Item Number (GTIN) based on GS1 global standards.
The Benefits of Bar Coding
There are many benefits to the use of bar codes on vaccine products in Canada. Standardized vaccine product identification based on GS1 global standards on all levels of vaccine product packaging enables efficient and complete electronic health record keeping by the immunizer or clerical staff; reduces the number of immunization errors through improved completeness and accuracy of records; and expedites the follow up of adverse events after immunization. Additional benefits include improved inventory management, analytics and forecasting throughout the vaccine supply chain and improved record keeping, resulting in the ability to obtain accurate immunization coverage rates.
Developing the Standards
The bar code standards for vaccine products in Canada were identified and approved by the Automated Identification of Vaccine Products (AIVP) Advisory Task Group. This group was formed in 2007 and is a collaborative effort between all stakeholder groups in the area of immunization and is co-chaired by the Public Health Agency of Canada (the Agency) and the vaccine industry with representation from vaccine manufacturers, jurisdictions, health authorities, health professional associations, regulators, international standard setting agencies, and electronic health record (EHR) and clinical management software developers.
The AIVP Advisory Task Group reports to the Canadian Immunization Registry Network as part of the Public Health Network infrastructure. The Canadian Immunization Registry Network is a working group made up of immunization experts from all 13 Canadian provinces and territories who are involved in the development of immunization registries and monitoring vaccine uptake in their respective jurisdictions.
Global Standards
The GS1 system of standards is utilized for the bar coding of vaccine products. GS1 standards are open, global and non-proprietary, and any organization from anywhere around the world can subscribe to this system. GS1 bar codes are the only non-proprietary bar codes based on an open system of standards developed by industry, allowing organizations of all sizes to participate.
The Global Trade Item Number (GTIN) is the GS1 standard for product identification. It is a unique product identifier recognized globally. For pharmaceutical products including vaccines, the GTIN is permanently assigned by the manufacturer of the product. GTINs on vaccine products enable consistent and reliable identification from point of manufacturing to immunization in a healthcare setting. The use of the GTIN on vaccine products also allows for efficient and accurate updating of electronic health records, and avoids the need for re-labelling vaccines with proprietary identifiers.
In addition to the GTIN, secondary or variable information can also be represented in the GS1 system of standards, referred to as Application Identifiers (AIs). For example, the GTIN follows (01), the expiry date follows (17), the lot number follows (10), and the serial number follows (21). Figure 1 shows examples of how the Application Identifiers are portrayed in bar codes.
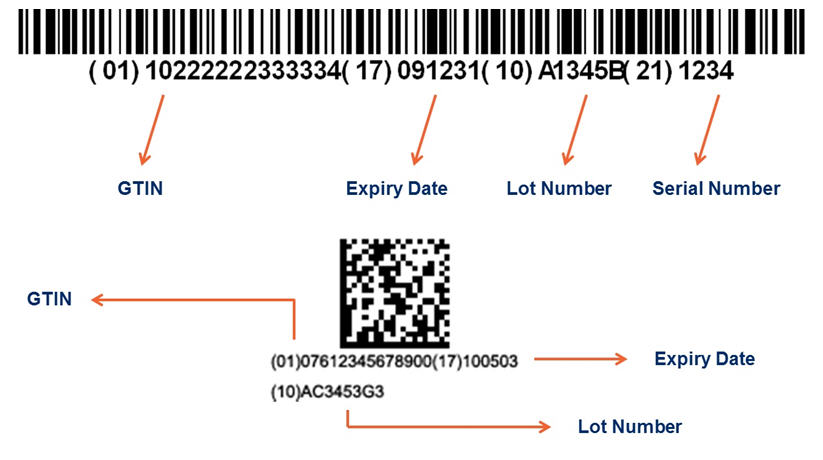
Note: these images are enlarged for illustrative purposes. The actual bar codes on the products are smaller.
Information on GS1 global standards can be found at GS1 Canada.
Harmonizing Vaccine Bar Code Standards
Canada and the United States have harmonized bar code standards for vaccine products using GS1 global standards. The exception is that the United States require the expiry date on the primary packaging to be encoded in the bar code whereas Canada has left the expiry date as optional because the information can be referenced using the lot number.
The vaccine bar code initiative has also worked closely with the Canadian pharmaceutical bar coding project, and as a result the standards and requirements for the two projects have been harmonized.
The Bar Code Standards for Levels of Packaging
Bar codes can be assigned at any level of packaging by the manufacturer of the product. Figure 2 shows a typical packaging hierarchy. In this example, the bar code on the primary (and occasionally the secondary) level of packaging is used for entry into health care records at the point of immunization, and also has the potential to be used for traceability to the patient. Secondary, case and pallet level bar codes are used for inventory management, tracking and traceability. Additional information on the levels of packaging can be found in Appendix A.

Figure 2 - Text Description
Bar codes can be assigned at any level of packaging by the manufacturer of the product. The image in figure 2 shows a typical packaging hierarchy, consisting of four separate pictures. The first picture, labeled “primary”, is a picture of a single dose vaccine syringe in sterile packaging. The second picture, labeled “secondary”, is a picture of a small box containing four of the single dose vaccine syringes, or primary packaging. The third picture, labeled “case / shipper”, is a picture of a larger cardboard box containing three smaller boxes of the secondary level packaging of syringes. The final fourth picture, labeled “pallet”, is a picture of a wooden pallet containing four larger cardboard boxes (case) taped together with clear plastic shipping tape. On every image there is a visible example of a bar code, thus depicting how each level of packaging contains a bar code.
Standard for Primary Packaging
The standard for primary packaging (e.g. single or multidose vials, pre-filled syringes or sprayers of vaccine products) is detailed below.
Standard
A two dimensional (2D) bar code is applied onto the primary package. This bar code includes the Global Trade Item Number (GTIN) and the lot number. The expiry date in the bar code is recommended but optional for the primary package as it can be determined through the lot number.
Lot number and expiry date will continue to appear in human readable form on the primary packaging as per Canadian labelling requirements.
Description
The 2D bar code has the ability to provide a significant amount of information on a very small surface (e.g. a vial or pre-filled syringe), which is its main advantage. In addition, 2D bar codes are easier to read on curved surfaces and are more resilient. For example, when handled multiple times, they will still maintain high scanning efficiency.
For an example of a GS1 Data Matrix (2D bar code), see Figure 3.
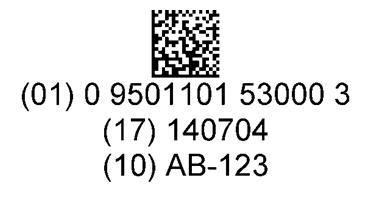
Note: this image is enlarged for illustrative purposes. The actual bar code on the product is smaller.
Standard for Secondary Packaging
The standard for secondary packaging (e.g. the box or carton containing vials or pre-filled syringes of vaccine products) is detailed below.
Standard
Either a two dimensional (2D) or linear (also known as one dimensional or 1D) bar code is applied onto the secondary package. This bar code includes the Global Trade Item Number (GTIN), the lot number and the expiry date.
The lot number and expiry date will continue to appear in human readable form on the secondary packaging as per Canadian labelling requirements.
Description
The GS1 DataBar (formerly referred to as Reduced Space Symbology or RSS) is a family of compact 1D or linear bar code symbols. GS1 DataBars are appropriate for vaccines as they are much smaller than International Article Number (EAN) or Universal Product Code (UPC) linear bar codes. They can carry additional information such as serial numbers, lot numbers and expiry dates.
For an example of a GS1 Data Matrix (2D bar code), see Figure 3 above. For examples of the linear GS1 DataBar (1D bar code), see Figure 4 below.
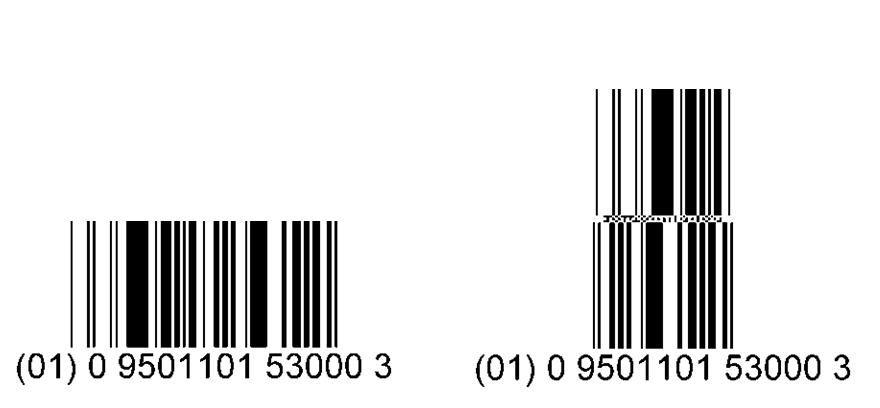
Note: this image is enlarged for illustrative purposes. The actual bar code on the product is smaller.
Standard for Case Level Packaging
The standard for the case level packaging (e.g. the shipping container or box which contains one or more secondary packages) is detailed below.
Standard
A linear (1D) bar code is applied onto the case level container. This bar code includes the Global Trade Item Number (GTIN), the lot number and the expiry date.
The bar code may also contain a serial number at the manufacturer’s discretion. Serial numbers are being introduced in many countries as a means of meeting anti-counterfeiting requirements.
Description
The GS1-128 is a linear (1D) bar code appropriate for case level identification of vaccines. The GS1-128 symbology can carry a 14 digit GTIN and additional information such as serial numbers, lot numbers, and expiry dates. For an example of a GS1-128 (1D bar code), see Figure 5.

Note: this image is enlarged for illustrative purposes. The actual bar code on the product is smaller.
Vaccine Identification Database System (VIDS)
The Agency has developed the Vaccine Identification Database System (VIDS), a web based repository of information for all vaccine products currently licensed for human use in Canada, to support the use of bar codes on vaccine products in a healthcare setting. Vaccine manufacturers provide data to the Agency using GS1 Canada’s ECCnet Registry to provide GTIN and standardized product data. The Agency requires specific product data for VIDS which is identified in Appendix B. The Agency then combines this information with vaccine lot number, expiry date and recall information within VIDS to support frontline healthcare professionals. When the bar code on a vaccine vial is scanned, it is linked to the specific product information downloaded from VIDS which then can be recorded into the electronic immunization or inventory record, eliminating the need for manual entry or paper based recording. The goal of VIDS is to contain information on all vaccine products currently licensed for use in Canada, and does not contain historical information or information on vaccines available internationally.
Vaccine product information in VIDS is aligned with product information found in Health Canada’s Drug Product Database (DPD).
ECCnet Registry
GS1 Canada's ECCnet Registry is Canada's national product registry. This industry product information registry is the most comprehensive standards-based and perpetually cleansed registry in Canada. ECCnet Registry offers a central point of access for industry to exchange vital, GS1 standards-based product data – from pharmaceutical, to food product, to medical device information – used in everyday operations. By centralizing and standardizing the critical product data shared between healthcare organizations, ECCnet Registry enables improved accuracy and clarity of product data used by the healthcare sector for operational and patient care processes.
Bar Code Standard Implementation Status
The bar code standards for vaccine products in Canada are being implemented on a voluntary basis by vaccine manufacturers. Implementing bar code standards entails both a commitment from the vaccine manufacturers to manage production lines, as well as a commitment by end users (health care settings) to ensure camera-ready scanners are in place and software is ready to take full advantage of the bar code and vaccine information from VIDS. While great progress has been made by manufacturers and some health care settings, an increase in the availability and use of bar codes on vaccine products as described in this document is expected by 2017, as this is the target timeline agreed upon for implementation by all stakeholders for the majority of vaccines.
In 2009, the AIVP Advisory Task Group reached a consensus on vaccine bar code standards in Canada, recommending the placement of a GTIN, a lot number and an optional expiry date on the primary package. In order for this information to fit on the primary package (the vial or the syringe), vaccine labelling must be changed on production lines to put a two dimensional (2D) bar code on the product. Canadian manufacturers have committed to adhering to these standards for most of their products by 2017.
As of early 2011, the first vaccine labelling production lines in Canada and the United States were able to print 2D bar codes on vaccine vial labels. Several manufacturers had adopted the standards by the end of 2014 and it is expected that most major manufacturers will be in compliance with the new standards on most of their products by 2017. Products manufactured in Europe will be the last to have the 2D bar codes as European standards have not yet been harmonized with those in Canada and the United States.
To support the implementation and use of bar codes in Canada, the AIVP Advisory Task Group has supported early adopters of bar code technology through funding and technical support since 2010. Support has been, and continues to be provided, to a variety of health care delivery areas such as private health, public health and pharmacy settings. In these settings, bar coding has been successful in reducing errors and increasing the accuracy of vaccine data captured at the point of care. Despite the success of the technology when implemented, there remain significant barriers to widespread adoption.
Only half of immunization registries are currently able to connect to the internet and none of the systems at the jurisdictional level are using bar code scanning technology. Nevertheless, many small clusters of populations, such as the First Nations communities in western and central Canada, are scanning vaccine bar codes at the point of administration, as are many physicians. Some public health clinics, particularly for mass immunization clinics, and some pharmacy settings, particularly those in western Canada, are using bar code scanning technology. A quarter of vaccine inventory settings are using bar code scanning, as are many hospitals and clinics to assist with inventory keeping of vaccines. The scanners are most commonly used for pharmaceutical products, medical supplies and patient identification.
While there is potential for immunization surveillance systems to connect to VIDS via a direct live link, there has been minimal uptake on this feature. However, users are still able to access VIDS and download an Excel table, and in turn use this table to update their systems. In addition, many private and public clinics as well as inventory management systems use the VIDS data to update their catalogues on a regular basis.
The Canadian Immunization Registry Network was established to coordinate the development of standards and facilitate the sharing of knowledge and experience to develop a national network of immunization registries. The AIVP Advisory Task Group continues to report to and work closely with the Canadian Immunization Registry Network.
The AIVP Advisory Task Group continues to work with both the manufacturers and health care settings in the jurisdictions to support the implementation and adoption of the vaccine bar code standards in Canada.
For more information on the Bar Code Standards for Vaccine Products in Canada please contact AIVP_IAPV@phac-aspc.gc.ca. For access to VIDS visit the VIDS website.
Appendix A: Packaging Summary
| Packaging Level | Symbology | Information contained in the bar code | Implementation Target Date | Status |
|---|---|---|---|---|
| Primary | 2D (i.e. GS1 DataMatrix) | GTIN, Lot # Expiry date recommended but optional Lot # and Expiry date must appear in human readable form as per Canadian labelling requirements |
December 31, 2016 | As of January 1st, 2017, 80% of vaccine products will have 2D bar codes on the primary packaging. |
| Secondary | 2 D or linear (i.e. GS1 DataMatrix, GS1 DataBar, or GS1-128) | GTIN, Lot #, Expiry date Lot # and Expiry date must appear in human readable form as per Canadian labelling requirements |
December 31, 2017 | As of January 1, 2018 80% of vaccine products will have 2D or linear bar codes on the secondary package. |
| Case level | Linear (i.e. GS1-128) | GTIN, Lot #, Expiry date | December 31, 2017 | As of January 1, 2018, 80% of case level vaccine products will have the GS1-128 bar code on the case level package. |
Appendix B: Attributes Included in VIDS
| Attributes directly from ECCnet Registry | Manual Entry by the Agency | |
|---|---|---|
| Active Ingredient Name | Temperature Control Range | Lot Number |
| Active Ingredient Strength | Size | Expiry Date |
| Active Ingredient Strength | Size UOM | Product Recall |
| Active Ingredient Strength per Typical Dose | Contraindications | Product Hold |
| Active Ingredient Strength per Typical Dose UOM | Precautions | Provincial/Territorial Immunization Schedules |
| Global Trade Item Number (GTIN) | ECCnet Classification Code | |
| Drug Identification Number (DIN) | Drug Type Identifier | |
| Brand Name | Discontinue Date | |
| American Hospital Formulary System Code (AHFSC) | Reinstatement Date | |
| Anatomical Therapeutic Chemical (ATC) Classification Code | Global Product Classification Code | |
| Dosage Form | Hazardous Material & Transportation Handling | |
| Extended Description | Drug Strength and Unit of Measure (UOM) as per label | |
| Generic Name | Manufacturer/ Supplier | |
| Label Storage Condition | Type | |
| Route of Administration | Total units | |
| Short Description | ||