Enhanced Surveillance of Invasive Meningococcal Disease in Canada:
1 January, 2004, through 31 December, 2005
1 June 2007
Volume 33
Number 10
Invasive meningococcal disease (IMD) has been a nationally notifiable disease in Canada since 19241 . The Immunization and Respiratory Infections Division of the Centre for Infectious Disease Prevention and Control and the National Microbiology Laboratory (NML), Public Health Agency of Canada (PHAC), have published enhanced IMD surveillance reports since 19952-5 . This surveillance report provides information on IMD from 1 January, 2004, through 31 December, 2005.
Methods
As of 1 January, 2000, clinical cases of IMD were excluded from national reporting, and the case definition used for cases reported during the period covered by this publication was as follows6:
Confirmed case: invasive disease* with laboratory confirmation of infection:
- isolation of Neisseria meningitidis from a normally sterile site (blood, cerebrospinal fluid [CSF], joint, pleural or pericardial fluid) or
- demonstration of N. meningitidis antigen in CSF.
*Invasive meningococcal disease usually manifests itself as meningitis and/or septicemia, although other manifestations may be observed. Invasive disease may progress rapidly to purpura fulminans, shock and death.
Provincial and territorial departments of health report non-nominal epidemiologic data to the PHAC on all confirmed cases of IMD meeting the national case definition. Provincial and territorial public health and/or hospital laboratories send N. meningitidis isolates to the NML in Winnipeg for confirmation of serogroup and further bacteriologic studies. These consist of serotyping and subtyping for all isolates and multilocus enzyme electrophoresis or multilocus sequence typing for all serogroup C isolates. Probabilistic matching is conducted to link epidemiologic and laboratory data. Matching is done using the following variables: province/territory, date of birth (or age), sex, onset date and serogroup. Data are entered into an Access database (v 2000) and analyzed using the Statistical Package for the Social Sciences (v 12.0).
Population estimates for the provinces and territories (P/T) were obtained from Statistics Canada and were based on the 2001 Census7. These estimates were used to calculate overall, age group- and serogroup-specific incidence rates. All incidence rates are per 100,000 population per year. Chi-square tests, t tests, or the non-parametric Kruskall-Wallis H test were used to analyze associations between variables.
At the time of publication, the PHAC does not have a systematic method of collecting information on IMD outbreaks at the national level. In order to describe the number of IMD outbreaks during this surveillance period, e-mail surveys were sent to IMD representatives in all P/T in May 2005 and December 2006.
Results
Number of cases
In 2004 and 2005, the P/T reported a total of 190 and 178 IMD cases, respectively. Ninety percent of these were successfully matched to laboratory data in both years; 19 and 15 cases were not matched to laboratory data in 2004 and 2005, respectively.
The NML reported an additional 5 and 3 cases in 2004 and 2005, respectively, that could not be matched to those reported by the P/T (3 from Quebec, 2 from Ontario and 1 from each of Alberta, Prince Edward Island and New Brunswick). Therefore, the total numbers of cases included during 2004 and 2005 were 195 and 181, respectively. Tables 1 and 2 provide detailed incidence, case fatality rates (CFR), and serogroup data by age group and year. Figures 1 and 2 show the annual number of cases and incidence rates by province and territory for 2004 and 2005. These rates may be unstable because of small numbers.
Table 1.Incidence, case fatality and serogroup data for invasive meningococcal disease by age group, Canada, 2004
| Age group, years | < 1 | 1-4 | 5-9 | 10-14 | 15-19 | 20-24 | 25-64 | 65+ | Total |
|---|---|---|---|---|---|---|---|---|---|
| Overall incidence rate per 100,000 population (# cases) | 5.03 (17) | 1.76 (24) | 0.74 (14) | 0.62 (13) | 1.21 (26) | 1.07 (24) | 0.27 (48) | 0.36 (15) | 0.56 (181) |
| Case fatality rate, % (# deaths) | 0 (0) | 4.2 (1) | 0 (0) | 0 (0) | 7.7 (2) | 8.3 (2) | 6.3 (3) | 13.3 (2) | 5.5 (10) |
| Mortality rate per 100,000 population | 0 | 0.07 | 0 | 0 | 0.09 | 0.09 | 0.02 | 0.05 | 0.03 |
| Serogroup B incidence rate per 100,000 population (# of cases) | 3.55 (12) | 1.17 (16) | 0.58 (11) | 0.43 (9) | 0.56 (12) | 0.40 (9) | 0.12 (22) | 0.12 (5) | 0.30 (96) |
| Serogroup C incidence rate per 100,000 population (# of cases) | 0.30 (1) | 0.07 (1) | 0.11 (2) | 0.09 (2) | 0.28 (6) | 0.45 (10) | 0.08 (14) | 0.05 (2) | 0.12 (38) |
| Serogroup Y incidence rate per 100,000 population (# of cases) | 0 (0) | 0.15 (2) | 0.05 (1) | 0.05 (1) | 0.33 (7) | 0.18 (4) | 0.03 (6) | 0.07 (3) | 0.07 (24) |
| Serogroup W135 incidence rate per 100,000 population (# of cases) | 0.89 (3) | 0.29 (4) | 0 (0) | 0 (0) | 0.05 (1) | 0.04 (1) | 0.02 (3) | 0.09 (4) | 0.05 (16) |
| No. of other cases (e.g. serogroup 29E, nongroupables)* | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 3 |
| No. of cases with serogroup missing | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 4 |
| *Includes 2 cases of serogroup 29E and 1 non-groupable case. | |||||||||
Table 2. Incidence, case fatality and serogroup data for invasive meningococcal disease by age group, Canada, 2005
| Age group, years | < 1 | 1-4 | 5-9 | 10-14 | 15-19 | 20-24 | 25-64 | 65+ | Total |
|---|---|---|---|---|---|---|---|---|---|
| Overall incidence rate per 100,000 population (# cases) | 5.03 (17) | 1.76 (24) | 0.74 (14) | 0.62 (13) | 1.21 (26) | 1.07 (24) | 0.27 (48) | 0.36 (15) | 0.56 (181) |
| Case fatality rate, % (# deaths) | 0 (0) | 4.2 (1) | 0 (0) | 0 (0) | 7.7 (2) | 8.3 (2) | 6.3 (3) | 13.3 (2) | 5.5 (10) |
| Mortality rate per 100,000 population | 0 | 0.07 | 0 | 0 | 0.09 | 0.09 | 0.02 | 0.05 | 0.03 |
| Serogroup B incidence rate per 100,000 population (# of cases) | 3.55 (12) | 1.17 (16) | 0.58 (11) | 0.43 (9) | 0.56 (12) | 0.40 (9) | 0.12 (22) | 0.12 (5) | 0.30 (96) |
| Serogroup C incidence rate per 100,000 population (# of cases) | 0.30 (1) | 0.07 (1) | 0.11 (2) | 0.09 (2) | 0.28 (6) | 0.45 (10) | 0.08 (14) | 0.05 (2) | 0.12 (38) |
| Serogroup Y incidence rate per 100,000 population (# of cases) | 0 (0) | 0.15 (2) | 0.05 (1) | 0.05 (1) | 0.33 (7) | 0.18 (4) | 0.03 (6) | 0.07 (3) | 0.07 (24) |
| Serogroup W135 incidence rate per 100,000 population (# of cases) | 0.89 (3) | 0.29 (4) | 0 (0) | 0 (0) | 0.05 (1) | 0.04 (1) | 0.02 (3) | 0.09 (4) | 0.05 (16) |
| No. of other cases (e.g. serogroup 29E, nongroupables)* | 0 | 1 | 0 | 1 | 0 | 0 | 1 | 0 | 3 |
| No. of cases with serogroup missing | 1 | 0 | 0 | 0 | 0 | 0 | 2 | 1 | 4 |
| *Includes 2 cases of serogroup 29E and 1 non-groupable case. | |||||||||
Figure 1. Number of cases of IMD by province/territory by year
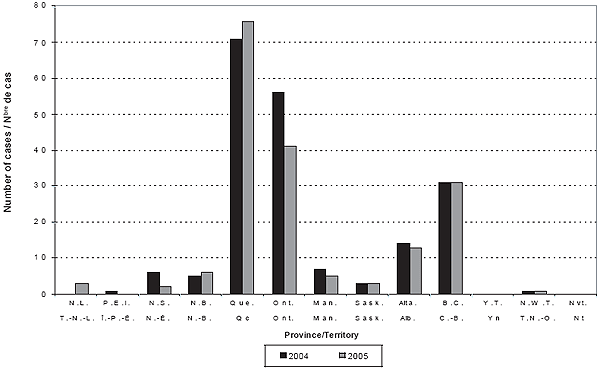
Figure 2. Incidence of IMD by province/territory by year
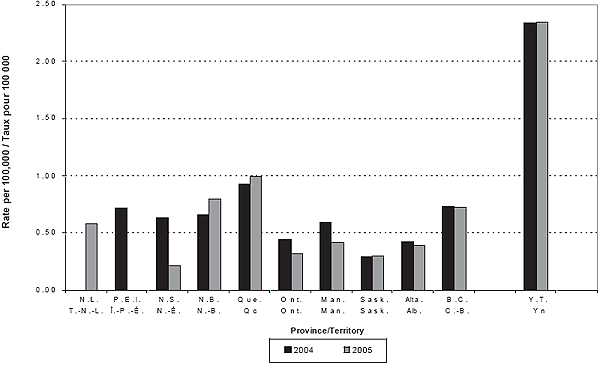
Incidence
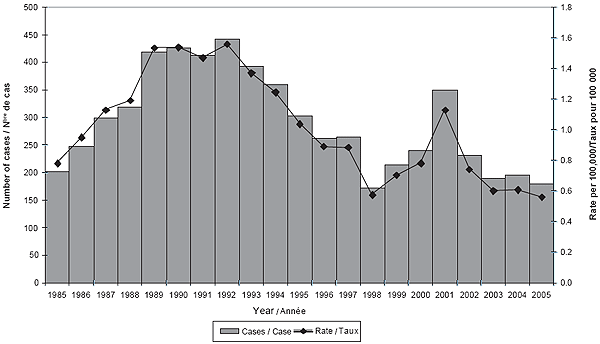
The overall incidence rates of IMD in 2004 and 2005 were 0.61 and 0.56 per 100,000 population, respectively. Figure 3 shows the annual number of cases and incidence rates since 1985 and illustrates the cyclical nature of meningococcal disease.
Figure 3. Number of cases and incidence rate of IMD in Canada, 1985-2005
Age and sex distribution
In 2004, the median age of IMD cases was 22 years (range 0 to 91 years); 23% of cases were under 5 years of age, an age group that accounts for 5.3% of the Canadian population. The highest incidence rates occurred among children: infants under 1 year and children 1 to 4 years old had rates of 5.34 and 1.97 cases per 100,000 population, respectively. Adolescents 15 to 19 years of age had the third highest rate, of 1.36 per 100,000 population.
In 2005, the median age of IMD cases was 19 years (range 0 to 96 years), and 23% of cases were under 5 years of age. The highest incidence rates were seen among infants under 1 year, at 5.03 per 100,000 population. Children between 1 and 4 years had the second highest rate, of 1.76 per 100,000 population, and adolescents 15 to 19 years old had the third highest rate, of 1.21 per 100,000 population.
Females accounted for 56% and 46% of IMD cases in 2004 and 2005, respectively. There was no significant difference in the median age of cases between males and females in either year.
Seasonal distribution
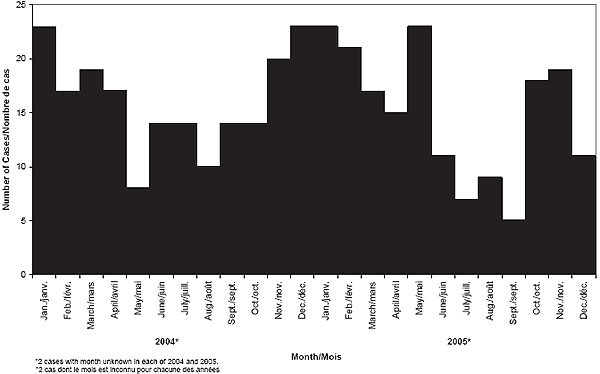
The occurrence of IMD cases in 2004 showed typical seasonal variation, with peaks occurring in the winter months, November to April. Interestingly, in 2005, IMD cases extended beyond the typical peak seen in the winter months, with a large number of cases occurring in May. Figure 4 shows the seasonal distribution of IMD cases.
Figure 4. Distribution of IMD cases by month, 2004-2005
Case fatality and mortality
IMD is associated with a relatively high case fatality. CFRs were 8.7% and 5.5% in 2004 and 2005, respectively (Tables 1 and 2). The median age of fatal cases was 46 years in 2004 and 28 years in 2005, both of which were higher than the median age of all cases in the respective years. In 2004, adults 65 years and older had the highest CFR, at 21.7%, followed by children aged 10 to 14 years, at 16.7%; note that the latter CFR was as a result of one death in that age range, demonstrating the instability of these CFRs. In 2005, adults 65 years and older had the highest CFR, at 13.3%, followed by those 20 to 24 years, at 8.3%.
Rates of mortality from IMD were 0.05 and 0.03 per 100,000 population in 2004 and 2005, respectively. In 2004, adults 65 years and older had the highest mortality rate, of 0.12 per 100,000 population. In 2005, persons 15 to 19 years and 20 to 24 years had the highest mortality rates, of 0.09 per 100,000 population. Case fatality and mortality rates could be unstable because of small numbers.
Serogroups
Serogroup information was available for 98% of confirmed cases in both 2004 (191/195) and 2005 (177/181). In both years, the incidence was highest for serogroup B disease (0.27 and 0.30 per 100,000 population, respectively). In 2004, incidence rates of serogroup C, Y and W135 were 0.18, 0.08 and 0.04 per 100,000 population, respectively. In 2005, these incidence rates were 0.12, 0.07 and 0.05 per 100,000 population, respectively. Figure 5 shows the annual incidence of serogroups B, C, Y and W135 cases by year since 1993.
In 2004, the median ages of IMD cases caused by serogroups B, C and Y were 19, 23 and 49 years, respectively. The median ages were statistically different for cases caused by serogroups B and Y (p = 0.002), and C and Y (p = 0.008) but not for B and C. In 2005, the median ages of cases caused by serogroups B, C and Y were 15, 22 and 22 years, respectively. The median ages were statistically different for serogroups B and C (p = 0.001) and B and Y (p = 0.009).
Although children under 5 years of age account for 5.3% of the total population, this age group accounts for the greatest proportion (27.6%) of serogroup B cases. Young children have the highest average annual incidence (per 100,000 population) of serogroup B disease, with rates of 3.26 and 3.55 for infants under 1 year of age and 0.95 and 1.17 for children 1 to 4 years of age in 2004 and 2005, respectively.
The highest annual incidence rates (per 100,000 population) for serogroup C disease in 2004 and 2005 were among infants under 1 year of age (1.19 and 0.30, respectively) and adults 20 to 24 years old (0.45 and 0.45, respectively). For serogroup Y disease, the highest annual incidence rates (per 100,000 population) in 2004 were among children 1 to 4 years of age and adults 65 years and older (0.22 and 0.17, respectively), whereas in 2005 annual rates were highest among adolescents 15 to 19 years old and adults 20 to 24 years of age (0.33 and 0.18, respectively).
Serogroup C had the highest CFR in both years, followed by serogroup B. In 2004, the CFR for serogroups C and B were 19.6 % and 4.6%, and in 2005 10.5% and 6.3%, respectively. There were no deaths reported among serogroup Y cases in 2004 or 2005.
Serotypes and serosubtypes
In 2004, 25% of the 72 serogroup B cases with serotype information were serotype 17, 22% were non-serotypable, and 21% were serotype 4. The most common serotype and subtype combinations for serogroup B cases were B:17:P1.19 (18/72) and B:14:P1.14 (7/72). Eighty percent of the 46 serogroup C cases with serotype information were serotype 2a, and 15% were non-serotypable. The most common combinations for serogroup C cases were C:2a:P1.5 (17/46) and C:2a:P1.1,7 (13/46). Forty-six percent of the 24 serogroup Y cases with serotype information were serotype 14, and 29% were non-serotypable. The most common serogroup Y combinations were Y:NT:P1.5 (5/24) and Y:14:P1. (5/24).
In 2005, 26% of the 81 serogroup B cases with serotype information were non-serotypable, 22% were serotype 17, and 22% were serotype 4. The most common serotype and subtype combinations for serogroup B cases were B:17:P1.19 (18/81) and B:4:P1.4 (9/81). Thirty-six of the 38 serogroup C cases (95%) had serotype information. Of these, 83% were serotype 2a, and 8% were non-serotypable. The most common combinations for serogroup C cases were C:2a:P1.5 (11/36) and C:2a:P1.1,7 (10/36). Forty-eight percent of the 21 serogroup Y cases with serotype information were serotype 14, and 33% were serotype 2c. The most common serogroup Y combinations were Y:14:P1.2,5 (6/21) and Y:2c:P1.2,5 (4/21).
Multilocus enzyme electrophoretic typing
The NML conducts multilocus enzyme electrophoretic (MLEE) typing on serogroup C isolates received from the P/T. In 2004, 70% of serogroup C isolates (39/56) from IMD cases available for MLEE typing were found to belong to the hypervirulent clone of ET-37, all of these being the ET-15 variant. Twenty-five percent of C isolates (14/56) did not have ET profile data, and 5% (3/56) belonged to neither the ET-15 nor other members of the ET-37 clonal complex. In 2005, 68% of serogroup C strains (26/38) from IMD cases available for MLEE typing were found to belong to the hypervirulent clone of ET-37, 92% of these (24/26) being the ET-15 variant. Twenty-nine percent (11/38) did not have ET profile data.
Outbreaks
All 13 P/T responded to e-mail surveys regarding IMD outbreaks. Survey results indicated that there were two IMD outbreaks in Canada between 1 January, 2004, and 31 December, 2005. Both were community-based outbreaks involving serogroup C meningococci (serosubtype 2a:P1.5).
An outbreak in British Columbia among men who have sex with men (MSM) began in September 2004. There were 9 confirmed cases of meningococcal C (5 deaths) and 1 confirmed case of non-typable IMD (0 deaths). All cases had lived close to, or visited, Vancouver Lower Mainland. In response to the outbreak, approximately 8,000 doses of meningococcal C conjugate vaccine were distributed in an immunization campaign targeting all gay and bisexual men in the province. The outbreak was declared over in August 2005 (Dr. D. Patrick, British Columbia Centre for Disease Control, Vancouver: personal communication, December 2006).
The serogroup C outbreak in Moncton, New Brunswick, occurred between March and May 2005. The outbreak affected persons between 10 and 19 years of age, and there were 2 confirmed cases (1 death). As part of the prevention and control strategy, a meningococcal vaccination program was targeted for persons from grade 5 to 19 years of age. A total of 15,993 doses of meningococcal C conjugate vaccine were distributed (X.Teng, New Brunswick Department of Health, Fredericton: personal communication, December 2006).
Discussion
Polymerase chain reaction
Increasingly, polymerase chain reaction (PCR) technology is being used as an alternative laboratory method of detecting N. meningitidis. Although some P/T have been using PCR techniques for diagnosing IMD, cases diagnosed using only this method did not meet the case definition in 2004 and 2005, and were therefore not included in the national numbers. The national case definition underwent revision in 2005 and now includes PCR testing. The case definition, effective 1 January 2006(8), is as follows:
Confirmed case: invasive disease* with laboratory confirmation of infection:
- isolation of Neisseria meningitidis from a normally sterile site (blood, CSF, joint, pleural or pericardial fluid) or
- demonstration of N. meningitidis DNA by appropriately validated nucleic acid test (NAT†) from a normally sterile site.
Probable case: invasive disease* with purpura fulminans or petechiae and no other apparent cause:
- with demonstration of N. meningitidis antigen in the CSF or
- in the absence of isolation of N. meningitidis or demonstration of DNA by appropriately validated NAT† from a normally sterile site.
*Invasive meningococcal disease usually manifests itself as meningitis and/or septicemia, although other manifestations may be observed. Invasive disease may progress rapidly to purpura fulminans, shock and death. †Each jurisdiction will have a validation process for the NAT that they have in place.
Serogroups
As seen in Figure 5, there has been much fluctuation in serogroup C disease over time, the most recent peak occurring between 2000 and 2001. There has been less fluctuation in serogroup B disease, which tends to predominate in non-outbreak years. Serogroup B disease has been the most common serogroup in Canada since 2002. The incidence of serogroup Y remained relatively stable in Canada between 1995 and 2005; neither serogroup A nor W135 disease is commonly reported in Canada.
Figure 5. IMD Incidence, Serogroups B, C, Y, W 135, 1993-2005, Canada
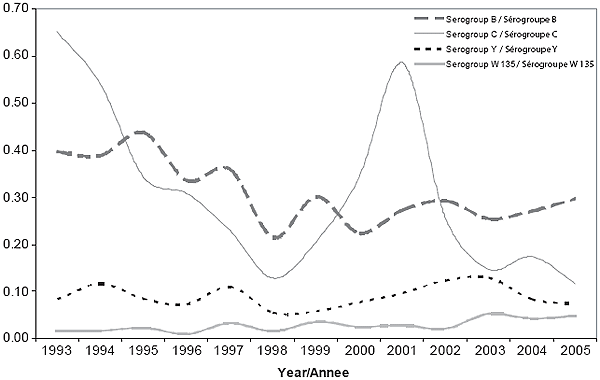
Incidence rates of serogroup B disease observed in 2004 and 2005 were comparable to the mean annual incidence rate for 1993 to 2003 (0.32 per 100,000 population). The annual median age of serogroup B cases since 2001 has been in the adolescent range, which is higher than that reported for 1999 (4 years of age) and 2000 (5 years)4,5. Despite this, the age-specific incidence is still highest among infants. Serogroup B disease was genetically heterogenous in most of the country during 2004 and 2005. However, a cluster of disease caused by a clone of ST-269 serogroup B meningococci (serotype 17:P1.19) was identified in a region of Quebec between October 2004 and March 2005. Retrospective analysis showed that the clone first emerged in the province in 20039. In 2004-2005, it was the most common circulating serogroup strain in Quebec but during the surveillance period was not detected in other P/T. Compared with all other serogroup B cases, a smaller proportion of cases with this clone were less than 5 years of age (32.7% and 11.1%, respectively; p = 0.01). There were no other significant differences in sex distribution or outcome between cases due to this clone and all other serogroup B cases.
Serogroup C disease had the second highest incidence rate, with outbreaks due to the strain C:2a:P1.5 reported in two provinces during 2004 and 2005. The strain associated with the outbreak among MSM in British Columbia was found to have the same pulsed-field gel electrophoresis pattern as the strain that caused an outbreak among adolescents in Abbottsford, BC, in 200110.
The incidence of serogroup Y disease was slightly lower in 2004 and 2005 than in 2001, 2002 and 2003 (0.10, 0.12 and 0.13 per 100,000 population, respectively). This suggests that the upward trend in serogroup Y disease reported previously, particularly in Ontario11, may have been due to the cyclical nature of the disease rather than a shift in disease epidemiology; however, continued monitoring is necessary.
The proportion of reported cases for which serogroup data are available has risen over time, from 60% in 1985 to 88% in 1992 and 98% in 2005. There were 4 non-groupable cases in 2004 and 1 non-groupable case in 2005. One of these patients, a healthy 13-year-old girl from British Columbia, was recently described by Hoang et al 12. Conventional serogrouping methods yielded negative results, but additional molecular methods confirmed the identity of the bacteria as an acapsular strain of N. meningitidis containing the capsular null locus (cnl strain, serotype 15, ST-198). Unencapsulated strains of N. meningitidis are generally associated with a carrier state. This was the first reported case of fatal meningococcal disease caused by an acapsular cnl strain isolated from an immunocompetent host.
Immunization programs
Meningococcal C conjugate (Men-C C) vaccine was approved for use in Canada in 2001. The National Advisory Committee on Immunization (NACI) recommends the vaccine for all children under 5 years, adolescents and young adults13. Routine Men-C C programs at various ages have now been implemented in all jurisdictions in Canada: 2002 (2 jurisdictions), 2003 (3), 2004 (5), 2005 (2) and 2007 (1)14.
The National Goals and Recommendations Consensus Conference for Vaccine-Preventable Diseases in Canada took place in Québec City, 12-14 June, 2005. Participants included federal and P/T representatives, international experts and representatives of key non-government groups. Disease-specific working groups reviewed current evidence, identified key issues and developed national goals and recommendations for disease reduction and immunization coverage targets, which were voted on in a plenary session. The proposed goal for invasive meningococcal disease is to reduce illness and death due to serogroup C disease through immunization. The proposed recommendations include the following:
- Prevent N. meningitidis serogroup C outbreaks in those under 25 years by 2015.
- Achieve a sustained reduction of 90% in the incidence of N. meningitidis serogroup C in children under 5 years of age by 2010.
- Achieve a sustained reduction of 95% in the incidence of N. meningitidis serogroup C in adolescents 12 to 19 years of age by 2010.
- Achieve a sustained reduction of 70% in the incidence of N. meningitidis serogroup C by 2010.
- Achieve and maintain age-appropriate immunization coverage with meningococcal C conjugate vaccine in 100% of N. meningitidis serogroup C close contacts of cases by 2010.
- Achieve and maintain age-appropriate immunization coverage with meningococcal C conjugate vaccine in 95% of high-risk groups for N. meningitidis serogroup C disease.
- Achieve and maintain age-appropriate immunization coverage with meningococcal C conjugate vaccine in 97% of children by their 2nd birthday by 2010.
- Achieve and maintain age-appropriate immunization coverage with meningococcal C conjugate vaccine in 90% of adolescents by their 17th birthday by 2012.
The mean incidence of serogroup C disease during the baseline period (1995-2001) and the immunization program implementation phase (2002-2005) was 0.31 per 100,000 and 0.18 per 100,000, respectively, representing a 43% decline in incidence between the two periods. These initial results suggest that the national recommendation to achieve a sustained reduction in the incidence of serogroup C disease by 2010 may be achievable with full implementation of routine Men-C C vaccination programs in Canada. However, it is not known whether this reduction is due to the universal programs, mass campaigns implemented during an outbreak, or the cyclical nature of IMD; further years of enhanced surveillance data are needed.
Limitations
Reporting was not complete for some data elements, including date of birth (40% missing), geolocator (6% missing) and clinical diagnosis (68% missing). Thirty-two percent of cases were missing immunization status information; among cases for which immunization status was reported, these data were not captured consistently (e.g. yes/no; complete/incomplete for age). Complete and consistent reporting of data elements is important for monitoring changes in disease epidemiology.
The scope of underreporting is unknown. However, 2% of confirmed IMD cases were reported by the NML and could not be matched to epidemiologic reports from the P/T, presenting a minimum estimate of the magnitude of underreporting. The epidemiologic information on these cases was either taken from the laboratory requisition or is missing. Beginning 1 January, 2006, probable cases are nationally notifiable8. Inclusion of probable cases will increase the sensitivity of enhanced IMD surveillance.
Because of the instability of results based on small numbers, caution should be used when interpreting these results.
Acknowledgements
We thank our colleagues from the provincial and territorial ministries of health for providing epidemiologic data and the public health and hospital laboratories from across Canada for submitting isolates to the NML for further studies. We also thank Dennis Law, Jan Stoltz and Averil Henderson for their technical expertise in conducting laboratory testing of isolates submitted to the NML.
References
Public Health Agency of Canada. Notifiable diseases on-line. <http://dsol-smed.phac-aspc.gc.ca/dsol-smed/ndis/index_e.html>.
Deeks S, Kertesz D, Ryan A et al. Surveillance of inva- sive meningococcal disease in Canada, 1995-1996. CCDR 1997;23(16):121-25.
Squires SG, Pelletier L, Mungai M et al. Invasive meningococcal disease in Canada, 1 January, 1997, to 31 December, 1998. CCDR 2000;26(21):177-82.
Squires SG, Deeks SL, Tsang RSW. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 1999, through 31 December, 2001. CCDR 2004;30(3):17-28.
Watkins KW, Deeks SL, Medaglia A, Tsang RSW. Enhanced surveillance of invasive meningococcal disease in Canada: 1 January, 2002, through 31 December, 2003. CCDR 2006;32(8):97-107.
Public Health Agency of Canada. Case definitions for diseases under national surveillance. CCDR 2000;26(S3):49.
Statistics Canada. Population estimates, 2004 and 2005 revised post-censal. Ottawa: Demography Division, Statistics Canada.
Public Health Agency of Canada. Guidelines for the prevention and control of meningococcal disease. CCDR 2005;31(S1):1-20.
Law DKS, Lorange M, Ringuette L et al. Invasive meningococcal disease in Québec Canada, due to an emerging clone of ST-269 serogroup B meningococci with serotype antigen and serosubtype antigen P1.19 (B:17:P1.19). J Clin Microbiol 2006;44:2743-49.
David ST, Gilbert M, Patrick DM et al. Outbreak of Neisseria meningitidis serogroup C in men who have sex with men, British Columbia. Can J Infect Dis Med Microbiol 2005;16:124 [P1.15].
Tsang RSW, Squires SG, Zollinger WD et al. Distribution of serogroups of Neisseria meningitidis and antigenic characterization of serogroup Y meningococci in Canada, January 1, 1999 to June 30, 2001. Can J Infect Dis 2002;13:391-96.
Hoang LMN, Thomas E, Tyler S et al. Rapid and fatal meningococcal disease due to a strain of Neisseria meningitidis containing the capsule null locus. Clin Infect Dis 2005;40:e38-42.
Meningococcal vaccine. In: National Advisory Committee on Immunization. Canadian immunization guide. 7th ed. Ottawa: Public Health Agency of Canada, 2006:237-50. Catalogue No. HP40-3/2006E.
Public Health Agency of Canada. Provincial and territorial immunization programs. <http://www. phac-aspc.gc.ca/im/ptimprog-progimpt/index-eng.php>.
Source: C Navarro, MSc, SL Deeks, MD, MHSc, A Medaglia, Immunization and Respiratory Infections Division, Centre for Infectious Disease Prevention and Control, Public Health Agency of Canada, Ottawa, Ontario; RSW Tsang, MMedSc, PhD, CNS Infection Division, National Microbiology Laboratory, Public Health Agency of Canada, Winnipeg, Manitoba.
Page details
- Date modified:
