CATMAT statement: Meningococcal disease and international travel

 Download this article as a PDF (873 KB - 8 pages)
Download this article as a PDF (873 KB - 8 pages) Published by: The Public Health Agency of Canada
Issue: Volume 41-5: Visiting friends and relatives
Date published: May 7, 2015
ISSN: 1481-8531
Submit a manuscript
About CCDR
Browse
Volume 41-5, May 7, 2015: Visiting friends and relatives
Advisory committee statements
Statement on Meningococcal Disease and the International Traveller
McCarthy A1 on behalf of the Committee to Advise on Tropical Medicine and Travel (CATMAT)*
Affiliation
1 The Ottawa Hospital General Campus, Ottawa, Ontario
Correspondence
DOI
https://doi.org/10.14745/ccdr.v41i05a02
Abstract
Background: Meningococcal meningitis occurs globally and the predominant serogroups vary by geographic region. Vaccines against serogroups A, B, C, Y and W-135 are available in Canada.
Objective: To provide guidance to health care professionals for the prevention of invasive meningococcal disease in international travellers from Canada.
Methods: This Statement was developed by the Committee to Advise on Tropical Medicine and Travel (CATMAT) to compliment the Canadian Immunization Guide. It considers the need for protection and the potential for adverse effects of vaccination.
Results: Meningococcal vaccine recommendations vary by traveller characteristics and travel destination. Meningococcal meningitis occurs globally and the predominant serogroup varies by geographic region. Areas of particular risk are the “meningitis belt” in Sub-Saharan Africa, Saudi Arabia during the Hajj and Umrah pilgrimages and places with current epidemics or heightened disease activity. For healthy travellers see the Canadian Immunization Guide. Quadrivalent vaccine should be given to individuals at increased risk for invasive meningococcal disease due to medical conditions with booster doses every five years. Meningococcal B vaccine should be considered.
Conclusion: Vaccination is the most effective measure for preventing invasive meningococcal disease. The Government of Canada’s travel health notices identify areas of new and recent meningococcal activity and are updated regularly.
Preamble
The Committee to Advise on Tropical Medicine and Travel (CATMAT) provides the Public Health Agency of Canada with ongoing and timely medical, scientific, and public health advice relating to tropical infectious disease and health risks associated with international travel. The Agency acknowledges that the advice and recommendations set out in this statement are based upon the best current available scientific knowledge and medical practices and is disseminating this document for information purposes to both travellers and the medical community caring for travellers.
Persons administering or using drugs, vaccines, or other products should also be aware of the contents of the product monograph(s) or other similarly approved standards or instructions for use. Recommendations for use and other information set out herein may differ from that set out in the product monograph(s) or other similarly approved standards or instructions for use by the licensed manufacturer(s). Manufacturers have sought approval and provided evidence as to the safety and efficacy of their products only when used in accordance with the product monographs or other similarly approved standards or instructions for use.
Introduction
The goal of this Statement is to provide guidance to health care professionals for the prevention of meningococcal disease in international travellers from Canada.
Methods
This Statement was developed by a working group of the Committee to Advise on Tropical Medicine and Travel (CATMAT). It was developed to compliment a thorough literature review and analysis which was conducted as part of the development of the National Advisory Committee on Immunization’s (NACI) recommendations in the Canadian Immunization GuideFootnote 1. CATMAT has considered the need for protection and the potential for adverse effects of vaccination. The Statement represents a narrative review of the travel medicine literature on meningococcal vaccines and CATMAT’s expert opinion. The recommendations do not include a description of the strength of the recommendation or grade of the quality of evidence as has been done in previous CATMAT statements. Each member of CATMAT is a volunteer and none declared a relevant conflict of interest.
Background
Meningococcal disease is caused by a Gram negative bacterium, Neisseria meningitidis. Neisseria are divided into 12 serogroups according to the immunological reactivity of their capsular polysaccharideFootnote 2. The five major serogroups most commonly associated with invasive disease are A, B, C, Y and W-135Footnote 2Footnote 3Footnote 4.
Person-to-person transmission occurs by close contact with respiratory secretions or saliva of infected personsFootnote 1Footnote 4. Humans are the only reservoirFootnote 5. Asymptomatic carriage occurs, and at any time, 5%–10% of the population may be carriers of N. meningitidisFootnote 1Footnote 3Footnote 4. Invasive disease is an infrequent consequence of nasopharyngeal colonizationFootnote 6.
Invasive meningococcal disease generally occurs one to fourteen days after exposure and usually presents as an acute febrile illness with rapid onset and features of meningitis or septicemia (meningococcemia), or both and a characteristic non-blanching petechial or purpuric rash. Symptoms of meningococcal meningitis include intense headache, fever, nausea, vomiting, photophobia and stiff neck. Meningococcemia often involves hypotension, acute renal failure, hemorrhage and multi organ failureFootnote 1Footnote 4. Fatality rates are approximately five to ten percent even with prompt antimicrobial treatment in healthcare facilitiesFootnote 5. Up to one-third of survivors may have long-term sequelae including hearing loss, neurologic disabilities and digit or limb amputationsFootnote 1Footnote 2Footnote 4.
Epidemiology
N. meningitidis is found worldwide. In most countries, N. meningitidis is recognized as a leading cause of meningitis and fulminant septicemia and is a significant public health problem. However, population-based surveillance with laboratory confirmation and strain characterization is still not attainable in many countries throughout the worldFootnote 7. Surveillance data from many countries are incomplete or lacking and there is currently no reliable global burden estimateFootnote 8.
Meningococcal meningitis occurs globally and the predominant serogroup varies by geographic region. In Australia, New Zealand and Europe, serogroup B predominates, followed by serogroup CFootnote 7Footnote 9. Serogroups B and C predominate in the United States and Canada, followed by serogroup YFootnote 7Footnote 9. The serogroup distribution across Latin America, South America and the Caribbean varies, with serogroups B and C predominating in some countries and W-135 and Y in othersFootnote 9. Little is known about the epidemiology of meningococcal disease in Asia and neighbouring areasFootnote 7Footnote 9.
Different patterns of invasive meningococcal disease are seen across AfricaFootnote 7. This is the case in the African meningitis belt, a region in sub-Saharan Africa extending from Senegal in the west to Ethiopia in the East (Figure 1), with a population of approximately 400 millionFootnote 10. During the dry season (approximately December through June), the incidence rate of meningococcal disease can reach as high as 1,000 cases per 100,000 population. In non-epidemic periods, the rate of meningococcal disease in this region is roughly five to ten cases per 100,000 populationFootnote 4Footnote 8. Risk is highest in travellers to the meningitis belt who have prolonged contact with local populations during an epidemic.
Because of the crowded conditions of the Hajj and Umrah pilgrimage to Saudi Arabia and high carrier rates of N. meningitidis among pilgrims, outbreaks of meningococcal disease have historically been a problemFootnote 4Footnote 11. The Hajj pilgrimages in 2000 and 2001, for example, were associated with large outbreaks of serogroup W-135 in returning pilgrims and their contacts.
Although people of any age can develop disease, endemic disease occurs most often in children and adolescents, whereas in meningococcal epidemics, rates may rise in older children and young adultsFootnote 1Footnote 8.
Figure 1: Map ofAfrican Meningitis Belt Figure 1 footnote 1
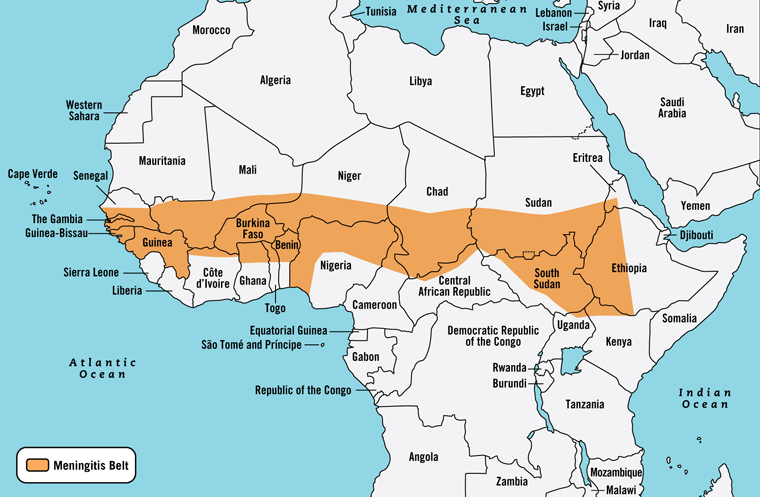
Text description: Figure 1
Figure 1: Map ofAfrican Meningitis Belt Figure 1 footnote 1
This is a map of Africa showing that the Meningitis Belt extends from west to east in mid-Africa. Starting in the west, it begins in the southern half of Senegal, includes all of the Gambia, Guinea Bissau and Guinea and then extends in a band across southern Mali, Niger, Chad, Sudan, and includes the western tip of Eritrea. The Meningitis Belt includes all of Burkina Faso and the northern parts of Cote d’Ivoire, Ghana, Togo, Benin, Nigeria (with the western portion extending to the coast), Central African Republic, most of South Sudan, and the northern tip of Kenya. The Meningitis Belt ends in East Africa with the western half of Ethiopia.
Prevention
Vaccination is the most effective measure for preventing invasive meningococcal disease. Vaccines for serogroups A, B, C, Y and W-135 are available in Canada. A comprehensive description of vaccines for serogroups A, C, Y and W-135 and associated recommendations is available in the most recent version of the Canadian Immunization GuideFootnote 1. A comprehensive description of the vaccine for serogroup B can be found in the NACI Statement on the Advice for the use of 4CMenB VaccineFootnote 9.
Vaccine recommendations for Canadian travellers
Meningococcal vaccine recommendations vary by characteristics of the traveller (e.g., age, medical conditions, etc.) and the travel destination. Specific recommendations for travellers from Canada are outlined below. For healthy travellers to a destination where risk of meningococcal transmission is high, please see Table 1 in Part 4 Active Vaccines: Meningococcal Vaccine: Meningococcal Vaccine of the Canadian Immunization Guide for the vaccination schedulesFootnote 1.
Routine vaccination programs with conjugate serogroup C vaccine have been implemented in every Canadian province and territoryFootnote 1. Independent of travel plans, the traveller should be up-to-date for age in accordance with their provincial/territorial immunization scheduleFootnote 12.
Travellers with underlying medical conditions
Vaccination is recommended for children and adults at increased risk of invasive meningococcal disease regardless of destination (Table 1). Refer to the Canadian Immunization Guide for detailed information on recommended products, scheduling and dosageFootnote 1.
| Individuals at increased risk for IMD due to medical conditions | Vaccine recommendations | |
|---|---|---|
| Serogroups A, C, Y, W-135 | Serogroup B | |
Persons with:
|
Individuals between two months to less than two years of ageFootnote 1 Men-C-ACYW-CRM (Menveo™) should be preferentially used. For infants two months to less than one year of age, a schedule using two or three doses with each dose given eight weeks apart with another dose at 12 to 23 months of age is recommended (and at least eight weeks from the previous dose). For children one to less than two years of age, a two-dose series is recommended with each dose given at least eight weeks apart. |
Individuals between two months and 17 years of age Multicomponent meningococcal serogroup B (4CMenB) vaccine should be considered. For infants two to five months, three doses given with an interval of at least one month between doses is recommended. A fourth dose (a booster) is recommended between 12 and 23 months of age. For infants six to 11 months, the first two doses should be separated by an interval of two months and a third dose is recommended between 12 and 23 months of age, no less than two months after the second dose. For children one to ten years, two doses separated by a two month interval is recommended. For individuals 11 to 17 years, two doses given at least one month apart is recommended. |
| Individuals two years of age and olderTable 1 footnote 2 Any of the quadrivalent conjugate vaccines (Men-C- ACYW-135-CRM, Menveo™; Men-C-ACYW-135-D, Menactra®; or Men-C-ACYW-135-TT, Nimenrix™) can be given as a two-dose series at least eight weeks apart. The use of polysaccharide meningococcal vaccine is not routinely recommended in Canada. Conjugate vaccines possess significant advantages over polysaccharide vaccines including better immune memory, longer duration of efficacy, lack of hyporesponsiveness with booster doses and possible reduction of bacterial carriage ratesFootnote 4Footnote 5. |
Individuals 18 to 55 years of ageTable 1 footnote 3 Although the manufacturer of the vaccine approved for use in Canada currently does not provide an adult schedule, in clinical trials of individuals from 18 to 55 years of age, two doses given at least one month apart have shown to be immunogenic and safe. |
|
| Booster dosesTable 1 footnote 4: Booster doses should be given every five years after the last dose. |
Booster doses: Because of unknown duration of protection after immunization, the need for a booster dose is yet to be determined. |
|
Travellers to the Hajj and Umrah pilgrimages
In the aftermath of two large invasive meningococcal disease (IMD) outbreaks during the 2000 and 2001 pilgrimages, the Kingdom of Saudi Arabia Ministry of Health implemented a requirement for all pilgrims to receive meningococcal vaccine. A certificate of vaccination against meningococcal meningitis is required for all visitors arriving for the purpose of Hajj or Umrah. Hajj or Umrah visas cannot be issued without a valid proof of vaccination.
- Adults and children aged two years and older must be vaccinated with quadrivalent meningococcal vaccine (serogroups A, C, Y and W-135)Footnote 13.
- Children between three months and two years of age must be vaccinated with two doses of meningococcal A vaccine, with a three-month interval between the two dosesFootnote 13.
- The vaccination must have been received not more than three years and not less than ten days before arrival in Saudi ArabiaFootnote 13.
In general, travellers to this region do not need to receive 4CMenB vaccine unless there is evidence of a hyperendemic strain or an outbreak that is known to be caused by serogroup B that can be prevented by the vaccine.
Refer to the recommendations in the Government of Canada’s travel health notices for pilgrims to the Hajj and Umrah posted each year in late summer to early fall for the most up-to-date information to ensure that individual travellers have appropriate vaccine documentationFootnote 14.
Travellers to sub-Saharan Africa
Quadrivalent meningococcal vaccine (serogroups A, C, Y, W-135) is recommended for travellers to the African meningitis belt (Figure 1) or countries outside the usual boundaries where epidemics have occurred, especially those who will be living or working there, or may be in close contact with the local population through school, accommodation, etc.
Immunization against serogroup C alone is not considered adequate for individuals travelling to this region. A single dose of any of the available quadrivalent conjugate meningococcal vaccines may be used for the immunization of individuals two years of age and olderFootnote 2. Men-C-ACYW-135-CRM (Menveo™) is recommended for immunization of individuals two months to less than two years of age. Refer to the Canadian Immunization Guide for detailed information and schedulesFootnote 1.
In general, travellers to this region do not need to receive 4CMenB vaccine unless there is evidence of a hyperendemic strain or an outbreak that is known to be caused by serogroup B that can be prevented by the vaccine.
Other considerations for travellers
- Travellers to areas with current epidemics or heightened disease activity should be vaccinated, regardless of duration of exposure. The Government of Canada’s travel health notices for recognized areas of new and recent meningococcal activity are released and updated regularly on travel.gc.caFootnote 14.
- IMD has historically occurred in schools, colleges and other places where large numbers of adolescents and young adults congregate. Individuals travelling to these settings may consider receipt of vaccine for serogroups B and A,C,Y, W-135 at least two weeks prior to arrivalFootnote 5.
- Individuals travelling to engage in research, industrial and/or clinical laboratory settings with the potential for routine exposure to N. meningitidis should be vaccinated with Men-C-ACYW-135 vaccine and 4CMenB vaccine. Re-vaccination of laboratory staff at ongoing risk of exposure should be done at routine five-year intervalsFootnote 1. Laboratory staff at ongoing risk of exposure should be re-vaccinated at routine five-year intervals with Men-C-ACYW-135 vaccineFootnote 1. Because of unknown duration of protection after immunization, the need for a booster dose of 4CMenB vaccine is yet to be determinedFootnote 9.
- There is no evidence to recommend routine meningococcal immunization of individuals travelling to work as health care providers; nosocomial transmission of IMD is very uncommonFootnote 1.
- Travellers to developed countries should follow the meningococcal immunization recommendations of the destination countryFootnote 15.
Additional vaccine characteristics and usage
Table 2 identifies additional vaccine information, such as common adverse reactions, contraindications, precautions and use in special populations. For complete and detailed information, refer to the Canadian Immunization GuideFootnote 1.
| Vaccine feature | Serogroup A, C, Y, W-135 vaccine | Serogroup B vaccine |
|---|---|---|
| Adverse reactions | Mild injection site (e.g., redness, tenderness and swelling) and systemic reactions (e.g., headache, malaise) have been reported. Serious adverse events are rare. | Among infants and children up to 12 months of age, the most commonly reported adverse reactions included erythema, induration, fever and sleepiness or irritability. A review of the evidence can be found in the NACI Statement on the Advice for the use of 4CMenB VaccineFootnote 9. |
| Contraindications and precautions | Contraindicated for individuals with a history of anaphylaxis after a previous dose of the vaccine or individuals with a proven anaphylactic reaction to any component of the vaccine or its containerFootnote 1. Administration of meningococcal vaccine should be postponed in individuals with moderate or severe acute illness; individuals with minor acute illnesses (with or without fever) may be vaccinatedFootnote 1. |
Contraindicated for individuals with a serious allergy to any vaccine component or previous doseFootnote 2. |
| Pregnancy and breastfeeding | Conjugate meningococcal vaccines have not been studied in pregnancy; however, the use of the vaccine may be considered, if indicatedFootnote 1Footnote 3. Inactivated vaccines, such as meningococcal vaccines, may be administered to women who are breastfeedingFootnote 1. | There are no studies of 4CMenB vaccine in pregnant or lactating women. |
| Immunocompromised travellers | Meningococcal vaccine is recommended for certain high-risk individuals as outlined above. When considering immunization of an immunocompromised person, consultation with the individual’s attending physician may be of assistance. For complex cases, referral to a physician with expertise in immunization and/or immunodeficiency is advised. | |
| Concurrent administration with other vaccines | Quadrivalent conjugate vaccines may be administered concomitantly with adolescent and adult age-appropriate vaccines at different injection sites using separate needles and syringesFootnote 1. | A review of the concomitant use of 4CMenB with other vaccines can be found in the NACI Statement on the Advice for the use of 4CMenB VaccineFootnote 9. |
| Interchangeability of vaccines | Any of the quadrivalent conjugate vaccines may be used for re-vaccination, regardless of which meningococcal vaccine was used for initial vaccinationFootnote 1. When possible, the infant series should be completed with the same vaccine. | Not applicable. |
General precautions
Travellers should be advised to practice good hand hygiene interventions and avoid activities that promote the exchange of respiratory secretions, such as sharing drinks, cigarettes, lipstick, etc. Avoid overcrowding in confined spaces. Following close contact with an individual infected with meningococcal disease, medical advice should be sought regarding possible chemoprophylaxis and vaccinationFootnote 5.
Conclusion
Meningococcal vaccination is the most effective measure for preventing IMD. The Government of Canada’s travel health notices identify areas of new and recent meningococcal activity and are updated regularlyFootnote 14.
Acknowledgements
This Statement was developed by the Meningococcal Working Group: McCarthy A (Chair), Bryson M, Bui Y, Cutler J, and Geduld J.
CATMAT members: McCarthy A (Chair), Boggild A, Brophy J, Bui Y, Crockett M, Greenaway C, Libman M, Teitelbaum P, Vaughan S.
Liaison members: Gershman M (US Centers for Disease Control and Prevention) and Pernica J (Association of Medical Microbiology and Infectious Disease Canada).
Ex-officio members: Marion D (Canadian Forces Health Services Centre, Department of National Defence), McDonald P (Division of Anti-Infective Drugs, Health Canada), Schofield S (Directorate of Force Health Protection, Department of National Defence) and Tepper M (Directorate of Force Health Protection, Department of National Defence).
Conflict of interest
None
Funding
This work was supported by the Public Health Agency of Canada.