Canada-U.S. joint white paper: Substance use and harms during the covid-19 pandemic and approaches to federal surveillance and response
U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health, Washington, DC
U.S. Department of Health and Human Services, Office of the Assistant Secretary for Planning and Evaluation, Washington, DC
U.S Department of Health and Human Services, Centers for Disease Control and Prevention, Atlanta, GA
U.S. Department of Health and Human Services, National Institutes of Health, Bethesda, MD
Health Canada, Controlled Substances and Cannabis Branch, Ottawa, Ontario
Public Health Agency of Canada, Health Promotion and Chronic Disease Prevention Branch, Ottawa, Ontario
Suggested Citation
Health Canada, Public Health Agency of Canada, and U.S. Department of Health and Human Services. Canada-U.S. Joint White Paper: Substance Use and Harms During the COVID-19 pandemic and Approaches to Federal Surveillance and Response. Ottawa, Ontario; Washington, D.C.: U.S. Department of Health and Human Services, Office of the Assistant Secretary for Health, 2022.
Executive Summary
Substance use and substance use disorders (SUDs), especially those that lead to drug overdose deaths, are prominent public health issues that extend beyond borders. For many countries, reducing the use of illicit drugs, the misuse of prescription medications, and drug overdoses have been longstanding challenges. The public health emergency (PHE) due to the COVID-19 pandemic has presented additional, unique challenges to efforts to reduce substance use, overdose, and drug-related harms.
This white paper is an initial collaborative product of the Canada and United States (U.S.) Health Working Group under the Joint Action Plan, an initiative under the 2021 Roadmap for a Renewed Canada-U.S. Partnership by President Biden and Prime Minister Trudeau, which establishes cooperation between the two countries to find effective approaches to addressing the opioid overdose crisis across three working groups covering border security, health, and law enforcement. The aims of this white paper are to provide information on substance use and drug overdose deaths in the United States and Canada, the impact of COVID-19 on this public health crisis, and actions the governments of both countries took to respond to the challenges posed by COVID-19. Additionally, this paper will describe the innovative or rapid surveillance approaches taken to track and understand impacts on substance use and overdose during the COVID-19 pandemic.
The overdose crisis in the U.S. remains unabated. From 1999 to 2019, the overdose death rate in the U.S. increased more than 250%. While the overdose crisis has evolved over time, it is now largely characterized as one fueled by deaths involving illicitly-manufactured synthetic opioids, including fentanyl, and resurgent stimulants such as methamphetamine. During the COVID-19 pandemic, the U.S. observed a 30% increase in overdose deaths in 2020 compared to 2019, and provisional estimates have indicated a continued increase of drug overdose deaths in 2021. Not only has the pandemic had a worsening effect on substance use and its related harms, but also it has highlighted the disproportionate impact on persons who are Black, Indigenous, and People of Color (BIPOC). As with many other medical conditions, disparities exist for BIPOC persons in both outcomes and treatment for substance use.
Similarly, Canada has seen a steady increase in unintentional poisoning deaths from 2007 to 2014, with a steeply inclining mortality beginning in 2015 to present. While illicitly manufactured fentanyl and fentanyl analogues continue as major drivers of the crisis in Canada, the unregulated drug supply is highly toxic and unpredictable in composition, and polysubstance use is common. In the majority of deaths, various combinations of potent opioids, stimulants, and/or benzodiazepines are detected. Also similar to the U.S. overdose crisis is the disproportionate impact of drug toxicity on certain racial and ethnic minority groups in Canada, particularly Indigenous communities.
The COVID-19 pandemic has impacted substance use and overdose deaths, which both Canada and the U.S. have worked to track using a variety of surveillance systems including: conceptual and dynamic models, substance-related harms surveillance, and rapid situational reporting in Canada and online surveys, syndromic surveillance, and use of urine drug testing and mortality data in the U.S. Noticing the alarming trends in drug and opioid overdose deaths and substance use, both governments implemented specific policy and programmatic actions. For the U.S., this included the expansion of telehealth, issuance of the Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder, and easing of restrictions around medication dispensing and delivery for opioid treatment programs (OTPs). For Canada, this included class exemptions and the development of a series of national guidance documents related to substance use in the context of COVID-19.
This white paper is just the beginning of collaborative efforts. More exchange of information and other joint efforts, including ones involving other countries, will be necessary to share lessons learned and effectively meet these complex and evolving public health challenges. Beyond information exchange is the need to describe the newly developed surveillance systems that have emerged and study how they can be refined for continued use and for appropriately capturing and focusing efforts on those disproportionately impacted.
Acknowledgements
This joint white paper was prepared by the U.S. Department of Health and Human Services under the general direction of the Office of the Assistant Secretary for Health, the Office of the Assistant Secretary for Planning and Evaluation, the Centers for Disease Control and Prevention, and the National Institutes of Health and by the Public Health Agency of Canada/Health Canada.
Editors of the white paper were
Commander (CDR) Gayle Tuckett, PharmD, U.S. Public Health Service, Policy and Special Projects Officer, Counter-Terrorism Emergency Coordination Staff, Office of the Center Director, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland.
Margot Kuo, MPH, Manager, Epidemiologist, Interjurisdictional Capacity Building, Substance-Related Harms Division, Centre for Surveillance and Applied Research, Health Promotion and Chronic Disease Prevention Branch, Public Health Agency of Canada, Ottawa, Ontario.
Captain (CAPT) Christopher M. Jones, PharmD, DrPH, MPH, U.S. Public Health Service, Acting Director, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, Georgia.
Wilson M. Compton, MD, MPE, Deputy Director, National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland.
Meriam Mikre, MPH, Senior Advisor, Immediate Office, Office of the Assistant Secretary for Health, Office of the Secretary, U.S. Department of Health and Human Services, Washington, D.C.
Contributing authors were
Margot Kuo, MPH, Manager, Epidemiologist, Interjurisdictional Capacity Building, Substance-Related Harms Division, Centre for Surveillance and Applied Research, Health Promotion and Chronic Disease Prevention Branch, Public Health Agency of Canada, Ottawa, Ontario.
Captain (CAPT) Christopher M. Jones, PharmD, DrPH, MPH, U.S. Public Health Service, Acting Director, National Center for Injury Prevention and Control, Centers for Disease Control and Prevention, Atlanta, Georgia.
Wilson M. Compton, MD, MPE, Deputy Director, National Institute on Drug Abuse, National Institutes of Health, Bethesda, Maryland.
Michele Musgrove, Acting Associate Director, Controlled Substances and Cannabis Branch, Health Canada, Ottawa, Ontario.
Commander (CDR) Gayle Tuckett, PharmD, U.S. Public Health Service, Policy and Special Projects Officer, Counter-Terrorism Emergency Coordination Staff, Office of the Center Director, Center for Drug Evaluation and Research, Food and Drug Administration, Silver Spring, Maryland.
Daniel Schwartz, BS, Behavioral Health Policy Analyst, Office of Behavioral, Health, Disability, and Aging Policy, Office of the Assistant Secretary for Planning and Evaluation, Office of the Secretary, U.S. Department of Health and Human Services, Washington, D.C.
Tisamarie B. Sherry, MD, PhD, Deputy Assistant Secretary, Office of Behavioral Health, Disability, and Aging Policy, Office of the Assistant Secretary for Planning and Evaluation, Office of the Secretary, U.S. Department of Health and Human Services, Washington, D.C.
Contents
- Executive Summary
- Acknowledgements
- COVID-19 and Substance Use and Drug Toxicity Deaths
- References
- Appendix
COVID-19 and Substance Use and Drug Toxicity Deaths
Background
Addressing substance use and deaths due to opioids and other drugs is a priority shared by many countries. The countries of focus in this paper are Canada and the United States (U.S.). Prior to exploring the impacts of COVID-19 on this significant public health issue in both countries, it is important to provide context on where both countries were in tackling substance use and overdose before the COVID-19 pandemic.
In Canada, deaths due to the toxicity of the street drug supply began to increase in 2015, with unprecedented mortality rates acknowledged in 2016, particularly in the Western provinces and Ontario. In April 2016, British Columbia declared a provincial public health emergency for accidental, illegal drug toxicity deaths.Footnote 1
The Public Health Agency of Canada (PHAC) national surveillance estimates that there were 29,052 deaths from apparent opioid toxicity between January 2016 and December 2021 across Canada. Footnote 1 The majority of deaths occurred in the provinces of British Columbia (BC), Alberta, and Ontario, with elevated rates in other areas such as Yukon and Saskatchewan. No province or territory has been unaffected, with deaths increasing in urban, rural, and remote regions. From 2016 – 2018, mortality trends were extreme and increasing annually, followed by a slight decrease in 2019.Footnote 1 This was attributed to a moderate reduction in street drug toxicity as well the expansion of public health and harm reduction services.Footnote 2 Unfortunately, there was a reversal of this downward trend following the onset of the COVID-19 pandemic.
The majority of drug toxicity deaths have been in males and individuals aged 20 to 59 years, making this a leading cause of death in Canada and the biggest driver of potential years-of-life lost. Footnote 1 While fentanyl and fentanyl analogs continue to be major drivers of the crisis, polysubstance use is the norm, and the unregulated drug supply is both highly toxic and unpredictable, with deaths involving co-occurrence of potent opioids, stimulants, and/or benzodiazepines.
In the U.S., there has been a steady rise in drug overdose deaths since the 1980s.Footnote 3 In the past 20 years, the U.S. overdose crisis has been characterized by three waves, with morbidity and mortality accounted for predominantly by prescription opioids initially (1999-2010), then by heroin (2010-2013), and most recently since 2013 by the introduction of highly potent illicit synthetic opioids such as illicitly manufactured fentanyl (IMF) and fentanyl analogs. Although there was a small decline in overdose deaths between 2017 and 2018, due to the proliferation of synthetic opioids and a resurgence of psychostimulants such as methamphetamine, the overdose death rate again rose in 2019 prior to the COVID-19 pandemic in the U.S.Footnote 4
Among the nearly 70,630 drug overdose deaths in the United States in 2019, 70% involved an opioid. Further, 72.9% of opioid-involved overdose deaths involved synthetic opioids other than methadone (e.g., IMFs) making them the main driver of these drug overdose deaths.Footnote 5 The largest increase in death rates involving synthetic opioids, from 2018-2019, occurred in the West, at 67.9%; and the largest increase involving psychostimulants occurred in the Northeast at 43.8%. This demonstrated a geographic shift in overdose deaths involving synthetic opioids and methamphetamine, where previously the highest increases in synthetic opioid-involved deaths were in the East and the highest increase for psychostimulant-involved deaths were in the Midwest.Footnote 6,Footnote 7,Footnote 8
For both countries, a high proportion of deaths involve some combination of opioids, stimulants, and/or benzodiazepines, and this tendency has only increased during the COVID-19 pandemic.
Impact of COVID-19
Since the onset of the COVID-19 pandemic in 2020, community mitigation measures such as stay-at-home and shelter-in-place orders have been widely implemented in the U.S. collateral consequences of these mitigation (e.g., economic stress, social isolation), coupled with changes to service delivery and fear of virus transmission have impacted patterns of substance use, mental health, and social support, including the risk of harms from substance use.Footnote 9
During the pandemic, people increasingly used drugs alone, both outdoors as well as in private residences. Due to the highly toxic supply, using drugs alone has been identified as a risk factor for serious outcomes and fatalities. Since 2020, drug toxicity deaths and harms have indeed resurged beyond previous levels. Specific contributing factors may include: the increasingly toxic drug supply; increasing feelings of isolation, stress, and anxiety; and diminished availability and accessibility of services for people who use drugs.Footnote 10,Footnote 11,Footnote 12,Footnote 13,Footnote 14,Footnote 15,Footnote 16
Measures enacted during the COVID-19 pandemic impacted delivery of services for people who use drugs, such as safe consumption sites, overdose prevention services, and withdrawal and treatment services. Changes included closure of services, reductions in hours, diminished capacity of in-person care, increased virtual care, longer waiting times, and reduced availability of supplies.Footnote 9, Footnote 17 These changes may be linked to harms through reduced access to services, increased use of drugs alone, no assisted injections, sharing and re-using supplies (e.g., syringes), and reduced health and wellbeing. Further, as a result of community mitigation measures, access to substance use disorder (SUD) treatment, including medications for opioid use disorder (MOUD), cognitive behavioral and other overdose prevention services, harm reduction services, and recovery support services may have been limited. This is due to clinician office and treatment facility closures, closure of community-based programs offering harm reduction services, and discontinuation of in-person treatment and recovery support services, as well as delays in seeking care due to concerns about exposure to COVID-19 during medical visits.
Border closures, travel restrictions, and associated disruptions in drug production and trafficking may also have impacted the availability and composition of the drug supply in Canada.Footnote 13 The Canadian Centre on Substance Use and Addiction suggested in May 2020 that fewer drugs may have been entering Canada due to disruption of precursor supply, shutdown of overseas drug synthesis facilities, and disruption of shipping routes.Footnote 18 Changes in the availability of substances may contribute to drug shortages, increased toxicity, stockpiling, and changes in tolerance among people who use drugs, which may increase substance-related harms.Footnote 19 The composition of the drug supply may also have changed, as fentanyl was a larger contributor to opioid-related deaths in Ontario and Alberta during COVID-19 compared to pre-COVID-19, and BC reported higher fentanyl concentrations in the drug supply in 2020 and 2021.Footnote 5, Footnote 19,Footnote 21,Footnote 22
Other health and social services have also been impacted during COVID-19, including medical services, pharmacies, mental health and addiction treatment programs, and supports such as housing and food programs.Footnote 23,Footnote 24 Disruptions to these services may result in lower quality of health care and difficulty accessing regular care and medications. Changes to health and social services may also lead to difficulty accessing necessities such as food, shelter, washrooms, and clothing, contributing to poorer physical and mental health. Footnote 9, Footnote 16
Similar to other medical conditions, disparities exist in both acquisition and treatment of various diseases with BIPOCs being disproportionately impacted. The same is true as it relates to substance use among these individuals both before and during COVID-19. In Canada, Indigenous people have historically been disproportionately affected by substance-related harms and, leading into the drug toxicity crisis and followed by the COVID-19 pandemic, this continued to be a reality.Footnote 25 In the U.S., COVID-19 has unequally affected many racially and ethnic minority groups putting them more at risk of getting sick, severe illness, and death from this disease. The addition of substance use further compounds this disproportionate impact. While rates of illicit opioid use among Black persons and Hispanic/Latino persons are similar to that of the general population, Black persons have experienced the greatest increase in rate for overdose deaths from non-methadone synthetic opioids (e.g., illicitly manufactured fentanyl) due to limited access to prevention, treatment, and recovery services for SUDs.Footnote 26 In addition, recent research has found that African American persons with a recent opioid use disorder diagnosis were over four times more likely to develop COVID-19, compared to whites.Footnote 27
Impact of Substance Use on COVID-19
While the main focus of this paper is to explore the impacts of the COVID-19 pandemic on substance use and overdose, it is important to highlight any impacts that substance use has on COVID-19. SUDs are considered an underlying medical condition; people who have a SUD are more susceptible to COVID-19 and its outcomes including an increased risk of severe illness and death.Footnote 23
In the U.S., a study funded by the National Institutes of Health found higher COVID-19 hospitalization and death rates among people with a history of SUDs compared to those without, 41.0% versus 30.1% and 9.6% versus 6.6%, respectively. After analyzing millions of electronic health records, investigators found that while individuals with an SUD constituted 10.3% of the total study population, they represented 15.6% of the COVID-19 cases. Further, those with a recent SUD on record were more likely than those without to develop COVID-19, an effect that was strongest for those with opioid use disorder, followed by tobacco use disorder.Footnote 23
A major objective of public health efforts during the pandemic was to reduce the likelihood of an individual developing severe disease. Those with pre-existing conditions or comorbidities at increased risk for severe disease were a primary focus of intervention strategies. While increasing age was the foremost predictor of severe outcomes, some Canadian and U.S. studies examining the effects of various comorbidities identified SUD as well other substance use related issues as risk factors.Footnote 24, Footnote 28 A study of laboratory-diagnosed COVID-19 cases in British Columbia found that substance use increased the risk of hospitalization for COVID-19.Footnote 24 Comorbidities associated with chronic substance use such as arrhythmias, cardiac insufficiency, myocardial infarction, COPD, pulmonary hypertension, and diabetes are also risk factors for COVID-19 infection and more severe outcomes.Footnote 20, Footnote 29, Footnote 30
Government Policy and Programmatic Actions Taken
Canadian Policy Actions
The Government of Canada has taken a number of targeted actions to reduce the risk of harm for people who use substances.
Regulatory actions to increase access to medications, services and care:
Prior to the pandemic, there were a number of supervised consumption services (SCS) sites in several provinces across Canada. These sites vary in physical space, operational models, associated services, and staffing, but all have personnel trained to support safer drug use, respond to an overdose, and provide referrals to other services. These sites were granted federal exemptions to the Controlled Substances Act and meet basic reporting and operational requirements. SCS sites have long provided safe consumptions spaces for people using illegal drugs, led harm reduction and overdose prevention activities, and have no deaths to date.
In response to the pandemic, Health Canada (HC) allowed each province and territory to establish new temporary spaces where people can consume drugs under supervision to reduce risk of overdose death within existing SCS, shelters or other temporary sites as needed. These temporary spaces aimed to reduce the risk of overdose due to using alone while allowing people to respect physical distancing measures. To help ensure people could maintain access to critical medications and harm reduction services, HC issued further class exemptions to allow pharmacists to: extend and renew prescriptions, transfer prescriptions to other pharmacies, and allow other individuals to deliver prescriptions containing controlled substances to patients. This ensured continued access to medications for treatment of SUDs and management of other health conditions, such as chronic pain.
Providing information and guidance:
Through funding from the Canadian Institutes of Health Research (CIHR), the Canadian Research Initiative in Substance Misuse (CRISM) has developed a series of national guidance documents related to substance use in the context of COVID-19 which are available on the CRISM website:
- Telemedicine Support for Addiction Services
- Supporting people who use substances in shelter settings during the COVID 19 pandemic
- Supporting people who use substances in acute care settings during the COVID-19 pandemic
- Medications and other clinical approaches to support physical distancing for people who use substances during the COVID-19 pandemic
- Strategies to reduce SARS-CoV-2 transmission in supportive recovery programs and residential addiction treatment services
- Harm reduction worker safety during the COVID-19 global pandemic
CRISM is also undertaking a rapid assessment of the issues that people who use drugs are experiencing during the COVID-19 crisis and the health service interventions to support them.
Indigenous Services Canada supported the development of culturally appropriate online resources for people who use substances during the COVID-19 pandemic, including fact sheets and online resources.
The Government of Canada provides funding to organizations like the Canadian Centre on Substance Use and Addiction (CCSA), which has developed an Impacts of COVID-19 on Substance Use online information hub. The hub includes publications from CCSA experts and links to other resources about substance use and COVID-19.
Building evidence:
Many stakeholders in Canada are calling for a safer alternative to the toxic illegal drug supply. HC funded pilot projects through the Substance Use and Addictions Program (SUAP) that provide prescribed pharmaceutical-grade drugs as a safer alternative to the toxic illegal drug supply to people who are at high risk of overdose, under the supervision of an authorized health care practitioner, with the primary goal of saving lives and preventing overdoses. During the pandemic, the number of safer supply projects the federal government is supporting increased -- as of July 6, 2022, there are over 20 projects supporting a range of service delivery, research/knowledge transfer and exchange projects and national safer supply community of practice, for a total investment of over $67M. Two million dollars has been provided to CRISM by the CIHR to conduct a rigorous, multi-year arms-length evaluation of several safer-supply pilot projects, and an independent qualitative preliminary assessment of ten federally-funded pilots, released in early 2022, was funded by HC.
In April 2020, the Government of Canada announced a $115 million investment in Canada's rapid research response to COVID-19, including funding for a CIHR COVID-19 and Mental Health Initiative in the following areas: implementation science and population-level intervention research to address the impacts of the COVID-19 pandemic and its containment measures on mental health and substance use, in collaboration with four provincial partners; rapid knowledge synthesis and mobilization of current evidence on mental health and substance use services, delivery, and related guidelines, in the COVID-19 context; and regional evaluations on the impact of COVID-19 on supervised consumptions services.
US Policy Actions
The U.S. government introduced several policies during the COVID-19 pandemic that aimed to facilitate access to OUD treatment.Note [1] Some policies are temporary in nature, including those tied to the public health emergency (PHE) declaration for COVID-19, while others are permanent. This section focuses on select policy actions that pertain specifically to medications for OUD—methadone and buprenorphine, in particular—taken by the U.S. Department of Health and Human Services (HHS) and the U.S. Department of Justice (DOJ).
Background on methadone and buprenorphine regulation
The use of methadone and buprenorphine to treat OUD is jointly regulated by HHS's Substance Abuse and Mental Health Services Administration (SAMHSA) and DOJ's Drug Enforcement Administration (DEA). These medications are generally only available to individuals with OUD via settings or healthcare practitioners that meet specific requirements, as described below.
Opioid treatment programs (OTPs) are specialty healthcare facilities that offer a comprehensive array of medical and psychosocial services for patients with OUD, including the dispensing of methadone and buprenorphine.Footnote 31 OTPs must be accredited, certified by SAMHSA, and registered with DEA. Methadone is only available for individuals with OUD through OTPs, with limited exceptions.
Prescribing buprenorphine for OUD is allowed for practitioners (physicians, physician assistants, nurse practitioners, clinical nurse specialists, certified registered nurse anesthetists, and certified nurse midwives) who receive a waiver from DEA after being approved by SAMHSA.Footnote 32 Practitioners are generally limited to treating 30 patients initially and can progressively increase their limit to 100 and 275 based on the amount of time with a waiver.
Medication dispensing and delivery
Current federal regulations restrict the dispensing of methadone doses for unsupervised (“take-home”) use based on the length of time a patient has been receiving treatment, and individual OTPs have discretion to limit take-home supply.Footnote 33 As a result, many individuals are typically required to visit an OTP daily to receive their dose. In response to the COVID-19 pandemic, SAMHSA has permitted states to request blanket exceptions to time-in-treatment requirements in order to minimize the risk of COVID-19 transmission at OTPs and preserve the supply of personal protective equipment.Footnote 34 These exceptions have allowed OTPs to dispense up to 28 days of take-home methadone doses to “stable” patients and up to 14 days of take-home doses for “less stable” patients. Forty-three states and the District of Columbia have received exceptions. In November 2021, SAMHSA announced that it was extending the modified take-home dose standards for one year beyond the eventual expiration of the COVID-19 PHE declaration while it pursues rulemaking to make these flexibilities permanent.Footnote 35
For the duration of the COVID-19 PHE, DEA also eased several OTP requirements to mitigate potential disruptions to treatment. Under these relaxed standards, OTP clinical staff, other authorized OTP staff, law enforcement officers, and National Guard members are permitted to make doorstep deliveries of methadone and buprenorphine to clients undergoing quarantine or isolation due to COVID-19.Footnote 36 DEA has also allowed OTPs to repeatedly use the same off-site location to dispense methadone and buprenorphine without separately registering the location, subject to certain requirements.Footnote 37,Footnote 38 Additionally, in June 2021, DEA permanently eliminated a moratorium on approving mobile components of OTPs (e.g., vans) and waived separate registration requirements.Footnote 39
One exception to the requirement that methadone be dispensed at OTPs and buprenorphine be dispensed at OTPs or prescribed by waived providers exists during emergency situations. Current rules allow DEA-registered practitioners working outside of an OTP and who lack a waiver for buprenorphine prescribing to dispense methadone for one day or buprenorphine for up to three days while their patient is being connected with long-term OUD treatment.Footnote 40 In March 2022, DEA began issuing exceptions that allow practitioners to dispense three days of medication at one time while the agency works to incorporate this change into their rules.Footnote 41
Telehealth
Historically, practitioners must conduct at least one in-person medical evaluation with a patient prior to issuing a prescription for a controlled substance via telehealth, with several exceptions.Footnote 42 HHS and DOJ exercised one exception for the COVID-19 PHE, which allows the prescribing of controlled substances via telehealth using a real-time, two-way, audio-visual communications device without having an initial in-person visit.Footnote 43 For the prescribing of buprenorphine for OUD, DEA further relaxed requirements by allowing the use of audio-only devices (e.g., telephone). Footnote 44
SAMHSA has similarly allowed OTPs to initiate treatment with buprenorphine after a telehealth visit and continue treating existing patients with buprenorphine or methadone via telehealth (using either audio-visual or audio-only devices).Footnote 45 Initiating treatment with methadone, however, continues to require an in-person medical evaluation. Additionally, the Centers for Medicare & Medicaid Services has modified Medicare coverage requirements during the COVID-19 PHE to allow the therapy and counseling components of OTP services to be conducted via telehealth (including use of audio-only devices).Footnote 46
Buprenorphine waiver requirements
The process to obtain a waiver to prescribe buprenorphine has traditionally involved completing training (with some exceptions) and submitting a notice of intent to SAMHSA, in which a practitioner certifies their capacity to provide or refer patients to counseling and other ancillary services.Footnote 47 Recognizing that some requirements to obtain a waiver may pose a barrier to treatment availability, HHS released Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorderin April 2021 that simplify the process for eligible practitioners to receive waivers to treat up to 30 patients with OUD.Footnote 48 Per the Guidelines, practitioners are now exempted from requirements related to training and certification of counseling and other ancillary service capacity if they wish to treat no more than 30 patients. Training and certification of counseling and ancillary service capacity is still required to obtain a waiver to treat more than 30 patients.
Efforts to Understand the Impacts on Substance Use and Overdose during COVID-19
Unites States Data Sources
Given these developments in the U.S., there was great interest early on in understanding how the COVID-19 pandemic was affecting substance use and overdose patterns. However, for multiple reasons many of the traditionally relied upon surveillance systems and data sources in the U.S. were not able to provide timely insight into these outcomes. First, findings from key substance use prevalence surveys such as the National Survey on Drug Use and Health (NSDUH) are only published once a year and have a one-year data lag, meaning findings related to 2020 substance use patterns would not be published until late 2021. Further, NSDUH is an in-person survey, and data collection was paused during the early months of the pandemic, further delaying data collection and release. Other key data sources also experience lags in data that have made real-time or near-real time surveillance challenging in the context of the COVID-19 pandemic. For example, final mortality data used to track overdose deaths have a 12-month data lag, and emergency department (ED) visit data to track non-fatal overdose through the Healthcare Cost and Utilization Project have at least a 24-month lag.
Faced with these realities, staff in HHS pursued new approaches to collecting, synthesizing, and disseminating data in order to monitor the impact of the pandemic. National data sources used in the U.S. during the COVID-19 pandemic have included multiple systems. Tracking overdose deaths include both final and early-release provisional data from the Centers for Disease Control and Prevention's (CDC) National Vital Statistics System (NVSS) as well as detailed death data from the State Unintentional Drug Overdose Reporting System (SUDORS). ED data are from the CDC's Drug Overdose Surveillance and Epidemiology (DOSE) system, which leverages electronic health record data in CDC's National Syndromic Surveillance Program in addition to hospital discharge/billing data. Proprietary urine toxicology test data have provided information from large scale testing systems. Cross-sectional surveys have provided information about mental health and substance-use-related outcomes in adults and youth. Each of these sources are described more fully below.
Canadian Data Sources
Regular surveys on alcohol and drug use among the Canadian general population and youth were either not at the point of collecting data or were delayed in the earlier days of the COVID-19 pandemic. The latter was the case for the school-based in-person Canadian Student Tobacco, Alcohol and Drugs Survey. The Canadian Cannabis Survey, however, continued to collect information electronically, including impacts of COVID-19 on cannabis use. Further, the Supervised Consumption Site (SCS) Pandemic Survey administered by HC gathered information on the impacts of COVID-19 on SCS service delivery (closing sites, changing delivery models, etc.) and on substance use behaviors among clients, among other objectives. Two periods of data collection were completed during the pandemic, and this is complemented by data collected monthly by HC on the SCS.
The provisioning of data on apparent opioid- and stimulant-related toxicity deaths to PHAC for national reporting is facilitated by the presence of Substance-Related Harms Public Health Officers (SRHs PHOs) in most Canadian Provinces and Territories. These are federal epidemiologists deployed since 2018 to support jurisdictional surveillance capacity building. They may support public health, coroner, and/or health ministry offices. At the outset of the pandemic, rapid data and intelligence to support growing awareness of the exacerbation of fatal and non-fatal drug toxicity events in the P/Ts was provided, coordinated, and/or ground-checked by the SRHs PHOs. The pre-positioning of these skilled human resources became pivotal in detecting early signals, to inform effective federal actions to support the P/Ts urgent response activities.
Prior to the pandemic, Canadian provincial and territorial coroners' and medical examiners' (C/ME) were already collaborating on improving standardization of case classification and reporting practices to augment the both quality and comprehensiveness of national representative data. In addition to this initiative, mortality data for national collation and reporting was already being obtained through various data-sharing agreements between PHAC and the P/Ts, often by the SRHs PHOs, and submitted to the Substance-Related Overdose and Mortality Surveillance (SOMS) Task Group and additional data elements shared as part of the National Chart Review Study.
- Provisional Mortality Data – In most Canadian provinces/territories (P/T), Coroners and Medical Examiners (C/MEs) offices report on drug toxicity deaths. Data are provided to PHAC for national reporting on apparent opioid toxicity and stimulant toxicity deaths.
- Hospitalization Data - Data collected by the Canadian Institute for Health Information (CIHI), and prepared by HC are used for national reporting on hospitalizations for opioid- and stimulant-related poisoning.
- Emergency Medical Services (EMS) Data – Provincial EMS are variably able to provide paramedic-attended overdose event data for national reporting.
United States Surveillance Structure: Pivoting during COVID-19
To address the need for rapid information to guide practice and policy development and to address the difficulties in collecting certain types of data during the COVID-19 pandemic, surveillance tools utilized in the U.S. were adjusted to circumvent data lags from traditional surveillance systems.
U.S. Mortality Data
Given the typical lag time of 12 months for final mortality data nationally in the U.S., CDC and other public health entities relied on provisional estimates of predicted drug overdose deaths in the U.S., overall and by state, from CDC's NVSS to understand the impact of the pandemic on overdose deaths. NVSS data capture all deaths, including drug overdose deaths, documented on death certificates submitted by state and jurisdiction vital records offices in the U.S. These provisional data have typically lagged by only 6 months and thus provide early indicators of emerging trends in overdose deaths. In December 2020, there was sufficient data from the early months of the pandemic to make an initial assessment that although overdose deaths were increasing before the COVID-19 pandemic, this increase accelerated in the first few months of the pandemic. Based on these provisional data, CDC issued a Health Alert Network (HAN) Notice – CDC's primary method of sharing information about urgent public health incidents – to raise awareness of the issue and provide action steps that could be taken to help mitigate the rise in overdose deaths.Footnote 49
CDC has now accelerated the availability of provisional data with a data dashboard that now provides predicted overdose death counts nationally and by state with a 4-month lag time from the date of death. CDC is also providing provisional mortality data in its Wide-ranging Online Data for Epidemiologic Research system – a point-and-click system that allows the public to analyze mortality data by various demographic and geographic variables with no more than a 6-month lag. As shown below, the latest provisional data from CDC predict continued increases in overdose deaths, largely driven by synthetic opioids such as IMF and resurgent psychostimulants such as methamphetamine.
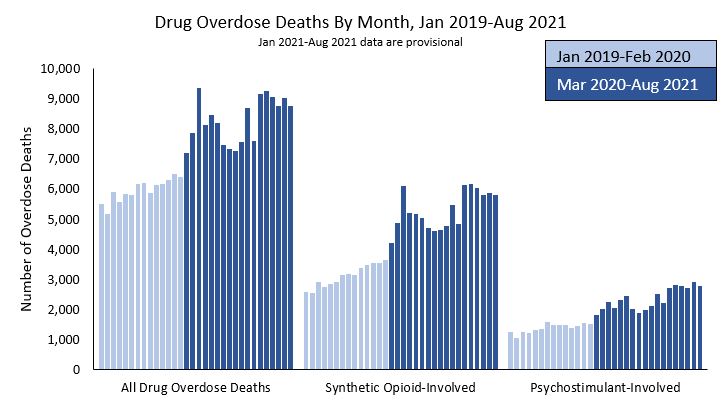
Figure 1 - Text description
This bar graph shows drug overdose deaths by month, from January 2019 through August 2021. The y-axis depicts the number of overdose deaths scaled from 0 to 10,000. The x-axis breaks the monthly data into three categories: all drug overdose deaths, synthetic opioid-involved overdose deaths, and psychostimulant-involved overdose deaths. For each category, drug overdose deaths were higher in the period between March 2020 and August 2021 (an average of 8,293 for all drug overdose deaths; 5,302 for synthetic opioid-involved overdose deaths and 2,343 for psychostimulant-involved overdose deaths) than between January 2019 and February 2020 (an average of 5,965 for all drug overdose deaths; 3,111 for synthetic opioid-involved overdose deaths; and 1,372 for psychostimulant-involved overdose deaths). All drug overdose deaths and synthetic opioid-involved overdose deaths reached a peak in May of 2020, decreased and spiked again in March 2021. Psychostimulant-involved overdose deaths spiked between May and August 2020, decreased and then spiked again in March 2021.
- Footnote * figure 1
-
Data from CDC WONDER as of May 12, 2022
State Unintentional Drug Overdose Reporting System (SUDORS) – Mortality Data
Although NVSS data have been instrumental in tracking overdose deaths, the data included on death certificates are limited, and many contextual factors related to the circumstances and characteristics of overdose decedents are not included. CDC's SUDORS captures data on unintentional and undetermined-intent drug overdose deaths from 47 states and the District of Columbia, providing comprehensive details about overdose deaths not available from other data sources. Data sources include death certificates, full postmortem toxicology testing results, and medical examiner/coroner reports, which include data from death scene investigations and witness findings, for example.
In December 2021, CDC reported on trends in overdose deaths involving IMFs prior to and during the pandemic from July 2019–December 2020. Key findings included:
1) deaths increased sharply in midwestern, southern, and western jurisdictions during 2019–2020;
2) approximately 4 in 10 deaths also involved stimulants;
3) approximately one half of decedents had no pulse when first responders arrived; and
4) evidence of injection was the most frequently documented route of drug use, but substantial percentages of deaths likely involved other routes of administration (e.g., oral or intranasal), especially in the West.Footnote 50
U.S. Syndromic Surveillance
One of the timeliest data sources that provided insight into substance use patterns during the COVID-19 pandemic came from CDC's National Syndromic Surveillance Program (NSSP). NSSP is a collaboration of the CDC, local and state health departments, and healthcare facilities that supports the collection of electronic health record patient encounter data received from EDs, urgent and ambulatory care centers, inpatient facilities, and laboratories. More than 3,500 active emergency facilities that represent portions of 50 states Washington, DC, and Guam, contribute data to NSSP, accounting for approximately 71% of all U.S. ED facilities.
The first public dissemination in the scientific literature using NSSP data to better understand the impact of the COVID-19 response on substance use patterns was in a June 2020 CDC Morbidity and Mortality Weekly Report. [49] In the study, CDC compared the volume of ED visits for four weeks early in the pandemic March 29–April 25, 2020 (weeks 14 to 17; the early pandemic period) to that during March 31–April 27, 2019 (the comparison period). Using the ED visit prevalence ratio (PR) to compare the proportion of ED visits for a specific condition out of all ED visits at that time, CDC found that the ED visit PR was higher in the early pandemic period compared to the pre-pandemic period for mental and SUDs in remission (PR=1.69, 95% CI: 1.64-1.75), stimulant-related disorders (PR=1.65, 95% CI: 1.62-1.67), and tobacco-related disorders (PR=1.19, 95% CI: 1.18-1.19).Footnote 51 A subsequent study using NSSP examining ED visits for drug overdose from December 30, 2018 to October 10, 2020, found ED visits for all drug overdoses slightly decreased from March 29 to April 11 2020 (range, 3.4%-4.3%) compared with the same weeks in 2019, but otherwise weekly counts of all drug and opioid overdoses ranged from 1% to 45% higher in 2020 compared with the same week in 2019. Overdoses exhibited great increases in weekly counts in 2020 compared with 2019.Footnote 52 Using updated NSSP data through January 2021, CDC researchers found that the PR for ED visits for symptoms of mental and substance use conditions among adults was higher in December 2020-January 2021 compared to December 2019-January 2020 (PR=1.28, 95% CI: 1.25-1.30).Footnote 53
The figures below detail weekly ED visit counts (Figure 2) and rates (i.e., the proportion of ED visits related to overdose; Figure 3) for suspected drug, opioid, heroin, and stimulant overdoses from January 2019 prior to the pandemic through December 2021. Consistent with prior research, there is a drop in ED visit counts for the overdose outcomes in the early part of the pandemic in March-April 2020, followed by a rise in ED visits that have remained elevated above pre-pandemic levels (Figure 2).
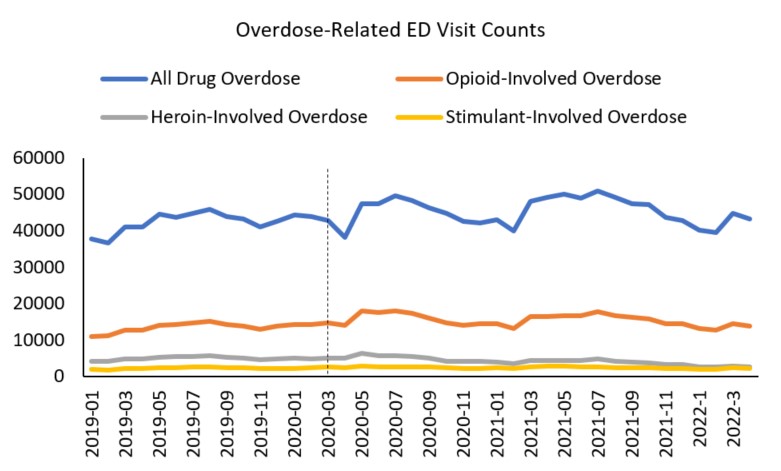
Figure 2 - Text description
This line graph details monthly overdose-related emergency department visit counts for suspected drug-involved, opioid-involved, heroin-involved, and stimulant-involved overdoses from January 2019 through April 2022. A vertical dashed line at March 2020 indicates the start of the COVID-19 pandemic. There is a drop in all drug overdose involved emergency department visit counts between March and April 2020, followed by a rise in all drug-involved overdose emergency department visits, which subsequently remained elevated above pre-pandemic levels. The top line showing all drug-involved overdose-related emergency department visits is 37,891 in January 2019; 45,821 in August 2019; dips to 38,261 in April 2020, peaks at 50,865 in July 2021, and declined to 43,164 in April 2022. The line for opioid-involved overdose-related emergency department visit counts follows a similar trend starting at 11,125 in January 2019, 15,243 in August 2019 dips to 14,185 in April 2020 peaking at 18,130 in May 2021 and declined to 13,932 in April 2022. The line for heroin-involved overdose-related emergency department visit counts starts at 4253 in January 2019, peaks at 6358 in May 2020, and was 2725 in April 2022. The line for stimulant-involved overdose-related emergency department visit counts starts at 1960 in January 2019, was at 2950 in May 2020, and was 2262 in April 2022.
For ED visit rates for overdose outcomes (i.e., the proportion of ED visits related to overdose), there was a spike in ED visit rates in March-June 2020 corresponding to a drop in overall ED visits and then generally a sustained elevation in rate of ED visits throughout most of the pandemic time period (Figure 3). The original sharp increase in rates is most likely a function of the overall decrease in ED visits as people may have delayed or avoided seeking medical care to avoid risk of COVID-19 exposure.
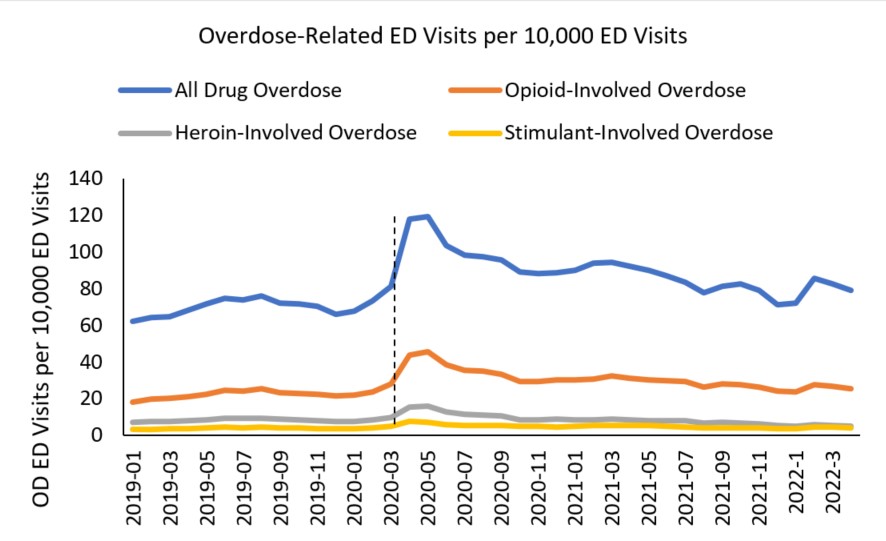
Figure 3 - Text description
This line graph details monthly overdose-related emergency department visits per 10,000 emergency department visits for suspected drug-involved, opioid-involved, heroin-involved, and stimulant-involved overdoses from January 2019 through April 2022. A vertical dashed line at March 2020 indicates the start of the COVID-19 pandemic, after which there is a sharp increase in all-drug overdose and opioid-involved overdose visits. The top line showing the rate of all drug-involved overdose emergency department visit rates is 62.3 in January 2019, 81.5 in March 2020, spikes to 119.1 in May of 2020, and decreases to 79.2 in April 2022. The line for opioid-involved overdose emergency department visit rates follows the same trend starting at 18.3 in January 2019, 28.3 in March 2020 peaking at 45.5 in May 2020, and decreases to 25.6 in April 2022. The line for heroin-involved emergency department visit rates starts at 7.0 in January 2019, is 9.9 in March 2020, peaks at 15.9 in May 2020, and decreases to 4.2 in April 2022. The line for stimulant-involved emergency department visit rates starts at 3.2. in January 2019, is 5.1 in March 2020, peaks at 7.7 in April 2020, and decreases to 4.2 in April 2022.
U.S. Urine Drug Testing Data
An additional timely data source that can be used to track changing substance use patterns is urine drug testing data. Several large proprietary data sets that capture urine drug testing results were used during the COVID-19 pandemic. First, a study conducted by HHS researchers used Millennium Health data on urine drug tests ordered between November 14, 2019 and July 10, 2020 for patients diagnosed with or at risk for SUD to compare results for non-prescribed fentanyl, heroin, cocaine, and methamphetamine before and during the pandemic.Footnote 52 Compared with the period before COVID-19, the percentage of specimens testing positive during the COVID-19 period increased from 3.80% to 7.32% for fentanyl (adjusted OR, 1.67 [95% CI, 1.55-1.81]; P < .001), from 1.29% to 2.09% for heroin (adjusted OR, 1.33 [95% CI, 1.11-1.61]; P = .002), from 3.59% to 4.76% for cocaine (adjusted OR, 1.19 [95% CI, 1.11-1.29]; P < .001), and from 5.89% to 8.16% for methamphetamine (adjusted OR, 1.23 [95% CI, 1.14-1.32]; P < .001).Footnote 54
A study by CDC researchers using urine drug testing data examined trends in testing for fentanyl and the percentage of positive test results before and during the pandemic.Footnote 55 Data were from Quest Diagnostics and included clinical drug monitoring of urine specimens from patients residing in all U.S. states and the District of Columbia. Among the 373,946 included specimens, 57,749 (15.4%) were from patients receiving MOUD. As seen in Figure 4 below, the numbers of specimens tested among patients receiving and not receiving MOUD declined 65% and 72%, respectively, during March 29–April 11, 2020 (weeks 14–15), compared with the same weeks in 2019. During September–December 2020 (weeks 35–52), the numbers of specimens tested among patients receiving and not receiving MOUD were 43% and 13% lower, respectively, compared with the same period in 2019.
In the first 2 full weeks of January 2019 (well before the beginning of the COVID-19 pandemic), 9.6% of specimens from patients receiving MOUD tested positive for nonprescribed fentanyl; the percentage testing positive approximately doubled by the end of 2019 (weeks 51–52) to 26.7% (Figure 4, Part A). During 2020, positive test result rates were highest during March 29–April 11 (weeks 13–14) when the percentage of positive test results peaked at 40.5%. During April 26–May 9, 2020 (weeks 17–18), the percentage of positive test results declined to 24.3% and continued to decline during September– December 2020 (weeks 35–52; range = 11.9%–18.5% positive per week), levels considerably lower than the corresponding period during 2019 (range = 18.5%–26.7% per week). Among patients not receiving MOUD (Figure 4, Part B), the prevalence of positive nonprescribed fentanyl test results did not increase significantly during the early pandemic months compared with that in 2019. A limited but significant increase during the second half of 2020 (weeks 27–52; range = 1.4%–1.8%) was observed, compared with the corresponding period during 2019 (range = 1.1%–1.7%).
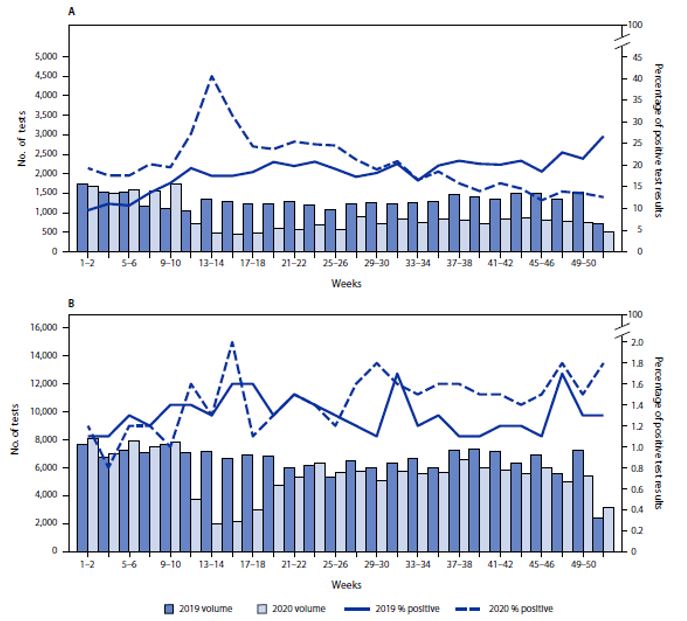
Figure 4 - Text description
This is a two-panel figure. Each panel shows the total volume of urine tests for fentanyl on the left-hand y-axis, the percentage of positive test results on the right-hand y-axis, and weeks from one to 52 in two weeks increments on the x axis. Dark and light blue bars show the volume of urine tests for each two-week increment for 2019 and 2020, respectively. A solid line and a dashed line show the percent positive tests for each two-week increment in 2019 and 2020, respectively. The top panel reflects data from patients receiving MOUD; the bottom panel reflects data from patients not receiving MOUD.
The top panel shows that in the first two full weeks of January 2019, 9.6% of specimens from patients receiving MOUD tested positive for nonprescribed fentanyl; the percentage testing positive approximately doubled by weeks 51–52 to 26.7%. During 2020, positive test result rates were highest during weeks 13–14, peaking at 40.5%. During weeks 17–18, the percentage of positive test results declined to 24.3% and continued to decline during weeks 35–52 to levels considerably lower than the corresponding period during 2019. Among patients not receiving MOUD, the prevalence of positive nonprescribed fentanyl test results did not increase significantly during the early pandemic months compared with that in 2019. There was a significant increase in the percent of positive test results during the second half of 2020, between weeks 27–52, compared with the corresponding period during 2019.
Abbreviation: ICD-10-CM= International Classification of Diseases, Tenth Revision, Clinical Modification
- Footnote * figure 4
-
Primary and secondary y-axis scales are different between panels.
- Footnote † figure 4
-
Patients receiving medication for opioid use disorder were defined as those with an ICD-10-CM code F11 (opioid related disorders) and a positive urine drug test result for buprenorphine or methadone indicated as prescribed.
- Footnote § figure 4
-
Patients not receiving medication for opioid use disorder had no ICD-10-CM F11 code and a negative urine test result for buprenorphine and methadone.
Source: Niles JK, Gudin J, Vivolo-Kantor AM, Gladden RM, Mustaquim D, Seth P, Kaufman HW. Notes from the Field: Testing for Nonprescribed Fentanyl and Percentage of Positive Test Results Among Patients with Opioid Use Disorder—United States, 2019–2020. Morbidity and Mortality Weekly Report. 2021 Nov 26;70(47):1649-51.
U.S. Online Surveys of Adults
One approach to addressing the delayed collection of in-person substance use survey data was through use of internet-based and/or phone-based surveys, which do not rely on in-person data collection. The CDC reported on substance use and mental-health-related findings among U.S. adults during the COVID-19 pandemic based on data from an online respondent panel.Footnote 56,Footnote 57,Footnote 58, Footnote 59 In the first published survey, which reflected respondents surveyed between June 24-30, 2020, 40.9% of overall respondents reported an adverse mental health or behavioral health symptoms, including 13.3% of respondents who started or increased substance use to cope with stress or emotions related to COVID-19.Footnote 56 A subsequent survey found similar rates of substance use and mental health challenges among respondents surveyed in August-September 2020.Footnote 57 Importantly, prevalence of substance use and mental health challenges varied by demographic characteristics in these surveys (Table 1).Footnote 56,Footnote 57,Footnote 58, Footnote 59 Prevalence of adverse mental or behavioral health symptoms was also higher among respondents with disabilities; caregivers for adults; essential workers and unemployed respondents; and respondents who were lesbian, gay, or bisexual.Footnote 57
| Prevalence ratio (95% CI) | ||||
|---|---|---|---|---|
| Characteristic | Symptoms of anxiety disorder or depressive disorder† | Symptoms of a TSRD related to COVID-19§ | Started or increased substance use to cope with stress or emotions related to COVID-19 | Serious consideration of suicide in past 30 days |
| Gender | ||||
| Female vs. male | 1.04 (0.96-1.12) | 0.88 (0.81-0.97) | 0.85 (0.75-0.98) | 0.70 (0.60-0.82)** |
| Age group (yrs) | ||||
| 18-24 vs. 25-44 | 1.56 (1.44-1.68)** | 1.28 (1.16-1.41)** | 1.31 (1.12-1.53)** | 1.59 (1.35-1.87)** |
| 18-24 vs. 45-64 | 3.10 (2.79-3.44)** | 2.67 (2.35-3.03)** | 3.35 (2.75-4.10)** | 6.66 (5.15-8.61)** |
| 18-24 vs. ≥65 | 7.73 (6.19-9.66)** | 5.01 (4.04-6.22)** | 8.77 (5.95-12.93)** | 12.51 (7.88-19.86)** |
| 25-44 vs. 45-64 | 1.99 (1.79-2.21)** | 2.09 (1.86-2.35)** | 2.56 (2.14-3.07)** | 4.18 (3.26-5.36)** |
| 25-44 vs. ≥65 | 4.96 (3.97-6.20)** | 3.93 (3.18-4.85)** | 6.70 (4.59-9.78)** | 7.86 (4.98-12.41)** |
| 45-64 vs. ≥65 | 2.49 (1.98-3.15)** | 1.88 (1.50-2.35)** | 2.62 (1.76-3.9)** | 1.88 (1.14-3.10) |
| Race/Ethnicity†† | ||||
| Hispanic vs. non-Hispanic black | 1.35 (1.18-1.56)** | 1.15 (1.00-1.33) | 1.19 (0.97-1.46) | 1.23 (0.98-1.55) |
| Hispanic vs. non-Hispanic Asian | 2.27 (1.73-2.98)** | 1.59 (1.24-2.04)** | 3.29 (2.05-5.28)** | 2.82 (1.74-4.57)** |
| Hispanic vs. non-Hispanic other race or multiple races | 1.23 (0.98-1.55) | 1.24 (0.96-1.61) | 1.99 (1.27-3.13)** | 1.89 (1.16-3.06) |
| Hispanic vs. non-Hispanic white | 1.40 (1.27-1.54)** | 1.50 (1.35-1.68)** | 2.09 (1.79-2.45)** | 2.35 (1.96-2.80)** |
| Non-Hispanic black vs. non-Hispanic Asian | 1.68 (1.26-2.23)** | 1.38 (1.07-1.78) | 2.75 (1.70-4.47)** | 2.29 (1.39-3.76)** |
| Non-Hispanic black vs. non-Hispanic other race or multiple races | 0.91 (0.71-1.16) | 1.08 (0.82-1.41) | 1.67 (1.05-2.65) | 1.53 (0.93-2.52) |
| Non-Hispanic black vs. non-Hispanic white | 1.03 (0.91-1.17) | 1.30 (1.14-1.48)** | 1.75 (1.45-2.11)** | 1.90 (1.54-2.36)** |
| Non-Hispanic Asian vs. non-Hispanic other race or multiple races | 0.54 (0.39-0.76)** | 0.78 (0.56-1.09) | 0.61 (0.32-1.14) | 0.67 (0.35-1.29) |
| Non-Hispanic Asian vs. non-Hispanic white | 0.62 (0.47-0.80)** | 0.95 (0.74-1.20) | 0.64 (0.40-1.02) | 0.83 (0.52-1.34) |
| Non-Hispanic other race or multiple races vs. non-Hispanic white | 1.14 (0.91-1.42) | 1.21 (0.94-1.56) | 1.05 (0.67-1.64) | 1.24 (0.77-2) |
Reproduced from Czeisler ME et al., Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic – United States, June 24-30, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(32):1049-1057. TSRD = trauma- and stress-related disorder.
U.S. Surveys of Youth
As with online surveys of adults, surveillance to document youth substance use was also implemented. Data comparing the first 3 months of 2020 (just before the COVID-19 pandemic in the U.S.) to 2021 (during the pandemic) are available from the Monitoring the Future (MTF) survey of 8th, 10th, and 12th graders conducted annually by the University of Michigan with support from the National Institute on Drug Abuse. MTF found that rates of substance use by teens in the U.S. decreased significantly in 2021 compared to early 2020 (prior to the COVID-19 pandemic) across nearly every substance category (Figure 5). Past-12-month use of cannabis, any illicit drug other than cannabis, and nicotine vaping declined for all three grades, and past-12-month use of alcohol declined for 10th and 12th graders.Footnote 60 In 2021, MTF also asked students about whether they experienced increases or decreases in a variety of mental health issues since the start of the pandemic.Footnote 60 Students in all three grades reported increases in all of these mental health symptoms and signs during the pandemic.
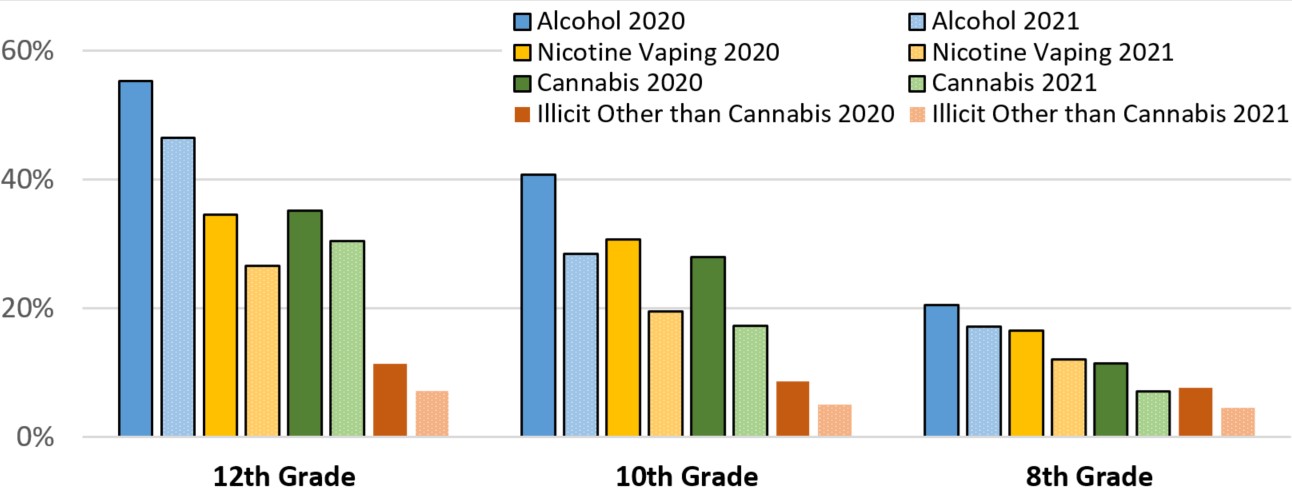
Figure 5 - Text description
This is a bar chart comparing pre-pandemic 2020 to 2021 levels of past-year alcohol use, nicotine vaping, cannabis use, and illicit substance use other than cannabis use among 12th, 10th, and 8th graders. The y-axis shows the percentage from 0 to 60; the x-axis shows 8 bars each for 12th, 10th, and 8th grade students corresponding to pre-pandemic 2020 and 2021 alcohol use, nicotine vaping, cannabis use, and illicit substance use other than cannabis use. In all cases, rates of substance use decreased in 2021 compared to early 2020. The percentage of 12th graders using each category of drugs in the pre-pandemic 2020 vs 2021 periods are as follows: alcohol – 55.3 vs 46.5; nicotine vaping – 34.5 vs 26.6; cannabis – 35.2 vs 30.5; and illicit other than cannabis – 11.4 vs 7.2. The percentage of 10th graders using each category of drugs in the pre-pandemic 2020 vs 2021 periods are as follows: alcohol – 40.7 vs 28.5; nicotine vaping – 30.7 vs 19.5; cannabis – 28.0 vs 17.3; and illicit other than cannabis – 8.6 vs 5.1. The percentage of 10th graders using each category of drugs in the pre-pandemic 2020 vs 2021 periods are as follows: alcohol – 20.5 vs 17.2; nicotine vaping – 16.6 vs 12.1; cannabis – 11.4 vs 7.1; and illicit other than cannabis –7.7 vs 4.6.
Source: Johnston LD, Miech RA, O'Malley PM, Bachman JG, Schulenberg JE, Patrick ME. Monitoring the Future national survey results on drug use 1975-2021: Overview, key findings on adolescent drug use. Ann Arbor: Institute for Social Research, University of Michigan, 2022.
In addition, CDC conducted the Adolescent Behavior and Experiences Survey (ABES) during January–June 2021 to assess student behaviors and experiences during the COVID-19 pandemic. ABES was a one-time, probability-based online survey of U.S. high school students. ABES found that approximately one third of students who used vaping products did so daily, and 22.4% of students who drank alcohol did so ≥6 times per month. Preventing adolescent nicotine use is important because research suggests that adolescent exposure to nicotine increases susceptibility to addiction to other substances,Footnote 61,Footnote 62 including opioids.Footnote 62 Approximately one in three students who ever used alcohol or other drugs reported using these substances more during the pandemic. The prevalence of substance use was typically higher among non-Hispanic American Indian and Alaska Native students, older students, and gay, lesbian, or bisexual students than among students of other racial or ethnic groups, younger students, and heterosexual students. The prevalence of alcohol use also was higher among non-Hispanic White students than among those of other racial or ethnic groups. Students only attending school virtually had a lower prevalence of using most of the substances examined than did students attending schools with in-person or hybrid models.Footnote 63
Canadian Surveillance Structure: Pivoting during COVID-19
Surveillance, modelling, and applied research evidence has been critical to understanding the scope and nature of Canada's opioid crisis, and in guiding public health action. Some of the pieces were in place prior to COVID-19 and others evolved in response to the exacerbation of morbidity and mortality due to substances since March 2020.
Existing national and provincial surveillance structure that was leveraged during the COVID-19 response and exacerbation of drug toxicity events and deaths:
- Federal epidemiologists deployed to the provinces and territories by the Substance-Related Harms Division of the Public Health Agency of Canada, specifically, the Office of Public Health Field Service and Training in the Emergency Management Branch, with content expertise, surveillance, and research support provided by the Centre for Surveillance and Applied Research of PHAC's Health Promotion and Chronic Disease Prevention Branch.
- This cross-branch model links the SRHs PHOs with support for harm reduction and drug policy knowledge, substance-related harms surveillance and research activities at a national level while supporting technical skills and expertise for setting up SRHs surveillance that serve the provinces and territories.
- SRHs PHOs often prepare the provincial data for submission for national reporting. These resources were pivotal in being able to understand what was happening in the jurisdictions at the start of COVID-19 measures and begin to get some data and intelligence to describe and assess the increases in harms, overdose events and deaths that were being seen.
Conceptual Model
At the outset of the COVID-19 outbreak in Canada, PHAC analysts led the development of a conceptual model to synthesize the available evidence on substance related harms and provide a visual representation of how the pandemic may intersect with these harms.Footnote 64 The model was developed through an iterative process of literature review, discussion within the research group that developed it, and consultation with public health professionals and experts.
The conceptual model incorporates five main interrelated domains (Figure 6). Importantly, these domains likely intersect and interact, which may increase the risk of harms. Also, the concepts and relationships described in this model likely intersect with sociodemographic and socioeconomic factors, reflecting the disproportionate impacts of both substance-related harms and COVID-19 on certain population groups.
The five interrelated domains are:
-
Substance use as a means of coping – people may turn to substances to cope with the effects of the pandemic, including mental health impacts; stigmatization and discrimination; hardships related to employment, finances, and access to basic needs; increased demands on essential workers; and increased barriers to accessing mental health services. [Domain 1]
-
Changes in social support and networks – physical distancing, closures of facilities, limits on gatherings, increased caregiving demands may affect social supports and resources which would otherwise help people cope with stress or overcome a SUD. Changes in supply and access to substances and patterns of substance use with others also increase risk of harms. [Domain 2]
-
Changes in availability and accessibility of services related to substance use and harms – the pandemic has changed the availability and accessibility of treatment services, outreach, and substance-related therapies; harm reduction services; and the capacity of hospitals, EDs, and first responders to treat overdose and other substance-related harms. [Domain 3]
-
Increased risk of COVID-19 transmission among people who use substances - reduced access to harm reduction services may result in the sharing of substance use equipment; stigma may compound this by acting as a barrier to accessing services or being provided high quality services or may increase the likelihood in engaging in harmful behaviors or coping methods. Incarcerated populations experience crowding as well as substance use and associated comorbidities which may elevate the risk of COVID-19 infection and the likelihood of severe outcomes. People experiencing homelessness, instability or inadequate housing may have less regular access to hygiene supplies, may not be able to practice physical distancing, may experience barriers to risk reduction measures for both substance use and COVID-19, and have poorer access to health services. [Domain 4]
-
Increased risk of severe outcomes among people who use substances – many of the conditions identified as risk factors for severe outcomes disproportionately affect people who use substances (e.g., chronic lung disease, cardiovascular disease, weakened immune system); the use of long-acting and immunosuppressive opioids as well as withdrawal (which could result from disruptions in access to opioids during the pandemic) have been associated with increased risk of respiratory infections; withdrawal may mask COVID-19 symptoms; the risk of overdose may be particularly high during the pandemic due to impacts on respiratory function, drug supplies, and access to harm reduction and treatment services. [Domain 5]
The conceptual model has helped inform a dynamic model of opioid use and overdose death, particularly in informing the parameterization of future scenarios during the pandemic, before data were available.
It also offers guidance for areas of investigation where there is currently limited evidence. It has been used to inform data development efforts within PHAC, including content on special surveys implemented during the pandemic period, which have generated data relevant to understanding impacts of the pandemic on substance related harms, pointing to potential public health intervention needs. There is a need for further research investigating the far-reaching implications of COVID-19 on how people use substances in Canada and the unique harms to which they are exposed. Further research investigating how the intersections between the pandemic and substance-related harms affect different groups and communities across Canada is needed to document health inequalities and inequities.
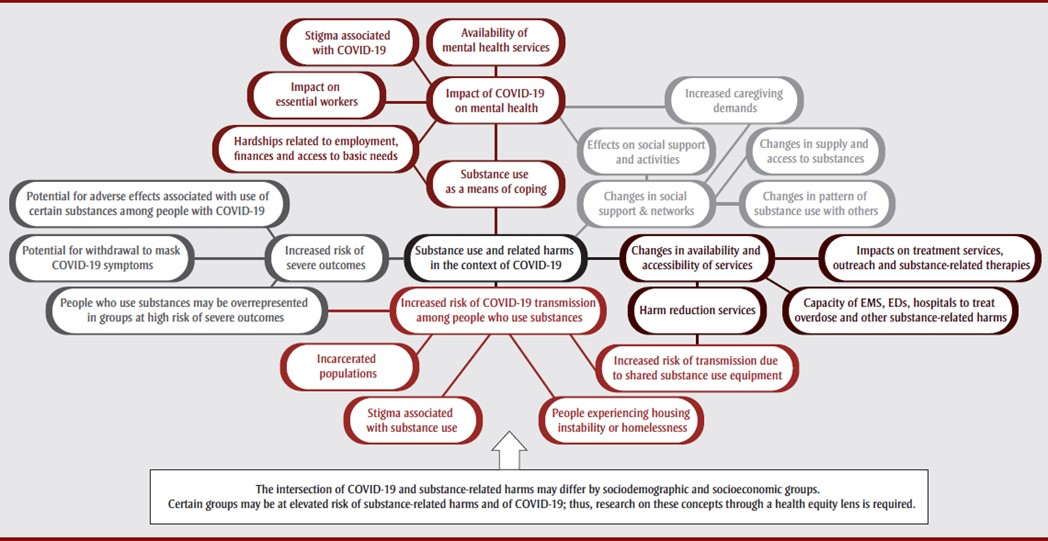
Figure 6 - Text description
| Assigned ID | Text | Heading | Connects to |
|---|---|---|---|
| 0.0 | Substance use and related harms in the context of COVID-19 | Main heading | 1.0, 2.0, 3.0, 4.0, 5.0 |
| 1.0 | Substance use as a means of coping | Domain 1 heading | 0.0, 1.1, 1.2 |
| 1.1 | Hardships related to employment, finances and access to basic needs | Subheading within domain 1 | 1.0, 1.2 |
| 1.2 | Impact of COVID-19 on mental health | Subheading within domain 1 | 1.0, 1.1, 1.3, 1.4, 1.5 |
| 1.3 | Impact on essential workers | Subheading within domain 1 | 1.2 |
| 1.4 | Stigma associated with COVID-19 | Subheading within domain 1 | 1.2 |
| 1.5 | Availability of mental health services | Subheading within domain 1 | 1.2 |
| 2.0 | Changes in social support & networks | Domain 2 heading | 0.0, 2.1, 2.2, 2.3, 2.4 |
| 2.1 | Effects on social support and activities | Subheading within domain 2 | 2.0, 1.2 |
| 2.2 | Increased caregiving demands | Subheading within domain 2 | 2.0, 1.2 |
| 2.3 | Changes in supply and access to substances | Subheading within domain 2 | 2.0 |
| 2.4 | Change in patterns of substance use with others | Subheading within domain 2 | 2.0 |
| 3.0 | Changes in availability and accessibility of services | Domain 3 heading | 0.0, 3.1, 3.2, 3.3 |
| 3.1 | Impacts on treatment services, outreach and substance-related therapies | Subheading within domain 3 | 3.0 |
| 3.2 | Capacity of EMS, EDs, hospitals to treat overdose and other substance-related harms | Subheading within domain 3 | 3.0 |
| 3.3 | Harm reduction services | Subheading within domain 3 | 3.0, 4.1 |
| 4.0 | Increased risk of COVID-19 transmission among people who use substances | Domain 4 heading | 0.0, 4.1, 4.2, 4.3, 4.4, 5.1 |
| 4.1 | Increased risk of transmission due to shared substance use equipment | Subheading within domain 4 | 4.0, 3.3 |
| 4.2 | People experiencing housing instability or homelessness | Subheading within domain 4 | 4.0 |
| 4.3 | Stigma associated with substance use | Subheading within domain 4 | 4.0 |
| 4.4 | Incarcerated populations | Subheading within domain 4 | 4.0 |
| 5.0 | Increased risk of severe outcomes | Domain 5 heading | 0.0, 5.1, 5.2, 5.3 |
| 5.1 | People who use substances may be overrepresented in groups at high risk of severe outcomes | Subheading within domain 5 | 5.0, 4.0 |
| 5.2 | Potential for withdrawal to mask COVID-19 symptoms | Subheading within domain 5 | 5.0 |
| 5.3 | Potential for adverse effects associated with use of certain substances among people with COVID-19 | Subheading within domain 5 | 5.0 |
Note: The intersection of COVID-19 and substance-related harms may differ by sociodemographic and socioeconomic groups. Certain groups may be at elevated risk of substance-related harms and of COVID-19; thus, research on these concepts through a health equity lens is required.
Dynamic Model
Modelling opioid-related deaths during the COVID-19 outbreak
PHAC's Centre for Surveillance and Applied Research (CSAR) developed a simulation model of opioid-related deaths to project the number of these deaths that might occur as the COVID-19 outbreak evolved. Projections covered the period from 2020 through to December 2022 through various releases and updates of the model incorporating newer data and other changes. The model leverages the existing quarterly reporting on opioid-related harms, the model helped to provide a national picture, inform decision-making, and guide response efforts.
The model is a simulation model, or a system dynamics model of opioid-related deaths, using mathematical equations to estimate and project how many cases may occur in the coming weeks or months, simulating real-world possibilities in a virtual environment. Models are not reliable predictors, but they can help understand and plan for what might happen in certain scenarios.
Simulations were based on several scenarios focusing on the level of toxic fentanyl and its analogues in the drug supply and the proportion of opioid-related deaths prevented by health interventions, like supervised consumption sites and naloxone use. The results of the model continued to suggest that, under some scenarios, the number of opioid-related deaths would remain high or increase through to December 2022.
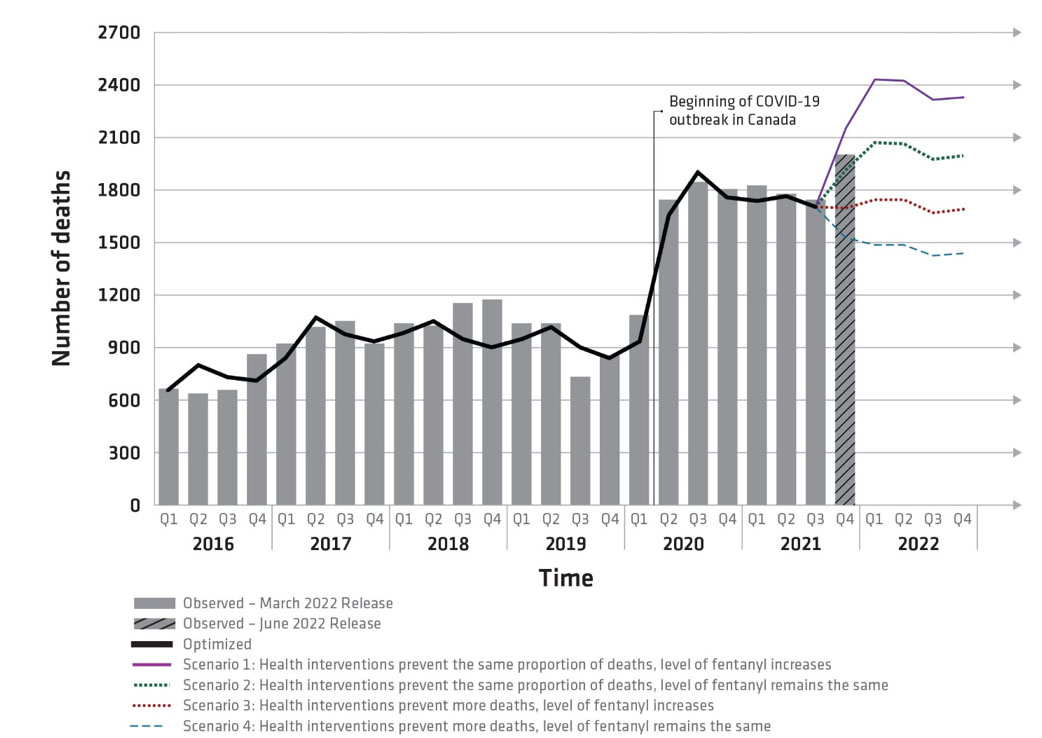
Figure 7 - Text description
Observed and projected opioid-related deaths, Canada, January 2016 to December 2022
- Scenario 1 (purple line): If health interventions prevent 30% of opioid-related deaths from October 2021 through December 2022 and the level of fentanyl in the drug supply increases after December 2021, then a further increase in deaths may occur through December 2022.
- Scenario 2 (green line): If health interventions prevent 30% of opioid-related deaths from October 2021 through December 2022, and the level of fentanyl in the drug supply remains the same as in December 2021, then deaths through December 2022 may remain high.
- Scenario 3 (red line): If health interventions improve after September 2021 to prevent 50% of opioid-related deaths, and the level of fentanyl in the drug supply increases after December 2021, then deaths through December 2022 may remain relatively consistent with levels observed from April 2020 through September 2021.
- Scenario 4 (blue line): If health interventions improve after September 2021 to prevent 50% of opioid-related deaths, and the level of fentanyl in the drug supply remains the same as in December 2021, then deaths through December 2022 may decrease but not to below levels seen at the peak of the opioid overdose crisis before the onset of COVID-19.
- Solid black line: represents the model output for the period from January 2016 to September 2021, which was calibrated to observed data shown by the grey bars. Comparing the real data from the past to the simulated deaths from the model shows us that the model is well set up.
When interpreting model results, it is important to recognize that if the model structure, parameter values, or assumptions are incorrect, the simulations may not be an accurate reflection of what may happen. The model considers Canada as a whole and does not account for regional variation in the parameter values in the model. For example, changes to the drug supply may have started earlier in some jurisdictions, and some provinces and territories may have taken steps early in the COVID-19 outbreak to adapt health interventions to prevent opioid-related deaths. As with all models, if the projections result in the introduction of measures by officials to reduce the number of opioid-related deaths, the projected numbers become less accurate.
Model development
Model parameter values were identified from various data and information sources, including Statistics Canada, CIHI, PHAC, HC, Provinces and Territories, and peer-reviewed literature. In the model, people using opioids can die from an opioid overdose at different rates depending on the type of opioid use. Mortality rates for non-medical opioid use are also affected by seasonality, level of fentanyl in the drug supply, and proportion of deaths prevented by health interventions designed to reduce opioid-related deaths.
One notable change in the developing iterations of the model was a switch away from using the PHAC Opioid-and Stimulant-related Harms Surveillance for information on the level of fentanyl in the drug supply. Instead, HC's Drug Analysis Service data are used for this.
National Substance-Related Harms Surveillance Reporting
Canadian national-level reporting on substance-related harms currently includes data on opioid- and stimulant-related toxicity deaths, emergency medical services (EMS) responses to suspected opioid-related overdoses, and opioid- and stimulant-related poisoning hospitalizations. National data are updated on a quarterly basis, through public-facing reports and interactive data products. Surveillance data are submitted by provincial and territorial partners (for mortality and EMS), and administrative data from the Canadian Institute of Health Information (for hospitalizations), supported by HC.
The surveillance system was initiated in late 2016 with mortality data and was incrementally expanded over time to include other data elements and data sources. National surveillance was most recently published in June 2022, reflecting data spanning January 2016 to December 2021. National data are published with a six-month time lag, and individual provinces and territories may also independently report on their data with a shorter lag.
National reporting has confirmed that the COVID-19 pandemic exacerbated the already severe and ongoing public health overdose crisis.
Mortality
Across Canada, surveillance estimates that there were 29,052 apparent opioid toxicity deaths between January 2016 and December 2021. During the first year of the pandemic, there was a 95% increase in apparent opioid toxicity deaths (April 2020 – March 2021, 7,362 deaths), compared to the year before (April 2019 – March 2020, 3,747 deaths). Since then, deaths have remained high.
In 2021, there were 7,560 deaths from apparent opioid toxicity. This is approximately 21 deaths per day. In the years prior to the pandemic, there were between 8 (in 2016) and 12 (in 2018) deaths per day. A number of factors may have contributed to a worsening of the toxic drug events and deaths over the course of the pandemic, including the increasingly toxic drug supply; increased feelings of isolation, stress, and anxiety; and changes in the availability or accessibility of services for people who use drugs, including fewer safe spaces to consume drugs.
The majority of deaths have occurred in British Columbia, Alberta and Ontario. In 2021, these three provinces accounted for 88% of all apparent opioid toxicity deaths. Elevated rates have also been observed in other areas, including Yukon and Saskatchewan.
Most apparent opioid toxicity deaths were among young- to middle-aged males. Males accounted for the majority of accidental apparent opioid toxicity deaths (74%) in 2021. For males and for females, the majority of accidental apparent opioid toxicity deaths were among individuals aged 20 to 59 years.
Toxicity of supply continues to be a major driver of the crisis. Of all accidental apparent opioid toxicity deaths in 2021, 86% involved fentanyl, and 81% involved opioids that were only non-pharmaceutical.
The number of apparent stimulant toxicity deaths in 2021 was high; almost all (98%) of those deaths were accidental. More than half (59%) of accidental apparent opioid toxicity deaths in 2021 also involved a stimulant, reflecting the polysubstance nature of this crisis. Of the accidental apparent stimulant toxicity deaths in those months, 62% involved cocaine, while 55% involved methamphetamines. Of the accidental apparent stimulant toxicity deaths, 86% involved an opioid.
It is important to note that stimulant data were only available from six provinces and territories in 2021. Furthermore, data on opioid and stimulant toxicity deaths are not mutually exclusive.
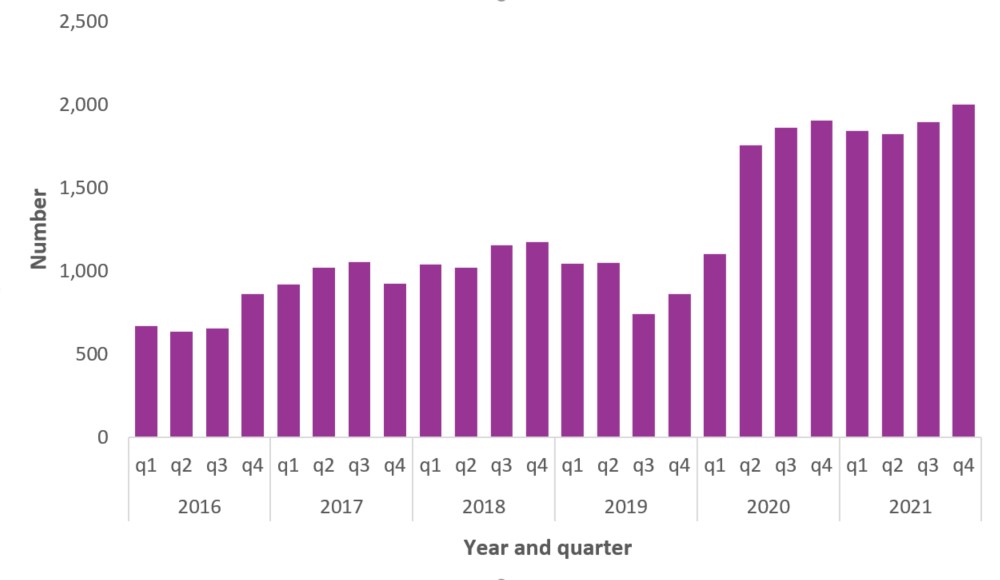
Figure 8 - Text description
| Year | Quarter | Number of deaths |
|---|---|---|
| 2016 | 1st quarter | 668 |
| 2nd quarter | 637 | |
| 3rd quarter | 654 | |
| 4th quarter | 860 | |
| 2017 | 1st quarter | 920 |
| 2nd quarter | 1,021 | |
| 3rd quarter | 1,054 | |
| 4th quarter | 921 | |
| 2018 | 1st quarter | 1,039 |
| 2nd quarter | 1,021 | |
| 3rd quarter | 1,154 | |
| 4th quarter | 1,172 | |
| 2019 | 1st quarter | 1,042 |
| 2nd quarter | 1,048 | |
| 3rd quarter | 739 | |
| 4th quarter | 860 | |
| 2020 | 1st quarter | 1,100 |
| 2nd quarter | 1,756 | |
| 3rd quarter | 1,860 | |
| 4th quarter | 1,905 | |
| 2021 | 1st quarter | 1,841 |
| 2nd quarter | 1,821 | |
| 3rd quarter | 1,893 | |
| 4th quarter | 2,001 |
Emergency Medical Services (Paramedic-Attended Overdose Events)
A total of 41,686 EMS responses to suspected opioid-related overdoses occurred in 2021, based on available data from nine provinces and territories. For a similar timeframe in 2019, before the pandemic, there were 21,759 EMS responses; this represents a 92% increase.
Of the EMS responses for suspected opioid-related overdoses between in 2021, 73% were among males. The majority of EMS responses for suspected opioid-related overdoses in that period were among those aged 20 to 49 years; however, variations are apparent between P/Ts.
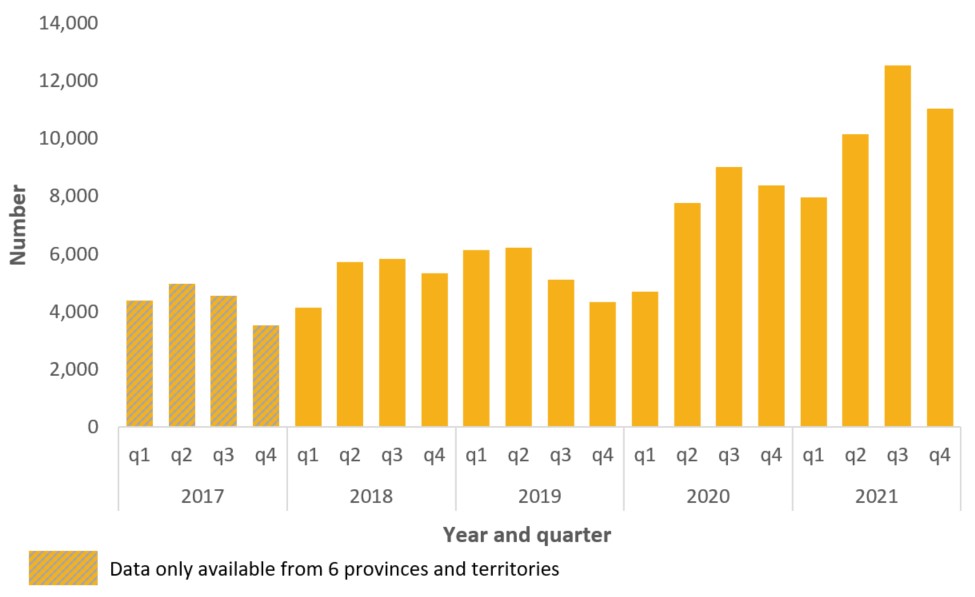
Figure 9 - Text description
| Year | Quarter | Number of EMS responses |
|---|---|---|
| 2017 | 1st quarter | 4,382 |
| 2nd quarter | 4,973 | |
| 3rd quarter | 4,552 | |
| 4th quarter | 3,506 | |
| 2018 | 1st quarter | 4,120 |
| 2nd quarter | 5,721 | |
| 3rd quarter | 5,819 | |
| 4th quarter | 5,320 | |
| 2019 | 1st quarter | 6,120 |
| 2nd quarter | 6,220 | |
| 3rd quarter | 5,102 | |
| 4th quarter | 4,312 | |
| 2020 | 1st quarter | 4,675 |
| 2nd quarter | 7,756 | |
| 3rd quarter | 8,998 | |
| 4th quarter | 8,367 | |
| 2021 | 1st quarter | 7,960 |
| 2nd quarter | 10,135 | |
| 3rd quarter | 12,544 | |
| 4th quarter | 11,047 |
Hospitalizations Related to Opioids and/or Stimulants
There was a total of 30,860 opioid-related and 13,575 stimulant-related poisoning hospitalizations from January 2016 to December 2021 in Canada (excluding Quebec).
During the first year of the pandemic, there was a 27% increase in opioid-related poisoning hospitalizations (April 2020 – March 2021, 5,599 hospitalizations), compared to the year before (April 2019 – March 2020, 4,426 hospitalizations). Since then, opioid-related poisoning hospitalizations have continued to increase.
A total of 6,164 opioid-related poisoning hospitalizations occurred in 2021. This is approximately 17 hospitalizations per day. In the years prior to the pandemic, there were between 12 in 2019 and 14 in 2017.
Of all opioid-related poisoning hospitalizations in 2021, 32% involved fentanyl or fentanyl analogues. Among accidental opioid-related poisoning hospitalizations, a larger proportion involved fentanyl (35%) than among intentional poisonings (18%).
Most accidental opioid-related poisoning hospitalizations occurred among males (65%) and among individuals aged 20 to 49 years (58%) in 2021.
Of all opioid-related poisoning hospitalizations, 28% involved co-poisoning with a non-opioid substance; 17% involved co-poisoning with a stimulant. Among intentional opioid-related poisoning hospitalizations, a larger proportion involved a co-occurring non-opioid substance (43%) than among accidental poisonings (24%).
Rates of both opioid- and stimulant-related poisoning hospitalizations continue to remain high in British Columbia, Saskatchewan and Alberta, followed by Territories and Ontario. In 2021, 88% of poisoning hospitalizations involving opioids and 85% of poisoning hospitalizations involving stimulants occurred in British Columbia, Alberta, and Ontario.
A total of 2,387 stimulant-related poisoning hospitalizations occurred in 2021. This is approximately seven hospitalizations per day. In the years prior to the pandemic, there were between five in 2016 and six in 2019.
Most accidental stimulant-related poisoning hospitalizations occurred among males (68%) and among individuals aged 20 to 49 years (72%) in 2021.
Of all stimulant-related poisoning hospitalizations, 61% involved co-poisoning with a non-stimulant substance; 43% of all stimulant-related poisoning hospitalizations involved co-poisoning with opioids (including fentanyl or fentanyl analogues), while 24% involved specifically fentanyl or fentanyl analogues. Among accidental stimulant-related poisoning hospitalizations, a larger proportion involved fentanyl or fentanyl analogues (32%) than among intentional (8%) poisonings.
Similar to data on opioid- and stimulant-related toxicity deaths, data on opioid-related and stimulant-related poisoning hospitalizations are not mutually exclusive. A proportion of poisoning hospitalizations involving a stimulant also involved an opioid.

Figure 10 - Text description
| Year | Quarter | Number of hospitalizations |
|---|---|---|
| 2016 | 1st quarter | 1,092 |
| 2nd quarter | 1,186 | |
| 3rd quarter | 1,172 | |
| 4th quarter | 1,217 | |
| 2017 | 1st quarter | 1,256 |
| 2nd quarter | 1,333 | |
| 3rd quarter | 1,359 | |
| 4th quarter | 1,252 | |
| 2018 | 1st quarter | 1,132 |
| 2nd quarter | 1,344 | |
| 3rd quarter | 1,326 | |
| 4th quarter | 1,237 | |
| 2019 | 1st quarter | 1,142 |
| 2nd quarter | 1,216 | |
| 3rd quarter | 1,140 | |
| 4th quarter | 998 | |
| 2020 | 1st quarter | 1,067 |
| 2nd quarter | 1,315 | |
| 3rd quarter | 1,412 | |
| 4th quarter | 1,434 | |
| 2021 | 1st quarter | 1,438 |
| 2nd quarter | 1,529 | |
| 3rd quarter | 1,649 | |
| 4th quarter | 1,531 |
Source: Health Canada Data Partners: provincial and territorial offices of Chief Coroners and Chief Medical Examiners, P/T public health, health partners, Emergency Medical Services data providers, and the Canadian Institute for Health Information (CIHI)
Citation: Special Advisory Committee on the Epidemic of Opioid Overdoses. Opioid and Stimulant-related Harms in Canada. Ottawa: Public Health Agency of Canada; June 2022. https://health-infobase.canada.ca/substance-related-harms/opioids-stimulants/
Rapid Situational Reporting
At the beginning of the COVID-19 pandemic, concerns were raised regarding the impacts of public health measures for COVID-19 on the drug toxicity crisis. In addition, media reports from several jurisdictions indicated an increase in opioid-related overdoses and deaths.
However, surveillance data was not available to describe the evolving situation or inform decision-making, as is it delayed by approximately six months. Therefore, PHAC reached out to provincial and territorial partners for preliminary information, in order to fill this gap.
A table was created to capture preliminary information from each province and territory, covering data and information on any changes, particularly increases, in existing substance related harms indicators, or new observations. The main areas reported on were near-real time mortality, hospitalizations, ED visits, emergency medical services, or other data sources (e.g., naloxone distribution, safe consumption sites) as available. PHAC collated the returned information and provided a summary of recent media articles, covering local and regional trends, to give context.
Based on existing relationships with provincial and territorial partners, preliminary information was collected and collated to assess the changes and geographic distribution of risk related to toxic substances. It was shared in a manner that safeguarded the information as preliminary and subject to change, correction, and requiring ongoing monitoring, but allowing it to be leveraged as early as possible. Emerging trends and issues of note were shared with senior management in PHAC, and with the members of the Special Advisory Committee on the Epidemic of Opioid Overdoses, and SOMS-TG.
While Canadian national surveillance improved understanding of the situation since 2016, with a focus on mortality, there are ongoing evidence gaps limiting understanding the full magnitude of the crisis and impact on Canadians. Key areas for ongoing development are:
- Polysubstance use
- Circumstances involving non-fatal overdoses (e.g., ED visits)
- Risk factors among sub-populations who have been affected
Enhanced data and surveillance on fatal and non-fatal overdoses would enable a more comprehensive picture of harms across the country and enable us to better inform and target interventions. Finally, there is a need to take a coordinated approach to leveraging toxicology data sources (e.g., targeted wastewater testing, urine drug screening, forensic toxicology testing, drug analysis and community drug checking) along with survey data to monitor the toxicity of the unregulated drug supply while providing context on key patterns and experience related to substance harms.
Surveys assessing impacts of COVID-19 on substance use and service delivery
Supervised consumption sites continue to remain open during the COVID-19 pandemic. From September 2020 to June 2021, 52% of sites reported no operational impacts, while 42% were operating at reduced hours, 7% however, are operating at increased hours. The SCS observed an increased use of different substances than normally used and a decrease in dangerous drug practices, as noted in Figure 11.
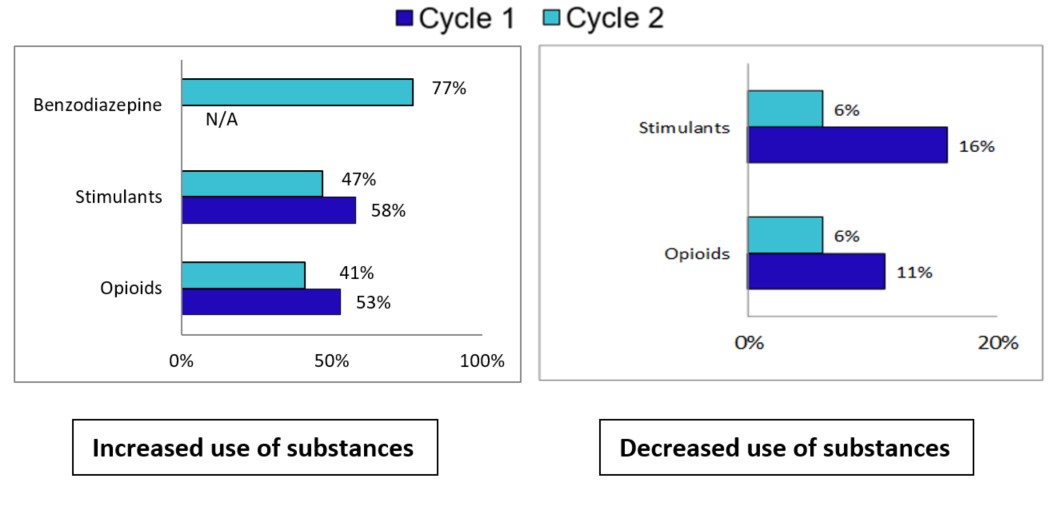
Figure 11 - Text description
| Survey Cycle | Type of Drug | Percentage of Sites Reporting a change |
|---|---|---|
| Cycle 1 | Stimulants | 16% |
| Opioids | 11% | |
| Cycle 2 | Stimulant | 6% |
| Opioids | 6% |
| Survey Cycle | Type of Drug | Percentage of Sites Reporting a change |
|---|---|---|
| Cycle 1 | Benzodiazepine | N/A |
| Stimulants | 58% | |
| Opioids | 53% | |
| Cycle 2 | Benzodiazepine | 77% |
| Stimulants | 47% | |
| Opioids | 41% |
* This is multi-select question, some respondents selected both increases and decreases in the types of substances used.
Source: Health Canada
Conclusions, Implications, and Future Opportunities
In the U.S. and Canada, the pandemic presented unique challenges for many substance use surveillance systems that have historically captured data from in-person surveys or used data from systems that make final data or data sets available months to years after the data were originally collected. In addition, the regular lag time for data availability from these surveillance systems, ranging from 6 months to more than 24 months made them obsolete during the early part of the pandemic when stay-at-home orders and other mitigations measures were in full-effect, constraining understanding by policymakers, public health authorities, and clinical providers of how the pandemic was impacting substance use and overdose patterns. However, because of these challenges, new innovations emerged, and federal scientists utilized other systems such as online panel surveys, syndromic surveillance, urine drug testing, and provisional mortality and forecasting models to identify emerging trends and provide recommendations for action in communities.
With the COVID-19 pandemic highlighting the significant lag times and information demands, further exploration of how to shorten lag times is warranted. Exploration of how new systems and surveillance innovations that arose during the pandemic can be leveraged, refined, and integrated into permanent surveillance systems after the COVID-19 pandemic subsides is also warranted. Similarly, with the COVID-19 pandemic limiting or eliminating access to OUD treatment programs and other harm reduction measures, policy and programmatic actions (e.g.., telehealth exceptions, remote initiation and prescribing of buprenorphine and other controlled substances, safer-supply pilot projects in Canada, and OTP exceptions) were introduced to circumvent the issue of access and reduce the risk of harm for people who use substances. Continued evaluation of these policies introduced during the COVID-19 pandemic is necessary to determine whether the temporary provisions and exemptions should remain.
The rapid spread of COVID-19 drove home the vast interconnectedness of countries. Given that diseases are not limited by borders, countries must take on a worldwide approach working together to prevent, control, and respond to a myriad of interconnected public health challenges. This paper is one step in learning how two countries have taken various approaches in addressing substance use and associated overdose deaths, particularly the opioid crisis, within our borders and observing how our systems and approaches are similar and different.
Building on the collaborations, shared learning, and shared goals of our countries, a logical next step stemming from our efforts to share information about surveillance systems and the impact of the COVID-19 pandemic on substance use and related harms is to continue to engage bilaterally across subject matter experts. This could include engaging in panel discussions, presentations, and additional collaborative papers and publications with the potential of reaching a broader audience. Recognizing the differences in surveillance approaches, another step forward would be in assessing the feasibility of integrating innovative surveillance approaches used by our partner country during the pandemic. Finally, opportunities exist to explore how Canada and the U.S. can continue to work collaboratively and support research efforts on innovative programmatic and policy interventions to address the ongoing overdose crisis.
Notes
- Note 1
-
The policies described here do not pertain to the use of methadone or buprenorphine to treat medical conditions other than OUD, unless otherwise specified. Additionally, the information included below is current as of March 31, 2022.
- Note 2
-
Data from Manitoba were not available for 2021
- Note 3
-
Data for 2017 only includes 6 provinces and territories
- Note 4
-
Data from Quebec were not available
References
- Footnote 1
-
Special Advisory Committee on the Epidemic of Opioid Overdoses. Apparent Opioid and Stimulant Toxicity Deaths--January 2016 to December 2021 [Internet]. Ottawa, On; 2022. Available from: https://health-infobase.canada.ca/src/doc/SRHD/Update_Deaths_2022-06.pdf
- Footnote 2
-
Irvine, M. A., Kuo, M., Buxton, J. A., Balshaw, R., Otterstatter, M., Macdougall, L., Milloy, M-J., Bharmal, A., Henry, B., Tyndall, M., Coombs, D., and Gilbert, M. (2019) Modelling the combined impact of interventions in averting deaths during a synthetic-opioid overdose epidemic. Addiction, 114: 1602– 1613. https://doi.org/10.1111/add.14664
- Footnote 3
-
W.M. Compton, E.B. Einstein and C.M. Jones, Exponential increases in drug overdose: Implications for epidemiology and research, International Journal of Drug Policy, https://doi.org/10.1016/j.drugpo.2022.103676
- Footnote 4
-
Jones CM, Bekheet F, Park JN, Alexander GC. The Evolving Overdose Epidemic: Synthetic Opioids and Rising Stimulant-Related Harms. Epidemiol Rev. 2020 Jan 31;42(1):154-166.
- Footnote 5
-
Centers for Disease Control and Prevention. Drug Overdose Deaths. Updated February 22, 2022. Available from: https://www.cdc.gov/drugoverdose/deaths/index.html. Accessed March 27, 2022.
- Footnote 6
-
Shover CL, Falasinnu TO, Dwyer CL, et al. Steep increases in fentanyl-related mortality west of the Mississippi River: Recent evidence from county and state surveillance. Drug Alcohol Depend. 2020;216:108314. https://www.sciencedirect.com/science/article/pii/S037687162
- Footnote 7
-
Hedegaard H, Bastian BA, Trinidad JP, Spencer MR, Warner M. Regional Differences in the Drugs Most Frequently Involved in Drug Overdose Deaths: United States, 2017. National Vital Statistics Reports; vol 68 no 12. Hyattsville, MD: National Center for Health Statistics. 2019. https://www.cdc.gov/nchs/data/nvsr/nvsr68/nvsr68_12-508.pdf
- Footnote 8
-
O'Donnell J, Gladden RM, Mattson CL, Hunter CT, Davis NL. Vital Signs: Characteristics of Drug Overdose Deaths Involving Opioids and Stimulants — 24 States and the District of Columbia, January–June 2019. MMWR Morb Mortal Wkly Rep 2020;69:1189–1197. http://dx.doi.org/10.15585/mmwr.mm6935a1
- Footnote 9
-
Russell C, Ali F, Nafeh F, Rehm J, LeBlanc S, Elton-Marshall T. Identifying the impacts of the COVID-19 pandemic on service access for people who use drugs (PWUD): A national qualitative study. J Subst Abuse Treat [Internet]. 2021;129:108374. Available from: https://www.sciencedirect.com/science/article/pii/S0740547221001008
- Footnote 10
-
The Ontario Drug Policy Research Network, The Office of the Chief Coroner for Ontario/Ontario Forensic Pathology Service, Public Health Ontario, Centre on Drug Policy Evaluation. Preliminary Patterns in Circumstances Surrounding Opioid-Related Deaths in Ontario during the COVID-19 Pandemic [Internet]. Toronto, On; 2020. Available from: https://www.publichealthontario.ca/-/media/documents/o/2020/opioid-mortality-covid-surveillance-report.pdf?la=en
- Footnote 11
-
CBC News. More people died of an illicit drug overdose in first 8 months of 2020 than all of 2019: B.C. coroner [Internet]. CBC News. 2020. Available from: https://www.cbc.ca/news/canada/british-columbia/overdose-deaths-bc-august-2020-1.5735247
- Footnote 12
-
BC Coroners Service. Illicit drug toxicity deaths in BC January 1, 2020—December 31, 2020 [Internet]. Burnaby, BC; 2022. Available from: https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-and-divorce/deaths/coroners-service/statistical/illicit-drug.pdf
- Footnote 13
-
Nguyen T, Buxton JA. Pathways between COVID-19 public health responses and increasing overdose risks: A rapid review and conceptual framework. Int J Drug Policy [Internet]. 2021;93:103236. Available from: https://www.sciencedirect.com/science/article/pii/S0955395921001419
- Footnote 14
-
Palis H, Bélair M-A, Hu K, Tu A, Buxton J, Slaunwhite A. Overdose deaths and the COVID-19 pandemic in British Columbia, Canada. Drug Alcohol Rev [Internet]. 2021 Dec 15;n/a(n/a). Available from: https://doi.org/10.1111/dar.13424
- Footnote 15
-
Gomes T, Kitchen SA, Murray R. Measuring the Burden of Opioid-Related Mortality in Ontario, Canada, During the COVID-19 Pandemic. JAMA Netw Open [Internet]. 2021 May 26;4(5):e2112865–e2112865. Available from: https://doi.org/10.1001/jamanetworkopen.2021.12865
- Footnote 16
-
CCSA. Impacts of the COVID-19 Pandemic on Substance Use Treatment Capacity in Canada [Internet]. 2020. Available from: https://www.ccsa.ca/sites/default/files/2020-12/CCSA-COVID-19-Impacts-Pandemic-Substance-Use-Treatment-Capacity-Canada-2020-en.pdf
- Footnote 17
-
Galarneau LR, Hilburt J, O’Neill ZR, Buxton JA, Scheuermeyer FX, Dong K, et al. Experiences of people with opioid use disorder during the COVID-19 pandemic: A qualitative study. PLoS One. 2021;16(7):e0255396.
- Footnote 18
-
CCENDU, CCSA. Changes Related to COVID-19 in the Illegal Drug Supply and Access to Services and Resulting Health Harms [Internet]. CCENDU Alert. Ottawa, On; 2020. Available from: https://www.ccsa.ca/sites/default/files/2020-05/CCSA-COVID-19-CCENDU-Illegal-Drug-SupplyAlert-2020-en.pdf
- Footnote 19
-
Ali F, Russell C, Nafeh F, Rehm J, LeBlanc S, Elton-Marshall T. Changes in substance supply and use characteristics among people who use drugs (PWUD) during the COVID-19 global pandemic: A national qualitative assessment in Canada. Int J Drug Policy [Internet]. 2021;93:103237. Available from: https://www.sciencedirect.com/science/article/pii/S0955395921001420
- Footnote 20
-
Alberta Health Services. COVID-19 Scientific Advisory Group Rapid Evidence Report Risk Factors for Severe COVID-19 Outcomes [Internet]. 2021. Available from: https://www.albertahealthservices.ca/assets/info/ppih/if-ppih-covid-19-sag-risk-factors-for-severe-covid-19-outcomes-rapid-review.pdf
- Footnote 21
-
CCSA. Impacts of the COVID-19 Pandemic on People Who Use Substances: What We Heard [Internet]. Ottawa, On; 2020. Available from: https://www.ccsa.ca/impacts-covid-19-pandemic people-who-use-substances-what-we-heard
- Footnote 22
-
Gomes T, Murray R, Kolla G, Leece P, Bansal S, Besharah J, et al. Changing Circumstances Surrounding Opioid-Related Deaths in Ontario during the COVID-19 Pandemic [Internet]. Toronto, On; 2021. Available from: https://www.publichealthontario.ca/-/media/documents/c/2021/changing-circumstances-surrounding-opioid-related-deaths.pdf?la=en
- Footnote 23
-
National Institutes of Health. News Release: Substance use disorders linked to COVID-19 susceptibility. Posted September 14, 2020. Available from: https://www.nih.gov/news-events/news-releases/substance-use-disorders-linked-covid-19-susceptibility#:~:text=A%20National%20Institutes%20of%20Health,on%20Drug%20Abuse%20(NIDA). Accessed March 27, 2022.
- Footnote 24
-
Velásquez García HA, Wilton J, Smolina K, Chong M, Rasali D, Otterstatter M, et al. Mental Health and Substance Use Associated with Hospitalization among People with COVID-19: A Population-Based Cohort Study. Viruses [Internet]. 2021 Oct 31;13(11):2196. Available from: https://pubmed.ncbi.nlm.nih.gov/34835002
- Footnote 25
-
First Nations Health Authority. First Nations Toxic Drug Deaths Doubled During the Pandemic in 2020. Posted May 27, 2021. Available from: https://www.fnha.ca/about/news-and-events/news/first-nations-toxic-drug-deaths-doubled-during-the-pandemic-in-2020
- Footnote 26
-
Substance Abuse and Mental Health Services Administration: The Opioid Crisis and the Black/African American Population: An Urgent Issue. Publication No. PEP20-05-02-001. Office of Behavioral Health Equity. Substance Abuse and Mental Health Services Administration, 2020.
- Footnote 27
-
National Institute on Drug Abuse. News Releases: Substance use disorders linked to COVID-19 susceptibility. Posted September 14, 2020. Available from: https://www.nih.gov/news-events/news-releases/substance-use-disorders-linked-covid-19-susceptibility#:~:text=A%20National%20Institutes%20of%20Health,on%20Drug%20Abuse%20
- Footnote 28
-
CDC. Underlying Medical Conditions Associated with Higher Risk for Severe COVID-19: Information for Healthcare Professionals [Internet]. cdc.gov. 2022. Available from: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-care/underlyingconditions.html#print
- Footnote 29
-
Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol [Internet]. 2006 Aug 1;47(3):330–42. Available from: https://doi.org/10.1111/j.1574-695X.2006.00097.x
- Footnote 30
-
Wang QQ, Kaelber DC, Xu R, Volkow ND. COVID-19 risk and outcomes in patients with substance use disorders: analyses from electronic health records in the United States. Mol Psychiatry. 2021 Jan;26(1):30–9.
- Footnote 31
-
21 USC 823(g); 42 CFR 8.
- Footnote 32
-
21 USC 823(g)(2).
- Footnote 33
-
42 CFR 8.12(i).
- Footnote 34
-
Substance Abuse and Mental Health Services Administration. Opioid Treatment Program (OTP) Guidance. Updated March 19, 2020. Available from: https://www.samhsa.gov/sites/default/files/otp-guidance-20200316.pdf. Accessed March 11, 2022.
- Footnote 35
-
Substance Abuse and Mental Health Services Administration. SAMHSA Extends the Methadone Take-Home Flexibility for One Year While Working Toward a Permanent Solution. Published November 18, 2021. Available from: https://www.samhsa.gov/newsroom/press-announcements/202111181000. Accessed March 11, 2022.
- Footnote 36
-
Drug Enforcement Administration. Letter regarding doorstep delivery. Published March 16, 2020. Available from: https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-015)%20SAMHSA%20Exemption%20NTP%20Deliveries%20(CoronaVirus).pdf. Accessed March 11, 2022.
- Footnote 37
-
Drug Enforcement Administration. Letter regarding unregistered off-site locations for methadone. Published April 7, 2020. Available from: https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-025)(DEA078)_Off-site_OTP_delivery_method_(Final)+_esign.pdf. Accessed March 11, 2022.
- Footnote 38
-
Drug Enforcement Administration. Letter regarding unregistered off-site locations for buprenorphine. Published April 28, 2020. Available from: https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-030)(DEA087)_off_site_otp_delivery_buprenorphine_(esign).pdf. Accessed March 11, 2022.
- Footnote 39
-
Drug Enforcement Administration. Registration Requirements for Narcotic Treatment Programs with Mobile Components. 86 FR 33861. Federal Register. Published June 28, 2021. Available from: https://www.federalregister.gov/documents/2021/06/28/2021-13519/registration-requirements-for-narcotic-treatment-programs-with-mobile-components. Accessed March 11, 2022.
- Footnote 40
-
21 CFR 1306.07(b).
- Footnote 41
-
Drug Enforcement Administration. Instructions to request exception to 21 CFR 1306.07(b) 3-day rule (EO-DEA248). Published March 23, 2022. Available from: https://www.deadiversion.usdoj.gov/drugreg/Instructions-to-request-exception-to-21CFR1306.07(b)-3-day-rule-(EO-DEA248)-Clean.pdf. Accessed March 31, 2022.
- Footnote 42
-
21 USC 829(e); 21 USC 802(54).
- Footnote 43
-
Drug Enforcement Administration. How to Prescribe Controlled Substances to Patients During the COVID-19 Public Health Emergency. Available from: https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-023)(DEA075)Decision_Tree_(Final)_33120_2007.pdf. Accessed March 11, 2022.
- Footnote 44
-
Drug Enforcement Administration. Letter regarding prescribing and dispensing of buprenorphine via telemedicine. Published March 31, 2020. Available from: https://www.deadiversion.usdoj.gov/GDP/(DEA-DC-022)(DEA068)%20DEA%20SAMHSA%20buprenorphine%20telemedicine%20%20(Final)%20+Esign.pdf. Accessed March 11, 2022.
- Footnote 45
-
Substance Abuse and Mental Health Services Administration. FAQs: Provision of methadone and buprenorphine for the treatment of Opioid Use Disorder in the COVID-19 emergency. Updated April 21, 2020. Available from: https://www.samhsa.gov/sites/default/files/faqs-for-oud-prescribing-and-dispensing.pdf. Accessed March 12, 2022.
- Footnote 46
-
Centers for Medicare & Medicaid Services. Medicare and Medicaid Programs; Policy and Regulatory Revisions in Response to the COVID-19 Public Health Emergency. 85 FR 19230. Federal Register. Published April 6, 2020. Available from: https://www.federalregister.gov/documents/2020/04/06/2020-06990/medicare-and-medicaid-programs-policy-and-regulatory-revisions-in-response-to-the-covid-19-public. Accessed March 11, 2022.
- Footnote 47
-
Substance Abuse and Mental Health Services Administration. Become a Buprenorphine Waivered Practitioner. Updated January 3, 2022. Available from: https://www.samhsa.gov/medication-assisted-treatment/become-buprenorphine-waivered-practitioner. Accessed March 12, 2022.
- Footnote 48
-
U.S. Department of Health and Human Services. Practice Guidelines for the Administration of Buprenorphine for Treating Opioid Use Disorder. 86 FR 22439. Federal Register. Published April 28, 2021. Available from: https://www.federalregister.gov/documents/2021/04/28/2021-08961/practice-guidelines-for-the-administration-of-buprenorphine-for-treating-opioid-use-disorderrr. Accessed March 11, 2022.
- Footnote 49
-
Centers for Disease Control and Prevention. Increase in Fatal Drug Overdoses Across the United States Driven by Synthetic Opioids Before and During the COVID-19 Pandemic. Distributed via the CDC Health Alert Network December 17, 2020. Available from: https://emergency.cdc.gov/han/2020/han00438.asp. Accessed March 11, 2022.
- Footnote 50
-
Trends in and Characteristics of Drug Overdose Deaths Involving Illicitly Manufactured Fentanyls — United States, 2019–2020. O’Donnell J, Tanz LJ, Gladden RM, Davis NL, Bitting J. MMWR Morb Mortal Wkly Rep. 2021 Dec 17;70(50):1740-1746.
- Footnote 51
-
Impact of the COVID-19 Pandemic on Emergency Department Visits - United States, January 1, 2019-May 30, 2020. Hartnett KP, Kite-Powell A, DeVies J, Coletta MA, Boehmer TK, Adjemian J, Gundlapalli AV; National Syndromic Surveillance Program Community of Practice. MMWR Morb Mortal Wkly Rep. 2020 Jun 12;69(23):699-704.
- Footnote 52
-
Trends in US Emergency Department Visits for Mental Health, Overdose, and Violence Outcomes Before and During the COVID-19 Pandemic. Holland KM, Jones C, Vivolo-Kantor AM, Idaikkadar N, Zwald M, Hoots B, Yard E, D'Inverno A, Swedo E, Chen MS, Petrosky E, Board A, Martinez P, Stone DM, Law R, Coletta MA, Adjemian J, Thomas C, Puddy RW, Peacock G, Dowling NF, Houry D. JAMA Psychiatry. 2021 Apr 1;78(4):372-379.
- Footnote 53
-
Update: COVID-19 Pandemic-Associated Changes in Emergency Department Visits - United States, December 2020-January 2021. Adjemian J, Hartnett KP, Kite-Powell A, DeVies J, Azondekon R, Radhakrishnan L, van Santen KL, Rodgers L.MMWR Morb Mortal Wkly Rep. 2021 Apr 16;70(15):552-556.
- Footnote 54
-
Wainwright JJ, Mikre M, Whitley P, et al. Analysis of drug test results before and after the US declaration of a national emergency concerning the COVID-19 outbreak. JAMA 2020;324:1674–7. PMID:32945855 https://doi.org/10.1001/jama.2020.17694.
- Footnote 55
-
Notes from the Field: Testing for Nonprescribed Fentanyl and Percentage of Positive Test Results Among Patients with Opioid Use Disorder - United States, 2019-2020. Niles JK, Gudin J, Vivolo-Kantor AM, Gladden RM, Mustaquim D, Seth P, Kaufman HW. MMWR Morb Mortal Wkly Rep. 2021 Nov 26;70(47):1649-1651.
- Footnote 56
-
Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic - United States, June 24-30, 2020. Czeisler MÉ, Lane RI, Petrosky E, Wiley JF, Christensen A, Njai R, Weaver MD, Robbins R, Facer-Childs ER, Barger LK, Czeisler CA, Howard ME, Rajaratnam SMW.MMWR Morb Mortal Wkly Rep. 2020 Aug 14;69(32):1049-1057.
- Footnote 57
-
Follow-up Survey of US Adult Reports of Mental Health, Substance Use, and Suicidal Ideation During the COVID-19 Pandemic, September 2020. Czeisler MÉ, Lane RI, Wiley JF, Czeisler CA, Howard ME, Rajaratnam SMW.JAMA Netw Open. 2021 Feb 1;4(2):e2037665.
- Footnote 58
-
Mental Health and Substance Use Among Adults with Disabilities During the COVID-19 Pandemic - United States, February-March 2021. Czeisler MÉ, Board A, Thierry JM, Czeisler CA, Rajaratnam SMW, Howard ME, Clarke KEN.MMWR Morb Mortal Wkly Rep. 2021 Aug 27;70(34):1142-1149.
- Footnote 59
-
Mental health, substance use, and suicidal ideation among unpaid caregivers of adults in the United States during the COVID-19 pandemic: Relationships to age, race/ethnicity, employment, and caregiver intensity. Czeisler MÉ, Drane A, Winnay SS, Capodilupo ER, Czeisler CA, Rajaratnam SM, Howard ME.J Affect Disord. 2021 Dec 1;295:1259-1268.
- Footnote 60
-
Miech RA, Schulenberg JE, Johnston LD, Bachman JG, O'Malley PM, Patrick ME. National press release, "Teen use of illicit drugs decreased in 2021, as the COVID-19 pandemic continued" University of Michigan News Service: Ann Arbor, MI, 2021. Available at: https://news.umich.edu/teen-use-of-illicit-drugs-decreased-in-2021-as-the-covid-19-pandemic-continued/ Accessed March 13, 2022.
- Footnote 61
-
US Department of Health and Human Services. Health effects of e cigarette use among U.S. youth and young adults [chapter 3]. In: E-cigarette use among youth and young adults: a report of the Surgeon General. Atlanta, GA: US Department of Health and Human Services, CDC; 2016:97–126.
- Footnote 62
-
Klein LC. Effects of adolescent nicotine exposure on opioid consumption and neuroendocrine responses in adult male and female rats. Exp Clin Psychopharmacol 2001;9:251–61.
- Footnote 63
-
Brener ND, Bohm MK, Jones CM, Puvanesarajah S, Robin L, Suarez N, et. al. Use of Tobacco Products, Alcohol, and Other Substances Among High School Students During the COVID-19 Pandemic — Adolescent Behaviors and Experiences Survey, United States, January–June 2021 MMWR Morb Mortal Wkly Rep. Forthcoming.
- Footnote 64
-
Enns A, Pinto A, Venugopal J, Grywacheski V, Gheorghe M, Kakkar T, et al. Evidence-informed policy brief – Substance use and related harms in the context of COVID-19: a conceptual model. Published September 16, 2020. https://doi.org/10.24095/hpcdp.40.11/12.03
Canadian Conceptual Model References:
DOMAIN 1:
- Nanos. COVID-19 and increased alcohol consumption [Internet]. Ottawa (ON): Canadian Centre on Substance Abuse; 2020 Apr [cited 2020 May 4]. Available from: https://www.ccsa.ca/sites/default/files/2020-04/CCSA-NANOS-Alcohol-Consumption-During-COVID-19-Report-2020-en.pdf
- Rajkumar RP. COVID-19 and mental health: a review of the existing literature. Asian J Psychiatr. 2020;52:102066. doi:10.1016/j.ajp.2020.102066.
- Findlay L, Arim R. Canadians report lower self-perceived mental health during the COVID-19 pandemic [Internet]. Ottawa (ON): Statistics Canada; 2020 [cited 2020 May 11] Available from: https://www150.statcan.gc.ca/n1/en/pub/45-28-0001/2020001/article/00003-eng.pdf?st=3GIUQH_D
- Holmes EA, O’Connor RC, Perry VH, et al. Multidisciplinary research priorities for the COVID-19 pandemic: a call for action for mental health science. Lancet Psychiatry. 2020;7(6):547–60. doi:10.1016/S2215-0366(20)30168-1.
- Budhwani H, Sun R. Creating COVID-19 stigma by referencing the novel coronavirus as the “Chinese virus” on Twitter: quantitative analysis of social media data. J Med Internet Res. 2020;22(5). doi:10.2196/e19301
- Jimenez‐Sotomayor MR, Gomez‐Moreno C, Soto‐Perez‐de‐Celis E. Coronavirus, ageism, and Twitter: an evaluation of tweets about older adults and COVID‐19. J Am Geriatr Soc. 2020;68(8):1661-5. doi:10.1111/jgs.16508.
- Logie CH, Turan JM. How do we balance tensions between COVID-19 public health responses and stigma mitigation? Learning from HIV research. AIDS Behav. 2020;24(7):2003-6. doi:10.1007/s10461-020-02856-8.
- Sinha R. How does stress increase risk of drug abuse and relapse? Psychopharmacology (Berl). 2001;158(4):343-59. doi:10.1007/s002130100917.
- Statistics Canada. Labour force survey, April 2020 [Internet]. Ottawa (ON): Statistics Canada; 2020 May 8 [cited 2020 May 14]. Available from: https://www150.statcan.gc.ca/n1/daily-quotidien/200508/dq200508a-eng.htm
- Lai J, Ma S, Wang Y, et al. Factors associated with mental health outcomes among health care workers exposed to coronavirus disease 2019. JAMA Netw Open. 2020;3(3):e203976. doi:10.1001/jamanetworkopen.2020.3976.
- Zhou X, Snoswell CL, Harding LE, et al. The role of telehealth in reducing the mental health burden from COVID-19. Telemed J E Health. 2020;26(4):377-9. doi:10.1089/tmj.2020.0068.
DOMAIN 2:
- Douglas M, Katikireddi SV, Taulbut M, McKee M, McCartney G. Mitigating the wider health effects of covid-19 pandemic response. BMJ. 2020;369:m1557. doi:10.1136/bmj.m1557.
- Chou KL, Liang K, Sareen J. The association between social isolation and DSM-IV mood, anxiety, and substance use disorders: wave 2 of the National Epidemiologic Survey on Alcohol and Related Conditions. J Clin Psychiatry. 2011;72(11):1468-76. doi:10.4088/JCP.10m06019gry.
- Peirce RS, Frone MR, Russell M, Cooper ML. Financial stress, social support, and alcohol involvement: a longitudinal test of the buffering hypothesis in a general population survey. Health Psychol. 1996;15(1):38-47. doi:10.1037//0278-6133.15.1.38.
- Gausman J, Langer A. Sex and gender disparities in the COVID-19 pandemic. J Womens Health. 2020;29(4):465-6. doi:10.1089/jwh.2020.8472.
- BC Coroners Service. Illicit drug overdose deaths in B.C.; Findings of coroners’ investigations [Internet]. British Columbia: Government of British Columbia; 2018 Sep 27 [cited 2020 Jun 10]. Available from: https://www2.gov.bc.ca/assets/gov/birth-adoption-death-marriage-and-divorce/deaths/coroners-service/statistical/illicitdrugoverdosedeathsinbc-findingsofcoronersinvestigations-final.pdf
- Harris K. CBC News. Halt drug possession charges during pandemic to stem spike in overdose deaths, advocates say [Internet]. Toronto (ON): Canadian Broadcasting Corporation; 2020 May 14 [cited 2020 May 15]. Available from: https://www.cbc.ca/news/politics/drug-possession-covid19-1.5568631
- City News. Dwindling drug supply on DTES drives prices up, leaves users desperate as COVID-19 closes border [Internet]. Vancouver (BC): City News; 2020 Mar 24 [cited 2020 May 4]. Available from: https://www.citynews1130.com/2020/03/24/drug-supply-bc-covid-19-border/
- Bula F. Safe supply of opioids needed ‘right away’ to avoid overwhelming hospitals in COVID-19 pandemic: Vancouver Mayor. Vancouver (BC): Globe and Mail; 2020 Mar 24 [cited 2020 May 4]. Available from: https://www.theglobeandmail.com/canada/british-columbia/article-safe-supply-of-opioids-needed-right-away-to-avoid-overwhelming/
- Canadian Centre on Substance Abuse. Changes related to COVID-19 in the illegal drug supply and access to services and resulting health harms (CCENDU Alert) [Internet]. Ottawa (ON): Canadian Centre on Substance Abuse; 2020 May [cited 2020 Jun 10]. Available from: https://www.ccsa.ca/changes-related-covid-19-illegal-drug-supply-and-access-services-and-resulting-health-harms
- BC Coroners Service. Illicit drug toxicity deaths in BC: January 1, 2010 – May 31, 2020 [Internet]. British Columbia: Government of British Columbia; 2020 Jun 11 [cited 2020 Jun 12]. Available from: https://www2.gov.bc.ca/gov/content/life-events/death/coroners-service/statistical-reports
- Ministry of Finance. Ontario supports Ontario’s beverage alcohol sector during COVID-19 [Internet]. Toronto (ON): Government of Ontario; 2020 Jun 17 [cited 2020 Aug 18]. Available from: https://news.ontario.ca/mof/en/2020/06/ontario-supports-ontarios-beverage-alcohol-sector-during-covid-19.html
- O. Reg. 128/20: Pick-up and delivery of cannabis. Toronto (ON): Government of Ontario; 2020 [cited 2020 Aug 18]. Available from: https://www.ontario.ca/laws/regulation/200128?_ga=2.115126085.334318074.1597752485-584514098.1593817007
DOMAIN 3:
- Alexander GC, Stoller KB, Haffajee RL, Saloner B. An epidemic in the midst of a pandemic: opioid use disorder and COVID-19. Ann Intern Med. 2020;173(1):57-8. doi:10.7326/M20-1141.
- Becker WC, Fiellin DA. When epidemics collide: coronavirus disease 2019 (COVID-19) and the opioid crisis. Ann Intern Med. 2020;173(1):59-60. doi:10.7326/M20-1210.
- CTV News. Vancouver: B.C. offering safe supply to some drug users to minimize harm during COVID-19 crisis. Vancouver (BC): CTV News; 2020 Mar 26 [cited 2020 May 19]. Available from: https://bc.ctvnews.ca/b-c-offering-safe-supply-to-some-drug-users-to-minimize-harm-during-covid-19-crisis-1.4870530
- Health Canada. Government of Canada approves new treatment options for opioid use disorder and supports research, treatment and harm reduction projects in Ontario [Internet]. Ottawa (ON): Government of Canada; 2019 [cited 2020 May 19]. Available from: https://www.canada.ca/en/health-canada/news/2019/05/government-of-canada-approves-new-treatment-options-for-opioid-use-disorder-and-supports-research-treatment-and-harm-reduction-projects-in-ontario.html
- Jeffords S. Toronto: “Two crises”: Ontario’s opioid problem worsens during COVID-19 as services for drug users scale back [Internet]. Toronto (ON): Canadian Broadcasting Corporation; 2020 Apr 30 [cited 2020 May 11]. Available from: https://www.cbc.ca/news/canada/toronto/ontario-opioid-covid19-1.5551368
- Polewski L. Hamilton: Coronavirus: opioid crisis escalating in Hamilton due to naloxone restrictions, doctor says [Internet]. Hamilton (ON): Global News; 2020 Apr 23 [cited 2020 May 19]. Available from: https://globalnews.ca/news/6855439/coronavirus-hamilton-opioid-crisis-naloxone-restrictions/
- Little L-A. North Bay: North Bay emergency responders to stop using naloxone nasal spray during pandemic. North Bay (ON): CTV News; 2020 Apr 3 [cited 2020 May 19]. Available from: https://northernontario.ctvnews.ca/north-bay-emergency-responders-to-stop-using-naloxone-nasal-spray-during-pandemic-1.4881599
DOMAIN 4:
- Jenkins WD, Bolinski R, Bresett J, et al. COVID-19 during the opioid epidemic-exacerbation of stigma and vulnerabilities. J Rural Health. 2020;10.1111/jrh.12442. doi:10.1111/jrh.12442.
- Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-2. doi:10.7326/M20-1212.
- Nanos. COVID-19 and increased alcohol consumption [Internet]. Ottawa (ON): Canadian Centre on Substance Abuse; 2020 Apr [cited 2020 May 4]. Available from: https://www.ccsa.ca/sites/default/files/2020-04/CCSA-NANOS-Alcohol-Consumption-During-COVID-19-Report-2020-en.pdf
- Public Health Agency of Canada. The Chief Public Health Officer’s report on the state of public health in Canada 2019. Addressing stigma: towards a more inclusive health system [Internet]. Ottawa (ON): Public Health Agency of Canada; 2019 [cited 2020 May 11]. Available from: https://www.canada.ca/en/public-health/corporate/publications/chief-public-health-officer-reports-state-public-health-canada/addressing-stigma-toward-more-inclusive-health-system.html
- European Monitoring Centre for Drugs and Drug Addiction. COVID-19 and people who use drugs [Internet]. Lisbon (PT): European Monitoring Centre for Drugs and Drug Addiction; 2020 Mar 25 [cited 2020 May 11]. Available from: https://www.emcdda.europa.eu/publications/topic-overviews/covid-19-and-people-who-use-drugs_en
- Kouyoumdjian F, Schuler A, Matheson FI, Hwang SW. Health status of prisoners in Canada: narrative review. Can Fam Physician. 2016;62(3):215-22.
- Blair A, Parnia A, Siddiqi A. Testing lags and emerging COVID-19 outbreaks in federal penitentiaries in Canada. medRxiv. 2020 Jan 1. doi:10.1101/2020.05.02.20086314.
- Tsai J, Wilson M. COVID-19: a potential public health problem for homeless populations. Lancet Public Health. 2020;5(4):e186-7. doi:10.1016/S2468-2667(20)30053-0.
DOMAIN 5:
- Volkow ND. Collision of the COVID-19 and addiction epidemics. Ann Intern Med. 2020;173(1):61-2. doi:10.7326/M20-1212.
- CDC COVID-19 Response Team. Preliminary estimates of the prevalence of selected underlying health conditions among patients with coronavirus disease 2019 - United States, February 12–March 28, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(13):382‐6. doi:10.15585/mmwr.mm6913e2.
- Slaunwhite AK, Gan WQ, Xavier C, Zhao B, Buxton JA, Desai R. Overdose and risk factors for coronavirus disease 2019. Drug Alcohol Depend. 2020;212:108047. doi:10.1016/j.drugalcdep.2020.108047.
- Brack A, Rittner HL, Stein C. Immunosuppressive effects of opioids—clinical relevance. J Neuroimmune Pharmacol. 2011;6(4):490-502. doi:10.1007/s11481-011-9290-7.
- Farkas A, Lynch MJ, Westover R, et al. Pulmonary complications of opioid overdose treated with naloxone. Ann Emergency Med. 2020;75(1):39-48. doi:10.1016/j.annemergmed.2019.04.006.
- Szabo G, Saha B. Alcohol’s effect on host defense. Alcohol Res. 2015;37(2):159-70.
- Carrico AW, Horvath KJ, Grov C, et al. Double jeopardy: methamphetamine use and HIV as risk factors for COVID-19. AIDS Behav. 2020;1-4. doi:10.1007/s10461-020-02854-w.
- Leece P, Cavacuiti C, Macdonald EM, et al.; Canadian Drug Safety and Effectiveness Research Network. Predictors of opioid-related death during methadone therapy. J Subst Abuse Treat. 2015;57:30-5. doi:10.1016/j.jsat.2015.04.008.
- Peterson C, Liu Y, Xu L, Nataraj N, Zhang K, Mikosz C. U.S. national 90-day readmissions after opioid overdose discharge. Am J Prev Med. 2019;56(6):875-81. doi:10.1016/j.amepre.2018.12.003.
Appendix
Abbreviations
- ABES
- Adolescent Behaviors and Experiences Survey
- BC
- British Columbia
- BIPOC
- Black, Indigenous, People of Color
- C/MEs
- Coroners and Medical Examiners
- CDC
- Centers for Disease Control and Prevention
- CDSA
- Controlled Drugs and Substances Act
- CIHI
- Canadian Institute of Health Information
- CIHR
- Canadian Institutes of Health Research
- COPE
- COVID-19 Outbreak Public Evaluation
- COVID-19
- 2019 Novel Coronavirus Disease
- CRISM
- Canadian Research Initiative in Substance Misuse
- CSSA
- Canadian Centre on Substance Use and Addiction
- DEA
- Drug Enforcement Administration
- DOJ
- U.S. Department of Justice
- DOSE
- Drug Overdose Surveillance and Epidemiology
- ED
- Emergency Department
- EMS
- Emergency Management Services
- HAN
- Health Alert Network
- HC
- Health Canada
- HHS
- U.S. Department of Health and Human Services
- IMF
- Illicitly-manufactured fentanyl
- MTF
- Monitoring the Future
- MMWR
- CDC’S Morbidity and Mortality Weekly Report
- MOUD
- Medications for Opioid Use Disorders
- NSDUH
- National Survey on Drug Use and Health
- NSSP
- National Syndromic Surveillance Program
- NVSS
- National Vital Statistics System
- OAT
- Opioid Agonist Therapy
- OPHFST
- Office of Public Health Field Service and Training
- OTP
- Opioid Treatment Program
- OUD
- Opioid Use Disorder
- PHAC
- Public Health Agency of Canada
- PHE
- Public Health Emergency
- P/Ts
- Provinces and Territories
- SAMHSA
- Substance Abuse and Mental Health Services Administration
- SARS-CoV-2
- Severe Acute Respiratory Syndrome Coronavirus 2
- SCS
- Supervised Consumption Site
- SOMS-TG
- Substance-Related Overdose and Mortality Surveillance Task Group
- SRHs PHOs
- Substance-Related Harms Public Health Officers
- SUAP
- Substance Use and Addictions Program
- SUD
- Substance Use Disorders
- SUDORS
- State Unintentional Drug Overdose Reporting System
- US
- United States