Adverse Events Following Immunization (AEFI) Bi-annual Report from July 1 to December 31, 2018
For COVID-19 data, refer to COVID-19 adverse events following immunization.
Safety assessment summary for July 1 to December 31, 2018
- No vaccine safety signals were identified from July 1 to December 31, 2018.
- This report presents 1,372 AEFI reports routinely received from federal, provincial and territorial jurisdictions between July and December 2018.
Background
Vaccines are closely monitored in Canada at all phases of the vaccine product 'life cycle' from discovery through market authorization (pre-market) and beyond, as people begin using them (post-market). Many stakeholders are involved in vaccine safety surveillance, including the federal government, provincial, territorial and local public health authorities, health care providers, vaccine industry and the public. Provincial and territorial vaccination programs monitor AEFIs and report them to the Public Health Agency of Canada (the Agency). The Agency conducts post-market safety surveillance through the Canadian Adverse Events Following Immunization Surveillance System (CAEFISS). CAEFISS is managed by the Agency and is unique in that it includes both passive (spontaneous reports from federal, provincial and territorial (FPT) public health authorities) and active surveillance. Active surveillance is conducted by Immunization Monitoring Program ACTive (IMPACT), a network of 12 pediatric hospitals across Canada that screens hospital admissions for specific AEFIs. Vaccine safety concerns are monitored and addressed through the Vaccine Vigilance Working Group (VVWG).
An Adverse Event Following Immunization (AEFI) is defined as any untoward medical occurrence which follows immunization and which does not necessarily have a causal relationship with the usage of the vaccine. The adverse event may be any unfavourable or unintended sign, abnormal laboratory finding, symptom or disease. Serious AEFIs are those which are life-threatening, result in hospitalization or a prolongation of hospitalization, result in persistent or significant disability, or where the outcome is a birth defect or death, as defined by the World Health Organization (WHO) (PDF). AEFIs not meeting the definition of a serious event are classified as non-serious.
WHO defines a vaccine safety signal as information (from one or multiple sources) that indicates a new and potentially causal association, or a new aspect of a known association, between a vaccine and an event previously unknown or incompletely documented that could affect health. Epidemiological studies are usually needed to assess the causal relationship between the vaccine and the signal. The primary purpose of vaccine post-market surveillance is to detect safety concerns. These concerns include a possible increase in the severity or frequency of expected AEFIs, or occurrence of one or more unexpected events (i.e., an event that is not consistent with Canadian product information or labeling). This allows vaccination providers and public health vaccination program providers to take public health action at the level of the:
- individual (such as further investigations to confirm a diagnosis and determine possible causes, consultation to rule out allergy to one or more vaccine components, or evaluate whether or not to give subsequent doses of a vaccine), and/or
- vaccination program (such as investigation of a cluster of adverse events, review of procedures to ensure that vaccine storage requirements have been strictly followed, or consideration of a change in policy to adopt a less reactogenic vaccine)
The Agency also shares AEFI data with Health Canada's Health Products and Food Branch, the national regulatory authority for vaccines in Canada. This enables formal action related to vaccines marketed in Canada to take place if needed. These actions may include issuing communications to vaccination providers or the public regarding the safety concern or requiring additional information or investigation by the vaccine distributor, or changes to the product labeling.
Vaccine safety surveillance reports summarizing CAEFISS data are released by the Agency on a routine basis. The bi-annual reports, similar to the previous Quarterly Reports, summarize all AEFI reports received by the Agency from January to June and July to December, regardless of the date the vaccine was given.
In this report, the data for July to December 2018 as well as the 4-year averages for the same time period are shown. However, because these data reflect the date the reports were received and not the date the vaccine was given, the ability to compare and interpret patterns is limited. The report does provide a data snapshot that highlights serious and non-serious AEFI reports received for descriptive purposes.
Notes on interpretation: AEFI reports submitted to the Public Health Agency of Canada represent a suspicion, opinion, or observation by the reporter as opposed to an assertion or proof that the vaccine may have caused the event. Additional limitations to AEFI report data include potential underreporting, lack of certainty regarding the diagnostic validity of a reported event, missing information regarding other potential causes and other reporting biases. These biases are mitigated by the Agency through use of a reporting form for AEFI (PDF) with a guide to its use, standardized medical coding using the Medical Dictionary for Regulatory Activities (MedDRA), follow-up with the jurisdictions for completeness of information, high reporting rates and inclusion of an active pediatric surveillance component in CAEFISS.
Results highlighted for July 1 to December 31, 2018
Data presented in this bi-annual report include AEFI reports routinely received from July 1 to December 31, 2018, and comparisons are made to the average number of reports received in the same time period over the previous 4 calendar years (2014-2017). The data analysed were extracted from the CAEFISS database on February 20, 2019, by the Agency.
It is important to note that in previous reports, one jurisdiction was excluded due to data transmission technical issues. As of January 2018, the issues have been resolved and all jurisdictions are now reporting in full. This is reflected by the increase of the total number of reports and in the results of the analysis for this reporting period.
All reports are processed and coded using MedDRA, a standardized medical terminology that supports data entry, retrieval, evaluation and presentation of clinical information and further coded with a main reason for reporting through a detailed review of individual case safety reports. Therefore, if more than one event is described, the one that is determined to have led to reporting is coded as the primary AEFI. In addition, all reports describing a serious event were reviewed and, unless highlighted in this report, found either to be expected (based on known vaccine-related adverse reactions), to have alternate explanations not related to vaccination, or are currently being monitored or investigated further.
Number of AEFI and serious AEFI reports
A total of 1,372 AEFI reports were submitted to the Agency from July to December 2018. During the same time period of 2014, 2015, 2016 and 2017, the Agency received an average of 1,266 (range: 1,051-1,433) [Figure 1].
A total of 114 AEFI reports received by the Agency between July and December of 2018 were classified as serious (8% of all AEFI reports). During the same time period for 2014, 2015, 2016 and 2017, the Agency received an average of 116 (range: 94-143) serious AEFI reports (9% to 10% of all AEFI reports). As noted above, all jurisdictions are now reporting full data as of January 2018, which led to a slight increase in the number of AEFI reports received during this period. The proportion of serious reports was similar to previous periods.
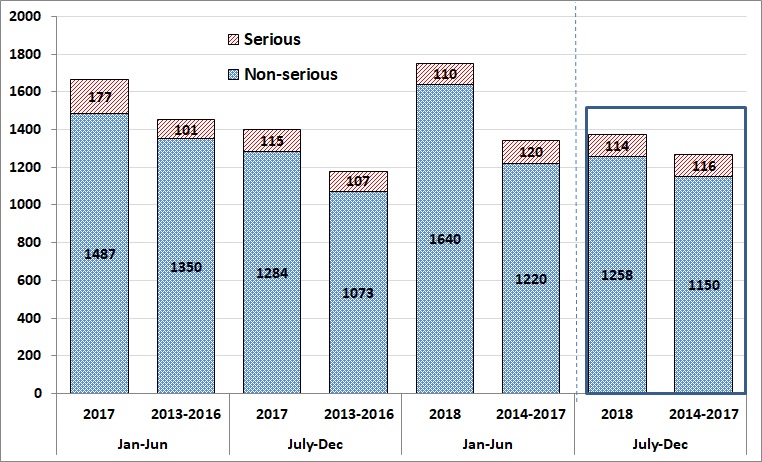
Figure 1 - Text description
| Half year | Year | Number of AEFI reports | |
|---|---|---|---|
| Non serious | Serious | ||
| Jan-Jun | 2017 | 1,487 | 177 |
| July-Dec | 2017 | 1,284 | 115 |
| Jan-Jun | 2018 | 1,640 | 110 |
| July-Dec | 2018 | 1,258 | 114 |
| Half year | Year | Average number of AEFI reports | |
|---|---|---|---|
| Non serious | Serious | ||
| Jul-Dec | 2013-2016 | 1,350 | 101 |
| Jan-Jun | 2013-2016 | 1,073 | 107 |
| Jul-Dec | 2014-2017 | 1,220 | 120 |
| Jan-Jun | 2014-2017 | 1,150 | 116 |
Frequency of serious and non-serious AEFI reports by age group
Table 1 shows the number of serious and non-serious AEFI reports by age group from July to December 2018 and comparison with the 2014-2017 average for the same time period. The majority of serious adverse events (SAEs) were in those less than 18 years of age (83%, n=95) with approximately two-thirds (66%, n=75) of total serious reports being reported in children under the age of two. Possible reasons for the greater proportion of SAEs in the less than 18 age group may be due to the IMPACT surveillance system, which actively searches for specific surveillance targets in children admitted to pediatric tertiary care hospitals. Also, the number of vaccines received by children less than 2 years of age creates more opportunities for adverse events temporarily associated with immunization and to report to a health care provider. As noted above, all jurisdictions are now reporting full data as of January 2018, which led to a slight increase in the number of reports received during this period. The proportion of serious reports was similar to previous periods.
| Age group | Serious reports | Non-serious reports | ||
|---|---|---|---|---|
| 2018 | Average | 2018 | Average | |
| 2014-17 | 2014-17 | |||
| 0 to <1 year | 38 (33%) | 41 (35%) | 182 (14%) | 154 (13%) |
| 1 to <2 years | 37 (32%) | 35 (30%) | 197 (16%) | 151 (13%) |
| 2 to <7 years | 10 (9%) | 14 (12%) | 126 (10%) | 111 (10%) |
| 7 to <18 years | 10 (9%) | 9 (8%) | 161 (13%) | 203 (18%) |
| 18 to <65 years | 11 (10%) | 13 (11%) | 394 (31%) | 389 (34%) |
| 65 years and over | 6 (5%) | 5 (4%) | 167 (13%) | 138 (12%) |
| Unknown | 2 (2%) | 1 (0%) | 31 (2%) | 6 (1%) |
| Total | 114 | 116 | 1,258 | 1,150 |
AEFI reports received by reporter type
Figure 2 presents the originating reporter of the AEFI reports (both serious and non-serious) received during July to December 2018. The majority of reports received were reported by Registered Nurses (RN) (73%), followed by Medical Doctors (MD) (12%) and consumers (6%).
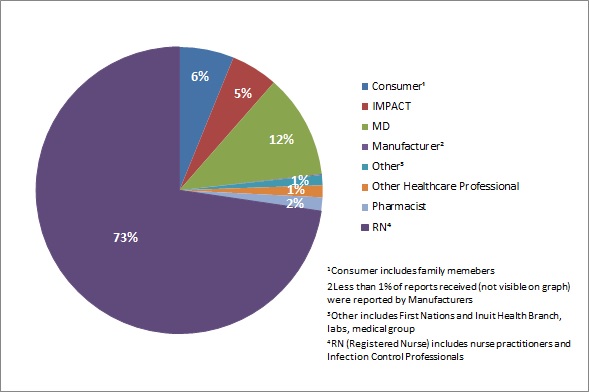
Figure 2 - Text description
| AEFI reports received by type of originating reporter | Percentage of total AEFI reports |
|---|---|
| ConsumerFootnote 1 | 6% |
| IMPACT | 5% |
| MD | 12% |
| ManufacturerFootnote 2 | 0% |
| OtherFootnote 3 | 1% |
| Other healthcare professional | 1% |
| Pharmacist | 2% |
| RNFootnote 4 | 73% |
|
|
AEFIs by major classification
Figure 3 presents the main types of AEFIs (both serious and non-serious) reported during July to December 2018. Excluding the "Other events" category, the majority of the AEFIs reported were vaccination site reactions, followed by allergic or allergic-like events.
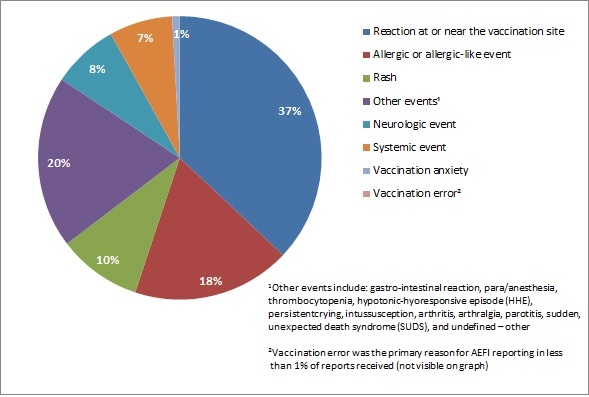
Figure 3 - Text description
| Main reason for reporting an adverse event following immunization | Percentage of total AEFI reports |
|---|---|
| Reaction at or near the vaccination site | 37% |
| Allergic or allergic-like event | 18% |
| Other eventsFootnote 1 | 20% |
| Rash | 10% |
| Systemic event | 7% |
| Neurologic event | 8% |
| Vaccination anxiety | 1% |
| Vaccination errorFootnote 2 | 0% |
|
|
The main types of AEFI by level of seriousness reported between July-December 2018 compared to the 2014-2017 average for the same time period are shown in Table 2. Between July and December of 2018 as well as in the past 4 years, excluding the "Other events" category, reactions at or near the vaccination site, allergic or allergic-like events, and rash were the main AEFIs reported for non-serious cases. Excluding the "Other events" category, neurologic events (which are most often seizures triggered by fever), reactions at or near the vaccination site, and systemic events (i.e., events involving many body systems often accompanied by fever) were the most frequent AEFIs reported for the serious cases.
| Primary AEFI reported | Serious reports | Non-serious reports | ||
|---|---|---|---|---|
| 2018 | Average | 2018 | Average | |
| 2014-17 | 2014-17 | |||
| Reaction at or near the vaccination site | 16 (14%) | 12 (10%) | 492 (39%) | 501 (44%) |
| Allergic or allergic-like event | 4 (4%) | 10 (8%) | 244 (19%) | 183 (16%) |
| Rash | 1 (1%) | 1 (1%) | 130 (10%) | 172 (15%) |
| Systemic event | 13 (11%) | 26 (23%) | 86 (7%) | 100 (9%) |
| Neurologic event | 42 (37%) | 39 (34%) | 62 (5%) | 37 (3%) |
| Vaccination anxiety | 1 (1%) | 0 (0%) | 11 (1%) | 16 (1%) |
| Vaccination error | 0 (0%) | 0 (0%) | 0 (0%) | 2 (0%) |
| Other eventsFootnote 1 | 37 (32%) | 28 (24%) | 233 (19%) | 140 (12%) |
| Total | 114 | 116 | 1,258 | 1,149 |
|
||||
Vaccines administered in AEFI reports
Table 3 lists the most commonly administered vaccines among AEFI reports between July-December 2018. Pneumococcal vaccines had the most AEFI reports (serious and non-serious combined) followed by measles, mumps, rubella, varicella (MMRV and MMR + V) vaccines. As seen in previous years, the highest proportion of serious reports were following pneumococcal, MMRV and MMR + V, Diphtheria, Tetanus and acellular Pertussis (DTaP) infant series, and meningococcal vaccines. An increase in the number of reports received for some vaccines was noted during the reporting period including zoster vaccines. This could be explained by changes in jurisdictional immunization programs (e.g.: Recombinant Zoster Vaccine (RVZ) was approved for marketing in Canada in November 2017 and covered in the province of Ontario for individuals aged 65 and over). As noted above, all jurisdictions are now reporting full data as of January 2018, which led to a slight increase in the number of reports received during this period. The proportion of serious reports was similar to previous periods.
| Vaccines administered | Serious reports | Non-serious reports | ||
|---|---|---|---|---|
| 2018 | Average | 2018 | Average | |
| 2014-17 | 2014-17 | |||
| 1. DTaP booster | 1 (0%) | 1 (0%) | 6 (0%) | 19 (1%) |
| 2. DTaP infant series | 46 (17%) | 46 (18%) | 260 (12%) | 198 (11%) |
| 3. Hepatitis B | 9 (3%) | 6 (2%) | 66 (3%) | 87 (5%) |
| 4. Human papillomavirus (HPV) | 5 (2%) | 3 (1%) | 89 (4%) | 83 (5%) |
| 5. Influenza | 16 (6%) | 22 (9%) | 322 (15%) | 309 (17%) |
| 6. Measles, mumps, rubella, varicella (MMRV and MMR + V) | 48 (18%) | 41 (16%) | 323 (15%) | 240 (13%) |
| 7. Meningococcal | 41 (15%) | 39 (15%) | 233 (11%) | 188 (10%) |
| 8. Other vaccines | 0 (0%) | 1 (0%) | 26 (1%) | 19 (1%) |
| 9. Pneumococcal | 59 (22%) | 60 (24%) | 352 (16%) | 316 (17%) |
| 10. Rotavirus | 29 (11%) | 24 (10%) | 113 (5%) | 75 (4%) |
| 11. Tdap booster | 9 (3%) | 6 (2%) | 204 (9%) | 181 (10%) |
| 12. Travel vaccines | 5 (2%) | 4 (2%) | 56 (3%) | 76 (4%) |
| 13. Zoster virus | 5 (2%) | 1 (0%) | 106 (5%) | 52 (3%) |
| Total | 273 | 254 | 2,156 | 1,842 |
|
||||
Summary
This bi-annual report for 2018 is based on reports of adverse events received at the Agency from FPT public health authorities and active, pediatric hospital based surveillance. Detailed evaluation of the reports and reporting patterns in collaboration with provincial/territorial vaccine safety focal points of the VVWG and Health Canada have not identified any vaccine safety signals of concern. The tables and figures in this report provide a snapshot of the data provided to, and reviewed by, the Agency.