Update on Quadrivalent Meningococcal Vaccines available in Canada
An Advisory Committee Statement (ACS) National Advisory Committee on Immunization (NACI)
Preamble
The National Advisory Committee on Immunization (NACI) provides the Public Health Agency of Canada (hereafter referred to as the Agency) with ongoing and timely medical, scientific, and public health advice relating to immunization. The Agency acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and is disseminating this document for information purposes. People administering the vaccine should also be aware of the contents of the relevant product monograph(s). Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) of the Canadian manufacturer(s) of the vaccine(s). Manufacturer(s) have sought approval of the vaccine(s) and provided evidence as to its safety and efficacy only when it is used in accordance with the product monographs. NACI members and liaison members conduct themselves within the context of the Agency's Policy on Conflict of Interest, including yearly declaration of potential conflict of interest.
Table of Contents
Summary of information contained in this NACI statement
The following table highlights key information for immunization providers. Please refer to the remainder of the Statement for details

Download the alternative format
(PDF format, 1,723 KB, 78 pages)
Organization: Public Health Agency of Canada
Publication Date: April 2015
Cat.: HP40-125/2014E-PDF
ISBN: 978-1-100-23447-2
Pub.: 140308
Related Topics
1. What
Invasive meningococcal disease (IMD) is a potentially fatal disease usually presenting as an acute febrile illness with rapid onset and features of meningitis or septicemia (meningococcemia), or both, and a characteristic non-blanching rash.
Nimenrix™ is a newly licensed quadrivalent (serogroups A, C, Y and W135) meningococcal vaccine conjugated to the tetanus toxoid (TT).
This Statement provides information regarding the immunogenicity, safety and recommended use of Nimenrix™, and provides updated immunogenicity and safety information about Menactra® (Men-C-ACYW-DT) with respect to its expanded age indication.
2. Who
For routine vaccination in adolescents, either monovalent meningococcal C conjugate or quadrivalent conjugate meningococcal vaccines can be used depending on local epidemiology and other programmatic considerations. If using a quadrivalent product, any of the three available products can be chosen.
For high risk individuals 2 years of age and older or for travellers 2 years and older going to areas where the meningococcal vaccine is recommended, any of the available quadrivalent products can be used.
For high risk children 2 months to less than 2 years of age or travellers 2 months to less than 2 years of age who are going to areas where the meningococcal vaccine is recommended, Menveo™ (Men-C-ACYW-CRM) is the recommended product.
Schedules are provided in the Meningococcal Chapter of the Canadian Immunization Guide.
3. How
Nimenrix™ is authorized for use for immunization of individuals from 12 months to 55 years of age, but can be used for those ≥ 56 years of age if indicated. It should not be administered to subjects with known hypersensitivity to any component of the vaccine.
Nimenrix™ may be administered alone or concomitantly with other routinely administered vaccines in Canada. The National Advisory Committee on Immunization (NACI) recommends periodic boosters for individuals at high risk for meningococcal disease or who have ongoing increased risk of exposure. Additional information is provided in the Meningococcal Chapter of the Canadian Immunization Guide..
Nimenrix™ has a safety profile similar to other monovalent and quadrivalent conjugated meningococcal vaccines that are authorized for use in Canada.
4. Why
IMD mortality is approximately 10%. Of IMD survivors, up to a third may experience long term sequelae.
Approximately one-third of all IMD is associated with Neisseria meningitidis serogroups A, C, Y and W-135; immunization against vaccine preventable IMD serogroups is important, particularly for those at higher risk for infection.
I. Introduction
The purpose of this Statement is to supplement previous conjugate meningococcal vaccine statementsFootnote 1,Footnote 2,Footnote 3,Footnote 4 which have outlined the use of monovalent meningococcal C and quadrivalent conjugate meningococcal vaccines. This statement will:
- Review existing NACI recommendations on the use of conjugate meningococcal vaccines;
- Update the epidemiology of meningococcal disease in Canada;
- Review and update information on quadrivalent conjugate meningococcal vaccines and vaccination schedules used in Canada following the approval of a new quadrivalent conjugate meningococcal vaccine Nimenrix™ (GlaxoSmithKline [GSK]) and of a new age indication for Menactra® (Sanofi Pasteur).
Background:
Three monovalent conjugate meningococcal vaccines (using CRM197 or TT as carrier proteins) are available in Canada, and with the authorization of Nimenrix™, three quadrivalent conjugate meningococcal vaccines (using CRM197, diphtheria toxoid [DT], or TT as carrier proteins) are also available in Canada. NACI recommends that healthy children be immunized with monovalent conjugate meningococcal C vaccine vaccine routinely at 12 months of age; however, they may begin meningococcal immunization earlier depending on provincial/territorial schedules. In addition, NACI recommends the routine use of a conjugate vaccine, either monovalent or quadrivalent (depending on local epidemiology and other programmatic considerations), for a routine adolescent booster dose at around 12 years of age. For specific vaccine recommendations, please refer to the Canadian Immunization Guide.
NACI recommends the use of quadrivalent conjugate meningococcal vaccine for serogroups A, C, W135 and Y for immunization in the following groups:
Those at high risk due to medical conditions:
- Persons with functional or anatomic asplenia (including sickle cell disease);
- Persons with congenital complement, properdin, factor D or primary antibody deficiencies;
- Persons with acquired complement deficiency due to receipt of the terminal complement inhibitor eculizumab (Soliris™);
- In addition, immunization should be considered for individuals with HIV, especially if congenitally acquired.
Those at increased risk due exposures:
- Travellers when meningococcal vaccine is recommended or required, including travellers to sub-Saharan Africa and pilgrims to the Hajj in Mecca, Saudi Arabia;
- Laboratory personnel who are potentially routinely exposed to N. meningitidis;
- Military personnel during recruit training and on certain deployments.
For high risk individuals due to medical conditions, 2 years of age and over, either Men-C-ACYW-CRM or Men-C-ACYW-DT vaccine should be given as a 2 dose series at least 8 weeks apart. Men-C-ACYW-135 vaccines are not authorized for use in those 56 years of age and older; however, based on limited evidence and expert opinion their use is considered appropriate. For high risk children from 2 months to less than 2 years of age, Men-C-ACYW-CRM should be preferentially used; for children 1 to less than 2 years of age, a 2 dose series is recommended with each dose given at least 8 weeks apart, and for infants 2 months to less than 1 year of age a schedule using 2 or 3 doses with another dose at 12 to 23 months of age is recommended.Footnote 5
Those at high risk for meningococcal disease due to medical conditions should receive periodic boostersFootnote 5 for those vaccinated at 6 years of age or younger, a booster dose should be provided 3-5 years after the last dose and then every 5 years thereafter; for those vaccinated at 7 years of age or older, booster doses should be provided every 5 years after the last dose. Schedules for those at high risk due to medical conditions are provided in Table 3 of the Meningococcal Chapter of the Canadian Immunization Guide.
Those at increased risk due to exposures, including travellers to areas where meningococcal vaccine is recommended, should receive the quadrivalent conjugate meningococcal vaccine, along with periodic boosters if they remain at ongoing increased risk of exposure. Schedules for travellers are provided in Table 1 of the Meningococcal Chapter of the Canadian Immunization Guide. Revaccinated for close contacts of an IMD case is recommended as per Table 2 of the Meningococcal Chapter of the Canadian Immunization Guide.
In December 2012, Health Canada (HC) approved the expansion of the age indication for Menactra® (Men-C-ACYW-DT) starting at 9 months of age. In March 2013, HC authorized the use of a new quadrivalent conjugate vaccine for individuals 12 months to 55 years of age, Nimenrix™ (Men-C-ACYW-TT) produced by GlaxoSmithKline (GSK). The purpose of this statement is to provide recommendations for the new product Nimenrix™ and for the new age indication for Menactra®.
II. Methods
NACI reviewed key questions for the literature review as proposed by the Meningococcal Working Group, including such considerations as the burden of disease to be prevented; the target population, safety, immunogenicity, efficacy and effectiveness of the vaccine; vaccine schedules; and other aspects of the overall immunization strategy. The knowledge synthesis was performed by medical specialists at the Agency and supervised by the Working Group. Following critical appraisal of individual studies, summary tables were prepared with ratings of the quality of the evidence using NACI's methodological hierarchy (Tables 4 and 5), and proposed recommendations for vaccine use were developed. The Working Group chair and Agency medical specialist presented the evidence and proposed recommendations to NACI on October 8, 2013. Following thorough review of the evidence and consultation at the NACI meeting, the Committee voted on specific recommendations. The description of relevant considerations, rationale for specific decisions, and knowledge gaps are described in the text.
III. Epidemiology
N. meningitidis (meningococcus) is a potentially serious pathogen that colonizes up to 10% of healthy individuals. Meningococci can be classified based on the immunologic reactivity of the polysaccharide capsule into at least 12 different serogroups, of which five (A, B, C, W-135 and Y) are associated most frequently with IMD.
IMD usually presents as an acute febrile illness with rapid onset and features of meningitis or septicemia (meningococcemia), or both, and a characteristic non-blanching rash. Overall case fatality is approximately 10%, and up to a third of survivors may have long-term sequelae, which can include hearing loss, neurologic disabilities and digit or limb amputations.Footnote 4,Footnote 6 All probable and confirmed IMD cases are reported from the provinces and territories to the Agency's Enhanced IMD Surveillance System. Provincial and territorial public health and/or hospital laboratories send all meningococcal isolates to the Agency’s National Microbiology Laboratory for strain characterization, including confirmation of serogroup and determination of serotype, serosubtype and sequence type/clonal complex.
Although IMD is reported year round, there is considerable variation in geographical and temporal incidence, with the majority of cases occurring between November and March. The incidence of IMD in Canada has ranged from 0.45 to 1.18 cases per 100,000 population from 1995 to 2011. Between 2007 and 2011, an average of 193.8 cases of IMD were reported annually in Canada, with an average incidence of 0.58 cases per 100,000 population.
Figure 1. Incidence of IMD (per 100 000 population) in Canada by serogroup and year, 1995 to 2011
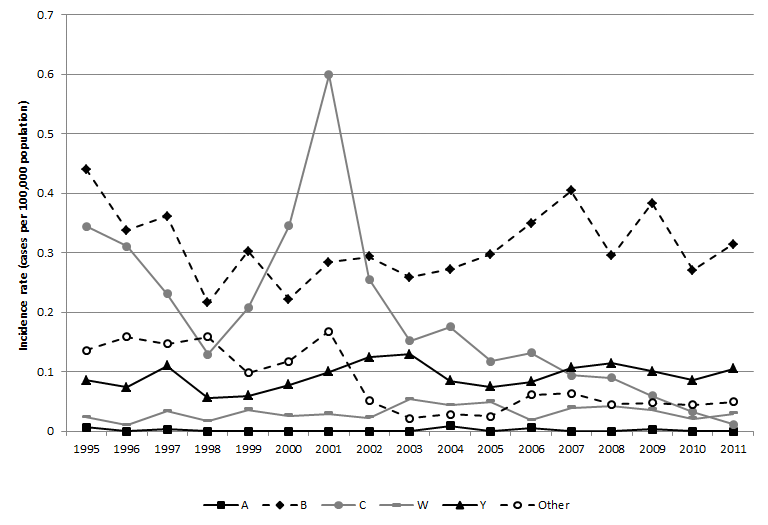
*“Other” is defined as all serogroups other than those listed, ungroupable and unknown serogroup.
Figure 1. Incidence of IMD (per 100 000 population) in Canada by serogroup and year, 1995 to 2011 - Text Description
Figure 1 shows a line graph depicting the annual incidence rate of reported IMD cases in Canada per 100,000 population, by serogroup and year from 1995 to 2011. The x-axis contains the years from 1995 to 2011. The y-axis on the left displays reported cases per 100,000 population, starting from 0.00 at the bottom to 0.70 at the top. The annual incidence rates of serogroups A, B, C, W-135, Y, and other serogroups are each depicted by a line. The other serogroup category includes serogroups 29E, X, and Z as well as non-groupable cases and cases with unknown serogroup.
For the majority of years, serogroup B has had the highest incidence rates, ranging from 0.22 to 0.44 cases per 100,000 population. The incidence of serogroup C decreases from 0.35 to 0.13 cases per 100,000 population from 1995 to 1998, then increases to a peak of 0.60 cases per 100,000 population in 2001, and then decreases over the next ten years to a low of 0.01 cases per 100,000 population in 2011. The incidence of serogroups W-135 and Y is relatively stable, ranging from 0.01 to 0.05 cases per 100,000 population for W-135 and from 0.06 to 0.13 cases per 100,000 population for Y. The incidence due to serogroup A is very low for all years at 0.009 cases per 100,000 population or less. From 1995 to 2001, IMD due to other, non-groupable, and unknown serogroups ranges from 0.10 to 0.17 cases per 100,000 population. After this IMD due to other serogroups decreases, ranging from 0.02 to 0.06 cases per 100,000 population from 2002 to 2011.
In Canada, serogroups B is now the most common cause of IMD followed by serogroup Y. Following the occurrence of localized serogroup C outbreaks in the late 1990s and early 2000s, conjugate serogroup C vaccination programs were implemented in all Canadian provinces and territories between 2002 and 2006 (Table 1), resulting in significant decreases in serogroup C incidence in all age groups and regions.Footnote 6,Footnote 7,Footnote 8,Footnote 9,Footnote 10,Footnote 11,Footnote 12,Footnote 13,Footnote 14,Footnote 15,Footnote 16,Footnote 17,Footnote 18,Footnote 19,Footnote 20,Footnote 21,Footnote 22,Footnote 23,Footnote 24,Footnote 25,Footnote 26,Footnote 27 With the declining incidence, cases of serogroup C are rare, and serogroup B now makes up the greatest proportion of reported IMD cases in Canada (62% due to serogroup B versus 2% due to serogroup C in 2011). Serogroup A is exceedingly rare in Canada, and tends to be associated with travel to areas of the world where it remains endemic (e.g. the African meningitis belt).
| P/T | Year of initial implementation of routine meningococcal C conjugate program | Current infant schedule using meningococcal C conjugate | Current adolescent schedule using meningococcal C conjugate (C) or ACYW-135 conjugate (Q) |
|---|---|---|---|
| BC | 2003 | 2, 12 months (since 2005) | (C) Grade 6 (since 2003) |
| AB | 2002 | 2, 4, 12 months (since 2007) | (Q) Grade 9 (since 2011) |
| SK | 2004 | 12 months (since 2004) | (Q) Grade 6 (since 2011) |
| MB | 2004 | 12 months (since 2009) | (C) Grade 4 (since 2004) |
| ON | 2004 | 12 months (since 2004) | (Q) Grade 7 (since 2009) |
| QC | 2002 | 12 months (since 2002) | (C) Grade 9 (since 2013) |
| NL | 2005 | 12 months (since 2005) | (Q) Grade 4 (since 2007) |
| NB | 2004 | 12 months (since 2004) | (Q) Grade 9 (since 2007) |
| NS | 2005 | 12 months (since 2005) | (C) Grade 7 (since 2010) |
| PE | 2003 | 12 months (since 2003) | (Q) Grade 9 (since 2006) |
| YK | 2003 | 2, 12 months (since 2009) | (C) Grade 6 (since 2006) |
| NT | 2004 | 2, 12 months (since 2004) | (C) Grade 9 (since 2008) |
| NU | 2006 | 12 months (since 2007) | (C) Grade 6 (since 2006) |
| Serogroup | Average annual number (range) |
Average annual incidence (per 100 000) |
Median age | Case fatality ratio |
|---|---|---|---|---|
| A | 0.2 (0-1) |
0.00 | 16 | 0.0% |
| B | 112 (92-133) |
0.33 | 16 | 6.0% |
| C | 19.2 (4-31) |
0.06 | 44.5 | 15.3% |
| W-135 | 11.2 (7-14) |
0.03 | 38 | 8.5% |
| Y | 34.4 (29-38) |
0.10 | 47 | 11.9% |
| OtherTable 2 footnote * | 16.8 (15-21) |
0.05 | 20 | 7.7% |
| All serogroups | 193.8 (154-233) |
0.58 | 20 | 8.2% |
|
||||
Table 2 presents the average annual number and range of IMD cases and incidence rates for 2007 to 2011. It also indicates the median age and case fatality ratio (CFR) by serogroup from 2007 to 2011.
Figure 2. Percent distribution and average number of IMD cases occurring in Canada between 2007 and 2011 by serogroup and province/territory
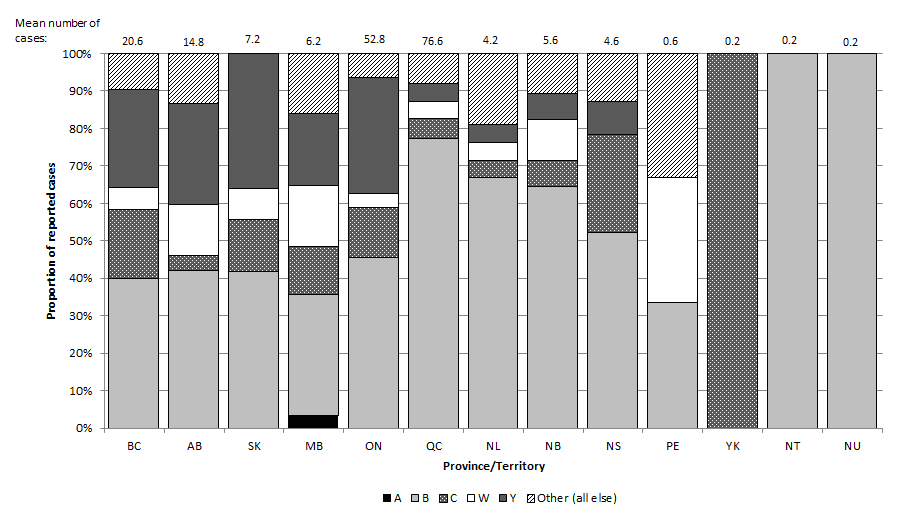
Figure 2. Percent distribution and average number of IMD cases occurring in Canada between 2007 and 2011 by serogroup and province/territory - Text Description
Figure 2 shows a stacked bar graph depicting the percent distribution and average number of IMD cases occurring in Canada between 2007 and 2011 by serogroup and province/territory. The x-axis contains each province and territory, running from British Columbia to Nunavut. The y-axis on the left represents the overall proportion of reported cases during that five year period of 2007 to 2011. The y-axis starts with 0 at the bottom and reaches 100% at the top. Each stacked bar depicts the percent distribution of cases due to serogroups A, B, C, W-135, Y, and other serogroups for each province or territory. The other serogroup category includes serogroups 29E, X, and Z as well as non-groupable cases and cases with unknown serogroup. The average number of cases occurring during this period is portrayed to one decimal place on top of each province and territory's bar.
Very few cases were reported in the three territories and Prince Edward Island (1 to 3 cases per year) from 2007 to 2011. In the Yukon Territory serogroup C accounts for 100% of cases, while serogroup B accounts for 100% of cases in the Northwest Territories and Nunavut. In Prince Edward Island, cases are equally divided across serogroups B, W-135, and other. The small numbers mean that proportions may vary greatly from year to year, and should thus be interpreted with caution.
Among the remaining provinces, serogroup B accounts for the largest proportion of cases, ranging from 32% in Manitoba to 77% in Quebec. The proportion of cases due to serogroup C ranges from 4% in Alberta to 26% in Nova Scotia. The proportion of cases due to serogroup Y ranges from 5% in Quebec and Newfoundland to 36% in Saskatchewan. The proportion of cases due to W-135 ranges from 0% in Nova Scotia to 16% in Manitoba. The proportion of serogroup A cases is 0% for all groups with the exception of Manitoba (3%). The proportion of non-groupable cases and cases due to other or unknown serogroups, ranges from 0% in Saskatchewan to 19% in Newfoundland.
The average number of cases in each province and territory was 20.6 for British Columbia (serogroup distribution: 40% B, 18% C, 6% W-135, 26% Y, 10% all others), 14.8 for Alberta (serogroup distribution: 42% B, 4% C, 14% W-135, 27% Y, 14% all others), 7.2 for Saskatchewan (serogroup distribution: 42% B, 14% C, 8% W-135, 36% Y), 6.2 for Manitoba (serogroup distribution: 3% A, 32% B, 13% C, 16% W-135, 19% Y, 16% all others), 52.8 for Ontario (serogroup distribution: 45% B, 13% C, 4% W-135, 31% Y, 6% all others), 76.6 for Quebec (serogroup distribution: 77% B, 5% C, 4% W-135, 5% Y, 8% all others), 4.2 for Newfoundland (serogroup distribution: 67% B, 5% C, 5% W-135, 5% Y, 19% all others), 5.6 for New Brunswick (serogroup distribution: 64% B, 7% C, 11% W-135, 7% Y, 11% all others), 4.6 for Nova Scotia (serogroup distribution: 52% B, 26% C, 9% Y, 13% all others), 0.6 for Prince Edward Island (serogroup distribution: 33% B, 33% W-135, 33% all others), 0.2 for the Yukon Territory (all serogroup C), 0.2 for the Northwest Territories (all serogroup B), and 0.2 for Nunavut (all serogroup B).
As demonstrated in Figure 2, geographic differences in the serogroup distribution of IMD exist across Canada. The highest incidence of IMD and serogroup B-specific IMD occurred in Québec where, on average, 77% of cases were due to serogroup B from 2007 to 2011. Among remaining provinces, the serogroup distribution varied, with serogroup B making up between 32% and 67% of cases from 2007 to 2011, depending on the province. Very few cases were reported in the three territories and Prince Edward Island (1 to 3 cases per year) from 2007 to 2011. The small numbers mean that proportions may vary greatly from year to year, and should thus be interpreted with caution.
Figure 3. Percent distribution and average number of IMD cases occurring in Canada between 2007 and 2011 by serogroup and age group
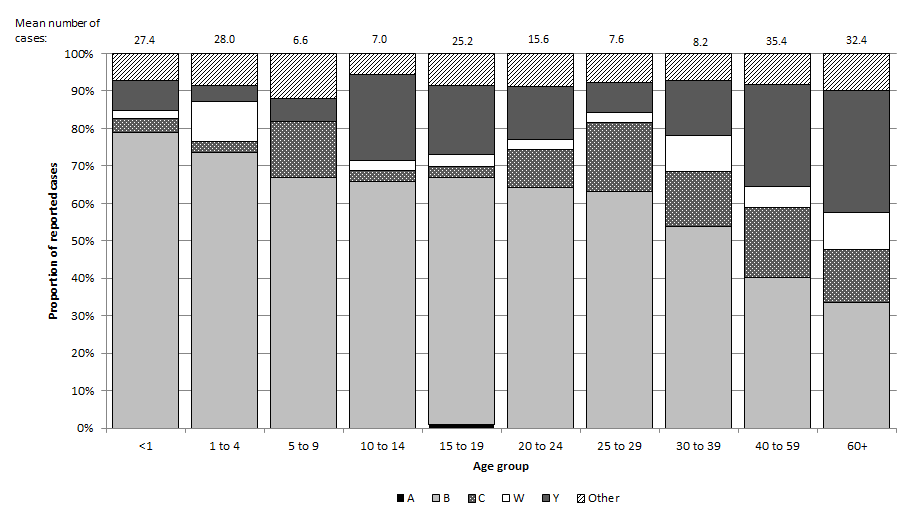
Figure 3. Percent distribution and average number of IMD cases occurring in Canada between 2007 and 2011 by serogroup and age group - Text Description
Figure 3 shows a stacked bar graph depicting the percent distribution and average number of IMD cases occurring in Canada between 2007 and 2011 by serogroup and age group. The x-axis contains the age groups and includes infants less than one year of age, one to four year olds, five to nine year olds, ten to 14 year olds, 15 to 19 year olds, 20 to 24 year olds, 25 to 29 year olds, 30 to 39 year olds, 40 to 59 year olds, and adults 60 years of age and older. The y-axis on the left represents the overall proportion of reported cases during that five year period of 2007 to 2011. The y-axis starts with 0 at the bottom and reaches 100% at the top. Each stacked bar depicts the percent distribution of cases due to serogroups A, B, C, W-135, Y, and other serogroups for each age group. The other serogroup category includes serogroups 29E, X, and Z as well as non-groupable cases and cases with unknown serogroup. The average number of cases occurring during this period is portrayed to one decimal place on top of each age group's bar.
Serogroup B accounts for the largest proportion of cases in all age groups, although the proportion of cases due to serogroup B decreases with age, ranging from 33% in those 60 years of age and greater to 79% in less than one year olds. The proportion of cases due to serogroups C and Y tends to increase with age. The proportion due to serogroup C ranges from 3% in ten to 14 year olds to 19% in adults 40 to 59 years old. The proportion due to serogroup Y ranges from 4% in one to four year olds to 33% in adults 60 years of age and older. The proportion of cases due to W-135 is low in each age group, ranging from 0% in five to nine year olds to 11% in one to four year olds. The proportion of serogroup A cases is 0% for all age groups except 15 to 19 year olds (1%) and the proportion of cases due to non-groupable cases and cases due to other or unknown serogroups ranges from 6% in ten to 14 year olds to 12% in five to nine year olds.
The mean number of cases in each province and territory was 27.4 for less than one year olds (serogroup distribution: 79% B, 4% C, 2% W-135, 8% Y, 7% all others), 28.0 for one to four year olds (serogroup distribution: 74% B, 3% C, 11% W-135, 4% Y, 9% all others), 6.6 for five to nine year olds (serogroup distribution: 67% B, 15% C, 6% Y, 12% all others), 7.0 for ten to 14 year olds (serogroup distribution: 66% B, 3% C, 3% W-135, 23% Y, 6% all others), 25.2 for 15 to 19 year olds (serogroup distribution: 1% A, 66% B, 3% C, 3% W-135, 18% Y, 9% all others), 15.6 for 20 to 24 year olds (serogroup distribution: 64% B, 10% C, 3% W-135, 14% Y, 9% all others), 7.6 for 25 to 29 year olds (serogroup distribution: 63% B, 18% C, 3% W-135, 8% Y, 8% all others), 8.2 for 30 to 39 year olds (serogroup distribution: 54% B, 15% C, 10% W-135, 15% Y, 7% all others), 35.4 for 40 to 59 year olds (serogroup distribution: 40% B, 19% C, 6% W-135, 27% Y, 9% all others), 32.4 for those 60 years of age and older (serogroup distribution: 33% B, 14% C, 10% W-135, 33% Y, 10% all others).
The serogroup distribution of IMD also differs by age, with serogroup Y cases having the highest median age from 2007 to 2011 (47 years), followed by C (44.5 years) and W-135 (38 years). As seen in Figure 3, the proportion of cases due to serogroup B decreases with age while conversely, the proportion of cases due to serogroups C and Y tends to increase with age.
IV. Vaccine
| Nimenrix™ | Menveo™ | Menactra® | |
|---|---|---|---|
| Type of Vaccine | Conjugate A,C,Y,W-135 (Men-C-ACYW-TT) | Conjugate A,C,Y,W-135 (Men-C-ACYW-CRM) | Conjugate A,C,Y,W-135 (Men-C-ACYW-DT) |
| Manufacturer | GlaxoSmithKline | Novartis | Sanofi Pasteur |
| Concentration of Polysaccharide | 5 µg of each serogroup | 10 µg of serogroup A 5 µg each of serogroups C, Y, W-135 |
4 µg of each serogroup |
| Protein Carrier | 44 µg of tetanus toxoid (TT) W-135 and Y directly conjugated to TT; A and C conjugated to an adipicdihydrazide spacer and indirectly to TT |
Varying amounts of CRM197 conjugated to each polysaccharide: 16.7 to 33.3 µg to A 7.1 to 12.5 µg to C 3.3 to 8.3 µg to W-135 5.6 to 10 µg to Y | 48 µg of diphtheria toxoid (DT) |
| Preservative | N/A | N/A | N/A |
| Adjuvant | N/A | N/A | N/A |
| AdministrationTable IV footnote * | 0.5ml IM Requires reconstitution | 0.5ml IM Requires reconstitution | 0.5ml IM |
| Manufacturer Schedule | 12 months to 55 year of age | 2 years to 55 years | 9 months to 55 years |
|
|||
A note about carrier proteins: While vaccines containing CRM197 and TT in general demonstrate greater immunogenicity compared to those containing DT, controversy remains concerning the comparative superiority of these two proteins.Footnote 6,Footnote 7,Footnote 8,Footnote 9,Footnote 10,Footnote 11
IV.2 Efficacy or effectiveness
Pre-authorization efficacy trials are difficult to conduct because of the relative rarity of meningococcal disease; therefore, antibody-dependent, complement-mediated, bactericidal activity measured using serum bactericidal assay (SBA) is used as an immunological correlate of protection.
The efficacy of new conjugate meningococcal vaccines can be inferred from the demonstration of immunologic non-inferiority to authorized meningococcal vaccines. Regulatory approval therefore is based on post-vaccination immunogenicity data only. Post-licensure effectiveness of conjugate vaccines has relied on the screening methodFootnote 28 which requires accurate coverage data as well as the immunization status of cases. In Canada, the number of cases of meningococcal disease is small and it would take many years to have sufficient cases to be able to estimate vaccine effectiveness (VE). In addition, the current quality of data from surveillance of coverage and cases is not high enough to enable VE to be measured. Immunogenicity is therefore the best method available.
IV.3 Immunogenicity (include evidence for adequacy of correlates of protection)
Conjugate meningococcal vaccines available in Canada are licensed based on the induction of seroresponse measured by SBA using human (hSBA) or baby rabbit serum (rSBA) as the source of exogenous complement. Because human serum that lacks bactericidal antibodies against meningococci is difficult to obtain, infant rabbit sera that lack intrinsic bactericidal activity is a preferred source of complement for assessing bactericidal responses to meningococcal vaccines. However, the assessment of immune response to conjugated meningococcal vaccines with the use of rSBA results in much higher bactericidal titres and demonstrates various levels of correlation with hSBA for different serotypes. Seroresponse for the purpose of licensure is most often defined as rSBA titre ≥1:8 or a fourfold titre increase in individuals with an rSBA titre ≥1:4 at baseline. For the purpose of Nimenrix™ licensure, seroresponse was defined as:
- For subjects < 2 years of age: rSBA antibody titre ≥ 1:8
- For subjects ≥ 2 years of age: rSBA titre ≥ 1:32 for individuals with pre-vaccination titre < 1:8, or a 4-fold increase in titre for individuals with pre-vaccination titre ≥ 1:8
There is currently no consensus on the preferential use of rSBA or hSBA in determining short-term protection against IMD. Because of the rapid onset of illness, protection is more reliant on circulating antibody rather than immunologic memory. At this time, the GMT titre required for long term protection is unknown.Footnote 14 Non-functional quantification of Immunoglobulin G (IgG) antibodies is measured using a standard enzyme-linked immunosorbent assay (ELISA) and provides supplementary information to SBA results; however, these are not a direct correlate of protection. For more information about the correlates of protection refer to the previous NACI Update on the use of quadrivalent conjugate meningococcal vaccines.
Although suitable for within-study comparison of vaccines, due to differences in methodologies, results of SBA assays using human or rabbit complement are not comparable between studies. Similarly, because of inter-laboratory differences in methodology, caution is needed when comparing results generated from different laboratories.Footnote 15,Footnote 16,Footnote 17 The impact of vaccination on carriage and herd immunity cannot be predicted using data available from immunogenicity studies.
IV.3.1 Immunogenicity of Nimenrix™
Immunogenicity of Nimenrix™ in children less than 12 months of age
The immunogenicity of the one- or two-dose (second dose at 12 months) Men-C-ACYW-TT schedule was assessed in 149 infants 9 months of age and compared with vaccination only at 12 months of age.Footnote 18 Following initial vaccination, an rSBA titre ≥1:8 was elicited in all infants against all serogroups except for serogroup C where 1 infant did not reach the predefined seroprotection level. Although the proportion of patients with rSBA titres ≥1:8 was comparable between 9 months and 12 months groups (that had received 1 vaccine dose), the proportion with hSBA titre ≥1:8 was significantly higher in the 12-month age group (1 dose of vaccine) for all serogroups except C; similarly rSBA geometric mean titres (GMTs) were also significantly higher after a single vaccine dose at 12-months of age than at 9 months of age for all serogroups except A. The study did not include a comparator vaccine because no meningococcal vaccine was licensed for this age group at the time of the study.
Immunogenicity of Nimenrix™ in children 12 to 23 months of age
Immunogenicity of Men-C-ACYW-TT in toddlers 12-23 months of age was reported in approximately 1,000 children in five phase II and III clinical trials 4-6 weeks following vaccination.Footnote 18,Footnote 19,Footnote 20,Footnote 21,Footnote 22 An rSBA titre ≥1:8 was elicited in more than 99% of toddlers against all four groups. Non-inferiorityFootnote 19)Footnote 20 was demonstrated in all studies which investigated the immunogenicity of Men-C-ACYW-TT versus monovalent Men-C-CRM197. In two studies that measured hSBA at 4 and 6 weeks post-vaccination, individuals who had received Men-C-ACYW-TT achieved significantly higher bactericidal antibody levels, as measured by hSBA titre and GMT, compared with those elicited by vaccination with Men-C-CRM197.
Immunogenicity of Nimenrix™ in children 2 to 10 years of age
A total of four published studies evaluated immunogenicity 1 month following a single dose of Nimenrix™ in over 1,400 children 2-10 years of age. In the immunogenicity cohort of the randomized controlled trial (RCT) study by Memish et al.,Footnote 23 children were randomized to receive either a single dose of Men-C-ACYW-TT or Men-P-ACYW-135. Immune protection, as measured with rSBA>1:8 was non-inferior in the Men-C-ACYW-TT group when compared to the Men-P-ACYW-135 group. The GMTs were statistically significantly higher for all four serogroups in the Men-C-ACYW-TT group. Seroresponse was also significantly higher in the Men-C-ACYW-TT than the Men-P-ACYW-135 group. Similarly, using the same comparator vaccine, Vesikari et al.,Footnote 24 demonstrated non-inferiority (rSBA >1:8) of Men-C-ACYW-TT for all four serogroups. Compared with Men-P-ACYW-135, rSBA GMT values were also higher for each serogroup in the Men-C-ACYW-TT group, after adjusting for pre-vaccination measurements. An exploratory analysis also demonstrated a significantly higher seroresponse for serogroups A and C in the Men-C-ACYW-TT group than in the Men-P-ACYW-135 group.
In an RCT study done by Knuf et al.,Footnote 25 non-inferiority to Men-C-CRM197 was demonstrated with greater than 99% of subjects in the Men-C-ACYW-TT group achieving>1:8 rSBA titres. The rSBA GMT values were significantly higher in the monovalent Men-C-CRM197 group for serogroup C, suggesting a more robust antibody response.
Baxter et al.Footnote 26 assessed the immunogenicity of Men-C-ACYW-TT in 79 healthy children 10 years of age and compared vaccine response to that elicited in adolescents and adults 11-25 years of age. Immune response was similar except for relatively higher but statistically non-significant hSBA-MenA GMT values in the 10 year old group.
Immunogenicity of Nimenrix™ in individuals 11 to 19 years of age
Although several studies investigated the immunogenicity of Men-C-ACYW-TT in individuals over 10 years of age, only five reported results for the 11 -19 year age group. In a study of 115 individuals 11-17 years of age who received Men-C-ACYW-TT conducted by Østergaard et al.Footnote 27 all achieved an rSBA titre ≥1:8 one month following vaccination. In another RCT trial conducted by Østergaard et al.Footnote 29Footnote 30 50 individuals received Men-C-ACYW-TT or Men-P-ACYW-135. All individuals achieved rSBA titres ≥1:8 for all serogroups and there were no significant differences in rSBA GMT between groups, except for that of serogroup W-135 at month 42 which was significantly higher in the Men-C-ACYW-TT group (n=19) than in the Men-P-ACYW-135 group (n=17). Seroresponse rates in the Men-C-ACYW-TT group were higher than 90.2% for all serogroups. In both groups, most subjects (58.8%-90.9%) had high pre-vaccination rSBA titres for all serogroups. Similarly, a study of 1,025 individuals by Bermal et al.Footnote 31Footnote 32 using the same comparison vaccine demonstrated non-inferiority of Men-C-ACYW-TT to Men-P-ACYW-135. Prior to vaccination, the percentage of subjects in each group with rSBA titres ≥1:8 was between 78.6% and 90.9% for serogroups A, W-135 and Y, and was between 58.6% and 59.4% for serogroup C. One month following vaccination, at least 99.6% of subjects in both groups had rSBA titres of ≥1:8 against each meningococcal vaccine serogroup; seroresponse rates were significantly higher in the Men-C-ACYW-TT group for serogroups A, W-135 and Y, and non-inferior to serogroup C compared with the Men-P-ACYW-135 group. After vaccination, rSBA GMTs, against each serogroup increased by between 29.2-fold and 274.3-fold in Men-C-ACYW-TT vaccinees, and by 16.4-fold to 185.9-fold in Men-P-ACYW-135 vaccinees. Unlike the study conducted by Østergaard et al., rSBA GMTs were significantly higher following Men-C-ACYW-TT than Men-P-ACYW-135 for all serogroups; however, the Østergaard study was much smaller and underpowered to detect differences.
In two trials, the immunogenicity of Men-C-ACYW-TT was compared against another conjugate quadrivalent vaccine (Men-C-ACYW-DT, Menactra®). Baxter et al.Footnote 26 randomized 784 healthy individuals between the ages of 11- and 25 years. Both vaccines led to similar responses in terms of hSBA >1:4 for serogroup C after vaccination. For serogroups A, W-135 and Y, Men-C-ACYW-TT led to a greater response than Men-C-ACYW-DT. Adjusted GMT values were higher for all serogroups in the Men-C-ACYW-TT group. The percentage of vaccine responders was also higher in the Men-C-ACYW-TT group. Halperin et al.Footnote 33 randomized 1,016 healthy individuals between the ages of 10- and 25 to receive a single dose of 1 of 2 lots (A or B) of Men-C-ACYW‐TT or a single lot of Men-C-ACYW‐DT. In the study, the Men-C-ACYW‐TT vaccine elicited a non-inferior immune response compared with the Men-C-ACYW‐DT vaccine.
Immunogenicity of Nimenrix™ in individuals 19 to 55 years of age
Dbaibo et al.Footnote 34 compared the immunogenicity of Men-C-ACYW-TT to Men-P-ACYW-135 in a study of over 1,100 individuals. In the Men-C-ACYW-TT group 99.3% of vaccine recipients achieved rSBA >1:8 demonstrating non-inferiority to Men-P-ACYW-135. After vaccination with Men-C-ACYW-TT, rSBA GMTs increased by at least 20-fold for serogroups A, W-135 and Y, and 109-fold for serogroup C. Based on GMT values and exploratory analysis, statistically significantly higher GMTs were found in the Men-C-ACYW-TT group for all serogroups except C, in which there was no significant difference. In a study by Borja-Tabora et al.Footnote 35, a total of 500 individuals aged 11-55 years received either Men-C-ACYW-TT or Men-P-ACYW-135. One month following vaccination more than 99.7% of Men-C-ACYW-TT recipients achieved rSBA titres of >1:8. Based on their rSBA titres, Men-C-ACYW-TT was non-inferior to Men-P-ACYW-135; GMT values 1 month post vaccination were higher in the Men-C-ACYW-TT group for each serogroup. Seroresponse rates ranged from 82.7% to 96.3% in the Men-C-ACYW-TT group and 69.7% to 91.7% in the Men-P-ACYW-135 group, with a statistically significant response demonstrated for serogroups A and Y following the administration of Men-C-ACYW-TT.
Immunogenicity of Nimenrix™ in individuals more than 55 years of age
In a study by Dbaibo et al.Footnote 36 immunogenicity of MenACWY-TT was compared with that of Men-P-ACYW-135 in 369 healthy adults between 56 and 103 years of age. One month following vaccination there were no significant differences in rSBA >1:8; however, those vaccinated with Men-P-ACYW-135 did achieve a significantly higher vaccine response for serogroup A and rSBA GMT values for serogroups A and C than those receiving the conjugate vaccine. Lower vaccine response was primarily observed in individuals receiving MenACWY-TT who had very high titres (≥1:128) pre-vaccination.
Persistence of the immune response
Five studies (N=1,306) looked at the persistence of immune response after administration of Men-C-ACYW-TT 12 to 48 months after immunization. All studies are extension studies of RCT aimed at assessing non-inferiority of Men-C-ACYW-TT against other meningococcal vaccines (Men-C-CRM197 or Men-P-ACYW-135) among age-appropriate comparators. In all studies antibody persistence measured by rSBA was similar or higher in the Men-C-ACYW-TT vaccine recipients than other vaccines for all serogroups.
The Knuf et al.Footnote 37 study followed toddlers aged 12-14 months at vaccination and children aged 3-5 years at the time of vaccination for a total of 15 months to determine antibody persistence. The percentage achieving a rSBA titre ≥ 1:8 for serogroup C was higher in the Men-C-ACYW-TT group than in the control group receiving Men-C-CRM197 (12-14 month group). Comparing the Men-C-ACYW-TT group for serogroups C and Y, the Men-C-ACYW-TT group also had higher rSBA titres than in the control group which received Men-P-ACYW-135 (3-5 year group). GMT values were also reported to be higher.
In two studies Vesikari et al.Footnote 19Footnote 38 reported immune responses following Men-C-ACYW-TT administration in toddlers aged 12-23 months. In a study of 170 toddlers, 3 years post vaccination 90.8% of Men-C-ACYW-TT recipients retained rSBA titres ≥ 1:8 for all serogroups, and 73.6% hSBA titres ≥ 1:4 for serogroups C, W and Y; only 28.1% had hSBA titres ≥ 1:4 for serogroup A. There was no significant difference in rSBA titre between Men-C-ACYW-TT and Men-C-CRM197 recipients 3 years following vaccination. In the first 2 years post vaccination the GMTs were higher among the group immunized with Men-C-ACYW-TT than Men-C-CRM197, whereas in year 3 no significant difference was observed. Similarly, in a study that followed 225 infants, rSBA titres and GMT values between Men-C-ACYW-TT and Men-C-CRM197 vaccine recipients were comparable 4 years following initial vaccination.Footnote 38
Vesikari et al.Footnote 24 also followed children aged 2-10 years for a total of 3 years post vaccination. Children in the Men-C-ACYW-TT group showed higher rSBA GMT values for all serogroups in year 1 and 2 post vaccination when compared to those who received Men-P-ACYW-135. In year 3, all serogroups except C, showed higher GMT values in the Men-C-ACYW-TT group compared to the Men-P-ACYW-135 group.
In the Østergaard et al.Footnote 30 study, 36 adolescents who received Men-C-ACYW-TT or Men-P-ACYW-135 were followed for a total of 42 months. In both groups, rSBA titres ≥1:8 and GMT values were similar for all serogroups except for that of serogroup W-135 at month 42 which was significantly higher in the Men-C-ACYW-TT group.
In a larger study of 535 adolescents reported by Bermal et alFootnote 31 and Quiambao et al.Footnote 43 using Men-P-ACYW-135 as the comparator vaccine, in years 3 and 4 following immunization, the Men-C-ACYW-TT group showed higher rSBA titres ≥ 1:8 and GMT values for all serogroups except C than the Men-P-ACYW-135 comparator group.
In a persistence study done by Borja-Tabora et al.,Footnote 35 500 individuals 11-55 years of age were randomized to receive either Men-C-ACYW-TT or Men-P-ACYW-135. The rSBA titres were higher in the Men-C-ACYW-TT compared with the Men-P-ACYW-135 group in year 3 and 4, with more than 82.8% of Men-C-ACYW-TT recipients retaining titres ≥1:8 for all serogroups at year 4.
In the only study that reported data using hSBA, Baxter et al.Footnote 44 compared the persistence of immune response in 403 adolescents and young adults 11-25 years of age following immunization with Men-C-ACYW-TT and Men-C-ACYW-DT. Three years following vaccination, the proportion of the Men-C-ACYW-TT group with hSBA titres >1:8 was marginally superior for serogroup C and W-135, while GMT values were significantly higher for all serogroups except A.
Based on the available immune persistence data, Men-C-ACYW-TT appears to have superior immune persistence when compared with non-conjugated, and non-inferior when compared with conjugated meningococcal vaccines up to 4 years after immunization. No peer-reviewed articles of individuals over the age of 1 year related to boosting were available at the time of the conducted literature review. There have been no studies that have looked at the three meningococcal quadrivalent conjugate vaccines head to head, and Menveo™ has not been compared directly with Nimenrix™.
IV.3.2 Immunogenicity of Menactra®
Pina et al.Footnote 39 reported immunogenicity data from two studies (A and B) following vaccination of infants and toddlers with Menactra® at 9 and 12 months of age respectively. The vaccine was provided in a two-dose schedule either separately or together with one or more licensed childhood vaccines (measles, mumps, rubella and varicella vaccine [MMRV], Haemophilus influenzae type b [Hib], pneumococcal conjugate vaccine [Pneu-C-7] and hepatitis A) at 12 months of age. All studies used human serum (hSBA) as the source of complement with the primary immunogenicity endpoint being the percentage of participants who achieved a titre >1:8 30 days following the administration of the second dose.
Immunogenicity was assessed in 724 children. After two doses of Men-C-ACYW-DT in Study A the percentage of participants achieving hSBA titres ≥1:8 ranged from 90.5% to 100% for serogroups A, C and Y, and 81.2% to 88.2% for serogroup W-135. Concomitant administration with MMRV and Pneu-C-7 demonstrated non inferiority compared with administration of Men-C-ACYW-DT alone (upper limit of the 95% confidence interval [CI] of the difference was <10%) for all serogroups except W-135 when Men-C-ACYW-DT was administered along with the fourth dose of Pneu-C-7. In Study B, responses to the MMRV and Hib vaccine antigens were similar between groups that received MMRV and Hib alone or concomitantly with Men-C-ACYW-DT. In the case of Pneu-C-7, statistical non-inferiority was not demonstrated for serotypes 4, 6B and 18C (upper limit of the 95% CI of the antipneumococcal antibody ratio was >2); when given with Men-C-ACYW-DT; however, more than 98% of participants achieved pneumococcal multiplex opsonophagocytic assay titres ≥1:8, and ELISA antibody concentrations ≥35 µg/mL were achieved for all serotypes. When using an hSBA titre ≥1:4, seroprotection was achieved by more than 91% of those who received Men-C-ACYW-DT.
In a poster presented at the 2013 European Society for Paediatric Infectious Diseases meeting, Noya et alFootnote 40 reported an RCT of 122 children who received either Men-C-ACYW-DT or meningococcal C conjugate vaccine. The rSBA titres following a two-dose schedule were reported at 18 and 19 months of age in 55 children. One month following second dose (19 months of age), more than 96% of those who had received Men-C-ACYW-DT achieved rSBA titres ≥1:8 for all serogroups (≥96%).
IV.4 Vaccine administration and schedule
Schedule: Nimenrix™ has been approved for use in individuals aged 12 months to 55 years.
Dosage: 0.5ml of reconstituted vaccine should be administered.
Route of administration: This vaccine is intended for intramuscular injection. It may be given in the deltoid muscle or for children 12-23 months of age in the anterolateral thigh.
Booster doses: The manufacturer has not yet determined the need for a booster dose, however NACI recommends periodic boosters doses for individuals at high risk for meningococcal disease or who have ongoing increased risk of exposure.
IV.5 Storage requirements
As per the product monograph, Nimenrix™ should be stored in a refrigerator between 2˚C to 8˚C, the diluent can be stored at room temperature (25oC). This product should not be frozen and should be protected from light.
IV.6 Simultaneous Administration with other Vaccines
Men-C-ACYW-TT vaccine has been given simultaneously with a 10-valent pneumococcal conjugate vaccine, hexavalent infant vaccine, MMRV vaccine, seasonal influenza vaccine, and combined hepatitis A and B vaccine.
In a study by Ruiz-Palacios et al.Footnote 21 infants aged 12 to 23 months received Men-C-CYW-TT and Pneu-C-10. A comparable immunogenicity was demonstrated when these products were co-administered except for a reduced response for pneumococcal serotype 18C which is conjugated to TT. This may suggest potential interference requiring further research. Pneu-C-10 is licensed for use in Canada, but at this time all provinces and territories are using Pneu-C-13.
Knuf et al Footnote 22 investigated the use of Men-C-ACYW-TT co-administered with DTaP-HBV-IPV/Hib with additional arms of the study which include participants who received both vaccines with 1 month separating each shot as well as a control group that only received Men-C-CRM197. The study reported a similar safety profile and immune response measured by rSBA titres for separate and concomitant administration of Men-C-ACYW-TT with DTaP-HBV-IPV/Hib. However, rSBA GMTs for serogroups A, C and W-135 were significantly lower in the group that received Men-C-ACYW-TT 1 month after DTaP-HBV-IPV/Hib compared with the group that received Men-C-ACYW-TT first. However, this finding is likely to be of limited clinical significance since more than 97.3% of these individuals achieved rSBA titres ≥ 1:8.
In a Finnish study by Vesikari et al.Footnote 20 Men-C-ACYW-TT was administered with MMRV in children aged 12 - 23 months; comparison groups included Men-C-ACYW-TT alone, MMRV alone and Men-C-CRM197 alone. Based on this study, MMRV with Men-C-ACYW-TT was found to have similar immune responses as MMRV alone except for rubella for which GMT values were lower in the co-administration group 1 month after vaccination. However, all children in both groups reached the protective immune cut off of >4IU/ml. The Men-C-ACYW-TT+MMRV group and Men-C-ACYW-TT groups were similar in their ability to elicit an immune response to all meningococcal serogroups. The rSBA GMT values were greater for serogroup C in both groups given Men-C-ACYW-TT than in the Men-C-CRM197 group 1 month after vaccination. This information suggests that based on one study, co-administration of these two vaccines is possible, bearing in mind that there may be lower GMTs for rubella.
In adolescents, a study by Østergaard et al.Footnote 27 investigated providing Men-C-ACYW-TT along with hepatitis A and B vaccines. Co-administration was found to be non-inferior in terms of immunogenicity and safety.
Reyes et al.Footnote 41 investigated the co-administration of Men-C-ACYW-TT with an unadjuvanted seasonal influenza vaccine in adults age 18-55. Non-inferiority was demonstrated in terms of predefined rSBA GMT ratios for all serogroups except C (only marginally exceeded, therefore did not meet non-inferiority criteria). However, more than 99% of subjects in both groups achieved post-vaccination rSBA titers ≥ 1:8 for all serogroups. Individual and simultaneous administration of the two vaccines had a similar safety profile.
NACI has reviewed the available data on the co-administration of Men-C-ACYW-TT with the above products. The product monograph from the manufacturer recommends co-administration with additional vaccine products (hepatitis A and hepatitis B vaccines, MMR). There are no clinical studies available that specifically address the co-administration of these products with Men-C-ACYW-TT. However, based on expert opinion, co-administration of Men-C-ACYW-TT with products containing single or fewer antigens than the products cited in the above studies should not compromise safety or efficacy of either product.
IV.7 Adverse Events
IV.7.1 Safety of Nimenrix™
Safety of Nimenrix™ in children less than 12 months of age
Two safety profiles reported by Klein et al.Footnote 18 in a group of 160 infants who had received Men-C-ACYW-TT at 9 months of age showed a safety profile similar to that following vaccine administration at 12 months of age.
Safety of Nimenrix™ in children 12-23 months of age
The Safety of Men-C-ACYW-TT in this age group was reported in four studies. In an RCT of 304 healthy toddlers Vesikari et al.Footnote 19 reported similar reactogenicity and safety profiles of the Men-C-ACYW-TT and the Men-C-CRM197 vaccines. Unsolicited symptoms were reported by approximately half of the participants, the most common being rhinitis in the Men-C-ACYW-TT group (8.3%) and pyrexia in the Men-C-CRM197 group (13.3%). Solicited local and general symptoms of grade 3 intensity were reported in no more than 3.5% of toddlers in both groups, and no serious adverse events (SAEs) considered causally related to the vaccination were reported throughout the study. Grade 3 was defined as redness and swelling > 30mm in diameter, pain/cried when limb was moved spontaneously, loss of appetite to not eating at all, fever >39.5 C and/or all other symptoms that prevent normal activity. Another RCT of 749 toddlers conducted by Vesikari et al.Footnote 20 reported similar safety results in the group that received only the Men-C-ACYW-TT vaccine.
In a study conducted Ruiz-Palacios et al.Footnote 21 that randomized 343 toddlers to receive Pneu-C-10 and Men-C-ACYW-TT vaccines, pain at the injection site and irritability were the most frequently reported solicited symptoms. Grade 3 intensity was reported in less than 2.2% of toddlers.
The study by Klein et al.Footnote 18 that compared the safety of 1 versus 2 doses of Men-C-ACYW-TT reported a tendency toward an increase in the percentage of subjects with fever and loss of appetite in the two dose group. A higher percentage of asthma, emergency department visits and rash was also noted in those receiving two doses.
Safety of Nimenrix™ in children 2-10 years of age
Three studies assessed the safety of Nimenrix™ in children aged 2-10 years. In the study conducted by Memish et al,Footnote 23 Men-C-ACYW-TT and Men-P-ACYW-135 vaccines administered to 1125 children resulted in a similar safety profile, including fewer than 1% of grade 3 solicited and unsolicited general symptoms in both treatment groups. There were no SAEs due to vaccination in this study. In the RCT done by Knuf et al.,Footnote 25 which included 414 children, fewer grade 3 adverse events were seen in participants who had received Men-C-ACYW-TT (6.8% of participants) versus Men-C-CRM197 (15.1%). In this study, fewer overall grade 3 adverse events were noted in either group. Among 309 children in the study by Vesikari et al.Footnote 24 the most common adverse event was pain at the injection site (45.1% Men-C-ACYW-TT vs 71.8% Men-P-ACYW-135). Grade 3 unsolicited adverse events occurred in (6.5% vs 5.1% of vaccine recipients). Only one grade 3 symptom was considered related to immunization: a hematoma reported in a child who had received Men-C-ACYW-TT.
Safety of Nimenrix™ in individuals 11-17 years of age
In the study by Østergaard et al.,Footnote 12 which included only 50 individuals, pain was reported by half of Men-C-ACYW-TT recipients and was the most common adverse event, with headache being reported by one third of subjects. In the Bermal et al.Footnote 31 study, in which Men-C-ACYW-TT was compared with Men-P-ACYW-135, results were reported for 1,025 individuals aged 10-18 years: grade 3 general symptoms were reported by 1.6% vs. 0.4% of vaccine recipients respectively. The most common adverse event reported was pain at the injection site (26.2% vs 26.8%). Of the other local adverse events, redness at the injection site was reported by 12.3% vs 9.6% (p=0.0075), which was the only statistically significant event. Of the general symptoms, fatigue and headaches were the most commonly reported (up to 14.3% and 13.4%, respectively). There was no symptom that was statistically significantly different when compared. No SAEs were associated with the administration of either vaccine.
Safety of Nimenrix™ in individuals 18-55 years of age
Three studies evaluated the safety of Men-C-ACYW-TT in this age group. Dbaibo et al.Footnote 34 reported pain and redness at the site of injection (19.5% [16.9%-22.1%] and 13.5% [9.9%-17.9%]) as the most common adverse events. Grade 3 general symptoms were reported rarely (less than 2% of study participants) with the most common being headache. Only two SAEs that were reported in one subject (0.6%) were considered to be related to vaccination; these were abdominal pain and gastritis 5 days after vaccination. No new-onset chronic illnesses (NOCI) were reported.
In a pooled analysis done by Bermal et al.,Footnote 31 that compared Men-C-ACYW-TT against Men-P-ACYW, the pre-specified primary objective (non-inferiority in terms of the grade 3 solicited and unsolicited systemic symptoms within 4 days of vaccination) was not met. This may have been due to a poor estimation of the incidence of grade III adverse events, therefore the sample size was inadequate. Further analysis did not show statistically significant differences in the percentage of subjects with any general symptom. Although this study did not do a separate analysis on individuals 18-55 years of age, there were no significant differences between rates of any solicited symptoms in the 11-17 and 18-55 study cohorts with either the Men-C-ACYW-TT or the Men-P-ACYW group.
In the study by Borja-Tabora,Footnote 35 very similar results were found as those outlined above. Grade 3 general symptoms were reported in 1.3% of participants in the Men-C-ACYW-TT group, with none in the Men-P-ACYW-135 group. The most common symptoms were pain at the site of injection and headache. Both vaccines demonstrated a comparable safety profile, although injection site redness and swelling were more frequent in Men-C-ACYW-TT recipients. In the 6 month follow-up phase, one case of new onset of chronic illness was reported (food allergy) and two SAEs (costochondiritis and mental disorder). None of these were considered related to vaccination by the investigators.
Safety of Nimenrix™ in individuals more than 55 years of age
In the study by Dbaibo et al.Footnote 36 fewer than 3.0% of subjects reported adverse events; no SAEs were considered vaccine related. Overall, few adverse events were reported in the studies available, and most of these were local pain, redness and swelling. Other reported adverse events included irritability, drowsiness, headache, loss of appetite, fatigue, gastrointestinal symptoms and fever. No cases of idiopathic thrombocytopenic purpura, Guillain-Barré Syndrome (GBS), nephrotic syndrome or brachial neuritis were reported. Compared with vaccines used as controls, adverse events in the Men-C-ACYW-TT groups, although clinically not relevant, were more commonly cited, possibly due to the TT component of the vaccine which is known to be more reactogenic than other protein carriers.
IV.7.2 Safety of Menactra®
Pina et al.Footnote 39 reported safety data in infants and toddlers given Men-C-ACYW-DT at 9 and 12 months of age from three studies (A, B and C) following separate and concomitant administration of Men-C-ACYW-DT with one or more licensed childhood vaccines (MMRV, Hib, Pneu-C-7, Hep A) at 12 months of age. The reported safety parameters included solicited and unsolicited adverse events in more than 3000 children up to 6 months after the last vaccination.
The majority of solicited injection-site reactions were similar across all study groups and included grade 1 tenderness, erythema and swelling. Irritability was the most frequently reported adverse event, with abnormal crying or drowsiness being reported by approximately one third to one half of the participants. Unsolicited adverse events were reported by 40%-50% of children, but were mostly grade 1 or 2 in intensity and comparable across groups. Fever was more frequently reported in subjects when Men-C-ACYW-DT was administered concomitantly with MMRV and Pneu-C-7 (20.7%-24.5%) than when it was given alone (12.4%-13.7%). Rates of grade 3 fever were similar across all groups. SAEs were uncommon.
IV.8 Contraindications and Precautions
As per the product monograph, Nimenrix™ should not be given to patients with known hypersensitivity to any component of the vaccine.
IV.9 Other Considerations
In a study conducted by Dbaibo et al.Footnote 42 of 271 subjects, immune response following administration of Men-C-ACYW-TT was tested in individuals aged 4.5-34 years who had 30-42 months earlier received Men-P-ACYW-135 vaccination; a separate group of controls (had not received Men-P-ACYW-135 in past 10 years) was also recruited. For all four serogroups, 1 month post vaccination, all study participants achieved rSBA titres >1:8. However, rSBA GMT values and vaccine response rates were significantly higher in the group that had not previously received Men-P-ACYW-135, for all serogroups, indicating better immune response in those naïve to polysaccharide vaccine. In evaluating the safety, Grade 3 symptoms were noted in 11.5% of the Men-P-ACYW-135/Men-C-ACYW-TT group as compared with 3.8% of the non Men-P-ACYW-135/Men-C-ACYW-TT group. Pain, related fever, related headache and fatigue were reported more frequently in the Men-P-ACYW-135/Men-C-ACYW-TT group (p<0.05).
Knuf et al.Footnote 37 assessed the induction of immune memory with a challenge dose of Men-P-ACYW-135 (one fifth of a dose) to children primed with Men-C-ACYW-TT or Men-C-CRM197 15 months earlier. After 1 month following vaccination with Men-P-ACYW-135, all study participants achieved rSBA titres >1:8; rSBA GMT values were significantly higher for serogroups A, W-135 and Y in the Men-C-ACYW-TT compared with the Men-C-CRM197 group and were comparable for serogroup C in both study groups, suggesting a similar memory induction by both vaccines. Safety post Men-P-ACYW-135 administration was similar in both groups.
V. Recommendations
Please note that provinces and territories must consider economic factors and other local programmatic/operational factors when considering inclusion of the following recommendations in publicly funded immunization programs.
Recommendation #1
For routine immunization of adolescents, any of the quadrivalent or monovalent C conjugate meningococcal vaccines registered in Canada may be used. The choice between quadrivalent and monovalent C conjugate vaccines is dependent on local epidemiology and other programmatic considerations. (NACI Recommendation Grade B)
Recommendation #2
For the immunization of high risk individuals 2 years of age and older, any of the quadrivalent conjugate meningococcal vaccines registered in Canada may be used. (NACI Recommendation Grade B)
Recommendation #3
For the immunization of high risk individuals between 2 months and less than 2 years of age, Men-C-ACYW-CRM (Menveo™) is the recommended product. Schedules are provided in Table 3 of the Meningococcal Chapter of the Canadian Immunization Guide. (NACI Recommendation Grade B)
Recommendation #4
For immunization of individuals 2 years of age and older travelling to areas where meningococcal vaccine is recommended, any of the quadrivalent conjugate meningococcal vaccines may be used. (NACI Recommendation Grade B)
Men-C-ACYW-CRM (Menveo™) is recommended for immunization of individuals 2 months to less than 2 years of age who are travelling to areas where meningococcal vaccine is recommended. Refer to Table 1 of the Meningococcal Chapter of the Canadian Immunization Guide.
Proof of meningococcal immunization may be required by certain countries. For example, Saudi Arabia requires proof of meningococcal immunization for pilgrims to the Hajj in Mecca. Please refer to the yearly Hajj recommendations and requirements published by the Agency to ensure that individual travellers have appropriate vaccine documentation. Information on countries experiencing outbreaks of meningococcal disease is kept up to date by the World Health Organization.
Additional travel-related recommendations are available at and in the CATMAT statement on Meningococcal Vaccination in Travellers.
VI. Research Priorities
Research to address the following outstanding questions is encouraged:
- Duration of projection/immunity to allow assessment of recommendations for booster doses of quadrivalent vaccines.
- Comparative study of the three available conjugate quadrivalent vaccines.
- Efficacy, effectiveness, immunogenicity and safety of quadrivalent vaccines in high risk groups, including splenectomized individuals.
- The immunogenicity and safety of co-administration of quadrivalent vaccines with routine age appropriate vaccines, including the newly authorized meningococcal B vaccine.
VII. Surveillance Issues
Surveillance should also be encouraged and conducted to address the following outstanding issues:
- The impact of meningococcal vaccination programs on the epidemiology of IMD in Canada.
- Immunization coverage of individual quadrivalent meningococcal vaccines and the impact on carriage and herd immunity.
- The potential occurrence of serogroup replacement.
- Potential alterations in the ecological niche of meningococcus such that other bacteria that will replace meningococcus as carriage of this organism decreases.
- The impact on rubella and invasive pneumococcal disease following co-administration with Nimenrix™ and the infant vaccines PCV and MMR.
Tables
| Level | Description |
|---|---|
| I | Evidence from randomized controlled trial(s). |
| II-1 | Evidence from controlled trial(s) without randomization. |
| II-2 | Evidence from cohort or case-control analytic studies, preferably from more than one centre or research group using clinical outcome measures of vaccine efficacy. |
| II-3 | Evidence obtained from multiple time series with or without the intervention. Dramatic results in uncontrolled experiments (such as the results of the introduction of penicillin treatment in the 1940s) could also be regarded as this type of evidence. |
| III | Opinions of respected authorities, based on clinical experience, descriptive studies and case reports, or reports of expert committees. |
| Quality Rating | Description |
|---|---|
| Good | A study (including meta-analyses or systematic reviews) that meets all design- specific criteriaTable 4 footnote * well. |
| Fair | A study (including meta-analyses or systematic reviews) that does not meet (or it is not clear that it meets) at least one design-specific criterionTable 4 footnote * but has no known "fatal flaw". |
| Poor | A study (including meta-analyses or systematic reviews) that has at least one design-specificTable 4 footnote * "fatal flaw", or an accumulation of lesser flaws to the extent that the results of the study are not deemed able to inform recommendations. |
|
|
| Grade | Recommendation |
|---|---|
| A | NACI concludes that there is good evidence to recommend immunization. |
| B | NACI concludes that there is fair evidence to recommend immunization. |
| C | NACI concludes that the existing evidence is conflicting and does not allow making a recommendation for or against immunization; however other factors may influence decision-making. |
| D | NACI concludes that there is fair evidence to recommend against immunization. |
| E | NACI concludes that there is good evidence to recommend against immunization. |
| I | NACI concludes that there is insufficient evidence (in either quantity and/or quality) to make a recommendation, however other factors may influence decision-making. |
| Evidence for administration of conjugate vaccine after the receipt of polysaccharide vaccine | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Dbaibo, G. et al. 2012aFootnote 42 |
Men-C-ACWY-TT |
Open-label controlled trial Lebanon (extension of Men-P-ACYW study conducted in 2005) |
N=271 individuals divided in two groups based on prior receipt of Men-P-ACYW 30-42 months prior to study (Men-P-ACYW and no Men-P-ACYW groups) All individuals received one dose of Men-C-ACWY-TT |
rSBA titer >1:8 used as measure of immunity Vaccine response was defined as an titer of at least 1:32 at 1 month previously seronegative subjects (rSBA titer <1:8) and at least a four-fold increase in previously seropositive subjects All subjects in all groups achieved seroprotection (rSBA titer >1:8 ) for all serogroups one month post vaccination; All subjects in the no Men-P-ACYW group achieved rSBA titers ≥1:128 for all serogroups; proportion of subjects with rSBA titers ≥1:128 in the Men-P-ACYW S group ranged from 97.0% to 100% Vaccine response was significantly different between groups and ranged from 41.1% to 83.0% in the Men-P-ACYW group and from 76.9% to 97.3% in the no Men-P-ACYW group Pre/post vaccination rSBA GMT increase ranged from 3.9- to 30.1-fold in the Men-P-ACYW group and from 11.8- to 246-fold in the no Men-P-ACYW group rSB; post-vaccination rSBA GMT was significantly lower in the Men-P-ACYW group than in the no Men-P-ACYW group for all serogroups Post-vaccination anti-PS GMC, adjusted for age strata, was significantly higher in the Men-P-ACYW group compared to the no Men-P-ACYW group for all serogroups except A. Listed below are rSBA titers for Meningococcal serogroups (≥ 1:8) 1 month after Men-C-ACWY-TT vaccination: No previous Men-P-ACYW dose (95% CI) |
Level I |
Good |
| Evidence for Immunogenicity of Nimenrix™ in children less than 12 months of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Klein, N.P. et al., 2013Footnote 18 |
Alternate dosing schedules of MenACWY-TT |
Open-label randomized controlled trial Phase II |
N=349 infants 9 months old ACWY-1 group: MenACWY-TT at 12 months of age ACWY-2 group: |
Seroprotection was defined as hSBA titers ≥1:4, or rSBA titers ≥1:8 for all serotypes. Following 1 immunization, rSBA titre ≥1:8 was elicited in all infants against all serogroups except for serogroup C where 1 infant did not reach seroprotection level. Although proportion of patients with rSBA titres ≥1:8 was comparable between 9 months and 12 months groups (that had received 1 vaccine dose), the proportion with hSBA titre ≥1:8 was significantly higher in 12-month age group (1 dose of vaccine) for all serogroups except C. rSBA geometric mean titres (GMTs) also significantly higher after a single vaccine dose at 12-months of age than at 9 months of age for all serogroups except A. The study did not include a comparator vaccine because no meningococcal vaccine was licensed for this age group at the time of the study. Listed below are % hSBA titers ≥1:4 [95%CI] for all serotypes 1 month post-vaccination with 1 dose (ACWY-1) or 2 doses (ACWY-2). |
Level 1 |
Good |
| Evidence for Safety of Nimenrix™ in children less than 12 months of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Klein, N.P. et al., 2013Footnote 18 |
Alternate dosing schedules of MenACWY-TT |
Open-label randomized controlled trial Phase II 16 medical centers in USA |
N=349 infants 9 months old ACWY-1 group: MenACWY-TT at 12 months of age ACWY-2 group: |
After any dose, solicited symptoms (within 8 days) of Grade 3 intensity reported by less than 3.0% of subjects. Fever reported by 3.3% of subjects vaccinated at 9 months of age and 9.6% and 10.8% of subjects vaccinated at 12 months of age (Men-ACWY-2 and Men-ACWY-1 group, respectively). No child reported fever >40°C. Fever and loss of appetite reported more often after second vs. first dose in Men-ACWY-2 group. Unsolicited (within 6 months after last dose) SAEs reported by 3.1% of subjects in ACWY-1 group and 1.1% in ACWY-2 group, none considered by investigators as related to vaccination. |
Level 1 |
Good |
| Evidence for Immunogenicity of Nimenrix™ in children 12-23 months of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Knuf, M et al. 2010Footnote 13 |
Nimenrix™ |
Blinded Randomized Control Trial Multiple sites in 2 countries (Germany, Austria) Phase II |
N=240, 12-14 month old |
Summary of Results: Only data for the Men-ACWY-TT F3 group (12-14 months) are included, as this represents the only relevant formulation approved for use in toddlers. Vaccine response defined as a post-vaccination rSBA titre of ≥1:32 in initially seronegative subjects and a ≥4-fold increase in rSBA titre from pre- to post-vaccination in initially seropositive subjects 1 month post-vaccine. Vaccine responses rates for serogroup C in toddlers were similar in each Men-ACWY-TT group (95.1-97.7%) and the MenCCRM197 control group (91.1%). Response rates for serogroup A and Titers were also assessed ≥1:8 and ≥1:128. All Men-P-ACWY serogroup responses (except serogroup C) were significantly greater than control MenC-CRM-197 responses for both ≥1:8 and ≥1:128. The % rSBA titers ≥1:8 [95%CI] one month post-vaccination listed below: Serogroup C: Serogroup W-135: Serogroup Y: |
Level I |
Good |
Ruiz-Palacois, G.M., et al. 2013Footnote 21 |
MenACWY-TT and PHiD-CV |
Open randomized controlled trial 2 centers in Mexico and 3 in Taiwan Phase IIIb |
N=363 |
Seroprotection defined as rSBA titer ≥1:8. Immunogenicity of PHID-CV and MenACWY-TT co-administered (Coad) was non-inferior to individual administrations, except for a reduced response to pneumococcal serotype 18C. One month after vaccination with MenACWY-TT, 97.5-100% of toddlers had rSBA titers ≥128 for each serogroup, GMTs increased between prevaccination and postvaccination (range 75.4-fold to 439.1-fold). Below are results for Meningococcal serotypes 1 month post Meningococcal vaccination: % rSBA ≥1:8 (95%CI) Serogroup C Serogroup W-135 Serogroup Y GMT (95% CI) Serogroup C Serogroup W-135 Serogroup Y |
Level I |
Good |
Knuf, M., et al. 2011Footnote 22 |
MenACWY-TT and DTaP-HBV-IPV/Hib or MenC (Meningitic) |
Open label randomized controlled trial 72 centers in Austria, Germany and Greece |
N=793 12-23 month old toddlers |
Seroprotection defined as rSBA titer ≥1:8. MenACWY-TT co-administered with DTaP-HBV-IPV/Hib was non-inferior to MenACWY-TT alone. rSBA GMTs for serogroups A, C and W-135 were significantly lower in the group that received Men-C-ACWY-TT 1 month after DTaP-HBV-IPV/Hib compared with the group that received Men-C-ACYW-TT first. Listed below are % rSBA ≥1:8 (95% CI) for subjects immunized under various protocols. Serogroup C Serogroup Y GMT (95%CI) Serogroup W-135 Serogroup Y |
Level I |
Good |
Vesikari, T. et al., 2011Footnote 20 |
Coadminis-tered |
Open randomized controlled trial 14 centres in Finland Phase III |
N=1000 children 12-23 months old |
Seroprotection defined as rSBA titer ≥1:8. Non-inferiority demonstrated for Men-C-ACYW-TT versus monovalent Men-C-CRM197 Listed below are % rSBA ≥1:8 (95% CI) for each serotype 1 month post-vaccination: Serotype A Serotype C Serotype W-135 Serotype Y |
Level I |
Good |
| Evidence for Safety of Nimenrix™ in children 12-23 months of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Vesikari, T. et al., 2012bFootnote 19 |
MenACWY-TT |
Open randomized controlled trial Phase II 11 centres in Finland |
N=304 children 12-23 months old |
Grade 3 symptom if limb spontaneously Men-ACYW-TT and Men-C-CRM vaccines resulted in similar safety profiles, with no SAEs considered causally related to vaccination throughout the study. Injection site redness most common solicited (within 4 days) local symptom in both groups (36.8% and 32.9% in Men-ACWY-TT and Men-C-CRM group, respectively). Irritability most common solicited general symptom in both groups (38.6% and 39.7% in Men-ACWY-TT and Men-C-CRM group, respectively). Grade 3 solicited symptoms reported in less than 3.5% toddlers in each group. Unsolicited symptoms (within 31 days) reported in 52.8% of toddlers in the MenACWY-TT and 50.7% of toddlers in the MenC-CRM group within one month post-vaccination, with rhinitis being most commonly reported (less than 13.3% in both groups). Unsolicited AEs of grade 3 intensity reported in 11.4% of toddlers in Men-ACWY-TT and 14.7% of toddlers in Men-PS group. |
Level I |
Good |
Vesikari, T. et al., 2011Footnote 20 |
Coadminis-tered |
Open randomized controlled trial Phase III 14 centres in Finland |
N=1000 children 12-23 months old |
Grade 3 symptom if limb spontaneously Injection site redness most common solicited (within 4 days) local symptom; Grade 3 intensity: Men-ACWY-TT+MMRV group (4.1%) and Men-ACWY-TT group (4.4%). General solicited symptoms of Grade 3 intensity infrequently reported (less than 1.6% of subjects in each group). Unsolicited (within 43 days) symptoms reported by 64.8% of subjects in Men-ACWY-TT+MMRV group, 60.2% in Men-ACWY-TT group and 68.3% in MMRV group (30.7%, 15.0% and 28.6%, respectively considered related to vaccination). Irritability most frequently reported unsolicited symptom considered related to vaccination in Men-ACWY-TT+MMRV group (8.8%) and in MMRV group (7.9%). No unsolicited SAEs considered causally related to vaccination throughout the study. |
Level I |
Good |
Ruiz-Palacios, G.M. et al., 2013Footnote 21 |
MenACWY-TT and PHiD-CV |
Open randomized controlled trial 2 centers in Mexico and 3 in Taiwan Phase IIIb |
N=363 |
Pain at injection site most frequently reported solicited (within 4 days) local symptom, with 6.8 to 8.8% in PHiD-CV group and 2.4-7.8% in Men-ACWY-TT group reporting Grade 3 intensity. Irritability most common solicited general symptom, with less than 1.2% of toddlers in either group reporting Grade 3 intensity (one toddler in Men-ACWY-TT group reporting fever ≥40.0°C). Unsolicited (within 31 days) symptoms after first vaccination similar between groups (42.9-46.7%); Grade 3 intensity in 6.0% in Coad group and 2.2% in Men-ACWY-TT and PHiD-CV groups. After second vaccination, unsolicited symptoms reported in 44.0% and 34.4% of the toddlers in MenACWY-TT and PHiD-CV groups, respectively; Grade 3 intensity in 6.6% in Men-ACWY-TT group and 3.3% in PHiD-CV group. No Grade 3 symptoms considered causally related to vaccination. SAEs within one month reported in: 2 toddlers in Coad group, 3 toddlers in Men-ACWY-TT group and and 1 toddler in PHiD-CV group. No SAEs considered by investigators as related to vaccination, and all resolved without sequelae. |
Level I |
Good |
Klein, N.P. et al., 2013Footnote 18 |
Alternate dosing schedules of MenACWY-TT |
Open-label randomized controlled trial Phase II |
N=349 infants 9 months old ACWY-1 group: MenACWY-TT at 12 months of age ACWY-2 group: |
After any dose, solicited symptoms (within 8 days) of Grade 3 intensity reported by less than 3.0% of subjects. Fever reported by 3.3% of subjects vaccinated at 9 months of age and 9.6% and 10.8% of subjects vaccinated at 12 months of age (Men-ACWY-2 and Men-ACWY-1 group, respectively). No child reported fever >40°C. Fever and loss of appetite reported more often after second vs. first dose in Men-ACWY-2 group. Unsolicited (within 6 months after last dose) SAEs reported by 3.1% of subjects in ACWY-1 group and 1.1% in ACWY-2 group, none considered by investigators as related to vaccination. |
Level 1 |
Good |
| Evidence for Immunogenicity of Nimenrix™ in children 2-10 years of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Memish, ZA et al. 2011Footnote 23 |
Nimenrix™ (MenACWY-TT) versus Mencevax (Men-PS) |
Open-label randomized controlled trial |
N=1504 Both groups received one dose of either MenACWY-TT or Men-PS |
Seroprotection was defined as rSBA ≥1:8. Seroprotection was non-inferior in the Men-C-ACYW-TT group compared to the Men-P-ACYW-135 group. The GMTs were statistically significantly higher for all four serogroups in the Men-C-ACYW-TT group. Seroresponse was also significantly higher in the Men-C-ACYW-TT than the Men-P-ACYW-135 group. Listed below are rSBA levels for Meningococcal serogroups (> 1:8) 1 mos post vaccination: MenACWY-TT (95% CI) Men-PS (95% CI) rSBA GMT : Men-PS (95% CI) |
Level I |
Good |
Vesikari, T. et al. 2012aFootnote 24 |
MenACWY-TT versus MenACWY-PS |
Open label randomized controlled trial 11 centers in Finland |
N=309 2-5 year olds Both received MenACWY-TT or MenACYW-PS |
Seroprotection was defined as rSBA ≥1:8. One month after vaccination, vaccine vaccine was non-inferior to the MenACWY-PS vaccine. Exploratory analysis also demonstrated a significantly higher seroresponse for serogroups A and C in the Men-C-ACYW-TT group than in the Men-P-ACYW-135 group. Exploratory analysis suggested that rSBA GMTs were higher in MenACWY-TT recipients compared to MenACWY-PS for all 4 serotypes. Listed below are rSBA titers ≥1:8 at 1 month post-vaccination. MenACWY-TT (95% CI) rSBA GMT Men-PS (95% CI) |
Level I |
Good |
Knuf, M., et al 2013Footnote 25 |
MenACWY-TT versus |
Open label randomized controlled trial 31 centers in two countries (Germany, France) |
N=414 2-5 year olds, Both received either MenACWY-TT or MenC-CRM197 |
Seroprotection was defined as rSBA ≥1:8. rSBA vaccine response was defined as an rSBA titre of at least 1:32 in initially seronegative children (i.e. rSBA titre <1:8) and a four-fold increase in titre from pre- to postvaccination in initially seropositive children (i.e. rSBA titre ≥1:8). MenACWY-TT was shown to be non-inferior to MenC-CRM197. Vaccine response rates for rSBA-MenC were 94.8 and 95.7 % in the ACWY-TT and MenC-CRM197 groups, respectively. Listed below are rSBA titers ≥1:8 at 1 month post-vaccination. MenC-CRM197 rSBA GMT MenC-CRM197 (95% CI) |
Level I |
Good |
Baxter, R. et al., 2011Footnote 26 |
MenACWY-TT vs. MenACWY-DT |
Randomized controlled parallel-group study Phase II Single-blind for 11-25 year old subjects, open for 10 year olds |
N=827 subjects 10-25 years old (N=587 MenACWY-TT; N=197 MenACWY-DT; N=88 MenACWY-TT/10 years old) |
Serum hSBA titer ≥1:4 considered protective against disease caused by serogroup C. It is common practice to extend the 1:4 threshold to other serogroups. Seroconversion defined as hSBA titers ≥1:8 one month postvaccination in subjects who had titers <1:4 prior to vaccination. Vaccine response defined as postvaccination hSBA antibody titer ≥1:16 in subjects with prevaccination hSBA antibody titer <1:4 or ≥4-fold increase in hSBA antibody titer postvaccination in subjects with prevaccination hSBA antibody titer ≥1:4. Immune response was similar except for relatively higher but statistically non-significant hSBA-MenA GMT values in the 10 year old group. Listed below are %hSBA titers ≥1:4 [95%CI] 1 month post-vaccination for each serotype. Serotype A Serotype C Serotype W-135 Serotype Y |
Level II-1 |
Fair (low sample size in 10 year group; no appropriate control) |
| Evidence for Safety of Nimenrix™ in children 2-10 years of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Memish, ZA et al. 2011Footnote 23 |
Nimenrix™ |
See Above Parents recorded local and systematic reactions within 4 days of vaccination Unsolicited events within 31 days were also recorded Serious Adverse events up to 6 months after vaccination |
N=1504 Both groups received one dose of either ACWY-TT or Men-PS |
Men-C-ACYW-TT and Men-P-ACYW-135 vaccines resulted in similar safety profiles, including fewer than 1% of grade 3 solicited and unsolicited general symptoms in both treatment groups. There were no SAEs due to vaccination. Insufficient power to meet primary non-inferiority safety objective. Exploratory comparison identified higher incidence of injection site pain with Men-PS compared to MenACWY-TT in ≥6 year olds (p=0.0309). No serious adverse events related to injection, no deaths. Frequencey of grade 3 general symptoms: 2-5 yr olds: 6-10 yrs olds: Serious Adverse Events: |
Level I |
Fair (Insuffi-cient power to meet primary non-inferiority safety objective) |
Vesikari, et al. 2012Footnote 24 |
MenACWY-TT versus Men-PS |
Open label randomized controlled trial 11 centers in Finland |
N=309 2-5 year olds Both received MenACWY-TT or Men-P-ACYW |
Within 4 days following vaccination, pain was most frequent solicited local symptom. Most common adverse event was pain at injection site. Grade 3 unsolicited adverse events occurred in (6.5% vs 5.1% of vaccine recipients). Only one grade 3 symptom was considered related to immunization: hematoma reported in a child who received Men-C-ACYW-TT. Solicited Events: SAEs and NOCs were recorded up to 6 months post-vaccination. |
Level I |
Fair (non-inferiority of safety was not assessed) |
Knuf, M., et al. 2013Footnote 25 |
MenACWY-TT versus |
Open label randomized controlled trial 31 centers in two countries (Germany, France) |
N=414 2-5 year olds, Both received either MenACWY-TT or MenC-CRM197 |
The occurrence of solicited local symptoms and solicited general symptoms were recorded up to 4 days post-vaccination. SAEs were reported during 6 months post-vaccination. In 2-5 year olds, redness was the most common solicited local symptom (35.2% ACWY-TT, or 39.6% in MenC-CRM). In 6-10 year olds, pain was most common solicited local symptom (43.9% ACWY-TT, 54.0% MenC-CRM). Fewer grade 3 adverse events were seen in participants who had received Men-C-ACYW-TT (6.8% of participants) versus Men-C-CRM197 (15.1%). Solicited symptoms: 6-10 year Unsolicited symptoms: SAE: |
Level I |
Fair (non-inferiority of safety was not assessed) |
| Evidence for Immunogenicity of Nimenrix™ in children 11-19 years age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Ostergaard, L., et al., 2012Footnote 27 |
MenACWY-TT, HepA/B, or coadminis-tered |
Open, randomised, controlled study Phase III 5 centres in Sweden and 1 centre in Denmark |
N=611 participants 11-17 years old N=122 MenACWY-TT |
Seroprotection was defined as rSBA titers ≥1:8. MenACWY-TT co-administered with HepA/B was non-inferior to MenACWY-TT alone. Listed below are % rSBA GMTs ≥ 1:8 at 1 month post-vaccination [95% CI]. Serogroup C Serogroup W-135 Serogroup Y |
Level I |
Good |
Ostergaard, L., et al., 2013Footnote 30 |
MenACWY-TT vs Men-PS |
Extension study Single center in Denmark Phase II |
N=50 immune persistence at 42 months post vaccination |
Seroprotection was defined as rSBA titers ≥1:8. All individuals achieved rSBA titres ≥1:8 for all serogroups and there were no significant differences in rSBA GMT between groups, except for that of serogroup W-135 at month 42 which was significantly higher in the Men-C-ACYW-TT group (n=19) than in the Men-P-ACYW-135 group (n=17). Listed below are rSBA GMTs at 42 months post-vaccination.Serogroup A Serogroup C Serogroup Y |
Level I |
Fair, (95% CI not provided for all rSBA titers; small sample size) |
Bermal, N. et al., 2011Footnote 31 |
MenACWY-TT vs Men-PS |
Open randomized controlled trial Multiple Centers in India, the Philippines, Taiwan Phase III |
N=1025 Blood samples collected prior to and one month after vaccination. |
Seroprotection was defined as rSBA titers ≥1:8. One month post-vaccination, at least 99.6% of subjects in both groups had rSBA titres ≥1:8 against each meningococcal vaccine serogroup; seroresponse rates significantly higher in Men-C-ACYW-TT group for serogroups A, W-135 and Y, and non-inferior to serogroup C compared with the Men-P-ACYW-135 group. Listed below are % rSBA GMTs ≥ 1:8 at 1 month post-vaccination [95% CI]. Serogroup C Serogroup W-135 Serogroup Y |
Level I |
Good |
Baxter, R. et al., 2011Footnote 26 |
MenACWY-TT vs. MenACWY-DT |
Randomized controlled parallel-group study Phase II Single-blind for 11-25 year old subjects, open for 10 year olds 10 centers in USA |
N=827 subjects 10-25 years old (N=587 MenACWY-TT; N=197 MenACWY-DT; N=88 MenACWY-TT/10 years old) |
Serum hSBA titer ≥1:4 considered protective against disease caused by serogroup C. It is common practice to extend the 1:4 threshold to other serogroups. Seroconversion defined as hSBA titers ≥1:8 one month postvaccination in subjects who had titers <1:4 prior to vaccination. Vaccine response defined as postvaccination hSBA antibody titer ≥1:16 in subjects with prevaccination hSBA antibody titer <1:4 or ≥4-fold increase in hSBA antibody titer postvaccination in subjects with prevaccination hSBA antibody titer ≥1:4. Immune response was similar except for relatively higher but statistically non-significant hSBA-MenA GMT values in the 10 year old group. Listed below are %hSBA titers ≥1:4 [95%CI] 1 month post-vaccination for each serotype. Serotype A Serotype C Serotype W-135 Serotype Y |
Level I |
Good |
| Evidence for Safety of Nimenrix™ in children 11-17 years age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Ostergaard, L., et al., 2009Footnote 12 |
5 different formulations of MenACWY-TT |
Double-blind randomized controlled trial |
N=50, 18-25 years old |
Grade 3 intensity symptoms defined as redness and swelling >50mm in diameter, axillary/oral body temperature >39.5 C, or any other event preventing normal everyday activity. Pain most commonly reported solicited (within 8 days) symptom at the injection site. Fatigue and headache most commonly reported systemic symptom. Fever reported by six subjects (two in group MenACWY-TT 5C, one in group MenACWY-TT 2.5 and three in control group) Grade 3 intensity local and general solicited symptoms reported six and nine subjects, respectively; incidence of symptoms following Men-ACWY-TT administration similar to control vaccine, except for pain and swelling. |
Level I |
Fair (low sample size for safety assess-ment) |
Bermal, N. et al., 2011Footnote 31 |
MenACWY-TT vs Men-PS |
Open randomized controlled trial Multiple Centers in India, the Philippines, Taiwan Phase III |
N=1025, 11-17 years of age N=1247, 18-55 years of age |
Pain at injection site most common solicited (within 4 days) local symptom reported by adolescents (26.2% in Men-ACWY-TT and 26.8% in Men-PS group). Redness and swelling reported by 12.3% and 9.3% (respectively) in ACWY-TT group, and by 6.3% in Men-PS group recipients. Local symptoms of Grade 3 intensity reported by no more than 1.2% of subjects in ACWY-TT group and by no subjects in Men-PS group. Solicited general symptom after vaccination reported by adolescents within the same range in both groups; less than 0.9% of subjects in either treatment group reported solicited general symptoms of Grade 3 intensity after vaccination. At least one unsolicited (within 31 days) reported by 9.4% participants in Men-ACWY-TT group and 10.1% participants in Men-PS group. Unsolicited AEs considered causally related to vaccination by investigators reported by 1.4% and 1.2% by Men-ACWY-TT and Men-PS recipients, respectively; none of Grade 3 intensity. No cases of new onset chronic illness reported throughout the entire study period. Five subjects experienced one or more SAEs, none of which considered causally related to vaccination by investigators. |
Level I |
Fair (under-powered for inferiority analysis) |
| Evidence for Immunogenicity of Nimenrix™ in adults age 18-55 years | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Dbaibo, G. et al. 2012bFootnote 34 |
Men-C-ACWY-TT Men-P- ACWY |
Open randomized controlled trial Lot to lot consistency (3 lots) 4 centers in Lebanon and the Philippines |
N=1247 individuals 18-55 years of agerandomi-zed in to receive Men-C-ACWY-TT (3 groups) or |
rSBA titer >1:8 used as measure of immunity Vaccine response was defined as an titer of at least 1:32 at 1 month previously seronegative subjects (rSBA titer <1:8) and at least a four-fold increase in previously seropositive subjects Consistency and non-inferiority was demonstrated between the 3 lots of ACWY-TT tested. Percentage of subjects with rSBA titers >1:128 increased from between 48.8% and 79% to between 98.9% and 99.5% post vaccination with Men-C-ACWY-TT for all serogroups Vaccine response in Men-C-ACWY-TT group (pooled) was >80% for all serogroups; non-inferiority in vaccine response was demonstrated for all serogroups (significantly higher in in Men-C-ACWY-TT group than in Men-P- ACWY group for all serogroups except C) rSBA GMT was significantly higher in the Men-C-ACWY-TT group than in Men-P- ACWY group for all serogroups except C) |
Level I |
Good |
Borja-Tabora, C., et al. (2013)Footnote 35 |
Men-C-ACWY-TT Men-P- ACWY |
Open randomized controlled trial Three centers in the Philippines and Saudi Arabia Three year follow-up reported Phase IIb |
N=500 individuals 11 to 55 years old randomized to receive Men-C-ACWY-TT or Men-P- ACWY vaccine All individuals received one dose of either Men-C-ACWY-TT or Men-P- ACWY vaccine Blood samples were collected at Month 1, Year 1, Year 2, and Year 3. |
rSBA titer >1:8 used as measure of immunity Vaccine response was defined as an titer of at least 1:32 at 1 month previously seronegative subjects (rSBA titer <1:8) and at least a four-fold increase in previously seronpositive subjects More than 99.4% of subjects attained rSBA titers >1:8 and >1:128, with pre/post vaccination rSBA GMT increasing at least 9 fold one month following immunization Men-C-ACWY-TT for all serogroups. Vaccine response in Men-C-ACWY-TT group ranged from 82.7% to 96.3%; non-inferiority in vaccine response was demonstrated for all serogroups (significantly higher in in Men-C-ACWY-TT group than in Men-P- ACWY group for serogroups A and Y) At Year 3, ≥99.1% and ≥92.9% of the participants in the Men-C-ACWY-TT group retained rSBA titres ≥8 and ≥128 for each of the four serogroups; rSBA GMTs at Year 3 remained higher than pre-vaccination values and were statistically significantly higher than in the Men-P-ACWY group for all serogroups Listed below are rSBA titers for Meningococcal serogroups (≥ 1:8) 1 month post vaccination: MenACWY-TT (95% CI) A 100 [98.9, 100] C 99.7 [98.4, 100] W-135 99.7 [98.4, 100] Y 100 [98.9, 100] Men-PS (95% CI) A 100 [96.8, 100] Y 100 [96.8, 100] |
Level I |
Good |
Bermal, N., et al (2011)Footnote 31 |
MenACWY-TT vs Men-PS |
Open randomized controlled trial Multiple Centers in India, the Philippines, Taiwan Phase III |
N=1025 11-17 years of age for immunogenic-city, pooled with N=1247 18-55 years of age for safety Blood samples collected prior to and one month after vaccination. |
Vaccine response defined as rSBA titer of at least 1:32 in subjects initially seronegative [i.e. rSBA titer <1:8] and a four-fold increase in titer in subjects initially seropositive (i.e. rSBA titer ≥1:8). One month post-vaccination, ≥85.4%-97.1% had a vaccine response (post-titer ≥1:8 in initially seronegative and ≥4-fold increase in seropositive), versus 78.0%-96.6% after Men-PS, against each vaccine serogroup. rSBA GMTs, against each serogroup increased between 29.2- to 274.3-fold in ACWY-TT vaccinees after vaccination, and by 16.4- to 185.9-fold in Men-PS vaccinees. Comparisons showed that the percentage of subjects with a vaccine response against serogroups A, W-135 and Y was statistically significantly higher following ACWY-TT than Men-PS, even when adjusted for pre-vaccination values. |
Level I |
Good |
| Safety of Nimenrix™ in adults age 19-55 years | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Bermal, N., et al (2011)Footnote 31 |
MenACWY-TT vs Men-PS |
Open randomized controlled trial Multiple Centers in India, the Philippines, Taiwan Phase III |
N=1247 (Pooled with 1-17) 18-55 years of age Blood samples collected prior to and one month after vaccination. |
Non-inferiority of MenACWY-TT for grade 3 solicited and unsolicited systemic symptoms could not be demonstrated. The ratio of incidence in the ACWY-TT group over the Men-PS group between groups in grade 3 symptoms symptoms was 1.42% [95% CI 0.67; 3.00]. No symptoms were significantly different between the two vaccines. Exploratory comparisons between groups revealed no significant differences in general symptom frequency (solicited or unsolicited). |
Level I |
Fair (insuffi-cient power for safety non-inferiority test) |
Dbaibo, G. et al. 2012bFootnote 34 |
MenACWY-TT vs Men-PS |
Partially blinded randomized controlled trial Lot to lot consistency 4 centers in Lebanon and the Philippines Phase III |
N=1025 11-17 years of age for immunogeni-city, pooled with 18-55 years of age for safety |
Percentage of subjects reporting pain, redness and swelling at the injection site was higher in ACWY-TT recipients than MenPS recipients, but occurrence of general symptoms was similar in both groups. |
Level I |
Good |
Borja-Tabora, C., et al. 2013Footnote 35 |
See above |
See above |
See above |
During four-day post-vaccination period, grade 3 general symptoms were reported by five participants (1.3%) in the ACWY-TT group and by no participant in the Men-PS group. Any grade 3 symptoms were reported by 12 participants (3.2%) in the ACWY-TT group and by one participant (0.8%) in the Men-PS group. Non-inferiority of the MenACWY-TT vaccine over the MenACWY polysaccharide vaccine in terms of any grade 3 general symptom was demonstrated. Pain was most frequent local symptom, headache most frequent general symptom for both groups. During 6-month extended safety follow-up period two SAEs were reported in the ACWY-TT group, but none were considered related to vaccination by the investigators. |
Level I |
Good |
| Evidence Immunogenicity of Nimenrix™ in adults more than 55 years of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Dbaibo, G., et al., 2013Footnote 36 |
Nimenrix™ (MenACWY-TT) versus Mencevax (MenPS) |
phase IIIb, open-label, randomized |
N=400 healthy adults 56-103 years old |
Vaccine response defined as: post-vaccination rSBA titer C1:32 in initially seronegative subjects; four-fold increase in initially seropositive subjects with rSBA titer 1:8 and <1:128; two-fold increase in initially seropositive subjects with rSBA titer ≥1:128 One month following vaccination there were no significant differences in rSBA >1:8; however, those vaccinated with Men-P-ACYW-135 did achieve a significantly higher vaccine response for serogroup A and rSBA GMT values for serogroups A and C than those receiving the conjugate vaccine. Lower vaccine response was primarily observed in individuals receiving MenACWY-TT who had very high titres (≥1:128) pre-vaccination. Listed below are % rSBA titers ≥ 1:8 for Meningococcal serogroups 1 month post vaccination: MenACWY-TT (95% CI) Serogroup A: Serogroup C: Serogroup W-135: Serogroup Y: |
Level I |
Good |
| Evidence for Safety of Nimenrix™ in adults more than 55 years of age | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Dbaibo, G., et al., 2013Footnote 36 |
Nimenrix™ (MenACWY-TT) versus Mencevax (MenPS) |
phase IIIb, open-label, randomized Single center in Lebanon |
N=400 healthy adults 56-103 years old |
Fewer than 3.0% of subjects reported adverse events; no SAEs were considered vaccine related. |
Level 1 |
Good |
| Evidence for Antibody Persistence | ||||||
|---|---|---|---|---|---|---|
| Study Details | Summary | |||||
| Study | Vaccine | Study Design | Participants | Summary of Key Findings | Level of Evidence | Quality |
Vesikari, T. et al., 2012aFootnote 24 |
MenACWY-TT versus Men-PS |
Open label randomized controlled trial 11 centers in Finland Phase II |
N=309 2-5 year olds Both received MenACWY-TT or Men-P-ACYW |
Vaccine response was defined as post-vaccination rSBA titer ≥ 1:32 for initially seronegative children (rSBA titer < 1:8 at pre-vaccination) and at least a 4-fold increase in rSBA titer from pre- to post-vaccination for initially seropositive Children in Men-C-ACYW-TT group showed higher rSBA GMT values for all serogroups year 1 and 2 post vaccination when compared to those who received Men-P-ACYW-135. In year 3, all serogroups except C, showed higher GMT values in the Men-C-ACYW-TT group compared to the Men-P-ACYW-135 group rSBA GMT Serogroup A Men-PS (95% CI) Serogroup C Men-PS (95% CI) Serogroup W-135 Men-PS (95% CI) Serogroup Y Men-PS (95% CI) Listed below are rSBA titers ≥ 1:8 for Meningococcal serogroups 3 years post-vaccination: Men-PS (95% CI) |
Level I |
Good |
Knuf, M., et al. (2012)Footnote 37 |
MenACWY-TT versus Men-PS or MenC-CRM197 |
Extension study of open label randomized controlled trial 35 centers in Germany and Austria |
N=203 12-14 month olds -both groups tested for persistence 15 months post vaccination |
Seroprotection was defined as rSBA titers ≥1:8. The percentage achieving rSBA titre ≥ 1:8 for serogroup C was higher in the Men-C-ACYW-TT group than in the control group receiving Men-C-CRM197 (12-14 month group). Comparing the Men-C-ACYW-TT group for serogroups C and Y, the Men-C-ACYW-TT group also had higher rSBA titres than in the control group which received Men-P-ACYW-135 (3-5 year group). GMT values were also reported to be higher. MenC-CRM197 (95% CI) : 75.0 [58.8, 87.3] 3-5 yr olds rSBA ≥ 1:8 titres at 16 months post immune challenge MenACWY-TT (95% CI): 100 [89.1, 100] |
Level I |
Good |
Ostergaard et al. 2013Footnote 30 |
MenACWY-TT vs Men-PS |
Extension study Single center in Denmark Phase II |
N=50 -immune persistence at 42 months post vaccination |
Seroprotection was defined as rSBA titers ≥1:8. At 42 months post-vaccination, 100% (95% CI 82.4-100%) of subjects in the MenACWYTT group (19/19) had rSBA titers ≥ 1:8 compared with 88.2% (95% CI 63.6-98.5%) in the MenPS group (15/17). In both groups, rSBA titres ≥1:8 and GMT values were similar at month 42 for all serogroups except serogroup W-135, which was significantly higher in the Men-C-ACYW-TT group. Listed below are rSBA GMTs ≥ 1:8 at 42 months post-vaccination. Serogroup C Serogroup W-135 Serogroup Y |
Level I |
Fair, (95% CI not provided for all rSBA titers; small sample size) |
Vesikari, T. et al. 2012bFootnote 19 |
MenACWY-TT |
Open randomized controlled trial |
N=304 individuals 12-23 months old: N=177 (MenACWY-TT) + 37 (MenC-CRM197) |
Seroprotection was defined as rSBA titers ≥1:8, or hSBA titers ≥1:4. 3 years post-vaccination, 90.8% of Men-C-ACYW-TT recipients retained rSBA titres ≥ 1:8 for all serogroups, and 73.6% hSBA titres ≥ 1:4 for serogroups C, W and Y; only 28.1% had hSBA titres ≥ 1:4 for serogroup A. There was no significant difference in rSBA titre between Men-C-ACYW-TT and Men-C-CRM197 recipients 3 years post- vaccination. In first 2 years post-vaccination the GMTs were higher among the group immunized with Men-C-ACYW-TT than Men-C-CRM197, whereas in year 3 no significant difference was observed. Listed below are rSBA titers (≥ 1:8) and hSBA titers (≥ 1:4) for Meningococcal serogroups 3 years post-vaccination: Serogroup C (95% CI) Serogroup W-135 (95% CI) Serogroup Y (95% CI) |
Level I |
Good |
Borja-Tabora, C., et al. 2013Footnote 35 |
Men-C-ACWY-TT |
Open randomized controlled trial Three centers in the Philippines and Saudi Arabia Three year follow-up reported Phase IIb |
N=500 individuals 11 to 55 years old randomized to receive Men-C-ACWY-TT or Men-P- ACWY vaccine All individuals received one dose of either Men-C-ACWY-TT or Men-P- ACWY vaccine Blood samples were collected at Month 1, Year 1, Year 2, and Year 3. |
Seroprotection was defined as rSBA titers ≥1:8. At Year 3, ≥99.1% and ≥92.9% of the participants in the Men-C-ACWY-TT group retained rSBA titres ≥8 and ≥128 for each of the four serogroups; rSBA GMTs at Year 3 remained higher than pre-vaccination values and were statistically significantly higher than in the Men-P- ACWY group for all serogroups Listed below are rSBA titers (≥ 1:8) for Meningococcal serogroups 3 years post-vaccination: Men-PS (95% CI) |
Level I |
Good |
List of Abbreviations
Acknowledgments
†NACI Members: Dr. I. Gemmill (Chair), Dr. C. Quach-Thanh (Vice-Chair), Dr. S. Deeks, Dr. B. Henry, Dr. D. Kumar, Dr. M. Salvadori, Dr. B. Seifert, Dr. N. Sicard, Dr. W. Vaudry, Dr. R. Warrington.
Former NACI Members: Dr. N. Crowcroft, Dr. B. Warshawsky (Chair).
Liaison Representatives: Dr. J. Blake (Society of Obstetricians and Gynaecologists of Canada), Dr. J. Brophy (Canadian Association for Immunization Research and Evaluation), Dr. J. Emili (College of Family Physicians of Canada), Dr. M. Lavoie (Council of Chief Medical Officers of Health), Dr. C. Mah (Canadian Public Health Association), Dr. D. Moore (Canadian Paediatric Society), Dr. A. Pham-Huy (Association of Medical Microbiology and Infectious Disease Canada), Ms. E. Sartison (Canadian Immunization Committee).
Former Liaison Representative: Dr. A. Mawle (Centers for Disease Control and Prevention, United States)
Ex-Officio Representatives: Ms. G. Charos (Centre for Immunization and Respiratory Infectious Diseases [CIRID], Public Health Agency of Canada [PHAC], Dr. G. Coleman (Biologics and Genetic Therapies Directorate [BGTD], Health Canada [HC]), Dr. (LCol) P. Eagan (National Defence and the Canadian Armed Forces), Dr. J. Gallivan (Marketed Health Products Directorate [MHPD], HC), Dr. B. Law (CIRID, PHAC), Ms. M. St-Laurent (CIRID, PHAC), Dr. T. Wong (First Nations and Inuit Health Branch [FNIHB], HC).
Former Ex-Officio Representatives: Dr. M. Carew (FNIHB, HC), Dr. D. Garcia (FNIHB, HC), Dr. A. Klein (BGTD, HC), Dr. B. Raymond (CIRID, PHAC), Dr. E. Taylor (MHPD, HC).
†This statement was prepared by Dr. S. Desai, Dr. O. Baclic, Dr. N. Crowcroft, Dr. B. Henry, Ms. J. Rotondo, Dr. M. Tunis and approved by NACI.
NACI also gratefully acknowledges the contribution of Dr. J. Bettinger, Dr. P. De Wals, Dr. J. Embree.